Abstract
Atopic dermatitis (AD) is a chronic inflammatory skin disease, accompanied by itching and swelling. The main pathological mechanism of AD is related to the imbalance between Type 2 helper cells (Th2 cells) and Type 1 helper cells (Th1 cells). Currently, no safe and effective means to treat and prevent AD are available; moreover, some treatments have side effects. Probiotics, such as some strains of Lactobacillus, can address these concerns via various pathways: i) facilitating high patient compliance; ii) regulating Th1/Th2 balance, increasing IL-10 secretion, and reducing inflammatory cytokines; iii) accelerating the maturation of the immune system, maintaining intestinal homeostasis, and improving gut microbiota; and iv) improving the symptoms of AD. This review describes the treatment and prevention of AD using 13 species of Lactobacillus. AD is commonly observed in children. Therefore, the review includes a higher proportion of studies on AD in children and fewer in adolescents and adults. However, there are also some strains that do not improve the symptoms of AD and even worsen allergies in children. In addition, a subset of the genus Lactobacillus that can prevent and relieve AD has been identified in vitro. Therefore, future studies should include more in vivo studies and randomized controlled clinical trials. Given the advantages and disadvantages mentioned above, further research in this area is urgently required.
Keywords: atopic dermatitis, Lactobacillus, type 2 helper cells, gut microbiota, immunomodulation
1. Introduction
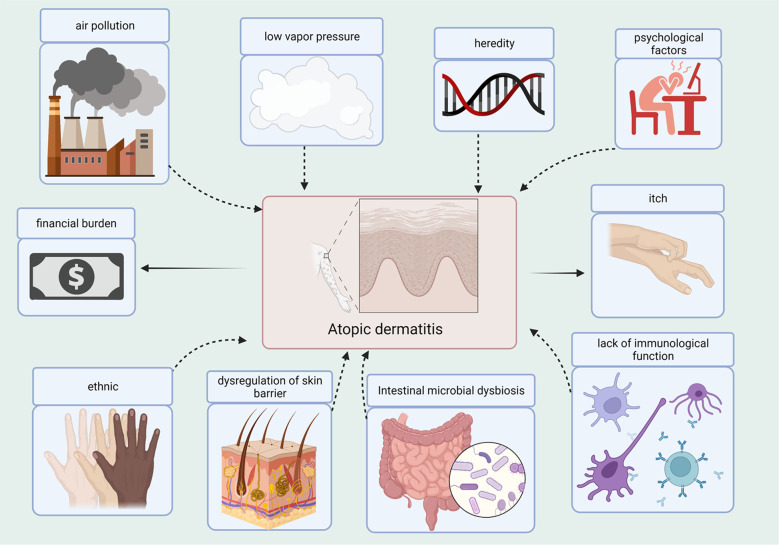
Atopic dermatitis (AD) is a chronic inflammatory skin disease, and patients frequently experience complications from concurrent allergic conditions. The annual incidence of AD has increased in the recent years, particularly in children. Based on the Finnish national database, the prevalence of AD varies by age group, with the highest prevalence in the age group 0-14 years (47.46%), followed by that in 15-60 year olds (43.74%) (Salava et al., 2022). The actual prevalence of AD between 6 months and 12 years has been reported at 11% in Israel (Weil et al., 2022). One report showed that the incidence of AD in infants aged 3 months in China was 40.81% (Guo et al., 2019). Patient quality of life can be severely affected, as AD causes scratching, itching, and a rash. The financial burden on households increases with disease severity (Weil et al., 2022). The incidence of AD is strongly correlated with heredity and environment ( Figure 1 ). In other words, people with AD often have a family history of AD, and the incidence of AD may increase when the father or mother has a particular allergy. Although the exact mechanisms of AD have not yet been elucidated, impaired immunological function and dysregulation of the skin barrier are considered to be the primary pathogenic mechanisms (Yang et al., 2020). Concurrently, environmental factors such as poor eating habits, sudden lifestyle changes, and certain allergenic stimuli are also associated with AD. A climatic conditions closely associated with an elevated risk of AD is low vapor pressure (Yokomichi et al., 2022). In Chongqing, China, infants exposed to polluted air are at an increased risk of developing AD (Luo et al., 2022). Psychological factors also play an important role in AD development; subsequently, AD leads to fluctuating depressive symptoms (Chatrath et al., 2022). As a result, individuals with AD can be caught in a vicious cycle.
Figure 1.
Causes and Consequences of the AD. Several factors contribute to the development of AD, such as air pollution, low vapor pressure, heredity, psychological factors, ethnicity, dysregulation of the skin barrier, dysbiosis of gut microbes, and lack of immune function. AD contributes to the itchiness and financial burden.
Although the pathogenesis of AD is not clear, decades of research indicate that the mechanisms of AD can potentially be attributed to genetic factors, environmental exposures, impaired skin barrier, abnormal immune function, and microbial imbalances. This suggests that AD is a systemic organ-related allergic disease. One study demonstrated an increased probability of early AD in maternal offspring due to dysregulated interferon signaling cascade (Schedel et al., 2023). Patients with high-risk genes tend to develop AD earlier (Hikino et al., 2022). A notable manifestation of AD is pruritus, which causes patients to scratch vigorously (Yosipovitch et al., 2020; Steinhoff et al., 2022). After skin rupture, the epidermal barrier is damaged, and antigens penetrate the skin, producing chemokines and inflammatory mediators (IL-25, IL-33, and thymic stromal lymphopoietin). Th cells polarize to Th2 and produce IL-4, IL-13, and IL-5 (Cosmi et al., 2019; Renert-Yuval and Guttman-Yassky, 2020). Th1, Th17, Th22, and other pathways are activated; a variety of cytokines and growth factors involved in inflammatory immune responses through the Janus kinase (JAK) pathway are produced and enhance Th2 cell differentiation (Bao et al., 2013; Rerknimitr et al., 2017). Scratching coupled with endogenous and other exogenous triggers, such as histamine, proteases, substance P, various interleukins, and environmental allergens leads to keratinocyte activation, and intensified skin inflammation. Inflammatory mediators and multi-pathway inflammation cause intensive scratching and further damage to the skin barrier. Moreover, bacteria take advantage of the situation and the vicious circle continues. Under physiological conditions, the skin microenvironment maintains immune homeostasis and reduces skin colonization by pathogenic bacteria. Diversity of the gut microbiome is significantly lower in infants with AD (Abrahamsson et al., 2012; Penders et al., 2013). The presence of Staphylococcus aureus was detected on the skin of 90% of patients with AD, and this pathogen can lead to disease progression (Powers et al., 2015; Blicharz et al., 2019). Staphylococcus aureus secretes staphylococcal enterotoxins A, B, and C and toxic shock syndrome toxin 1 (TSST-1), which activate lymphocytes and macrophages (Nakatsuji and Gallo, 2019). In addition, staphylococcal enterotoxin B promotes the expression of IL-31. IL-31 inhibits the expression of polyfilament proteins and antimicrobial peptides, which favor Staphylococcus aureus. Importantly, IL-31 is a key factor in itching (Meng et al., 2018). Previous studies showed that mediators produced by Staphylococcus aureus promotes adhesion, colonization, and spread to the skin. These mechanisms are complex and interact. In future, scientists may also identify new mechanisms that are yet to be discovered.
The gut plays an important role in the immune response. At the same time, Natural killer (NK) T cells, innate lymphoid cells, and intestinal flora regulate each other to maintain intestinal homeostasis and normal immune function (Cairo and Webb, 2022). In addition, healthy gut flora has a protective effect against food allergies (Méndez et al., 2021). Disturbances of the intestinal microbiome in early infancy worsen immune dysfunction in children with AD (Lee et al., 2022). In addition, a study showed that transplanting fecal microbiota to restore gut ecology provides a new method for treating AD (Kim et al., 2021). Lower microbial diversity has been associated with a higher incidence of AD (Galazzo et al., 2020). Moreover, greater severity of clinical manifestations in patients with AD has been associated with a lower number of Bifidobacteria in the intestine (Watanabe et al., 2003). Conversely, higher amounts of pathogenic Clostridium difficile have been detected in the stool of patients with AD (Penders et al., 2007). In one region of Brazil, children with AD have a higher prevalence of Clostridium difficile and a lower abundance of Lactobacillus (Melli et al., 2020). Clostridium difficile causes a decrease in beneficial bacteria, loss of immune function, and increased intestinal permeability. Studies have shown that colonization of the gut flora precedes AD changes (Galazzo et al., 2020); therefore, a timely intervention in the gut microbiota could be a promising preventive approach. Diet has an important impact on the colonization of gut microbes in early infancy (Galazzo et al., 2020). In early infancy, microbes are primarily affected by type of delivery; however, food becomes an important factor starting at 13 weeks (Galazzo et al., 2020). Food allergies and AD are closely related, and approximately one-third of children have both AD and food allergies (Hui-Beckman et al., 2023). Food-induced AD most likely occurs in children with severe AD (Robison and Singh, 2019). Food allergies increase the permeability of the intestine, making it easier for allergens to trigger the submucosal immune system through the intestinal barrier (Lee et al., 2013; Gao et al., 2021). Cytokines and inflammatory mediators produced after the activation of the immune system further increase intestinal permeability. The interaction between the gut microbiota and skin has been called the gut-skin axis by some authors (Mahmud et al., 2022; Varela-Trinidad et al., 2022). Healthy gut flora is beneficial for healthy skin.
Various treatments have been used in AD, including topical glucocorticoids and immunosuppressive agents, phototherapy, and narrow-spectrum ultraviolet radiation B. AD is a chronic inflammatory disease that affects patients with impaired skin barrier function. The long-term administration of topical corticosteroids carries a high risk. Topical corticosteroids are the mainstream treatment for moderate to severe AD; however, they have side effects, such as hormonal dermatitis, when used in large, long-term doses in combination with potent hormonal creams. In addition, prolonged use of topical corticosteroids may cause serious side effects, such as adrenal insufficiency (Böckle et al., 2014). Additionally, patients hesitate to use glucocorticoids (Maghen et al., 2019). Immunosuppressant treatment for AD may cause conjunctivitis (Wollenberg et al., 2018) or lymphopenia (Bakker et al., 2018) in some patients. Narrow-spectrum ultraviolet radiation B has a relieving effect on AD (Ben Mordehai et al., 2022); however, long-term use may cause abnormal skin reactions. Moreover, some biological drugs are expensive and have side effects (Puar et al., 2021). Patients receiving dupilumab treatment were reported to suffer from eye discomfort (Miniotti et al., 2022). Owing to the above reasons, safe and effective treatments for AD remain limited. Recently, treatment with probiotics has been proposed to regulate the gut microbiome in AD. The gut microbiome plays an essential role in the maintenance of host homeostasis and immunomodulation. Imbalances in microbial flora can contribute to many diseases. Intestinal microbial dysbiosis is the leading cause of AD-like symptoms (Kim et al., 2020a). Oral administration of L. sakei proBio65 (Rather et al., 2021) and L. salivarius LS01 can improve the quality of life of children (Niccoli et al., 2014) and adults (Drago et al., 2011) with AD. In an experimental model, maternal mice and their offspring orally supplemented with L. reuteri Fn041 maintained the balance of the immune response to prevent AD (Qi et al., 2022; Zhao et al., 2022; Zhou et al., 2022).
Lactobacillus is a naturally occurring rod-shaped bacterium that is a part of the normal flora in some organs of humans, animals, and plants. The storage conditions for Lactobacillus are simple. L. sakei proBio65 live and inactivated bacteria can improve AD symptoms and enhance the function of skin barrier (Jeong et al., 2020a; Rather et al., 2021). Administration L. paracasei KBL382 alleviated AD by modulating immune responses (Kim et al., 2020a). L. paracasei KBL382 reduced serum levels of immunoglobulin E (IgE) and immune cell infiltration (Kim et al., 2020a). Moreover, supplementation with L. rhamnosus HN001 substantially reduced the cumulative prevalence of AD (Wickens et al., 2008). These findings suggest that Lactobacillus may provide an alternative strategy for the treatment and prevention of AD. In this review, we focus on the role of Lactobacillus as a novel therapeutic agent for AD.
2. Classification of Lactobacillus and its mechanism of prevention and treatment of AD
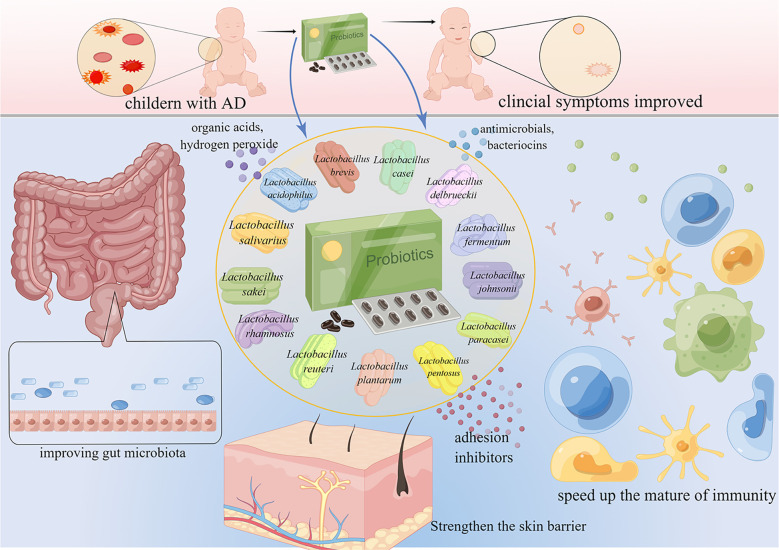
Probiotics are living microorganisms such as Lactobacillus spp., Bifidobacterium spp., Enterococcus spp., Streptococcus spp., Propionibacterium spp., Bacillus cereus spp., and Saccharomyces boulardii that benefit the host. Lactobacillus spp. is the most widely used probiotic microorganism. The genus Lactobacillus includes more than 200 species (Sun et al., 2015), and can be subdivided into at least 24 phylogenetic groups (Zheng et al., 2015). Several Lactobacillus species have been studied for AD prevention and treatment ( Figure 2 ), including L. rhamnosus, L. plantarum, L. acidophilus, L. sakei, L. reuteri, L. salivarius, L. paracasei, L. casei, L. delbrueckii, L. fermentum, L. johnsonii, L. pentosus, and L. brevis. These Lactobacillus species have been reported to produce a variety of substances, such as organic acids, hydrogen peroxide, low-molecular-weight antimicrobials, bacteriocins, and adhesion inhibitors. Lactobacillus products stimulate innate immunity and promote balanced microbial communities through the competitive rejection and antimicrobial activity against pathogenic bacteria (Goldstein et al., 2015). Administration of Lactobacillus decreased the serum levels of IgE (Sunada et al., 2008; Wakabayashi et al., 2008; Tanaka et al., 2009; Prakoeswa et al., 2017), and achieved a balance of Th1/Th2 (Wickens et al., 2008; Kwon et al., 2018; Kim et al., 2019a; Zhao et al., 2022). Moreover, the intestinal barrier (Wickens et al., 2008; Kim et al., 2020a; Zhao et al., 2022), immune function (Wickens et al., 2008; Kim et al., 2019a; Kim et al., 2020a) and skin barrier (Mariman et al., 2016) have been improved after the administration of Lactobacillus. The mechanisms of the 13 kinds of Lactobacillus are listed in Table 1 . Lactobacillus shows certain effects on both animals and humans with AD ( Tables 2 , 3 ). Lactobacillus has high economic value in biotechnology, food production, and therapeutic applications.
Figure 2.
Lactobacillus for the treatment and prevention of AD. AD is common in children. Lactobacillus accelerates the maturation of the immune system, maintains intestinal homeostasis, improves the gut microbiome, and ultimately improves the symptoms of AD.
Table 1.
Mechanism of action of Lactobacillus in the treatment of AD.
| Probiotic | strain | Mechanism of action | Reference |
|---|---|---|---|
| L. rhamnosus | RHT3201 | decrease of eosinophil cationic protein, eosinophil count, IL-31, and serum IgE concentration | (Lee et al., 2016; Jeong et al., 2020a) |
| HN001 | enhanced gut barrier function, influence on the immune system and a more balanced Th1/Th2 immune profile | (Wickens et al., 2008) | |
| LGG | decreased IL-10 protection against pathogenic macromolecules in the gut and accelerated immunological maturation, regulation of the immune response, stimulation of peripheral blood cells to secrete IL-10 and transforming growth factor β |
(Kalliomäki et al., 2003; Sawada et al., 2007; Boyle et al., 2008; Marsella, 2009; Nermes et al., 2011; Marsella et al., 2012; Carucci et al., 2022) | |
| CGMCC 1.3724 (LPR) | reduction of plasma total IgE, upregulation of IFN-γ production at the skin level | (Tanaka et al., 2009) | |
| L. acidophilus | L-92 | activation of regulatory T cells and Th1 cells, decrease of eosinophil count and increase of change ratio for serum TGF-β, prevention of IgE-mediated hypersensitivity, modulation of Th1/Th2 balance | (Shah et al., 2010; Torii et al., 2011; Inoue et al., 2014; Yamamoto et al., 2016) |
| L-55 | decrease of serum total IgE level | (Sunada et al., 2008) | |
| LAVRI-A1 | increase of allergen sensitization in infants | (Taylor et al., 2007) | |
| L. plantarum | MG4221 | inhibition of inflammatory and allergic reactions and regulation of the NF-κB/MAPK pathways in keratinocytes | (Hong et al., 2021a) |
| LM1004 | decrease of mRNA levels of Th2 and Th17 cell transcription factors, increase of transcription factors of Th1 and Treg cells, galactin-9, filaggrin, enhanced immunomodulation | (Kim et al., 2019a) | |
| CJLP133 | upregulation of total IgE levels, increased TGF-β expression, increased proportion of regulatory T cells at baseline | (Kim et al., 2017) | |
| IS-10506 | decreased levels of serum IgE, IL-4, and IL-17, increased the levels of Foxp3+ to IL-10 ratio, downregulate Th2 adaptive immune response | (Prakoeswa et al., 2017) | |
| NCIMB8826 | amelioration of skin pathology, improvement of skin barrier integrity, skin thickening, and diminished excoriations | (Mariman et al., 2016) | |
| L. sakei | ProBio65 | increased expression of Foxp3+ transcription factor, Modulation of the expression levels of inflammatory cytokines IL-10 and IL-12, inhibition of mast cell activation, improvement of allergen-induced skin inflammation, downregulation of IgE and IL-4 production | (Park et al., 2008; Kim et al., 2013; Rather et al., 2021) |
| KCTC 10755BP | decreased chemokine levels | (Woo et al., 2010) | |
| WIKIM30 | modulation of allergic Th2 responses, enhanced Treg generation and increased relative abundance of intestinal bacteria that are positively related to Treg generation | (Kwon et al., 2018) | |
| L. reuteri | Fn041 | regulation of systemic Th1 and Th2 cytokine ratios and promotion of CD4+CD25+Foxp3+ regulatory T cell proliferation in mesenteric lymph nodes, regulation of intestinal microbiota | (Zhao et al., 2022) |
| JCM 1112 | dependent on the presence of Toll-like receptor 2 and the induction of TNF-α-induced protein 3 and cylindromatosis in HaCaT cells | (Kawahara et al., 2018) | |
| ATCC55730 | modulation of the in vivo the cytokine pattern at an extra-intestinal site | (Miniello et al., 2010) | |
| L. salivarius | LS01 | Initiation of intestinal immunity, rebalancing of the changed intestinal microbiota, modulation of Thl/Th2 cytokine profiles | (Drago et al., 2011; Drago et al., 2012; Niccoli et al., 2014) |
| PM-A0006 | immune-modulating effects | (Wu et al., 2012) | |
| L. paracasei | CBAL74 | steroid sparing effect | (D'Auria et al., 2021) |
| KBL382 | increased immunosuppressive response and modified metabolic functions of gut microbiota | (Kim et al., 2020a) | |
| WK3001 | diminished mast cell infiltration and plasma IgE levels, suppression of immediate hypersensitivity reactions and IL-4 mRNA expression in the auricles | (Wakabayashi et al., 2008) | |
| L. casei | KCTC 12398BP | isolation of P14 protein decreased the levels of IL-4 in RAW264.7 and Balb/c splenocytes in vitro and ex vivo | (Kim et al., 2015b) |
| DN-114001 | effect on gut microbiota | (Klewicka et al., 2011) | |
| JCM 1134T | modulation of the serum levels of IgE and cytokines and eosinophil count | (Ogawa et al., 2006) | |
| L. delbrueckii | subsp. Bulgaricus LB-2 | influence on both Th1 and Th2 through induction of Treg cells and secretion of IL-10 | (Sheikhi et al., 2017) |
| OLL1073R-1 | attenuation of IL-6 secretion from lymph node cells and reduced IL-6 levels in inflamed tissues, such as auricles | (Kano et al., 2013) | |
| R-037 | induce IL-12 and Th1 | (Watanabe et al., 2009) | |
| L. fermentum | VRI 003 PCC™ | increased TNF-α responses to both heat-killed Staphylococcus aureus and heat-killed Lactobacillus | (Prescott et al., 2005) |
| L. johnsonii | NCC533 | reduction of the gene expression of proinflammatory cytokines (IL-8, IL-12 and IL-23) and CD86 | (Inoue et al., 2007) |
| L. brevis | NS1401 | restoration of the Th1/Th2 balance through enhancing Th1-mediated immunity | (Choi et al., 2017) |
| SBC8803 | increased IgE production, increased production of immunosuppressive cytokines such as IL-10 and TGF-b1 | (Segawa et al., 2008a) |
Table 2.
Effects of Lactobacillus on the clinical manifestations of AD.
| Probiotic | strain | Participants | Interventions | Outcome | Reference |
|---|---|---|---|---|---|
| L. rhamnosus | RHT3201 | 100 children (aged 1–12 years) with moderate AD | 1.0 × 1010 CFU (no details were provided) | decreased SCORAD total score and levels of eosinophil cationic protein | (Jeong et al., 2020a) |
| HN001 | mothers from 14–16 weeks gestation | 6×109 CFU/d, from 14-16 weeks gestation until 6 months post-partum if breastfeeding | no significant differences | (Wickens et al., 2018) | |
| High risk infants | 6×109 CFU/d, 9×109 CFU/d, indirectly from 35 weeks gestation until 6 months after birth if breastfeeding, and directly from birth until 2 years | decreased cumulative prevalence of eczema, SCORAD score, and skin prick tests sensitization | (Wickens et al., 2013) | ||
| 425 children | 6 ×109 CFU, 9 ×109 CFU/d, from 35 weeks gestation until birth, continued to 6 months after birth in mothers if breastfeeding, and from birth until 2 years in all infants | reduced cumulative prevalence of eczema at 4 years | (Wickens et al., 2012) | ||
| Pregnant women, 474 infants at risk of allergic disease | 6×109 CFU/d, pregnant women from 35 weeks gestation until 6 months if breastfeeding, infants receive the same treatment from birth to 2 years | reduced risk of eczema | (Wickens et al., 2008) | ||
| LGG | 250 pregnant women carrying infants at high risk of allergic disease | 1.8×1010 CFU/d, from 36 weeks gestation until delivery | no significant differences | (Boyle et al., 2011) | |
| 100 AD patients (aged 6– 36 months) | 1×1010 CFU/d, for 12 weeks | reduced SCORAD scores | (Carucci et al., 2022) | ||
| 159 mothers, 132 children at high risk | 1×1010 CFU/d, for 4 weeks | improved AD | (Kalliomäki et al., 2003) | ||
| 39 infants with AD | 3.4×109 CFU/d | increased proportions of CD19+ CD27+ B cells and fewer infection | (Nermes et al., 2011) | ||
| 131 children (6–24 months old) | 1010 CFU, 6 months | no significant differences | (Rose et al., 2010) | ||
| 35 infants (aged less than 1 year) with atopic eczema | 4 weeks | no significant differences | (Salmi et al., 2010) | ||
| 11 adults and 73 infants | 1.8×1010 CFU/d, for 7 days. 1.8×1010 CFU/d, from 36 weeks gestation until delivery | no significant differences | (Boyle et al., 2008) | ||
| 105 pregnant women | 5×109 CFU, twice daily | no significant differences | (Kopp et al., 2008) | ||
| infants (aged 3–12 months) with mild-to-moderate AD | 5×109 CFU, for 12 weeks | no significant differences | (Grüber et al., 2007) | ||
| 54 infants (aged 1–55 months) with moderate to severe AD | 10×109 CFU/d, for 8 weeks | no significant differences | (Fölster-Holst et al., 2006) | ||
| L. acidophilus | L-92 | 57patients with AD(≥ 16 years) | 20.7 mg/d, for 24 weeks | decreased investigator global assessment, eczema area and severity index, and AD score | (Yamamoto et al., 2016) |
| 49 AD patients (aged ≥ 16 years) | 20.7 mg/d, for 8 weeks | decreased SCORAD scores and eosinophil count | (Inoue et al., 2014) | ||
| 20 children (age 4–15 years) | 3×1010 CFU/d, for 8 consecutive weeks | ameliorated symptoms of AD in Japanese children and effect on serum concentrations of thymus | (Torii et al., 2011) | ||
| LAVRI-A1 | 153 children | 3×109/d, from birth to 6 months. | no significant differences | (Prescott et al., 2008) | |
| 231 pregnant atopic women and babies | 3×109/d, for the first 6 months of life | no significant differences | (Taylor et al., 2007) | ||
| 178 children | 3 × 109 CFU/d, for 6 months | no significant differences | (Jensen et al., 2012) | ||
| L. plantarum | CJLP133 | 76 children (median age of 7.1 years) with moderate-to-severe AD | 1×1010 CFU/d, for 12 weeks | increased proportion of Treg cells with concurrent decrease in TGF-β mRNA expression | (Kim et al., 2017) |
| children (aged 12 months to 13 years) | 0.5×1010 CFU, twice a day for 12 weeks | lower SCORAD score at week 14 in the probiotic group than that in the placebo group; higher SCORAD score in the probiotic group from weeks 2 to 14 than that in the placebo group; L. plantarum CJLP133 significantly decreased total eosinophil count, logarithmic IFN-γ and IL-4 | (Han et al., 2012) | ||
| IS-10506 | 22 children with mild and moderate AD | 1010 CFU, twice daily for 12 weeks | decreased SCORAD and levels of IL-4, IFN-γ, and IL-17; upregulation of Foxp3+ to IL-10 ratio | (Prakoeswa et al., 2017) | |
| L. sakei | ProBio65 | Children (aged 3–9 years) and adolescents (aged 10 to 18) with AD | 1 × 1010 cells/d per sachet (400 mg) | decreased SCORAD total score when compared with baseline and potential improvement of skin barrier functions | (Rather et al., 2021) |
| KCTC 10755BP | 88 children (aged 2–10 years) with AEDS | 5×109 CFU, twice daily for 12 weeks | decreased total SCORAD scores | (Woo et al., 2010) | |
| L. reuteri | ATCC55730 | patients (aged 4–10 years) | 108 CFU/d, for 8 weeks were prescribed as 1 tablet once per day (2 hours before meals) | no significant differences | (Miniello et al., 2010) |
| mothers and their babies | 1×108 CFU/d, from gestational week 36 until delivery | diminished IgE-associated eczema and skin prick test reactivity | (Abrahamsson et al., 2007) | ||
| L. salivarius | LS01 | patients (aged 25–63 years) | 5×109 CFU/d, for a month | decreased SCORAD index and the count of Staphylococcus Aureus. | (Drago et al., 2014) |
| 43 patients (aged 0–11 years) with AD | 1×109 CFU/sachet, 2 sachets/d, for 8 weeks, and 1 sachet/day for the following 8 weeks | improved SCORAD score and itching | (Niccoli et al., 2014) | ||
| 38 patients (aged 18–46 years) with moderate/severe AD | 1×109 CFU/g, twice daily, for 16 weeks | decreased SCORAD score | (Drago et al., 2012) | ||
| 38 patients (aged from 18 to 46 years) with moderate/severe AD | 1×109 CFU/g, twice daily, for 16 weeks | improved SCORAD score and dermatology life quality index | (Drago et al., 2011) | ||
| PM-A0006 | 60 children (aged 2–14 years) with moderate to severe AD | 2×109 CFU, twice daily, for 8 weeks | decreased SCORAD scores range 8 weeks and 10 weeks. L. salivarius PM-A0006 significantly reduced medication use frequency and eosinophil cationic protein levels at 8 weeks. Lactobacillus salivarius PM-A0006 reduced AD intensity | (Wu et al., 2012) | |
| L. paracasei | CBAL74 | 58 infants and young children with moderate to severe AD (aged 6–36 months) | 8 g/d for 12 weeks | decreased SCORAD index | (D'Auria et al., 2021) |
| GM-080 | infants with AD (aged 4–30 months) | 1× 1,010 equivalent CFU, for 16 weeks | decreased CCL17 levels and TEWL in lesions and unaffected skin | (Yan et al., 2019) | |
| K71 | 34 adults with AD | 100 mg/d (~2 × 1011 bacteria), over 12 weeks | decreased skin severity scores compared with baseline | (Moroi et al., 2011) | |
| L. casei | DN-114001 | 40 children (aged 6–18 months) with AD | 109 cells/d, for 3 months | decreased SCORE index | (Klewicka et al., 2011) |
| L. delbrueckii | subsp. Bulgaricus LB-2 | 20 children (age 1–12 years) with AD | co-cultured with different concentrations of UV killed bacteria in RPMI-1640 plus 10% FCS for 48/72 h | upregulated the secretion of IL-10, IL-12, and IFN-γ, and decreased secretion of IL-4. | (Sheikhi et al., 2017) |
| L. fermentum | VRI 003 PCC™ | 53 children with moderate or severe AD | 1×109 twice daily for 8 weeks | increased Th1-type cytokine IFN-γ responses to PHA and SEB | (Prescott et al., 2005) |
Table 3.
Effects of Lactobacillus on the experimental AD.
| Probiotic | strain | Experimental animal | Interventions | Outcome | Reference |
|---|---|---|---|---|---|
| L. rhamnosus | RHT3201 | six-week-old female NC/Nga mice | 1×108, 1×109, or 1×1010 cells/d, for 8 weeks. | improved dermatitis scores and frequency of scratching | (Lee et al., 2016) |
| LGG | two litters of Beagles (same sire and dam) with AD | 200 × 109 CFU/d, ten Culturelle® capsule; offspring from the second pregnancy, LGG, 100 × 109 CFU/d |
decreased allergen-specific IgE and partially prevented AD | (Marsella et al., 2012) | |
| 2 adult beagles with severe AD and 16 pups | One capsule containing a minimum of LGG 20×109 CFUs; first litter female dogs did not receive LGG. During the second pregnancy, a dosage of 10 capsules/d LGG from week 3 of gestation and continued throughout lactation. During the third pregnancy, 5 capsules/d from 3 weeks to 6 months of age |
decreased serum titer of allergen-specific IgE and moderated reaction to intradermal testing | (Marsella, 2009) | ||
| specific pathogen-free NC/Nga mice | 30–50 mg/d | increased plasma IL-10 levels and enhanced IL-10 mRNA expression in both Peyer’s patches and mesenteric lymph nodes | (Sawada et al., 2007) | ||
| CGMCC 1.3724 (LPR) | specific-pathogen free pregnant NC / NgaTnd mice, pups until 12 weeks of age | 5 ×108 CFU/ ml | decreased clinical symptoms of dermatitis, reduced scratching frequency | (Tanaka et al., 2009) | |
| L. acidophilus | L-92 | ICR mice BALB/c mice BALB/c and NC/Nga mice |
3 and 30 mg/kg | inhibited vascular permeability in both passive cutaneous anaphylaxis | (Shah et al., 2010) |
| L-55 | female NC/Nga mice (5 weeks old) with AD-like skin lesions | 1 and 10 mg/d, for 75 days | inhibited dermatitis score, ear swelling, scratching behavior | (Sunada et al., 2008) | |
| L. plantarum | MG4221 | NC/Nga mice (male, 4 weeks old) | a single dose (7 μg·cm−2) of 200 μL of PM2.5 (500 μg·mL−1) with 2% dinitrochlorobenzene another single dose (7 μg·cm−2) of 200 μL of PM2.5 (500 μg·mL−1) with 0.2% dinitrochlorobenzene |
decreased transepidermal water loss and erythema; decreased scratching behavior | (Hong et al., 2021a) |
| LM1004 | AD-induced rat (histamine-induced vasodilation) and mouse (pruritus and contact dermatitis) | 2 × 1012 cells, for 28 days | reduced vasodilation, pruritus, edema, and serum histamine | (Kim et al., 2019a) | |
| NCIMB8826 | APOC1+/+ mice | 3×108 CFU, three times a week, for 8 weeks | ameliorated skin pathology, improved skin barrier integrity, eliminated of skin thickening, and fewer excoriations | (Mariman et al., 2016) | |
| L. sakei | ProBio65 | dogs with CAD | 2 × 109 CFU/g, for 2 months | reduced disease severity index, CASESI score values | (Kim et al., 2015a) |
| 25 male 6-week-old NC/Nga mice | 5×109 CFU/ml, 200 μL/d, for 2 weeks | improved condition of skin and reduced scratching frequency | (Kim et al., 2013) | ||
| mice triggered by allergen | 1 ×108 CFU/mL, 200 μL/d, for 2 weeks | faster recovery of AD | (Park et al., 2008) | ||
| WIKIM30 | wild-type male BALB/c mice | 2 × 109 CFU bacteria, 200 μL/d | reduced AD-like skin lesions | (Kwon et al., 2018) | |
| L. reuteri | Fn041 | seven-week-old male and female BALB/C mice | 1×109 CFU/d, once a day, 100 μL each time, each time | suppressed AD symptoms such as skin swelling, mast cell and eosinophil infiltration | (Zhao et al., 2022) |
| Japan Collection of Microorganisms 1112 | specific pathogen-free male NC/Nga mice (aged 10 weeks) | 0.1% (w/v) Lactobacillus water extract (LW)-treated, and 1.0% (w/v) LW-treated; 0.1% LW in 80% ethanol, or 1.0% LW in 80% ethanol, twice weekly for one week |
suppressed the development of house dust mite-induced atopic skin lesions and thymus and activation-regulated chemokine expression | (Kawahara et al., 2018) | |
| L. paracasei | KBL382 | mice with Dermatophagoides farinae extract -induced AD | 1 × 109 CFU/d, for 4 weeks | reduced AD-associated skin lesions and epidermal thickening | (Kim et al., 2020a) |
| K71 | 41 dogs with mild to moderate cAD | 5 mg/kg, once daily, for 12 weeks | decreased CADESI, and pruritus scores; the reduced medication scores | (Ohshima-Terada et al., 2015) | |
| WK3001 | five-week-old male NC/Nga mice | basic diet at concentrations of 0.03% (low dose) or 0.3% (high dose) | reduced development of AD-like skin lesions | (Wakabayashi et al., 2008) | |
| L. casei | KCTC 12398BP | male NC/Nga mice | 1, 10, and 100 μg/mL of P14 0.1, 0.2, 1, 5, and 10 μg/mL of P14 |
downregulated AD index and scratching score in AD-like NC/Nga mice | (Kim et al., 2015b) |
| JCM 1134T | six-week-old male NC/Nga mice | NC/Nga mice were divided into four groups of six each and administered CD, DD (500 mg of dextran per day) LD (1×107 CFU of lyophilized L. casei subsp. casei per day) LDD (1×107 CFU of lyophilized L. casei subsp. casei and 500 mg of dextran per day) (control diet; CD) (dextran diet; DD) (L. casei subsp. casei diet; LD) (L. casei subsp. casei and dextran diet; LDD) |
decreased clinical skin severity scores and total IgE levels | (Ogawa et al., 2006) | |
| L. delbrueckii | OLL1073R-1 | specific-pathogen-free female NC/Nga mice and BALB/c mice, (4- or 5-wk-old). | bacterial, 1 mg/d | inhibited development of dermatitis and elevation of an acute inflammation marker, serum amyloid A | (Kano et al., 2013) |
| R-037 | female BALB/c mice (5-weeks-old) and male NC/Nga mice (7-weeks-old) | 5 g/d/mouse, from day 0 to day 55 | reduced inflammatory auricular thickness and alleviated the AD clinical score | (Watanabe et al., 2009) | |
| L. johnsonii | NCC533 | NC/NgaTnd mice | 4 weeks | suppressed exacerbation of the clinical severity of dermatitis and suppressed epidermal hyperplasia and infiltration of inflammatory cells in skin | (Tanaka et al., 2008) |
| pups of 4 pregnant NC/Nga mice. | 1010 cells, from 20 to 22 days of age via oral administration | enhanced gene expression of the proinflammatory cytokines [interleukin-8 (IL-8), IL-12 and IL-23] and decreased gene expression of CD86 | (Inoue et al., 2007) | ||
| L. brevis | NS1401 | female NC/Nga mice (24 Six-weeks old) | 5 × 108 CFU/d per mouse, for 8 weeks | reduced skin thickness and infiltration of mast cells and eosinophils in skin lesions and the size and number of immune cells in draining lymph nodes | (Choi et al., 2017) |
| SBC8803 | male 5-week-old NC/Nga mice | 0%, 0.05% or 0.5%, once a week for 9 weeks. | inhibited ear swelling, and suppressed the development of dermatitis. | (Segawa et al., 2008a) |
3. Monostrain Lactobacillus in the treatment and prevention of AD
3.1. Lactobacillus rhamnosus -effective prevention and treatment of AD in both animal and clinical experiments
L. rhamnosus is the most studied species of Lactobacillus (Petrova et al., 2021). The peptidoglycan of L. rhamnosus CRL1505 can regulate the immune function of human intestinal epithelial and dendritic cells (Salva et al., 2021). Early exposure to LGG in dogs with AD had long-term immune effects and significantly reduced allergen-specific IgE despite the lack of a clear clinical effect (Marsella, 2009; Marsella et al., 2012). In clinical trials, infants aged 0–2 years had a reduced incidence of atopic eczema when their mothers have been administered LGG during pregnancy (Kalliomäki et al., 2003). The preventive effect of LGG extended to 4 years (Kalliomäki et al., 2003). LGG may enhance the gut barrier function and promote immune response development in infants with AD (Nermes et al., 2011). In 2016, researchers observed that L. rhamnosus IDCC 3201 tyndallizate (RHT3201) had the potential to treat AD. The mast cell count and serum IgE concentration in axillary lymph node cells were decreased in RHT3201-fed NC/Nga mice compared with those in the control group (Lee et al., 2016). Another animal experiment concluded that heat-treated LGG could improve the symptoms of NC/Nga mice with AD (Sawada et al., 2007). In 2020, Jeong and colleagues (Jeong et al., 2020a) found that RHT3201 had a therapeutic effect on AD in children. L. rhamnosus GG (LGG) is a strain of L. rhamnosus that regulates gut flora and reduces conditional pathogenic bacteria (Chen et al., 2020b). When children with AD were supplemented with LGG, the clinical severity and quality of life improved (Carucci et al., 2022). Simultaneously, the use of topical steroids was reduced (Carucci et al., 2022).
L. rhamnosus decreases the concentrations of eosinophilic cationic protein, eosinophil count, IL-31 (Jeong et al., 2020a), and serum IgE (Tanaka et al., 2009; Lee et al., 2016); supports a better Th1/Th2 balance (Wickens et al., 2008); prevents pathogenesis caused by large molecules in the intestine; accelerates immune maturation (Nermes et al., 2011); stimulates peripheral blood cells to secrete IL-10 (Kalliomäki et al., 2003; Sawada et al., 2007); converts growth factor β (Kalliomäki et al., 2003), and upregulates the production of IFN-γ at the skin level (Tanaka et al., 2009). However, not all L. rhamnosus strains are equally effective. Wickens et al. (2012) studied the efficacy of L. rhamnosus HN001 and HN019 in atopic diseases and demonstrated that L. rhamnosus HN001 was more effective in improving AD than L. rhamnosus HN019 (Wickens et al., 2012). Moreover, the protective effect on eczema can persist two years when L. rhamnosus HN001 is administered to children with AD (Wickens et al., 2008). In addition, L. rhamnosus HN001 can prevent atopic sensitization in the long term (Wickens et al., 2013). Moreover, several studies have shown that the protective effect of LGG against AD requires further investigation. Two randomized controlled trials have shown that prenatal LGG treatment was not associated with a reduced risk of eczema (Boyle et al., 2008; Boyle et al., 2011). No clear causal relationship between the positive effects of LGG and infantile eczema has been reported (Fölster-Holst et al., 2006; Grüber et al., 2007; Kopp et al., 2008; Rose et al., 2010).
Some probiotics affect multiple immune pathways through different mechanisms and protect against the pathogenesis of eczema (Wickens et al., 2008). However, supplementation mothers with L. rhamnosus HN019, was not effective in preventing eczema in infants, indicating that L. rhamnosus HN019 is less likely to be passed on to infants through breast milk (indirect supplementation route) (Wickens et al., 2018). One of the reasons that prenatal LGG was not shown to prevent eczema in infants may be that the impact of prenatal probiotic therapy on fetal B cell development was not excluded (Boyle et al., 2008). Prenatal LGG intake may not directly contribute to the effects of postpartum breast milk regulation. It is possible that postpartum intake by nursing mothers can alter immunity and/or microbiota to benefit breast milk composition (Boyle et al., 2011). Probiotics are commonly added to dairy products (Grüber et al., 2007), which can also affect the efficacy of L. rhamnosus in AD. It is important to note that the number of participants who completed the study and the subgroups analyzed were insufficient. In addition, the complexity of human life makes it challenging to achieve homogeneity. In addition, eczema in early infancy can naturally improve, and more than 40% of patients with AD recover around the age of 3 years (Illi et al., 2004). Moreover, it is more difficult for clinical trials to recruit sufficient human participants than for animal studies to include a sufficient number of animals.
L. rhamnosus has been shown to be effective in the prevention and treatment of AD in both animal and clinical experiments. However, future research needs to continue to explore different strains of L. rhamnosus and use strains with excellent laboratory efficacy in clinical trials. Effective strains of L. rhamnosus can be passed on to the offspring from the mother, and subsequently, infants may potentially not develop AD.
3.2. Some strains of Lactobacillus acidophilus alleviate symptoms of AD and show good safety
L. acidophilus is a commercially significant probiotic isolated from the human gastrointestinal tract (Bull et al., 2013). Moreover, it is an important class of bioprotective agents (Anjum et al., 2014). Supplementation with L. acidophilus L-92 significantly reduced vascular permeability in diseased mice and attenuated the clinical symptoms of AD (Shah et al., 2010). The administration of L. acidophilus L-92 not only significantly attenuated AD symptoms in children (Torii et al., 2011), but also improved the symptoms in adults (Inoue et al., 2014). L. acidophilus triggers an anti-inflammatory response (Goh et al., 2021). L. acidophilus L-92 inhibited the inflammatory response dominated by Th2 cells by activating regulatory T (Treg) and Th1 cells (Yamamoto et al., 2016). L. acidophilus L-55 decreased the occurrence of anaphylactic dermatitis-like skin lesions in NC/Nga mice by decreasing serum total IgE levels (Sunada et al., 2008). However, the findings on several strains of L. acidophilus have been inconsistent. For example, there is no evidence that L. acidophilus NCFM can improve AD (Larsen et al., 2011). Supplementation with L. acidophilus LAVRI-A1 in children with allergies did not reduce the risk of dermatitis (Taylor et al., 2007; Prescott et al., 2008; Jensen et al., 2012), and the sensitization rate in the L. acidophilus LAVRI-A1 group was significantly higher than that in the control group (Taylor et al., 2007).
In early studies, L. acidophilus demonstrated good safety and efficacy in children with AD, and future studies in mothers are ongoing or are expected to begin soon. In addition, the time required to evaluate clinical outcomes is insufficient. The detailed interplay between the early microbial environment and the developing immune system remains largely unknown and requires further exploration. Overall, the findings suggest that not every strain of L. acidophilus has the potential to prevent or treat AD. The effect of L. acidophilus on the prevention of AD requires further study.
3.3. Lactobacillus plantarum regulates the host immune system to improve AD symptoms
L. plantarum is a rod-shaped lactic acid-producing bacterium that is used in probiotics and silage production. L. plantarum has the potential to be a highly effective immunomodulatory probiotic in the human gut microbiome. In recent years, an increasing number of studies have shown the health benefits of L. plantarum. (Seddik et al., 2017). The extract of fermented blueberry black rice containing L. plantarum MG4221 had an effect similar to that of dexamethasone, but with fewer side effects; oral administration of FBBBR in NC/Nga mice reduced skin dryness, erythema, and scratch behavior (Hong et al., 2021a). L. plantarum NCIMB8826 can soothe the skin of mice with AD, strengthen the skin barrier, and alleviate scratching (Mariman et al., 2016). Supplementation with L. plantarum CJLP133 and IS-10506 has been shown to be beneficial for treating AD in children (Han et al., 2012; Kim et al., 2017; Prakoeswa et al., 2017). In addition, L. plantarum IS-10506 has an immunomodulatory effect and can effectively relieve AD symptoms in adults (Prakoeswa et al., 2022).
L. plantarum BF_15 can successfully colonize murine intestines by rebalancing intestinal microbiota (Zhang et al., 2020). The extract of fermented blueberry black rice containing L. plantarum MG4221 inhibited the production of serum IgE and Th2 cell-related cytokines, suggesting that fermented blueberry black rice may be an essential functional food in AD (Hong et al., 2021a). Additionally, oral administration of L. plantarum NCIMB8826 reduced the number of mast cells in the colon. Finally, Staphylococcus aureus infection is the main reason for the exacerbation of AD-like symptoms, but L. plantarum can alleviate AD-like symptoms by inhibiting Staphylococcus aureus (Kim et al., 2020b). Additionally, lipoteichoic acids isolated from L. plantarum and Staphylococcus aureus have shown anti-AD effects. Lipoteichoic acid combination therapy can alleviate AD by reducing the formation of membrane attack complexes and inhibiting Th1 reactions (Kim et al., 2019c). Notably, L. plantarum LM1004 not only regulates the host immune system and gut microbiota, but is also promising for the treatment of AD and obesity in humans (Kim et al., 2019a). Collectively, the preliminary evidence suggests L. plantarum is a potential therapeutic strategy. Moreover, it has an acceptable safety profile in adults. L. plantarum improves the symptoms of AD, although it is ineffective in preventing AD. Future research should focus on the preventive effects of L. plantarum on AD. L. plantarum is a promising Lactobacillus strain that can be further explored to improve treatment options and efficacy.
3.4. Lactobacillus sakei has potential as a supplement for the treatment of AD due to its anti-inflammatory and skin barrier protective properties
L. sakei was isolated from fermented meat, fish, and kimchi. L. sakei KDP is a potent antioxidant and antibacterial agent (Ghoneum and Abdulmalek, 2021). L. sakei 07, combined with Bifidobacterium bifidum B10, regulates immunity and the gut microbiota (Wang et al., 2019). Oral administration of live and inactivate (Kim et al., 2013; Rather et al., 2021) L. sakei probio65 can increase skin sebum content and improve the function of the skin barrier (Rather et al., 2021). L. sakei probio65 inhibits AD-like skin lesions and may serve as an influential novel anti-inflammatory medication that resolves AD symptoms in mice (Kim et al., 2013). In experimental dogs (Kim et al., 2015a) and mice (Park et al., 2008) with AD, orally administered L. sakei probio65 significantly reduced the disease severity index without clear side effects. Supplemental treatment with L. sakei KCTC 10755BP has the potential to alleviate the clinical severity of AD syndrome in children (Woo et al., 2010).
L. sakei WIKIM30 was isolated from kimchi and can significantly reduce AD-like skin lesions, regulate allergic Th2 responses, increase the relative abundance of intestinal bacteria positively correlated with Treg production, and has potential in AD treatment (Kwon et al., 2018). Current research shows that L. sakei can be anti-inflammatory and can protect the skin barrier. L. sakei has the potential to be used as a therapeutic supplement in AD. L. sakei originates from fermented foods, and direct intake of fermented foods may have the same effect. As mentioned above, both live and inactivated L. sakei have been shown to be effective. Fermented foods such as kimchi are popular and readily available, and patients can effortlessly benefit and achieve improvement of AD. Future research can further apply L. sakei in clinical practice and investigate its preventive effect on AD. In addition, the therapeutic effects of other strains of L. sakei can also be explored.
3.5. Lactobacillus reuteri supplementation is effective in preventing AD
L. reuteri has been reported to occur naturally in the intestines of all vertebrates and mammals. L. reuteri induces neonatal IgA production (Mu et al., 2021). L. reuteri survives in the gastrointestinal tract of mammals and benefits host health (Engevik et al., 2021). L. reuteri NK33 can be used to improve gut dysbiosis (Han et al., 2020). L. reuteri strain, the Japan Collection of Microbiology 1112, significantly inhibited the expression of allergic lesions and thymus and activation-regulated chemokines at the site of lesions in NC/Nga mice (Kawahara et al., 2018). Prenatal and postnatal supplementation with L. reuteri Fn041 effectively prevented the fetus from developing AD, remodeled the intestinal ecology, and improved the immune function of Peyer’s patches (Qi et al., 2022; Zhou et al., 2022). L. reuteri Fn041 regulated the intestinal flora and significantly inhibited AD symptoms by regulating the systemic ratio of Th1 and Th2 cytokines in mice (Zhao et al., 2022). L. reuteri DYNDL22M62 attenuated AD symptoms by modulating gut bacteria in mice (Fang et al., 2022). Mothers and their babies supplied with L. reuteri ATCC 55730 had a lower prevalence of IgE-associated eczema at 2 years of age (Abrahamsson et al., 2007). However, in another clinical trial, L. reuteri ATCC 55730 did not improve clinical symptoms (Miniello et al., 2010). This may be because the small sample size. Alternatively, the duration of the experiment may have been too short. L. reuteri has been extracted from the gastrointestinal tract of all mammals, and future studies could explore the reasons for an absence of this L. and whether higher abundance of L. reuteri is beneficial. L. reuteri appears to have preventive and therapeutic effects in animals. Further clinical research on L. reuteri is required to explore the treatment and prevention of AD.
3.6. Lactobacillus salivarius actively improves the quality of life in children and adult patients with AD
L. salivarius is a Lactobacillus species that occurs in the human gastrointestinal tract and oral mucosa. It produces bacteriocins, and is used as a probiotic. It modifies the gastrointestinal system to alleviate intestinal diseases and promote host health (Neville and O'Toole, 2010). L. salivarius is valuable for both animals and humans. L. salivarius can reduce pathogen colonization of the gastrointestinal tract of animals (Hong et al., 2021b). Administration of L. salivarius can prevent and treat a variety of chronic diseases in humans (Chaves et al., 2017), including AD, asthma, cancer, and bad breath (Drago et al., 2014). The combined administration of L. salivarius PM-A0006 and fructooligosaccharides exhibited a notable anti-AD effect compared with either therapy alone in the treatment of children with moderate-to-severe AD (Wu et al., 2012). L. salivarius LS01 can help manage AD in children and improve their quality of life; moreover, partial effect remains after termination of medication (Niccoli et al., 2014). L. salivarius LS01 actively improves the quality of life in adult patients with AD by regulating the balance of Th1/Th2 (Drago et al., 2011; Drago et al., 2012; Kim et al., 2014). Additionally, L. salivarius has shown efficacy in improving AD in existing studies. As the name suggests, L. salivarius is present in the oral cavity. The study of the oral environment is of great significance for increasing L. salivarius. The preventive effect of L. salivarius on AD requires further study. However, the long-term safety and persistence of L. salivarius remains to be studied. More potent subspecies of L. salivarius are yet to be discovered.
3.7. Lactobacillus paracasei has anti-inflammatory properties that can reduce the development of AD-like skin lesions
L. paracasei originates from the healthy human gastrointestinal tract and is widely distributed in food. L. paracasei has anti-inflammatory properties that can reduce antigenic pro-inflammatory responses. L. paracasei improves immune function by enhancing NK cell function and IFN-γ concentrations (Lee et al., 2017). In addition to a preventive effect on AD-like skin changes in mice L. paracasei KW3110 demonstrated inhibitory effects even when supplementation was started after symptoms have appeared (Wakabayashi et al., 2008). Supplementation with L. paracasei KW3110 can significantly reduce the development of AD-like skin lesions in mice, while regulating immunity (Wakabayashi et al., 2008). Oral supplementation with L. paracasei K71 can be used to treat dogs with AD (Ohshima-Terada et al., 2015). A diet with added K71 can be used as a complementary therapy for adult AD patients (Moroi et al., 2011). Moreover, L. paracasei NL41 reduced inflammation by improving the intestinal environment and maintaining intestinal integrity in rats (Zeng et al., 2021). Daily oral administration of L. plantarum HEAL9 and L. paracasei 8700 has been shown to regulate the peripheral immune response in children with celiac disease autoimmunity (Håkansson et al., 2019). L. paracasei KBL382 can significantly reduce AD-related lesions and epidermal thickening in mice by modulating the immune response and changing intestinal microbiota composition(Kim et al., 2020a).
Additionally, heat-killed L. paracasei CBA L74 has a minimal effect on steroid use, but its effect on reducing the severity of AD needs to be further studied (D'Auria et al., 2021). However, there is no evidence that L. paracasei GM-080 has a retention effect equivalent to that of glucocorticoids (Yan et al., 2019). This may be due to the inappropriate selection of L. paracasei strains, timing of administration, timing of exposure, and failure to achieve appropriate dosing levels. In conclusion, L. paracasei enhances NK cell function and IFN-γ concentrations to regulate immune mechanisms and achieve anti-inflammatory effects. Concurrently, L. paracasei can improve the intestinal environment and maintain intestinal homeostasis to achieve anti-inflammatory effects. The duration of L. paracasei administration does not require special emphasis, and intervention in patients with AD before and after the appearance of symptoms can achieve the desired effect. Hence, these studies might help clarify whether L. paracasei can improve intestinal barrier function and maintain immune system balance. Oral administration of L. paracasei can prevent and treat AD. However, more clinical experiments are needed to explore prevention of AD with the administration of L. paracasei.
3.8. Lactobacillus casei treats AD by balancing the gut microbiota and immune responses
L. casei is found in many fermented foods and coexists with gut microbiota. It is involved in housekeeping functions, metabolism, cell wall biogenesis, and environmental adaptation (Licandro-Seraut et al., 2014). L. casei CCFM1074 can balance gut microbiota and immune responses (Fan et al., 2021). L. casei regulates the host immune response (Aktas et al., 2016) and can be used to treat AD. During the Japanese cedar pollen season, NC/Nga mice orally administered L. casei Japan Collection of Microorganisms (JCM) 1134T combined with dextran experienced a possible effect on the prevention and treatment of allergic reactions (Ogawa et al., 2006). Furthermore, researchers screened an active ingredient, protein P14, from the L. casei extract, which specifically lowered IgE and IL-4 levels in AD-like NC/Nga mice, suggesting potential therapeutic effects in AD (Kim et al., 2015b). Similarly, the administration of L. casei DN–114001 to the diet of children with AD was beneficial to the increase in the count of intestinal flora and its maintenance for five months after the cessation of probiotics (Klewicka et al., 2011). L. casei DN—114001 can improve clinical symptoms in children with AD long term (Klewicka et al., 2011). Overall, these studies provide a good foundation for developing future therapeutic or preventive approaches using L. casei in individuals with AD. L. casei has an extended effect after stopping supplementation; therefore, the effect of permanent colonization of the intestine after regular supplementation should be studied. Few studies have been conducted on L. casei for the treatment of AD. More subspecies of L. casei are yet to be identified.
3.9. Lactobacillus delbrueckii alleviates AD by maintaining and improving intestinal barrier function by stimulating immune cells to reduce inflammatory responses
L. delbrueckii is one of the most economically valuable fermented Lactobacillus species. L. delbrueckii maintains and improves the intestinal barrier function by stimulating immune cells (Kobayashi et al., 2019). L. delbrueckii also improved intestinal integrity and immune responses in piglets (Chen et al., 2020a). L. delbrueckii subsp. bulgaricus may be involved in regulation of immune factor secretion in patients with AD (Sheikhi et al., 2017). IL-6 is a leading cause of dermatitis; whereas oral administration with L. delbrueckii subspecies bulgaricus OLL1073R-1 attenuated dermatitis by inhibiting the IL-6 response and restoring the elevation of serum amyloid levels in the NC/Nga mouse model of AD (Kano et al., 2013). In addition, oral supplementation with heat-treated L. delbrueckii R-037 can inhibit the rise of serum total IgE in allergic model mice, decrease inflammation, and alleviate AD; however, its effect on serum total IgE levels needs to be further studied (Watanabe et al., 2009). Studies in animal (mouse) models and have shown that L. delbrueckii plays a role in the management of AD. Experimental samples are easier to obtain in animal experiments than in clinical studies. However, only results of clinical studies that show the efficacy of L. delbrueckii will enable its widespread use in the management of patients with AD. In future, it will be necessary to further investigate the use of L. delbrueckii in patients with AD, including factors such as the time of supplementation, dosage, and strain activity. Moreover, the understanding of the preventive function of L. delbrueckii requires further experiments in both animals and humans.
3.10. Lactobacillus fermentum regulates the immune response and benefits children with AD
L. fermentum is a gram-positive bacterium. It can improve the functionality and nutritional value of foods (Naghmouchi et al., 2020). L. fermentum can restore homeostasis of the intestinal microflora and regulate the immune response in mice (Rodríguez-Nogales et al., 2017). Mice were immunized with L. fermentum NWS29 and exposed to ovalbumin. L. fermentum NWS29 inhibited the expression of certain inflammatory factors to achieve an anti-inflammatory effect (Nawaz et al., 2015). Mice were inoculated with the Salmonella vaccine and L. fermentum PC2. When mice were challenged with live Salmonella typhimurium, L. fermentum enhanced mucosal and immune responses and played a protective role (Esvaran and Conway, 2012). L. fermentum KBL374 and KBL375 can modulate the innate immune response by improving intestinal barrier function and reducing leukocyte infiltration in mice (Jang et al., 2019). L. fermentum CJL-112 protected mice from the deadly influenza virus infection by stimulating macrophages, activating Th1 cells, and increasing immunoglobulin A production (Yeo et al., 2014). In a clinical trial, L. fermentum PCCTM strengthened Th1 IFN-γ responses and achieved clinical benefits in children with AD(Prescott et al., 2005).
L. fermentum MS15 inhibited exogenous IL-10 induced human β-defensin-2 and regulated the response to the inflammatory stimulus (Habil et al., 2014). In future, L. fermentum can be combined with other vaccines to enhance their protective effect (Esvaran and Conway, 2012). These different strains of L. fermentum have been shown to regulate immunity and may also have some effect in the prevention of AD. In future, more research is required to explore the potential of L. fermentum in the treatment of AD.
3.11. Lactobacillus johnsonii improves intestinal inflammation and alleviates the severity of AD
L. johnsonii is a probiotic that can be isolated from dairy products. Notably, L. johnsonii BS15 can regulate intestinal inflammation (Charlet et al., 2020; Xin et al., 2020a; Xin et al., 2020b; Wang et al., 2021). Oral administration of L. johnsonii NC553 can relieve the severity of AD and inhibit epidermal hyperplasia and infiltration of inflammatory cells into the skin (Inoue et al., 2007). Additionally, it relieves skin damage by inhibiting pro-inflammatory cytokines and CD86 (Inoue et al., 2007). Therefore, early administration of L. johnsonii NC553 in mice with allergies may help reduce AD exacerbations (Tanaka et al., 2008). L. johnsonii has been shown to improve intestinal inflammation in animal models. L. johnsonii is present in fermented dairy products, and it is worth investigating whether an effective dose can be achieved through daily yogurt intake in children. L. johnsonii can also ameliorate skin damage in mice. However, there have been few experiments related to the treatment of AD with L. johnsonii, and further research is needed to determine its effectiveness in the prevention and treatment of AD.
3.12. Lactobacillus pentosus regulates the host immune system and improves systemic inflammatory response
L. pentosus regulates the host immune system and plays an integral role in intestinal health (Ma et al., 2020). L. pentosus KF340 regulates systemic immunity and improves systemic inflammatory response (Kim et al., 2019b). L. pentosus S-PT84 may be involved in the modulation of immune mechanisms to alleviate clinical allergy symptoms (Majumder et al., 2020). Although administration of L. pentosus and placebo can improve symptoms, L. pentosus significantly improved the average subjective ratings evaluated using the SCORAD index for allergen-sensitizing AD (Ahn et al., 2020). L. pentosus KF340 reduced cell infiltration and serum IgE levels at the site of lesions in mice by inducing type 1 regulatory T cells (Tr1 cells) that produce IL-10 (Kim et al., 2019b). L. pentosus S-PT84 reduced the concentrations of histamine in the serum, mouse mast cell protease, total IgE, and IgG (Majumder et al., 2020). The detailed function of L. pentosus remains largely unknown and requires further investigation. L. pentosus has been shown to exert anti-inflammatory effects and benefit intestinal health. However, there is limited evidence on the efficacy of L. pentosus in the treatment and prevention of AD, and further research is needed.
3.13. Lactobacillus brevis alleviates symptoms of AD by regulating the immune response
L. brevis is a gram-positive, rod-shaped Lactobacillus that is frequently used as a starter culture in silage fermentation, sourdough, and lactic acid-fermented beer and wine. L. brevis is widely used in the fermentation industry. Orally administered L. brevis SBC8803 can significantly inhibit the production of IgE and severity of AD symptoms, and long-term use can inhibit AD development. However, it did not affect the production of cytokines produced by Th1 and Th2 (Segawa et al., 2008a). L. brevis NS1401, isolated from kimchi, stimulated immune cells to secrete Th1 or Th2 cytokines, balance Th1/Th2, and alleviate AD symptoms (Choi et al., 2017). L. brevis stabilizes the gut microbiota, prevents the growth of pathogenic bacteria, and reduces intestinal inflammation (Han et al., 2021). L. brevis can not only improve disease but also regulate immunity. In nine-week-old female BALB/c mice managed with L. brevis KB290 (3 × 109 CFU/g), cytotoxicity mediated by mouse splenocytes increased (Fukui et al., 2013). Similarly, the spleen cells of mice treated with L. brevis KCTC12777BP also expressed high levels of TNF-α. L. brevis 12777BP improves immunity in mice and prevents organisms from being invaded by pathogens (Jeong et al., 2020b). The supernatant of L. brevis BGZLS10-17 can be divided into two components: a GABA-containing and a GABA-free component (Bajić et al., 2020). The supernatant containing GABA relies on ATG5 autophagy to stimulate Foxp3+, IL-10, and transforming growth factor-β, CTLA4 and Sirp-α isoimmunoregulatory molecule expression (Bajić et al., 2020). The GABA-free supernatant can also regulate the immune response through other mechanisms (Bajić et al., 2020). Heated L. brevis KB290 accelerated the secretion of IL-8, induced ERK1/2 phosphorylation, increase p38MAPK phosphorylation, and enhanced the expression of IL-8 mRNA (Yakabe et al., 2013). Heated L. fermentans SBC8803 inhibited IgE production and histamine secretion (Segawa et al., 2008b). L. brevis regulates immunity through various mechanisms and has anti-inflammatory effects. Unfortunately, the prevention and treatment of AD by L. brevis remain unclear. L. brevis may be an innate probiotic to inhibit AD development, and is a promising Lactobacillus strain that requires further research.
4. Multi-strain Lactobacillus for the treatment and prevention of AD
By critically reviewing the current literature, we also address the advantages and major disadvantages of the simultaneous use of two or more strains of probiotics. Lactobacillus supplementation can improve AD by regulating the intestinal microbiome. Intestinal flora has the potential to improve AD. In two experiments, administration of a Lactobacillus mixture had a preventive effect on AD in hairless mice (Holowacz et al., 2018a; Holowacz et al., 2018b). L. plantarum CJLP55, CJLP133, and CJLP136 isolated from kimchi inhibited AD-like skin lesions, reduced serum IgE levels, and restored the condition of the skin (Won et al., 2011). Moreover, multi-strain probiotics have been shown to have immunomodulatory effects and prevent AD in high-risk infants (Kukkonen et al., 2007). A mixture of heat-inactivated L. casei LOCK 0900, L. casei LOCK 0908, and L. paracasei LOCK 0919 modulated in vitro cytokine profiles of allergic children to produce anti-allergic Th1 reactions (Cukrowska et al., 2010). More specifically, prenatal and postnatal supplementation with a mixture of Bifidobacterium BGN4, L. aD011, and Acidophilus A031 can substantially reduce the probability of developing AD before the age of one year (Kim et al., 2010). Compared to a single strain, mixed lactic acid bacteria significantly enhanced the ability of Th1 cells to respond. Multi-strain probiotics help maintain good skin function, are beneficial for the treatment of AD (Rosenfeldt et al., 2003; Wang and Wang, 2015), and maintain intestinal barrier function in children with AD (Rosenfeldt et al., 2004). In addition, a mixture of Lactobacillus spp. improves the clinical symptoms of adults with AD (Iemoli et al., 2012).
However, benefits of the use of mixed probiotics should be interpreted with caution. Consumption of formula containing probiotics (Bifidobacterium longum BL999 and L. rhamnosus LPR) before the age of one year did not effectively prevent eczema in infants at high risk of allergies in Asia (Soh et al., 2009). Eczema symptoms in infants did not change when L. paracasei CNCM I-2116 or Bifidobacterium lactate CNCM I3446 were used as an adjunct to basic topical therapy (Gore et al., 2012). Moreover, there is no evidence that L. NFCM and L. Bi-07 affect the intestinal flora of children with AD (Larsen et al., 2011). The combination of probiotics did not have a positive effect on AD treatment, which may be due to several reasons. First, there were differences between strains, different combinations of strains, and individual differences in the study subjects. Second, although the researchers prudently selected the infants, irreversible immune-related events occurred. Therefore, probiotic supplementation had no effect on AD. Moreover, of the reason for the probiotic mixture not achieving the desired effect may be because the mother did not supplement with probiotics before antenatal administration. Asian populations may differ, as this is the first randomized controlled trial to be conducted in Asia (Soh et al., 2009). In addition, the lack of effect may be due to a smaller bacterial population and a smaller number of experimental subjects (Larsen et al., 2011). Lactobacillus species are complex, the strains are diverse, and their combinations are random and varied. Countless combinations of different strains of the same or different species occur. Perhaps, we can find an effective way to explore the therapeutic effects of these different combinations of strains on AD. Inappropriate strains can have negative effects. Perhaps strains with side effects negate the efficacy of beneficial strains, which needs to be explored further. Moreover, research in Asian populations is limited. The efficacy of mixed strains in AD treatment and prevention should be investigated in Asia, a region with a large population.
5. Conclusion
Based on reviews and meta-analyses, probiotics can prevent or treat AD, and perinatal administration of probiotics can prevent AD (Kuitunen, 2013; Mansfield et al., 2014; Panduru et al., 2015). Children (Tan-Lim et al., 2021) and adults with moderate to severe AD can also be administered probiotics to treat AD (Kim et al., 2014). In addition, the preventive effect of probiotics on pediatric AD is better than that of treatment (Lee et al., 2008). A mixture of Lactobacillus and Bifidobacteria can effectively reduce the incidence of eczema in infants and young children during the first three years of life (Sun et al., 2021). The preventive effects of probiotics on eczema appear to last until age of two (Dang et al., 2013) and extend to the age four years (Kuitunen, 2013). The variety and specificity of probiotic strains enriches therapeutic options. Nevertheless, there is substantial evidence that LGG supplementation does not reduce the prevalence of eczema (Kim et al., 2014). Not all Lactobacillus strains can be used to treat AD, and further experiments are needed to screen for the most effective Lactobacillus strains. Additionally, probiotics require repeated experiments to determine the mechanism of their efficacy against AD, effective dose, optimal administration time, and other characteristics.
In conclusion, since the discovery of Lactobacillus, numerous studies have demonstrated that this bacterium has a positive effect on the host ( Tables 2 , 3 ). In addition, previous research demonstrated the robust anti-inflammatory and homeostatic effects of Lactobacillus. The various health properties of Lactobacillus have been confirmed by different research groups. The mechanisms and beneficial effects of Lactobacillus are diverse (e.g., inflammation, immunity, gut health, brain function). Importantly, various types, species, and strains of Lactobacillus exist. Different strains from the same species have different functions. Therefore, research findings must be considered with utmost caution. The effect of some Lactobacillius, such as L. rhamnosus HN001 and L. casei, is retained for longer after supplementation; therefore, extending their function and permanent colonization of the intestine after regular supplementation can be studied. A portion of Lactobacillus is isolated from human organs. The absence of normal Lactobacillus colonization in some patients and options for restoration of these Lactobacillus strains are worth examining. In addition, different subtypes of the same Lactobacillus strain may have opposite effects; therefore, the positive and negative effects of the subtypes remain to be studied. A more potent subtype may suppress the effects of the effective subtype. However, most of the Lactobacillus strains are commensal or are present in food. Thus, ruling out an external interference in experiments is challenging. Finally, as a promising step towards precision and personalized medicine, Lactobacillus may become a food supplement to improve future AD treatments. Additional research is needed to explore other varieties of probiotic strains to enrich treatment options. Moreover, effective methods to preserve the activity and effects of probiotics should be explored. Importantly, additional human studies are needed to support the growing evidence of the beneficial effects observed in animal models of various diseases such as cancer, depression, and obesity.
Author contributions
AX is responsible for the collection of data and writing of the original manuscript. AC and YC are responsible for the organization of the original manuscript. ZL and SJ are responsible for editing. DC and RY are responsible for the concept development, review of the manuscript and revision. RY is responsible for funding acquisition. All authors contributed to the article and approved the submitted version.
Acknowledgments
We would like to thank Editage (www.editage.cn) for English language editing.
Funding Statement
This work was supported by the Jiangsu Provincial Department of Science and Technology (No. BE2022698), the Wuxi Science and Technology Bureau (No.Y20222003), the Wuxi Municipal Medical Innovation Team (No.CXTD2021013), the Wuxi Commission of Health and Family Planning (Nos. SW202201 and M202171) and the Wuxi Young and Middle-aged Medical Talents Project (Nos. BJ2020075 and BJ2020079).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Abrahamsson T. R., Jakobsson H. E., Andersson A. F., Björkstén B., Engstrand L., Jenmalm M. C. (2012). Low diversity of the gut microbiota in infants with atopic eczema. J. Allergy Clin. Immunol. 129 434-40, 440.e1–440.e2. doi: 10.1016/j.jaci.2011.10.025 [DOI] [PubMed] [Google Scholar]
- Abrahamsson T. R., Jakobsson T., Böttcher M. F., Fredrikson M., Jenmalm M. C., Björkstén B., et al. (2007). Probiotics in prevention of IgE-associated eczema: A double-blind, randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 119, 1174–1180. doi: 10.1016/j.jaci.2007.01.007 [DOI] [PubMed] [Google Scholar]
- Ahn S. H., Yoon W., Lee S. Y., Shin H. S., Lim M. Y., Nam Y. D., et al. (2020). Effects of lactobacillus pentosus in children with allergen-sensitized atopic dermatitis. J. Korean Med. Sci. 35, e128. doi: 10.3346/jkms.2020.35.e128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aktas B., De Wolfe T. J., Safdar N., Darien B. J., Steele J. L. (2016). The impact of lactobacillus casei on the composition of the cecal microbiota and innate immune system is strain specific. PloS One 11, e0156374. doi: 10.1371/journal.pone.0156374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anjum N., Maqsood S., Masud T., Ahmad A., Sohail A., Momin A. (2014). Lactobacillus acidophilus: Characterization of the species and application in food production. Crit. Rev. Food Sci. Nutr. 54, 1241–1251. doi: 10.1080/10408398.2011.621169 [DOI] [PubMed] [Google Scholar]
- Bajić S. S., Đokić J., Dinić M., Tomić S., Popović N., Brdarić E., et al. (2020). GABA potentiate the immunoregulatory effects of lactobacillus brevis BGZLS10-17 via ATG5-dependent autophagy in vitro . Sci. Rep. 10, 1347. doi: 10.1038/s41598-020-58177-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker D. S., Garritsen F. M., Leavis H. L., Van Der Schaft J., Bruijnzeel-Koomen C., Van Den Broek M. P. H., et al. (2018). Lymphopenia in atopic dermatitis patients treated with oral immunosuppressive drugs. J. Dermatol. Treat 29, 682–687. doi: 10.1080/09546634.2018.1451619 [DOI] [PubMed] [Google Scholar]
- Bao L., Zhang H., Chan L. S. (2013). The involvement of the JAK-STAT signaling pathway in chronic inflammatory skin disease atopic dermatitis. Jakstat 2, e24137. doi: 10.4161/jkst.24137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blicharz L., Rudnicka L., Samochocki Z. (2019). Staphylococcus aureus: an underestimated factor in the pathogenesis of atopic dermatitis? Postepy Dermatol. Alergol. 36, 11–17. doi: 10.5114/ada.2019.82821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böckle B. C., Jara D., Nindl W., Aberer W., Sepp N. T. (2014). Adrenal insufficiency as a result of long-term misuse of topical corticosteroids. Dermatology 228, 289–293. doi: 10.1159/000358427 [DOI] [PubMed] [Google Scholar]
- Boyle R. J., Ismail I. H., Kivivuori S., Licciardi P. V., Robins-Browne R. M., Mah L. J., et al. (2011). Lactobacillus GG treatment during pregnancy for the prevention of eczema: a randomized controlled trial. Allergy 66, 509–516. doi: 10.1111/j.1398-9995.2010.02507.x [DOI] [PubMed] [Google Scholar]
- Boyle R. J., Mah L. J., Chen A., Kivivuori S., Robins-Browne R. M., Tang M. L. (2008). Effects of lactobacillus GG treatment during pregnancy on the development of fetal antigen-specific immune responses. Clin. Exp. Allergy 38, 1882–1890. doi: 10.1111/j.1365-2222.2008.03100.x [DOI] [PubMed] [Google Scholar]
- Bull M., Plummer S., Marchesi J., Mahenthiralingam E. (2013). The life history of lactobacillus acidophilus as a probiotic: A tale of revisionary taxonomy, misidentification and commercial success. FEMS Microbiol. Lett. 349, 77–87. doi: 10.1111/1574-6968.12293 [DOI] [PubMed] [Google Scholar]
- Cairo C., Webb T. J. (2022). Effective barriers: The role of NKT cells and innate lymphoid cells in the gut. J. Immunol. 208, 235–246. doi: 10.4049/jimmunol.2100799 [DOI] [PubMed] [Google Scholar]
- Carucci L., Nocerino R., Paparo L., De Filippis F., Coppola S., Giglio V., et al. (2022). Therapeutic effects elicited by the probiotic lacticaseibacillus rhamnosus GG in children with atopic dermatitis. the results of the ProPAD trial. Pediatr. Allergy Immunol. 33, e13836. doi: 10.1111/pai.13836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlet R., Bortolus C., Sendid B., Jawhara S. (2020). Bacteroides thetaiotaomicron and lactobacillus johnsonii modulate intestinal inflammation and eliminate fungi via enzymatic hydrolysis of the fungal cell wall. Sci. Rep. 10, 11510. doi: 10.1038/s41598-020-68214-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatrath S., Lei D., Yousaf M., Chavda R., Gabriel S., Silverberg J. I. (2022). Longitudinal course and predictors of depressive symptoms in atopic dermatitis. J. Am. Acad. Dermatol. 87, 582–591. doi: 10.1016/j.jaad.2022.04.061 [DOI] [PubMed] [Google Scholar]
- Chaves B. D., Brashears M. M., Nightingale K. K. (2017). Applications and safety considerations of lactobacillus salivarius as a probiotic in animal and human health. J. Appl. Microbiol. 123, 18–28. doi: 10.1111/jam.13438 [DOI] [PubMed] [Google Scholar]
- Chen L., Li H., Chen Y., Yang Y. (2020. b). Probiotic lactobacillus rhamnosus GG reduces mortality of septic mice by modulating gut microbiota composition and metabolic profiles. Nutrition 78, 110863. doi: 10.1016/j.nut.2020.110863 [DOI] [PubMed] [Google Scholar]
- Chen F., Wang H., Chen J., Liu Y., Wen W., Li Y., et al. (2020. a). Lactobacillus delbrueckii ameliorates intestinal integrity and antioxidant ability in weaned piglets after a lipopolysaccharide challenge. Oxid. Med. Cell Longev 2020, 6028606. doi: 10.1155/2020/6028606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi C. Y., Kim Y. H., Oh S., Lee H. J., Kim J. H., Park S. H., et al. (2017). Anti-inflammatory potential of a heat-killed lactobacillus strain isolated from kimchi on house dust mite-induced atopic dermatitis in NC/Nga mice. J. Appl. Microbiol. 123, 535–543. doi: 10.1111/jam.13515 [DOI] [PubMed] [Google Scholar]
- Cosmi L., Maggi L., Mazzoni A., Liotta F., Annunziato F. (2019). Biologicals targeting type 2 immunity: Lessons learned from asthma, chronic urticaria and atopic dermatitis. Eur. J. Immunol. 49, 1334–1343. doi: 10.1002/eji.201948156 [DOI] [PubMed] [Google Scholar]
- Cukrowska B., Rosiak I., Klewicka E., Motyl I., Schwarzer M., Libudzisz Z., et al. (2010). Impact of heat-inactivated lactobacillus casei and lactobacillus paracasei strains on cytokine responses in whole blood cell cultures of children with atopic dermatitis. Folia Microbiol. (Praha) 55, 277–280. doi: 10.1007/s12223-010-0041-6 [DOI] [PubMed] [Google Scholar]
- D'Auria E., Panelli S., Lunardon L., Pajoro M., Paradiso L., Beretta S., et al. (2021). Rice flour fermented with lactobacillus paracasei CBA L74 in the treatment of atopic dermatitis in infants: A randomized, double- blind, placebo- controlled trial. Pharmacol. Res. 163, 105284. doi: 10.1016/j.phrs.2020.105284 [DOI] [PubMed] [Google Scholar]
- Dang D., Zhou W., Lun Z. J., Mu X., Wang D. X., Wu H. (2013). Meta-analysis of probiotics and/or prebiotics for the prevention of eczema. J. Int. Med. Res. 41, 1426–1436. doi: 10.1177/0300060513493692 [DOI] [PubMed] [Google Scholar]
- Drago L., De Vecchi E., Toscano M., Vassena C., Altomare G., Pigatto P. (2014). Treatment of atopic dermatitis eczema with a high concentration of lactobacillus salivarius LS01 associated with an innovative gelling complex: A pilot study on adults. J. Clin. Gastroenterol. 48 Suppl 1, S47–S51. doi: 10.1097/MCG.0000000000000249 [DOI] [PubMed] [Google Scholar]
- Drago L., Iemoli E., Rodighiero V., Nicola L., De Vecchi E., Piconi S. (2011). Effects of lactobacillus salivarius LS01 (DSM 22775) treatment on adult atopic dermatitis: a randomized placebo-controlled study. Int. J. Immunopathol. Pharmacol. 24, 1037–1048. doi: 10.1177/039463201102400421 [DOI] [PubMed] [Google Scholar]
- Drago L., Toscano M., De Vecchi E., Piconi S., Iemoli E. (2012). Changing of fecal flora and clinical effect of l. salivarius LS01 in adults with atopic dermatitis. J. Clin. Gastroenterol. 46 Suppl, S56–S63. doi: 10.1097/MCG.0b013e318265ef38 [DOI] [PubMed] [Google Scholar]
- Engevik M. A., Ruan W., Esparza M., Fultz R., Shi Z., Engevik K. A., et al. (2021). Immunomodulation of dendritic cells by lactobacillus reuteri surface components and metabolites. Physiol. Rep. 9, e14719. doi: 10.14814/phy2.14719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esvaran M., Conway P. L. (2012). Strain dependent protection conferred by lactobacillus spp. administered orally with a salmonella typhimurium vaccine in a murine challenge model. Vaccine 30, 2654–2661. doi: 10.1016/j.vaccine.2012.02.011 [DOI] [PubMed] [Google Scholar]
- Fang Z., Pan T., Wang H., Zhu J., Zhang H., Zhao J., et al. (2022). Limosilactobacillus reuteri attenuates atopic dermatitis via changes in gut bacteria and indole derivatives from tryptophan metabolism. Int. J. Mol. Sci. 23. doi: 10.3390/ijms23147735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Z., Ross R. P., Stanton C., Hou B., Zhao J., Zhang H., et al. (2021). Lactobacillus casei CCFM1074 alleviates collagen-induced arthritis in rats via balancing Treg/Th17 and modulating the metabolites and gut microbiota. Front. Immunol. 12, 680073. doi: 10.3389/fimmu.2021.680073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fölster-Holst R., Müller F., Schnopp N., Abeck D., Kreiselmaier I., Lenz T., et al. (2006). Prospective, randomized controlled trial on lactobacillus rhamnosus in infants with moderate to severe atopic dermatitis. Br. J. Dermatol. 155, 1256–1261. doi: 10.1111/j.1365-2133.2006.07558.x [DOI] [PubMed] [Google Scholar]
- Fukui Y., Sasaki E., Fuke N., Nakai Y., Ishijima T., Abe K., et al. (2013). Effect of lactobacillus brevis KB290 on the cell-mediated cytotoxic activity of mouse splenocytes: a DNA microarray analysis. Br. J. Nutr. 110, 1617–1629. doi: 10.1017/S0007114513000767 [DOI] [PubMed] [Google Scholar]
- Galazzo G., Van Best N., Bervoets L., Dapaah I. O., Savelkoul P. H., Hornef M. W., et al. (2020). Development of the microbiota and associations with birth mode, diet, and atopic disorders in a longitudinal analysis of stool samples, collected from infancy through early childhood. Gastroenterology 158, 1584–1596. doi: 10.1053/j.gastro.2020.01.024 [DOI] [PubMed] [Google Scholar]
- Gao G., Li C., Fan W., Zhang M., Li X., Chen W., et al. (2021). Brilliant glycans and glycosylation: Seq and ye shall find. Int. J. Biol. Macromol. 189, 279–291. doi: 10.1016/j.ijbiomac.2021.08.054 [DOI] [PubMed] [Google Scholar]
- Ghoneum M., Abdulmalek S. (2021). KDP, a lactobacilli product from kimchi, enhances mucosal immunity by increasing secretory IgA in mice and exhibits antimicrobial activity. Nutrients 13. doi: 10.3390/nu13113936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh Y. J., Barrangou R., Klaenhammer T. R. (2021). In vivo transcriptome of lactobacillus acidophilus and colonization impact on murine host intestinal gene expression. mBio 12. doi: 10.1128/mBio.03399-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein E. J., Tyrrell K. L., Citron D. M. (2015). Lactobacillus species: Taxonomic complexity and controversial susceptibilities. Clin. Infect. Dis. 60 Suppl 2, S98–107. doi: 10.1093/cid/civ072 [DOI] [PubMed] [Google Scholar]
- Gore C., Custovic A., Tannock G. W., Munro K., Kerry G., Johnson K., et al. (2012). Treatment and secondary prevention effects of the probiotics lactobacillus paracasei or bifidobacterium lactis on early infant eczema: Randomized controlled trial with follow-up until age 3 years. Clin. Exp. Allergy 42, 112–122. doi: 10.1111/j.1365-2222.2011.03885.x [DOI] [PubMed] [Google Scholar]
- Grüber C., Wendt M., Sulser C., Lau S., Kulig M., Wahn U., et al. (2007). Randomized, placebo-controlled trial of lactobacillus rhamnosus GG as treatment of atopic dermatitis in infancy. Allergy 62, 1270–1276. doi: 10.1111/j.1398-9995.2007.01543.x [DOI] [PubMed] [Google Scholar]
- Guo Y., Zhang H., Liu Q., Wei F., Tang J., Li P., et al. (2019). Phenotypic analysis of atopic dermatitis in children aged 1-12 months: Elaboration of novel diagnostic criteria for infants in China and estimation of prevalence. J. Eur. Acad. Dermatol. Venereol. 33, 1569–1576. doi: 10.1111/jdv.15618 [DOI] [PubMed] [Google Scholar]
- Habil N., Abate W., Beal J., Foey A. D. (2014). Heat-killed probiotic bacteria differentially regulate colonic epithelial cell production of human β-defensin-2: Dependence on inflammatory cytokines. Benef. Microbes 5, 483–495. doi: 10.3920/BM2013.0061 [DOI] [PubMed] [Google Scholar]
- Håkansson Å., Andrén Aronsson C., Brundin C., Oscarsson E., Molin G., Agardh D. (2019). Effects of lactobacillus plantarum and lactobacillus paracasei on the peripheral immune response in children with celiac disease autoimmunity: A randomized, double-blind, placebo-controlled clinical trial. Nutrients 11. doi: 10.3390/nu11081925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X., Ding S., Ma Y., Fang J., Jiang H., Li Y., et al. (2021). Lactobacillus plantarum and lactobacillus brevis alleviate intestinal inflammation and microbial disorder induced by ETEC in a murine model. Oxid. Med. Cell Longev 2021, 6867962. doi: 10.1155/2021/6867962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y., Kim B., Ban J., Lee J., Kim B. J., Choi B. S., et al. (2012). A randomized trial of lactobacillus plantarum CJLP133 for the treatment of atopic dermatitis. Pediatr. Allergy Immunol. 23, 667–673. doi: 10.1111/pai.12010 [DOI] [PubMed] [Google Scholar]
- Han S. K., Kim J. K., Joo M. K., Lee K. E., Han S. W., Kim D. H. (2020). Lactobacillus reuteri NK33 and bifidobacterium adolescentis NK98 alleviate escherichia coli-induced depression and gut dysbiosis in mice. J. Microbiol. Biotechnol. 30, 1222–1226. doi: 10.4014/jmb.2002.02058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikino K., Tanaka N., Koido M., Tomizuka K., Koike Y., Ito S., et al. (2022). Genetic architectures underlie onset age of atopic dermatitis. J. Invest. Dermatol. 142, 3337–3341.e7. doi: 10.1016/j.jid.2022.06.010 [DOI] [PubMed] [Google Scholar]
- Holowacz S., Blondeau C., Guinobert I., Guilbot A., Hidalgo S., Bisson J. F. (2018. a). Lactobacillus salivarius LA307 and lactobacillus rhamnosus LA305 attenuate skin inflammation in mice. Benef. Microbes 9, 299–309. doi: 10.3920/BM2017.0084 [DOI] [PubMed] [Google Scholar]
- Holowacz S., Guinobert I., Guilbot A., Hidalgo S., Bisson J. F. (2018. b). A mixture of five bacterial strains attenuates skin inflammation in mice. Antiinflamm. Antiallergy Agents Med. Chem. 17, 125–137. doi: 10.2174/1871523017666180813123823 [DOI] [PubMed] [Google Scholar]
- Hong S. M., Kang M. C., Jin M., Lee T. H., Lim B. O., Kim S. Y. (2021. a). Fermented blueberry and black rice containing lactobacillus plantarum MG4221: A novel functional food for particulate matter (PM(2.5))/dinitrochlorobenzene (DNCB)-induced atopic dermatitis. Food Funct. 12, 3611–3623. doi: 10.1039/D0FO02966A [DOI] [PubMed] [Google Scholar]
- Hong Y., Zhou Z., Yu L., Jiang K., Xia J., Mi Y., et al. (2021. b). Lactobacillus salivarius and lactobacillus agilis feeding regulates intestinal stem cells activity by modulating crypt niche in hens. Appl. Microbiol. Biotechnol. 105, 8823–8835. doi: 10.1007/s00253-021-11606-2 [DOI] [PubMed] [Google Scholar]
- Hui-Beckman J. W., Goleva E., Berdyshev E., Leung D. Y. M. (2023). Endotypes of atopic dermatitis and food allergy. J. Allergy Clin. Immunol. 151, 26–28. doi: 10.1016/j.jaci.2022.07.021 [DOI] [PubMed] [Google Scholar]
- Iemoli E., Trabattoni D., Parisotto S., Borgonovo L., Toscano M., Rizzardini G., et al. (2012). Probiotics reduce gut microbial translocation and improve adult atopic dermatitis. J. Clin. Gastroenterol. 46 Suppl, S33–S40. doi: 10.1097/MCG.0b013e31826a8468 [DOI] [PubMed] [Google Scholar]
- Illi S., Von Mutius E., Lau S., Nickel R., Grüber C., Niggemann B., et al. (2004). The natural course of atopic dermatitis from birth to age 7 years and the association with asthma. J. Allergy Clin. Immunol. 113, 925–931. doi: 10.1016/j.jaci.2004.01.778 [DOI] [PubMed] [Google Scholar]
- Inoue Y., Kambara T., Murata N., Komori-Yamaguchi J., Matsukura S., Takahashi Y., et al. (2014). Effects of oral administration of lactobacillus acidophilus l-92 on the symptoms and serum cytokines of atopic dermatitis in Japanese adults: a double-blind, randomized, clinical trial. Int. Arch. Allergy Immunol. 165, 247–254. doi: 10.1159/000369806 [DOI] [PubMed] [Google Scholar]
- Inoue R., Otsuka M., Nishio A., Ushida K. (2007). Primary administration of lactobacillus johnsonii NCC533 in weaning period suppresses the elevation of proinflammatory cytokines and CD86 gene expressions in skin lesions in NC/Nga mice. FEMS Immunol. Med. Microbiol. 50, 67–76. doi: 10.1111/j.1574-695X.2007.00233.x [DOI] [PubMed] [Google Scholar]
- Jang Y. J., Kim W. K., Han D. H., Lee K., Ko G. (2019). Lactobacillus fermentum species ameliorate dextran sulfate sodium-induced colitis by regulating the immune response and altering gut microbiota. Gut Microbes 10, 696–711. doi: 10.1080/19490976.2019.1589281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen M. P., Meldrum S., Taylor A. L., Dunstan J. A., Prescott S. L. (2012). Early probiotic supplementation for allergy prevention: Long-term outcomes. J. Allergy Clin. Immunol. 130, 1209–1211.e5. doi: 10.1016/j.jaci.2012.07.018 [DOI] [PubMed] [Google Scholar]
- Jeong K., Kim M., Jeon S. A., Kim Y. H., Lee S. (2020. a). A randomized trial of lactobacillus rhamnosus IDCC 3201 tyndallizate (RHT3201) for treating atopic dermatitis. Pediatr. Allergy Immunol. 31, 783–792. doi: 10.1111/pai.13269 [DOI] [PubMed] [Google Scholar]
- Jeong M., Kim J. H., Lee J. S., Kang S. D., Shim S., Jung M. Y., et al. (2020. b). Heat-killed lactobacillus brevis enhances phagocytic activity and generates immune-stimulatory effects through activating the TAK1 pathway. J. Microbiol. Biotechnol. 30, 1395–1403. doi: 10.4014/jmb.2002.02004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalliomäki M., Salminen S., Poussa T., Arvilommi H., Isolauri E. (2003). Probiotics and prevention of atopic disease: 4-year follow-up of a randomised placebo-controlled trial. Lancet 361, 1869–1871. doi: 10.1016/S0140-6736(03)13490-3 [DOI] [PubMed] [Google Scholar]
- Kano H., Kita J., Makino S., Ikegami S., Itoh H. (2013). Oral administration of lactobacillus delbrueckii subspecies bulgaricus OLL1073R-1 suppresses inflammation by decreasing interleukin-6 responses in a murine model of atopic dermatitis. J. Dairy Sci. 96, 3525–3534. doi: 10.3168/jds.2012-6514 [DOI] [PubMed] [Google Scholar]
- Kawahara T., Hanzawa N., Sugiyama M. (2018). Effect of lactobacillus strains on thymus and chemokine expression in keratinocytes and development of atopic dermatitis-like symptoms. Benef. Microbes 9, 643–652. doi: 10.3920/BM2017.0162 [DOI] [PubMed] [Google Scholar]
- Kim S. O., Ah Y. M., Yu Y. M., Choi K. H., Shin W. G., Lee J. Y. (2014). Effects of probiotics for the treatment of atopic dermatitis: A meta-analysis of randomized controlled trials. Ann. Allergy Asthma Immunol. 113, 217–226. doi: 10.1016/j.anai.2014.05.021 [DOI] [PubMed] [Google Scholar]
- Kim W. K., Jang Y. J., Han D. H., Jeon K., Lee C., Han H. S., et al. (2020. a). Lactobacillus paracasei KBL382 administration attenuates atopic dermatitis by modulating immune response and gut microbiota. Gut Microbes 12, 1–14. doi: 10.1080/19490976.2020.1819156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. H., Kim K., Kim W. (2021). Gut microbiota restoration through fecal microbiota transplantation: A new atopic dermatitis therapy. Exp. Mol. Med. 53, 907–916. doi: 10.1038/s12276-021-00627-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M. S., Kim J. E., Yoon Y. S., Kim T. H., Seo J. G., Chung M. J., et al. (2015. b). Improvement of atopic dermatitis-like skin lesions by IL-4 inhibition of P14 protein isolated from lactobacillus casei in NC/Nga mice. Appl. Microbiol. Biotechnol. 99, 7089–7099. doi: 10.1007/s00253-015-6455-y [DOI] [PubMed] [Google Scholar]
- Kim J. Y., Kwon J. H., Ahn S. H., Lee S. I., Han Y. S., Choi Y. O., et al. (2010). Effect of probiotic mix (Bifidobacterium bifidum, bifidobacterium lactis, lactobacillus acidophilus) in the primary prevention of eczema: A double-blind, randomized, placebo-controlled trial. Pediatr. Allergy Immunol. 21, e386–e393. doi: 10.1111/j.1399-3038.2009.00958.x [DOI] [PubMed] [Google Scholar]
- Kim Y., Lee Y. D., Kim M., Kim H., Chung D. K. (2019. c). Combination treatment with lipoteichoic acids isolated from lactobacillus plantarum and staphylococcus aureus alleviates atopic dermatitis via upregulation of CD55 and CD59. Immunol. Lett. 214, 23–29. doi: 10.1016/j.imlet.2019.08.005 [DOI] [PubMed] [Google Scholar]
- Kim J., Lee B. S., Kim B., Na I., Lee J., Lee J. Y., et al. (2017). Identification of atopic dermatitis phenotypes with good responses to probiotics (Lactobacillus plantarum CJLP133) in children. Benef. Microbes 8, 755–761. doi: 10.3920/BM2017.0034 [DOI] [PubMed] [Google Scholar]
- Kim I. S., Lee S. H., Kwon Y. M., Adhikari B., Kim J. A., Yu D. Y., et al. (2019. a). Oral administration of β-glucan and lactobacillus plantarum alleviates atopic dermatitis-like symptoms. J. Microbiol. Biotechnol. 29, 1693–1706. doi: 10.4014/jmb.1907.07011 [DOI] [PubMed] [Google Scholar]
- Kim Y., Park J. Y., Kim H., Chung D. K. (2020. b). Differential role of lipoteichoic acids isolated from staphylococcus aureus and lactobacillus plantarum on the aggravation and alleviation of atopic dermatitis. Microb. Pathog. 147, 104360. doi: 10.1016/j.micpath.2020.104360 [DOI] [PubMed] [Google Scholar]
- Kim J. Y., Park B. K., Park H. J., Park Y. H., Kim B. O., Pyo S. (2013). Atopic dermatitis-mitigating effects of new lactobacillus strain, lactobacillus sakei probio 65 isolated from kimchi. J. Appl. Microbiol. 115, 517–526. doi: 10.1111/jam.12229 [DOI] [PubMed] [Google Scholar]
- Kim H., Rather I. A., Kim H., Kim S., Kim T., Jang J., et al. (2015. a). A double-blind, placebo controlled-trial of a probiotic strain lactobacillus sakei probio-65 for the prevention of canine atopic dermatitis. J. Microbiol. Biotechnol. 25, 1966–1969. doi: 10.4014/jmb.1506.06065 [DOI] [PubMed] [Google Scholar]
- Kim J. E., Sharma A., Sharma G., Lee S. Y., Shin H. S., Rudra D., et al. (2019. b). Lactobacillus pentosus modulates immune response by inducing IL-10 producing Tr1 cells. Immune Netw. 19, e39. doi: 10.4110/in.2019.19.e39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klewicka E., Cukrowska B., Libudzisz Z., Slizewska K., Motyl I. (2011). Changes in gut microbiota in children with atopic dermatitis administered the bacteria lactobacillus casei DN–114001. Pol. J. Microbiol. 60, 329–333. doi: 10.33073/pjm-2011-047 [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Honme Y., Sashihara T. (2019). Lactobacillus delbrueckii subsp. bulgaricus 2038 and streptococcus thermophilus 1131 induce the expression of the REG3 family in the small intestine of mice via the stimulation of dendritic cells and type 3 innate lymphoid cells. Nutrients 11. doi: 10.3390/nu11122998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp M. V., Hennemuth I., Heinzmann A., Urbanek R. (2008). Randomized, double-blind, placebo-controlled trial of probiotics for primary prevention: No clinical effects of lactobacillus GG supplementation. Pediatrics 121, e850–e856. doi: 10.1542/peds.2007-1492 [DOI] [PubMed] [Google Scholar]
- Kuitunen M. (2013). Probiotics and prebiotics in preventing food allergy and eczema. Curr. Opin. Allergy Clin. Immunol. 13, 280–286. doi: 10.1097/ACI.0b013e328360ed66 [DOI] [PubMed] [Google Scholar]
- Kukkonen K., Savilahti E., Haahtela T., Juntunen-Backman K., Korpela R., Poussa T., et al. (2007). Probiotics and prebiotic galacto-oligosaccharides in the prevention of allergic diseases: A randomized, double-blind, placebo-controlled trial. J. Allergy Clin. Immunol. 119, 192–198. doi: 10.1016/j.jaci.2006.09.009 [DOI] [PubMed] [Google Scholar]
- Kwon M. S., Lim S. K., Jang J. Y., Lee J., Park H. K., Kim N., et al. (2018). Lactobacillus sakei WIKIM30 ameliorates atopic dermatitis-like skin lesions by inducing regulatory T cells and altering gut microbiota structure in mice. Front. Immunol. 9, 1905. doi: 10.3389/fimmu.2018.01905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen N., Vogensen F. K., Gøbel R., Michaelsen K. F., Abu Al-Soud W., Sørensen S. J., et al. (2011). Predominant genera of fecal microbiota in children with atopic dermatitis are not altered by intake of probiotic bacteria lactobacillus acidophilus NCFM and bifidobacterium animalis subsp. lactis bi-07. FEMS Microbiol. Ecol. 75, 482–496. doi: 10.1111/j.1574-6941.2010.01024.x [DOI] [PubMed] [Google Scholar]
- Lee A., Lee Y. J., Yoo H. J., Kim M., Chang Y., Lee D. S., et al. (2017). Consumption of dairy yogurt containing lactobacillus paracasei ssp. paracasei, bifidobacterium animalis ssp. lactis and heat-treated lactobacillus plantarum improves immune function including natural killer cell activity. Nutrients 9. doi: 10.3390/nu9060558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. J., Park Y. M., Kim B., Tae I. H., Kim N. E., Pranata M., et al. (2022). Disordered development of gut microbiome interferes with the establishment of the gut ecosystem during early childhood with atopic dermatitis. Gut Microbes 14, 2068366. doi: 10.1080/19490976.2022.2068366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H., Park J. H., Park D. I., Kim H. J., Cho Y. K., Sohn C. I., et al. (2013). Mucosal mast cell count is associated with intestinal permeability in patients with diarrhea predominant irritable bowel syndrome. J. Neurogastroenterol. Motil. 19, 244–250. doi: 10.5056/jnm.2013.19.2.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Seto D., Bielory L. (2008). Meta-analysis of clinical trials of probiotics for prevention and treatment of pediatric atopic dermatitis. J. Allergy Clin. Immunol. 121, 116–121.e11. doi: 10.1016/j.jaci.2007.10.043 [DOI] [PubMed] [Google Scholar]
- Lee S. H., Yoon J. M., Kim Y. H., Jeong D. G., Park S., Kang D. J. (2016). Therapeutic effect of tyndallized lactobacillus rhamnosus IDCC 3201 on atopic dermatitis mediated by down-regulation of immunoglobulin e in NC/Nga mice. Microbiol. Immunol. 60, 468–476. doi: 10.1111/1348-0421.12390 [DOI] [PubMed] [Google Scholar]
- Licandro-Seraut H., Scornec H., Pédron T., Cavin J. F., Sansonetti P. J. (2014). Functional genomics of lactobacillus casei establishment in the gut. Proc. Natl. Acad. Sci. U.S.A. 111, E3101–E3109. doi: 10.1073/pnas.1411883111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo P., Wang D., Luo J., Li S., Li M. M., Chen H., et al. (2022). Relationship between air pollution and childhood atopic dermatitis in chongqing, China: A time-series analysis. Front. Public Health 10, 990464. doi: 10.3389/fpubh.2022.990464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maghen P., Unrue E. L., Oussedik E., Cline A., Cardwell L. A., Feldman S. R. (2019). Regardless of how risks are framed, patients seem hesitant to use topical steroids for atopic dermatitis. Br. J. Dermatol. 181, 842–844. doi: 10.1111/bjd.17929 [DOI] [PubMed] [Google Scholar]
- Mahmud M. R., Akter S., Tamanna S. K., Mazumder L., Esti I. Z., Banerjee S., et al. (2022). Impact of gut microbiome on skin health: Gut-skin axis observed through the lenses of therapeutics and skin diseases. Gut Microbes 14, 2096995. doi: 10.1080/19490976.2022.2096995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Hu C., Yan W., Jiang H., Liu G. (2020). Lactobacillus pentosus increases the abundance of akkermansia and affects the serum metabolome to alleviate DSS-induced colitis in a murine model. Front. Cell Dev. Biol. 8, 591408. doi: 10.3389/fcell.2020.591408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder K., Jin Y., Shibata H., Mine Y. (2020). Oral intervention of lactobacillus pentosus s-PT84 attenuates the allergenic responses in a BALB/C mouse model of egg allergy. Mol. Immunol. 120, 43–51. doi: 10.1016/j.molimm.2020.01.025 [DOI] [PubMed] [Google Scholar]
- Mansfield J. A., Bergin S. W., Cooper J. R., Olsen C. H. (2014). Comparative probiotic strain efficacy in the prevention of eczema in infants and children: A systematic review and meta-analysis. Mil Med. 179, 580–592. doi: 10.7205/MILMED-D-13-00546 [DOI] [PubMed] [Google Scholar]
- Mariman R., Reefman E., Tielen F., Persoon-Deen C., Van De Mark K., Worms N., et al. (2016). Lactobacillus plantarum NCIMB8826 ameliorates inflammation of colon and skin in human APOC1 transgenic mice. Benef. Microbes 7, 215–225. doi: 10.3920/BM2015.0074 [DOI] [PubMed] [Google Scholar]
- Marsella R. (2009). Evaluation of lactobacillus rhamnosus strain GG for the prevention of atopic dermatitis in dogs. Am. J. Vet. Res. 70, 735–740. doi: 10.2460/ajvr.70.6.735 [DOI] [PubMed] [Google Scholar]
- Marsella R., Santoro D., Ahrens K. (2012). Early exposure to probiotics in a canine model of atopic dermatitis has long-term clinical and immunological effects. Vet. Immunol. Immunopathol. 146, 185–189. doi: 10.1016/j.vetimm.2012.02.013 [DOI] [PubMed] [Google Scholar]
- Melli L., Carmo-Rodrigues M. S. D., Araújo-Filho H. B., Mello C. S., Tahan S., Pignatari A. C. C., et al. (2020). Gut microbiota of children with atopic dermatitis: Controlled study in the metropolitan region of são paulo, Brazil. Allergol. Immunopathol. (Madr) 48, 107–115. doi: 10.1016/j.aller.2019.08.004 [DOI] [PubMed] [Google Scholar]
- Méndez C. S., Bueno S. M., Kalergis A. M. (2021). Contribution of gut microbiota to immune tolerance in infants. J. Immunol. Res. 2021, 7823316. doi: 10.1155/2021/7823316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J., Moriyama M., Feld M., Buddenkotte J., Buhl T., Szöllösi A., et al. (2018). New mechanism underlying IL-31-induced atopic dermatitis. J. Allergy Clin. Immunol. 141, 1677–1689.e8. doi: 10.1016/j.jaci.2017.12.1002 [DOI] [PubMed] [Google Scholar]
- Miniello V. L., Brunetti L., Tesse R., Natile M., Armenio L., Francavilla R. (2010). Lactobacillus reuteri modulates cytokines production in exhaled breath condensate of children with atopic dermatitis. J. Pediatr. Gastroenterol. Nutr. 50, 573–576. doi: 10.1097/MPG.0b013e3181bb343f [DOI] [PubMed] [Google Scholar]
- Miniotti M., Lazzarin G., Ortoncelli M., Mastorino L., Ribero S., Leombruni P. (2022). Impact on health-related quality of life and symptoms of anxiety and depression after 32 weeks of dupilumab treatment for moderate-to-severe atopic dermatitis. Dermatol. Ther. 35, e15407. doi: 10.1111/dth.15407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordehai Y., Barzilai A., Dalal A., Pavlotsky F. (2022). Long-term narrowband UV-b efficacy in moderate to severe atopic dermatitis. Dermatitis 33, 282–286. doi: 10.1097/DER.0000000000000810 [DOI] [PubMed] [Google Scholar]
- Moroi M., Uchi S., Nakamura K., Sato S., Shimizu N., Fujii M., et al. (2011). Beneficial effect of a diet containing heat-killed lactobacillus paracasei K71 on adult type atopic dermatitis. J. Dermatol. 38, 131–139. doi: 10.1111/j.1346-8138.2010.00939.x [DOI] [PubMed] [Google Scholar]
- Mu Q., Swartwout B. K., Edwards M., Zhu J., Lee G., Eden K., et al. (2021). Regulation of neonatal IgA production by the maternal microbiota. Proc. Natl. Acad. Sci. U.S.A. 118. doi: 10.1073/pnas.2015691118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naghmouchi K., Belguesmia Y., Bendali F., Spano G., Seal B. S., Drider D. (2020). Lactobacillus fermentum: A bacterial species with potential for food preservation and biomedical applications. Crit. Rev. Food Sci. Nutr. 60, 3387–3399. doi: 10.1080/10408398.2019.1688250 [DOI] [PubMed] [Google Scholar]
- Nakatsuji T., Gallo R. L. (2019). The role of the skin microbiome in atopic dermatitis. Ann. Allergy Asthma Immunol. 122, 263–269. doi: 10.1016/j.anai.2018.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawaz M., Ma C., Basra M. A., Wang J., Xu J. (2015). Amelioration of ovalbumin induced allergic symptoms in balb/c mice by potentially probiotic strains of lactobacilli. Benef. Microbes 6, 669–678. doi: 10.3920/BM2014.0141 [DOI] [PubMed] [Google Scholar]
- Nermes M., Kantele J. M., Atosuo T. J., Salminen S., Isolauri E. (2011). Interaction of orally administered lactobacillus rhamnosus GG with skin and gut microbiota and humoral immunity in infants with atopic dermatitis. Clin. Exp. Allergy 41, 370–377. doi: 10.1111/j.1365-2222.2010.03657.x [DOI] [PubMed] [Google Scholar]
- Neville B. A., O'Toole P. W. (2010). Probiotic properties of lactobacillus salivarius and closely related lactobacillus species. Future Microbiol. 5, 759–774. doi: 10.2217/fmb.10.35 [DOI] [PubMed] [Google Scholar]
- Niccoli A. A., Artesi A. L., Candio F., Ceccarelli S., Cozzali R., Ferraro L., et al. (2014). Preliminary results on clinical effects of probiotic lactobacillus salivarius LS01 in children affected by atopic dermatitis. J. Clin. Gastroenterol. 48 Suppl 1, S34–S36. doi: 10.1097/MCG.0000000000000233 [DOI] [PubMed] [Google Scholar]
- Ogawa T., Hashikawa S., Asai Y., Sakamoto H., Yasuda K., Makimura Y. (2006). A new synbiotic, lactobacillus casei subsp. casei together with dextran, reduces murine and human allergic reaction. FEMS Immunol. Med. Microbiol. 46, 400–409. doi: 10.1111/j.1574-695X.2006.00046.x [DOI] [PubMed] [Google Scholar]
- Ohshima-Terada Y., Higuchi Y., Kumagai T., Hagihara A., Nagata M. (2015). Complementary effect of oral administration of lactobacillus paracasei K71 on canine atopic dermatitis. Vet. Dermatol. 26(350-3), e74–e75. doi: 10.1111/vde.12224 [DOI] [PubMed] [Google Scholar]
- Panduru M., Panduru N. M., Sălăvăstru C. M., Tiplica G. S. (2015). Probiotics and primary prevention of atopic dermatitis: a meta-analysis of randomized controlled studies. J. Eur. Acad. Dermatol. Venereol. 29, 232–242. doi: 10.1111/jdv.12496 [DOI] [PubMed] [Google Scholar]
- Park C. W., Youn M., Jung Y. M., Kim H., Jeong Y., Lee H. K., et al. (2008). New functional probiotic lactobacillus sakei probio 65 alleviates atopic symptoms in the mouse. J. Med. Food 11, 405–412. doi: 10.1089/jmf.2007.0144 [DOI] [PubMed] [Google Scholar]
- Penders J., Gerhold K., Stobberingh E. E., Thijs C., Zimmermann K., Lau S., et al. (2013). Establishment of the intestinal microbiota and its role for atopic dermatitis in early childhood. J. Allergy Clin. Immunol. 132, 601–607.e8. doi: 10.1016/j.jaci.2013.05.043 [DOI] [PubMed] [Google Scholar]
- Penders J., Thijs C., Van Den Brandt P. A., Kummeling I., Snijders B., Stelma F., et al. (2007). Gut microbiota composition and development of atopic manifestations in infancy: the KOALA birth cohort study. Gut 56, 661–667. doi: 10.1136/gut.2006.100164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrova M. I., Reid G., Ter Haar J. A. (2021). Lacticaseibacillus rhamnosus GR-1, a.k.a. lactobacillus rhamnosus GR-1: Past and future perspectives. Trends Microbiol. 29, 747–761. doi: 10.1016/j.tim.2021.03.010 [DOI] [PubMed] [Google Scholar]
- Powers C. E., Mcshane D. B., Gilligan P. H., Burkhart C. N., Morrell D. S. (2015). Microbiome and pediatric atopic dermatitis. J. Dermatol. 42, 1137–1142. doi: 10.1111/1346-8138.13072 [DOI] [PubMed] [Google Scholar]
- Prakoeswa C. R. S., Bonita L., Karim A., Herwanto N., Umborowati M. A., Setyaningrum T., et al. (2022). Beneficial effect of lactobacillus plantarum IS-10506 supplementation in adults with atopic dermatitis: A randomized controlled trial. J. Dermatol. Treat 33, 1491–1498. doi: 10.1080/09546634.2020.1836310 [DOI] [PubMed] [Google Scholar]
- Prakoeswa C. R. S., Herwanto N., Prameswari R., Astari L., Sawitri S., Hidayati A. N., et al. (2017). Lactobacillus plantarum IS-10506 supplementation reduced SCORAD in children with atopic dermatitis. Benef. Microbes 8, 833–840. doi: 10.3920/BM2017.0011 [DOI] [PubMed] [Google Scholar]
- Prescott S. L., Dunstan J. A., Hale J., Breckler L., Lehmann H., Weston S., et al. (2005). Clinical effects of probiotics are associated with increased interferon-gamma responses in very young children with atopic dermatitis. Clin. Exp. Allergy 35, 1557–1564. doi: 10.1111/j.1365-2222.2005.02376.x [DOI] [PubMed] [Google Scholar]
- Prescott S. L., Wiltschut J., Taylor A., Westcott L., Jung W., Currie H., et al. (2008). Early markers of allergic disease in a primary prevention study using probiotics: 2.5-year follow-up phase. Allergy 63, 1481–1490. doi: 10.1111/j.1398-9995.2008.01778.x [DOI] [PubMed] [Google Scholar]
- Puar N., Chovatiya R., Paller A. S. (2021). New treatments in atopic dermatitis. Ann. Allergy Asthma Immunol. 126, 21–31. doi: 10.1016/j.anai.2020.08.016 [DOI] [PubMed] [Google Scholar]
- Qi C., Tu H., Zhao Y., Zhou J., Chen J., Hu H., et al. (2022). Breast milk-derived limosilactobacillus reuteri prevents atopic dermatitis in mice via activating retinol absorption and metabolism in peyer's patches. Mol. Nutr. Food Res. 67, e2200444. doi: 10.1002/mnfr.202200444 [DOI] [PubMed] [Google Scholar]
- Rather I. A., Kim B. C., Lew L. C., Cha S. K., Lee J. H., Nam G. J., et al. (2021). Oral administration of live and dead cells of lactobacillus sakei proBio65 alleviated atopic dermatitis in children and adolescents: A randomized, double-blind, and placebo-controlled study. Probiotics Antimicrob. Proteins 13, 315–326. doi: 10.1007/s12602-020-09654-7 [DOI] [PubMed] [Google Scholar]
- Renert-Yuval Y., Guttman-Yassky E. (2020). New treatments for atopic dermatitis targeting beyond IL-4/IL-13 cytokines. Ann. Allergy Asthma Immunol. 124, 28–35. doi: 10.1016/j.anai.2019.10.005 [DOI] [PubMed] [Google Scholar]
- Rerknimitr P., Otsuka A., Nakashima C., Kabashima K. (2017). The etiopathogenesis of atopic dermatitis: Barrier disruption, immunological derangement, and pruritus. Inflammation Regener. 37, 14. doi: 10.1186/s41232-017-0044-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison R. G., Singh A. M. (2019). Controversies in allergy: Food testing and dietary avoidance in atopic dermatitis. J. Allergy Clin. Immunol. Pract. 7, 35–39. doi: 10.1016/j.jaip.2018.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Nogales A., Algieri F., Garrido-Mesa J., Vezza T., Utrilla M. P., Chueca N., et al. (2017). Differential intestinal anti-inflammatory effects of lactobacillus fermentum and lactobacillus salivarius in DSS mouse colitis: Impact on microRNAs expression and microbiota composition. Mol. Nutr. Food Res. 61. doi: 10.1002/mnfr.201700144 [DOI] [PubMed] [Google Scholar]
- Rosenfeldt V., Benfeldt E., Nielsen S. D., Michaelsen K. F., Jeppesen D. L., Valerius N. H., et al. (2003). Effect of probiotic lactobacillus strains in children with atopic dermatitis. J. Allergy Clin. Immunol. 111, 389–395. doi: 10.1067/mai.2003.389 [DOI] [PubMed] [Google Scholar]
- Rosenfeldt V., Benfeldt E., Valerius N. H., Paerregaard A., Michaelsen K. F. (2004). Effect of probiotics on gastrointestinal symptoms and small intestinal permeability in children with atopic dermatitis. J. Pediatr. 145, 612–616. doi: 10.1016/j.jpeds.2004.06.068 [DOI] [PubMed] [Google Scholar]
- Rose M. A., Stieglitz F., Köksal A., Schubert R., Schulze J., Zielen S. (2010). Efficacy of probiotic lactobacillus GG on allergic sensitization and asthma in infants at risk. Clin. Exp. Allergy 40, 1398–1405. doi: 10.1111/j.1365-2222.2010.03560.x [DOI] [PubMed] [Google Scholar]
- Salava A., Rieppo R., Lauerma A., Salo V. (2022). Age-dependent distribution of atopic dermatitis in primary care: A nationwide population-based study from Finland. Acta Derm. Venereol. 102, adv00738. doi: 10.2340/actadv.v102.2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmi H., Kuitunen M., Viljanen M., Lapatto R. (2010). Cow's milk allergy is associated with changes in urinary organic acid concentrations. Pediatr. Allergy Immunol. 21, e401–e406. doi: 10.1111/j.1399-3038.2009.00881.x [DOI] [PubMed] [Google Scholar]
- Salva S., Tiscornia I., Gutiérrez F., Alvarez S., Bollati-Fogolín M. (2021). Lactobacillus rhamnosus postbiotic-induced immunomodulation as safer alternative to the use of live bacteria. Cytokine 146, 155631. doi: 10.1016/j.cyto.2021.155631 [DOI] [PubMed] [Google Scholar]
- Sawada J., Morita H., Tanaka A., Salminen S., He F., Matsuda H. (2007). Ingestion of heat-treated lactobacillus rhamnosus GG prevents development of atopic dermatitis in NC/Nga mice. Clin. Exp. Allergy 37, 296–303. doi: 10.1111/j.1365-2222.2006.02645.x [DOI] [PubMed] [Google Scholar]
- Schedel M., Leach S. M., Strand M. J., Danhorn T., Macbeth M., Faino A. V., et al. (2023). Molecular networks in atopic mothers impact the risk of infant atopy. Allergy 78, 244–257. doi: 10.1111/all.15490 [DOI] [PubMed] [Google Scholar]
- Seddik H. A., Bendali F., Gancel F., Fliss I., Spano G., Drider D. (2017). Lactobacillus plantarum and its probiotic and food potentialities. Probiotics Antimicrob. Proteins 9, 111–122. doi: 10.1007/s12602-017-9264-z [DOI] [PubMed] [Google Scholar]
- Segawa S., Hayashi A., Nakakita Y., Kaneda H., Watari J., Yasui H. (2008. a). Oral administration of heat-killed lactobacillus brevis SBC8803 ameliorates the development of dermatitis and inhibits immunoglobulin e production in atopic dermatitis model NC/Nga mice. Biol. Pharm. Bull. 31, 884–889. doi: 10.1248/bpb.31.884 [DOI] [PubMed] [Google Scholar]
- Segawa S., Nakakita Y., Takata Y., Wakita Y., Kaneko T., Kaneda H., et al. (2008. b). Effect of oral administration of heat-killed lactobacillus brevis SBC8803 on total and ovalbumin-specific immunoglobulin e production through the improvement of Th1/Th2 balance. Int. J. Food Microbiol. 121, 1–10. doi: 10.1016/j.ijfoodmicro.2007.10.004 [DOI] [PubMed] [Google Scholar]
- Shah M. M., Miyamoto Y., Yamada Y., Yamashita H., Tanaka H., Ezaki T., et al. (2010). Orally supplemented lactobacillus acidophilus strain l-92 inhibits passive and active cutaneous anaphylaxis as well as 2,4-dinitroflurobenzene and mite fecal antigen induced atopic dermatitis-like skin lesions in mice. Microbiol. Immunol. 54, 523–533. doi: 10.1111/j.1348-0421.2010.00251.x [DOI] [PubMed] [Google Scholar]
- Sheikhi A., Giti H., Heibor M. R., Jafarzadeh A., Shakerian M., Baharifar N., et al. (2017). Lactobacilus delbrueckii subsp. bulgaricus modulates the secretion of Th1/Th2 and treg cell-related cytokines by PBMCs from patients with atopic dermatitis. Drug Res. (Stuttg) 67, 724–729. doi: 10.1055/s-0043-117612 [DOI] [PubMed] [Google Scholar]
- Soh S. E., Aw M., Gerez I., Chong Y. S., Rauff M., Ng Y. P., et al. (2009). Probiotic supplementation in the first 6 months of life in at risk Asian infants–effects on eczema and atopic sensitization at the age of 1 year. Clin. Exp. Allergy 39, 571–578. doi: 10.1111/j.1365-2222.2008.03133.x [DOI] [PubMed] [Google Scholar]
- Steinhoff M., Ahmad F., Pandey A., Datsi A., Alhammadi A., Al-Khawaga S., et al. (2022). Neuroimmune communication regulating pruritus in atopic dermatitis. J. Allergy Clin. Immunol. 149, 1875–1898. doi: 10.1016/j.jaci.2022.03.010 [DOI] [PubMed] [Google Scholar]
- Sunada Y., Nakamura S., Kamei C. (2008). Effect of lactobacillus acidophilus strain l-55 on the development of atopic dermatitis-like skin lesions in NC/Nga mice. Int. Immunopharmacol. 8, 1761–1766. doi: 10.1016/j.intimp.2008.08.011 [DOI] [PubMed] [Google Scholar]
- Sun Z., Harris H. M., Mccann A., Guo C., Argimón S., Zhang W., et al. (2015). Expanding the biotechnology potential of lactobacilli through comparative genomics of 213 strains and associated genera. Nat. Commun. 6, 8322. doi: 10.1038/ncomms9322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M., Luo J., Liu H., Xi Y., Lin Q. (2021). Can mixed strains of lactobacillus and bifidobacterium reduce eczema in infants under three years of age? a meta-analysis. Nutrients 13. doi: 10.3390/nu13051461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A., Fukushima Y., Benyacoub J., Blum S., Matsuda H. (2008). Prophylactic effect of oral administration of lactobacillus johnsonii NCC533 (La1) during the weaning period on atopic dermatitis in NC/NgaTnd mice. Eur. J. Dermatol. 18, 136–140. doi: 10.1684/ejd.2008.0350 [DOI] [PubMed] [Google Scholar]
- Tanaka A., Jung K., Benyacoub J., Prioult G., Okamoto N., Ohmori K., et al. (2009). Oral supplementation with lactobacillus rhamnosus CGMCC 1.3724 prevents development of atopic dermatitis in NC/NgaTnd mice possibly by modulating local production of IFN-gamma. Exp. Dermatol. 18, 1022–1027. doi: 10.1111/j.1600-0625.2009.00895.x [DOI] [PubMed] [Google Scholar]
- Tan-Lim C. S. C., Esteban-Ipac N. A. R., Mantaring J. B. V., 3rd, Chan Shih Yen E., Recto M. S. T., Sison O. T., et al. (2021). Comparative effectiveness of probiotic strains for the treatment of pediatric atopic dermatitis: A systematic review and network meta-analysis. Pediatr. Allergy Immunol. 32, 124–136. doi: 10.1111/pai.13305 [DOI] [PubMed] [Google Scholar]
- Taylor A. L., Dunstan J. A., Prescott S. L. (2007). Probiotic supplementation for the first 6 months of life fails to reduce the risk of atopic dermatitis and increases the risk of allergen sensitization in high-risk children: a randomized controlled trial. J. Allergy Clin. Immunol. 119, 184–191. doi: 10.1016/j.jaci.2006.08.036 [DOI] [PubMed] [Google Scholar]
- Torii S., Torii A., Itoh K., Urisu A., Terada A., Fujisawa T., et al. (2011). Effects of oral administration of lactobacillus acidophilus l-92 on the symptoms and serum markers of atopic dermatitis in children. Int. Arch. Allergy Immunol. 154, 236–245. doi: 10.1159/000321110 [DOI] [PubMed] [Google Scholar]
- Varela-Trinidad G. U., Domínguez-Díaz C., Solórzano-Castanedo K., Íñiguez-Gutiérrez L., Hernández-Flores T. J., Fafutis-Morris M. (2022). Probiotics: Protecting our health from the gut. Microorganisms 10. doi: 10.3390/microorganisms10071428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi H., Nariai C., Takemura F., Nakao W., Fujiwara D. (2008). Dietary supplementation with lactic acid bacteria attenuates the development of atopic-dermatitis-like skin lesions in NC/Nga mice in a strain-dependent manner. Int. Arch. Allergy Immunol. 145, 141–151. doi: 10.1159/000108139 [DOI] [PubMed] [Google Scholar]
- Wang H., He S., Xin J., Zhang T., Sun N., Li L., et al. (2021). Psychoactive effects of lactobacillus johnsonii against restraint stress-induced memory dysfunction in mice through modulating intestinal inflammation and permeability-a study based on the gut-brain axis hypothesis. Front. Pharmacol. 12, 662148. doi: 10.3389/fphar.2021.662148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang I. J., Wang J. Y. (2015). Children with atopic dermatitis show clinical improvement after lactobacillus exposure. Clin. Exp. Allergy 45, 779–787. doi: 10.1111/cea.12489 [DOI] [PubMed] [Google Scholar]
- Wang W., Xing W., Wei S., Gao Q., Wei X., Shi L., et al. (2019). Semi-rational screening of probiotics from the fecal flora of healthy adults against DSS-induced colitis mice by enhancing anti-inflammatory activity and modulating the gut microbiota. J. Microbiol. Biotechnol. 29, 1478–1487. doi: 10.4014/jmb.1807.06061 [DOI] [PubMed] [Google Scholar]
- Watanabe T., Hamada K., Tategaki A., Kishida H., Tanaka H., Kitano M., et al. (2009). Oral administration of lactic acid bacteria isolated from traditional south Asian fermented milk 'dahi' inhibits the development of atopic dermatitis in NC/Nga mice. J. Nutr. Sci. Vitaminol. (Tokyo) 55, 271–278. doi: 10.3177/jnsv.55.271 [DOI] [PubMed] [Google Scholar]
- Watanabe S., Narisawa Y., Arase S., Okamatsu H., Ikenaga T., Tajiri Y., et al. (2003). Differences in fecal microflora between patients with atopic dermatitis and healthy control subjects. J. Allergy Clin. Immunol. 111, 587–591. doi: 10.1067/mai.2003.105 [DOI] [PubMed] [Google Scholar]
- Weil C., Sugerman P. B., Chodick G., Liang H., Wang H., Calimlim B. M., et al. (2022). Epidemiology and economic burden of atopic dermatitis: Real-world retrospective data from a Large nationwide Israeli healthcare provider database. Adv. Ther. 39, 2502–2514. doi: 10.1007/s12325-022-02120-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens K., Barthow C., Mitchell E. A., Stanley T. V., Purdie G., Rowden J., et al. (2018). Maternal supplementation alone with lactobacillus rhamnosus HN001 during pregnancy and breastfeeding does not reduce infant eczema. Pediatr. Allergy Immunol. 29, 296–302. doi: 10.1111/pai.12874 [DOI] [PubMed] [Google Scholar]
- Wickens K., Black P., Stanley T. V., Mitchell E., Barthow C., Fitzharris P., et al. (2012). A protective effect of lactobacillus rhamnosus HN001 against eczema in the first 2 years of life persists to age 4 years. Clin. Exp. Allergy 42, 1071–1079. doi: 10.1111/j.1365-2222.2012.03975.x [DOI] [PubMed] [Google Scholar]
- Wickens K., Black P. N., Stanley T. V., Mitchell E., Fitzharris P., Tannock G. W., et al. (2008). A differential effect of 2 probiotics in the prevention of eczema and atopy: A double-blind, randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 122, 788–794. doi: 10.1016/j.jaci.2008.07.011 [DOI] [PubMed] [Google Scholar]
- Wickens K., Stanley T. V., Mitchell E. A., Barthow C., Fitzharris P., Purdie G., et al. (2013). Early supplementation with lactobacillus rhamnosus HN001 reduces eczema prevalence to 6 years: Does it also reduce atopic sensitization? Clin. Exp. Allergy 43, 1048–1057. doi: 10.1111/cea.12154 [DOI] [PubMed] [Google Scholar]
- Wollenberg A., Ariens L., Thurau S., Van Luijk C., Seegräber M., De Bruin-Weller M. (2018). Conjunctivitis occurring in atopic dermatitis patients treated with dupilumab-clinical characteristics and treatment. J. Allergy Clin. Immunol. Pract. 6, 1778–1780.e1. doi: 10.1016/j.jaip.2018.01.034 [DOI] [PubMed] [Google Scholar]
- Won T. J., Kim B., Lim Y. T., Song D. S., Park S. Y., Park E. S., et al. (2011). Oral administration of lactobacillus strains from kimchi inhibits atopic dermatitis in NC / Nga mice. J. Appl. Microbiol. 110, 1195–1202. doi: 10.1111/j.1365-2672.2011.04981.x [DOI] [PubMed] [Google Scholar]
- Woo S. I., Kim J. Y., Lee Y. J., Kim N. S., Hahn Y. S. (2010). Effect of lactobacillus sakei supplementation in children with atopic eczema-dermatitis syndrome. Ann. Allergy Asthma Immunol. 104, 343–348. doi: 10.1016/j.anai.2010.01.020 [DOI] [PubMed] [Google Scholar]
- Wu K. G., Li T. H., Peng H. J. (2012). Lactobacillus salivarius plus fructo-oligosaccharide is superior to fructo-oligosaccharide alone for treating children with moderate to severe atopic dermatitis: a double-blind, randomized, clinical trial of efficacy and safety. Br. J. Dermatol. 166, 129–136. doi: 10.1111/j.1365-2133.2011.10596.x [DOI] [PubMed] [Google Scholar]
- Xin J., Zeng D., Wang H., Sun N., Khalique A., Zhao Y., et al. (2020. a). Lactobacillus johnsonii BS15 improves intestinal environment against fluoride-induced memory impairment in mice-a study based on the gut-brain axis hypothesis. PeerJ 8, e10125. doi: 10.7717/peerj.10125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin J., Zeng D., Wang H., Sun N., Zhao Y., Dan Y., et al. (2020. b). Probiotic lactobacillus johnsonii BS15 promotes growth performance, intestinal immunity, and gut microbiota in piglets. Probiotics Antimicrob. Proteins 12, 184–193. doi: 10.1007/s12602-018-9511-y [DOI] [PubMed] [Google Scholar]
- Yakabe T., Shimohata T., Takahashi A. (2013). Lactobacillus brevis KB290 enhances IL-8 secretion by vibrio parahaemolyticus-infected caco-2 cells. J. Microbiol. Biotechnol. 23, 118–124. doi: 10.4014/jmb.1207.07020 [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Yokoyama K., Matsukawa T., Kato S., Kato S., Yamada K., et al. (2016). Efficacy of prolonged ingestion of lactobacillus acidophilus l-92 in adult patients with atopic dermatitis. J. Dairy Sci. 99, 5039–5046. doi: 10.3168/jds.2015-10605 [DOI] [PubMed] [Google Scholar]
- Yang G., Seok J. K., Kang H. C., Cho Y. Y., Lee H. S., Lee J. Y. (2020). Skin barrier abnormalities and immune dysfunction in atopic dermatitis. Int. J. Mol. Sci. 21. doi: 10.3390/ijms21082867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan D. C., Hung C. H., Sy L. B., Lue K. H., Shih I. H., Yang C. Y., et al. (2019). A randomized, double-blind, placebo-controlled trial assessing the oral administration of a heat-treated lactobacillus paracasei Supplement in infants with atopic dermatitis receiving topical corticosteroid therapy. Skin Pharmacol. Physiol. 32, 201–211. doi: 10.1159/000499436 [DOI] [PubMed] [Google Scholar]
- Yeo J. M., Lee H. J., Kim J. W., Lee J. B., Park S. Y., Choi I. S., et al. (2014). Lactobacillus fermentum CJL-112 protects mice against influenza virus infection by activating T-helper 1 and eliciting a protective immune response. Int. Immunopharmacol. 18, 50–54. doi: 10.1016/j.intimp.2013.10.020 [DOI] [PubMed] [Google Scholar]
- Yokomichi H., Mochizuki M., Shinohara R., Kushima M., Horiuchi S., Kojima R., et al. (2022). Association of the incidence of atopic dermatitis until 3 years old with climate conditions in the first 6 months of life: Japan environment and children's study (JECS). PloS One 17, e0268204. doi: 10.1371/journal.pone.0268204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yosipovitch G., Berger T., Fassett M. S. (2020). Neuroimmune interactions in chronic itch of atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 34, 239–250. doi: 10.1111/jdv.15973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Z., Guo X., Zhang J., Yuan Q., Chen S. (2021). Lactobacillus paracasei modulates the gut microbiota and improves inflammation in type 2 diabetic rats. Food Funct. 12, 6809–6820. doi: 10.1039/D1FO00515D [DOI] [PubMed] [Google Scholar]
- Zhang N., Li C., Niu Z., Kang H., Wang M., Zhang B., et al. (2020). Colonization and immunoregulation of lactobacillus plantarum BF_15, a novel probiotic strain from the feces of breast-fed infants. Food Funct. 11, 3156–3166. doi: 10.1039/C9FO02745A [DOI] [PubMed] [Google Scholar]
- Zhao Y., Qi C., Li X., Lu M., Zhang H., Zhou J., et al. (2022). Prevention of atopic dermatitis in mice by lactobacillus reuteri Fn041 through induction of regulatory T cells and modulation of the gut microbiota. Mol. Nutr. Food Res. 66, e2100699. doi: 10.1002/mnfr.202100699 [DOI] [PubMed] [Google Scholar]
- Zheng J., Ruan L., Sun M., Gänzle M. (2015). A genomic view of lactobacilli and pediococci demonstrates that phylogeny matches ecology and physiology. Appl. Environ. Microbiol. 81, 7233–7243. doi: 10.1128/AEM.02116-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Xu G., Li X., Tu H., Li H., Chang H., et al. (2022). Limosilactobacillus reuteri FN041 prevents atopic dermatitis in pup mice by remodeling the ileal microbiota and regulating gene expression in peyer's patches after vertical transmission. Front. Nutr. 9, 987400. doi: 10.3389/fnut.2022.987400 [DOI] [PMC free article] [PubMed] [Google Scholar]