Abstract
Background
Cefepime/taniborbactam is a cephalosporin/bicyclic boronate β-lactamase inhibitor combination in clinical development for nosocomial pneumonia due to MDR Gram-negative bacteria. A murine pneumonia model was used to characterize cefepime/taniborbactam in vivo pharmacodynamics against Enterobacterales and Pseudomonas aeruginosa strains.
Methods
Clinical cefepime-non-susceptible Enterobacterales and P. aeruginosa strains expressing serine carbapenemases and/or other cefepime-hydrolysing β-lactamases with cefepime/taniborbactam combination MICs of 0.12–16 mg/L were used. Cefepime and taniborbactam human-simulated regimens equivalent to clinical doses (i.e. 2/0.5 g q8h) were established in the pneumonia model. The in vivo activity of the cefepime human-simulated regimen given alone or concomitantly with escalating taniborbactam exposures against eight Enterobacterales and four P. aeruginosa strains was assessed. Taniborbactam pharmacokinetics were evaluated to determine systemic exposures of regimens used; taniborbactam fAUC0–24/MIC values required for efficacy were estimated using the Hill equation. In addition, the in vivo activity of the cefepime/taniborbactam combination human-simulated regimen was assessed against 18 strains.
Results
Among Enterobacterales, median taniborbactam fAUC0–24/MIC values associated with stasis and 1 log kill were 0.96 and 4.03, respectively, while for P. aeruginosa, requirements were 1.35 and 3.02 for stasis and 1 log kill, respectively. The cefepime/taniborbactam human-simulated regimen produced >2 log kill in 14/18 strains and >1 log kill in 18/18 strains.
Conclusions
Cefepime/taniborbactam produced marked in vivo bactericidal activity against cefepime-non-susceptible Enterobacterales and P. aeruginosa isolates with cefepime/taniborbactam MICs up to and including 16 mg/L in the pneumonia model. Assessments of the probability of clinical attainment of the identified targets should be undertaken to support the selected cefepime/taniborbactam dose for treatment of nosocomial pneumonia.
Introduction
Hospital-acquired bacterial pneumonia (HABP) and ventilator-associated bacterial pneumonia (VABP) are serious nosocomial infections prevalent among patients requiring critical care. HABP/VABP due to carbapenem-resistant and MDR Gam-negative strains are particularly challenging to treat and associated with high morbidity and mortality.1 Toxicity to some of the antibiotics of last resort, notably polymyxins, in addition to poor therapeutic outcomes, creates further challenges when utilizing these agents for management of HABP/VABP in critically ill patients.
New β-lactam/β-lactamase inhibitor (BLBLi) combinations have played pivotal roles in enriching the antibiotic armamentarium as they are generally well tolerated and offer coverage against MDR Gram-negative strains, minimizing the need for less-tolerable last-line agents. Several BLBLi combinations have recently been approved for HABP/VABP caused by Enterobacterales and Pseudomonas aeruginosa2 and are active against carbapenem-resistant subsets, including ceftazidime/avibactam, ceftolozane/tazobactam and imipenem/cilastatin/relebactam. Other BLBLis in the pipeline include cefepime/taniborbactam, a combination of cefepime, a fourth-generation cephalosporin, and taniborbactam, a novel bicyclic boronic acid-containing β-lactamase inhibitor. Taniborbactam inhibits most clinically relevant serine β-lactamases belonging to Ambler classes A, C and D, and MBLs (class B) of VIM, NDM, SPM-1 and GIM-1 types.3
The objective of this study was to investigate cefepime/taniborbactam pharmacodynamics against clinical cefepime-non-susceptible Enterobacterales and P. aeruginosa isolates expressing serine β-lactamases (such as CTX-M, KPC and OXA-48-like) as well as inducible AmpC-expressing P. aeruginosa in a translational murine pneumonia model4 to support clinical dose selection for HABP/VABP treatment with cefepime/taniborbactam (2/0.5 g every 8 h as 4 h infusion) in Phase III studies.
Methods
Antimicrobial test agents
Taniborbactam (batch numbers CA19-1355 and CA20-0265; Venatorx Pharmaceuticals Inc., Malvern, PA, USA) was used for in vivo and in vitro testing. For in vitro testing, taniborbactam master stock (10 mg/mL) was prepared in DMSO. For in vivo testing, taniborbactam was reconstituted to 10 mg/mL using sterile water for injection (Hospira, Inc., Lake Forest, IL, USA). Subsequent dilutions in sterile 0.9% normal saline (NS) solution (B. Braun Medical Inc., Irvine, CA, USA) were made to attain final concentrations that would deliver the required doses based on study mice population’s mean weight. Cefepime HCl (batch number LRAB8503, Sigma–Aldrich, Inc., St. Louis, MO, USA) was used for in vitro testing. Cefepime 1 g vials (lots 108307C and 108725C, WG Critical Care, LLC, Paramus, NJ, USA) were reconstituted and diluted with 0.9% NS for in vivo testing. Taniborbactam and cefepime were administered via subcutaneous injections of 0.1 mL volumes.
Bacterial isolates and susceptibility testing
Thirteen Enterobacterales and five P. aeruginosa were provided by Venatorx Pharmaceuticals, Inc.5 or selected from the FDA-CDC Antimicrobial Resistance Isolate Bank (Atlanta, GA, USA) or the Hartford Hospital Center for Anti-Infective Research and Development isolate repository. Isolates harboured genes for serine carbapenemases, ESBLs and/or inducible AmpC cephalosporinases. Cefepime and cefepime/taniborbactam combination MICs at a fixed taniborbactam concentration of 4 mg/L were determined in triplicate using reference broth microdilution methodology with concurrent quality control as outlined by CLSI.6,7 Modal MICs were utilized to characterize the isolates for final analyses.
Neutropenic murine pneumonia model
Specific pathogen-free, female ICR mice weighing 20–22 g were obtained from Charles River Laboratories, Inc. (Wilmington, MA, USA) and housed in HEPA-filtered cages in groups of six at controlled room temperature, provided with food and water ad libitum and allowed to acclimatize for a minimum of 48 h before experimentation commencement. A 12 h light/12 h dark cycle was maintained. The protocol was approved by the Institutional Animal Care and Use Committee at Hartford Hospital (Assurance #A3185-01).
Mice were rendered transiently neutropenic by injecting cyclophosphamide intraperitoneally at 250 and 100 mg/kg 4 days and 1 day before inoculation, respectively. Uranyl nitrate 5 mg/kg was administered intraperitoneally 3 days before inoculation to produce a controlled degree of renal impairment reducing the dosing requirement for humanizing the regimens of the test agents.
Prior to mice lung inoculation, two transfers of the organisms (previously frozen at −80°C in skimmed milk) were performed onto Trypticase soy agar plates with 5% sheep blood (TSA II™; Becton, Dickinson & Co.; Sparks, MD, USA) and incubated at 37°C for approximately 24 h. After 18–24 h incubation of the second bacterial transfer, a bacterial suspension of approximately 107 cfu/mL in NS with 3% mucin was made for inoculation. Inoculum bacterial densities were confirmed by serial dilution and culture of an aliquot for quality control. Mice were anaesthetized using vaporized isoflurane (2%–3% v/v in an oxygen carrier) until respiratory rate visually decreased. Pneumonia was induced via intranasal inoculation with 50 μL of the bacterial suspension 2 h prior to the initiation of antimicrobial therapy. Mice were allowed to fully recover from anaesthesia in an oxygen-enriched chamber prior to randomization into treatment and control groups. All experiments were conducted in a non-blinded design, i.e. the handling staff were aware of group allocation at the different experimental stages.
Plasma and bronchopulmonary pharmacokinetics
The purpose of these studies was to establish human-simulated regimens of cefepime and taniborbactam in the murine pneumonia model equivalent to clinical doses of 2 and 0.5 g, respectively, administered every 8 h as 4 h infusions based on the unbound (free) plasma exposures. Following the selection and the confirmation of the cefepime and taniborbactam human-simulated regimens, additional studies were conducted to quantify the plasma and bronchopulmonary exposures achieved following the administration of the taniborbactam dosages utilized in the dose-ranging studies in combination with the cefepime human-simulated regimen.
Cefepime pharmacokinetic data in healthy adult volunteers collected in Phase I studies upon taniborbactam co-administration8 and cefepime and taniborbactam pharmacokinetic parameters in the murine model were utilized for simulation. Cefepime protein-binding percentages utilized in the simulations were 20% and 0% in humans and mice, respectively,9 while taniborbactam protein-binding percentages were 0% and 19.4% in humans10 and mice,11 respectively.
Infected mice (6–10 groups of six mice each) received the estimated human-simulated regimen of cefepime as monotherapy or in combination with that of taniborbactam or fractions of the established taniborbactam human-simulated regimen: 1.56% or 12.5% of the taniborbactam human-simulated regimen doses (equivalent to 7.8 or 62.5 mg every 8 h as 4 h infusion, respectively).
At 6–10 different timepoints, groups of six mice were euthanized by CO2 asphyxiation followed by blood collection via intracardiac puncture and cervical dislocation. Following blood collection, but prior to cervical dislocation, bronchoalveolar lavage (BAL) fluid was collected from the mice at the same timepoints using methods previously described.12 Formic acid in water (2% v/v) was added to BAL samples (equal parts) prior to freezing. All sample tubes were stored at −80°C until drug and urea concentration determination. Cefepime, taniborbactam and urea concentrations in plasma and BAL fluid were assayed by either Keystone Bioanalytical, Inc. (North Wales, PA, USA) or by Venatorx Pharmaceuticals, Inc. using qualified LC-MS/MS methods.
Cefepime and taniborbactam concentrations in the epithelial lining fluid (ELF) were estimated by correcting the drug concentration in BAL fluid for the dilution with NS during lavage using the following formula13:
CompoundELF = CompoundBAL × (Ureaplasma/UreaBAL), where CompoundBAL is the measured concentration of either cefepime or taniborbactam in the BAL fluid sample and Ureaplasma and UreaBAL are the concentrations of urea in paired plasma and BAL fluid samples from each mouse, respectively.
Statistical outliers for each respective analyte were removed by the IQR method. A pharmacokinetic model was fitted to the plasma and ELF concentrations of each of cefepime and taniborbactam and the best-fit estimate parameters were determined by the non-linear least-squares techniques (WinNonlin, Version 8.3, Pharsight Corp., Mountain View, CA, USA). Compartment model selection was based on visual inspection of the fit and comparison of model diagnostics.
These parameters were utilized to estimate the plasma and ELF exposures and the ELF penetration ratios as the ratio of the ELF AUC0–24 to unbound (free) plasma AUC0–24.
Taniborbactam dose-ranging studies
The purpose of these studies was to assess the in vivo bactericidal activity of the human-simulated exposure of cefepime monotherapy (2 g every 8 h as 4 h infusion) and of cefepime in combination with escalating taniborbactam exposures against cefepime-non-susceptible β-lactamase-producing Enterobacterales and P. aeruginosa.
Eight Enterobacterales and four P. aeruginosa isolates were selected with a range of cefepime/taniborbactam MICs (0.12 to 16 mg/L), expressing various types of Class A, C and D β-lactamases.
Mice (six per group) were prepared and inoculated, and treatment commenced 2 h later with cefepime monotherapy human-simulated regimen or cefepime human-simulated regimen in combination with taniborbactam as 1.56%, 6.25%, 12.5% or 100% of the taniborbactam human-simulated regimen.
For each isolate tested, six untreated mice were used as 0 h controls to determine the pre-treatment inoculum density, and six additional mice as 24 h controls. The 24 h control animals received NS in the same volume and schedule as the most frequently dosed drug regimen. Dosing order of different treatments and control groups was kept consistent to minimize confounding. After 24 h, all animals were euthanized by CO2 asphyxiation followed by cervical dislocation then lungs were removed aseptically and individually homogenized in NS. Serial dilutions were plated onto TSA plates with 5% sheep blood for cfu enumeration. Bacterial densities at 24 h were evaluated to determine the change in log10 cfu/lungs achieved with each control/treatment. Statistical outliers were excluded systematically after application of the IQR rule.
We had previously reported that fAUC0–24/MIC was the pharmacokinetic/pharmacodynamic (PK/PD) driver of taniborbactam activity.11 Thus, for taniborbactam pharmacodynamic analyses, fAUC0–24/MIC values were estimated for the different taniborbactam exposures for each isolate using the bioactive, free-drug exposures and isolate-specific cefepime/taniborbactam combination MIC value. A sigmoidal inhibitory Emax model was fitted to the data using WinNonlin and the effective taniborbactam indices (fAUC0–24/MIC) required to achieve net stasis, 1 log and 2 log bacterial cell killing from the starting bacterial burden (0 h groups) for each isolate were estimated. The goodness of fit for each of these relationships was characterized using the coefficient of determination (R2).
In vivo activities of cefepime and cefepime/taniborbactam human-simulated exposures
The purpose of these studies was to assess the in vivo bactericidal activity of cefepime human-simulated exposure as monotherapy (2 g every 8 h as 4 h infusion) and cefepime in combination with taniborbactam human-simulated exposure (cefepime/taniborbactam, 2/0.5 g every 8 h as 4 h infusion) against cefepime-non-susceptible β-lactamase-producing Enterobacterales (n = 13) and P. aeruginosa (n = 5).
Mice (six per group) were inoculated then treatment was commenced 2 h later. For each isolate tested, six untreated mice were used as 0 h controls, and six mice (receiving NS) as 24 h controls. Dosing order of different treatments and control groups was kept consistent to minimize confounding. After 24 h, animals were euthanized, and lung homogenates were processed as described before for cfu enumeration. Bacterial densities at 24 h were evaluated to define the changes in log10 cfu/lungs. Statistical outliers were excluded systematically after application of the IQR rule.
Results
In vitro susceptibility
Cefepime and cefepime/taniborbactam combination MICs at a fixed taniborbactam concentration of 4 mg/L for the tested isolates are shown in Table 1. One isolate of P. aeruginosa (PSA 1715) was intermediate to cefepime and all other isolates were cefepime-resistant based on the MIC breakpoints defined by the CLSI.6 Extent of cefepime MIC potentiation by taniborbactam was 32- to >4096-fold for Enterobacterales and 4- to >256-fold for P. aeruginosa.
Table 1.
A summary of all the Enterobacterales and P. aeruginosa isolates selected for the in vivo efficacy studies. All isolates were examined in the in vivo efficacy of cefepime/taniborbactam human-simulated exposures studies. Isolates designated by bolded CAIRD IDs were also examined in taniborbactam dose-ranging studies
| Isolate | MIC (mg/L) | β-Lactamase encoded | |||
|---|---|---|---|---|---|
| CAIRD ID | Source | Source ID | Cefepime | Cefepime/taniborbactam | |
| EC 747 | ATCC | BAA 2340 | 512 | 2 | KPC |
| EC 753 a | IHMA | 1904038 | >512 | 2 | CTX-M-15 |
| EC 755 a,b | IHMA | 1886025 | >512 | 16 | CTX-M-15, EC-TYPE, OXA-1, TEM-1 |
| ECL 108 | CDCe | 0163 | >512 | 2 | KPC-2, ACT-7, CTX-M-15, OXA-1, TEM-1B |
| KP 647 | CDCe | 0112 | >512 | 0.12 | KPC-3 |
| KP 679 | CDCe | 0066 | >512 | 4 | OXA-232, OXA-9, TEM-1A, CTX-M-15, OXA-1 |
| KP 681 c | CDCe | 0075 | >512 | 1 | CTX-M-15, OXA-1, OXA-232, SHV-1 |
| KP 731 d | CAIRD | >512 | 1 | SHV-11, TEM-1, KPC-3 | |
| PSA 1711 | CDCe | 0518 | 128 | 16 | PDC-103, KPC-2 |
| PSA 1714 | CDCe | 0528 | 32 | 4 | PDC-1 |
| PSA 1715 | CDCe | 0529 | 16 | 4 | PDC-5 |
| PSA 1844 | CDCe | 0356 | >512 | 2 | KPC-2, PDC-42 |
| EC 720 | CDCe | 0437 | >512 | 16 | CTX-M-15, OXA-1 |
| EC 748 | CDCe | 0001 | 512 | 2 | ESC-35, OXA-1, KPC-3 |
| ECL 124d | CAIRD | >512 | 8 | TEM-OSBL, CTX-M-15, ACT-7 | |
| KP 579d | CAIRD | >512 | 16 | SHV-11, TEM-1, CTX-M-15, OXA-48 | |
| KP 744d | CAIRD | >512 | 4 | SHV-32, TEM-1, CTX-M-15, OXA-48 | |
| PSA 1681d | CAIRD | >512 | 8 | OXA-486, PDC-8, OXA-10, CTX-M-2 | |
CAIRD, Center for Anti-Infective Research and Development; EC, E. coli; ECL, Enterobacter cloacae; KP, K. pneumoniae; PSA, P. aeruginosa; OSBL, original-spectrum β-lactamase.
PBP3 characterized by a 4-amino-acid (YRIK) insertion after position 333 and an A413V substitution.
G137D substitution in OmpC known to be present.
Mutation in OmpK35 porin known to be present.
MICs were previously reported.11
Molecular mechanisms of β-lactam resistance listed as posted on the FDA-CDC Antimicrobial Resistance Isolate Bank website at the time of study execution.
Cefepime and taniborbactam plasma and bronchopulmonary pharmacokinetics
Cefepime and taniborbactam were detected in mouse plasma and BAL fluid following subcutaneous administration in all pharmacokinetic studies. Cefepime and taniborbactam plasma and ELF concentration–time profiles are shown in Figures 1 and 2. Cefepime and taniborbactam plasma and ELF pharmacokinetics were described satisfactorily using one-compartment models with first-order elimination. Best-fit pharmacokinetic parameters in the neutropenic pneumonia model are shown in Tables S1 to S4 (available as Supplementary data at JAC Online). Best-fit parameters in plasma were utilized to develop a cefepime human-simulated regimen of 2 g every 8 h as 4 h infusion as monotherapy and in combination with a taniborbactam human-simulated regimen of 0.5 g every 8 h as 4 h infusion or deescalating proportions of the latter established regimen. The murine dosing regimens are summarized in Table S5.
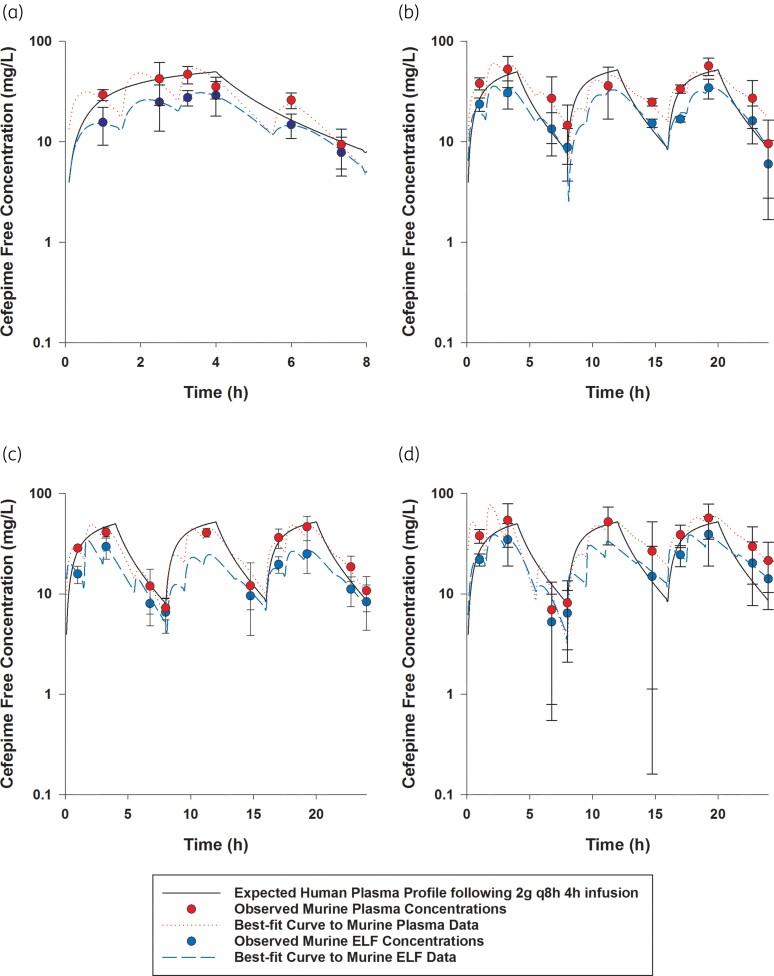
Figure 1.
Cefepime free plasma and ELF concentrations achieved in mice receiving human-simulated regimen of cefepime (2 g every 8 h as 4 h infusion): (a) as monotherapy; (b) in combination with 1.56% of the doses of the human-simulated regimen of taniborbactam; (c) in combination with 12.5% of the doses of the human-simulated regimen of taniborbactam; and (d) in combination with 100% of the doses of the human-simulated regimen of taniborbactam. Data are means ± SDs. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
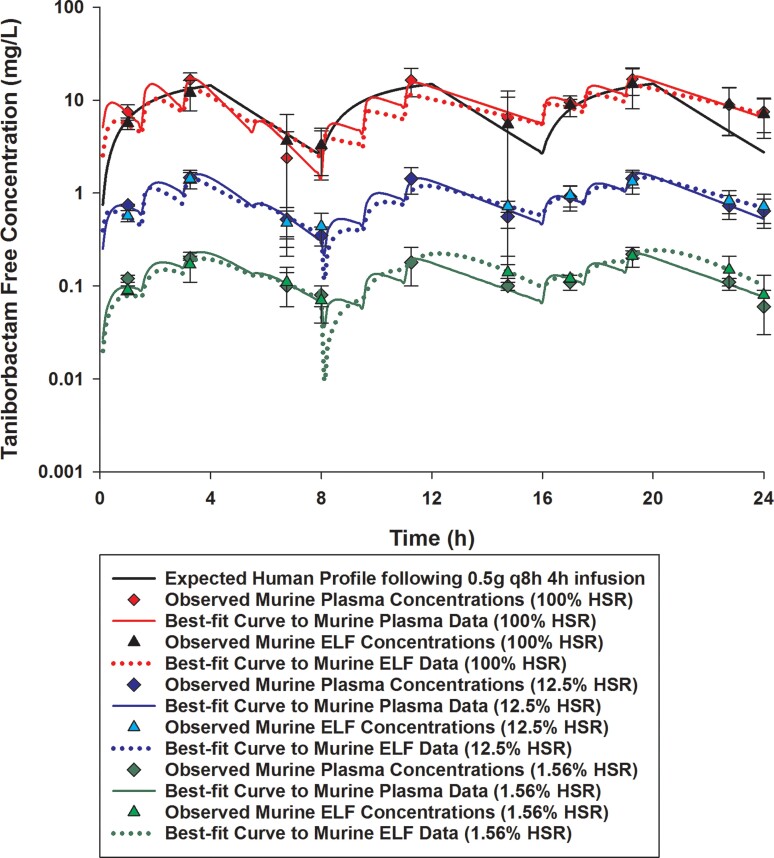
Figure 2.
Taniborbactam free plasma and ELF concentrations achieved in mice receiving 1.56%, 12.5% or 100% of the doses of the human-simulated regimen of taniborbactam (0.5 g every 8 h as 4 h infusion) in combination with cefepime human-simulated regimen (2 g every 8 h as 4 h infusion). Data are means ± SDs. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Comparisons of cefepime and taniborbactam fAUC0–24, fCmax and %fT>MIC values estimated in plasma and ELF at each MIC doubling dilution from 2 to 128 mg/L in humans and mice receiving the examined regimens are presented in Tables 2 and 3. The established murine cefepime and taniborbactam human-simulated regimens reasonably approximated the targeted human plasma exposures. Across the doses examined, taniborbactam plasma and ELF AUC0–24 values were dose-proportional across the range of dose studies (R2 = 1).
Table 2.
Comparison of cefepime plasma and ELF exposures achieved in humans receiving 2 g every 8 h as 4 h infusion versus infected mice receiving cefepime human-simulated regimen subcutaneously
| Matrix/regimen | Species | %fT>MIC for an MIC (mg/L) of: | fAUC0–24 (mg·h/L) | fC max (mg/L) | ELF:plasma penetration | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 4 | 8 | 16 | 32 | 64 | 128 | |||||
| Plasma | |||||||||||
| Human | 100 | 100 | 99 | 73 | 45 | 0 | 0 | 700.9 | 52.0 | ||
| ȃMonotherapy | Mouse | 100 | 100 | 93 | 77 | 35 | 0 | 0 | 656.8 | 54.0 | |
| ȃIn combination with taniborbactam 1.56% HSR | Mouse | 100 | 100 | 100 | 97 | 52 | 0 | 0 | 821.0 | 60.6 | |
| ȃIn combination with taniborbactam 12.5% HSR | Mouse | 100 | 100 | 98 | 80 | 37 | 0 | 0 | 668.2 | 48.9 | |
| ȃIn combination with taniborbactam 100% HSR | Mouse | 100 | 99 | 95 | 88 | 59 | 3 | 0 | 858.4 | 76.9 | |
| ELF | |||||||||||
| ȃMonotherapy | Mouse | 100 | 100 | 90 | 50 | 0 | 0 | 0 | 428.4 | 31.0 | 0.65 |
| ȃIn combination with taniborbactam 1.56% HSR | Mouse | 100 | 100 | 98 | 70 | 14 | 0 | 0 | 516.7 | 35.8 | 0.63 |
| ȃIn combination with taniborbactam 12.5% HSR | Mouse | 100 | 100 | 93 | 51 | 2 | 0 | 0 | 406.1 | 34.2 | 0.61 |
| ȃIn combination with taniborbactam 100% HSR | Mouse | 100 | 100 | 95 | 74 | 17 | 0 | 0 | 545.2 | 39.0 | 0.64 |
HSR, human-simulated regimen.
Table 3.
Comparison of taniborbactam plasma and ELF exposures achieved in humans receiving 0.5 g every 8 h as 4 h infusion versus infected mice receiving taniborbactam in combination with cefepime human-simulated regimen subcutaneously
| Matrix/regimen | Species | %fT>MIC for an MIC (mg/L) of: | fAUC0–24 (mg·h/L) | fC max (mg/L) | ELF:plasma penetration | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 4 | 8 | 16 | 32 | 64 | 128 | |||||
| Plasma | |||||||||||
| Human | 99 | 83 | 53 | 0 | 0 | 0 | 0 | 205.2 | 15.0 | ||
| ȃTaniborbactam 1.56% HSR | Mouse | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3.2 | 0.2 | |
| ȃTaniborbactam 12.5% HSR | Mouse | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 22.1 | 1.6 | |
| ȃTaniborbactam 100% HSR | Mouse | 98 | 93 | 65 | 5 | 0 | 0 | 0 | 229.9 | 18.1 | |
| ELF | |||||||||||
| ȃTaniborbactam 1.56% HSR | Mouse | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3.5 | 0.2 | 1.11 |
| ȃTaniborbactam 12.5% HSR | Mouse | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 21.0 | 1.5 | 0.95 |
| ȃTaniborbactam 100% HSR | Mouse | 100 | 89 | 51 | 0 | 0 | 0 | 0 | 195.2 | 15.1 | 0.85 |
HSR, human-simulated regimen.
Cefepime penetration ratio into the ELF was estimated to be 0.65. Penetration of cefepime into the ELF was unaltered by taniborbactam co-administration at any dosing level. Taniborbactam penetration ratio into the ELF was mostly consistent between different taniborbactam dosages and ranged between 0.85 and 1.11.
Taniborbactam dose-ranging studies
Enterobacterales
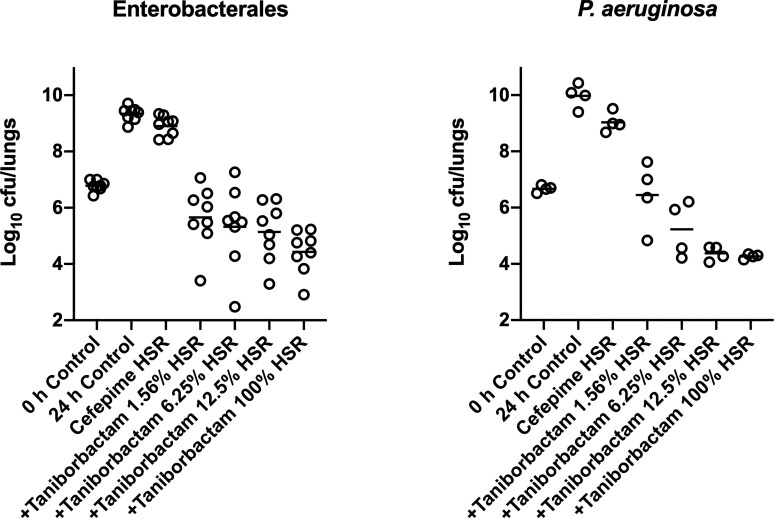
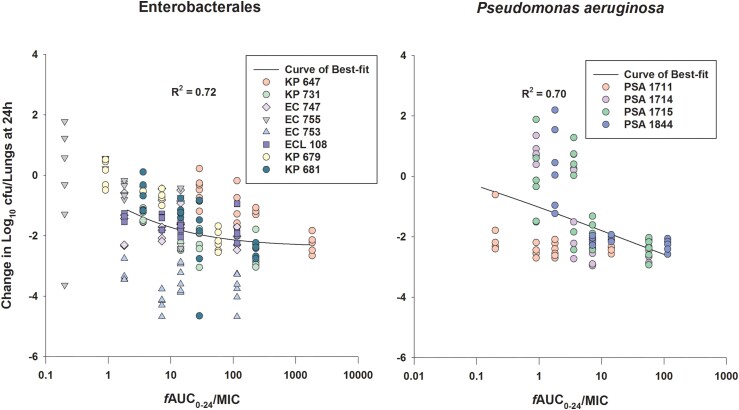
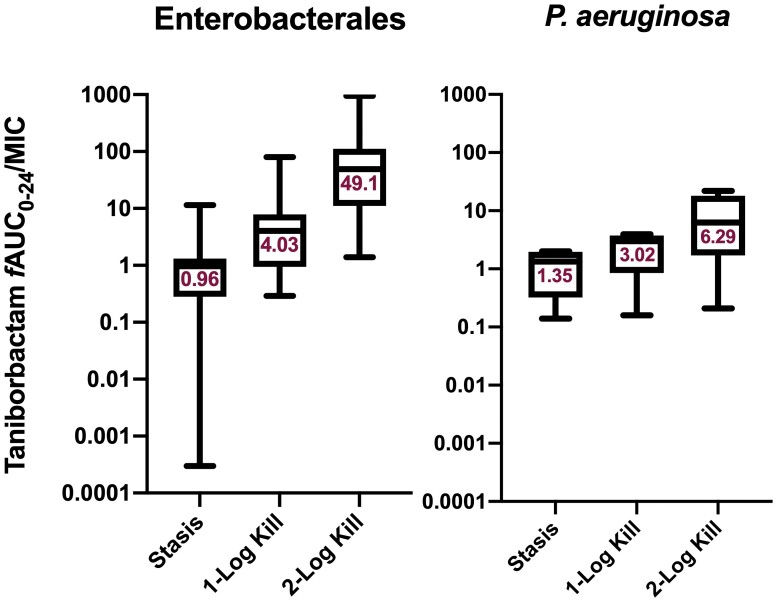
At 0 h, average (±SD) bacterial burdens were 6.78 ± 0.23 log10 cfu/lungs. For all the tested isolates, adequate growth in the lungs was achieved; the bacterial burdens increased over 24 h by an average magnitude of 2.57 ± 0.32 log10 cfu/lungs in the untreated controls. Consistent with the isolates’ non-susceptibility to cefepime, robust increase in the bacterial burden was observed in the lungs of the mice receiving the cefepime monotherapy human-simulated regimen, indicating lack of in vivo activity; the bacterial burdens increased over 24 h by an average magnitude of 2.12 ± 0.46 log10 cfu/lungs compared with the 0 h controls. Compared with the cefepime monotherapy human-simulated regimen, taniborbactam co-administration at all tested ratios enhanced the degree of bacterial killing. Average maximal reduction of burden (Imax) at 24 h achieved due to taniborbactam co-administration was 5.59 ± 1.09 log10 cfu/lungs, estimated relative to the bacterial densities in the lungs of the mice treated with the cefepime monotherapy human-simulated regimen (Figure 3). For the composite of eight Enterobacterales isolates, the plasma fAUC0–24/MIC ratios associated with static, 1 log kill and 2 log kill endpoints were 0.22, 1.45 and 36.46, respectively (Figure 4; R2 = 0.72). As for the individual isolates, exposure–response relationships were strong based upon the coefficient of determination (median R2 = 0.94). The median fAUC0–24/MIC associated with static, 1 log kill and 2 log kill endpoints were 0.96, 4.03, and 49.1, respectively (Figure 5).
Figure 3.
Taniborbactam dose-ranging efficacy in combination with cefepime human-simulated exposure against Enterobacterales (n = 8, left) and P. aeruginosa isolates (n = 4, right) in the neutropenic pneumonia model. Each dot represents the mean log10 cfu/lungs for one bacterial strain per regimen.
Figure 4.
The curves of best fit to the taniborbactam fAUC0–24/MIC and the changes in log10 cfu/lungs at 24 h relative to 0 h groups for the composites of examined Enterobacterales (n = 8) and P. aeruginosa (n = 4) isolates in the neutropenic pneumonia model. The solid lines represent the curves of best fit, while the circles represent the actual changes in bacterial burdens observed in the individual mice. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Figure 5.
Taniborbactam fAUC0–24/MIC required to achieve efficacy endpoints against Enterobacterales (n = 8) and P. aeruginosa (n = 4) when co-administered with the cefepime human-simulated regimen. Median values are displayed on the plots. Median 2 log kill target for Enterobacterales calculated based on seven isolates (target not achieved against one isolate). Hinges represent 25th and 75th percentiles. Whiskers represent 10th and 90th percentiles. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
P. aeruginosa
Across the four isolates, average initial bacterial burdens at 0 h were 6.66 ± 0.25 log10 cfu/lungs and increased over 24 h by 3.29 ± 0.41 and 2.36 ± 0.49 log10 cfu/lungs in the untreated controls and those that received the cefepime monotherapy human-simulated regimen, respectively.
Compared with cefepime monotherapy, taniborbactam co-administration enhanced the bacterial killing, resulting in average Imax of 5.07 ± 0.62 log10 cfu/lungs (Figure 3). For the composite of four P. aeruginosa isolates, plasma fAUC0–24/MIC associated with stasis, 1 log kill, and 2 log kill were 0.03, 0.93 and 18.22, respectively (Figure 4; R2 = 0.70). Across the fits for the individual isolates, median fAUC0–24/MIC associated with stasis, 1 log kill, and 2 log kill were 1.35, 3.02, and 6.29, respectively (median R2 = 0.89; Figure 5).
In vivo activities of cefepime and cefepime/taniborbactam human-simulated exposures
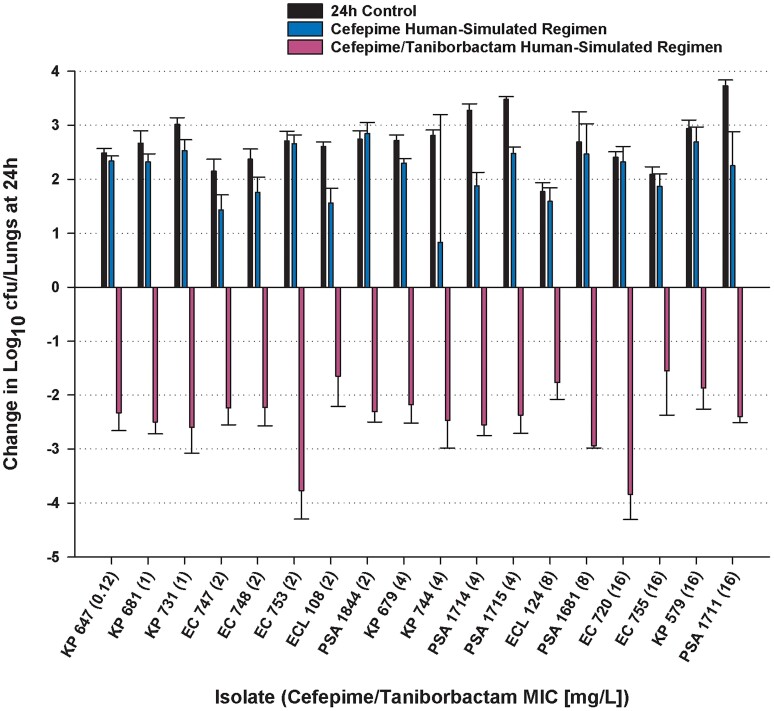
At 0 h, average bacterial burdens were 6.73 ± 0.18 log10 cfu/lungs and increased over 24 h by 2.49 ± 0.47 and 1.92 ± 1.17 log10 cfu/lungs in the untreated controls and those that received the cefepime monotherapy human-simulated regimen, respectively. Cefepime and taniborbactam human-simulated exposures co-administration resulted in ≥1 log reduction at 24 h compared with the initial bacterial burdens against all 18 isolates and >2 log reduction against 14 of 18 isolates (Figure 6). Using a Student’s t-test, cefepime and taniborbactam combination human-simulated exposures’ bactericidal activities against Enterobacterales and P. aeruginosa isolates were compared and found to be not significantly different between the two species (P = 0.70). Cefepime and taniborbactam combination human-simulated exposures’ magnitude of killing against the four isolates with cefepime/taniborbactam MICs = 16 mg/L was comparable to that achieved against isolates of lower MICs (P = 0.98).
Figure 6.
Comparative efficacy of the human-simulated exposure of cefepime monotherapy and in combination with taniborbactam human-simulated exposure against 13 Enterobacterales and 5 P. aeruginosa clinical isolates expressing serine β-lactamases. Data are means ± SDs. Value between parentheses following strain designation represents cefepime/taniborbactam MIC in mg/L. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Discussion
Taniborbactam ability to restore cefepime activity in vitro against clinical cephalosporin- and carbapenem-resistant Enterobacterales and P. aeruginosa was illustrated as part of a recent global surveillance study.5 A total of 99.5% of Enterobacterales (n = 13 731) and 94.2% of P. aeruginosa (n = 4619) isolates were inhibited at a cefepime/taniborbactam concentration of ≤8 mg/L, with MIC90 values of 0.25 and 8 mg/L, respectively. Importantly, cefepime/taniborbactam was active in vitro against a variety of resistant subsets including piperacillin/tazobactam, ceftazidime/avibactam, ceftolozane/tazobactam, meropenem/vaborbactam and MDR phenotypes.5 Cefepime/taniborbactam combination thus has the potential to fulfil the unmet need of combating infections due to carbapenem-resistant and MDR Gram-negative pathogens with a coverage that surpasses currently approved comparators.
We have previously demonstrated cefepime/taniborbactam combination’s potent in vivo activity against Enterobacterales and P. aeruginosa clinical isolates including carbapenem-resistant isolates in the murine thigh and complicated urinary tract infection models.11,14 Using a taniborbactam dose-fractionation design in the thigh infection model, we established that the PK/PD index that best correlated with taniborbactam inhibitory activity (i.e. protection of cefepime from β-lactamase hydrolysis) was fAUC0–24/MIC.11 The current study in the murine pneumonia model adds to the understanding of cefepime/taniborbactam in vivo pharmacodynamics, and the data have important implications for the clinical dose selection for the HABP/VABP indication.
A cefepime human-simulated regimen was included in all in vivo pharmacodynamics assessments as a negative control to demonstrate that the efficacy of the cefepime/taniborbactam combination was not due to unexpected in vivo killing with the β-lactam monotherapy. Model validation using a different antibiotic was not performed concomitantly in this investigation. Nevertheless, the neutropenic murine pneumonia model utilized is well validated for antibacterial PK/PD investigations as supported by literature,15,16 as the efficacies of various agents are generally predictable based on the MICs demonstrating the translational application of the model. An example from one of our recent investigations where the in vivo activity of a levofloxacin human-simulated regimen equivalent to 750 mg q24h was concordant with the in vitro MICs of Enterobacterales strains; bacteriostatic activity (−0.46 ± 0.42 log10 cfu/lung) and bacterial killing (−3.02 ± 0.31 log10 cfu/lung) were observed in lung-infected mice with isolates of susceptible MICs (1 and 0.12 mg/L, respectively), while >2 log10 cfu/lung bacterial growth was observed with seven levofloxacin-resistant isolates.4 While clinically relevant in vivo activity in pneumonia due to the target organisms studied in the current investigation is inferred by the use of this translational model, which incorporates humanized drug exposures, HABP/VABP trial data in man are required to fully assess the clinical utility of the compound for this pulmonary indication.
In the current investigation, we observed activity of cefepime/taniborbactam against a diverse group of cefepime-non-susceptible, serine-β-lactamase-producing Enterobacterales and P. aeruginosa isolates including carbapenemase producers. In order to identify the magnitude of taniborbactam fAUC0–24/MIC required to achieve efficacy endpoints that correlate with clinical outcome,16 a dose-ranging study design was implemented, in which a fixed cefepime clinical exposure was administered while varying taniborbactam exposure. This design allows the assessment of taniborbactam target threshold required to restore the in vivo efficacy of the pharmacodynamically optimized clinical cefepime exposure without the potential confounding effect of varying cefepime activity.
Against Enterobacterales, median taniborbactam plasma targets required for stasis and 1 log kill in the neutropenic pneumonia model were comparable to those previously reported in the neutropenic thigh infection model (0.96 and 4.03 versus 1.18 and 2.62, respectively).11 However, against P. aeruginosa, plasma target exposures in the pneumonia model were several folds higher than those observed in the thigh infection model (1.35 and 3.02 versus 0.29 and 0.46, respectively).11 Given the high extent of taniborbactam ELF penetration in the murine model, the difference in threshold plasma targets is unlikely the function of major discrepancies in taniborbactam exposures at infection sites between models. The higher threshold targets against P. aeruginosa in the pneumonia model might be attributed to differences in infection pathogenesis as the formation of biofilm on airway epithelial cells serves as a barrier to limit antibiotic access to bacterial cells.17 Other possible explanations are differences in starting inoculum between models (∼1 log higher in the pneumonia model), thus the higher exposure requirements for achieving the efficacy endpoints, and inter-isolate variabilities as the isolates examined were different between the two models. Nevertheless, given that the estimated average taniborbactam fAUC0–24 associated with a 0.5 g dose every 8 h in healthy volunteers is ∼205 mg·h/L, the targets derived in the pneumonia model predict that this taniborbactam dose, in combination with cefepime 2 g every 8 h as 4 h infusion, would provide sufficient exposure to achieve bactericidal activity against cefepime-resistant Enterobacterales and P. aeruginosa isolates with cefepime/taniborbactam MICs up to and including 16 mg/L in patients. Nonetheless, the impact of variable pharmacokinetics among critically ill patients on the probability of pharmacodynamic target attainment should be assessed to support clinical breakpoint determination.
Non-clinical PK/PD assessments, utilizing mean or median human concentration–time profiles from healthy subjects for establishing the murine human-simulated regimens, is a practical approach that provides reasonable approximation to the exposures observed among the majority of target patients. The assumption herein is that inter-subject variability in pharmacokinetic exposures exists among patients as well as among mice on a comparable scale and the mean murine profile typically falls within the reported coefficient of variation of the concentrations for the majority of the dosing interval.18 Efficacy assessments utilizing human-simulated regimens allow for screening antibiotics such as preclinical and clinical candidates against phenotypically and genotypically diverse isolates including those at the upper end of the MIC distribution not frequently isolated from patients during clinical trials. In the current investigation, administration of cefepime/taniborbactam human-simulated exposures resulted in ∼2 log kill against the majority of the examined Enterobacterales and P. aeruginosa isolates including isolates with combination MICs exceeding the MIC90 from recent surveillance.5 The high degree of killing observed was consistent with predictions from the dose-ranging studies and further supported cefepime/taniborbactam clinical dose selection where a stringent threshold (>1 log kill) is predictive of clinical efficacy for severe infections such as pneumonia.16 It should be noted that by humanizing cefepime/taniborbactam exposures according to plasma concentrations, the corresponding concentration–time profile in mouse ELF, the infection site, may be considerably different compared with the human ELF profile in pneumonia patients. Differences in ELF pharmacokinetic profile estimates and the inter-subject variability observed among infected mice, healthy humans and patients with pneumonia are to be expected, which should be taken into account when considering the translation applicability of the preclinical data. Nevertheless, the robustness of the dose-ranging study design through the use of a wide range of taniborbactam exposures may allow for informative PK/PD analyses when ELF data from cefepime/taniborbactam Phase I bronchopulmonary pharmacokinetic studies are considered.10,19
In summary, co-administration of taniborbactam with a cefepime plasma human-simulated regimen markedly enhanced the latter’s in vivo efficacy against clinical cefepime-non-susceptible Enterobacterales and P. aeruginosa isolates expressing a broad range of serine β-lactamases in the neutropenic pneumonia model with cefepime/taniborbactam MICs up to and including 16 mg/L. Taniborbactam fAUC0–24/MIC targets required for various efficacy endpoints were identified. Assessments of the probability of the clinical attainment of these PK/PD targets should be undertaken to support the proposed cefepime/taniborbactam dose (2/0.5 g every 8 h as 4 h infusion) for treatment of HABP/VABP due to Enterobacterales and P. aeruginosa with these resistant phenotypes.
Supplementary Material
Acknowledgements
This study was presented in part at ASM Microbe 2022, Washington, D.C., USA (Poster 2321). We thank our colleagues at the Center for Anti-Infective Research and Development, Hartford, CT, USA for assistance with the conduct of the study and Fan Yi, PhD at Venatorx Pharmaceuticals, Inc., Malvern, PA, USA for assistance with LC-MS/MS analysis.
Contributor Information
Kamilia Abdelraouf, Center for Anti-Infective Research and Development, Hartford Hospital, Hartford, CT, USA.
David P Nicolau, Center for Anti-Infective Research and Development, Hartford Hospital, Hartford, CT, USA; Division of Infectious Diseases, Hartford Hospital, Hartford, CT, USA.
Funding
This project was sponsored by Venatorx Pharmaceuticals, Inc., Malvern, PA, USA and funded in whole or in part with Federal funds from the Department of Health and Human Services; Administration for Strategic Preparedness and Response; and Biomedical Advanced Research and Development Authority, under Contract No. HHSO100201900007C.
Transparency declarations
D.P.N. and K.A. have received research grants from the study sponsor.
Supplementary data
Tables S1 to S5 are available as Supplementary data at JAC Online.
References
- 1. Kalil AC, Metersky ML, Klompas Met al. . Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 2016; 63: e61–e111. 10.1093/cid/ciw353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kidd JM, Kuti JL, Nicolau DP. Novel pharmacotherapy for the treatment of hospital-acquired and ventilator-associated pneumonia caused by resistant gram-negative bacteria. Expert Opin Pharmacother 2018; 19: 397–408. 10.1080/14656566.2018.1438408 [DOI] [PubMed] [Google Scholar]
- 3. Hamrick JC, Docquier J-D, Uehara Tet al. . VNRX-5133 (taniborbactam), a broad-spectrum inhibitor of serine- and metallo-β-lactamases, restores activity of cefepime in Enterobacterales and Pseudomonas aeruginosa. Antimicrob Agents Chemother 2020; 64: e01963-19. 10.1128/AAC.01963-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Asempa TE, Abdelraouf K, Nicolau DP. Metallo-β-lactamase resistance in Enterobacteriaceae is an artefact of currently utilized antimicrobial susceptibility testing methods. J Antimicrob Chemother 2020; 75: 997–1005. 10.1093/jac/dkz532 [DOI] [PubMed] [Google Scholar]
- 5. Hackel M, Wise MGG, Sahm DF. Antimicrobial activity of cefepime in combination with taniborbactam against resistant clinical isolates of Enterobacterales, Pseudomonas aeruginosa, and Stenotrophomonas maltophilia from 2018–2020 global surveillance. Thirty-Second European Congress of Clinical Microbiology and Infectious Diseases, Lisbon, Portugal, 2022. Abstract P0633.
- 6. CLSI . Performance Standards for Antimicrobial Susceptibility Testing—Thirty-Second Edition: M100. 2022.
- 7. CLSI . Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically—Eleventh Edition: M07. 2018.
- 8. Dowell JA, Marbury TC, Smith WBet al. . Safety and pharmacokinetics of taniborbactam (VNRX-5133) with cefepime in subjects with various degrees of renal impairment. Antimicrob Agents Chemother 2022; 66: e0025322. 10.1128/aac.00253-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mattie H, Sekh BA, van Ogtrop MLet al. . Comparison of the antibacterial effects of cefepime and ceftazidime against Escherichia coli in vitro and in vivo. Antimicrob Agents Chemother 1992; 36: 2439–43. 10.1128/AAC.36.11.2439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. ClinicalTrials.gov . Safety and pharmacokinetics of VNRX-5133 in the epithelial lining fluid of healthy adult subjects. https://clinicaltrials.gov/ct2/show/NCT03870490.
- 11. Abdelraouf K, Almarzoky Abuhussain S, Nicolau DP. In vivo pharmacodynamics of new-generation β-lactamase inhibitor taniborbactam (formerly VNRX-5133) in combination with cefepime against serine-β-lactamase-producing Gram-negative bacteria. J Antimicrob Chemother 2020; 75: 3601–10. 10.1093/jac/dkaa373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abdelraouf K, Nicolau DP. Comparative in vivo efficacies of tedizolid in neutropenic versus immunocompetent murine Streptococcus pneumoniae lung infection models. Antimicrob Agents Chemother 2016; 61: e01957-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rennard SI, Basset G, Lecossier Det al. . Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J Appl Physiol (1985) 1986; 60: 532–8. 10.1152/jappl.1986.60.2.532 [DOI] [PubMed] [Google Scholar]
- 14. Lasko MJ, Nicolau DP, Asempa TE. Clinical exposure–response relationship of cefepime/taniborbactam against Gram-negative organisms in the murine complicated urinary tract infection model. J Antimicrob Chemother 2022; 77: 443–7. 10.1093/jac/dkab405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Andes DR, Lepak AJ. In vivo infection models in the pre-clinical pharmacokinetic/pharmacodynamic evaluation of antimicrobial agents. Curr Opin Pharmacol 2017; 36: 94–9. 10.1016/j.coph.2017.09.004 [DOI] [PubMed] [Google Scholar]
- 16. Bulitta JB, Hope WW, Eakin AEet al. . Generating robust and informative nonclinical in vitro and in vivo bacterial infection model efficacy data to support translation to humans. Antimicrob Agents Chemother 2019; 63: e02307-18. 10.1128/AAC.02307-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pang Z, Raudonis R, Glick BRet al. . Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and alternative therapeutic strategies. Biotechnol Adv 2019; 37: 177–92. 10.1016/j.biotechadv.2018.11.013 [DOI] [PubMed] [Google Scholar]
- 18. Keel RA, Crandon JL, Nicolau DP. Efficacy of human simulated exposures of ceftaroline administered at 600 milligrams every 12 hours against phenotypically diverse Staphylococcus aureus isolates. Antimicrob Agents Chemother 2011; 55: 4028–32. 10.1128/AAC.00372-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. ClinicalTrials.gov . Safety and intrapulmonary pharmacokinetics of cefepime and taniborbactam in healthy subjects. https://clinicaltrials.gov/ct2/show/NCT04951505.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.