Abstract
OBJECTIVES
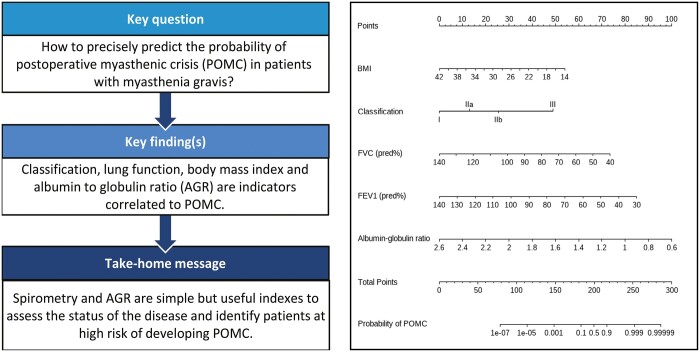
Thymectomy plays an important role in the comprehensive treatment of myasthenia gravis. The present study aimed to investigate the risk factors for postoperative myasthenic crisis (POMC) in these patients and then establish a predicting model based on preoperatively available indicators.
METHODS
The clinical records of 177 consecutive patients with myasthenia gravis who received extended thymectomy between January 2018 and September 2022 in our department were retrospectively reviewed. Patients were divided into 2 groups according to whether they developed POMC. Univariate and multivariate regression analyses were conducted to identify the independent risk factors of POMC. Then a nomogram was constructed to intuitively show the results. Finally, the calibration curve and bootstrap resampling were used to evaluate its performance.
RESULTS
POMC occurred in 42 (23.7%) patients. By multivariate analysis, body mass index (P = 0.029), Osserman classification (P = 0.015), percentage of predicted forced vital capacity (pred%) (P = 0.044), percentage of predicted forced expiratory volume in the first second (pred%) (P = 0.043) and albumin to globulin ratio (P = 0.009) were identified as independent risk factors and entered into the nomogram. The calibration curve showed good concordance between the predicted and actual probability of prolonged ventilation.
CONCLUSIONS
Our model is a valuable tool for predicting POMC in myasthenia gravis patients. For those high-risk patients, appropriate preoperative treatment is necessary to improve the symptoms and greater attention to postoperative complications is needed.
Keywords: Myasthenic crisis, Thymectomy, Spirometry, Albumin to globulin ratio, Body mass index
Myasthenia gravis (MG) is an acquired autoimmune disease induced by antibodies against the acetylcholine receptor, muscle-specific kinase or other acetylcholine receptor-related proteins [1].
INTRODUCTION
Myasthenia gravis (MG) is an acquired autoimmune disease induced by antibodies against the acetylcholine receptor, muscle-specific kinase or other acetylcholine receptor-related proteins [1]. Weakness of skeletal muscle is the predominant symptom caused by these antibodies. The weakness can be localized or generalized and usually includes eye muscles causing ptosis or diplopia [2]. Although the exact pathogenesis of MG has not been completely studied, more than 70% of patients with MG present with thymus gland abnormalities [3]. Therefore, thymectomy is believed to play a critical role in the comprehensive management of MG patients [4, 5].
In recent years, with the development of minimally invasive surgical techniques and the idea of fast-track surgery, early extubation after thymectomy in MG patients has been gradually accepted [6, 7]. This procedure could reduce the risk of postoperative pulmonary infection and the length of intensive care unit (ICU) stay [8]. However, the recovery of muscle strength could take several months. Thus, some patients might develop postoperative myasthenic crisis (POMC) and then required prolonged ventilation support or urgent reintubation because of respiratory failure caused by respiratory muscle weakness. Therefore, it is of great value to explore the determinants and then identify those high-risk patients preoperatively.
Several studies have explored relevant risk factors of POMC or prolonged ventilation support [9–12]. However, to our knowledge, few studies have focused on the specific parameters of spirometry tests and serum markers. Besides, few researchers provided intuitive and effective models to identify those high-risk patients. Nomogram has been accepted as a reliable tool to graphically depict the generating probability of a clinical event since its first application [13]. Hence, we carried out the present study.
MATERIALS AND METHODS
Ethical statement
This study was approved by the ethic committee of Xuanwu Hospital, Capital Medical University (approval number: 2021108). Written informed consent was obtained from all participants.
Patients and variables
We retrospectively reviewed 177 consecutive MG patients who underwent extended thymectomy from January 2018 to September 2022 in the department of thoracic surgery at our institution. The following variables were obtained: age, gender, classification, duration of the disease, body mass index [BMI, calculated as weight (in kg)/height2 (in m2)], preoperative medication, history of myasthenic crisis, parameters of preoperative spirometry test, preoperative albumin to globulin ratio (AGR) (calculated as the albumin divided by globulin values in serum), surgical approach, operative time and blood loss, thymic pathology, length of ventilation support and ICU stay.
The diagnosis of MG was based on some or all of the followings: clinical manifestation, physical examination, neostigmine test, electromyography results and positive antibodies. Even though the classification proposed by the Myasthenia Gravis Foundation of America has been more recommended in recent years [14]. The severity of the disease was still assessed using Osserman classification in this study because of our institutional guidelines [15]. Duration of the disease was defined as the period between the first onset of symptoms and admission. History of myasthenic crisis was characterized by worsening of muscle weakness and respiratory failure requiring intubation and mechanical ventilation. Forced vital capacity (FVC), forced expiratory volume in the first second (FEV1) and peak expiratory flow were collected. These parameters were presented as percentages of predicted values to eliminate the influence of age, sex and stature as far as possible. All blood samples were drawn in a fasting state within 5 days before the operation. All patients with anterior mediastinal mass underwent an enhanced chest computed tomography for better differential diagnosis of thymic cyst and thymoma [16]. Preoperative usage of anticholinesterase and corticosteroid was optimized and maintained until operation. Corticosteroids were gradually decreased to the minimal dose required to maintain remission. Immunosuppressant was only used preoperatively in a few patients with poor effect or contraindication to anticholinesterase or corticosteroid. Preoperative injection of immunoglobulin or plasma exchange was only used in a small number of patients with severe MG.
In the present study, all patients received preoperative multidisciplinary treatment (MDT) consisted of thoracic surgeons, neurologists and anaesthesiologists to determine the optimal timing for surgery and ensure that patients could tolerate the operation. General anaesthesia was conducted in all patients. It was induced with propofol or etomidate, sufentanil and rocuronium or cisatracurium. Total intravenous anaesthesia was used to maintain the appropriate depth of anaesthesia intraoperatively. The operation was conducted by video-assisted thoracoscopic surgery (VATS) or thoracotomy approach. Total thymectomy was performed, including the removal of all mediastinal soft tissue, especially bilateral cardiophrenic angle fat. The scope of the surgical resection was the inferior horns of thyroid gland, diaphragm and bilateral phrenic nerves. Lung wedge resection, pericardiotomy, phrenic nerve resection or innominate vein resection or reconstruction might be performed due to the involvement of the tumour. Most patients were transferred to the ICU with preserved endotracheal intubation when the surgery was finished to ensure safety and improve the efficiency of the operating room. Then critical care physicians gradually reduced respiratory support according to the patient’s condition. Whether to extubate was mainly based on spontaneous breathing trial, muscle strength and arterial blood gas analysis [17]. The specific criteria for extubation were as follows: (i) The patient was fully conscious and able to cooperate. (ii) Muscle strength recovered to grade 4 or preoperative level. (iii) Patients could autonomously lift their head off the bed surface for more than 15 s. (iv) A 120-min spontaneous breathing trial was performed under a low ventilator support condition (pressure support ventilation mode, positive end-expiratory pressure: 5 cm H2O and pressure support: 5 cm H2O, oxygen concentration: 35%). The tidal volume was over 4 ml/kg and the oxygen saturation, heart rate, respiratory rate and blood pressure were all unremarkable. Blood gas analysis was re-examined after the trial and there was no obvious rise in partial pressure of carbon dioxide. (v) Patients could pass cuff leak test. The duration of ventilation support was calculated as the period from the end of surgery to final extubation. All enrolled patients were divided into the following 2 groups: (i) group 1: patients required more than 24 hours of ventilation support or urgent reintubation despite successful extubation within 24 hours postoperatively (POMC) and (ii) group 2: patients who were extubated successfully within 24 hours without reintuabation (non-POMC).
Statistical analysis
IBM SPSS (version 26.0, IBM corp., Armonk, NY, USA) and R software (version 4.0.3, https://www.r-project.org) were used for statistical analysis. Continuous variables were provided as medians (interquartile ranges) if the distribution was nonnormal, and as means ± SDs if the distribution was normal. Categorical variables were provided as numbers and percentages. Mann–Whitney U-test or Student’s t-test was used for comparing the differences between the 2 groups for continuous variables and χ2 test or Fisher’s exact test was used for categorical variables. Univariate and multivariate logistic regression analyses were performed to identify the potential factors related to POMC. Odds ratio (OR) with a 95% confidence interval was provided to estimate correlation strength. Then the nomogram was established based on multivariable analysis. Its performance was assessed by calibration plot and a 1000 bootstrap resampling validation. A 2-sided P-value < 0.05 was considered statistically significant among all analysis methods.
RESULTS
Comparison of clinicopathological characteristics between 2 groups
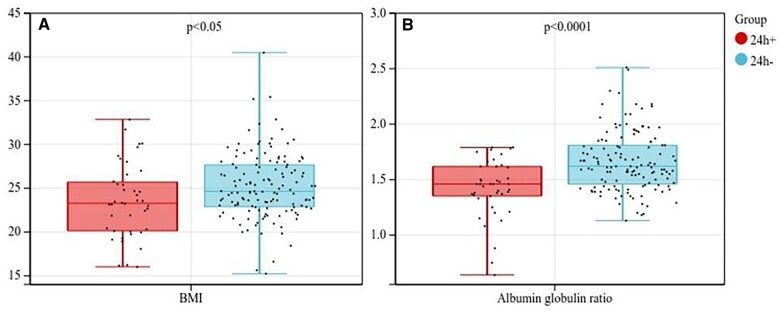
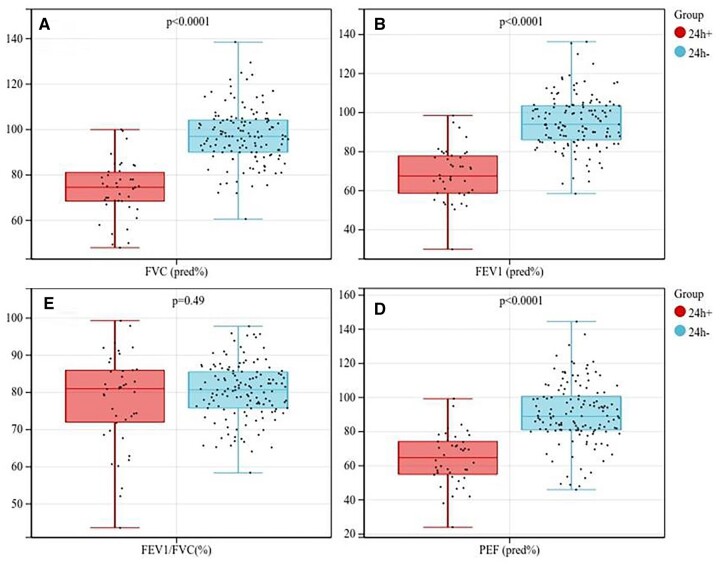
From January 2018 to September 2022, 177 patients were enrolled in the study. In total, 42 (23.7%) patients developed POMC and required more than 24 h of mechanical ventilation support in which 37 patients failed to extubate within 24 h, while 5 patients required urgent reintubation due to respiratory failure although successfully extubated within 24 h. Most patients developed POMC were treated with intravenous immunoglobulin, which was the standard strategy for POMC in our institution. Other alternative options included plasma exchange or high-dose corticosteroids [18]. Among the 42 POMC patients in this cohort, immunoglobulin, intravenous immunosuppressive agent (cyclophosphamide) and high dose of steroids were applied in 22 (52.4%), 1 (2.4%) and 6 (14.3%) cases, respectively. No patients underwent postoperative plasmapheresis. In addition, non-invasive ventilator sputum suction by fibre-optic bronchoscope, adjustment of pyridostigmine dose and escalation of antibiotics were other common measures in these patients. Other major complications included pneumonia in 14 cases (7.9%), atrial fibrillation in 10 cases (5.6%) and urinary tract infection in 2 cases (1.1%). No patients died within 30 days after surgery. The specific baseline characteristics are shown in Table 1. Most patients (83.6%) were treated with pyridostigmine preoperatively and there was no significant difference between the 2 groups. Compared with those developed POMC, non-POMC patients had higher BMI (25.26 ± 3.75 vs 23.52 ± 4.19, P = 0.012) and AGR (1.66 ± 0.27 vs 1.42 ± 0.26, P < 0.001). The box plots of these 2 indicators are shown in Fig. 1. For lung function indexes, non-POMC patients had a better percentage of predicted FVC (97.37 ± 12.27 vs 73.69 ± 12.31, P < 0.001), percentage of predicted FEV1 (95.19 ± 13.62 vs 68.54 ± 13.48, P < 0.001) and percentage of predicted peak expiratory flow (90.55 ± 17.99 vs 64.11 ± 15.31, P < 0.001). However, as for FEV1/FVC, there was no obvious difference between the 2 groups (80.37 ± 7.55 vs 77.89 ± 12.47, P = 0.228). The box plots of spirometry indexes are shown in Fig. 2.
Table 1:
Comparison of baseline variables between 2 groups
| Characteristics | Group 1 (n = 42) | Group 2 (n = 135) | P-value |
|---|---|---|---|
| Age (years), mean ± SD | 46.36 ± 15.14 | 47.62 ± 14.60 | 0.628 |
| Gender, n (%) | 0.573 | ||
| Male | 20 (47.6) | 71 (52.6) | |
| Female | 22 (52.4) | 64 (47.4) | |
| BMI (kg/m2), mean ± SD | 23.52 ± 4.19 | 25.26 ± 3.75 | 0.012* |
| Duration of the disease (months), median (IQR) | 3 (2–13.25) | 4 (2–12) | 0.553 |
| Osserman classification, n (%) | <0.001* | ||
| I | 4 (9.5) | 57 (42.2) | |
| IIa | 2 (4.8) | 15 (11.1) | |
| IIb | 33 (78.6) | 61 (45.2) | |
| III | 3 (7.1) | 2 (1.5) | |
| Dose of pyridostigmine (mg), median (IQR) | 180 (180–180) | 180 (90–180) | 0.123 |
| Application of steroid hormone, n (%) | 0.939 | ||
| Yes | 8 (19.0) | 25 (18.5) | |
| No | 34 (81.0) | 110 (81.5) | |
| Application of immunoglobulin, n (%) | 0.044* | ||
| Yes | 5 (11.9) | 5 (3.7) | |
| No | 37 (88.1) | 30 (96.3) | |
| Application of plasma exchange, n (%) | 0.38 | ||
| Yes | 1 (2.4) | 1 (0.7) | |
| No | 41 (97.6) | 134 (99.3) | |
| Application of immunosuppressant, n (%) | 0.341 | ||
| Yes | 7 (16.7) | 15 (11.1) | |
| No | 35 (83.3) | 120 (88.9) | |
| History of myasthenic crisis, n (%) | 0.13 | ||
| Yes | 7 (16.7) | 7 (5.2) | |
| No | 35 (83.3) | 128 (94.8) | |
| Serum albumin (g/l), mean ± SD | 38.82 ± 3.18 | 40.73 ± 3.25 | 0.001* |
| Serum globulin (g/l), mean ± SD | 28.36 ± 6.53 | 24.99 ± 3.52 | <0.001* |
| Albumin to globulin ratio, mean ± SD | 1.42 ± 0.26 | 1.66 ± 0.27 | <0.001* |
| FVC (pred%), mean ± SD | 73.69 ± 12.31 | 97.37 ± 12.27 | <0.001* |
| FEV1 (pred%), mean ± SD | 68.54 ± 13.48 | 95.19 ± 13.62 | <0.001* |
| FEV1/FVC (%), mean ± SD | 77.89 ± 12.47 | 80.37 ± 7.55 | 0.228 |
| PEF (pred%), mean ± SD | 64.11 ± 15.31 | 90.55 ± 17.99 | <0.001* |
P < 0.05.
BMI: body mass index; FEV1: forced expiratory volume in the first second; FVC: forced vital capacity; IQR: interquartile range; PEF: peak expiratory flow; SD: standard deviation.
Figure 1:
Box plots of BMI and AGR between 2 groups.
Figure 2:
Box plots of spirometry indexes between 2 groups.
Overall, 152 (85.9%) patients received VATS, including 72 right-side approaches, 14 bilateral approaches and 66 subxiphoid approaches. There was no significant difference in the surgical approach between the 2 groups. The presence of thymoma was confirmed in 81 (45.8%) patients, including 1 type A thymoma, 7 type AB thymomas, 18 type B1 thymomas, 42 type B2 thymomas and 13 type B3 thymomas. Thymic cyst was confirmed in 14 (7.9%) patients. A higher proportion of POMC patients presented with thymoma (73.8% vs 37.0%, P < 0.001). Besides, due to the longer ventilation support required, POMC patients had significant longer duration of ICU stay too. The specific surgical-related indicators are shown in Table 2.
Table 2:
Comparison of surgical-related variables between 2 groups
| Characteristics | Group 1 (n = 42) | Group 2 (n = 135) | P-value |
|---|---|---|---|
| Surgical procedure, n (%) | 0.12 | ||
| VATS | 33 (78.6) | 119 (88.1) | |
| Trans-sternal | 9 (21.4) | 16 (11.9) | |
| Operation time (min), mean ± SD | 189.29 ± 54.79 | 182.07 ± 46.08 | 0.398 |
| Blood loss (ml), mean ± SD | 45.74 ± 64.29 | 33.16 ± 58.51 | 0.237 |
| Pathology, n (%) | <0.001* | ||
| Thymoma | 31 (73.8) | 50 (37.0) | |
| Non-thymoma | 11 (26.2) | 85 (63.0) | |
| Postoperative ventilation time (h), median (IQR) | 59.5 (34.75–116.75) | 10 (3–16) | <0.001* |
| Duration of ICU stay (h), median (IQR) | 118.5 (78.75–181.75) | 32 (19–44) | <0.001* |
P < 0.05.
ICU: intensive care unit; IQR: interquartile range; SD: standard deviation; VATS: video-assisted thoracoscopic surgery.
Predictors of POMC based on multivariate analysis
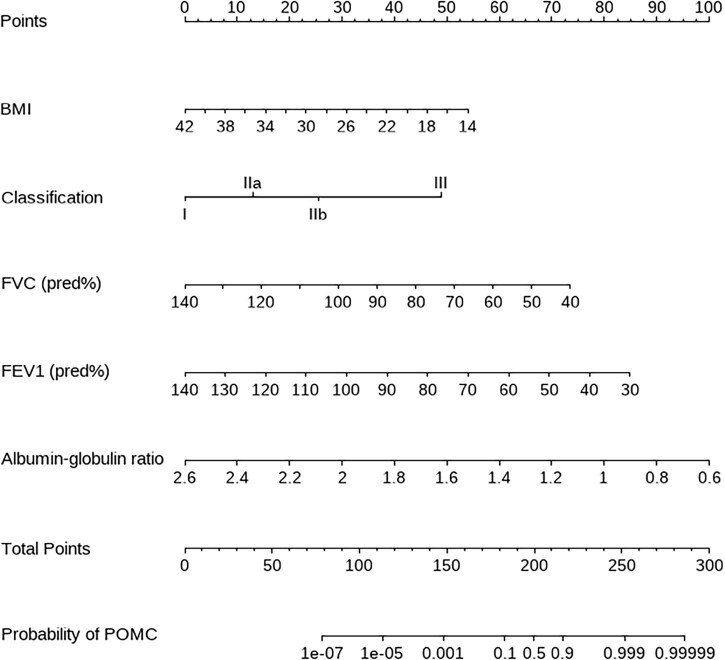
We conducted a multivariate logistic regression analysis including 7 preoperative available factors with a P-value < 0.05 identified by univariate analysis (Table 3). Of these, Osserman classification (P = 0.013), BMI (OR 0.792, P = 0.022), AGR (OR 0.002, P = 0.007), FVC (OR 0.906, P = 0.044) and FEV1 (OR 0.903, P = 0.043) were identified as independent predictors of POMC.
Table 3:
Multivariate analysis of preoperative characteristics related to POMC
| Characteristics | OR | 95% CI | P-value |
|---|---|---|---|
| Osserman classification | 0.015* | ||
| I | Ref | ||
| IIa | 4.496 | 0.168–120.577 | 0.370 |
| IIb | 39.345 | 3.237–478.234 | 0.004* |
| III | 628.645 | 6.258–63 152.149 | 0.006* |
| BMI | 0.787 | 0.634–0.976 | 0.029* |
| Albumin to globulin ratio | 0.002 | 0.000–0.213 | 0.009* |
| Application of immunoglobulin | 14.331 | 0.902–227.741 | 0.057 |
| FVC (pred%) | 0.906 | 0.823–0.997 | 0.044* |
| FEV1 (pred%) | 0.903 | 0.818–0.997 | 0.043* |
| PEF (pred%) | 0.966 | 0.919–1.014 | 0.16 |
| Presence of thymoma | 0.877 | 0.182–4.232 | 0.870 |
P < 0.05.
BMI: body mass index; CI: confidence interval; FVC: forced vital capacity; FEV1: forced expiratory volume in the first second; OR: odds ratio, PEF: peak expiratory flow.
Construction of the predicting nomogram
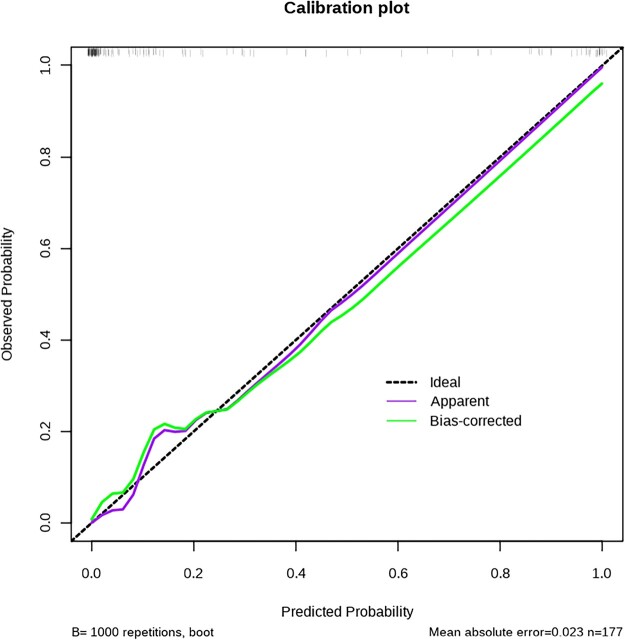
In order to intuitively show our results and easily identify those high-risk patients, we constructed a nomogram based on multivariate analysis (Fig. 3). The calibration of it was shown by a visual plot comparing the predicted and actual possibility of POMC (Fig. 4). Good concordance between the predicted and actual probability was obtained. Besides, the nomogram was subjected to a 1000 bootstrap resampling for internal validation and a low mean absolute error (0.023) was achieved.
Figure 3:
A nomogram to predict prolonged postoperative ventilation for MG patients after extended thymectomy. The value of each variable was given a score on the point scale axis. A total score could be easily calculated by adding every single score together. Locate it on the total points axis, then draw a line straight down to get the probability of prolonged ventilation.
Figure 4:
Calibration curve of the nomogram. The x-axis represented the predicted probability, and the y-axis represented the actual probability of prolonged postoperative ventilation. A perfect prediction would correspond to the black dashed line. The purple and the green lines, respectively, showed the apparent and bias corrected curve.
DISCUSSION
Extended thymectomy is an important approach for treating MG. However, POMC—a life-threatening complication occurs in some cases. It was defined as postoperative respiratory failure requiring prolonged ventilation support or urgent reintubation. Nevertheless, the specific threshold to diagnose POMC has not reached consensus yet. Some studies took 24 h as the standard [11, 19], while others usually used 48 h as the criteria [12]. In the present study, we adopted the former one as the diagnosis of POMC.
As the most important complication and the major cause of death in MG patients, it is critical to investigate the predictors of POMC and identify those high-risk patients as early as possible. In our study, POMC occurred in 42 (23.7%) patients, which was similar to the incidence reported in previous studies [9, 11, 19]. We found 5 preoperative available predictors for POMC: higher Osserman classification; lower BMI, FVC, FEV1 and AGR. Based on these indicators, we constructed a nomogram to calculate the exact possibility of POMC of each patient preoperatively. The distribution of the probabilities is uneven in the model, with a short length of the segment between 0.1 and 0.9. However, in our opinion, it is even more useful because it can discriminate between low- and high-risk patients better. For high-risk patients, greater attention to postoperative complications especially POMC are needed. High dose of corticosteroids or immunoglobulin could be used in advance. Besides, it is critical to improve the status of the disease in order to prevent the development of POMC. Increasing doses of pyridostigmine, application of steroid hormone, immunosuppressant or even immunoglobulin may be useful for these patients. Then the improvement of the disease could be confirmed by re-examination of AGR and spirometry test.
BMI is a broadly accepted measure for assessing obesity. The previous study had shown that MG patients with a higher BMI had a significantly higher risk of postoperative complications after thymectomy [20]. Similarly, Leuzzi et al. [21] found BMI > 28 an independent predictor of POMC. However, interestingly, we obtained totally opposite results in the present cohort. Higher BMI was identified as a protective factor. Therefore, studies with larger sample sizes are needed to further explore it.
Serum albumin is a typical indicator to evaluate the nutritional condition of patients. Besides, it can be used to evaluate the inflammatory status too. Therefore, it has been considered an attractive diagnostic or prognostic of lots of infectious or autoimmune disorders [22]. For MG, hypoalbuminemia was confirmed to be correlated with increased disease severity and poorer outcomes [23, 24]. Similarly, serum globulin responds to infection and elevates with inflammatory reactions [25]. Thus, AGR is a better indicator than albumin or globulin alone theoretically. However, few studies have focused on the role of AGR in predicting POMC. In our study, POMC patients had lower preoperative serum albumin and higher globulin. Therefore, preoperative AGR was significantly higher in non-POMC patients and was identified as an independent predictor of POMC.
Spirometry test is a simple but useful method for quantitative assessment and management of a variety of pulmonary conditions [26]. MG is an autoimmune disease characterized by weakness of various groups of skeletal muscles including respiratory muscles, resulting in impaired lung function [27]. Several previous studies had revealed that decreased lung function parameters were related to POMC or prolonged ventilation support [9, 10, 28, 29]. However, all these researches used the specific values of spirometry parameters. In our opinion, it is more reasonable to use the percentage of predicted indexes to eliminate the influence of age, sex or body habitus, especially since there was a significant difference in BMI between POMC and non-POMC patients in the present study.
History of myasthenic crisis is another factor frequently confirmed to be correlated with POMC or prolonged ventilation support in previous researches [10, 19, 21]. In our study, there was no significant difference between the proportion of patients with a history of myasthenic crisis between the 2 groups due to the relatively small sample size. However, more patients who developed POMC received preoperative intravenous immunoglobulin (11.9% vs 3.7%), which is usually only used to treat unstable preoperative MG patients [30]. In the multivariate analysis, the association between preoperative application of immunoglobulin and the development of POMC was not significant though a high OR value was obtained. So, in our opinion, the history of MC is a potential predictor of POMC and still needs to be evaluated in larger sample size studies.
Limitations
Several limitations should be pointed out in our study. First, the nature of retrospective and single-centre studies has resulted in limited sample size and the external validity of the results. Second, we do not have a specific protocol for anaesthesia. The method of anaesthesia, the dosage of anaesthetics and muscle relaxants used all depended on the anaesthetist’s favourite and experience. Third, as an important biomarker of MG, just a few of the enrolled patients received the quantitative detection of acetylcholine receptor antibody titers. Therefore, it was not included in the study. Above all, large-scale, multicentre and more rigorous studies are needed in the future.
CONCLUSIONS
Spirometry test and preoperative AGR are simple and useful indicators for assessing the severity of MG. Higher classification, impaired lung function, lower BMI and AGR are potential predictors for POMC. Appropriate preoperative treatment is necessary for improving the symptoms and greater attention to postoperative complications is needed for those high-risk patients.
Glossary
ABBREVIATIONS
- AGR
Albumin to globulin ratio
- BMI
Body mass index
- FEV1
Forced expiratory volume in the first second
- FVC
Forced vital capacity
- ICU
Intensive care unit
- MG
Myasthenia gravis
- OR
Odds ratio
- POMC
Postoperative myasthenic crisis
- VATS
Video-assisted thoracoscopic surgery
Contributor Information
Bohua Wei, Department of Thoracic Surgery, Xuanwu Hospital Capital Medical University, Beijing, China.
Gaojun Lu, Department of Thoracic Surgery, Xuanwu Hospital Capital Medical University, Beijing, China.
Yi Zhang, Department of Thoracic Surgery, Xuanwu Hospital Capital Medical University, Beijing, China.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest: none declared.
DATA AVAILABILITY
The data analysed during the current study are available from the corresponding author on reasonable request.
Author contributions
Bohua Wei: Conceptualization; Data curation; Formal analysis; Visualization; Writing—original draft. Gaojun Lu: Data curation; Resources; Software; Validation; Writing—review & editing. Yi Zhang: Methodology; Project administration; Supervision; Writing—review & editing.
Reviewer information:
Interdisciplinary CardioVascular and Thoracic Surgery thanks Larry R. Kaiser, Yoshimasa Maniwa, Paolo Scanagatta and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
REFERENCES
- 1. Gilhus NE, Tzartos S, Evoli A, Palace J, Burns TM, Verschuuren JJGM.. Myasthenia gravis. Nat Rev Dis Primers 2019;5:30. [DOI] [PubMed] [Google Scholar]
- 2. Gilhus NE. Myasthenia gravis. N Engl J Med 2016;375:2570–81. [DOI] [PubMed] [Google Scholar]
- 3. Meriggioli MN, Sanders DB.. Autoimmune myasthenia gravis: emerging clinical and biological heterogeneity. Lancet Neurol 2009;8:475–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wolfe GI, Kaminski HJ, Aban IB, Minisman G, Kuo H, Marx A. et al. ; MGTX Study Group. Long-term effect of thymectomy plus prednisone versus prednisone alone in patients with non-thymomatous myasthenia gravis: 2-year extension of the MGTX randomised trial. Lancet Neurol 2019;18:259–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhu K, Li J, Huang X, Xu W, Liu W, Chen J. et al. Thymectomy is a beneficial therapy for patients with non-thymomatous ocular myasthenia gravis: a systematic review and meta-analysis. Neurol Sci 2017;38:1753–60. [DOI] [PubMed] [Google Scholar]
- 6. Mohamed A, Shehada S, Aigner C, Ploenes T, Alnajdawi Y, Van Brakel L. et al. Anesthetic management during robotic-assisted minimal invasive thymectomy using the Da Vinci system: a single center experience. J Clin Med 2022;11:4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. O’Sullivan KE, Kreaden US, Hebert AE, Eaton D, Redmond KC.. A systematic review of robotic versus open and video assisted thoracoscopic surgery (VATS) approaches for thymectomy. Ann Cardiothorac Surg 2019;8:174–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen L, Xie W, Zheng D, Wang S, Wang G, Sun J. et al. Early extubation after thymectomy is good for the patients with myasthenia gravis. Neurol Sci 2019;40:2125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Du A, Li X, An Y, Gao Z.. Risk factors of prolonged ventilation after thymectomy in thymoma myasthenia gravis patients. J Cardiothorac Surg 2021;16:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chigurupati K, Gadhinglajkar S, Sreedhar R, Nair M, Unnikrishnan M, Pillai M.. Criteria for postoperative mechanical ventilation after thymectomy in patients with myasthenia gravis: a retrospective analysis. J Cardiothorac Vasc Anesth 2018;32:325–30. [DOI] [PubMed] [Google Scholar]
- 11. Xue L, Wang L, Dong J, Yuan Y, Fan H, Zhang Y. et al. Risk factors of myasthenic crisis after thymectomy for thymoma patients with myasthenia gravis. Eur J Cardiothorac Surg 2017;52:692–7. [DOI] [PubMed] [Google Scholar]
- 12. Li K, Qian K, Feng Y, Guo W, Tan Q, Deng B.. Predictive factors of prolonged mechanical ventilation, overall survival, and quality of life in patients with post-thymectomy myasthenic crisis. World J Surg Oncol 2017;15:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Van Zee KJ, Manasseh DE, Bevilacqua JLB, Boolbol SK, Fey JV, Tan LK. et al. A nomogram for predicting the likelihood of additional nodal metastases in breast cancer patients with a positive sentinel node biopsy. Ann Surg Oncol 2003;10:1140–51. [DOI] [PubMed] [Google Scholar]
- 14. Jaretzki A, Barohn RJ, Ernstoff RM, Kaminski HJ, Keesey JC, Penn AS. et al. Myasthenia gravis: recommendations for clinical research standards. Ann Thorac Surg 2000;70:327–34. [DOI] [PubMed] [Google Scholar]
- 15. Osserman KE, Genkins G.. Studies in myasthenia gravis: review of a twenty-year experience in over 1200 patients. Mt Sinai J Med 1971;38:497–537. [PubMed] [Google Scholar]
- 16. Zhonggao J, YiJiao W, Yongfeng W, Zhitao P, Jun W, Diansheng L. et al. Multislice computed tomography performance in differential diagnosis of high-density thymic cyst and thymoma in lesions less than 3 cm. Thorac Cancer 2018;9:1300–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boles J, Bion J, Connors A, Herridge M, Marsh B, Melot C. et al. Weaning from mechanical ventilation. Eur Respir J 2007;29:1033–56. [DOI] [PubMed] [Google Scholar]
- 18. Chaudhuri A, Behan PO.. Myasthenic crisis. QJM 2009;102:97–107. [DOI] [PubMed] [Google Scholar]
- 19. Ando T, Omasa M, Kondo T, Yamada T, Sato M, Menju T. et al. Predictive factors of myasthenic crisis after extended thymectomy for patients with myasthenia gravis. Eur J Cardiothorac Surg 2015;48:705–9. [DOI] [PubMed] [Google Scholar]
- 20. Liu X, Shao M, Sun L, Zhang L, Jia X, Li W.. Influence of body mass index on postoperative complications after thymectomy in myasthenia gravis patients. Oncotarget 2017;8:94944–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Leuzzi G, Meacci E, Cusumano G, Cesario A, Chiappetta M, Dall'Armi V. et al. Thymectomy in myasthenia gravis: proposal for a predictive score of postoperative myasthenic crisis. Eur J Cardiothorac Surg 2014;45:e76–88. [DOI] [PubMed] [Google Scholar]
- 22. Kratz F. Albumin as a drug carrier. Design of prodrugs, drug conjugates and nanoparticles. J Control Release 2008;132:171–83. [DOI] [PubMed] [Google Scholar]
- 23. Yoshimoto Y, Ishida S, Hosokawa T, Arawaka S.. Assessment of clinical factors affecting outcome of myasthenia gravis. Muscle Nerve 2021;64:90–4. [DOI] [PubMed] [Google Scholar]
- 24. Weng Y, Yang D, Qian M, Wei M, Yin F, Li J. et al. Low serum albumin concentrations are associated with disease severity in patients with myasthenia gravis. Medicine (Baltimore) 2016;95:e5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gabay C, Kushner I.. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med 1999;340:448–54. [DOI] [PubMed] [Google Scholar]
- 26. Liou TG, Kanner RE.. Spirometry. Clinic Rev Allerg Immunol 2009;37:137–52. [DOI] [PubMed] [Google Scholar]
- 27. Katzberg HD, Vajsar J, Vezina K, Qashqari H, Selvadurai S, Chrestian N. et al. Respiratory dysfunction and sleep-disordered breathing in children with myasthenia gravis. J Child Neurol 2020;35:600–6. [DOI] [PubMed] [Google Scholar]
- 28. Lee HS, Lee HS, Lee HE, Bae MK, Chung KY, Shin HY. et al. Predictive factors for myasthenic crisis after videoscopic thymectomy in patients with myasthenia gravis. Muscle Nerve 2015;52:216–20. [DOI] [PubMed] [Google Scholar]
- 29. Thieben MJ, Blacker DJ, Liu PY, Harper CM, Wijdicks EFM.. Pulmonary function tests and blood gases in worsening myasthenia gravis. Muscle Nerve 2005;32:664–7. [DOI] [PubMed] [Google Scholar]
- 30. Gamez J, Salvadó M, Carmona F, de Nadal M, Romero L, Ruiz D. et al. Intravenous immunoglobulin to prevent myasthenic crisis after thymectomy and other procedures can be omitted in patients with well-controlled myasthenia gravis. Ther Adv Neurol Disord 2019;12:1756286419864497. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data analysed during the current study are available from the corresponding author on reasonable request.
Author contributions
Bohua Wei: Conceptualization; Data curation; Formal analysis; Visualization; Writing—original draft. Gaojun Lu: Data curation; Resources; Software; Validation; Writing—review & editing. Yi Zhang: Methodology; Project administration; Supervision; Writing—review & editing.
Reviewer information:
Interdisciplinary CardioVascular and Thoracic Surgery thanks Larry R. Kaiser, Yoshimasa Maniwa, Paolo Scanagatta and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.