Abstract
Subjective cognitive complaints (SCCs) refer to self-perceived cognitive decline and are related to objective cognitive decline. SCCs in cognitively normal individuals are considered a preclinical sign of subsequent cognitive impairment due to Alzheimer’s disease, and SCCs in cognitively normal patients with Parkinson’s disease (PD) are also gaining attention. The aim of this review was to provide an overview of the current research on SCCs in cognitively normal patients with PD. A systematic search found a lack of consistency in the methodologies used to define and measure SCCs. Although the association between SCCs and objective cognitive performance in cognitively normal patients with PD is controversial, SCCs appear to be predictive of subsequent cognitive decline. These findings support the clinical value of SCCs in cognitively normal status in PD; however, further convincing evidence from biomarker studies is needed to provide a pathophysiological basis for these findings. Additionally, a consensus on the definition and assessment of SCCs is needed for further investigations.
Keywords: Mild cognitive impairment, Parkinson’s disease, Subjective cognitive complaints, Subjective cognitive decline, Subjective memory complaints
INTRODUCTION
Parkinson’s disease (PD) is characterized by motor symptoms. However, nonmotor symptoms also affect the daily activities of patients with PD. Cognitive impairment is very common and one of the most disabling nonmotor symptoms in patients with PD [1]. The prevalence of dementia in PD is estimated to be 30%–40% [2,3], and dementia eventually develops in 80% of patients [4]. The emergence of the concept of mild cognitive impairment (MCI) in PD (PD-MCI) as a predementia status [5] has led to the widely accepted diagnostic criteria for PD-MCI proposed by the Movement Disorder Society (MDS) Task Force [6].
Since early detection and diagnosis of MCI are important in managing cognitive impairment, attention to the pre-MCI stage has also increased [7,8]. Since, by definition, the pre-MCI status should include no objective cognitive impairment [9,10], researchers have focused on subjective feelings of cognitive decline. In fact, concern about changes in cognition reported by either the patient or informant is already one of the essential criteria for the diagnosis of MCI due to both Alzheimer’s disease (AD) and PD [6,11]. Subjective feelings about cognitive decline, herein referred to as subjective cognitive complaints (SCCs), exist at all cognitive levels, including normal cognition, MCI, and even dementia [12-14], and SCCs are known to be correlated with objective cognitive deteriorations in nondemented individuals with or without PD [13-18].
In AD spectrum disorders, the characteristics and predictive values of SCCs with normal cognition have been investigated for a long time, but there is a wide range of diversity in the terminology, definition, and assessment of SCCs. In 2014, a conceptual framework for SCCs was introduced, which unified the different terminologies used for indicating SCCs in preclinical AD into “subjective cognitive decline” (SCD) [10]. Currently, SCD is considered a potential at-risk status of AD, and the concept of SCD has come to the forefront in AD research.
Studies presenting results for SCCs in PD have been rapidly increasing; however, the methodologies for defining SCCs, selecting participants, and interpreting results have shown substantial inconsistencies. In particular, many studies have not distinguished SCCs in normal cognition from those in PD-MCI or PD with dementia [19-21]. This review aims to provide an overview of how SCCs in cognitively normal patients with PD have been defined and assessed and to examine the association between SCCs and objective cognitive performance. This review also explores the clinical significance of SCCs supported by their predictive power for subsequent cognitive decline and their association with biomarkers relevant to cognitive impairment.
METHODS
Search strategy
Using the electronic databases of PubMed, Scopus, Web of Science, and Embase, we searched for original articles published prior to March 31, 2022. The search was conducted in titles and abstracts using the following terms: “Parkinson’s disease” combined with either “cognitive complaints,” “memory complaints,” “subjective cognitive,” or “subjective memory.” Furthermore, relevant articles were identified from the reference lists of the selected articles or other sources. Articles not written in English were excluded from this study.
The search yielded 387 articles, and 14 articles were collected from other sources. After removing duplicates, 300 articles were identified.
Selection of studies
The studies were selected by JYH according to the following criteria: 1) studies were conducted in patients with PD; 2) normal cognition was defined using the diagnostic criteria for PD-MCI [6] proposed by the MDS Task Force; 3) methods for SCC assessment were described; 4) SCCs were reported by patients; and 5) data and results for cognitively normal patients with SCCs could be distinguished from those for cognitively impaired patients. Three hundred identified articles were screened, and 255 articles were excluded based on a review of the title and abstracts. The full text of the remaining 45 articles was then read and assessed for eligibility. Among them, 26 articles were excluded for the following reasons: 23 did not distinguish normal cognition from MCI or dementia, one evaluated individual with prodromal PD, one excluded patient with SCC, and one used duplicated data. Finally, 19 articles were included in this review. The selection process is illustrated in Figure 1.
Figure 1. FigureTitle.
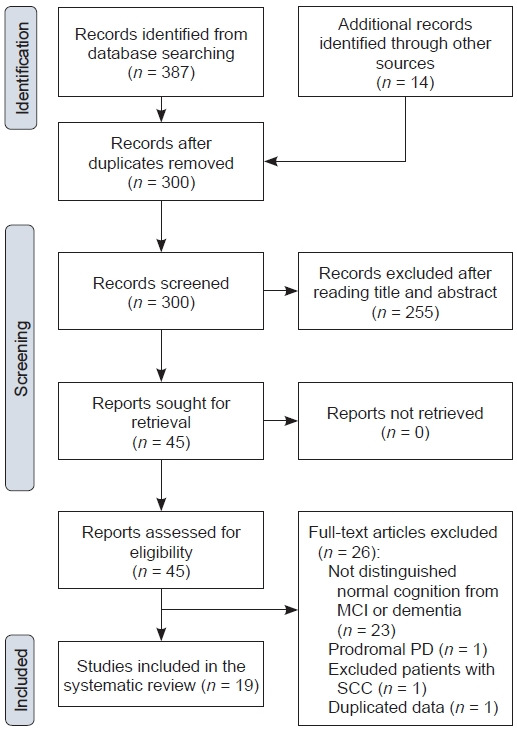
PRISMA flow diagram. MCI, mild cognitive impairment; PD, Parkinson’s disease; SCC, subjective cognitive complaint.
RESULTS AND DISCUSSION
Ten studies were cross-sectional observational studies, and eight studies had a longitudinal observational design (Table 1). The interval between baseline and follow-up in the longitudinal studies ranged from 2 to 7.5 years (mean, 3.3 years). Additionally, one randomized controlled study tested whether cognitive training improves cognitive function.
Table 1.
Details of the included studies
| Study | Design | Sample | Term for SCCs | Assessment of SCCs | Criteria for SCCs | Level of MCI criteria | Incidence of SCCs | Relationship between SCCs and objective cognition | Effects of depression or anxiety | Prediction of cognitive decline in the future | Neuroimaging findings |
|---|---|---|---|---|---|---|---|---|---|---|---|
| AlDakheel et al., [32] 2019 | Longitudinal, 2 years | 81 CN, 58 MCI | Subjective cognitive complaints | 1) NBI, 19-item questionnaire, yes/no question | 1) Answer “yes” in at least 1 item | II | - | - | - | No SCC method was associated with cognitive decline over time. | - |
| 2) A single question “Do you have any concerns about your memory or thinking?”, | 2) Answer “yes” | ||||||||||
| 3) MDS-UPDRS question 1.1 | 3) ≥ 1 | ||||||||||
| Barbosa et al., [33] 2019 | Cross-sectional | 76 CN, 31 MCI, 21 PDD | Subjective cognitive complaints | NMSS domain-5, 3 items, severity (0–3) × frequency (1–4) | NMSS domain-5 ≥ 1 | I | 82.9% of CN | In PD-CN, the presence of SCCs was associated with a lower MoCA score, but the association was lost when adjusted for depressive symptoms. | In PD-CN, the presence of SCCs was associated with a higher depression score. The SCC severity was associated with depression, apathy and anxiety severity. | - | - |
| Baschi et al., [42] 2018 | Cross-sectional | 42 CN without SMCs, 40 CN with SMCs, 48 MCI with SMCs, 17 MCI without SMCs | Subjective memory complaints | MAC-Q, 6-items, 1–5 Likert scale | MAC-Q ≥ 25 | II | 48.8% of CN | Patients with SMCs showed lower performance in visuospatial function tasks than those without SMCs. | SMCs were significantly associated with anxiety but not depression. | - | - |
| Chua et al., [34] 2021 | Cross- sectional | 29 MCI without SCCs, 13 MCI with SCCs, 45 CN without SCCs, 34 CN with SCCs | Subjective cognitive complaints | NMSS domain-5, 3 items, severity (0–3) × frequency (1–4) | NMSS domain-5 ≥ 1 | II | 28.1% of CN | - | SCCs were associated with anxiety, depression, and apathy in cognitively normal patients. | - | - |
| Erro et al., [43] 2014 | Longitudinal, 2 years | 10 CN with SMCs, 33 CN without SMCs, 13 MCI with SMCs, 20 MCI without SMCs | Subjective memory complaints | NMSQ Item 12, yes/no question, for memory | Answer “yes” | I | 23.3% | - | - | The presence of SMCs at baseline was an independent predictor of development of MCI at follow-up. | - |
| Galtier et al., [47] 2019 | Longitudinal, 7.5 years | 8 CN without SCD, 13 CN with SCD, 22 MCI, 20 healthy controls | Subjective cognitive decline | Questions for change in 5 cognitive functions | Answer “yes” in ≥ 1 domain | I | 61.9% of CN | No significant differences were found between healthy controls, CN without SCD, and CN with SCD. | No difference in depression score between CN with and without SCD. | Conversion to dementia during the follow-up was more frequent in patients with SCD (33.3%, 4/12) compared to patients without SCD (14.3%, 1/7). | - |
| Gasca-Salas et al., [35] 2020 | Longitudinal, 2 years | 81 CN, 57 MCI | Subjective cognitive complaints | NBI, 19 item questionnaire, yes/no questions | Used as a continuous variable | II | - | - | - | None of the NBI items predicted progression to MCI or dementia after 1 and 2 years in individuals who were PD-CN at baseline. | - |
| Han et al., [36] 2021 | Longitudinal, 3.19 years for PD without SCC and 2.97 years for PD with SCC | 59 CN with SCC, 189 CN without SCC, 135 MCI | Subjective cognitive complaint | MDS-UPDRS question 1.1, a single question, 0–4 scale | ≥ 1 | II | 23.8% of CN | At baseline, patients with SCC had worse performance in memory function than those without SCC. | At baseline, patients with SCC had a higher depression score than those without SCC. | Patients with SCC exhibited a greater reduction in attention and executive function than those without SCC. | - |
| Hong et al., [44] 2012 | Cross-sectional | 20 CN with SMCs, 15 CN without SMCs | Subjective memory complaints | A single question ‘‘Do you have any memory- related problems?’’ | Answer “yes” | I | 57.1% | Patients with SMCs showed poorer performance in semantic and phonemic fluency than those without SMCs. | Depression score did not differ between patients with and without SMCs. | - | Patients with SMCs showed decreased gray matter densities in the frontal and parietal areas than those without SMCs. |
| Hong et al., [49] 2014 | Cross-sectional | 49 SMCs with PD, 47 SMCs without PD, 23 healthy controls | Subjective cognitive decline | A single question “Do you feel that you have a declining memory?” | Answer “yes” | I | - | SMCs with PD had lower semantic fluency than SMCs without PD. | - | - | SMCs with PD had focal cortical thinning in the frontal, temporal, and occipital areas than SMCs without PD. |
| Hong et al., [48] 2014 | Longitudinal, 2.4 years | 25 CN with SMCs, 21 CN without SMCs | Subjective cognitive decline | Single question “Do you feel that you have a declining memory?” | Answer “yes” | I | 54.3% | No difference in cognitive performance at baseline between patients with and without SMCs | Depression score did not differ between patients with and without SMCs | Patients with SMCs converted to MCI more frequently and showed more rapid decline in semantic fluency and visual memory than those without SMCs. | - |
| Jenny et al., [45] 2020 | Cross-sectional | 46 CN | Subjective memory complaints | Questions for difficulty in cognitive abilities, 7 items | Answer “yes” in ≥ 1 item | II | Not reported | - | SMCs were significantly correlated with depression score. | - | - |
| Lehrner et al., [46] 2014 | Cross-sectional | 16 CN, 5 MCI (single domain), 83 MCI (multiple domains) | Subjective memory complaints | FAI, 16 items, 1-5 Likert scale | FAI z score ≤ -1.5 | I | 6.3% of CN | - | No significant difference between depressed and nondepressed patients with PD-CN. | - | - |
| Mills et al., [37] 2020 | Longitudinal, 3 years | 324 CN | Subjective cognitive complaints/ subjective cognitive decline | Neuro-QoL: GC and EF | Used the t-score as a continuous variable | I | - | MoCA memory domains were associated with Neuro-QoL GC and EF. | Depression scores were associated with lower Neuro-QoL-GC and -EF. | Increasing SCCs in Neuro-QoL-EF were associated with development of MCI over 3 years of follow-up. | - |
| Ophey et al., [50] 2022 | Cross-sectional | 18 CN with SCD, 12 CN without SCD | Subjective cognitive decline | SCD-Q, a complex scale (0–60) | ≥ 1 point in ≥ 2 domains | II | 60% | - | Depression score is a predictor of SCD. | - | Higher SCD score was correlated with decreased metabolism in many areas scattered in the frontal, temporal, parietal, and occipital areas, but it was not significant after FDR correction. |
| Pan et al., [38] 2021 | Cross- sectional | 46 CN, 53 MCI | Subjective cognitive complaints | CCI, 10-items, yes/no questions | CCI > 3 “yes” | II | 26.1% of CN | Time of Stroop color-word test was associated with presence of SCCs. | No difference in depression and anxiety between patients with and without SCCs. | - | - |
| Purri et al., [39] 2020 | Longitudinal, 5 years | 153 CN | Subjective cognitive complaint | Single question “Do you feel that your memory and thinking have gotten worse?” | Yes | I | 81 (53%) patients | No between-group differences in global cognition at baseline | The SCC groups had significantly higher depression scores at baseline. | The SCC group declined more than the non-SCC group on global cognition, processing speed, and executive function, although the results were not significant after correction for multiple testing. The SCC group was more likely to progress to cognitive impairment over time. | |
| van Balkom et al., [40] 2022 | Clinical trial | 28 CN, 16 MCI (single domain), 69 MCI (multiple domain), 23 PDD | Subjective cognitive complaints | PD-CFRS, 12 items, 0–2 Likert scale | PD-CFRS > 3 | II | Only patients with SCCs were enrolled. | - | - | Cognitive training did not improve cognitive function or reduce the severity of SCCs in cognitively normal patients with SCCs. | - |
| Xiao et al., [41] 2021 | Cross- sectional | 332 CN (134 EOPD, 198 LOPD) | Subjective cognitive complaints | MDS-UPDRS question 1.1, a single question, 0–4 scale | ≥ 1 | I | 22.3% (18.7% EOPD, 24.7% LOPD) | EOPD-SCCs had lower scores in MoCA domains of visuospatial/ executive abilities and memory and lower total MoCA scores. LOPD-SCCs had lower scores in MoCA memory domains. | Both EOPD and LOPD with SCCs had higher scores in anxiety, depression, and apathy scales than those without SCCs. | - | - |
SCC, subjective cognitive complaint; PD, Parkinson’s disease; CN, cognitively normal; MCI, mild cognitive impairment; PDD, Parkinson’s disease with dementia; SMC, subjective memory complaint; SCD, subjective cognitive decline; EOPD, early-onset PD < 50 years; LOPD, late-onset PD ≥ 50 years; NBI, Neurobehavioral Inventory; MDS-UPDRS, Movement Disorders Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale; NMSS, Non-Motor Symptom Scale for Parkinson’s Disease; MAC-Q, Memory Complaints Questionnaire; NMSQ, Nonmotor Symptoms Questionnaire; FAI, Forgetfulness Assessment Inventory; Neuro-QoL, Quality of Life in Neurological Disorders; GC, General Concerns; EF, Executive Function; SCD-Q, SCD-questionnaire; CCI, Cognitive Complaint Interview; PD-CFRS, PD-Cognitive Functional Rating Scale; MoCA, Montreal Cognitive Assessment; FDR, false discovery rate.
Terminologies for subjective cognitive complaints
Various terms have been used to indicate SCCs [22], which were originally introduced in the AD spectrum; hence, the subjective feeling of memory impairment was considered the preclinical stage of MCI due to AD [23-28]. However, the conceptual framework proposed by the SCD Initiative Working Group does not restrict subjective impairment to the memory domain [10]. Cognitive impairment in PD also affects multiple cognitive domains [29-31], and the term “cognitive” is recommended to indicate subjective impairment. In the selected studies of this review, the term “SCCs” was the most frequently used (10 studies) [32-41], and “subjective memory complaints (SMCs)” [42-46] and “SCD” [37,47-50] were both used in five studies each. Mills et al. [37] used both “SCCs” and “SCD.” Early studies used the term “memory,” [43,44,46] which seems to be influenced by the term SMCs from the AD field. However, since 2019, when studies on SCCs began to expand, all studies assessed diverse cognitive functions to define SCCs and used the term “cognitive” to indicate SCCs. One study that used “memory” also assessed cognitive functions, including memory; hence, it should have used the term “cognitive.” [45] The term “complaints” (15 times) was more frequently used than “decline” (five times), but there is no consensus on which is more appropriate. Self-perceived cognitive impairment has been studied at all cognitive levels, including MCI and dementia [13,14,17,33,51]. In studies of SCCs in PD, SCCs do not refer to a particular cognitive level but to a subjective symptom [32-35,38,42,43,46]. Therefore, when indicating SCCs in patients with PD, the cognitive level of the patients must be determined. Meanwhile, since a conceptual framework for SCD in preclinical AD was introduced, SCD is generally recognized to indicate a status affiliated with the preclinical stage of AD rather than a symptom, even if there is no expression of “pre-MCI.” [9,10] Since unification in terminology and concepts is important to set further research targets, a consensus for terminology and definition of SCCs in PD is needed.
Assessment of subjective cognitive complaints
Most studies assessed SCCs using a single question or questionnaire. Eight studies determined the presence of SCCs through a single question regarding participants’ memory [32,43,44,48,49] or general cognition [32,36,39,41]. Among them, four studies assessed SCCs using part of a large scale: the Nonmotor Symptoms Questionnaire [52] Item 12 [43] and the MDS-Unified PD Rating Scale (UPDRS) [53] question 1.1 [32,36,41]. Two studies used questions on patients’ performance in five [47] or seven [45] cognitive domains. Ten studies assessed SCCs using questionnaires administered to patients: Non-Motor Symptoms Scale for Parkinson’s Disease [54] domain 5 (two studies) [33,34], Memory Complaints Questionnaire [55] (one study) [42], Neurobehavioral Inventory (NBI) [56] (two studies) [32,35], Quality of Life in Neurological Disorders [57]- Cognition: General Concerns and Cognition: Executive Function (one study) [37], Forgetfulness Assessment Inventory [58] (one study) [46], Subjective Cognitive Decline Questionnaire (one study) [50], Cognitive Complaint Interview [59] (one study) [38], and Parkinson’s Disease Cognitive Functional Rating Scale [60] (one study) [40]. One study simultaneously examined the relationship between SCC and cognitive performance through NBI, a single question, and MDS-UPDRS question 1.1 [32]. To simplify the quantification of SCCs, several questionnaires for SCCs have recently been validated in patients with PD [13,14,16,60,61]. However, there is no evidence to support the superiority of the questionnaire over a single question. AlDakheel et al. [32] explored the predictive ability of three methods, but they failed to reach a conclusion because none of the methods predicted cognitive decline over time. Moreover, many questionnaires assess difficulties in daily activities due to cognitive dysfunction [14,51,60,62], which is similar to the method used to assess functional decline in cognitively impaired patients. Therefore, further research and discussion are needed to answer questions about the proper approach to assess SCCs and the more relevant aspects of the clinical implications of SCCs.
Frequency of subjective cognitive complaints
Thirteen studies provided frequency data. The studies included 438 patients with SCCs (36.3%) out of 1,207 cognitively normal patients. The reported frequencies varied widely, ranging from 6.3% to 82.9% (median, 48.8%).
Objective measurements for cognition
Details of the neuropsychological assessments performed in the included studies are summarized in Table 2. All studies assessed global cognition using the Mini-Mental State Examination (MMSE) [63], Montreal Cognitive Assessment (MoCA) [64], Parkinson Neuropsychometric Dementia Assessment (PANDA) [65], Scales for Outcomes in Parkinson’s Disease–COGnition (SCOPA-COG) [66], and Mattis Dementia Rating Scale-2 (MDRS-2) [67]. Seven and four studies used only the MMSE or MoCA, respectively, and three studies adopted both the MMSE and MoCA. SCOPA-COG was used in two studies, and MDRS-2 and PANDA were used in one study each. SCOPA-COG, MDRS-2, and PANDA were used combined with MMSE or MoCA.
Table 2.
Details of the neuropsychological assessment of the included studies
| Study | Global cognition | Attention and working memory | Executive function | Language | Memory | Visuospatial function | Anxiety, depression, and apathy |
|---|---|---|---|---|---|---|---|
| AlDakheel et al., [32] 2019 | SCOPA-COG, MoCA, MMSE | Color word interference color naming test, letter-number sequencing test | TMT-B minus A, visual verbal test | Category fluency test, BNT | RCFT delayed recall, CVLT delayed recall | JLOT, RCFT copy | - |
| Barbosa et al., [33] 2019 | MoCA | - | - | - | - | - | HADS-D, HADS-A, AS |
| Baschi et al., [42] 2018 | MoCA, MMSE | TMT-A, visual search | Raven’s progressive matrices, FAB | Token Test, Aachner Aphasie test –naming subtest | Story Recall Test, RAVLT | Constructional apraxia, Clock drawing test | HADS-D, HADS-A |
| Chua et al., [34] 2021 | MoCA, MMSE | WMS-IV Symbol Span, WAIS-IV Digit Span | Fruit Fluency tests, FAB | BNT, WAIS-IV Similarities | RCFT delayed recall, ADAS-Cog delayed recall | JLOT, RCFT copy | HADS-A, GDS, AS |
| Erro et al., [43] 2014 | - | (Attention/executive function domain), FAB, Phonemic/semantic fluency, RCFT copy, Corsi block test, Verbal span test, TMT-B minus A, Stroop color-word test | (Attention/executive function domain), FAB, Phonemic/semantic fluency, RCFT copy, Corsi block test, Verbal span test, TMT-B minus A, Stroop color-word test | - | RAVLT, RCFT recall | JLOT, Constructional apraxia test, Clock drawing test | HADS-D, HADS-A |
| Galtier et al., [47] 2019 | MMSE | Digit span–backward, Stroop color-word test | Verbal fluency test WCST | The Naming Test | CVLT, Spatial Recall Test | JLOT, WAIS-III Block design | BDI |
| Gasca-Salas et al., [35] 2020 | SCOPA-COG, MMSE | Color word interference color naming test, Letter-number sequencing test | TMT-B minus A, Visual verbal test | Category fluency test, BNT | RCFT delayed recall, CVLT delayed recall | JLOT, RCFT copy | GDS |
| Han et al., [36] 2021 | MMSE | SDMT, TMT-A | Stroop color-word test, TMT-B | BNT, Semantic fluency test | AVLT, RCFT delayed recall | RCFT, Clock drawing test | BDI |
| Hong et al., [44] 2012 | MMSE | Digit span–forward, Digit span–backward | Phonemic fluency test, Semantic fluency test, Stroop test | BNT | RCFT recall, SVLT | RCFT copy | BDI |
| Hong et al., [49] 2014 | MMSE | Digit span–forward, Stroop color test | Phonemic fluency test, Semantic fluency test | BNT | RCFT delayed recall, SVLT delayed recall | RCFT copy | BDI |
| Hong et al., [48] 2014 | MMSE | Digit span–forward, Stroop color test | Phonemic fluency test, Semantic fluency test | BNT | RCFT delayed recall, SVLT delayed recall | RCFT copy | BDI |
| Jenny et al., [45] 2020 | MMSE | Not declared | Not declared | Not declared | Not declared | Not declared | BDI, BAI |
| Lehrner et al., [46] 2014 | MMSE | AKT, Digit symbol test, Symbol counting, TMT-B, TMT-B minus A | TMT-A, Planning Maze test, Five Point test, Stroop test, Phonemic fluency test | Semantic fluency test, BNT | Verbal selective reminding test | - | BDI-II |
| Mills et al., [37] 2020 | MoCA | MoCA items–attention and language | (Visuospatial/executive function domain) MoCA items–TMT, cube copy, and clock copy | MoCA items–language, naming, and abstraction | MoCA items– delayed recall and orientation | (Visuospatial/ executive function domain) MoCA items–TMT, cube copy, and clock copy | BDI |
| Ophey et al., [50] 2022 | MMSE, PANDA | Digit span–forward, Digit span–backward | WCST, Alternating category fluency test, Phonemic fluency test | BNT, MMSE language task, Semantic fluency test | PANDA learning, PANDA delayed recall, MMSE delayed recall | Block span–forward, Block span– backward, MMSE pentagon, PANDA spatial imagery task | BDI-II |
| Pan et al., [38] 2021 | MoCA, MMSE | Digit span–backward, TMT-A, Stroop color-word test | TMT-B, Clock drawing test, Verbal fluency test | BNT, WAIS-III Similarities | AVLT, LMT | JLOT, HVOT | HADS-D, HADS-A |
| Purri et al., [39] 2020 | MoCA, MDRS-2 | TMT-A, SDMT | Letter-number sequencing, Phonemic fluency test, TMT-B | BNT, Semantic fluency test | HVLT-R | JLOT, Clock drawing test | GDS-15 |
| van Balkom et al., [40] 2022 | MoCA | Stroop color-word test, Digit span | Pentagon copy, Tower of London, Letter fluency test | BNT, Category fluency test | RAVLT, Location learning test | RCFT, Visual form discrimination test | PAS, AS, BDI |
| Xiao et al., [41] 2021 | MoCA | MoCA attention domain | MoCA visuospatial/ executive ability domain | MoCA language, naming domains | MoCA memory domain | MoCA visuospatial/ executive ability domain | HADS-D, HADS-A, LARS |
SCOPA-COG, Scales for Outcomes in Parkinson’s Disease-COGnition; MoCA, Montreal Cognitive Assessment; MMSE, Mini-Mental State Examination; PANDA, Parkinson Neuropsychometric Dementia Assessment; MDRS-2, Mattis Dementia Rating Scale-2; TMT, Trail-Making Test; WMS, Wechsler Memory Scale; WAIS, Wechsler Adult Intelligence Scale; FAB, Frontal Assessment Battery; RCFT, Rey-Osterrieth Complex Figure Test; SDMT, Symbol Digit Modality Test; AKT, Alters-Konzentrations-Test; WCST, Wisconsin Card Sorting Test; BNT, Boston Naming Test; CVLT, California Verbal Learning Test; RAVLT, Rey Auditory Verbal Learning Test; ADAS-Cog, Alzheimer’s Disease Assessment Scale-Cognitive; LMT, Logical Memory Test; HLVT-R, Hopkins Verbal Learning Test-Revised; JLOT, Judgment of Line Orientation Test; HVOT, Hooper Visual Organization Test; HADSD, Hospital Anxiety Depression Scale, depression subscale; HADS-A, Hospital Anxiety Depression Scale, Anxiety subscale; AS, Apathy Scale; GDS, Geriatric Depression Scale; BDI, Beck Depression Inventory; SVLT, Seoul Verbal Learning Test; BAI, Beck Anxiety Inventory; AVLT, Auditory Verbal Learning Test; PAS, Parkinson Anxiety Scale; LARS, Lille Apathy Rating Scale.
Sixteen studies measured objective cognition using a comprehensive neuropsychological battery. Among them, nine studies involved at least two tests for each of the five cognitive domains according to the recommendation for the level II criteria of PD-MCI. Jenny et al. [45] excluded patients in whom MCI was diagnosed according to the level II PD-MCI criteria, but the details of the neuropsychological assessment were not described in their report. Two studies identified cognitive domains using MoCA items [37,41], and Barbosa et al. [33] selected cognitively normal patients based only on the MoCA total score.
Association between subjective cognitive complaints and objective cognitive performance
SCCs are considered a potential risk factor for future cognitive decline, and many researchers have speculated about subtle cognitive decline in cognitively normal patients with SCCs, even when they show normal performance on standardized cognitive tests. Eleven of the selected studies reported a relationship between SCCs and objective cognitive function [33,36-39,41,42,44,47-49]. In six studies, patients with SCCs showed lower performance in visuospatial function [41,42], verbal fluency [44], inhibitory control ability [38], and memory function [36,37,41] in comparison with patients without SCCs. However, no significant differences between patients with and without SCCs were found in three studies [39,47,48]. In one study, the presence of SCC was associated with a lower MoCA score, but this association was lost when adjusted for depressive symptoms [33]. Hong et al. [49] also reported lower semantic fluency in PD patients with SCCs than in non-PD individuals with SCCs. It remains unclear whether there is minimal objective cognitive decline in cognitively normal patients with SCCs; however, it is noteworthy that three [36,37,41] of four studies with large sample sizes (n > 150) [36,37,39,41] commonly reported lower memory function in participants with SCCs than in those without SCCs.
The predictive value of subjective cognitive complaints for future cognitive decline
To demonstrate that SCCs can predict future cognitive deterioration, it is important to prove their clinical significance. Eight studies investigated whether SCCs are associated with future cognitive decline. Among them, five studies reported that SCCs were predictive of subsequent cognitive impairment. Patients with SCCs exhibited a greater reduction in attention [36,39], executive function [36,39,48], and memory function [48] than those without SCCs. The presence of SCCs at baseline was associated with more frequent development of MCI at follow-up assessments in the 2-year [43], 2.4-year [48], and 3-year [37] longitudinal observations. Purri et al. [39] demonstrated that patients with baseline SCCs were more likely to progress to MCI or dementia in a 5-year follow-up study. Galtier et al. [47] also reported that conversion to dementia was more frequent in patients with SCCs (4 of 12) than in those without SCCs (1 of 7), but the difference was not statistically significant due to the small sample size. However, two studies sharing cohort data showed conflicting results. AlDakheel et al. [32] assessed SCCs using three tools (NBI, single question, and MDS-UPDRS question 1.1), and none predicted cognitive decline. Moreover, none of the NBI items predicted the development of cognitive impairment in cognitively normal patients at baseline [35]. Although diversity in the measurement of SCCs, follow-up duration, assessment of cognitive performance, and sample size might have contributed to the conflicting results, the consistent results justify the significance of SCCs. Therefore, research focusing on defining and assessing SCCs for the best prediction of future decline is expected to be planned in the future.
Association between subjective cognitive complaints and psychiatric problems
Since the development of the concept of SCCs, the possibility that SCCs are another facet of depression has been debated. Memory complaints are a symptom of depression [68], which is common even in the early stages of PD [69]. Therefore, many studies on SCCs in PD have assessed depressive symptoms. Eight of the selected studies reported that SCCs were associated with depression [33,34,36,37,39,41,45,50], whereas six studies did not observe a significant association [38,42,44,46-48]. In particular, two studies reported that the impact of SCCs on lower cognitive performance [33] and faster cognitive decline [39] disappeared when adjusted for depressive scores. Although the association between depression and SCCs remains controversial, it has been suggested that depression is worth assessing in all research on SCCs in patients with PD.
Other psychiatric problems, including anxiety and apathy, also occur frequently in PD [70,71]. Anxiety and apathy were less frequently explored but appeared to be related to SCCs in most studies. Four studies reported an association between SCCs and anxiety [33,34,41,42], whereas two studies did not [38,45]. Only three studies [33,34,41] assessed apathy, but all reported a significant association between apathy and SCCs [33,34,40,41]. In addition, they related to SCCs in the same way as depression in four [33,34,38,41] out of six studies [33,34,38,41,42,45]; therefore, whether anxiety and apathy are independent of depression should be explored.
Biomarkers for subjective cognitive complaints
Biomarkers can provide convincing evidence that SCCs are associated with pathological changes in PD or other coexisting conditions. However, only three neuroimaging studies have been conducted in this regard. The first voxel-based morphometry study demonstrated decreased gray matter density in the frontal and parietal areas and poorer performance on verbal fluency and attention tasks in cognitively normal patients with SMCs than in patients without SMCs [44]. Another study reported that cognitively normal PD patients with SMCs showed less cortical thickness in the frontal, parahippocampal, and occipital areas and poorer performance in semantic fluency than cognitively normal nonPD subjects with SMCs [49]. A recent positron emission tomography study found a correlation between higher SCC scores and decreased metabolism in the right angular gyrus, bilateral middle temporal gyrus, bilateral occipital regions, and left middle frontal gyrus [50]. However, the significance of all three studies was observed in the uncorrected analyses and disappeared after false discovery rate correction. The observed changes in patients with SCCs seem to be related to PD-specific features; however, it is not evident that SCCs reflect a pathological burden related to cognitive impairment in PD.
Many biomarkers are linked to cognitive impairment in PD, including genetic mutations or variants in APOE [72,73], GBA [74,75], MAPT [76], amyloid beta 1–42 concentration in the cerebrospinal fluid [77,78], and positron emission tomography imaging findings for abnormal protein aggregation [79-81]. The concept of SCCs in PD as a prodromal symptom of cognitive impairment is controversial; therefore, research demonstrating a significant association between SCCs and these biomarkers could justify the clinical importance of SCCs.
Clinical trials for subjective cognitive complaints
Only one clinical trial has been conducted on patients with SCCs [40], and the trial aimed to assess the efficacy of cognitive training on cognitive function in patients with PD. Patients with SCCs, regardless of their cognitive level (n = 140), were enrolled and randomized. The experimental and active control groups were trained for eight weeks using online-based cognitive training or nonspecific cognitive engagement, respectively. The results showed no group differences in the Tower of London accuracy in both analyses with the entire population and the cognitively normal subgroup (15 cognitive training and 13 active control).
CONCLUSION
The techniques used for the assessment of SCCs show a wide range of methodological differences. Accordingly, the frequency of SCCs has varied, and the results from previous studies have been inconsistent. This inconsistency has been a major obstacle to the accumulation of evidence and reproduction of reported results. Thus, there is a clear need to reach a consensus on the definition and assessment of SCCs in cognitively normal PD patients.
Previous studies have reported relatively low performance in particular cognitive domains in cognitively normal patients with SCCs; however, the actual association of SCCs with subtle cognitive impairments remains unclear. Meanwhile, the presence of SCCs at baseline is related to faster cognitive decline or more frequent conversion to MCI or dementia in most studies, which strongly suggests the clinical importance of SCCs in the preclinical stage of cognitive decline. Only a few biomarker studies have described the organic changes relevant to cognitive impairment in PD. The accumulation of data from biomarker studies will provide powerful evidence for the existence of SCCs and reveal the pathophysiological characteristics of SCCs.
Footnotes
Conflicts of Interest
The authors have no financial conflicts of interest.
Funding Statement
This work was supported by a grant from the Korea Health Technology R&D Project through the Korean Healthy Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number HU21C0053).
Author Contributions
Conceptualization: Jin Yong Hong, Phil Hyu Lee. Data curation: Jin Yong Hong. Formal analysis: Jin Yong Hong. Funding acquisition: Phil Hyu Lee. Investigation: Jin Yong Hong, Phil Hyu Lee. Methodology: Jin Yong Hong. Supervision: Phil Hyu Lee. Writing—original draft: Jin Yong Hong. Writing review & editing: Phil Hyu Lee.
REFERENCES
- 1.Schrag A, Jahanshahi M, Quinn N. What contributes to quality of life in patients with Parkinson’s disease? J Neurol Neurosurg Psychiatry. 2000;69:308–312. doi: 10.1136/jnnp.69.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goetz CG, Emre M, Dubois B. Parkinson’s disease dementia: definitions, guidelines, and research perspectives in diagnosis. Ann Neurol. 2008;64 Suppl 2:S81–S92. doi: 10.1002/ana.21455. [DOI] [PubMed] [Google Scholar]
- 3.Riedel O, Klotsche J, Spottke A, Deuschl G, Förstl H, Henn F, et al. Frequency of dementia, depression, and other neuropsychiatric symptoms in 1,449 outpatients with Parkinson’s disease. J Neurol. 2010;257:1073–1082. doi: 10.1007/s00415-010-5465-z. [DOI] [PubMed] [Google Scholar]
- 4.Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov Disord. 2008;23:837–844. doi: 10.1002/mds.21956. [DOI] [PubMed] [Google Scholar]
- 5.Hoogland J, Boel JA, de Bie RMA, Geskus RB, Schmand BA, DalrympleAlford JC, et al. Mild cognitive impairment as a risk factor for Parkinson’s disease dementia. Mov Disord. 2017;32:1056–1065. doi: 10.1002/mds.27002. [DOI] [PubMed] [Google Scholar]
- 6.Litvan I, Goldman JG, Tröster AI, Schmand BA, Weintraub D, Petersen RC, et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Mov Disord. 2012;27:349–356. doi: 10.1002/mds.24893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kjeldsen PL, Damholdt MF. Subjective cognitive complaints in patients with Parkinson’s disease. Acta Neurol Scand. 2019;140:375–389. doi: 10.1111/ane.13158. [DOI] [PubMed] [Google Scholar]
- 8.Oedekoven C, Egeri L, Jessen F, Wagner M, Dodel R. Subjective cognitive decline in idiopathic Parkinson’s disease: a systematic review. Ageing Res Rev. 2022;74:101508. doi: 10.1016/j.arr.2021.101508. [DOI] [PubMed] [Google Scholar]
- 9.Jessen F, Amariglio RE, Buckley RF, van der Flier WM, Han Y, Molinuevo JL, et al. The characterisation of subjective cognitive decline. Lancet Neurol. 2020;19:271–278. doi: 10.1016/S1474-4422(19)30368-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jessen F, Amariglio RE, van Boxtel M, Breteler M, Ceccaldi M, Chételat G, et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement. 2014;10:844–852. doi: 10.1016/j.jalz.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Copeland JN, Lieberman A, Oravivattanakul S, Tröster AI. Accuracy of patient and care partner identification of cognitive impairments in Parkinson’s disease–mild cognitive impairment. Mov Disord. 2016;31:693–698. doi: 10.1002/mds.26619. [DOI] [PubMed] [Google Scholar]
- 13.Hong JY, Lee Y, Sunwoo MK, Sohn YH, Lee PH. Subjective cognitive complaints and objective cognitive impairment in Parkinson’s disease. J Clin Neurol. 2018;14:16–21. doi: 10.3988/jcn.2018.14.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ko J, Ha J, Lee JJ, Jin S, Lee J, Baek MS, et al. Reliability and validity of the subjective cognitive complaints questionnaire for Parkinson’s disease (SCCQ-PD) J Clin Neurol. 2022;18:171–178. doi: 10.3988/jcn.2022.18.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dujardin K, Duhamel A, Delliaux M, Thomas-Antérion C, Destée A, Defebvre L. Cognitive complaints in Parkinson’s disease: its relationship with objective cognitive decline. J Neurol. 2010;257:79–84. doi: 10.1007/s00415-009-5268-2. [DOI] [PubMed] [Google Scholar]
- 16.Koster DP, Higginson CI, MacDougall EE, Wheelock VL, Sigvardt KA. Subjective cognitive complaints in Parkinson disease without dementia: a preliminary study. Appl Neuropsychol Adult. 2015;22:287–292. doi: 10.1080/23279095.2014.925902. [DOI] [PubMed] [Google Scholar]
- 17.Rattanabannakit C, Risacher SL, Gao S, Lane KA, Brown SA, McDonald BC, et al. The cognitive change index as a measure of self and informant perception of cognitive decline: relation to neuropsychological tests. J Alzheimers Dis. 2016;51:1145–1155. doi: 10.3233/JAD-150729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sitek EJ, Sołtan W, Wieczorek D, Robowski P, Sławek J. Self-awareness of memory function in Parkinson’s disease in relation to mood and symptom severity. Aging Ment Health. 2011;15:150–156. doi: 10.1080/13607863.2010.508773. [DOI] [PubMed] [Google Scholar]
- 19.Castro PC, Aquino CC, Felício AC, Doná F, Medeiros LM, Silva SM, et al. Presence or absence of cognitive complaints in Parkinson’s disease: mood disorder or anosognosia? Arq Neuropsiquiatr. 2016;74:439–444. doi: 10.1590/0004-282X20160060. [DOI] [PubMed] [Google Scholar]
- 20.Santangelo G, Vitale C, Trojano L, Angrisano MG, Picillo M, Errico D, et al. Subthreshold depression and subjective cognitive complaints in Parkinson’s disease. Eur J Neurol. 2014;21:541–544. doi: 10.1111/ene.12219. [DOI] [PubMed] [Google Scholar]
- 21.Siciliano M, Trojano L, De Micco R, Russo A, Tedeschi G, Tessitore A. Subjective memory decline in Parkinson’s disease patients with and without fatigue. Parkinsonism Relat Disord. 2020;70:15–19. doi: 10.1016/j.parkreldis.2019.11.017. [DOI] [PubMed] [Google Scholar]
- 22.Abdulrab K, Heun R. Subjective memory impairment. A review of its definitions indicates the need for a comprehensive set of standardised and validated criteria. Eur Psychiatry. 2008;23:321–330. doi: 10.1016/j.eurpsy.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Barnes LL, Schneider JA, Boyle PA, Bienias JL, Bennett DA. Memory complaints are related to Alzheimer disease pathology in older persons. Neurology. 2006;67:1581–1585. doi: 10.1212/01.wnl.0000242734.16663.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jorm AF, Christensen H, Korten AE, Jacomb PA, Henderson AS. Memory complaints as a precursor of memory impairment in older people: a longitudinal analysis over 7-8 years. Psychol Med. 2001;31:441–449. [PubMed] [Google Scholar]
- 25.Kryscio RJ, Abner EL, Cooper GE, Fardo DW, Jicha GA, Nelson PT, et al. Self-reported memory complaints: implications from a longitudinal cohort with autopsies. Neurology. 2014;83:1359–1365. doi: 10.1212/WNL.0000000000000856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stewart R, Godin O, Crivello F, Maillard P, Mazoyer B, Tzourio C, et al. Longitudinal neuroimaging correlates of subjective memory impairment: 4-year prospective community study. Br J Psychiatry. 2011;198:199–205. doi: 10.1192/bjp.bp.110.078683. [DOI] [PubMed] [Google Scholar]
- 27.Striepens N, Scheef L, Wind A, Popp J, Spottke A, Cooper-Mahkorn D, et al. Volume loss of the medial temporal lobe structures in subjective memory impairment. Dement Geriatr Cogn Disord. 2010;29:75–81. doi: 10.1159/000264630. [DOI] [PubMed] [Google Scholar]
- 28.Wang L, van Belle G, Crane PK, Kukull WA, Bowen JD, McCormick WC, et al. Subjective memory deterioration and future dementia in people aged 65 and older. J Am Geriatr Soc. 2004;52:2045–2051. doi: 10.1111/j.1532-5415.2004.52568.x. [DOI] [PubMed] [Google Scholar]
- 29.Hanagasi HA, Tufekcioglu Z, Emre M. Dementia in Parkinson’s disease. J Neurol Sci. 2017;374:26–31. doi: 10.1016/j.jns.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 30.Kalbe E, Rehberg SP, Heber I, Kronenbuerger M, Schulz JB, Storch A, et al. Subtypes of mild cognitive impairment in patients with Parkinson’s disease: evidence from the LANDSCAPE study. J Neurol Neurosurg Psychiatry. 2016;87:1099–1105. doi: 10.1136/jnnp-2016-313838. [DOI] [PubMed] [Google Scholar]
- 31.Kehagia AA, Barker RA, Robbins TW. Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson’s disease. Lancet Neurol. 2010;9:1200–1213. doi: 10.1016/S1474-4422(10)70212-X. [DOI] [PubMed] [Google Scholar]
- 32.AlDakheel A, Gasca-Salas C, Armstrong MJ, Duff-Canning S, Marras C. Cognitive complaints in nondemented Parkinson’s disease patients and their close contacts do not predict worse cognitive outcome. Alzheimer Dis Assoc Disord. 2019;33:147–153. doi: 10.1097/WAD.0000000000000301. [DOI] [PubMed] [Google Scholar]
- 33.Barbosa RP, Mendonça MD, Caetano AP, Lampreia TM, Miguel R, Bugalho PM. Cognitive complaints in Parkinson’s disease patients: from subjective cognitive complaints to dementia and affective disorders. J Neural Transm (Vienna) 2019;126:1329–1335. doi: 10.1007/s00702-019-02042-8. [DOI] [PubMed] [Google Scholar]
- 34.Chua CY, Koh MRE, Chia NS, Ng SY, Saffari SE, Wen MC, et al. Subjective cognitive complaints in early Parkinson’s disease patients with normal cognition are associated with affective symptoms. Parkinsonism Relat Disord. 2021;82:24–28. doi: 10.1016/j.parkreldis.2020.11.013. [DOI] [PubMed] [Google Scholar]
- 35.Gasca-Salas C, Duff-Canning S, Armstrong MJ, Eslinger PJ, Schneider RB, Kennedy N, et al. Parkinson disease with mild cognitive impairment: domain-specific cognitive complaints predict dementia. Acta Neurol Scand. 2020;142:585–596. doi: 10.1111/ane.13326. [DOI] [PubMed] [Google Scholar]
- 36.Han LL, Wang L, Xu ZH, Liang XN, Zhang MW, Fan Y, et al. Disease progression in Parkinson’s disease patients with subjective cognitive complaint. Ann Clin Transl Neurol. 2021;8:2096–2104. doi: 10.1002/acn3.51461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mills KA, Schneider RB, Saint-Hilaire M, Ross GW, Hauser RA, Lang AE, et al. Cognitive impairment in Parkinson’s disease: associations between subjective and objective cognitive decline in a large longitudinal study. Parkinsonism Relat Disord. 2020;80:127–132. doi: 10.1016/j.parkreldis.2020.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pan C, Ren J, Hua P, Yan L, Yu M, Wang Y, et al. Subjective cognitive complaints in newly-diagnosed Parkinson’s disease with and without mild cognitive impairment. Front Neurosci. 2021;15:761817. doi: 10.3389/fnins.2021.761817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Purri R, Brennan L, Rick J, Xie SX, Deck BL, Chahine LM, et al. Subjective cognitive complaint in Parkinson’s disease patients with normal cognition: canary in the coal mine? Mov Disord. 2020;35:1618–1625. doi: 10.1002/mds.28115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Balkom TD, Berendse HW, van der Werf YD, Twisk JWR, Peeters CFW, Hoogendoorn AW, et al. Effect of eight-week online cognitive training in Parkinson’s disease: a double-blind, randomized, controlled trial. Parkinsonism Relat Disord. 2022;96:80–87. doi: 10.1016/j.parkreldis.2022.02.018. [DOI] [PubMed] [Google Scholar]
- 41.Xiao Y, Ou R, Yang T, Liu K, Wei Q, Hou Y, et al. Different associated factors of subjective cognitive complaints in early-and late-onset Parkinson’s disease patients. Front Neurol. 2021;12:749471. doi: 10.3389/fneur.2021.749471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baschi R, Nicoletti A, Restivo V, Recca D, Zappia M, Monastero R. Frequency and correlates of subjective memory complaints in Parkinson’s disease with and without mild cognitive impairment: data from the Parkinson’s disease cognitive impairment study. J Alzheimers Dis. 2018;63:1015–1024. doi: 10.3233/JAD-171172. [DOI] [PubMed] [Google Scholar]
- 43.Erro R, Santangelo G, Barone P, Picillo M, Amboni M, Longo K, et al. Do subjective memory complaints herald the onset of mild cognitive impairment in Parkinson disease? J Geriatr Psychiatry Neurol. 2014;27:276–281. doi: 10.1177/0891988714532015. [DOI] [PubMed] [Google Scholar]
- 44.Hong JY, Lee JE, Sohn YH, Lee PH. Neurocognitive and atrophic patterns in Parkinson’s disease based on subjective memory complaints. J Neurol. 2012;259:1706–1712. doi: 10.1007/s00415-011-6404-3. [DOI] [PubMed] [Google Scholar]
- 45.Jenny AL, Meyer A, Handabaka I, Calabrese P, Fuhr P, Gschwandtner U. Nonmotor-related quality of life in Parkinson’s patients with subjective memory complaints: comparison with PDQ-39. Parkinsons Dis. 2020;2020:7953032. doi: 10.1155/2020/7953032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lehrner J, Moser D, Klug S, Gleiß A, Auff E, Pirker W, et al. Subjective memory complaints, depressive symptoms and cognition in Parkinson’s disease patients. Eur J Neurol. 2014;21:1276–1284.:e77. doi: 10.1111/ene.12470. [DOI] [PubMed] [Google Scholar]
- 47.Galtier I, Nieto A, Lorenzo JN, Barroso J. Subjective cognitive decline and progression to dementia in Parkinson’s disease: a long-term follow-up study. J Neurol. 2019;266:745–754. doi: 10.1007/s00415-019-09197-0. [DOI] [PubMed] [Google Scholar]
- 48.Hong JY, Sunwoo MK, Chung SJ, Ham JH, Lee JE, Sohn YH, et al. Subjective cognitive decline predicts future deterioration in cognitively normal patients with Parkinson’s disease. Neurobiol Aging. 2014;35:1739–1743. doi: 10.1016/j.neurobiolaging.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 49.Hong JY, Yun HJ, Sunwoo MK, Ham JH, Lee JM, Sohn YH, et al. Cognitive and cortical thinning patterns of subjective cognitive decline in patients with and without Parkinson’s disease. Parkinsonism Relat Disord. 2014;20:999–1003. doi: 10.1016/j.parkreldis.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 50.Ophey A, Krohm F, Kalbe E, Greuel A, Drzezga A, Tittgemeyer M, et al. Neural correlates and predictors of subjective cognitive decline in patients with Parkinson’s disease. Neurol Sci. 2022;43:3153–3163. doi: 10.1007/s10072-021-05734-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rami L, Mollica MA, García-Sanchez C, Saldaña J, Sanchez B, Sala I, et al. The subjective cognitive decline questionnaire (SCD-Q): a validation study. J Alzheimers Dis. 2014;41:453–466. doi: 10.3233/JAD-132027. [DOI] [PubMed] [Google Scholar]
- 52.Martinez-Martin P, Schapira AH, Stocchi F, Sethi K, Odin P, MacPhee G, et al. Prevalence of nonmotor symptoms in Parkinson’s disease in an international setting; study using nonmotor symptoms questionnaire in 545 patients. Mov Disord. 2007;22:1623–1629. doi: 10.1002/mds.21586. [DOI] [PubMed] [Google Scholar]
- 53.Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease The Unified Parkinson’s Disease Rating Scale (UPDRS): status and recommendations. Mov Disord. 2003;18:738–750. doi: 10.1002/mds.10473. [DOI] [PubMed] [Google Scholar]
- 54.Chaudhuri KR, Martinez-Martin P, Brown RG, Sethi K, Stocchi F, Odin P, et al. The metric properties of a novel non-motor symptoms scale for Parkinson’s disease: results from an international pilot study. Mov Disord. 2007;22:1901–1911. doi: 10.1002/mds.21596. [DOI] [PubMed] [Google Scholar]
- 55.Crook TH 3rd, Larrabee GJ. A self-rating scale for evaluating memory in everyday life. Psychol Aging. 1990;5:48–57. doi: 10.1037//0882-7974.5.1.48. [DOI] [PubMed] [Google Scholar]
- 56.Blumer D. In: Neuropsychiatry of personality disorders. Boston. Ratey JJ, editor. MA: Blackwell Science; 1995. The neurobehavioral inventory: personality disorders in epilepsy; pp. 230–263. [Google Scholar]
- 57.HealthMeasures Neuro QoL [Internet] HealthMeasures. [accessed on 2022 Mar 31]. Available from: https://www.healthmeasures.net/explore-measurement-systems/neuro-qol.
- 58.Kogler B. Subjective memory complaint in mild cognitive impairment, Alzheimer’s disease and Parkinson’s disease. Vienna: University of Vienna; 2013. [Google Scholar]
- 59.Thomas-Anterion C, Honore-Masson S, Laurent B. The cognitive complaint interview (CCI) Psychogeriatrics. 2006;6:S18–S22. [Google Scholar]
- 60.Kulisevsky J, Fernández de Bobadilla R, Pagonabarraga J, Martínez-Horta S, Campolongo A, García-Sánchez C, et al. Measuring functional impact of cognitive impairment: validation of the Parkinson’s disease cognitive functional rating scale. Parkinsonism Relat Disord. 2013;19:812–817. doi: 10.1016/j.parkreldis.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 61.Dupouy J, Ory-Magne F, Mekies C, Rousseau V, Puel M, Rerat K, et al. Cognitive complaint in early Parkinson’s disease: a pilot study. Acta Neurol Scand. 2018;137:59–66. doi: 10.1111/ane.12808. [DOI] [PubMed] [Google Scholar]
- 62.Farias ST, Mungas D, Reed BR, Cahn-Weiner D, Jagust W, Baynes K, et al. The measurement of everyday cognition (ECog): scale development and psychometric properties. Neuropsychology. 2008;22:531–544. doi: 10.1037/0894-4105.22.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 64.Gill DJ, Freshman A, Blender JA, Ravina B. The Montreal cognitive assessment as a screening tool for cognitive impairment in Parkinson’s disease. Mov Disord. 2008;23:1043–1046. doi: 10.1002/mds.22017. [DOI] [PubMed] [Google Scholar]
- 65.Kalbe E, Calabrese P, Kohn N, Hilker R, Riedel O, Wittchen HU, et al. Screening for cognitive deficits in Parkinson’s disease with the Parkinson neuropsychometric dementia assessment (PANDA) instrument. Parkinsonism Relat Disord. 2008;14:93–101. doi: 10.1016/j.parkreldis.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 66.Marinus J, Visser M, Verwey NA, Verhey FR, Middelkoop HA, Stiggelbout AM, et al. Assessment of cognition in Parkinson’s disease. Neurology. 2003;61:1222–1228. doi: 10.1212/01.wnl.0000091864.39702.1c. [DOI] [PubMed] [Google Scholar]
- 67.Mattis S. Dementia rating scale: professional manual. Odessa, FL: Psychological Assessment Resources; 1988. [Google Scholar]
- 68.McIntyre RS, Xiao HX, Syeda K, Vinberg M, Carvalho AF, Mansur RB, et al. The prevalence, measurement, and treatment of the cognitive dimension/domain in major depressive disorder. CNS Drugs. 2015;29:577–589. doi: 10.1007/s40263-015-0263-x. [DOI] [PubMed] [Google Scholar]
- 69.Reijnders JS, Ehrt U, Weber WE, Aarsland D, Leentjens AF. A systematic review of prevalence studies of depression in Parkinson’s disease. Mov Disord. 2008;23:183–189. doi: 10.1002/mds.21803. quiz 313. [DOI] [PubMed] [Google Scholar]
- 70.Broen MP, Narayen NE, Kuijf ML, Dissanayaka NN, Leentjens AF. Prevalence of anxiety in Parkinson’s disease: a systematic review and metaanalysis. Mov Disord. 2016;31:1125–1133. doi: 10.1002/mds.26643. [DOI] [PubMed] [Google Scholar]
- 71.den Brok MG, van Dalen JW, van Gool WA, Moll van Charante EP, de Bie RM, Richard E. Apathy in Parkinson’s disease: a systematic review and meta-analysis. Mov Disord. 2015;30:759–769. doi: 10.1002/mds.26208. [DOI] [PubMed] [Google Scholar]
- 72.Jo S, Kim SO, Park KW, Lee SH, Hwang YS, Chung SJ. The role of APOE in cognitive trajectories and motor decline in Parkinson’s disease. Sci Rep. 2021;11:7819. doi: 10.1038/s41598-021-86483-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Williams-Gray CH, Goris A, Saiki M, Foltynie T, Compston DA, Sawcer SJ, et al. Apolipoprotein E genotype as a risk factor for susceptibility to and dementia in Parkinson’s disease. J Neurol. 2009;256:493–498. doi: 10.1007/s00415-009-0119-8. [DOI] [PubMed] [Google Scholar]
- 74.Alcalay RN, Caccappolo E, Mejia-Santana H, Tang MX, Rosado L, Orbe Reilly M, et al. Cognitive performance of GBA mutation carriers with early-onset PD: the CORE-PD study. Neurology. 2012;78:1434–1440. doi: 10.1212/WNL.0b013e318253d54b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Setó-Salvia N, Pagonabarraga J, Houlden H, Pascual-Sedano B, Dols-Icardo O, Tucci A, et al. Glucocerebrosidase mutations confer a greater risk of dementia during Parkinson’s disease course. Mov Disord. 2012;27:393–399. doi: 10.1002/mds.24045. [DOI] [PubMed] [Google Scholar]
- 76.Desikan RS, Schork AJ, Wang Y, Witoelar A, Sharma M, McEvoy LK, et al. Genetic overlap between Alzheimer’s disease and Parkinson’s disease at the MAPT locus. Mol Psychiatry. 2015;20:1588–1595. doi: 10.1038/mp.2015.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hall S, Surova Y, Öhrfelt A, Zetterberg H, Lindqvist D, Hansson O. CSF biomarkers and clinical progression of Parkinson disease. Neurology. 2015;84:57–63. doi: 10.1212/WNL.0000000000001098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Siderowf A, Xie SX, Hurtig H, Weintraub D, Duda J, Chen-Plotkin A, et al. CSF amyloid β 1-42 predicts cognitive decline in Parkinson disease. Neurology. 2010;75:1055–1061. doi: 10.1212/WNL.0b013e3181f39a78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gomperts SN, Locascio JJ, Makaretz SJ, Schultz A, Caso C, Vasdev N, et al. Tau positron emission tomographic imaging in the Lewy body diseases. JAMA Neurol. 2016;73:1334–1341. doi: 10.1001/jamaneurol.2016.3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gomperts SN, Locascio JJ, Rentz D, Santarlasci A, Marquie M, Johnson KA, et al. Amyloid is linked to cognitive decline in patients with Parkinson disease without dementia. Neurology. 2013;80:85–91. doi: 10.1212/WNL.0b013e31827b1a07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pappatà S, Santangelo G, Aarsland D, Vicidomini C, Longo K, Bronnick K, et al. Mild cognitive impairment in drug-naive patients with PD is associated with cerebral hypometabolism. Neurology. 2011;77:1357–1362. doi: 10.1212/WNL.0b013e3182315259. [DOI] [PubMed] [Google Scholar]


