Abstract
Background
the mySupport advance care planning intervention was originally developed and evaluated in Northern Ireland (UK). Family caregivers of nursing home residents with dementia received an educational booklet and a family care conference with a trained facilitator to discuss their relative’s future care.
Objectives
to investigate whether upscaling the intervention adapted to local context and complemented by a question prompt list impacts family caregivers’ uncertainty in decision-making and their satisfaction with care across six countries. Second, to investigate whether mySupport affects residents’ hospitalisations and documented advance decisions.
Design
a pretest–posttest design.
Setting
in Canada, the Czech Republic, Ireland, Italy, the Netherlands and the UK, two nursing homes participated.
Participants
in total, 88 family caregivers completed baseline, intervention and follow-up assessments.
Methods
family caregivers’ scores on the Decisional Conflict Scale and Family Perceptions of Care Scale before and after the intervention were compared with linear mixed models. The number of documented advance decisions and residents’ hospitalisations was obtained via chart review or reported by nursing home staff and compared between baseline and follow-up with McNemar tests.
Results
family caregivers reported less decision-making uncertainty (−9.6, 95% confidence interval: −13.3, −6.0, P < 0.001) and more positive perceptions of care (+11.4, 95% confidence interval: 7.8, 15.0; P < 0.001) after the intervention. The number of advance decisions to refuse treatment was significantly higher after the intervention (21 vs 16); the number of other advance decisions or hospitalisations was unchanged.
Conclusions
the mySupport intervention may be impactful in countries beyond the original setting.
Keywords: dementia, family caregivers, nursing homes, decision-making, older people
Key Points
This study implemented an existing intervention adapted to local context in six different countries, providing support for generalisability of the original findings.
Family caregivers reported less decision-making uncertainty and more positive perceptions of care after receiving the educational intervention.
This study did not find conclusive evidence about the impact of the intervention on the number of hospitalisations or advance decisions of nursing home residents.
Our findings may encourage nursing home staff to inform family caregivers about dementia and end-of-life care.
Introduction
Family caregivers, including relatives, friends or others, often serve as proxy decision makers to achieve person-centred care when a person with dementia is no longer able to contribute to care conversations with care providers [1, 2]. However, they may feel ill prepared for this task and experience decision-making as challenging [3]. This uncertainty can impact family caregivers’ wellbeing [4] and can affect the comfort of persons with advanced dementia [5]. Helping family caregivers reflect on values and preferences of the person with dementia and view dementia as a life limiting condition can be helpful in addressing these issues.
To address family caregivers’ uncertainty in decision-making about care and treatment, the Family Carer Decision Support Intervention was developed and implemented in Northern Ireland (UK) [6]. A randomised controlled trial (RCT) measured the efficacy of the advance care planning intervention across 24 nursing homes. The intervention consisted of an educational booklet for family caregivers and a structured conversation with a trained facilitator. Family caregivers in 12 nursing homes received the intervention and they experienced less decisional conflict and were more satisfied with the nursing home care compared with the family caregivers’ experiences in 12 control homes. However, the impact of the intervention when scaled up outside of the original context may differ. Therefore, six countries collaborated in the mySupport study consortium (https://mysupportstudy.eu/) aimed at adapting the intervention to their local contexts and implement the intervention in their own setting [7]. Two additional general adaptions were made. One, to facilitate upscaling by building internal capacity for family communication, a trained facilitator educated nursing home staff in conducting conversations themselves in their own nursing home (rather than an external facilitator). Two, family caregivers received a list of locally relevant example questions to prompt their engagement during the structured family care meeting, stimulating empowerment and personalised discussions.
This study aimed to assess the impact of the adapted Family Carer Decision Support intervention on family caregivers’ decision-making uncertainty and their satisfaction with nursing home care for their relative with dementia, across six countries. As a secondary aim, we studied the impact of the intervention on the number of advance decisions and hospitalisations. We thus aimed to determine if the collective results from six countries support the original findings and a successful scale-up beyond the original setting.
By studying the impact of an intervention that facilitates conversations about future decision-making in care and awareness of comfort care for people with dementia, this study attends to important nursing home research questions according to international experts [8] and contributes to a currently understudied area [9]. Furthermore, the intervention addresses some fundamental needs of nursing staff in palliative dementia care [10].
Methods
Design and ethical considerations
The mySupport study employs a hybrid effectiveness-implementation design [11] guided by the RE-AIM framework (Reach, Effectiveness, Adoption, Implementation, Maintenance) [12]. Since the original RCT indicated the efficacy of the Family Care Decision Support intervention, this design shifts focus to implementation and effectiveness in broader real-world settings [11]. The current study focused on the impact (Effectiveness) of the intervention across countries, after adapting to local context as needed, using a single group, pretest–posttest design. Ethics approval was obtained in each participating country according to local guidelines. Participants provided written informed consent before participation. We used the SQUIRE (2.0) guidelines to structure this report [13] and the TIDieR Checklist to report the intervention [14].
Context
Setting
Data were collected between November 2020 and May 2022 (during the COVID-19 pandemic) in the six countries of the mySupport study consortium: Canada, Czech Republic, Ireland, Italy, the Netherlands and the UK. In each country, two nursing homes were recruited with exception of the UK where six nursing homes were recruited (two in three different regions). Due to study drop-out, two nursing homes were finally included in the UK. Nursing homes were defined as long-term care facilities providing 24-hour nursing care. We specifically recruited nursing homes that provided care to people with dementia.
Population
The study’s target group were family caregivers of nursing home residents with advanced dementia (that is: residents who were unable to contribute to care conversations according to documented capacity assessments in the residents’ charts or the clinical judgement of the nursing home team). Family caregivers were 18 years or older, the primary family caregiver, and were able to understand and speak the local language. We aimed for a minimum of 10 family caregivers per nursing home (20 per country) as this allowed for analyses of the collective data [6] that is balanced across countries.
Recruitment
Eligible family caregivers were identified by nursing home staff and if agreed, contacted by the research team. After being presented an overview of the study, they were sent study information and an informed consent sheet.
Intervention
The previously developed Family Carer Decision Support [6] intervention was implemented in participating nursing homes. The intervention aims to inform family caregivers about end-of-life care options for people with advanced dementia to support them in end-of-life care decision-making. This reflects a broader definition of ‘advance care planning’ in dementia, including family caregiver engagement in advance care planning as proxy for the person with dementia who does not have the capacity to partake [15]. The intervention was adapted for implementation across countries by the international consortium [7, 16, 17]. A full description of the intervention has been published before [7]. In short, the intervention consisted of the following elements (Supplementary Figure S1).
Educational family booklet and question prompt list
Family caregivers received an educational booklet about comfort care for people with dementia at the end of life [17, 18]. The booklet discusses the dementia trajectory, possible symptoms and complications, shared decision-making, palliative care options and the dying phase and grief. In each country, we developed a question prompt list together with family caregivers (as part of our Patient and Public Involvement strategy) [16]. This list of sample questions served as a conversation aid for family caregivers during a meeting with a nursing home staff member.
Training and facilitation
In the original intervention, a facilitator external to the nursing homes was trained in conducting family care conferences. In the current study, a train-the-trainer model was implemented, involving external and internal facilitators. One or two external facilitators were trained per country, who then trained nursing home staff members in conducting family care conferences. In each participating nursing home, one to five nursing home staff members were trained as internal facilitators. Their professions ranged from nurse aide, nurse assistant, nurse, head nurse/clinical nurse unit manager, social worker, and nurse educator or nursing home manager.
Family care conference
The family care conference was a structured meeting of ~1 hour. The internal facilitator discussed the contents of the educational booklet, which the family caregiver had reviewed prior to the meeting, and any questions the family caregiver wanted to discuss. The aim was to inform and support family caregivers. The possibility of advance decisions and follow-up meetings were discussed.
Measures
Baseline assessments of primary outcomes took place before the family care conference (pre-implementation); secondary outcomes reflected the 3 months prior to the family care conference. Follow-up assessment of primary outcomes took place 6 weeks after the family care conference (post-implementation); secondary outcomes reflected the 3 months after the family care conference (Supplementary Figure S2).
Standardised surveys were used to assess the primary outcomes. The impact of the intervention on family caregivers’ decision-making uncertainty and satisfaction with care was measured with the Decisional Conflict Scale (DCS) and Family Perceptions of Care Scale (FPCS), respectively. The DCS consists of 16 items that are scored on a 5-point Likert scale, with a reported reliability of 0.78–0.92 for the total scale [19, 20]. The scale measures uncertainty and factors contributing to uncertainty and effective decision-making in five domains: (i) feeling informed; (ii) feeling clear about values that guide the decision; (iii) feeling supported in decision-making; (iv) feeling certain about choosing and (v) making an effective decision. Scores for the five domains were calculated, and a total score reflecting overall decisional conflict. Family caregivers were instructed to consider any decision that was made or discussed recently or to answer the questions ‘when considering their family member’s preferences of future care’. The FPCS consists of 25 items that are scored on a 7-point Likert scale [21]. The original scale measures perceptions of care provided in the last 4 weeks of life. To assess perception of care before and after the intervention, we adapted the scale with rephrased statements to assess perception of care during the resident’s stay [6]. We calculated a total score and scores for four subscales: (i) resident care; (ii) family support; (iii) communication and (iv) rooming. Additionally, family caregivers were asked about their gender, age, educational attainment, employment status and relationship to the resident.
The secondary outcomes, hospitalisations and documented advance decisions, were retrospectively obtained via chart review or reported by nursing home staff on a data extraction form that was designed and standardised for use across countries. The form included the location of death in case residents had died during the study within 3 months of the family care conference. Furthermore, it included length of stay and dementia severity assessed with the Functional Assessment Staging Tool (FAST) [22].
All measures were translated into the local language using steps 1–4 of [23].
The surveys were conducted via in-person interviews, by phone or videocall, via survey software or by pen-and-paper depending on participants’ preferences and possibilities considering COVID-19 regulations.
Analysis
Family caregivers’ baseline characteristics were compared between those who completed all study phases and those who did not, using independent t-tests for age and Fisher’s exact chi-square tests for gender, educational level, employment status and relationship to the resident. For all further analyses, we included family caregivers (and their relatives with dementia) who had completed all study phases (pre-implementation baseline assessment (T0), intervention and post-implementation follow-up assessment (T1); per protocol analysis) as we were interested in measuring changes in response to the intervention. We performed descriptive statistics to report family caregivers’ and residents’ baseline characteristics. Differences between baseline and follow-up DCS and FPCS total scores and subscale scores were assessed using linear mixed models for repeated measures, with assessment time (baseline or follow-up) as fixed effect. To minimise type I errors, subscale scores were compared only when total scale scores differed significantly, and Bonferroni correction for multiple subscale comparisons was applied. We performed additional sensitivity analyses with covariate adjustment to reduce bias in effect estimates when outcome data are missing [24, 25], adding age, gender and FAST score measured at baseline as covariates [6, 24].
Documented advance decisions and actual hospitalisations were compared with McNemar tests for paired data and Bonferroni corrected for multiple comparisons. All analyses were performed using SPSS version 25.0 (IBM Corporation, New York, 2017).
Results
Reach and implementation
Across 12 nursing homes in 6 countries, 163 family caregivers were eligible for participation and 109 provided informed consent (Figure 1). Of the enrolled family caregivers, 88 (81%) completed all study phases. Complete cases did not differ significantly from incomplete cases in age, gender, employment status or relationship to the resident with dementia (all P > 0.05). A difference in educational level was found between the two groups (P = 0.04), with incomplete cases having higher educational levels.
Figure 1.
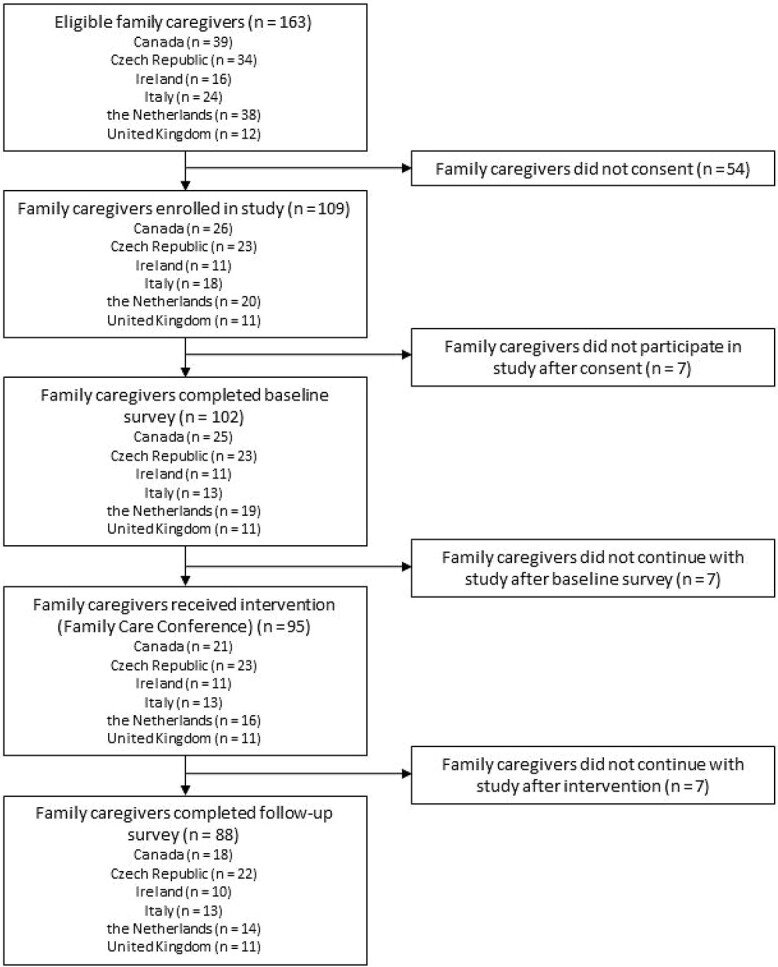
Participant flow diagram.
Two-thirds of the family caregivers were female, most (71/87) were children of the residents. The residents often (56/61) had moderately severe or severe dementia and were living in the nursing home for a median duration of 2 years and 1 month (Table 1).
Table 1.
Participants’ characteristics at baseline
| Characteristics | Total n [%] |
CA | CZ | IE | IT | NL | UK | |
|---|---|---|---|---|---|---|---|---|
| Family caregiver n | 88 | 18a | 22 | 10 | 13 | 14 | 11 | |
| Gender, male | 29 [33] | 5 | 8 | 3 | 5 | 5 | 3 | |
| Mean age (SD) | 61 (10) | 61 (9) | 62 (12) | 58 (9) | 57 (5) | 64 (11) | 61 (7) | |
| Educational attainment n | ||||||||
| Primary school | 1 [1] | 0 | 1 | 0 | 0 | 0 | 0 | |
| Secondary school | 50 [57] | 5 | 17 | 5 | 13 | 6 | 4 | |
| Undergraduate degree | 24 [28] | 8 | 4 | 3 | 0 | 6 | 3 | |
| Postgraduate degree | 12 [14] | 4 | 0 | 2 | 0 | 2 | 4 | |
| Employment status n | ||||||||
| Full time employment | 35 [40] | 8 | 11 | 2 | 8 | 3 | 3 | |
| Part time employment | 14 [16] | 1 | 1 | 3 | 1 | 5 | 3 | |
| Unemployed/Retired/Homemaker | 38 [44] | 8 | 10 | 5 | 4 | 6 | 5 | |
| Relationship to resident n | ||||||||
| Partner | 10 [11] | 1 | 2 | 1 | 0 | 4 | 2 | |
| Child | 71 [82] | 16 | 16 | 9 | 11 | 10 | 9 | |
| Sibling | 2 [2] | 0 | 2 | 0 | 0 | 0 | 0 | |
| Friend | 1 [1] | 0 | 1 | 0 | 0 | 0 | 0 | |
| Other | 3 [3] | 0 | 1 | 0 | 2 | 0 | 0 | |
| Nursing home resident n | 88 | 18‡ | 22 | 10‡ | 13 | 14‡ | 11‡ | |
| FAST score n | ||||||||
| 4) Mild dementia | 4 [7] | 0 | 0 | 0 | 0 | 3 | 1 | |
| 5) Moderate dementia | 1 [2] | 1 | 0 | 0 | 0 | 0 | 0 | |
| 6) Moderately severe dementia | 15 [25] | 3 | 5 | 2 | 0 | 5 | 0 | |
| 7) Severe dementia | 41 [67] | 2 | 17 | 4 | 13 | 4 | 1 | |
| Median length of stay, months (IQR) | 23 (15–37) | 40 (12–79) | 35 (16–69) | 27 (21–40) | 19 (9–28) | 23 (8–40) | NA | |
FAST: Functional Assessment Staging Tool, CA: Canada, CZ: the Czech Republic, IT: Italy, NL: the Netherlands, IE: Ireland, UK: the United Kingdom
aDemographics were missing for one family caregiver, ‡Missings per country for FAST score, CA: 12, IE: 4, NL: 2, UK: 9; and for Length of stay, CA: 4, NL: 2, UK: 11
Decisional conflict and perception of care
DCS total and subscale scores were significantly lower at follow-up (T1) compared with baseline (T0), indicating less decisional conflict for family caregivers after receiving the intervention. In the sensitivity analyses adjusted for covariates, this difference remained. However, the differences in the subscales ‘Support’ and ‘Uncertainty’ were no longer significant (Table 2).
Table 2.
DCS at baseline and follow-up
| Outcome | T0 | T1 | Unadjusted | Adjustedb | ||
|---|---|---|---|---|---|---|
| n | Mean (SD) | Mean (SD) | Difference in mean (95% CI) | Difference in mean (95% CI) | ||
| DCS total scorea | 88 | 32.2 (18.4) | 22.5 (16.7) | −9.6 (−13.3, −6.0)c | −9.2 (−14.1, −4.4)c | |
| DCS subscores | Difference in mean (99% CI) | Difference in mean (99% CI) | ||||
| Informed | 88 | 39.1 (26.4) | 25.2 (23.7) | −13.9 (−22.8, −5.1)c | −13.8 (−25.4, −2.1)c | |
| Values clarity | 86 | 34.1 (24.7) | 21.5 (19.9) | −12.8 (−20.1, −5.5)c | −12.1 (−20.7, −3.4)c | |
| Support | 87 | 25.5 (20.6) | 18.0 (18.7) | −7.5 (−13.2, −1.8)c | −6.0 (−13.2, 1.3) | |
| Uncertainty | 87 | 37.0 (21.1) | 30.1 (20.6) | −6.9 (−12.3, −1.5)c | −6.5 (−13.8, 0.8) | |
| Effective decision | 86 | 28.1 (20.9) | 19.9 (18.2) | −8.2 (−13.8, −2.6)c | −8.2 (−15.2, −1.3)c | |
aRange: 0–100 (0 = no conflict, 100 = high conflict).
bAdjusted for age, gender and FAST (n = 60).
cSignificant difference at P < 0.05 (total score) or Bonferroni corrected significance level of P < 0.01 (subscores).
Similarly, FPCS total and subscale scores differed significantly between T0 and T1, indicating a more positive perception of care after the intervention. In the sensitivity analyses adjusted for covariates, the positive effects remained for the total score and the subscale scores ‘Family support’ and ‘Communication’ (Table 3).
Table 3.
FPCS at baseline and follow-up
| Outcome | T0 | T1 | Unadjusted | Adjusted‡ | ||||
|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | % of max score | Mean (SD) | % of max score | Difference in mean (95% CI) | Difference in mean (95% CI) | ||
| FPCS total scoreb (range: 25–175) |
88 | 132.5 (25.9) | 75.7 | 143.9 (24.5) | 82.2 | 11.4 (7.8, 15.0)a | 10.4 (6.0, 14.9)a | |
| FPCS subscales | Difference in mean (99% CI) | Difference in mean (99% CI) | ||||||
| Resident Care (range: 11–77) |
88 | 60.0 (12.5) | 77.9 | 62.3 (12.2) | 80.9 | 2.3 (0.03, 4.5)a | 1.6 (−1.3, 4.6) | |
| Family Support (range: 6–42) |
88 | 28.0 (7.6) | 66.7 | 33.5 (7.0) | 79.8 | 5.5 (3.8, 7.2)a | 5.2 (3.2, 7.2)a | |
| Communication (range: 6–42) |
88 | 33.0 (6.1) | 78.6 | 36.2 (5.5) | 86.2 | 3.1 (1.9, 4.3)a | 3.3 (1.8, 4.7)a | |
| Rooming (range: 2–14) |
87 | 11.5 (3.0) | 82.1 | 12.1 (2.1) | 86.4 | 0.6 (0.08, 1.2)a | 0.5 (−0.2, 1.2) | |
aSignificant difference at P < 0.05 (total score) or Bonferroni corrected significance level of P < 0.0125 (subscale scores).
bHigher scores indicate more positive perception of care.
cAdjusted for age, gender and FAST (n = 60).
Advance decisions and actual hospitalisations
Table 4 shows that the number of documented advance decisions to refuse treatment (for example, do-not-intubate) was significantly higher after the intervention (32%) compared with before (24%). We did not find a difference in the number of other advance care plans, do-not-resuscitate orders or documented power of attorneys. Furthermore, we did not find a significant reduction in the number of hospital admissions after the intervention.
Table 4.
Advance decisions and actual hospitalisations (secondary outcomes)
| Outcome | T0 | T1 |
|---|---|---|
| Documented advance plans and decisions n (%) | ||
| Advance care plan | 25 (36) | 24 (36) |
| Advance decision to refuse treatmenta | 16 (24) | 21 (32) |
| Do not resuscitate | 35 (48) | 34 (47) |
| Power of attorney | 20 (31) | 20 (32) |
| Actual hospital admissions n (%) | ||
| Accident and emergency department | 6 (8) | 4 (5) |
| Inpatient ward | 3 (4) | 1 (1) |
| Outpatient department | 7 (9) | 1 (1) |
aSignificant different at Bonferroni corrected significance level of P < 0.00625.
Seven residents died during the study: six after all assessments and in one case, the family caregiver participated in the follow-up assessment after their death. One resident died in the emergency department and four in the nursing home; for two residents, the location of death was not reported.
Discussion
After family caregivers across six countries had received the adapted Family Carer Decision Support intervention, they experienced less uncertainty in decision-making and were more satisfied with the nursing home care for their relative. We did not find a difference in the number of hospitalisations and advance decisions, except for the number of advance decisions to refuse treatment which was higher after the intervention. These transnational results are similar to the results from the RCT in Northern Ireland [6].
Family caregivers were more positive about the nursing home’s support of and communication with family caregivers after the intervention. In a Canadian study that implemented a multidimensional intervention to improve end-of-life care and comfort supported by an educational family booklet, FPCS scores improved through a more positive perception of the care provided to the residents [26]. Furthermore, family caregivers in our study reported less decisional conflict after the intervention, with the strongest effects on feeling informed and clear about values. These findings mirror the changes on the DCS found by others after implementing an educational intervention to support end-of-life care decision-making [27]. While the mySupport intervention impacted family caregivers’ perception of support from nursing home staff (as evident from the FPCS scores), the perception of support in decision-making specifically was not significantly impacted. Possibly, family caregivers perceive other family members as more important for decision-making support than nursing home staff (as also implied by others [28]). Future studies should investigate effects of including more (close) family members in conversations about future care.
Regarding the impact on advance decisions and hospitalisations, no clear effect was found in the original RCT [6] nor in the current study. An umbrella review on advance care planning effectiveness reported some evidence for an increase in advance care planning documents and a decrease in hospitalisations [29]. In the current study, the number of hospitalisations was already low, leaving little room for reduction. The number of hospital admissions pre-covid was comparable to the number we found [30], but restrictions in hospital admissions during the COVID-19 pandemic [31] may have impacted our findings. Furthermore, the lack of a clear effect on the number of advance decisions may be related to local legislative frameworks. In the Netherlands, there is no clear distinction between ‘goals of care’ discussions and ‘advance care planning’; family caregivers can represent their relative in both. In Ireland and in some regions of Canada, advance care planning can only be legally performed by the person themselves, which is distinct from ‘goals of care’ discussions that can be conducted with proxies. The intervention may therefore have more impact on family caregivers’ preparedness for decisions they may need to make in the future, than on advance decisions. Changes in advance decisions and actual care may require interventions specifically targeting these outcomes in populations that have greater capacity to engage in documented advance care planning.
A strength of this study is the transnational setting that allowed assessment of whether findings from Northern Ireland (UK) generalise to other countries [32]. This study thus scales up previous findings, even during the COVID-19 pandemic. The study outcomes, less uncertainty in decision-making and more satisfaction with care resonate with family caregivers’ needs around the end of life: feeling prepared and supported while maintaining control of care [33], supporting the relevance of our findings. The sensitivity analyses indicate the findings’ robustness [25, 32]. Weaknesses of this study include the small sample size per country and the pretest–posttest design. The sample size per country was limited due to restricted access to nursing homes during COVID-19 and study dropout due to deaths, poor health of residents or family caregivers or other reasons common for this population [34]. Only in one country, the intended number of 20 participants was reached. It was therefore not possible to compare the outcomes between countries. Reporting data across countries may have masked intra-country differences and these are explored in forthcoming papers reporting on qualitative data. The study was conducted during the COVID-19 pandemic and restrictions varied during this time period. Participants’ responses may reflect influences other than the intervention, such as a more positive perception of communication with nursing home staff when visits were allowed [35], if visits were restricted during part of the data collection period. We cannot rule out contextual effects with a pretest–posttest design.
Our transnational findings provide evidence for the impact of structured conversations between family caregivers and trained nursing home staff, supported by written information, on family caregivers’ experiences of care and decision-making for residents with dementia. These findings may stimulate to incorporate communication training and education for nursing home staff about advance care planning, dementia and palliative care as core elements into curricula. Furthermore, nursing homes may be more encouraged to give information to family caregivers about dementia and palliative care, adapted to the local context, because of the demonstrated benefits of doing this. However, nursing home managers need to facilitate protected time for staff to communicate with families.
Future studies may target larger participant groups per country and evaluate the intervention beyond the current European-Canadian setting. This may provide further insight into the influence of local culture on the effectiveness of the intervention.
Supplementary Material
Acknowledgements
We want to thank all family caregivers participating in the study. We also thank the external facilitators and internal facilitators who supported and delivered the intervention. Furthermore, we acknowledge the mySupport study group who contributed to the acquisition of data or overall study design: Jack Lawrence, Bianca Tétrault, Danielle Just, Donny Li, Diandra Serrano, Emily Di Sante, Karolina Vlckova, Alan Connolly, Selena O’Connell, Molly Mattsson, Silvia Gonella, Karen Harrison Dening, Kay de Vries, Josie Dixon, Catherine Henderson, Adrienne McCann, Sophie Morris, Julie Doherty, Emma Loudon, Andrew Harding, Emily Cousins.
Contributor Information
Laura Bavelaar, Department of Public Health and Primary Care, Leiden University Medical Center, Leiden, the Netherlands.
Mandy Visser, Department of Public Health and Primary Care, Leiden University Medical Center, Leiden, the Netherlands.
Catherine Walshe, International Observatory on End of Life Care, Lancaster University, Lancaster, UK.
Nancy Preston, International Observatory on End of Life Care, Lancaster University, Lancaster, UK.
Sharon Kaasalainen, School of Nursing, McMaster University, Hamilton, Canada.
Tamara Sussman, School of Social Work, McGill University, Montreal, Canada.
Nicola Cornally, School of Nursing and Midwifery, University College Cork, Cork, Ireland.
Irene Hartigan, School of Nursing and Midwifery, University College Cork, Cork, Ireland.
Martin Loucka, Center for Palliative Care, Prague, Czech Republic.
Paola di Giulio, Department of Public Health and Pediatrics, University of Torino, Turin, Italy.
Kevin Brazil, School of Nursing and Midwifery, Queen’s University Belfast, Belfast, UK.
Wilco P Achterberg, Department of Public Health and Primary Care, Leiden University Medical Center, Leiden, the Netherlands.
Jenny T van der Steen, Department of Public Health and Primary Care, Leiden University Medical Center, Leiden, the Netherlands; Department of Primary and Community Care, Radboud university medical center, Nijmegen, the Netherlands.
Data Availability
Raw data (deidentified participant data) that support the findings of this study are available from the corresponding author, upon reasonable request.
Declaration of Conflicts of Interest
Prof Wilco Achterberg is an Associate Editor for Age and Ageing (care home section).
Declaration of Sources of Funding
This is an EU Joint Programme -Neurodegenerative Disease Research (JPND) project. This work was supported by the following funding organisations under the aegis of JPND -www.jpnd.eu: Canada, Canadian Institutes of Health Research [grant number 161462]; the Czech Republic, Ministry of Education, Youth and Sport [grant number 8F19005]; Netherlands, Netherlands Organisation for Health Research and Development [grant number 733051084]; Ireland, Health Research Board [grant number JPND-HSC-2018-002]; the United Kingdom, Alzheimer’s Society [grant number AS-IGF-17-001]. The funders played no role in the design, execution, analysis and interpretation of data, or writing of the study.
References
- 1. Jalbert JJ, Daiello LA, Lapane KL. Dementia of the Alzheimer type. Epidemiol Rev 2008; 30: 15–34. [DOI] [PubMed] [Google Scholar]
- 2. Cohen LW, Zimmerman S, Reed Det al. . Dementia in relation to family caregiver involvement and burden in long-term care. J Appl Gerontol 2014; 33: 522–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carter G, McLaughlin D, Kernohan WGet al. . The experiences and preparedness of family carers for best interest decision-making of a relative living with advanced dementia: a qualitative study. J Adv Nurs 2018; 74: 1595–604. [DOI] [PubMed] [Google Scholar]
- 4. Petriwskyj A, Parker D, Robinson A, Gibson A, Andrews S, Banks S. Family involvement in decision making for people with dementia in residential aged care: a systematic review of quantitative and qualitative evidence. JBI Database System Rev Implement Rep 2013; 11: 131–282. [DOI] [PubMed] [Google Scholar]
- 5. Steen van der JT, Onwuteaka-Philipsen BD, Knol DL, Ribbe MW, Deliens L. Caregivers’ understanding of dementia predicts patients’ comfort at death: a prospective observational study. BMC Med 2013; 11: 105–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brazil K, Carter G, Cardwell Cet al. . Effectiveness of advance care planning with family carers in dementia nursing homes: a paired cluster randomized controlled trial. Palliat Med 2018; 32: 603–12. [DOI] [PubMed] [Google Scholar]
- 7. Harding A, Doherty J, Bavelaar Let al. . A family carer decision support intervention for people with advance dementia residing in a nursing home: a study protocol for an international advance care planning intervention (mySupport study). BMC Geriatr 2022; 22: 822. 10.1186/s12877-022-03533-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morley JE, Caplan G, Cesari Met al. . International survey of nursing home research priorities. J An Med Dir Assoc 2014; 15: 309–12. [DOI] [PubMed] [Google Scholar]
- 9. Xie B, Berkley AS, Kwak J, Fleischmann KR, Dimmitt Champion J, Koltai KS. End-of-life decision making by family caregivers of persons with advanced dementia: a literature review of decision aids. SAGE Open Med 2019; 6: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bolt SR, Meijers JMM, Steen van der JT, Schols JMGA, Zwakhalen SMG. Nursing staff needs in providing palliative care persons with dementia at home or in nursing homes: a survey. J Nurs Scholarsh 2020; 52: 164–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bernet AC, Willens DE, Bauer MS. Effectiveness-implementation hybrid designs: implications for quality improvement science. Implement Sci 2013; 8: S2. 10.1186/1748-5908-8-S1-S2. [DOI] [Google Scholar]
- 12. Glasgow RE, Harden SM, Gaglio Bet al. . RE-AIM planning and evaluation framework: adapting to new science and practice with a 20-year review. Front Public Health 2019; 7: 64. 10.3389/fpubh.2019.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (standards for QUality improvement reporting excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf 2016; 25: 986–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hoffmann TC, Glasziou PP, Milne Ret al. . Better reporting of intervention: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014; 348: g1687. 10.1136/bmj.g1687. [DOI] [PubMed] [Google Scholar]
- 15. Steen van der JT. Developing guidance in addressing the challenges of advance care planning in dementia: an EAPC Delphi study. In: Abstracts from the 17th World Congress of the EAPC 2021. Pall Med. 2021; 35: 1, 243, 10.1177/02692163211035909. [DOI] [Google Scholar]
- 16. Bavelaar L, Nicula M, Morris Set al. . Developing country-specific questions about end-of-life care for nursing home residents with advanced dementia using the nominal group technique with family caregivers. Patient Educ Couns 2022; 105: 965–73. [DOI] [PubMed] [Google Scholar]
- 17. Bavelaar L, McCann A, Cornally Net al. . Guidance for family about comfort care in dementia: a comparison of an educational booklet adopted in six jurisdictions over a 15 year timespan. BMC Palliat Care 2022; 21: 76. 10.1186/s12904-022-00962-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Arcand M, Caron C. Comfort Care at the End of Life for Persons with Alzheimer’s Disease or Other Degenerative Diseases of the Brain - a Guide for Caregivers. Sherbrooke: Centre de santé et de services sociaux - institut universitaire de gériatrie de Sherbrooke, 2005. [Google Scholar]
- 19. O’Connor AM. Validation of a decisional conflict scale. Med Decis Making 1995; 15: 25–30. [DOI] [PubMed] [Google Scholar]
- 20. O’Connor AM. User Manual – Decisional Conflict Scale (16 Item Statement Format) [Internet]. Ottawa: Ottawa Hospital Research Institute; ©1993[updated 2010; cited 12 July 2022]. p. 16. Available from:https://decisionaid.ohri.ca/docs/develop/User_Manuals/UM_Decisional_Conflict.pdf [Google Scholar]
- 21. Vohra JU, Brazil K, Hanna S, Abelson J. Family perceptions of end-of-life care in long-term care facilities. J Palliat Care 2004; 20: 297–302. [PubMed] [Google Scholar]
- 22. Reisberg B, Ferris SH, Franssen E. An ordinal functional assessment tool for Alzheimer's-type dementia. Hosp Community Psychiatry 1985; 36: 593–5. [DOI] [PubMed] [Google Scholar]
- 23. Sousa VD, Rojjanasrirat W. Translation, adaptation and validation of instruments or scales for use in cross-cultural health care research: a clear and user-friendly guideline. J Eval Clin Pract 201, 17: 268–74. [DOI] [PubMed] [Google Scholar]
- 24. Groenwold RHH, Donders ART, Roes KCB, Harrell FE, Moons KGM. Dealing with missing outcome data in randomized trials in observational studies. Am J Epidemiol 2012; 175: 210–7. [DOI] [PubMed] [Google Scholar]
- 25. Thabane L, Mbuagbaw L, Zhang Set al. . A tutorial on sensitivity analyses in clinical trials: the what, why, when and how. BMC Med Res Methodol 2013; 13. 10.1186/1471-2288-13-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Verreault R, Arcand M, Misson Let al. . Quasi-experimental evaluation of a multifaceted intervention to improve quality of end-of-life care and quality of dying for patients with advanced dementia in long-term care institutions. Palliat Med 2018; 32: 613–21. [DOI] [PubMed] [Google Scholar]
- 27. Huang H-L, Lu W-R, Liu C-L, Chang H-J. Advance care planning information intervention for persons with mild dementia and their family caregivers: impact on end-of-life care decision conflicts. PLoS One 2020; 15: e0240684. 10.1371/journal.pone.0240684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schiffman IK, Werner P. Willingness of family caregivers of people with dementia to undertake advance care planning: examining an extended model of the theory of planned behavior. Dementia 2021; 20: 1044–57. [DOI] [PubMed] [Google Scholar]
- 29. Wendrich-van Dael A, Bunn F, Lynch J, Pivodic L, Van den Block L, Goodman C. Advance care planning for people living with dementia: an umbrella review of effectiveness and experiences. Int J Nurs Stud 2020; 107: 103576. 10.1016/j.ijnurstu.2020.103576. [DOI] [PubMed] [Google Scholar]
- 30. Afonso-Argilés FJ, Meyer G, Stephan Aet al. . Emergency department and hospital admissions among people with dementia living at home or in nursing homes: results of the European RightTimePlaceCare project on their frequency, associated factors and costs. BMC Geriatr 2020; 20: 453. 10.1186/s12877-020-01835-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Grimm F, Hodgson K, Brine R, Deeny SR. Hospital admissions from care homes in England during the COVID-19 pandemic: a retrospective, cross-sectional analysis using linked administrative data. Int J Popul Data Sci 2021; 5: 07. 10.23889/ijpds.v5i4.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nosek BA, Hardwicke TE, Moshontz Het al. . Replicability, robustness and reproducibility in psychological science. Annu Rev Psychol 2022; 73: 719–48. [DOI] [PubMed] [Google Scholar]
- 33. Davies N, Iliffe S, Hopwood Jet al. . The key aspects of online support that older family carers of people with dementia want at the end of life: a qualitative sudy. Aging Ment Health 2020; 24: 1654–61. [DOI] [PubMed] [Google Scholar]
- 34. Davies K, Collerton J, Jagger Cet al. . Engaging the oldest old in research: lessons from the Newcastle 85+ study. BMC Geriatr 2010; 10: 64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Smaling HJA, Tilburgs B, Achterberg WP, Visser M. The impact of social distancing due to the COVID-19 pandemic on people with dementia, family carers and healthcare professionals: a qualitative study. Int J Environ Res Public Health 2022; 19: 519. 10.3390/ijerph19010519. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data (deidentified participant data) that support the findings of this study are available from the corresponding author, upon reasonable request.


