Abstract
Background
The COVIH study is a prospective coronavirus disease 2019 (COVID-19) vaccination study in 1154 people with HIV (PWH), of whom 14% showed reduced antibody levels after primary vaccination. We evaluated whether an additional vaccination boosts immune responses in these hyporesponders.
Methods
The primary end point was the increase in antibodies 28 days after additional mRNA-1273 vaccination. Secondary end points included neutralizing antibodies, S-specific T-cell and B-cell responses, and reactogenicity.
Results
Of the 66 participants, 40 previously received 2 doses ChAdOx1-S, 22 received 2 doses BNT162b2, and 4 received a single dose Ad26.COV2.S. The median age was 63 years (interquartile range [IQR], 60–66), 86% were male, and median CD4+ T-cell count was 650/μL (IQR, 423–941). The mean S1-specific antibody level increased from 35 binding antibody units (BAU)/mL (95% confidence interval [CI], 24–46) to 4317 BAU/mL (95% CI, 3275–5360) (P < .0001). Of all participants, 97% showed an adequate response and the 45 antibody-negative participants all seroconverted. A significant increase in the proportion of PWH with ancestral S-specific CD4+ T cells (P = .04) and S-specific B cells (P = .02) was observed.
Conclusions
An additional mRNA-1273 vaccination induced a robust serological response in 97% of PWH with a hyporesponse after primary vaccination.
Clinical Trials Registration. EUCTR2021-001054-57-N.
Keywords: COVID-19, HIV, SARS-CoV-2 vaccines, additional dose, nonresponder
An additional 100 µg mRNA-1273 vaccination substantially increased SARS-CoV-2 S1-specific binding antibody levels in people with HIV with a serological hyporesponse after a primary vaccination regimen. This response was observed regardless of the primary vaccination regimen or patient characteristics.
People with human immunodeficiency virus (PWH) show diminished responses to a wide variety of vaccines compared to HIV-negative controls, such as hepatitis B [1] and seasonal influenza vaccines [2]. As we hypothesized that this also holds true for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines, we previously investigated the immunogenicity of SARS-CoV-2 vaccinations in PWH with the vaccines currently approved in the Netherlands. Whereas smaller studies showed variable antibody responses after SARS-CoV-2 vaccination in PWH compared to HIV-negative controls [3–9], our study in 1154 PWH clearly demonstrated a diminished antibody response [10]. In total, 165 (14%) of the participants had a hyporesponse (≤300 spike [S]-specific binding antibody units [BAU]/mL by chemiluminescence immunoassay [DiaSorin Liaison]) and in 33 (3%) of them the antibody level remained below the cutoff level of test positivity (<33.8 BAU/mL). In comparison with vaccinated HIV-negative controls, hyporesponse (≤300 BAU/mL) rates were 7% (59/884) versus none (0/94) after vaccination with BNT162b2, 4% (4/100) versus none (0/247) after mRNA-1273, 55% (82/150) versus 39% (10/26) after ChAdOx1-S, and all (20/20) versus 99% (72/73) after a single dose of Ad26.COV2.S. In our Dutch cohort, CD4+ T-cell counts under 250 cells/µL, age above 65 years, and male sex were associated with lower antibody levels (all P ≤ .001). The reduced response against SARS-CoV-2 in PWH compared to HIV-negative controls is in line with the higher breakthrough infection risk observed in PWH compared to HIV-negative controls (adjusted hazard ratio 1.28) [11]. In addition, a higher incidence of SARS-CoV-2 mortality was observed in PWH (adjusted hazard ratios 3.29 and 2.59) [12, 13]. It was reported that the magnitude of the antibody response after vaccination correlates with protection against symptomatic infection with the ancestral viral strain [14]. This correlation likely holds true for novel emerging variants, although it is known that the neutralization potency diminishes with every new variant [15] and that 2 doses of BNT162b2 vaccination are not very effective in preventing symptomatic Omicron BA.1 or BA.2 infection [16]. With the continuing emergence of novel variants, it is reasonable to assume that antibodies remain important for clinical protection against SARS-CoV-2 infection. Indeed, in healthy individuals, one additional vaccination after a primary vaccination regimen restored transient neutralization potency against the subvariants BA.2.12.1 and BA.4/5 [17]. Following from these observations and reasoning, PWH may require additional vaccinations to achieve adequate protection against SARS-CoV-2 infection, especially with the emergence of novel antigenically distinct variants like the currently circulating Omicron lineage.
The effect of an additional SARS-CoV-2 vaccination on the humoral and cellular immune responses in PWH with a low or absent serological response after completing a primary vaccination regimen is unknown. Three studies on additional SARS-CoV-2 vaccinations in PWH have been performed that showed an increase in antibody levels, without analysis of the cellular immune response [18–20]. All 3 studies did not focus on those who may benefit most from additional vaccinations, namely the subgroup of hyporesponders after a primary vaccination regimen.
The aim of this study was to evaluate the SARS-CoV-2 S-specific immune responses after an additional mRNA-1273 vaccination in PWH, who had a serological hyporesponse after a primary SARS-CoV-2 vaccination regimen.
METHODS
Study Design and Participants
We conducted a nested single arm intervention trial embedded within the prospective nationwide cohort study in 22 of the 24 HIV treatment centers in the Netherlands (coronavirus disease 2019 [COVID-19] vaccination response in people with HIV, COVIH, n = 1154). All 165 PWH from the COVIH study with a hyporesponse (defined as ≤300 S-specific BAU/mL, measured at 4–6 weeks after a primary vaccination regimen with either 2 doses of BNT162b2, mRNA-1273, ChAdOx1-S, or a single dose of Ad26.COV2.S) were eligible for participation. The serological assay cutoff definition followed the consensus by the Dutch national expert working group (HARMONY) on the harmonization of SARS-CoV-2 immunological assays. Participants with evidence of intercurrent SARS-CoV-2 infection, demonstrated by a history of a reported or documented positive polymerase chain reaction (PCR) or rapid antigen test, or with serological evidence of >300 BAU/mL shortly before the additional vaccination, were excluded.
Clinical Procedures
The intervention consisted of a single mRNA-1273 vaccination (100 µg) administered at the Erasmus University Medical Centre or Leiden University Medical Centre, both in the Netherlands. Blood samples were obtained immediately before the vaccination (at the same visit; T0) and 28 days later (T1) for collection of serum and peripheral blood mononuclear cells (PBMCs).
Clinical data were extracted from an electronic case record file. Recorded study variables included year of birth, sex assigned at birth, dates and type of primary SARS-CoV-2 vaccinations, current use of combination antiretroviral therapy (cART), most recent plasma HIV-RNA (copies/mL), most recent and nadir CD4+ T-cell count (cells/µL), and use of immunosuppressant medication. Local and systemic adverse events were evaluated via a standardized printed or electronic diary concerning vaccination related side-effects and medication use in the 7 days after the additional vaccination.
Laboratory Procedures
All serum samples were assessed at the Erasmus University Medical Centre (World Health Organization SARS-CoV-2 reference laboratory) for the presence of SARS-CoV-2 S1-specific binding antibodies (hereafter S1-specific antibodies) with a validated immunoglobulin G (IgG) trimeric chemiluminescence immunoassay (DiaSorin Liaison) with a lower limit of detection at 4.81 BAU/mL and a cutoff level for positivity at 33.8 BAU/mL.
SARS-CoV-2 S-specific neutralizing antibodies and S-specific T cells were measured on a selection of 40 participants of whom 20 had their primary vaccination with ChAdOx1-S and 20 with BNT162b2. Selection was based on the S1-specific antibodies 28 days after the additional vaccination (T1), to represent the whole range of antibody responses as closely as possible. Neutralizing functionality of antibodies was assessed by a plaque reduction neutralization test (PRNT) and the SARS-CoV-2 S-specific T cells by an activation induced marker assay (see Supplementary Methods for further details on laboratory tests).
SARS-CoV-2-S-fluorochrome–labelled tetramers were used to compare the S-specific B-cell compartment on a selection of 18 participants that were balanced for primary vaccination regimen (ChAdOx1-S or BNT162b2) and CD4+ T-cell counts. Four of these 18 participants were also included in a subgroup during the initial study, in which PBMCs were collected 21 days (± 3 days) after the first vaccination, and 4–6 weeks after completing a primary vaccination schedule. These PBMCs were used for longitudinal analysis of class-switching S-specific B cells.
Outcomes
The primary outcome was defined as the increase in S1-specific antibodies in PWH 28 days after the additional vaccination compared to the S1-specific antibodies immediately prior to additional vaccination. An adequate response was defined as the presence of S1-specific antibodies >300 BAU/mL [10, 21, 22]. Secondary outcomes included the association between participant characteristics and antibody responses, the detection of SARS-CoV-2 S-specific neutralizing antibodies targeting the ancestral SARS-CoV-2 (D614G) and Omicron (BA.1) variant, T-cell and B-cell responses targeting the ancestral SARS-CoV-2 (Wuhan-Hu1), and additionally T-cell responses targeting the Omicron (BA.1) variant. Lastly, we evaluated the tolerability by monitoring local and systemic vaccine-related adverse events. Severity of reactogenicity was measured as mild (symptoms present but no functional impairment or medication needed), moderate (necessitating medication, no functional impairment), or severe (impairing daily functioning).
Sample Size and Statistical Analysis Plan
The study was designed with the anticipation that 10% of the PWH would have a serological hyporesponse after the primary vaccination regimen in the COVIH study. If we were be able to include 80 PWH, we would have >95% power to detect a 20% increase in the proportion of PWH with an adequate serological response after the additional vaccination (1-sided α .05).
The baseline characteristics were described as number (percentage) or median (interquartile range [IQR]). The primary outcome was assessed as the difference between the S1-specific antibodies at T1 minus T0 with a 95% confidence interval (CI) by a paired t test. We evaluated the proportion with adequate serological responses by a McNemar test. To investigate factors associated with the absolute increase in antibody response at 28 days after the additional vaccination in PWH, we used unpaired t tests and a proportional odds generalized linear multivariable model with the covariates sex, age (subgroups 18–65 vs >65 years), most recent CD4+ T-cell count (subgroups <500 vs >500/mm3), nadir CD4+ T-cell count (subgroups <500 vs >500/mm3), and primary vaccination regimen (mRNA vs vector). For each coefficient in the regression model, 95% CIs and P values were reported. Coefficients with P values <.05 were considered significant. Undetectable serological responses (<4.81 BAU/mL) were reported as 4.81 in the statistical analyses, undetectable neutralizing antibodies (<10) as 10, and undetectable S-specific T-cell and B-cell responses (<0.01) as 0.01.
Data were analyzed using GraphPad Prism 9.4.1. Flow cytometry data were analyzed using FlowJo software version 10.8.1. FlowJo software was used to gate CD19+ B cells and OMIQ data analysis software was used for further analysis (www.omiq.ai). Cytonorm was used for batch corrections followed by uniform manifold approximation and projection (UMAP) dimensionality reduction to visualize the phenotypes of S-specific B cells. The study overview image was created with BioRender.com.
Ethics Committee Approval
The trial was performed in accordance with the principles of the Declaration of Helsinki, Good Clinical Practice guidelines, and in accordance with the Dutch Medical Research Involving Human Subjects Act (WMO). The trial was reviewed and approved by the Medical Research Ethics Committees United Nieuwegein (MEC-U, reference 20.125) and is registered in the International Clinical Trials Platform (EUCTR2021-001054-57-N). Participants signed an extra informed consent form for participation in the substudy.
RESULTS
Baseline Characteristics
Between 22 November 2021 and 28 December 2021, 75 of the 165 invited PWH were enrolled into this substudy. Before the additional vaccination, 5 (7%) participants had an increase in their S1-specific antibodies to >300 BAU/mL and were excluded. In 4 of these 5 participants nucleocapsid-specific antibodies were positive. The participant with negative nucleocapsid-specific antibodies received a single dose of Ad26.COV.S and the S1-specific antibodies may have increased after the measurement 4–6 weeks postvaccination. Two (3%) participants were excluded due to a PCR-confirmed SARS-CoV-2 infection within 28 days after the additional vaccination and 2 (3%) participants were lost to follow-up after having received the additional vaccination. Overall, 66 PWH were included in the analysis. Of the 66 participants, 40 (61%) had received 2 doses of ChAdOx1-S, 22 (33%) had received 2 doses of BNT162b2, and 4 (6%) had received a single dose of Ad26.COV2.S as primary vaccination regimen (Supplementary Figure 1).
Baseline characteristics of all participants are described in Table 1, including the characteristics of PWH with hyporesponse from the initial study who were not enrolled in this substudy. Participants had a median age of 63 years (IQR, 60–66 years), 86% were male, most recent median CD4+ T-cell count was 650 cells/μL (IQR, 423–941 cells/μL), and nadir CD4+ T-cell count was 230 cells/μL (IQR, 145–345 cells/μL). The majority (97%) were on cART and had a suppressed plasma HIV-RNA (96% < 50 copies/mL). No participants used immunosuppressant medication. Among the 66 participants analyzed, the median time between completing the primary vaccination regimen and the additional vaccination was 172 days (IQR, 154–195 days).
Table 1.
Baseline Characteristics of Participants
| Characteristics | COVIH Participants | COVIH-BOOST Participants | ||
|---|---|---|---|---|
| With Hyporesponse, Not Included n = 99 | With Hyporesponse, Included n = 66 | Primary mRNA Vaccinationa n = 22 | Primary Vector Vaccinationb n = 44 | |
| Sex assigned at birth | ||||
| ȃMale | 87 (87.9) | 57 (86.4) | 20 (90.9) | 37 (84.1) |
| ȃFemale | 12 (12.1) | 9 (13.6) | 2 (9.1) | 7 (15.9) |
| Age category, y | ||||
| ȃ18–55 | 33 (33.3) | 9 (13.6) | 5 (22.7) | 4 (9.1) |
| ȃ56–65 | 43 (43.4) | 34 (51.5) | 3 (13.6) | 31 (70.5) |
| ȃ65 + | 23 (23.2) | 23 (34.9) | 14 (63.6) | 9 (20.5) |
| SARS-CoV-2 vaccinations in primary schedule | ||||
| ȃBNT162b2 | 37 (37.4) | 22 (33.3) | 22 (100) | NA |
| ȃmRNA-1273 | 4 (4.0) | NA | NA | NA |
| ȃChAdOx1-S | 42 (42.4) | 40 (60.6) | NA | 40 (90.9) |
| ȃAd26.COV2.S | 16 (16.2) | 4 (6.1) | NA | 4 (9.1) |
| On cART | ||||
| ȃYes | 99 (100) | 64 (97.0) | 20 (90.9) | 44 (100) |
| Most recent plasma HIV viral load | ||||
| ȃ<50 copies/mL | 93 (93.9) | 63 (95.5) | 20 (90.9) | 43 (97.7) |
| ȃ≥50 copies/mL | 6 (6.1) | 3 (4.5) | 2 (9.1) | 1 (2.3) |
| Most recent CD4+ T-cell count | ||||
| ȃ<250 cells/µL | 13 (13.1) | 5 (7.6) | 4 (18.2) | 1 (2.3) |
| ȃ250–500 cells/µL | 21 (21.2) | 12 (18.2) | 5 (22.7) | 7 (15.9) |
| ȃ>500 cells/µL | 65 (65.7) | 49 (74.2) | 13 (59.1) | 36 (81.8) |
| Nadir CD4+ T-cell count | ||||
| ȃ<250 cells/µL | 45 (45.5) | 31 (47.0) | 12 (54.5) | 19 (43.2) |
| ȃ250–500 cells/µL | 32 (32.3) | 19 (28.8) | 5 (22.7) | 14 (31.8) |
| ȃ>500 cells/µL | 9 (9.1) | 7 (10.6) | 1 (4.5) | 6 (13.6) |
| ȃUnknown | 13 (13.1) | 9 (13.6) | 4 (18.2) | 5 (11.4) |
| Use of immunosuppressant medication | ||||
| ȃYes | 4 (4.0) | 0 | 0 | 0 |
| ȃDays since completing primary vaccination, median (IQR) | NA | 172 (154–195) | 192 (165–206) | 168 (152–181) |
Values are No. (%) for categorical variables or median (IQR) for continuous variables.
Abbreviations: cART, combination antiretroviral therapy; HIV, human immunodeficiency virus; IQR, interquartile range; NA, not applicable; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
BNT162b2, mRNA-1273.
ChadOx1-S, Ad26.COV2.S.
The mean S1-specific antibody level directly prevaccination was 35 BAU/mL (95% CI, 24–46 BAU/mL), including the 45 participants with serological responses below test positivity (<33.8 BAU/mL). Following ChAdOx1-S primary vaccination the antibody level directly prevaccination was mean 31 BAU/mL (95% CI, 20–42 BAU/mL), 46 BAU/mL (95% CI, 20–73 BAU/mL) after BNT162b2, and 19 BAU/mL (95% CI, −1 to 39 BAU/mL) after Ad26.COV2.S.
S1-Specific Antibodies
Twenty-eight days (IQR, 28–28 days) after the additional vaccination, all of the 45 antibody-negative participants seroconverted and S1-specific antibodies >300 BAU/mL were measured in 64/66 (97%) of the participants (P < .0001). All participants showed an increase in S1-specific antibodies after vaccination, with a mean of 4282 BAU/mL (95% CI, 3241–5323 BAU/mL, P < .0001; Figure 1).
Figure 1.
SARS-CoV-2 S1-specific binding antibody levels in PWH after additional mRNA-1273 vaccination. Levels of S1-specific binding antibodies measured 28 days after the additional mRNA-1273 vaccination in all 66 PWH (squares), in 22 PWH after primary vaccination with BNT162b2 (circles), in 40 PWH after primary vaccination with ChadOx1-S (triangles), and in 4 PWH after primary vaccination with Ad26.COV2.S (diamonds). The thick horizontal bar shows the mean S1-specific binding antibody level, also indicated above the graph, with error bars showing the standard error of the mean. The horizontal lines show the lower limit of detection of the performed test (4.81 BAU/mL), the positivity cutoff (33.8 BAU/mL), and the hyporesponse cutoff (300 BAU/mL). Comparisons of time points were performed by paired t test. Abbreviations: BAU, binding antibody unit; LLoD, lower limit of detection; PWH, people with human immunodeficiency virus; S, spike; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; T0, before additional vaccination; T1, 28 days after additional vaccination.
Only 2 participants with a well suppressed HIV load on cART did not reach >300 BAU/mL after the additional vaccination, with S1-specific antibodies increasing from 9 to 95 BAU/mL and from <4.81 to 107 BAU/mL. Both participants received BNT162b2 as primary vaccination and were men with low CD4+ T-cell nadirs <50 cells/µL and most recent CD4+ T-cell counts of 230 cells/µL and 313 cells/µL.
The mean increase in S1-specific antibodies was comparable between primary vaccination with ChAdOx1-S, BNT162b2, or Ad26.COV2.S. A mean increase of 3890 BAU/mL (95% CI, 2945–4835 BAU/mL) was measured after primary vaccination with ChAdOx1-S, 4549 BAU/mL (95% CI, 1986–7112 BAU/mL) after primary vaccination with BNT162b2, and 6744 BAU/mL (95% CI, −1978–15.465 BAU/mL) after primary vaccination with Ad26.COV2.S (P = .57). The mean increase in S1-specific antibodies was comparable between the most recent CD4+ T-cell count of <500 versus >500/mm3 (5400 vs 3895 BAU/mL, P = .85), age 18–65 versus >65 years (4336 vs 4183 BAU/mL, P = .52) and men versus women (4372 vs 3714 BAU/mL, P = .42).
A proportional odds generalized linear regression model was performed to investigate factors associated with the absolute increase in antibody response 28 days after the additional vaccination. This adjusted analysis did not identify significant associations between any of the participant characteristics of interest and S1-specific antibody responses (Supplementary Table 1).
Neutralizing Antibodies
After additional vaccination, neutralizing antibodies against the ancestral SARS-CoV-2 were present in all subgroup participants (40/40) and against the Omicron (BA.1) variant in 65% of participants (26/40) (Figure 2A). Neutralizing antibodies against the ancestral virus were higher when participants had primary vaccination with ChadOx1-S compared to primary vaccination with BNT162b2; mean PRNT50 was 3526 versus 1611 (P = .003). Neutralizing antibodies against the circulating Omicron variant were also numerically higher after ChadOx1-S; mean PRNT50 was 889 versus 442, but this not statistically significant (P = .46).
Figure 2.
Neutralizing antibodies to SARS-CoV-2 in subgroup participants (n = 40) after additional mRNA-1273 vaccination. A, PRNT50 titer measured 28 days after the additional mRNA-1273 vaccination against the ancestral SARS-CoV-2 (D614G) and Omicron (BA.1) variant after primary vaccination with ChAdOx1-S (triangles) and BNT162b2 (circles). The thick horizontal bar shows the mean neutralizing antibody titer, also indicated above the graph, with error bars showing the standard error of the mean. LLoD is 10. Comparisons between the 2 different primary vaccination groups were performed using unpaired t test. B, Correlation between the S1-specific binding antibody levels and neutralizing antibody levels targeting the ancestral SARS-CoV-2 by linear regression analysis on transformed data; R = 0.66, P < .0001. C, Correlation between the S1-specific binding antibody levels and neutralizing antibody levels targeting the Omicron BA.1 variant by linear regression analysis on transformed data; R = 0.45, P < .0001. Adequate responder cutoff is 300 BAU/mL (dotted line). Abbreviations: BAU, binding antibody unit; LLoD, lower limit of detection; PRNT50, 50% plaque reduction neutralization test; S, spike; SARS-CoV-2, severe acute respiratory syndrome coronavirus.
A positive correlation between S1-specific antibodies and the neutralizing antibodies against the ancestral variant (R = 0.66, P < .0001) was observed (Figure 2B). The correlation was less clear for neutralizing antibodies against the Omicron variant (R = 0.45, P < .0001; Figure 2C).
Frequency of SARS-CoV-2 S-Specific T Cells
Before the additional vaccination, high frequencies of ancestral S-specific T cells (median, 0.08%; IQR, 0.01%–0.21%) and Omicron S-specific T cells (median, 0.06%; IQR, 0.01%–0.24%) were detected. Additional vaccination led to a nonsignificant increase in ancestral S-specific CD4+ T cells (median, 0.08%; P = .51; Figure 3). However, the proportion of PWH with detectable ancestral S-specific CD4+ T cells significantly increased from 27/40 (68%) before additional vaccination to 34/39 (87%) after additional vaccination (P = .04). Omicron S-specific CD4+ T cells were comparable before and after additional vaccination (P = .95), and also the proportion of PWH with detectable Omicron S-specific CD4+ T cells did not differ between these time points (P = .95). S-specific CD8+ T cells were infrequently observed in study participants and no effect on S-specific CD8+ T cells was observed after additional vaccination for both the ancestral SARS-CoV-2 (P = .88) and the Omicron variant (P = .48).
Figure 3.
Frequency of SARS-CoV-2 S-specific T cells in subgroup participants (n = 40) before and 28 days after additional mRNA-1273 vaccination. CD4+ (CD4+CD40L+CD137+) and CD8+ (CD8+CD69+CD137+) T-cell responses to ancestral spike (Wuhan-Hu1) and Omicron spike (BA.1) measured by the AIM assay before additional vaccination (T0) compared to 28 days after additional vaccination (T1). Participants who received ChAdOx1-S as primary vaccination are shown as triangles, participants who received BNT162b2 as primary vaccination are shown as circles. The horizontal line shows the median, also indicated above the graph. The total numbers of participants with detectable S-specific T cells are indicated below the graphs. Comparisons of time points were performed by unpaired t test. Abbreviations: AIM, activation induced marker assay; S, spike; SARS-CoV-2, severe acute respiratory syndrome coronavirus; T0, before additional vaccination; T1, 28 days after additional vaccination.
Frequency and Phenotype of SARS-CoV-2 S-Specific B Cells
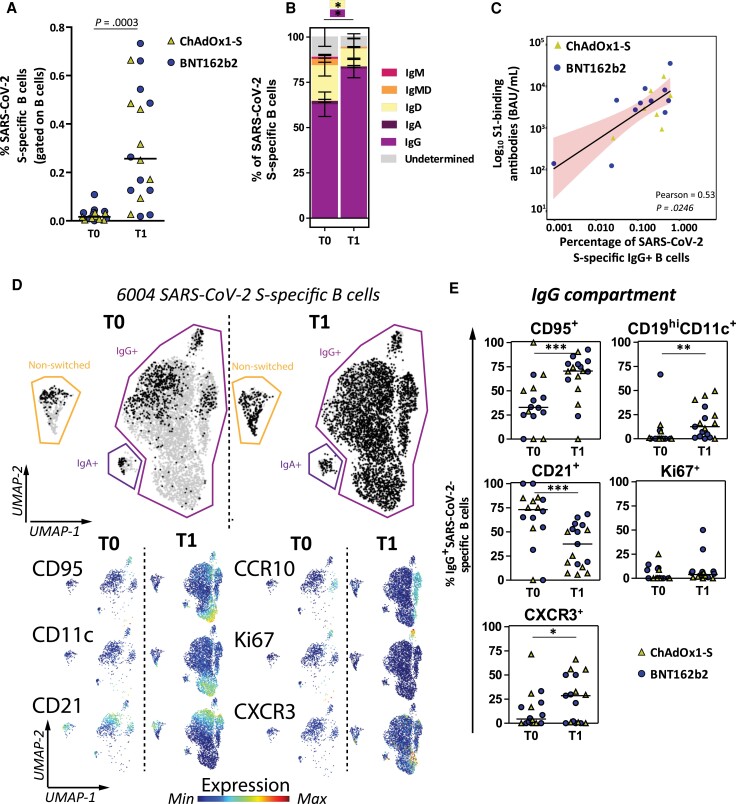
Low frequencies (median, 0.02%; IQR, 0.001%–0.03%) of S-specific B cells were detected at T0. S-specific B cells significantly increased (median, 0.26%; IQR, 0.13%–0.49%; P = .0003) 28 days after participants received the additional vaccination (Figure 4A). The proportion of PWH with detectable S-specific B cells also significantly increased from 13/18 (72%) at T0 to all participants (100%) at T1 (P = .02). To further investigate the phenotype of S-specific B cells, we compared the frequencies of unswitched (IgD, IgM, and IgMD) and switched (IgG and IgA) S-specific B cells (Supplementary Figure 4). Although not many S-specific B cells were present before the additional vaccination, the majority was already class-switched to IgG (mean 61%). However, a significant increase in switched IgG (mean, 82%; P = .01) was observed after the additional vaccination (Figure 4B). Longitudinal analysis of class-switching of S-specific B cells suggests a decrease in switched IgG+ S-specific B cells after a primary vaccination regimen in the PWH with initial hyporesponse (Supplementary Figure 5). Because the majority of S-specific B cells class-switched to IgG, we further focused on this memory IgG compartment. As expected, percentages of IgG+ S-specific B cells correlated with the S1-specific antibodies (Figure 4C). However, percentages of IgG+ S-specific memory B cells did not correlate with the most recent CD4+ T-cell count (Supplementary Figure 6). To investigate the phenotypical properties of IgG+ S-specific B cells (eg, migratory capacity [CXCR3], proliferating [Ki67], activation status [CD95], and recent germinal center graduation [CD21−] [23], and memory subsets, like CD45RB+ B cells and CD11c+CD19high cells [24]), we used a panel of 24 antibodies. In total, 6004 S-specific B cells were measured from all samples. To visualize how the phenotype of S-specific B cells changed after additional vaccination, we performed UMAP dimensionality reduction and depicted the S-specific B cells per time point (in black in Figure 4D; T0, 913 cells and T1, 5091 cells). Activated (CD95+), migrating (CXCR3+) and memory (CD11c+CD19high) S-specific IgG+ B cells were significantly more prevalent at T1, as were B cells recently graduating from the germinal centers (CD21− B cells). Proliferating (Ki67) S-specific IgG+ B cells were not significantly different between T0 and T1 (Figure 4E).
Figure 4.
Frequency and phenotype of SARS-CoV-2 S-specific B cells in subgroup participants (n = 18) before and 28 days after an additional mRNA-1273 vaccination. A, Percentages of S-specific B cells are shown as frequencies from total B cells per individual. Each individual is colored according to the primary vaccination regimen (triangles, ChAdOx1-s, n = 8; circles, BNT162b2, n = 10). The horizontal line shows the median. B, Isotype usage of S-specific B cells are shown as stacked bars at T0 and T1. C, Correlation plot between S1-specific binding antibody levels and IgG+ S-specific B cells. Pearson correlation analysis on nontransformed data is depicted and linear regression results shown as a black line with red shaded 95% confidence intervals. D, UMAP for all 6004 S-specific B cells (grey) to cluster cells based on 24 different markers. S-specific B cells are overlaid based on time point (black) on top of all cells (grey). Normalized expression of 6 selected markers is shown below the overlaid UMAP. Expression plots for all markers are in Supplementary Figure 7. E, Manually gated B-cell subsets are shown within the IgG+ B-cell compartment at each time point. A, B, and E, Statistical analyses, Wilcoxon matched pairs tests were performed to compare T0 versus T1. *P < .05, **P < .01, ***P < .001. Individuals and median values (A and E) and means with standard error of means (B) are shown. Abbreviations: IgG, immunoglobulin G; S, spike; SARS-CoV-2, severe acute respiratory syndrome coronavirus; T0, before additional vaccination; T1, 28 days after additional vaccination; UMAP, uniform manifold approximation and projection.
Reactogenicity
The additional dose was well tolerated and no serious adverse events were reported. Overall, 66% of the participants reported local or systemic adverse events, with pain at the injection site the most frequently reported local reaction and generalized myalgia and headache the most frequently reported systemic reactions (Supplementary Table 4).
DISCUSSION
To our knowledge, this study is the first to report the immunogenicity of an additional SARS-CoV-2 vaccination in PWH who had a low antibody response after a primary vaccination regimen. A substantial increase in S1-specific antibodies and memory B cells was shown, supporting the usefulness of a third vaccination dose in PWH and in particular for those with a documented hyporesponse after a primary vaccination regimen.
Remarkably, there was no significant association between the increase in S1-specific antibodies and the primary vaccination regimen, most recent CD4+ T-cell count, nadir CD4+ T-cell count, age, and sex. However, smaller differences between these variables cannot be ruled out because the study was powered on overall responsiveness and not for absolute differences in S1-specific antibodies between groups.
As expected, a positive correlation was observed between neutralization potency and S1-specific antibodies. This supports the observation that the correlation between the magnitude of the antibody levels and neutralization for the ancestral strain is also true in PWH for variants. Because neutralization was reported to be a correlate of protection against infection and disease, our data support a strategy to obtain a level of antibodies for optimal protection against SARS-CoV-2 infection in PWH.
S-specific T cells were detected before the additional vaccination in the majority of PWH, despite the low levels of S1-specific antibodies. This implies that although it is important to boost S1-specific antibodies in hyporesponders, there still is a second line of defense. After an additional vaccination, the proportion of participants with detected CD4+ T cells targeting the ancestral variant significantly increased. This increase was not observed for the Omicron variant; however, the responder rate was slightly higher at baseline compared to the ancestral response, leaving less room for a vaccination-induced increase. It was previously shown that S-specific T cells induced by vaccination can cross-react with the different Omicron sublineages [25, 26]. Similarly, no effect of additional vaccination on S-specific CD8+ T-cell responses was observed. CD8+ T-cell responses were low in general; however, this is in line with previous studies [25, 27], and could be attributed to an assay limitation using 15-mer peptides, whereas 8–10 mers are better suited for HLA class I presentation.
Furthermore, there was a significant induction of activated/homing and memory S-specific B cells after additional vaccination that correlated with the S1-specific antibodies. Combined with the longitudinal B-cell data from 4 participants, this implies that an additional vaccination is needed in these PWH with hyporesponse to induce activation and memory B cells for durable protection.
Other studies of additional SARS-CoV-2 vaccinations in different immunocompromised groups with a hyporesponse after a primary vaccination regimen also show that additional vaccination led to an increase in antibody levels. Nonetheless, lower seroconversion rates above a predefined cutoff were observed [28, 29]. However, the cutoffs for response differed between the studies and some studies only included participants with an antibody level <50 BAU/mL after a primary vaccination regimen, making the results less comparable with this study. A comparable study in participants receiving chemotherapy, immunotherapy, or both for solid tumors showed that 46 of the 48 hyporesponders reached S1-specific antibodies >300 BAU/mL 28 days after a third mRNA-1273 vaccination [30]. Therefore, compared to the literature on additional vaccinations in immunocompromised groups, PWH that are on an effective cART regimen with hyporesponse after a primary vaccination regimen appear to seroconvert more frequently and with higher increases in magnitude of S1-specific antibody response after an additional vaccine dose.
Our study had several limitations. First, our study population had an imbalance in sex distribution. Participants were generally virally suppressed on cART with CD4+ T-cell counts above 500 cells/μL, limiting the generalizability to the overall population of PWH. The study lacks a control group of immunocompetent participants, but as measuring S1-specific antibodies is not standard policy in the healthy population, these controls are difficult to find. Furthermore, our study only reports on additional vaccination with mRNA-1273, as this was standard policy in all the SARS-CoV-2 booster vaccination studies in immunocompromised groups in the Netherlands. We expect that this specific group of PWH with hyporesponse would also have seroconverted after either BNT162b2 or ChAdOx1 as additional vaccination, because the response with mRNA-1273 was so evidently positive. Although we did not meet our predefined sample size for a study power of >95%, the observed effect size was considerably higher than anticipated. As a consequence, the risk of an underpowered study is unlikely.
In conclusion, an additional mRNA-1273 vaccination substantially improved humoral and cellular immune response in PWH with low SARS-CoV-2 antibodies after a primary vaccination regimen. This shows that additional vaccinations are an effective approach in compensating for the reduced antibody responses in PWH. In addition, the results of this study suggest that also primary-vaccine–responsive people with waning vaccine-induced responses can expect to benefit from future boosters to reinforce protection against infection with viral variants.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. Foremost, we thank all participants of the study. We also thank the following people for recruitment of participants: Femke van Malsen, Roos van Heerde, Natasja van Holten, Willemien Dorama, Maartje Wagemaker, Ayten Karisli, René van Engen, Vincent Peters, Suzanne de Munnik, Vera Maas, Laura Laan, Jasmijn Steiner, Leontine van der Prijt, and Jolanda van der Swaluw; and Alessandro Sette and Alba Grifoni (La Jolla Institute for Immunology, La Jolla, San Diego, USA) for providing the peptide pools used in the AIM assay.
Author contributions. M. J. J., K. S. H., D. G., B. J. A. R., K. B., C. R., A. H. E. R., Y. M. M., P. D. K., R. D. d. V., and C. H. G. v. K. contributed conceptualization. M. J. J., D. G., W. H., and G. P. performed the formal analysis. B. J. A. R., K. B., C. R., A. H. E. R., and R. D. d. V. acquired funding. M. J. J., K. S. H., D. G., W. H., S. B., L. G., J. G. d. H., E. F. S., H. S. M. A., W. F. W. B., M. v. d. V., M. A. H. B., R. S., N. L., A. H. W. B., E. M. L., K. C. E. S., M. G. A. v. V., C. E. D., J. B., B. J. A. R., K. B., C. R., and A. H. E. R., performed investigations. M. J. J., K. S. H., B. J. A. R., K. B., C. R., A. H. E. R., Y. M. M., P. D. K., G. P., R. D. d. V., and C. H. G. v. K. contributed methodology. M. J. J., K. S. H., B. J. A. R., K. B., C. R., and A. H. E. R. administered the project. B. J. A. R., K. B., C. R., A. H. E. R., Y. M. M., R. D. d. V., and C. H. G. v. K. supervised the project. M. J. J., K. S. H., D. G., C. R., B. J. A. R., K. B., C. R., A. H. E. R., Y. M. M., R. D. d. V., and C. H. G. v. K. performed the validation. M. J. J., D. G., K. S. H., W. H., B. J. A. R., K. B., C. R., and A. H. E. R. contributed visualization. M. J. J., K. S. H., W. H., B. J. A. R., K. B., C. R., and A. H. E. R. wrote the original draft. All authors contributed to reviewing and editing of the manuscript.
Disclaimer. The funder of the study provided feedback on the study protocol but had no role in recruitment, data collection, data analysis, data interpretation, writing, or the decision to submit the manuscript.
Financial support. This work was supported by the Netherlands Organization for Health Research and Development (ZonMw) (grant number 10430072010008); and Health∼Holland (grant number EMCLHS20017 to D. G. and R. D. d. V.) cofunded by the PPP Allowance made available by the Health∼Holland, Top Sector Life Sciences and Health, to stimulate public-private partnerships.
Data sharing statement. Individual participant data that underlie the results reported in this article, after deidentification, will be made available to researchers who provide a methodologically sound study proposal.
Contributor Information
Marlou J Jongkees, Department of Internal Medicine, Section Infectious Diseases, and Department of Medical Microbiology and Infectious Diseases, Erasmus University Medical Centre, Rotterdam, the Netherlands.
Daryl Geers, Department of Viroscience, Erasmus University Medical Centre, Rotterdam, the Netherlands.
Kathryn S Hensley, Department of Internal Medicine, Section Infectious Diseases, and Department of Medical Microbiology and Infectious Diseases, Erasmus University Medical Centre, Rotterdam, the Netherlands.
Wesley Huisman, Department of Parasitology, Leiden University Centre for Infectious Diseases, Leiden University Medical Centre, Leiden, the Netherlands.
Corine H GeurtsvanKessel, Department of Viroscience, Erasmus University Medical Centre, Rotterdam, the Netherlands.
Susanne Bogers, Department of Viroscience, Erasmus University Medical Centre, Rotterdam, the Netherlands.
Lennert Gommers, Department of Viroscience, Erasmus University Medical Centre, Rotterdam, the Netherlands.
Grigorios Papageorgiou, Department of Biostatistics, Erasmus University Medical Centre, Rotterdam, the Netherlands.
Simon P Jochems, Department of Parasitology, Leiden University Centre for Infectious Diseases, Leiden University Medical Centre, Leiden, the Netherlands.
Jan G den Hollander, Department of Internal Medicine, Maasstad Hospital, Rotterdam, the Netherlands.
Emile F Schippers, Department of Infectious Diseases, Leiden University Medical Centre, Leiden, the Netherlands; Department of Internal Medicine, Haga Teaching Hospital, the Hague, the Netherlands.
Heidi S M Ammerlaan, Department of Internal Medicine, Catharina Hospital, Eindhoven, the Netherlands.
Wouter F W Bierman, Department of Internal Medicine and Infectious Diseases, University Medical Centre Groningen, Groningen, the Netherlands.
Marc van der Valk, Department of Internal Medicine and Infectious Diseases, DC Klinieken, Amsterdam, the Netherlands; Department of Infectious Diseases, Amsterdam Institute for Infection and Immunity, Amsterdam University Medical Centres, Amsterdam, the Netherlands.
Marvin A H Berrevoets, Department of Internal Medicine, Elisabeth-Tweesteden Hospital, Tilburg, the Netherlands.
Robert Soetekouw, Department of Internal Medicine and Infectious Diseases, Spaarne Gasthuis, Haarlem, the Netherlands.
Nienke Langebeek, Department of Internal Medicine and Infectious Diseases, Rijnstate Hospital, Arnhem, the Netherlands.
Anke H W Bruns, Department of Internal Medicine and Infectious Diseases, University Medical Centre Utrecht, Utrecht, the Netherlands.
Eliane M S Leyten, Department of Internal Medicine and Infectious Diseases, Haaglanden Medical Centre, the Hague, the Netherlands.
Kim C E Sigaloff, Department of Infectious Diseases, Amsterdam Institute for Infection and Immunity, Amsterdam University Medical Centres, Amsterdam, the Netherlands.
Marit G A van Vonderen, Department of Internal Medicine, Medical Centre Leeuwarden, Leeuwarden, the Netherlands.
Corine E Delsing, Department of Internal Medicine and Infectious Diseases, Medisch Spectrum Twente, Enschede, the Netherlands.
Judith Branger, Department of Internal Medicine, Flevo Hospital, Almere, the Netherlands.
Peter D Katsikis, Department of Immunology, Erasmus University Medical Centre, Rotterdam, the Netherlands.
Yvonne M Mueller, Department of Immunology, Erasmus University Medical Centre, Rotterdam, the Netherlands.
Rory D de Vries, Department of Viroscience, Erasmus University Medical Centre, Rotterdam, the Netherlands.
Bart J A Rijnders, Department of Internal Medicine, Section Infectious Diseases, and Department of Medical Microbiology and Infectious Diseases, Erasmus University Medical Centre, Rotterdam, the Netherlands.
Kees Brinkman, Department of Internal Medicine and Infectious Diseases, OLVG Hospital, Amsterdam, the Netherlands.
Casper Rokx, Department of Internal Medicine, Section Infectious Diseases, and Department of Medical Microbiology and Infectious Diseases, Erasmus University Medical Centre, Rotterdam, the Netherlands.
Anna H E Roukens, Department of Infectious Diseases, Leiden University Medical Centre, Leiden, the Netherlands.
References
- 1. van den Berg R, van Hoogstraten I, van Agtmael M. Non-responsiveness to hepatitis B vaccination in HIV seropositive patients; possible causes and solutions. AIDS Rev 2009; 11:157–64. [PubMed] [Google Scholar]
- 2. Pallikkuth S, De Armas LR, Pahwa R, et al. Impact of aging and HIV infection on serologic response to seasonal influenza vaccination. Aids 2018; 32:1085–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Madhi SA, Koen AL, Izu A, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in people living with and without HIV in South Africa: an interim analysis of a randomised, double-blind, placebo-controlled, phase 1B/2A trial. Lancet HIV 2021; 8:e568–e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Frater J, Ewer KJ, Ogbe A, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in HIV infection: a single-arm substudy of a phase 2/3 clinical trial. Lancet HIV 2021; 8:e474–e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Levy I, Wieder-Finesod A, Litchevsky V, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in people living with HIV-1. Clin Microbiol Infect 2021; 27:1851–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lombardi A, Butta GM, Donnici L, et al. Anti-spike antibodies and neutralising antibody activity in people living with HIV vaccinated with COVID-19 mRNA-1273 vaccine: a prospective single-centre cohort study. Lancet Reg Health Eur 2022; 13:100287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rahav G, Lustig Y, Lavee J, et al. BNT162b2 mRNA COVID-19 vaccination in immunocompromised patients: a prospective cohort study. EClinicalMedicine 2021; 41:101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Antinori A, Cicalini S, Meschi S, et al. Humoral and cellular immune response elicited by mRNA vaccination against SARS-CoV-2 in people living with HIV (PLWH) receiving antiretroviral therapy (ART) according with current CD4 T-lymphocyte count. Clin Infect Dis 2022; 75:e552–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Corma-Gómez A, Fernández-Fuertes M, García E, et al. Severe immunosuppression is related to poorer immunogenicity to SARS-CoV-2 vaccines among people living with HIV. Clin Microbiol Infect. 2022; 28:1492–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hensley KS, Jongkees MJ, Geers D, et al. Immunogenicity and reactogenicity of SARS-CoV-2 vaccines in people living with HIV in the Netherlands: a nationwide prospective cohort study. PLoS Med 2022; 19:e1003979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Coburn SB, Humes E, Lang R, et al. Analysis of postvaccination breakthrough COVID-19 infections among adults with HIV in the United States. JAMA Netw Open 2022; 5:e2215934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hippisley-Cox J, Coupland CA, Mehta N, et al. Risk prediction of COVID-19 related death and hospital admission in adults after COVID-19 vaccination: national prospective cohort study. BMJ 2021; 374:n2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bhaskaran K, Rentsch CT, MacKenna B, et al. HIV Infection and COVID-19 death: a population-based cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform. Lancet HIV 2021; 8:e24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 2021; 27:1205–11. [DOI] [PubMed] [Google Scholar]
- 15. Hachmann NP, Miller J, Collier AY, et al. Neutralization escape by SARS-CoV-2 Omicron subvariants BA.2.12.1, BA.4, and BA.5. N Engl J Med 2022; 387:86–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Altarawneh HN, Chemaitelly H, Ayoub HH, et al. Effects of previous infection and vaccination on symptomatic Omicron infections. N Engl J Med 2022; 387:21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Qu P, Faraone J, Evans JP, et al. Neutralization of the SARS-CoV-2 omicron BA.4/5 and BA.2.12.1 subvariants. N Engl J Med 2022; 386:2526–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lapointe HR, Mwimanzi F, Cheung PK, et al. People with HIV receiving suppressive antiretroviral therapy show typical antibody durability after dual COVID-19 vaccination, and strong third dose responses [published online ahead of print 7 June 2022]. J Infect Dis 10.1093/infdis/jiac229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tan Y, Zou S, Ming F, et al. Early efficacy and safety of the third dose inactivated COVID-19 vaccine among people living with HIV. J Acquir Immune Defic Syndr 2022; 90:e1–3. [DOI] [PubMed] [Google Scholar]
- 20. Yan Y, Davgadorj C, Lyu C, Zhang S, Qiu Y. Immunogenicity of a third dose of inactivated COVID-19 vaccine in people living with HIV-1, HBV, and tuberculosis during the Omicron variant epidemic: a cross-sectional study. J Infect 2022; 85:e109–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Oosting SF, van der Veldt AAM, GeurtsvanKessel CH, et al. mRNA-1273 COVID-19 vaccination in patients receiving chemotherapy, immunotherapy, or chemoimmunotherapy for solid tumours: a prospective, multicentre, non-inferiority trial. Lancet Oncol 2021; 22:1681–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Haggenburg S, Hofsink Q, Lissenberg-Witte BI, et al. Antibody response in immunocompromised patients with hematologic cancers who received a 3-dose mRNA-1273 vaccination schedule for COVID-19. JAMA Oncol 2022; 8:1477–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lau D, Lan LY, Andrews SF, et al. Low CD21 expression defines a population of recent germinal center graduates primed for plasma cell differentiation. Sci Immunol 2017; 2:eaai8153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Golinski ML, Demeules M, Derambure C, et al. CD11c+ B cells are mainly memory cells, precursors of antibody secreting cells in healthy donors. Front Immunol 2020; 11:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. GeurtsvanKessel CH, Geers D, Schmitz KS, et al. Divergent SARS-CoV-2 Omicron-reactive T and B cell responses in COVID-19 vaccine recipients. Sci Immunol 2022; 7:eabo2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tarke A, Coelho CH, Zhang Z, et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell 2022; 185:847–59.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Geers D, Shamier MC, Bogers S, et al. SARS-CoV-2 variants of concern partially escape humoral but not T-cell responses in COVID-19 convalescent donors and vaccinees. Sci Immunol 2021; 6:eabj1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee A, Wong SY, Chai LYA, et al. Efficacy of COVID-19 vaccines in immunocompromised patients: systematic review and meta-analysis. BMJ 2022; 376:e068632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Manothummetha K, Chuleerarux N, Sanguankeo A, et al. Immunogenicity and risk factors associated with poor humoral immune response of SARS-CoV-2 vaccines in recipients of solid organ transplant: a systematic review and meta-analysis. JAMA Netw Open 2022; 5:e226822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Oosting SF, van der Veldt AAM, Fehrmann RSN, et al. Immunogenicity after second and third mRNA-1273 vaccination doses in patients receiving chemotherapy, immunotherapy, or both for solid tumours. Lancet Oncol 2022; 23:833–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.