Abstract
Background
Monitoring changes in pharyngeal carriage of pneumococcus in children following 13-valent pneumococcal conjugate vaccine (PCV13) introduction in the United Kingdom in 2010 informs understanding of patterns of invasive pneumococcal disease (IPD) incidence.
Methods
Nasopharyngeal swabs from healthy children vaccinated with PCV13 according to schedule (2, 4, and 12 months) were cultured and serotyped. Results for children aged 13–48 months were compared between 2014–2015 and 2017–2019 and with children aged 6–12 months (2017–2020). Blood was obtained from a subset of children for pneumococcal serotype-specific immunoglobulin G (IgG).
Results
Total pneumococcal carriage at 13–48 months was 47.9% (473/988) in 2014–2015 and 51.8% (412/795) in 2017–2019 (P = .10); at age 6–12 months this value was 44.6% (274/615). In 2017–2019, 2.9% (95% confidence interval, 1.8%–4.3%) of children aged 13–48 months carried PCV13 serotypes (mainly 3 [1.5%] and 19A [0.8%]) and >20% carried the additional 20-valent PCV (PCV20) serotypes. Similar proportions of children had IgG ≥0.35 IU/mL for each serotype in 2014–2015 and 2017–2019. Serotype 7C carriage increased significantly (P < .01) between 2014–2015 and 2017–2019. Carriage of PCV20 serotypes 8 and 12F, both major causes of IPD, was rare.
Conclusions
Introduction of PCV20, if licensed for children, could significantly change the composition of pneumococcal serotypes carried in the pharynx of UK children.
Clinical Trials Registration
Keywords: pneumococcal, IPD, PCV, carriage, conjugate, invasive, nasopharyngeal, serotype, vaccine
Pneumococcal carriage in children aged 13–48 months remained stable between 2014–2015 and 2017–2019, both overall and for individual serotypes, with the exception of an increase in serotype 7C. More than 20% of children carried the additional serotypes contained in PCV20.
Despite the introduction of pneumococcal conjugate vaccines (PCVs), Streptococcus pneumoniae remains a common cause of pneumonia, meningitis, and septicemia (collectively termed invasive pneumococcal disease [IPD]), responsible for 318 000 deaths globally in children aged 1–59 months in 2015 [1]. Streptococcus pneumoniae is commonly carried in the nasopharynx of healthy children, who act as the main reservoir in the community. There are currently around 100 recognized pneumococcal serotypes [2], classified according to the polysaccharide capsule, with variable invasiveness (case-carrier ratios) [3–5].
In 2006, 7-valent PCV (PCV7) was introduced into the United Kingdom (UK) childhood vaccination program to protect against serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F, which were then common causes of IPD in the UK [6]. A subsequent reduction in PCV7 IPD cases and nasopharyngeal carriage was observed in the immunized cohort [7, 8], and a fall in PCV7 IPD in older age groups was observed due to reduced circulation of these serotypes [7]. These gains were partially offset by an increase in IPD due to other serotypes (“serotype replacement”) [9] and in April 2010, PCV7 was superseded by 13-valent PCV (PCV13), which includes an additional 6 serotypes (1, 3, 5, 6A, 7F, and 19A). Reductions in disease to near-negligible levels across all age groups have been observed for 4 of these additional 6 serotypes, with the exceptions being 19A (persisting at reduced levels), and 3 (no reduction observed) [10]. Further serotype replacement has followed the introduction of PCV13; 7 years later, the 6 main replacement serotypes were 8, 12F, 9N (together responsible for >40% of IPD cases in 2016–2017), 22F, 33F, and 15A [10]. Therefore, despite an estimated prevention (direct and indirect) of 28 631 cases of IPD between 2010–2011 and 2016–2017 in England and Wales, and a reduction in IPD in children, overall IPD rates (all ages) in 2016–2017 (10 per 100 000) were no lower than in 2010 [10]. Accordingly, PCVs against 15 (PCV15, additional 22F and 33F) and 20 (PCV20, additional 8, 10A, 11A, 12F, 15B) serotypes have been developed [11, 12].
The reduction of PCV13 impact by serotype replacement, and imminent availability of these novel vaccines, emphasizes the importance of understanding the distribution of serotypes circulating within a community.
Given the primary role young children have in pneumococcal transmission [13], understanding carriage in this group is important for understanding IPD epidemiology across all ages [14, 15].
Here we compare carriage and serotype-specific immunoglobulin G (IgG) concentrations in children at 2 time points following PCV13 introduction, and explore longer-term carriage and IPD trends over the past 12 years. Furthermore, we assess carriage and serotype-specific IgG concentrations in children aged 6–12 months to provide baseline data prior to the change in the UK vaccination schedule from 2, 4, and 12 months (2 + 1) to 3 and 12 months (1 + 1) in early 2020 [16, 17]. Together, these allow evaluation of potential indirect benefits to older age groups of introducing PCV15 and PCV20 into the UK infant schedule.
METHODS
Study Design
A cross-sectional observational study to establish the point prevalence of pneumococcal nasopharyngeal carriage in children from the UK Thames Valley region was conducted between June 2017 and March 2020.
Following informed consent, children were included in the study if they were in good health and had received 3 doses (children aged 13–48 months) or 2 doses (children aged 6–12 months) of PCV13, as per the UK infant immunization schedule. Children who had not received a complete course of PCV13, had received PCV13 within the last 28 days, had taken antibiotics in the preceding 30 days, were febrile, had a respiratory illness, or had a health condition that may have influenced the study were excluded. Demographic information (age, sex, ethnicity, number and ages of siblings, pattern of daycare attendance, and household smoking [living with an adult who smoked at home]) was recorded.
Data collected in this study were compared with data from a carriage study in children aged 13–48 months, vaccinated with PCV13 according to the same schedule, conducted in the Thames Valley between February 2014 and August 2015 [18].
All clinical investigation was conducted according to the principles expressed in the Declaration of Helsinki. Written ethics approval for the study was obtained from the Nottingham Research and Ethics Committee (identification number 17/EM/0158).
The study was registered at ClinicalTrials.gov (identifier NCT03102840).
Sample Size
A sample size of 1600 children aged 13–48 months was calculated to provide 80% power to detect an approximately 2-fold increase in serotype 19A point prevalence from 0.91% (2014–2015) to 1.62% at a significance of P < .05 (1-sided α). A futility analysis was planned when 750 participants had been enrolled; if the number of 19A-positive samples was ≤5 at this point, consideration would be given to stopping recruitment as it would be unlikely this degree of increase in serotype 19A carriage would be observed. Furthermore, a sample size of 813 children aged 6–12 months, based on serotype 19A carriage rate of 2.2%, was calculated to allow a subsequent study with the same sample size to be 80% powered to detect a doubling of serotype 19A carriage at a significance of P < .05 (1-sided α) in this age group.
Outcomes
The primary outcome was the presence of serotype 19A pneumococci. Secondary outcomes included the presence of (any) pneumococcal serotypes on swabs, molecular serotype of nasopharyngeal carriage isolates, and serotype-specific antibody concentrations.
Nasopharyngeal Swab Analysis
Swabs were collected and processed according to World Health Organization guidelines [19]. A single flexible aluminium shaft with rayon tip (MWE, Wiltshire, UK) was passed through the anterior nares as far as the posterior pharynx, rotated 360 degrees before removal, and placed into a tube of 0.5–1 mL of skim milk-tryptone-glucose-glycerin (STGG) transport medium.
Swabs were frozen on arrival at −80°C, before subsequent thawing, plating on Columbia blood agar, and overnight incubation to identify pneumococcal-positive samples, based on optochin susceptibility and bile solubility. “Sweeps” were performed of streptococcal selective culture plates from serial dilutions of STGG from swabs with pneumococcal-presumptive growth, which underwent DNA extraction followed by molecular serotyping using the Senti-SPv1.5 microarray (BUGS Bioscience, UK).
To enable comparisons between time periods, this approach was applied to both this study and a reanalysis of sweeps taken from cultures of stored swabs from 2014–2015 (previously analyzed as single isolates from each culture [18]). Based on secondary phenotyping conducted in 2014–2015 where all 24B/F isolates were confirmed to be 24F, all isolates identified as 24B/F in 2017–2020 were considered 24F. After review, given that 15B and 15C may switch, they were considered 15B/C and not distinguished further.
To resolve uncertainty regarding serotype 33A and 33F identification, secondary phenotyping by Quellung reaction (Statens Serum Institut, Denmark) was performed on a subset of 33A and 33F isolates from 2014–2015 and 2017–2020 which, along with analysis of genome sequences, confirmed identification of all 33A/F isolates as 33F [20].
Blood Collection and Measurement of Serum Pneumococcal Serotype-Specific IgG
Blood from capillary sampling was collected in a microtube (Multivette 600, Sarstedt, Leister, UK) from a subset of children whose parents gave consent. Pneumococcal serotype-specific serum IgG against PCV13 serotypes and 12 additional nonvaccine serotypes (2, 8, 9N, 10A, 11A, 12F, 15B, 17F, 20, 22F, 23B, 33F) was measured by multiplex immunoassay (Bio-Rad Laboratories, Hercules, California) with Luminex technology by the National Institute for Public Health and the Environment, the Netherlands [21].
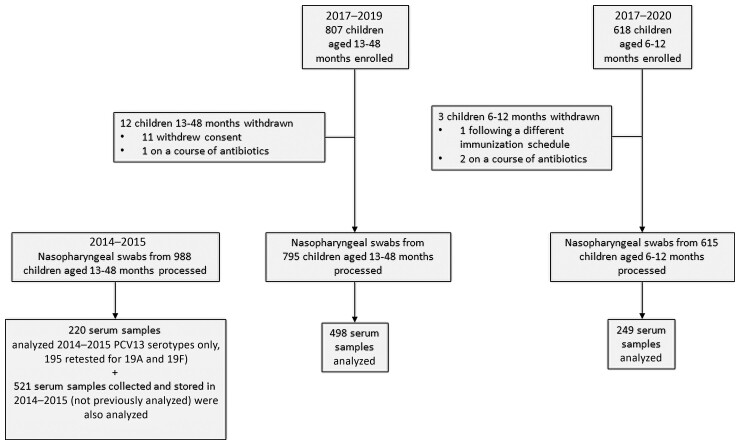
As well as data collected in this study, stored sera from 2014–2015 (batch 2, 521 samples) were tested at the same time (Figure 1).
Figure 1.
Overview of study recruitment. Abbreviation: PCV13, 13-valent pneumococcal conjugate vaccine.
Identification of Data for Carriage Study Trends
To explore longer-term trends, we searched PubMed for published data from other UK carriage studies conducted since 2006 in children aged <5 years using the terms (pneumococc*[Title/Abstract]) AND (carriage[Title/Abstract]) AND (England[Title/Abstract] OR Wales[Title/Abstract] OR Scotland[Title/Abstract] OR Northern Ireland[Title/Abstract] OR United Kingdom[Title/Abstract] OR UK[Title/Abstract]). Carriage studies from Southampton (Southampton University, 7 studies) [22], Gloucestershire and Hertfordshire (Public Health England [now UK Health Security Agency], 3 studies) [3, 4, 23] and Thames Valley (Oxford Vaccine Group, 3 studies) [8, 18] were included.
IPD Data
Serotype-specific IPD data for England and Wales collected by Public Health England were extracted from published tables, or figures using WebPlotDigitizer [24] for children <5 years and adults ≥65 years of age [7, 9, 10, 18, 25, 26]. Where necessary, rates were calculated using Office of National Statistics population data denominators [27].
Statistical Analysis
Pneumococcal carriage rates and 95% confidence intervals (CIs) were calculated using a binomial exact method. Associations between demographic features and carriage were explored with logistic regression.
Serotype-specific comparisons of carriage between time periods and age cohorts were performed using Fisher exact test, reported as odds ratios (ORs) with 99% CIs. P values < .01 were considered significant to allow for multiple comparisons.
Geometric mean concentrations (GMCs) of serotype-specific IgG and the proportion of children with IgG ≥0.35 µg/mL were compared for each age group and time period.
Serotype-specific IgG GMCs were calculated for carriers and noncarriers of pneumococcus and for carriers of particular serotypes. Log-transformed serum antibody concentrations were compared between children carrying different serotypes by calculating the geometric mean ratio adjusted for age and sex using linear regression.
Serum anti-pneumococcal IgG was analyzed to explore current and previous exposure to particular vaccine serotypes, which may have resulted in natural boosting. Three approaches were explored; Gaussian 2-component mixture models, best fit model, and linear regression mixture model (Supplementary Methods).
To fit long-term trends in overall pneumococcal carriage accounting for different carriage rates between studies, we calculated the percentage of each serotype as the proportion of total isolates for each study and found the best-fitting binomial regression model (Supplementary Methods, Supplementary Table 1).
All statistical analyses were performed using Stata version 17 software.
RESULTS
Following screening and informed consent, 1425 children were enrolled between June 2017 and August 2019 (children aged 13–48 months) and June 2017 and March 2020 (children aged 6–12 months, Figure 1). Following the withdrawal of 15 children, 1410 swabs were available for analysis. A further 988 stored nasopharyngeal swabs were reanalyzed from 2014–2015. Blood samples from 747 children in 2017–2020 were tested for pneumococcal antibodies.
Nasopharyngeal Carriage
Overall
Pneumococci were isolated from 473 of 988 swabs collected in 2014–2015 (47.9% [95% CI, 44.7%–51.0%]) and 412 of 795 children aged 13–48 months in 2017–2019 (51.8% [95% CI, 48.3%–55.3%]), with no significant change in carriage (P = .10). In 2014–2015, 481 carriers had been identified using a single-isolate approach, a similar proportion of carriers to this reanalysis (P = .72).
Carriage of pneumococci was lower in children aged 6–12 months (274/615, 44.6% [95% CI, 40.6%–48.6%]) in 2017–2019 compared with those aged 13–48 months (P = .01).
Risk Factors for Carriage
Carriers aged 13–48 months in 2017–2019 were similar to noncarriers in terms of age, sex, household size, number of siblings, and prevalence of smoking (Table 1). Mean hours per week of daycare or preschool attendance was significantly higher (P < .001) for carriers compared with noncarriers and remained significant after adjustment for other factors (P < .001; Table 1). The odds of carriage was significantly higher for White versus African heritage/Afro-Caribbean or Asian children, in those aged 13–48 months only, after adjustment for other factors.
Table 1.
Characteristics of Carriers and Noncarriers by Age Cohort, 2017–2020
| Characteristic | All | Carriers | Noncarriers | Univariate Analysis | Multivariable Analysis | |
|---|---|---|---|---|---|---|
| P Value | Odds Ratio (95% CI) | P Value | ||||
| 13–18 mo, No. | 795 | 412 | 383 | |||
| ȃAge | .739a | 0.84 (.70–1.00) | .053 | |||
| ȃȃMean age, y (SD) | 2.4 (0.9) | 2.4 (0.9) | 2.4 (0.8) | … | ||
| ȃSex (female, reference group) | .971b | 1.02 (.76–1.36) | .914 | |||
| ȃȃMale sex, No. (%) | 425 (53.5) | 220 (53.3) | 205 (53.5) | … | ||
| ȃRace/ethnicity, No. (%) | <.001b | <.001 | ||||
| ȃȃWhite (reference group) | 668 (84.0) | 367 (89.1) | 301 (78.6) | … | ||
| ȃȃAfrican heritage/Afro-Caribbean | 19 (2.4) | 4 (1.0) | 15 (3.9) | 0.24 (.08–.73) | ||
| ȃȃAsian | 42 (5.3) | 11 (2.7) | 31 (8.1) | 0.30 (.15–.62) | ||
| ȃȃMixed/other | 66 (8.3) | 30 (7.3) | 36 (9.4) | 0.63 (.38–1.07) | ||
| ȃHousehold size | .665c | 1.07 (.78–1.46) | .678 | |||
| ȃȃMean (SD) | 3.9 (0.9) | 3.9 (0.9) | 3.9 (0.9) | … | ||
| ȃȃMedian (min, max) | 4 (2, 8) | 4 (2, 8) | 4 (2, 8) | … | ||
| ȃNo. of siblings | .978a | 1.00 (.71–1.40) | .997 | |||
| ȃȃMean (SD) | 0.9 (0.8) | 0.9 (0.8) | 0.9 (0.9) | … | ||
| ȃȃMedian (min, max) | 1 (0, 5) | 1 (0, 5) | 1 (0, 5) | … | ||
| ȃSmoker in household (nonsmoker, reference group) | .665b | 0.97 (.57–1.65) | .903 | |||
| ȃȃChildren with a smoker in the house, No. (%) | 63 (7.9) | 31 (7.5) | 32 (8.4) | … | ||
| ȃDaycare or preschool attendance per week | <.001a | 1.03 (1.02–1.04) | <.001 | |||
| ȃȃMean, h (SD) | 17.2 (13.8) | 19.6 (13.6) | 14.6 (13.6) | … | ||
| ȃȃMedian, h (min, max) | 16 (0, 60) | 20 (0, 50) | 14 (0, 60) | … | ||
| 6–12 mo, No. | 615 | 274 | 341 | |||
| ȃAge | .005a | 2.42 (.69–8.50) | .168 | |||
| ȃȃMean age, y (SD) | 0.82 (0.1) | 0.84 (0.1) | 0.81 (0.1) | … | ||
| ȃSex (female, reference group) | .398b | 1.21 (.86–1.69) | .256 | |||
| ȃȃMale sex, No. (%) | 325 (52.8) | 150 (54.7) | 175 (51.3) | … | ||
| ȃRace/ethnicity, No. (%) | .726b | .600 | ||||
| ȃȃWhite (reference group) | 536 (87.2) | 242 (88.3) | 294 (86.2) | … | ||
| ȃȃAfrican heritage/Afro-Caribbean | 12 (2.0) | 4 (1.5) | 8 (2.3) | 0.57 (.16–2.00) | ||
| ȃȃAsian | 28 (4.6) | 13 (4.7) | 15 (4.4) | 1.00 (.44–2.27) | ||
| ȃȃMixed/other | 39 (6.3) | 15 (5.5) | 24 (7.0) | 0.69 (.34–1.39) | ||
| ȃHousehold size | <.001a | 0.89 (.63–1.25) | .491 | |||
| ȃȃMean (SD) | 3.7 (0.9) | 3.8 (0.9) | 3.5 (0.9) | … | ||
| ȃȃMedian (min, max) | 3 (2, 8) | 4 (2, 8) | 3 (2, 8) | … | ||
| ȃNo. of siblings | <.001a | 2.11 (1.43–3.11) | <.001 | |||
| ȃȃMean (SD) | 0.6 (0.8) | 0.8 (0.8) | 0.5 (0.8) | … | ||
| ȃȃMedian (min, max) | 0 (0, 4) | 1 (0, 4) | 0 (0, 4) | … | ||
| ȃSmoker in household (nonsmoker, reference group) | .555b | 0.68 (.37–1.26) | .215 | |||
| ȃȃChildren with a smoker in the house, No. (%) | 54 (8.8) | 22 (8.0) | 32 (9.4) | … | ||
| ȃDaycare or preschool attendance per week | .002a | 1.03 (1.01–1.05) | .001 | |||
| ȃȃMean, h (SD) | 4.9 (10.5) | 6.7 (12.0) | 3.4 (8.8) | … | ||
| ȃȃMedian, h (min, max) | 0 (0, 45) | 0 (0, 45) | 0 (0, 42) | … | ||
P values < .05 are in bold font.
Abbreviations: CI, confidence interval; OR, odds ratio; SD, standard deviation.
Mann–Whitney U test.
χ2 test; multivariable analysis includes all variables.
Household characteristics were more important for children aged 6–12 months, with significantly higher mean household size and number of siblings for carriers compared with noncarriers, in addition to higher mean age and number of hours per week of daycare or preschool attendance. However only increased number of siblings and hours of daycare or preschool attendance remained significant after adjustment for other factors (Table 1).
Serotype-Specific Carriage
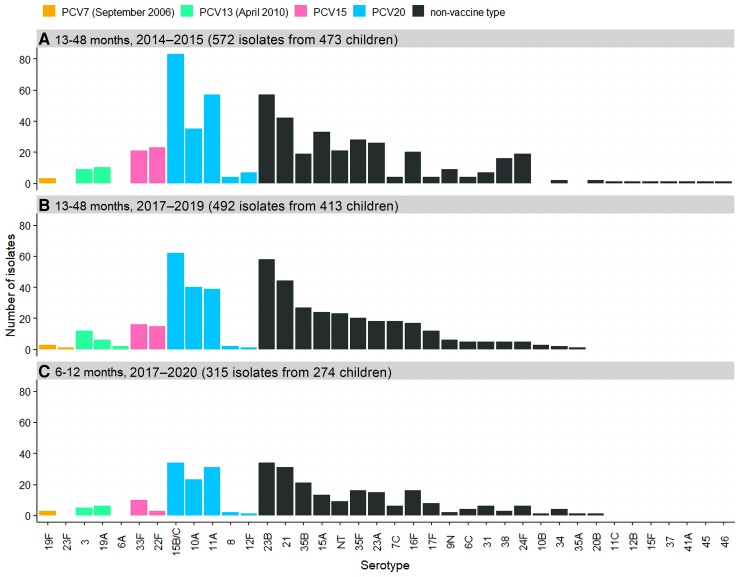
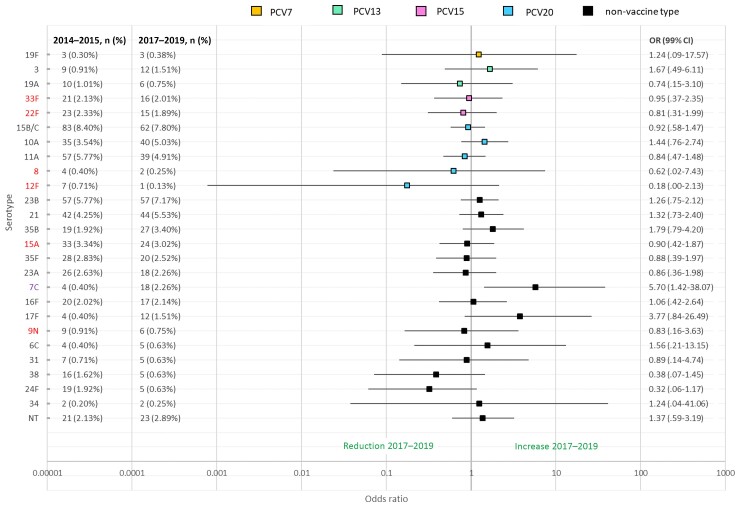
In 2017–2019, 23 of 795 children aged 13–48 months (2.9% [95% CI, 1.8%–4.3%]) were carrying a PCV13 serotype, most commonly serotypes 3 (12/795, 1.5% [95% CI, .8%–2.6%]) and 19A (6/795, 0.8% [95% CI, .3%–1.6%]) (Figure 2). The odds of carriage of individual serotypes in children aged 13–48 months were unchanged between 2014–2015 and 2017–2019 for all serotypes except for an increase in 7C (OR, 5.70 [95% CI, 1.87–23.22], P < .001; Figure 3). There was no significant difference in the odds of carriage for each individual serotype across the 2 age cohorts (Supplementary Figure 1).
Figure 2.
Serotypes isolated from nasopharyngeal swabs in 2014–2015 (A), 2017–2019 (B), and 2017–2020 (C). Month and year in parentheses refers to the date of introduction into the UK schedule. PCV15 and PCV20 are newly approved higher-valency vaccines. Abbreviations: NT, nontypeable; PCV7, 7-valent pneumococcal conjugate vaccine; PCV13, 13-valent pneumococcal conjugate vaccine; PCV15, 15-valent pneumococcal conjugate vaccine; PCV20, 20-valent pneumococcal conjugate vaccine.
Figure 3.
Serotype-specific odds ratios of nasopharyngeal carriage of pneumococci in children aged 13–48 mo in 2014–2015 (n = 988) compared to 2017–2019 (n = 795). Six main replacement serotypes for invasive pneumococcal disease (IPD) in 2016–2017 were 8, 12F, 9N, 22F, 33F, 15A [10]. Increase in IPD due to 7C was seen from 2016 to 2017 [26]. Lines denote 99% confidence intervals around each odds ratio. Due to multiple comparisons, P < .01 is considered significant. PCV15 and PCV20 are newly approved higher-valency vaccines. Abbreviations: CI, confidence interval; NT, nontypeable; OR, odds ratio; PCV7, 7-valent pneumococcal conjugate vaccine; PCV13, 13-valent pneumococcal conjugate vaccine; PCV15, 15-valent pneumococcal conjugate vaccine; PCV20, 20-valent pneumococcal conjugate vaccine.
The 5 most frequently isolated serotypes were 15B/C, 23B, 21, 10A, and 11A, though the order of prevalence varied between the 2 age groups and time periods (Figure 2).
Serotypes 22F and 33F (contained in PCV15) were carried by 4.5% (44/988) and 3.8% (30/795) of children aged 13–48 months 2014–2015 and 2017–2019, respectively. A further 17.5% (173/988) and 16.9% (134/795) of children aged 13–48 months were carrying serotypes 8, 10A, 11A, 12F, and 15B/C (also contained in PCV20) in 2014–2015 and 2017–2019, respectively. Invasiveness of these serotypes varies widely (Supplementary Figure 2).
Carriage of Multiple Serotypes
Overall, across all cohorts 2014–2015 and 2017–2020, the majority (83.4% [967/1159]) of pneumococcal carriers were carrying 1 serotype only, with the remaining carrying 2 (14.4% [167 children]), 3 (2.1% [24 children]), or 6 (0.1% [1 child]) serotypes.
Carriers of serotypes included in PCV13 were more likely to be carrying multiple serotypes; 47.5% (28/59) of vaccine-type carriers were carrying multiple pneumococcal serotypes compared with 16.9% (191/1127) of non-vaccine-type carriers (P < .001). A higher proportion of vaccine serotypes (36.7% [22/60]) compared with nonvaccine serotypes (15.8% [209/1319]) were minor serotypes in a mixed culture (as opposed to single serotype, or major serotype in a mixed culture) (P < .001). Vaccine serotypes had a lower median colony density overall (4850 colony-forming units [CFU]/mL; serotype 3, 2100 CFU/mL; serotype 19A, 9250 CFU/mL) than nonvaccine serotypes (10 000 CFU/mL) (Supplementary Figure 3).
Serology
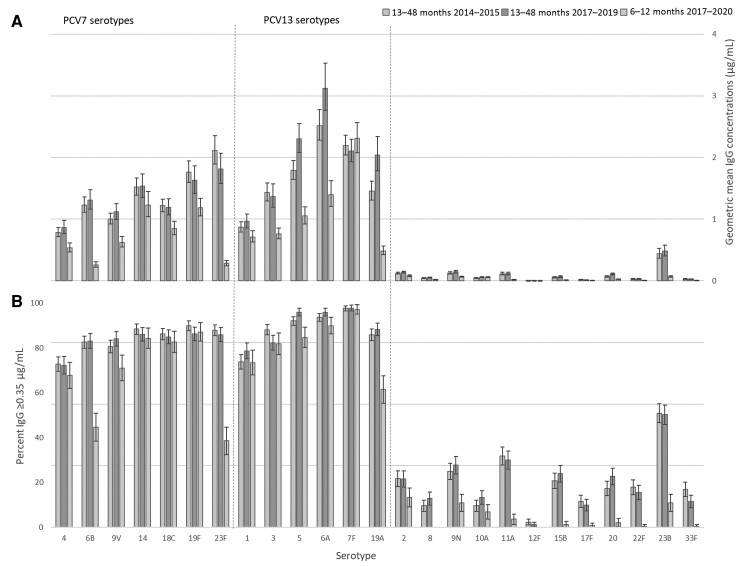
IgG GMCs were significantly higher (P < .002, Bonferroni correction 0.05/25) for children aged 13–48 months in 2017–2019 compared with 2014–2015 for PCV13 serotypes 5 and 19A and for nonvaccine serotypes 10A and 20 (Figure 4A), but there were no significant differences (P ≥ .002, Bonferroni correction 0.05/25) in the proportion of children with IgG titer ≥0.35 µg/mL in 2017–2019 versus 2014–2015 for any serotypes (Figure 4B).
Figure 4.
Serotype-specific geometric mean immunoglobulin G (IgG) concentrations (A) and proportion with serotype-specific IgG concentrations ≥0.35 µg/mL (B), for children aged 13–48 mo in 2014–2015 and 2017–2019 and 6–12 mo in 2017–2020. Error bars denote 95% confidence intervals. Abbreviations: IgG, immunoglobulin G; PCV7, 7-valent pneumococcal conjugate vaccine (introduced September 2006); PCV13, 13-valent pneumococcal conjugate vaccine (introduced April 2010).
As expected, given receipt of an additional dose of PCV13, serotype-specific IgG GMCs were significantly higher (as opposed to no difference) for children aged 13–48 months compared with children aged 6–12 months for 10 of 13 vaccine serotypes (Figure 4A); however, this was also observed for 11 of 12 nonvaccine serotypes. The proportion of children with serotype-specific IgG concentrations ≥0.35 µg/mL was significantly higher for children aged 13–48 months compared with children aged 6–12 months for 6 of 13 vaccine serotypes and 9 of 12 nonvaccine serotypes (Figure 4B).
There was no difference in serotype-specific IgG GMCs for carriers (any pneumococci) and noncarriers in either age cohort in 2017–2020. Carriers of vaccine serotypes 3 and 19A aged 13–48 months or 6–12 months (19A only) in 2017–2019 had higher serotype 3 or 19A IgG GMCs, respectively, compared with noncarriers and/or non-vaccine-type carriers after adjusting for age and sex, suggestive of natural boosting (Supplementary Figures 4 and 5, Supplementary Tables 2 and 3). Natural boosting of serotype-specific IgG was also observed for carriers of some nonvaccine serotypes in both age groups (Supplementary Tables 4 and 5, Supplementary Figures 6 and 7). There was no change in the proportion of natural boosting events identified for any vaccine serotypes between 2014–2015 and 2017–2019 across any of the 3 approaches (Supplementary Table 6).
Long-term Trends in Individual Serotypes as a Proportion of Total Isolates
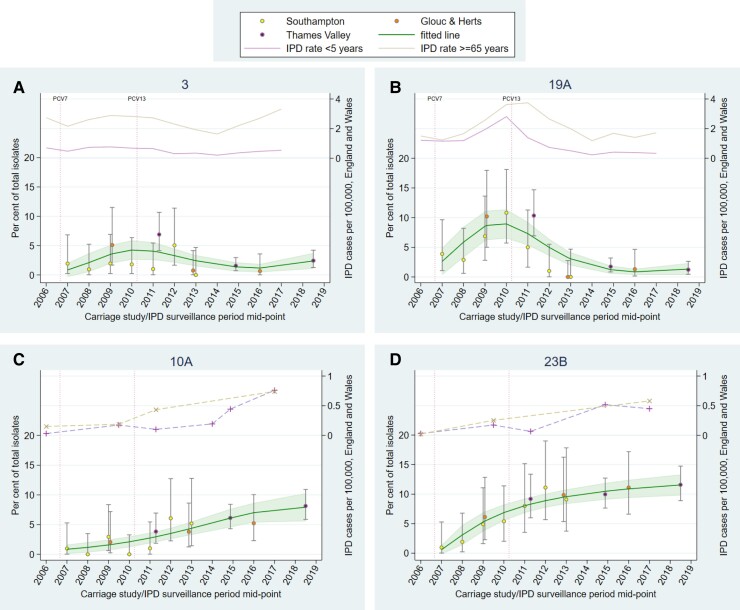
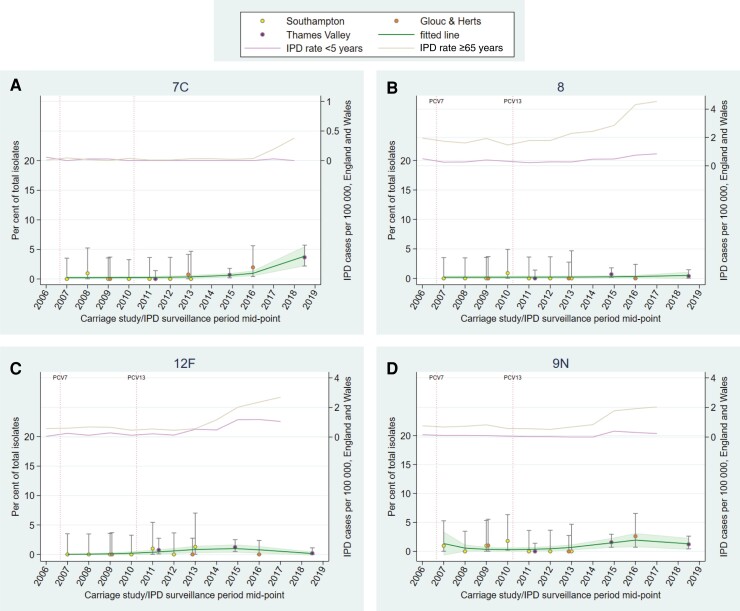
Trends in carriage generally aligned with trends in IPD for vaccine serotypes (PCV7, additional 6 PCV13 serotypes, and cross-reactive 6C), and showed reductions in carriage following vaccine introduction to low/negligible levels (Supplementary Figures 8–10). However, serotypes 3 and 19A can be seen persisting (albeit reduced for 19A) in both carriage and IPD (Figure 5A and 5B). Serotypes 10A and 23B have consistently increased in carriage and disease since 2007 (Figure 5C and 5D), with serotypes 15B/C and 35B showing similar trends with some fluctuation (Supplementary Figure 12E and 12H). Serotype 7C shows a more recent increase in both carriage (detected in this study) and IPD in the age group ≥65 years (Figure 6A). In contrast, carriage trends for serotypes 8, 12F, and 9N do not mirror upward IPD trends (particularly in those aged ≥65 years; Figure 6B–D). Most other serotypes appeared to stabilize, or had decreasing trends, following earlier increases during the time period (Supplementary Figures 8–13).
Figure 5.
Percentage of total isolates in recent carriage studies and annual invasive pneumococcal disease (IPD) rates in England and Wales for serotypes 3 (A), 19A (B), 10A (C), and 23B (D). A dashed line has been used where IPD data were not available for every year. Error bars denote 95% confidence intervals (CIs) for individual studies. Shading indicates 95% CI around fitted line. Serotype 10A is contained within PCV20. Abbreviations: Glouc & Herts, Gloucestershire and Hertfordshire; IPD, invasive pneumococcal disease; PCV7, 7-valent pneumococcal conjugate vaccine (introduced September 2006); PCV13, 13-valent pneumococcal conjugate vaccine (introduced April 2010).
Figure 6.
Percentage of total isolates in recent carriage studies and annual invasive pneumococcal disease (IPD) rates in England and Wales for serotypes 7C (A), 8 (B), 12F (C), and 9N (D). A dashed line has been used where IPD data were not available for every year. IPD data for serotype 7C is incomplete for 2017–2018. Error bars denote 95% confidence intervals (CIs) for individual studies. Shading indicates 95% CI around fitted line. Serotype 8 is contained within PCV20. Abbreviations: Glouc & Herts, Gloucestershire and Hertfordshire; IPD, invasive pneumococcal disease; PCV7, 7-valent pneumococcal conjugate vaccine (introduced September 2006); PCV13, 13-valent pneumococcal conjugate vaccine (introduced April 2010).
DISCUSSION
This is the second study in the UK Thames Valley to assess pneumococcal nasopharyngeal carriage in young children following PCV13 introduction in 2010. Key findings include a recent increase in serotype 7C carriage and disease, longer-term upward trends in carriage and disease of serotypes 10A, 23B, 15B/C, 35B, and low-level carriage of PCV13 serotypes 3 and 19A (and to a lesser extent, PCV7 serotypes 19F and 23F and PCV13 6A). The newly available PCV15 and PCV20 vaccines contain additional serotypes carried by 3.8% (22F and 33F) and 20.6% (22F, 33F, 8, 10A, 11A, 12F, 15B) of healthy children aged 13–48 months, respectively. Approximately 10% of total IPD cases in England and Wales are caused by 22F and 33F [25], and 40% by the additional 5 PCV20 serotypes (33% by 8 and 12F alone) [10], suggesting that PCV20 may have the potential to approximately halve IPD cases should distributions remain similar and there is no further rise in non-PCV15/20 types.
Overall pneumococcal carriage remained stable (around 50%) in children aged 13–48 months between 2014–2015 and 2017–2019, consistent with earlier UK carriage studies [3, 8]. Non-PCV13 serotypes continued to comprise the vast majority (>95%) of pneumococci isolated from PCV13-vaccinated children, in particular serotypes 23B, 21, 15B/C, 10A, and 11A (>49% of isolates in 2017–2019).
Carriage Prevalence
Vaccine Serotypes
Persistence of serotype 3 in carriage and disease is also seen in other countries with established PCV13 programs [28–30], implying low vaccine efficacy against this serotype.
The higher 19A-specific IgG concentrations in this study compared with 2014–2015 may indicate increased circulation of this serotype. However, the absence of significant increases in serologically defined natural boosting events, or nasopharyngeal carriage between the 2 time periods, and the relatively stable trend in carriage and IPD from 2015 onward, leaves uncertainty around interpretation of this result. Nevertheless, there is ongoing low-level circulation of 19A despite PCV13 introduction, a phenomenon that has also been reported in Sweden, France, and Ireland [31–33].
Serotype 5 has not been detected in carriage in this study or in the past decade [3, 4, 8, 18, 22, 23], suggesting that the increase in IgG between 2014–2015 and 2017–2019 may be a testing artefact.
Nonvaccine Serotypes
In the past 10 years, 7C carriage has been very low/negligible in England [8, 22, 23]; however, a sudden rapid increase in IPD cases in England and Wales caused by 7C was reported recently when cases increased from an average of 3 cases per year between 2000–2001 and 2015–2016 to 29 cases in 2016–2017, with the increase continuing into 2017–2018 [26]. This coincides with the increase in carriage seen in our study. The longer-term upward carriage trends observed for serotypes 10A and 23B (also seen in disease) were not detected by comparing 2 relatively close study time points.
Although serotypes 8 and 12F (responsible for 24% of cases <5 years and 25% of cases ≥65 years in 2016–2017 [10]) were infrequently carried, this does not rule out indirect protection from routine pediatric PCV20 introduction given that childhood PCV13 immunization against serotype 1 (also infrequently carried but highly invasive) reduced IPD incidence in both children and adults [7]. However, PCV20 also contains commonly carried, less invasive serotypes (10A, 11A, 15B), and the impact of reducing circulation of these serotypes, providing an ecological niche that other serotypes could fill, should also be considered [5, 33].
Strengths and Limitations of the Study
The cross-sectional design could only provide a point in time measurement; however, we have also explored long-term carriage study trends. To be comparable with previous studies, we selected a fully vaccinated cohort that may not fully represent carriage in the community. The sample size for the 6- to 12-month cohort was smaller than planned due to coronavirus disease 2019 (COVID-19)–related premature recruitment termination.
Strengths of the study were the large sample size overall, a 3-year study period reducing any potential impact of seasonality, the sensitivity of sweep and microarray approaches, and use of the same laboratories and methods throughout.
Methods used to explore natural boosting were experimental and results are taken in the context of other analyses presented here. However, it was reassuring that in general the serotypes with the greatest numbers of boosting events corresponded to the isolates detected in carriage.
Lockdown and other measures to prevent transmission of COVID-19 had a significant impact on IPD globally (reported across all age groups and serotypes in England, with a specific reduction in serotype 12F in children [34, 35]). Relatively stable carriage rates during the pandemic period (eg, in Belgium and Israel) suggest that disease reductions may have been due to suppression of viruses that can increase S. pneumoniae virulence/spread rather than reductions in carriage [36, 37]. Our data provide an important prepandemic baseline, though disentangling the impact of the pandemic from the change in schedule will be challenging.
CONCLUSIONS
The persistence of vaccine serotypes 3 and 19A in carriage and disease suggests that pediatric PCV13 immunization cannot further reduce disease due to these serotypes. Increased carriage of serotype 7C between 2014–2015 and 2017–2019, along with more gradual increases in serotypes such as 10A and 23B, highlights the need for ongoing monitoring.
The finding that 20% of children carry the additional 7 serotypes included in PCV20 suggests that introduction of this vaccine, if licensed in this age group, could significantly change the composition of pneumococcal serotypes carried in the pharynx of UK children, knowledge that is crucial for modeling the potential direct and indirect effects of PCV15 and PCV20 introduction.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Karen S Tiley, Oxford Vaccine Group, Department of Paediatrics, University of Oxford, Oxford, United Kingdom.
Helen Ratcliffe, Oxford Vaccine Group, Department of Paediatrics, University of Oxford, Oxford, United Kingdom.
Merryn Voysey, Oxford Vaccine Group, Department of Paediatrics, University of Oxford, Oxford, United Kingdom.
Kimberley Jefferies, Oxford Vaccine Group, Department of Paediatrics, University of Oxford, Oxford, United Kingdom.
Gemma Sinclair, Oxford Vaccine Group, Department of Paediatrics, University of Oxford, Oxford, United Kingdom.
Melanie Carr, Oxford Vaccine Group, Department of Paediatrics, University of Oxford, Oxford, United Kingdom.
Rachel Colin-Jones, Oxford Vaccine Group, Department of Paediatrics, University of Oxford, Oxford, United Kingdom.
David Smith, Oxford Vaccine Group, Department of Paediatrics, University of Oxford, Oxford, United Kingdom.
Jaclyn Bowman, Oxford Vaccine Group, Department of Paediatrics, University of Oxford, Oxford, United Kingdom.
Thomas Hart, Oxford Vaccine Group, Department of Paediatrics, University of Oxford, Oxford, United Kingdom.
Rama Kandasamy, Oxford Vaccine Group, Department of Paediatrics, University of Oxford, Oxford, United Kingdom.
Jason Hinds, Institute for Infection and Immunity, St George’s University, London, United Kingdom; BUGS Bioscience, London Bioscience Innovation Centre, London, United Kingdom.
Katherine Gould, Institute for Infection and Immunity, St George’s University, London, United Kingdom; BUGS Bioscience, London Bioscience Innovation Centre, London, United Kingdom.
Guy Berbers, Immunology, National Institute for Public Health and the Environment, Bilthoven, The Netherlands.
Irina Tcherniaeva, Immunology, National Institute for Public Health and the Environment, Bilthoven, The Netherlands.
Hannah Robinson, Oxford Vaccine Group, Department of Paediatrics, University of Oxford, Oxford, United Kingdom; National Institute for Health Research Oxford Biomedical Research Centre, Oxford, United Kingdom, and; National Institute for Health Research Clinical Research Network Thames Valley and South Midlands, Oxford, United Kingdom.
Emma Plested, Oxford Vaccine Group, Department of Paediatrics, University of Oxford, Oxford, United Kingdom; National Institute for Health Research Oxford Biomedical Research Centre, Oxford, United Kingdom, and; National Institute for Health Research Clinical Research Network Thames Valley and South Midlands, Oxford, United Kingdom.
Parvinder Aley, Oxford Vaccine Group, Department of Paediatrics, University of Oxford, Oxford, United Kingdom; National Institute for Health Research Oxford Biomedical Research Centre, Oxford, United Kingdom, and.
Matthew D Snape, Oxford Vaccine Group, Department of Paediatrics, University of Oxford, Oxford, United Kingdom; National Institute for Health Research Oxford Biomedical Research Centre, Oxford, United Kingdom, and.
Notes
Acknowledgments. The authors express their gratitude to all participating children and their families. The authors thank Sonu Shrestha for confirmatory Quellung testing on selected isolates, Vicky Harris for assistance with sample size calculations, Dan Phillips for his suggestions on the natural boosting methodology, Liberty Cantrell for data validation, and Andrew Pollard for helpful comments on the draft manuscript.
Disclaimer. The views expressed are those of the authors and not necessarily those of the National Health Service, the National Institute for Health Research (NIHR), or the UK Department of Health. Stored samples from the 2014–2015 carriage study were provided for reanalysis by Oxford Vaccine Centre Biobank.
Financial support. This investigator-initiated study was funded by Pfizer and sponsored by the University of Oxford. M. D. S. receives salary support from the Oxford NIHR Biomedical Research Centre and is an NIHR Senior Investigator. The research was supported by the National Institute for Health Research Oxford Biomedical Research Centre and NIHR Clinical Research Network Thames Valley and South Midlands.
References
- 1. Wahl B, O’Brien KL, Greenbaum A, et al. Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000–15. Lancet Glob Health 2018; 6:e744–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ganaie F, Saad JS, McGee L, et al. A new pneumococcal capsule type, 10D, is the 100th serotype and has a large cps fragment from an oral Streptococcus. mBio 2020; 11:e00937-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Southern J, Andrews N, Sandu P, et al. Pneumococcal carriage in children and their household contacts six years after introduction of the 13-valent pneumococcal conjugate vaccine in England. PLoS One 2018; 13:e0195799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Hoek AJ, Sheppard CL, Andrews NJ, et al. Pneumococcal carriage in children and adults two years after introduction of the thirteen valent pneumococcal conjugate vaccine in England. Vaccine 2014; 32:4349–55. [DOI] [PubMed] [Google Scholar]
- 5. Balsells E, Dagan R, Yildirim I, et al. The relative invasive disease potential of Streptococcus pneumoniae among children after PCV introduction: a systematic review and meta-analysis. J Infect 2018; 77:368–78. [DOI] [PubMed] [Google Scholar]
- 6. Trotter CL, Waight P, Andrews NJ, et al. Epidemiology of invasive pneumococcal disease in the pre-conjugate vaccine era: England and Wales, 1996–2006. J Infect 2010; 60:200–8. [DOI] [PubMed] [Google Scholar]
- 7. Waight PA, Andrews NJ, Ladhani NJ, Sheppard CL, Slack MP, Miller E. Effect of the 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease in England and Wales 4 years after its introduction: an observational cohort study. Lancet Infect Dis 2015; 15:629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hamaluba M, Kandasamy R, Ndimah S, et al. A cross-sectional observational study of pneumococcal carriage in children, their parents, and older adults following the introduction of the 7-valent pneumococcal conjugate vaccine. Medicine (Baltimore) 2015; 94:e335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Miller E, Andrews NJ, Waight PA, Slack MP, George RC. Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: an observational cohort study. Lancet Infect Dis 2011; 11:760–8. [DOI] [PubMed] [Google Scholar]
- 10. Ladhani SN, Collins S, Djennad A, et al. Rapid increase in non-vaccine serotypes causing invasive pneumococcal disease in England and Wales, 2000–17: a prospective national observational cohort study. Lancet Infect Dis 2018; 18:441–51. [DOI] [PubMed] [Google Scholar]
- 11. European Medicines Agency . Vaxneuvance.https://www.ema.europa.eu/en/medicines/human/EPAR/vaxneuvance. Accessed 25 May 2022.
- 12. European Medicines Agency . Apexxnar.https://www.ema.europa.eu/en/medicines/human/EPAR/apexxnar. Accessed 25 May 2022.
- 13. Weiser JN, Ferreira DM, Paton JC. Streptococcus pneumoniae: transmission, colonization and invasion. Nat Rev Microbiol 2018; 16:355–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weinberger DM, Grant LR, Weatherholtz RC, Warren JL, O’Brien KL, Hammitt LL. Relating pneumococcal carriage among children to disease rates among adults before and after the introduction of conjugate vaccines. Am J Epidemiol 2016; 183:1055–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mosser JF, Grant LR, Millar EV, et al. Nasopharyngeal carriage and transmission of Streptococcus pneumoniae in American Indian households after a decade of pneumococcal conjugate vaccine use. PLoS One 2014; 9:e79578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Public Health England . Changes to the infant pneumococcal conjugate vaccine schedule, 2019. https://www.gov.uk/government/publications/pneumococcal-vaccination-infant-schedule-changes-from-january-2020-letter. Accessed 21 September 2022.
- 17. Public Health England . Complete routine immunization schedule.https://www.gov.uk/government/publications/the-complete-routine-immunisation-schedule. Accessed 27 June 2022.
- 18. Kandasamy R, Voysey M, Collins S, et al. Persistent circulation of vaccine serotypes and serotype replacement after 5 years of infant immunization with 13-valent pneumococcal conjugate vaccine in the United Kingdom. J Infect Dis 2020; 221:1361–70. [DOI] [PubMed] [Google Scholar]
- 19. Satzke C, Turner P, Virolainen-Julkunen A, et al. Standard method for detecting upper respiratory carriage of Streptococcus pneumoniae: updated recommendations from the World Health Organization Pneumococcal Carriage Working Group. Vaccine 2013; 32:165–79. [DOI] [PubMed] [Google Scholar]
- 20. Statens Serum Institut . Streptococcus pneumoniae: textbook in serotyping, virulence factors and enzyme-linked immunosorbent assay (ELISA) for measuring pneumococcal antibodies. Copenhagen: Staten Serum Institut, 2013. [Google Scholar]
- 21. Elberse KE, de Greeff SC, Wattimena N, et al. Seroprevalence of IgG antibodies against 13 vaccine Streptococcus pneumoniae serotypes in the Netherlands. Vaccine 2011; 29:1029–35. [DOI] [PubMed] [Google Scholar]
- 22. Devine VT, Cleary DW, Jefferies JM, et al. The rise and fall of pneumococcal serotypes carried in the PCV era. Vaccine 2017; 35:1293–8. [DOI] [PubMed] [Google Scholar]
- 23. Flasche S, Van Hoek AJ, Sheasby E, et al. Effect of pneumococcal conjugate vaccination on serotype-specific carriage and invasive disease in England: a cross-sectional study. PLoS Med 2011; 8:e1001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rohatgi A. WebPlotDigitizer.https://automeris.io/WebPlotDigitizer/. Accessed 16 March 2021.
- 25. Amin-Chowdhury Z, Groves N, Sheppard CL, et al. Invasive pneumococcal disease due to 22F and 33F in England: a tail of two serotypes. Vaccine 2021; 39:1997–2004. [DOI] [PubMed] [Google Scholar]
- 26. Makwana A, Ladhani SN, Kapatai G, Campion E, Fry NK, Sheppard C. Rapid spread of pneumococcal nonvaccine serotype 7C previously associated with vaccine serotype 19F, England and Wales. Emerg Infect Dis 2018; 24:1919–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Office for National Statistics . Estimates for the population for the UK, England and Wales, Scotland and Northern Ireland.https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/datasets/populationestimatesforukenglandandwalesscotlandandnorthernireland. Accessed 16 March 2021.
- 28. Slotved HC, Dalby T, Harboe ZB, et al. The incidence of invasive pneumococcal serotype 3 disease in the Danish population is not reduced by PCV-13 vaccination. Heliyon 2016; 2:e00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wijayasri S, Hillier K, Lim GH, Harris TM, Wilson SE, Deeks SL. The shifting epidemiology and serotype distribution of invasive pneumococcal disease in Ontario, Canada, 2007–2017. PLoS One 2019; 14:e0226353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hanquet G, Krizova P, Dalby T, et al. Serotype replacement after introduction of 10-valent and 13-valent pneumococcal conjugate vaccines in 10 countries, Europe. Emerg Infect Dis 2022; 28:137–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Udden F, Runow E, Slotved HC, Fuursted K, Ahl J, Riesbeck K. Characterization of Streptococcus pneumoniae detected in clinical respiratory tract samples in southern Sweden 2 to 4 years after introduction of PCV13. J Infect 2021; 83:190–6. [DOI] [PubMed] [Google Scholar]
- 32. Corcoran M, Mereckiene J, Cotter S, et al. Using genomics to examine the persistence of Streptococcus pneumoniae serotype 19A in Ireland and the emergence of a sub-clade associated with vaccine failures. Vaccine 2021; 39:5064–73. [DOI] [PubMed] [Google Scholar]
- 33. Levy C, Varon E, Ouldali N, Bechet S, Bonacorsi S, Cohen R. Changes in invasive pneumococcal disease spectrum after 13-valent pneumococcal conjugate vaccine implementation. Clin Infect Dis 2020; 70:446–54. [DOI] [PubMed] [Google Scholar]
- 34. Amin-Chowdhury Z, Aiano F, Mensah A, et al. Impact of the coronavirus disease 2019 (COVID-19) pandemic on invasive pneumococcal disease and risk of pneumococcal coinfection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): prospective national cohort study, England. Clin Infect Dis 2021; 72:e65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Public Health England . Draft minutes of the Joint Committee on Vaccination and Immunisation Pneumococcal Sub-Committee meeting, Wednesday, 2 February 2022. By teleconference.2022. https://www.gov.uk/government/groups/joint-committee-on-vaccination-and-immunisation.
- 36. Danino D, Ben-Shimol S, Van Der Beek BA, et al. Decline in pneumococcal disease in young children during the COVID-19 pandemic in Israel associated with suppression of seasonal respiratory viruses, despite persistent pneumococcal carriage: a prospective cohort study. Clin Infect Dis 2022; 75:e1154–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Willen L, Ekinci E, Cuypers L, Theeten H, Desmet S. Infant pneumococcal carriage in Belgium not affected by COVID-19 containment measures. Front Cell Infect Microbiol 2022; 11:825427. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.