Abstract
Background
Raising awareness of antimicrobial resistance is a cornerstone of action plans to tackle this global One Health challenge. Tools that can reliably assess levels of awareness of antibiotic resistance (ABR) among human or animal healthcare professionals (HCPs) are required to guide and evaluate interventions.
Methods
We designed and tested an ABR awareness scale, a self-administered questionnaire completed by human and animal HCPs trained to prescribe and dispense antibiotics in six countries—Ghana, Nigeria, Tanzania, Vietnam, Thailand and Peru. Questionnaires also elicited demographic, practice, and contextual information. Psychometric analysis for the scale followed Rasch Measurement Theory. Bivariate analysis was carried out to identify factors associated with awareness scores.
Results
Overall, 941 HCPs (625 human and 316 animal) from Ghana, Nigeria, Tanzania, Vietnam, Thailand and Peru were included in the study. The 23-item ABR awareness scale had high-reliability coefficients (0.88 for human and 0.90 for animal HCPs) but performed better within countries than across countries. Median ABR awareness scores were 54.6–63.5 for human HCPs and 55.2–63.8 for animal HCPs (scale of 0–100). Physicians and veterinarians scored higher than other HCPs in every country tested. HCPs in this study reported working in contexts with limited laboratory infrastructures. More than 95% of HCPs were interested in receiving information or training on ABR and antimicrobial stewardship.
Conclusion
HCPs’ awareness of ABR can be reliably assessed with this validated 23-item scale within the countries tested. Using the scale alongside context questions and objective measurement of practices is recommended to inform interventions to improve antibiotic use.
Introduction
Antimicrobial resistance (AMR) has been signalled as one of the biggest threats to global health.1 Despite its importance, there is concern that awareness of AMR is low.2 The first objective of the World Health Organization’s Global Action Plan (GAP) on AMR, which operates as a blueprint for national action plans (NAPs) around the world, is to raise awareness about AMR among healthcare professionals (HCPs) and the public.1 Numerous local, national and international campaigns have been undertaken to raise awareness of AMR among prescribers and consumers—primarily in humans but also in veterinary medicine.2–8
Previously, a range of knowledge, attitudes and practices surveys have been used to assess AMR awareness among the public and HCPs—including veterinarians—worldwide.2,9–18 One challenge for these studies, however, is to create generalizable definitions of what people should know, think and do in relation to the complex topic of AMR. Among HCPs commonalities can be assumed, given medical and veterinary education include standard information on biological mechanisms and antimicrobials, however, variation in curricula and expectations is inevitable across specialities, places and over time.19 The relevance of different forms of microbial resistance also varies; for example, malaria, tuberculosis, HIV, typhoid and colibacillosis in animals, each has different epidemiological profiles as well as different mechanisms for developing drug resistance. AMR awareness and how to respond to it is also known to emerge through healthcare practice as much as through curriculum learning.20 While apparently simple questions such as ‘have you heard of antimicrobial resistance?’ may represent awareness of an abstract concept, capturing awareness of AMR as it bears relevance and meaning to a health practitioner requires more detailed and carefully designed questioning.
Psychometricians have established techniques for developing and validating scales that can be used to measure complex concepts.21–24 Such instruments intend to provide an ‘objective measurement of the skills, knowledge and abilities, as well as the subjective measurement of individuals’ interests, values and attitudes’.25 The process of development and validation of these measurement instruments entails three phases: item development (domains are identified and items generated), scale development (constructs are identified, questions are pretested and factors extracted) and scale evaluation (dimensionality, validity and reliability tests are performed).26
To aid the design and evaluation of AMR awareness-raising efforts, we developed a scale to quantitatively measure awareness of antibiotic resistance (ABR) among trained HCPs from low-and middle-income countries (LMICs) and upper-middle countries (UMICs). The questionnaires targeted both human and animal HCPs in recognition of AMR as a One Health problem.27 Our focus on LMICs and UMICs recognizes that manifestations of AMR and means to address it may differ from higher resource settings, with implications for what constitutes ‘awareness’, yet these data are urgently needed to inform the implementation of NAPs.
Methods
Study design
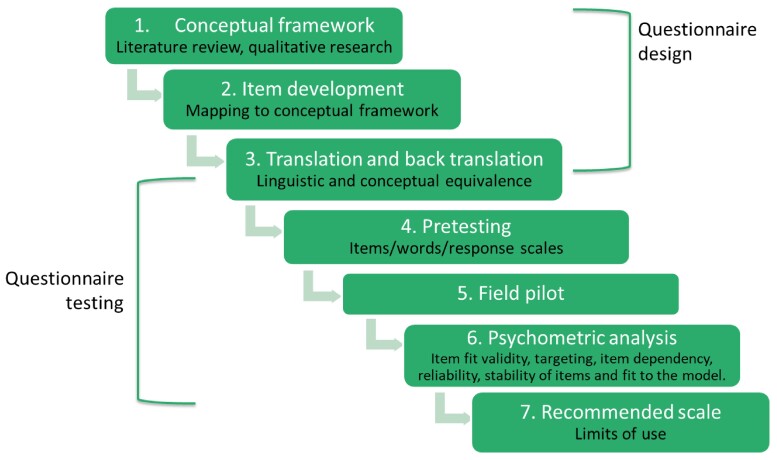
This was a multi-step multi-country study involving the design, testing and analysis of a scale to assess human and animal HCPs’ awareness of ABR (Figure 1). The first step involved qualitative research with human and animal HCPs across a range of settings in six LMICs to develop a conceptual framework, described elsewhere.20,28 In the questionnaire testing phase, items were then taken to six countries for translation, pretesting and piloting. In-country validation was done by local experts of the target population in each location. This allowed the adaptation of the wording and format of items and the assessment of the content validity and accuracy of translation of the tools to the different field sites. The sample size required for the survey response analysis was estimated to be 385 HCPs for each health sector (95% CI, 5% error, response distribution of 50%). Psychometric analysis was then carried out by two psychometricians, and the resulting scale examined for patterns of demographic and contextual factors that may have shaped ABR awareness scores of HCPs in each country. Data were collected between May and September 2018. Additional details on methods can be found in Table S1 (available as Supplementary data atJACOnline).
Figure 1.
Design and testing the HCP ABR awareness questionnaire. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Participants
Countries selected by building upon existing collaborations with local host institutions and aiming to represent different continents—were Ghana, Nigeria and Tanzania from West and East Africa, Vietnam and Thailand from Southeast Asia, and Peru from South America. From each country, human HCPs (HHCPs), animal HCPs (AHCPs) or both were invited to participate in the study and were eligible to participate if they were registered and/or licensed to prescribe or dispense antibiotics. Participants were mainly recruited from the capital cities of each country where the density of HCPs was highest. Printed, email or phone invitations to participate in the study were circulated at hospitals, private clinics, professional bodies and postgraduate programmes. Participation was confidential. Detailed information about the recruitment process in each country is included in the Supplementary material, p. 3.
Questionnaires and data collection
The questionnaires comprised four modules: demographics, ABR awareness; practice items and context items. ABR awareness-related items used a four-point Likert scale (‘strongly agree’, ‘agree’, ‘disagree’ and ‘strongly disagree’). Demographic, practice and contextual variables were multiple choice, Likert or binary formats (Table S1). The practice module was included to capture self-reported prescribing/dispensing practices, and the context module was included to situate the findings within the work settings of respondents.
Questionnaires were forward–back translated from English to local languages (including Swahili, Thai, Vietnamese and Spanish). Questionnaires for self-completion were delivered by email, including a link to the online version (with an Open Data Kit (ODK) platform) or in a paper printed version. The team manually entered completed paper-based questionnaires into the online ODK platform. Datasets were stored in a password-protected local server at the London School of Hygiene and Tropical Medicine (LSHTM) using FilR software.
Data analysis
Data were analysed using quantitative methodologies on SPSS software v.23.0 (IBM Corp., USA).
ABR awareness scale and scores
The ABR awareness module of the questionnaires was validated by applying psychometric techniques based on Rasch Measurement Theory.29 Analysis was performed separately for the human and AHCPs questionnaires, initially for separate countries and then combined across countries. Following psychometric evaluation, items were scored using the estimates from the Rasch model. Scores were produced in a standardized range of 0 to 100.30
Demographic and contextual factors
A descriptive analysis of demographic and context variables was carried out. The structure of the final version of the questionnaires included in the analysis comprised 58 items (Table S2). ABR awareness scores were calculated and compared to participants’ demographics and previous training on ABR, where scores were used as the main outcome to analyse any possible relationships with independent variables. Bivariate analysis included t-tests, one-way ANOVA with Tukey post hoc analysis or with Mann–Whitney U-test or Kruskal–Wallis depending on data distribution, at a significance level of 0.05.
A set of variables assessing prescription practices was initially included in the questionnaire. However, most questions were removed from the final analysis because these items performed poorly in relation to the qualitative research, which suggested a normative bias in respondent reporting. The items included in the final version of the questionnaires and analysed here are described in Supplementary Material, Table S3.
Ethical considerations
Consent was requested from participants either on paper or by including the consent form, designed in local language, as the first page of the online questionnaires. To encourage participation incentives were used in Peru and Vietnam, where participants could enter a prize draw for medical books or a book voucher, respectively, on survey completion through an anonymized link. Ethical approval was granted by the Ethics Committee of the London School of Hygiene and Tropical Medicine (United Kingdom) individually for each study site, reference numbers: 15167 (Ghana), 15053 (Nigeria), 15258 (Peru), 15105 (Tanzania), 15481 (Thailand) and 15255 (Vietnam), and by local ethics committees. Additional information on the local ethical approval is included in the Supplementary material p. 4.
Results
In total, 1091 participants (726 HHCPs and 365 AHCPs) agreed to participate in the study and filled in the questionnaires (Table 1). Response rates varied across countries from 12% to 88% depending on the country tested, health sector and survey delivery format. For AHCPs the sample size fell short by 20 participants. Only 941 (86.3%) respondents (625 HHCPs and 316 AHCPs) were included in the final analysis as a requirement to score the questionnaires was that participants did not have missing values in any of the 23 items related to the ABR awareness scale.30
Table 1.
Field sites and type of HCPs and total number of participants surveyed for every country
| Region | West Africa | East Africa | South America | Southeast Asia | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Country | Ghana | Nigeria | Tanzania | Peru | Vietnam | Thailand | ||||||||
| Site | Volta Region | Abuja | Arusha | Limaa | Ho Chi Minh | Bangkok | ||||||||
| Survey language | English | English | English and Kiswahili | Spanish | Vietnamese | Thai | ||||||||
| Delivery format | Paper | Paper | Paper | Online | Paper/Online | Online | ||||||||
| Number of participantsb | 106 H | 112 H | 164 H | 91 H/139 A | 253 H/183 A | 43 A | ||||||||
| Human health care professionalsc/(N/%) | ||||||||||||||
| Doctor | 48 | (46.2) | 55 | (64.7) | 32 | (25.4) | 53 | (63.1) | 73 | (32.3) | 261 | (41.8) | ||
| Dentist | 2 | (1.9) | 8 | (9.4) | 8 | (6.3) | 30 | (35.7) | 48 | (7.7) | ||||
| Nurse | 21 | (20.2) | 56 | (44.4) | 53 | (23.5) | 130 | (20.8) | ||||||
| Pharmacist | 15 | (14.4) | 21 | (24.7) | 14 | (11.1) | 62 | (27.4) | 112 | (17.9) | ||||
| Physician Assistant | 18 | (17.3) | 38 | (16.8) | 56 | (9.0) | ||||||||
| Medical/Clinical Officer | 16 | (12.7) | 16 | (2.6) | ||||||||||
| No answer | 1 | (1.2) | 1 | (1.2) | 2 | (0.3) | ||||||||
| Animal health care professionalsc/(N/%) | ||||||||||||||
| Veterinarian | 119 | (100) | 69 | (44.2) | 41 | (100) | 229 | (72.5) | ||||||
| Veterinary Drug seller | 18 | (11.5) | 18 | (5.7) | ||||||||||
| Veterinarian and drug seller | 64 | (41.0) | 64 | (20.3) | ||||||||||
| No answer | 5 | (3.2) | 5 | (1.5) | ||||||||||
Invitations to participate were sent to local participants, however, a snowballing technique was also used and participants from other parts of Thailand and Peru were included in the study.
Included ALL participants.
Only included participants with no missing values in the 23-item scale and for whom scores were calculated. H: HHCPs. A: AHCPs.
Performance of the HCP ABR awareness scale
The ABR awareness module of the questionnaires was reduced in dimension to 23 items, under four domains of ABR knowledge: mechanisms of ABR; antibiotic use (ABU) as a driver of resistance; transmission and infection control of ABR and detecting and recognizing ABR. The 23-items of the scale were those identified as contributing to the construct of ABR awareness whereas the items removed were those more closely related to practices. The domains identified as relevant for ABR awareness were the same for both versions of the questionnaire. Further details of the item reduction—including item fit validity, targeting, item dependency, reliability and stability of items—are described in detail in a separate report.30
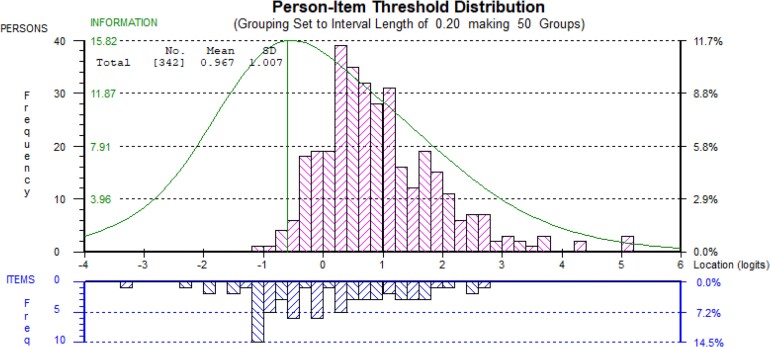
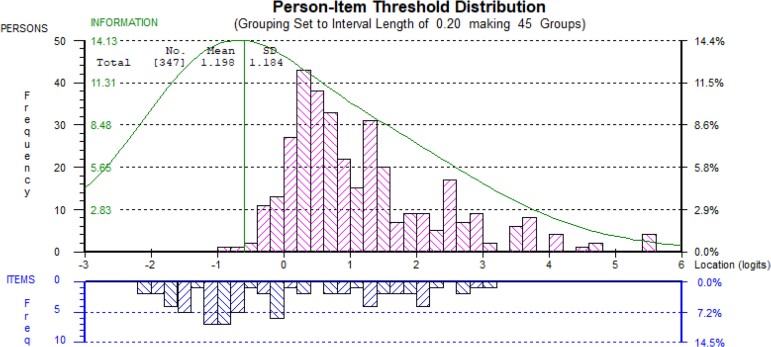
The Rasch analysis to assess and validate the scale was performed on each country’s data. Because not all items worked well in the scale across different languages and settings, questionnaires were validated in English language for HHCPs and in Spanish, Thai and Vietnamese language for AHCPs. The most coherent analysis included questionnaires administered in English language over 342 HHCPs from Ghana (n = 106), Nigeria (n = 112) and Tanzania (n = 124), and in Spanish, Thai and Vietnamese language over 348 AHCPs from Peru (n = 122), Thailand (n = 43) and Vietnam (n = 183), respectively. It was not possible to combine the HHCPs and AHCPs data and maintain a coherent analysis (Figures 2 and 3).
Figure 2.
Targeting of human HCP scale (23 items). This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Figure 3.
Targeting of animal HCP scale (23 items). This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Data from neither questionnaire, was a good fit to the Rasch model (P < 0.001) based on six and five class intervals. However, the reliability coefficients were high (Person Separation Index of 0.88 for HHCPs and 0.90 for AHCPs).30 Questionnaires were recommended to be used for analysis within rather than across countries and in the languages tested until further validation is undertaken. Versions of the developed scale can be found in English, Spanish, Vietnamese and Thai languages in the Supplementary material (Tables S4.1–S4.4). The output of the Rasch analysis was a scale that was used to calculate participants’ scores (Table S5). Items in the questionnaires were located on the scale from easiest to hardest (Tables S6 and S7).
Awareness scores
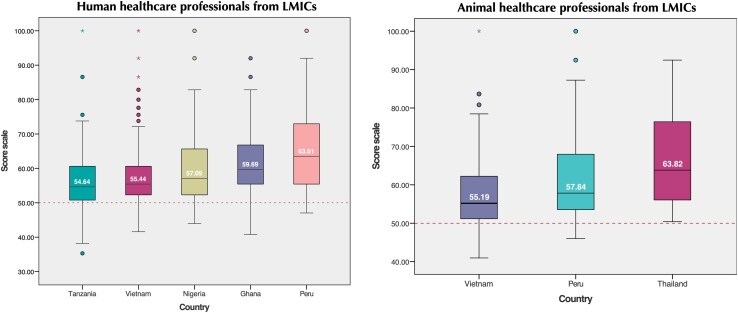
Results on awareness scores for professionals in each country are presented in Table 2 and distribution of scores in Figure 4. This figure should be considered as within-country scores that can form, for example, a baseline for subsequent surveys rather than interpreted comparatively across countries. Median scores ranged from 54.6 (IQR = 9.8) Tanzanian to 63.5 (IQR = 17.9) Peruvian HHCPs and 55.4 (IQR = 8.3) Vietnamese to 63.8 (IQR = 20.4) Thai AHCPs.
Table 2.
Descriptive statistics for participants scores by country
| Country | Type of HCP | Valid respondentsa | Scores mean | Scores SE | Scores median | IQR | Minimum score | Maximum score | 95% CI | |
|---|---|---|---|---|---|---|---|---|---|---|
| Lower bound | Upper bound | |||||||||
| Ghana | Human | 104 | 61.2 | 0.92 | 59.7 | 11.4 | 40.77 | 92.01 | 59.39 | 63.05 |
| Nigeria | Human | 85 | 59.7 | 1.15 | 57.1 | 13.4 | 43.97 | 100 | 57.40 | 61.96 |
| Tanzania | Human | 126 | 56.6 | 0.88 | 54.6 | 9.8 | 35.29 | 100 | 54.80 | 58.29 |
| Vietnam | Human | 226 | 57.5 | 0.67 | 55.4 | 8.3 | 41.59 | 100 | 56.18 | 58.83 |
| Animal | 156 | 58.2 | 0.84 | 55.2 | 11.3 | 40.95 | 100 | 56.58 | 59.91 | |
| Thailand | Animal | 41 | 66.1 | 1.85 | 63.8 | 20.4 | 50.4 | 92.47 | 62.37 | 69.85 |
| Peru | Human | 84 | 65.6 | 1.33 | 63.5 | 17.9 | 47.03 | 100 | 62.99 | 68.26 |
| Animal | 119 | 61.2 | 0.94 | 57.8 | 15.1 | 46 | 100 | 59.30 | 63.04 | |
Statistics based on participants with 23 valid answers in the ABR awareness scale. IQR = interquartile range.
Note that while the countries are positioned side by side, it is important to note that scores should be interpreted within-country rather than compared across countries, for example as baseline data within a given country.
Figure 4.
Distribution of scores in the ABR awareness scale by country for HHCPs (left) and AHCPs (right). Due to the presence of outliers, the median is used here as the main statistics reported. Note that while the countries are positioned side by side, it is important to note that scores should be interpreted within-country rather than compared across countries, for example as baseline data within a given country. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
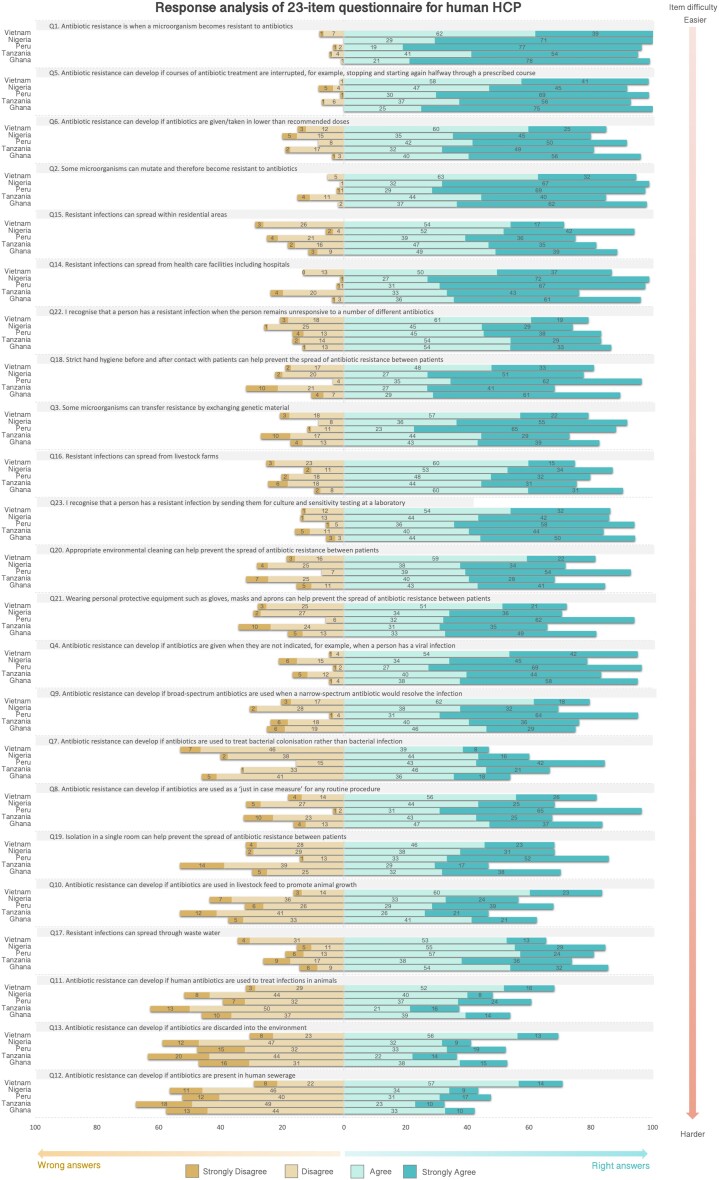
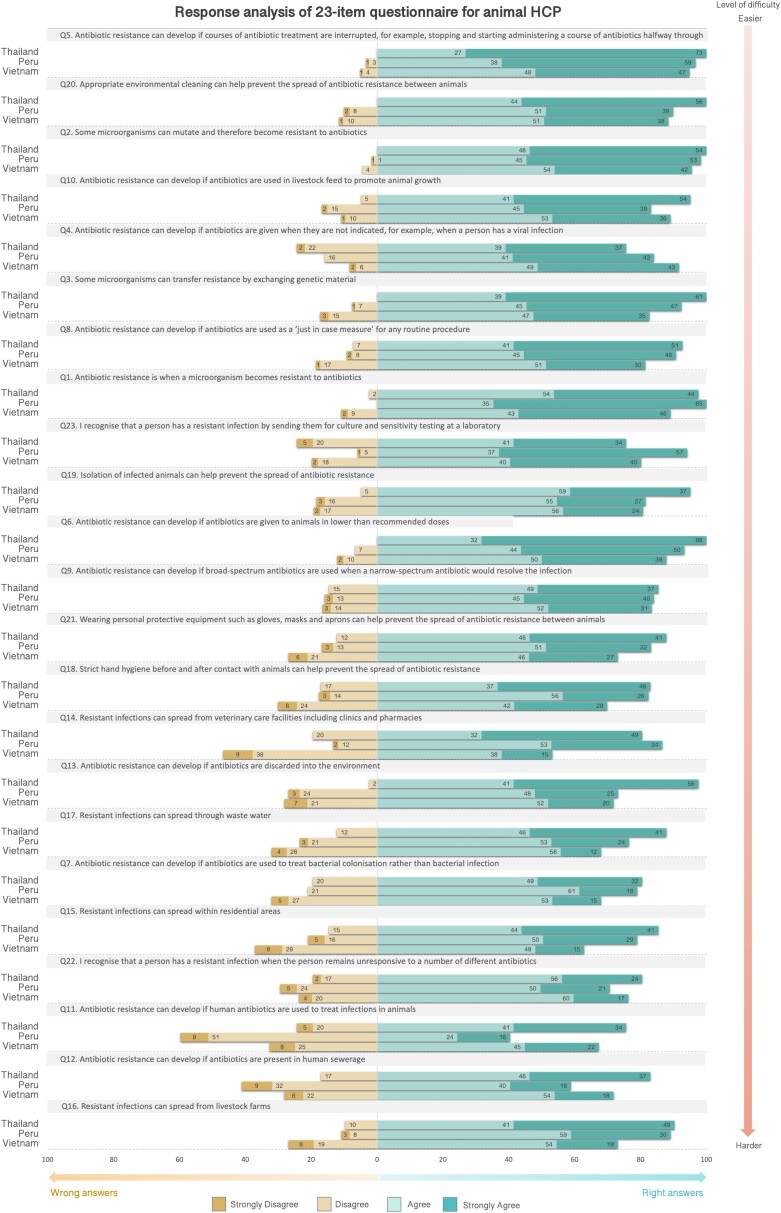
Participants’ responses to each item of the ABR awareness module mirrored the level of difficulty of the conceptual framework for both HHCPs and AHCPs. Items classified as easier by the Rasch model were those more commonly correctly answered, whereas those classified as more difficult showed more answer variations. The same trends were seen for all countries: however, small variations were observed between health sectors. Figure 5 (HHCPs) and Figure 6 (AHCPs) show the distribution of answers for each item of the ABR awareness module by country. For HHCPs, item 17 (spread of ABR through wastewater) was one of the most difficult items (position 20 in the scale) according to the Rasch model, however, the percentage of wrong answers for this item was smaller compared with other items in the scale around the same location. In Vietnam, item 7 (use of antibiotics to treat bacterial colonization) seemed to be the most difficult one (position 16 in the scale), in Nigeria item 13 (development of ABR if antibiotics are discarded into the environment) (position 22 in the scale), and in Peru, Ghana and Tanzania item 12 (development of ABR if antibiotics were present in human sewerage), as expected according to the prediction model.
Figure 5.
Response analysis of ABR awareness module used to calculate scores (23-item) for HHCPs. Items are ordered by level of difficulty, from easier to harder (vertical arrow on the right). Every bar in the graph represents the percentages of participant responses to every item in the Likert scale. To the right, there are the percentages of participants that ‘strongly agree’ or ‘agree’ with the items, and to the left, the percentages of participants that ‘strongly disagree’ and ‘disagree’ with the items. S1A (Mechanisms of ABR), S1B (ABU as a driver of ABR), S1C (Transmission and control of ABR infections) and S1D (Detecting/recognizing ABR) are the codes of the location of the items in the conceptual framework. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Figure 6.
Response analysis of ABR awareness module used to calculate scores (23-item) for AHCPs. Items are ordered by level of difficulty, from easier to harder (vertical arrow on the right). Every bar in the graph represents the percentages of participant responses to every item in the Likert scale. To the right, there are the percentages of participants that ‘strongly agree’ or ‘agree’ with the items, and to the left, the percentages of participants that ‘strongly disagree’ and ‘disagree’ with the items. S1A (Mechanisms of ABR), S1B (ABU as a driver of ABR), S1C (Transmission and control of ABR infections) and S1D (Detecting/recognizing ABR) are the codes of the location of the items in the conceptual framework. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Participants’ background and awareness scores
The analysis of demographic variables and ABR awareness scores showed statistically significant associations between scores and profession, training level and type of practice for HHCPs in some countries. No associations were seen between scores and gender or age (Table 3). For AHCPs, no associations were seen between scores and demographic variables, except by type of practice in Vietnam where those working in public practices scored statistically higher (P = 0.002) than those working in private practices (65.8 versus 58.41) (Table 4).
Table 3.
Demographic characteristics relationships with ABR awareness scores for human HCPsd
| Variable | Description | Ghana (n = 104) | Nigeria (n = 85) | Tanzania (n = 126) | Peru (n = 84) | Vietnam (n = 226) | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | Mean | SE | CI 95% | P value | N (%) | Mean | SE | CI 95% | P value | N (%) | Mean | SE | CI 95% | P value | N (%) | Mean | SE | CI 95% | P value | N (%) | Mean | SE | CI 95% | P value | |||||||
| Lower | Upper | Lower | Upper | Lower | Upper | Lower | Upper | Lower | Upper | ||||||||||||||||||||||
| Profession | Doctor | 48 (46.2) | 59.7b | 1.1 | 57.6 | 61.9 | 0.004c | 55 (64.7) | 59.27 | 1.4 | 56.5 | 62.0 | 0.156 | 32 (25.4) | 60.4a | 2.1 | 56.2 | 64.7 | 0.003c | 53 (63.1) | 70.4a | 1.6 | 67.2 | 73.7 | 0.000c | 73 (32.3) | 57.9 | 1.1 | 55.7 | 60.2 | 0.912 |
| Dentist | 2 (1.9) | 71.4 | 20.6 | 57.2 | 92.0 | 8 (9.4) | 54.04 | 3.0 | 47.0 | 61.1 | 8 (6.3) | 54.1 | 2.7 | 47.7 | 60.5 | 30 (35.7) | 57.5b | 1.4 | 54.6 | 60.4 | — | — | — | — | |||||||
| Nurse | 21 (20.2) | 60.7 | 1.8 | 56.8 | 64.5 | — | — | — | — | — | 56 (44.4) | 54.7 | 0.9 | 52.9 | 56.4 | — | — | — | — | — | 53 (23.5) | 57.1 | 1.5 | 54.1 | 60.0 | ||||||
| Pharmacist | 15 (14.4) | 68.7a | 2.9 | 62.4 | 75.0 | 21 (24.7) | 62.37 | 2.6 | 57.0 | 67.8 | 14 (11.1) | 61.9a | 3.9 | 53.6 | 70.3 | — | — | — | — | — | 62 (27.4) | 56.9 | 1.3 | 54.4 | 59.6 | ||||||
| Physician Assistant | 18 (17.3) | 58.5b | 2.0 | 54.2 | 62.7 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 38 (16.8) | 58.0 | 1.7 | 54.7 | 61.4 | ||||||
| Medical Officer | — | — | — | — | — | — | — | — | — | — | 16 (12.7) | 51.9b | 1.9 | 47.9 | 56.0 | — | — | — | — | — | — | — | — | — | — | ||||||
| Gender | Male | 66 (63.5) | 61.5 | 1.1 | 59.4 | 63.6 | 0.809 | 45 (52.9) | 61.42 | 1.7 | 57.9 | 64.9 | 0.106 | 57 (45.2) | 57.7 | 1.4 | 54.9 | 60.5 | 0.223 | 42 (50.0) | 68.2 | 1.9 | 64.3 | 72.0 | 0.055 | 73 (32.3) | 57.1 | 1.0 | 55.2 | 59.1 | 0.701 |
| Female | 37 (35.6) | 60.8 | 1.8 | 57.1 | 64.4 | 40 (47.1) | 57.71 | 1.4 | 54.9 | 60.5 | 69 (54.8) | 55.6 | 1.1 | 53.3 | 57.8 | 42 (50.0) | 63.1 | 1.8 | 59.5 | 66.7 | 153 (67.7) | 57.7 | 0.9 | 55.9 | 59.4 | ||||||
| Age | 18 to 25 | 10 (9.6) | 62.2 | 2.7 | 56.0 | 68.3 | 0.329 | 8 (9.4) | 57.28 | 2.4 | 51.7 | 62.9 | 0.711 | 16 (12.7) | 55.9 | 2.1 | 51.6 | 60.3 | 0.364 | 14 (16.7) | 61.4 | 1.9 | 57.2 | 65.6 | 0.067 | 16 (7.1) | 61.2 | 2.5 | 55.9 | 66.4 | 0.119 |
| 26 to 35 | 52 (50.0) | 62.3 | 1.3 | 59.7 | 65.0 | 52 (61.2) | 59.92 | 1.6 | 56.7 | 63.2 | 53 (42.1) | 58.6 | 1.6 | 55.4 | 61.9 | 39 (46.4) | 66.3 | 2.1 | 62.1 | 70.5 | 93 (41.2) | 57.3 | 1.1 | 55.1 | 59.5 | ||||||
| 36 to 45 | 16 (15.4) | 61.4 | 2.6 | 55.9 | 66.9 | 18 (21.2) | 61.22 | 2.3 | 56.3 | 66.1 | 27 (21.4) | 54.9 | 1.6 | 51.7 | 58.3 | 22 (26.2) | 63.5 | 2.4 | 58.5 | 68.5 | 43 (19) | 55.7 | 1.4 | 52.9 | 58.4 | ||||||
| 46 to 55 | 13 (12.5) | 56.4 | 2.7 | 50.6 | 62.2 | 7 (8.2) | 56.64 | 2.7 | 50.0 | 63.3 | 12 (9.5) | 53.3 | 1.6 | 49.8 | 56.7 | 3 (3.6) | 67.7 | 7.0 | 37.8 | 97.7 | 52 (23) | 57.7 | 1.3 | 55.2 | 60.2 | ||||||
| > 55 | 9 (8.7) | 58.9 | 2.9 | 52.3 | 65.7 | — | — | — | — | — | 10 (7.9) | 56.8 | 2.8 | 50.4 | 63.1 | 6 (7.1) | 77.8 | 5.7 | 63.1 | 92.6 | 5 (2.2) | 66.1 | 5.6 | 50.7 | 81.6 | ||||||
| Training level | Non-University | — | — | — | — | — | NC | — | — | — | — | — | 0.741 | 72 (57.1) | 54.1b | 1.1 | 52.0 | 56.2 | 0.001c | — | — | — | — | — | 0.776 | 95 (42) | 58.3 | 1.2 | 55.8 | 60.7 | 0.588 |
| University | 104 (100) | 61.2 | 0.9 | 59.4 | 63.0 | 29 (34.1) | 60.07 | 2.1 | 55.7 | 64.5 | 47 (37.3) | 60.1a | 1.5 | 57.1 | 63.2 | 38 (45.2) | 66.4 | 2.1 | 62.2 | 70.5 | 107 (47.3) | 56.8 | 0.9 | 55.1 | 58.5 | ||||||
| Specialist | — | — | — | — | — | 55 (64.7) | 59.27 | 1.4 | 56.5 | 62.0 | — | — | — | — | — | 21 (25) | 65.3 | 3.0 | 59.0 | 71.7 | — | — | — | — | — | ||||||
| Postgraduate (MSc, PhD) | — | — | — | — | — | — | — | — | — | — | 1 (0.8) | 70.7 | — | — | — | 21 (25) | 63.9 | 2.1 | 59.5 | 68.5 | 18 (8) | 57.7 | 1.8 | 54.0 | 61.5 | ||||||
| Type of caree | Primary Care | 32 (30.8) | 59.4 | 1.33 | 56.7 | 62.1 | 0.05 | — | — | — | — | — | NC | 33 (26.2) | 55.9 | 2.07 | 51.7 | 60.1 | 0.95 | 41 (48.8) | 64.2b | 1.73 | 60.7 | 67.7 | 0.01c | 141 (62.4) | 57.8 | 0.9 | 56 | 59.6 | 0.65 |
| Secondary Care | 54 (51.9) | 63.3 | 1.47 | 60.4 | 66.3 | — | — | — | — | — | 57 (45.2) | 56.5 | 1.16 | 54.2 | 58.8 | 19 (22.6) | 61.1b | 2.69 | 55.4 | 66.7 | 83 (36.7) | 57.2 | 0.99 | 55.2 | 59.1 | ||||||
| Tertiary Care | 18 (17.3) | 58.1 | 1.44 | 55 | 61.1 | 85 (100) | 59.7 | 1.14 | 57.4 | 62 | 14 (11.1) | 56.7 | 2.54 | 51.2 | 62.1 | 20 (23.8) | 72.5a | 2.96 | 66.3 | 78.7 | — | — | — | — | — | ||||||
| Type of Practice | Public practice | 78 (75) | 60.5 | 1.09 | 58.3 | 62.7 | 0.24 | 85 (100) | 59.7 | 1.14 | 57.4 | 62 | NC | 79 (62.7) | 55.7 | 0.84 | 54.1 | 57.4 | 0.09 | 31 (36.9) | 70.9a | 2.45 | 66 | 76 | 0.01c | 191 (84.5) | 57.5 | 0.77 | 56 | 59 | 0.98 |
| Private practice | 11 (10.6) | 61.2 | 1.93 | 56.9 | 65.5 | — | — | — | — | — | 32 (25.4) | 56.7 | 1.76 | 53.2 | 60.3 | 35 (41.7) | 61.6b | 1.73 | 58.1 | 65.1 | 20 (8.8) | 57.9 | 0.86 | 56.1 | 59.7 | ||||||
| Public and Private practice | 15 (14.4) | 65 | 2.46 | 59.7 | 70.3 | — | — | — | — | — | 12 (9.5) | 62.4 | 5.48 | 50.4 | 74.5 | 13 (15.5) | 63.8 | 2.85 | 57.6 | 70 | 12 (5.3) | 57.9 | 2.71 | 51.9 | 63.8 | ||||||
SE: Standard error, CI: Confidence interval, NC: Not calculated.
Different letters in different rows indicate statistical differences between groups.
Statistical differences (P < 0.05). All P values were calculated with ANOVA and Tukey post hoc comparison test. All significant values are two-tailed.
Includes only participants without missing values in the 23-item scale who replied to the question, therefore differences in numbers of participants across calculations may vary accordingly.
18 participants from Tanzania did not indicate the level of care of their working centres.
Note that while the countries are positioned side by side, it is important to note that scores should be interpreted within-country rather than compared across countries, for example as baseline data within a given country.
Table 4.
Demographic characteristics relationships with ABR awareness scores for AHCPsd
| Variable | Description | Thailand (n = 41) | Peru (n = 119) | Vietnam (n = 156) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | Score mean | SE | CI 95% | P value | N (%) | Score mean | SE | CI 95% | P value | N (%) | Score mean | SE | CI 95% | P value | |||||
| Lower | Upper | Lower | Upper | Lower | Upper | ||||||||||||||
| Profession | Veterinarian | 41 (100) | 66.4 | 1.9 | 62.6 | 70.2 | NC | 119 (100) | 61.2 | 1.0 | 59.3 | 63.1 | NC | 69 (44.2) | 60.2 | 1.4 | 57.5 | 63.0 | 0.077 |
| Veterinary drug seller | — | — | — | — | — | — | — | — | — | — | 18 (11.5) | 54.3 | 1.6 | 51.0 | 57.6 | ||||
| Veterinarian and drug seller | — | — | — | — | — | — | — | — | — | — | 64 (41.0) | 57.5 | 1.3 | 54.9 | 60.1 | ||||
| Gender | Male | 18 (43.9) | 66.8 | 2.3 | 61.8 | 71.7 | 0.53 | 65 (54.6) | 60.7 | 1.1 | 58.5 | 62.9 | 0.534 | 115 (73.7) | 58.7 | 1.0 | 56.6 | 60.7 | 0.409 |
| Female | 2 (48.8) | 67.0 | 3.1 | 60.6 | 73.5 | 53 (44.5) | 61.9 | 1.6 | 58.6 | 65.1 | 41 (26.3) | 57.1 | 1.4 | 54.2 | 60.0 | ||||
| Age | 18 to 25 | 3 (7.3) | 70.5 | 6.7 | 41.7 | 99.3 | 0.42 | 1 (0.8) | 76.4 | . | . | . | 0.126 | 4 (2.6) | 53.8 | 2.8 | 44.9 | 62.7 | 0.265 |
| 26 to 35 | 16 (39.0) | 67.3 | 3.1 | 60.7 | 73.8 | 43 (36.1) | 62.8 | 1.4 | 59.9 | 65.6 | 27 (17.3) | 60.2 | 2.4 | 55.3 | 65.1 | ||||
| 36 to 45 | 14 (34.1) | 67.4 | 3.4 | 60.1 | 74.7 | 37 (31.1) | 61.8 | 2.1 | 57.6 | 65.9 | 54 (34.6) | 58.0 | 1.4 | 55.2 | 60.8 | ||||
| 46 to 55 | 7 (17.1) | 58.2 | 3.3 | 50.3 | 66.2 | 21 (17.6) | 60.1 | 1.7 | 56.6 | 63.7 | 47 (30.1) | 57.3 | 1.5 | 54.2 | 60.4 | ||||
| > 55 | 1 (2.4) | 71.4 | — | — | — | 16 (13.4) | 56.3 | 2.3 | 51.3 | 61.2 | 14 (9.0) | 63.5 | 2.8 | 57.4 | 69.6 | ||||
| Training level | Non-University | ND | — | — | — | — | NC | 0.619 | 66 (42.3) | 56.3 | 1.2 | 53.9 | 58.6 | 0.071 | |||||
| University | ND | — | — | — | — | 48 (40.3) | 60.9 | 1.3 | 58.3 | 63.5 | 79 (50.6) | 59.3 | 1.3 | 56.8 | 61.8 | ||||
| Specialist | ND | — | — | — | — | 28 (23.5) | 60.5 | 2.0 | 56.3 | 64.6 | — | — | — | — | — | ||||
| Postgraduate (MSc, PhD) | ND | — | — | — | — | 40 (33.6) | 62.7 | 1.8 | 59.0 | 66.4 | 8 (5.1) | 63.9 | 3.7 | 55.2 | 72.7 | ||||
| Year prescribing/dispensinge | 0 a 5 | 14 (34.1) | 66.2 | 3.3 | 59.1 | 73.4 | 0.938 | 41 (34.5) | 61.9 | 1.5 | 58.9 | 65.0 | 0.801 | 10 (6.4) | 55.5 | 3.5 | 47.6 | 63.4 | 0.405 |
| 6 a 10 | 8 (19.5) | 67.5 | 3.9 | 58.3 | 76.6 | 33 (27.7) | 62.3 | 2.2 | 57.7 | 66.8 | 20 (12.8) | 58.3 | 1.8 | 54.4 | 62.1 | ||||
| 11 a 20 | 14 (34.1) | 66.1 | 3.6 | 58.4 | 73.9 | 24 (20.2) | 60.4 | 1.5 | 57.2 | 63.6 | 27 (17.3) | 60.0 | 2.1 | 55.6 | 64.3 | ||||
| 21 a 30 | 4 (9.8) | 61.7 | 5.1 | 45.4 | 78.0 | 7 (5.9) | 56.3 | 3.7 | 47.2 | 65.3 | 24 (15.4) | 55.8 | 1.6 | 52.5 | 59.1 | ||||
| 31 a 40 | 1 (2.4) | 71.4 | — | — | — | 3 (2.5) | 62.7 | 9.7 | 20.7 | 104.6 | 2 (1.3) | 51.4 | 4.7 | −7.9 | 110.7 | ||||
| 40+ | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | ||||
| Type of Practice | Public practice | 12 (29.3) | 72.1 | 3.9 | 63.6 | 80.6 | 0.07 | 20 (16.8) | 64.7 | 2.6 | 59.3 | 70.0 | 0.233 | 29 (18.6) | 64.12a | 2.4 | 59.3 | 69.0 | 0.002c |
| Private practice | 21 (51.2) | 62.4 | 2.2 | 57.7 | 67.0 | 63 (52.9) | 60.1 | 1.2 | 57.8 | 62.5 | 89 (57.1) | 56.27b | 1.0 | 54.3 | 58.2 | ||||
| Public and Private practice | 8 (19.5) | 67.0 | 3.9 | 57.9 | 76.1 | 35 (29.4) | 61.2 | 1.9 | 57.4 | 65.1 | 36 (23.1) | 58.5 | 1.7 | 55.0 | 62.0 | ||||
SE: Standard error, CI: Confidence interval, NR: Not relevant, NC: Not calculated.
Different letters in different rows indicate statistical differences between groups.
Statistical differences (P < 0.05). All P values were calculated with ANOVA with Tukey post hoc comparison test. All significant values are two-tailed.
Includes only participants without missing values in the 23-item scale who replied to the question; therefore, differences in the number of participants across calculations may vary accordingly.
11 and 73 participants from Peru and Vietnam, respectively, did not indicate the number of years prescribing/dispensing.
Note that while the countries are positioned side by side, it is important to note that scores should be interpreted within-country rather than compared across countries, for example, as baseline data within a given country.
Profession was statistically significantly associated with scores for Ghanaian (pharmacists 68.71 versus physicians 59.73; P = 0.004), Tanzania (physicians 60.43 versus pharmacists 61.93; P = 0.003) and Peruvian HHCPs (physicians 70.42 versus dentists 57.48; P < 0.001). Likewise, Tanzanian HHCPs with university degrees scored higher (60.12 versus 54.09, respectively; P = 0.002) than those trained at non-university institutions. Furthermore, Peruvian HHCPs working in tertiary care hospitals (72.5) or in public hospitals (70.9) had statistically significantly higher scores (P = 0.01) than those in primary (64.2) or secondary care (61.1) and in private practice (61.6).
Training on ABR
Overall, 34.7% of HHCPs said they had attended specific training on AMR or AMS (30.8% Ghana, 35.3% Nigeria, 42.9% Peru, 25.4% Tanzania and 38.5% Vietnam). In Peru and Vietnam, those who indicated that they were taught about ABR as part of their curricula had statistically significantly higher scores (P < 0.001 for HHCPs and P = 0.043 for AHCPs, respectively) than those who did not (Table 5). Among AHCPs, fewer received specific ABR training in Peru (25.2%) than in Thailand (78.0%) or Vietnam (55.1%) (Table 6).
Table 5.
Relationships between previous ABR training for HHCPs and awareness scores by countryb
| Items | Ghana | Nigeria | Peru | Tanzania | Vietnam | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | Score mean | P value | N | % | Score mean | P value | N | % | Score mean | P value | N | % | Score mean | P value | N | % | Score mean | P value | ||
| I was taught everything I needed to know about Antibiotic Resistance as part of my training curriculum | Agree | 64 | 61.5 | 61.7 | 0.491 | 54 | 63.5 | 61.3 | 0.103 | 46 | 54.8 | 69.4 | 0.001a | 99 | 78.6 | 56.9 | 0.326 | 169 | 74.8 | 57.9 | 0.748 |
| Disagree | 40 | 38.5 | 60.4 | 25 | 29.4 | 57.1 | 38 | 45.2 | 61 | 26 | 20.6 | 54.8 | 56 | 24.8 | 57.9 | ||||||
| The information and training I currently receive on Antibiotic Resistance are adequate for my day-to-day practice | Agree | 67 | 64.4 | 61.8 | 0.377 | 42 | 49.4 | 60.2 | 0.87 | 54 | 64.3 | 68.1 | 0.010a | 92 | 73 | 57.2 | 0.161 | 128 | 56.6 | 57.3 | 0.741 |
| Disagree | 37 | 35.6 | 60.1 | 37 | 43.5 | 59.8 | 30 | 35.7 | 61.1 | 33 | 26.2 | 54.4 | 97 | 42.9 | 57.8 | ||||||
| I have attended specific training on Antibiotic Resistance and/or Antibiotic Stewardship | Yes | 32 | 30.8 | 62 | 0.575 | 30 | 35.3 | 62.3 | 0.135 | 36 | 42.9 | 68.7 | 0.055 | 32 | 25.4 | 57.2 | 0.682 | 87 | 38.5 | 58.2 | 0.452 |
| No | 72 | 69.2 | 60.9 | 49 | 57.6 | 58.6 | 48 | 57.1 | 63.3 | 92 | 73 | 56.3 | 138 | 61.1 | 57.1 | ||||||
Statistical differences (P < 0.05). All significant values are two-tailed.
Includes only participants without missing values in the 23-item scale who replied to the question; therefore, differences in the number of participants across calculations may vary accordingly.
Note that while the countries are positioned side by side, it is important to note that scores should be interpreted within-country rather than compared across countries, for example, as baseline data within a given country.
Table 6.
Relationships between previous ABR training for AHCPs and awareness scores by countryb
| Items | Peru | Thailand | Vietnam | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | Score mean | P value | N | % | Score mean | P value | N | % | Score mean | P value | ||
| I was taught everything I needed to know about Antibiotic Resistance as part of my training curriculum | Agree | 55 | 46.2 | 59.6 | 0.116 | 35 | 85.4 | 66.64 | 0.943 | 122 | 78.2 | 59.06 | 0.043a |
| Disagree | 64 | 53.8 | 62.51 | 4 | 9.8 | 67.1 | 31 | 19.9 | 54.75 | ||||
| The information and training I currently receive on Antibiotic Resistance are adequate for my day-to-day practice | Agree | 46 | 38.7 | 61.78 | 0.475 | 27 | 65.9 | 66.07 | 0.632 | 86 | 55.1 | 58.26 | 0.979 |
| Disagree | 72 | 60.5 | 60.41 | 12 | 29.3 | 68.08 | 69 | 44.2 | 58.22 | ||||
| I have attended specific training on Antibiotic Resistance and/or Antibiotic Stewardship | Yes | 30 | 25.2 | 64 | 0.08 | 32 | 78 | 65.2 | 0.095 | 86 | 55.1 | 59.3 | 0.177 |
| No | 89 | 74.8 | 60.2 | 7 | 17.1 | 73.5 | 69 | 44.2 | 57 | ||||
Statistical differences (P < 0.05). All significant values are two-tailed.
Includes only participants without missing values in the 23-item scale who replied to the question; therefore, differences in the number of participants across calculations may vary accordingly.
Note that while the countries are positioned side by side, it is important to note that scores should be interpreted within-country rather than compared across countries, for example, as baseline data within a given country.
Context of antibiotic practice
Factual context factors such as the presence or absence of particular resources, presence of other diseases or receiving information appeared to provide reliable findings, when compared with the qualitative research carried out alongside the development of the questionnaires. Items that provided useful information were re-grouped together in three sections: ‘prioritization’, ‘resources’ and ‘information’.
AMR prioritization
Across most countries, both HHCPs and AHCPs reported that their medical decisions on prescribing antibiotics were driven by a fear of worse health outcomes (HHCPs: 62.5% Ghana, 75.3% Nigeria, 63.5% Tanzania, 68.1% Vietnam and 39.3% Peru. AHCPs: 68.9% Peru, 58.5% Thailand and 88.5% Vietnam). For example, they were more concerned about the level of hygiene and sanitation than ABR (HHCPs: 60.6% Ghana, 63.5% Nigeria, 60.7% Peru, 48.4% Tanzania and 38.5% Vietnam; AHCPs: 57.1% Peru, 65.9% Thailand and 81.4% Vietnam).
Various disease groups were also considered of higher concern than ABR among HHCPs in Africa. Infectious diseases such as TB, malaria and HIV were frequently considered more important, as well as trauma and accidents. Chronic diseases were considered more important by HHCPs across countries and malnutrition more so in Nigeria than elsewhere. Among AHCPs, more than half were more concerned about chronic diseases in Vietnam, Peru and Thailand. In Vietnam, malnutrition and infectious diseases were also a concern (Table S8).
Resource availability
The ‘context’ module asked HCPs about availability of relevant resources, receipt of information about ABR and potential ABR concerns. Among both HHCPs and AHCPs, many reported that the availability of laboratory services affected their decisions to prescribe/dispense antibiotics. Indeed, those who had nearby facilities where they could send their samples for culture and sensitivity testing tended to score higher in the scale than those without nearby facilities. However, the capacity of facilities to provide culture and sensitivity testing was often considered insufficient, although this varied between countries. Confidence in the equipment and staff availability at laboratories was lower for AHCPs than HHCPs, and lower in Vietnam than other settings. While in most countries over 80% participants did report having access to running water and over 88% had electricity at their workplace, only around two-thirds reported having access to the best antibiotic to treat particular infections at their workplace (Table S9).
Access to information about ABR
Around two-thirds of respondents felt the information received on ABR was not adequate to inform their day-to-day practice, especially among Peruvian AHCPs than elsewhere. In Vietnam and Thailand, access to local data on ABR was reported to be adequate by most respondents. However, in general, most participants felt they had inadequate access to local ABR patterns, with less than one-third reporting having access to them. In most settings, respondents reported that nobody at their workplace was monitoring ABR, except by AHCPs in Thailand. Among those AHCPs who reported workplace ABR monitoring their score was higher than those who do not.
Many respondents were aware of ABR campaigns, which was associated with a slightly higher awareness score among HHCPs but not AHCPs. Most participants across countries were interested in receiving more information about ABR, either online or at work, and indicated a willingness to participate in further training on ABR.
Overall, two-thirds of participants reported being exposed to advertising about antibiotics. Most respondents had sometimes or often received information and samples about antibiotics from medical representatives. Across both sectors, those who had often received samples from medical representatives scored higher in ABR awareness than others, suggesting that this could be a source of information about antibiotics (Table S10).
Discussion
ABR awareness raising is a cornerstone of the WHO GAP and, therefore, of many NAPs. Previous studies have attempted to develop surveys to measure factors associated with ABU among HCPs and the general public,31,32 however, these studies had specific target populations, and a full development and validation process of the tools had not been followed.32 In this study, two ABR awareness questionnaires were developed and validated to provide scores as a measurement of level of ABR awareness among HHCPs and AHCPs.
The development of a validated scale was a complex process that raised the question of whether ABR awareness is a single coherent and measurable construct. The similarities in biomedical training around the world, however, provided a platform for the development of the linear scale. We did not objectively measure HCPs’ prescribing practices and therefore the relationship between practice and awareness remains to be tested, and it is possible that better ABR awareness does not correlate with better antibiotic prescription.33 However, as good ABR awareness levels have been signalled as a prerequisite for the success of behavioural change activities regarding ABU,34,35 it was crucial to design a tool that enables such a measurement. Moreover, context-specific factors could influence antibiotic prescription/dispensing as much as awareness, highlighting the importance of their documentation as part of awareness surveys.
Validation of ABR awareness questionnaires
The developed scale comprises items in four domains of ABR knowledge, and these are similar for both human and AHCPs. This suggests that many of the concepts relevant to ABR awareness are shared by HCPs regardless of their background, and the minor differences might be related to the nature of their patients. Data misfits and challenges with some of the Rasch Measurement Theory criteria were seen during the validation process, although the questionnaires showed to be highly reliable and performed well enough to be used within the countries tested, and scores were recommended to be analysed as groups instead of individuals. Additionally, results obtained from respondents suggest that the level of item difficulty of the conceptual framework of ABR awareness questionnaire fitted well the Rasch model.
Even though the questionnaires were validated to produce ABR awareness scores in this study, it is recommended that, for optimal assessments across countries, these tools undergo further rounds of testing in different scenarios before using them as a globally standardized scale. Additionally, when implementing these versions of the questionnaires, the wording of the items should remain unchanged unless the rewording is validated.
ABR awareness scores
The results indicate that the levels of ABR awareness among HHCPs and AHCPs was reasonable but not high (median scores of 54.6 to 63.5 for HHCPs, and 55.4 to 63.8 for AHCPs) on a 0–100 scale. Scores for both health sectors were below the 75th percentile, which indicates that there are topics regarding ABR that remain unknown. In general, some variation in knowledge of ABR-related topics was observed in different countries, however, topics related to use of antibiotics in animals as a driver of ABR, and the spread of antibiotics residues and ABR through the environment, were incorrectly answered across many HCPs, regardless of their background. Interestingly, the item related to the potential of ABR development when human antibiotics are used in animals was one of the most unknown topics in all countries tested and both health sectors. Overall, while bacterial mechanisms of ABR and ABU in humans are reasonable well-known topics, ABU in animals and the impact of this in disseminating ABR in the environment are fairly unknown topics, indicating potential gaps in the training curricula of HCPs and highlighting the importance of applying a One Health approach when designing training programmes.
Previous studies have reported better than expected levels of awareness about ABR among HCPs and the public in different countries.2,20 Studies conducted in Peru,36 Ghana37 and Nigeria38 documented high awareness levels among physicians in some cities of these countries. However, methodologies used, questions asked, types of participant and topics included varied from the ones used in this study, making comparisons difficult.
The heterogeneity in awareness between different types of HCPs was unsurprising, considering that professions such as medicine and veterinary medicine that involve more years of study, responsibilities and wider scope of actions, are likely to have more content in their training curricula regarding diagnostic and treatment of diseases than other health professions. Nonetheless, all participants were prescribers/dispensers, and the findings indicate that preservice training about antibiotics and resistance may need to be enhanced particularly for nurses, pharmacists and veterinary drug sellers. Differences in scores between different types of HCPs also suggests that professionals’ backgrounds should be considered when planning AMR awareness campaigns, as diversity in the gaps of their knowledge could lead to information being absorbed differently.
Medical practices
Despite previous experiences with ABR cases, high awareness scores were not necessarily a good indicator of good practices when prescribing or dispensing antibiotics, as HCPs with high scores also reported agreeing with practices that could contribute to the development of ABR. We suggest that actual prescribing practices are captured through more reliable methods than self-reporting, such as point prevalence surveys or direct observation. Such objective practice measurement can then be compared with ABR awareness of individual practitioners.
Context of antibiotic practices
The working environments captured by the survey indicate additional factors that should be considered in understanding antibiotic practices, beyond AMR awareness. Many HCPs were working in facilities without laboratory testing facilities, and many reported a lack of confidence in the facilities they could access. Most HHCPs and AHCPs showed interest in receiving more information and participating in training about ABR. Access to information about ABR was statistically linked to scores, professionals being trained and/or having access to information scored significatively better. Taken together with the finding that participants from both health sectors reported fears of worse health outcomes if antibiotics were not prescribed, it is critical to ensure that HCPs are equipped with evidence about the likelihood common illnesses will benefit from antibiotic treatment—such as through the WHO’s Antibiotic Book,39 as well as information about sensitivity profiles of common pathogens of patients and animals in their care to guide choice of antibiotics.
Most participants did report access to running water and electricity in their workplace, suggesting a relatively privileged study sample given limited progress on WASH40 in health facilities in the study countries. Future awareness assessment efforts should aim for as representative a sample as possible from across different settings within a given country.
These findings suggest that there is not a single contextual variable shaping medical practices but rather a set that may be informing the ‘mindlines’ (internalized and collectively reinforced tacit guidelines) of HCPs and the practices that have become common sensical.41 Beyond improving access to information and the context of practice, addressing ABR as a complex problem will need to extend to wider determinants of infection and resistance including medical, infrastructural, social, economic and political dimensions.42
Some survey biases, such as non-response, social desirability and observer bias, were minimized by anonymization and the self-completion nature of the survey. Adjustments made to the inclusion criteria of some HCPs at each field site may have affected the results as the variability, and undefined lines between HCP roles between countries make drawing conclusions for this population difficult.
Conclusions
In this study, we developed a scale that provides a measurement of levels of ABR awareness at a group level, within countries and across HCPs from human and animal health sectors. This tool could be used to direct and track interventions to improve AMR awareness and to ascertain the success of these interventions, enabling cost-effectiveness evaluations. When combined with a module collecting information on contextual factors, and reliable data on prescribing practices, the tool could support the understanding of the ABR problem and the design of HCP-oriented interventions.
Supplementary Material
Acknowledgements
The authors would like to express their deepest thanks to everyone involved into making this study possible. To Elizabeth Tayler and Karen Mah at the World Health Organization (WHO), and Nandini Shetty at Public Health England for their direction and support. To Freddy Kitutu at the University of Makerere for his support during the development of the questionnaires. To all in-country supervisors and collaborators; Dr Evelyn Ansah, Edem Vidzro (Ghana), Tram Nguen Ngoc, Thu Tran Thi Anh, Katrina Lawson (Vietnam), Dr Cesar Ugarte, Dr Nestor Falcon, Dr Coralith Garcia, Dr Enrique Cornejo, Ailin Cabrera, Roberto Elias, Diego Diaz, Alfredo Delgado, and the Peruvian College of Veterinary Surgeons (Peru), Pelo Nyangusi, Beatrice Abraham, Elias Makoye, Dr Sadick Temu (Tanzania), Dr Olusesan Makinde (Nigeria) and Luechai Sringernyuang (Thailand). To London-based supervisors Dr Ana Mateus and Dr Javier Guitian, and to administrative and technical support staff Pat Ng and Chrissy Roberts.
Contributor Information
Chris Pinto Jimenez, Department of Global Health & Development, Faculty of Public Health and Policy, London School of Hygiene and Tropical Medicine, London WC1H 9SH, UK; Antimicrobial Resistance Centre, London School of Hygiene and Tropical Medicine, London WC1E 7HT, UK; MSc One Health Programme, The Royal Veterinary College, London NW1 0TU, UK.
Maddy Pearson, Department of Global Health & Development, Faculty of Public Health and Policy, London School of Hygiene and Tropical Medicine, London WC1H 9SH, UK.
Mathew Hennessey, MSc One Health Programme, The Royal Veterinary College, London NW1 0TU, UK; Veterinary Epidemiology, Economics and Public Health Group, Royal Veterinary College, Hatfield AL9 7TA, UK; Master’s Degrees, The London School of Hygiene and Tropical Medicine, London WC1E 7HT, UK.
Esin Nkereuwem, Master’s Degrees, The London School of Hygiene and Tropical Medicine, London WC1E 7HT, UK; Medical Research Council Unit, The Gambia at the London School of Hygiene and Tropical Medicine, Atlantic Boulevard, Fajara, PO Box 273, Banjul, The Gambia.
Chloe Crocker, Master’s Degrees, The London School of Hygiene and Tropical Medicine, London WC1E 7HT, UK.
Uzoamaka Egbujo, Master’s Degrees, The London School of Hygiene and Tropical Medicine, London WC1E 7HT, UK; Lagos State University Teaching Hospital (LASUTH), Street 101233, Ikeja, Nigeria.
Jolijn Hendriks, Department of Health Services Research and Policy, London School of Hygiene and Tropical Medicine, London WC1H 9SH, UK.
Sarah Smith, Department of Health Services Research and Policy, London School of Hygiene and Tropical Medicine, London WC1H 9SH, UK.
Phakha Whanpuch, Faculty of Social Sciences and Humanities, Mahidol University, Nakhon Pathom, 73170, Thailand.
Rachel Manongi, Kilimanjaro Christian Medical University College, M8HH+MQ4, Moshi, Tanzania.
Ngo Thi Hoa, Nuffield Department of Medicine, University of Oxford, Oxford OX3 7BN, UK; Oxford University Clinical Research Unit, District 5, Ho Chi Minh City, Vietnam; Department of Microbiology and Center for Biomed Research, Pham Ngoc Thach University of Medicine, ward 12, District 10, Ho Chi Minh City, Vietnam.
Clare I R Chandler, Department of Global Health & Development, Faculty of Public Health and Policy, London School of Hygiene and Tropical Medicine, London WC1H 9SH, UK; Antimicrobial Resistance Centre, London School of Hygiene and Tropical Medicine, London WC1E 7HT, UK.
Funding
This study was supported by the World Health Organization (WHO) with a fund granted to the London School of Hygiene and Tropical Medicine (LSHTM), United Kingdom. LSHTM grant number PHGHZL40. Chris Pinto J. and Mathew Hennessey received support towards travelling costs for data collection from the project fund of the MSc. One Health programme delivered jointly by the Royal Veterinary College and the London School of Hygiene and Tropical Medicine, University of London.
Transparency declarations
None to declare.
Supplementary data
Tables S1 to S10 is available as Supplementary data at JAC online.
References
- 1. WHO . Global Action Plan on Antimicrobial Resistance. WHO Library Cataloguing-in-Publication Data. Geneva, Switzerland: World Health Organization, 2016; 28.. https://www.who.int/publications/i/item/9789241509763. [Google Scholar]
- 2. WHO . Antibiotic resistance: multi-country public awareness survey. World Health Organization, 2015; 59. https://apps.who.int/iris/bitstream/handle/10665/194460/9789241509817_eng.pdf. [Google Scholar]
- 3. Hills H. Students help raise awareness of AMR. Vet Rec 2016; 179: 558. 10.1136/vr.i6445 [DOI] [PubMed] [Google Scholar]
- 4. Earnshaw S, Mancarella G, Mendez Aet al. European Antibiotic Awareness Day: a five-year perspective of Europe-wide actions to promote prudent use of antibiotics. Euro Surveill 2014; 19: 20928. 10.2807/1560-7917.ES2014.19.41.20928 [DOI] [PubMed] [Google Scholar]
- 5. Schrier L, Hadjipanayis A, del Torso Set al. European Antibiotic Awareness Day 2017: training the next generation of health care professionals in antibiotic stewardship. Eur J Pediatr 2018; 177: 279–83. 10.1007/s00431-017-3055-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huttner B, Saam M, Moja Let al. How to improve antibiotic awareness campaigns: findings of a WHO global survey. BMJ Glob Health 2019; 4: e001239. 10.1136/bmjgh-2018-001239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burstein VR, Trajano RP, Kravitz RLet al. Communication interventions to promote the public’s awareness of antibiotics: a systematic review. BMC Public Health 2019; 19: 899. 10.1186/s12889-019-7258-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cross ELA, Tolfree R, Kipping R. Systematic review of public-targeted communication interventions to improve antibiotic use. J Antimicrob Chemother 2017; 72: 975–87. 10.1093/jac/dkw520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mazinska B, Struzycka I, Hryniewicz W. Surveys of public knowledge and attitudes with regard to antibiotics in Poland: did the European antibiotic awareness day campaigns change attitudes? PLoS ONE 2017; 12: e0172146. 10.1371/journal.pone.0172146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Oh JM, Ming LC, Bakrin FSet al. Social aspects of antibiotic use in the South and East Asian students and general population. J Young Pharm 2018; 10: 66–73. 10.5530/jyp.2018.10.16 [DOI] [Google Scholar]
- 11. Scaioli G, Gualano MR, Gili Ret al. Antibiotic use: a cross-sectional survey assessing the knowledge, attitudes and practices amongst students of a school of medicine in Italy. PLoS ONE 2015; 10: e0122476. 10.1371/journal.pone.0122476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McCullough AR, Parekh S, Rathbone Jet al. A systematic review of the public’s knowledge and beliefs about antibiotic resistance. J Antimicrob Chemother 2016; 71: 27–33. 10.1093/jac/dkv310 [DOI] [PubMed] [Google Scholar]
- 13. McCullough AR, Rathbone J, Parekh Set al. Not in my backyard: a systematic review of clinicians’ knowledge and beliefs about antibiotic resistance. J Antimicrob Chemother 2015; 70: 2465–73. 10.1093/jac/dkv164 [DOI] [PubMed] [Google Scholar]
- 14. Saha SK, Barton C, Promite Set al. Knowledge, perceptions and practices of community pharmacists towards antimicrobial stewardship: a systematic scoping review. Antibiotics (Basel) 2019; 8: 263. 10.3390/antibiotics8040263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen X, Wu L, Xie X. Assessing the linkages between knowledge and use of veterinary antibiotics by pig farmers in rural China. Int J Environ Res Public Health 2018; 15: 1126. 10.3390/ijerph15061126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fowler H, Davis MA, Perkins Aet al. Survey of veterinary antimicrobial prescribing practices, Washington State 2015. Vet Rec 2016; 179: 651. 10.1136/vr.103916 [DOI] [PubMed] [Google Scholar]
- 17. Hughes LA, Williams N, Clegg Pet al. Cross-sectional survey of antimicrobial prescribing patterns in UK small animal veterinary practice. Prev Vet Med 2012; 104: 309–16. 10.1016/j.prevetmed.2011.12.003 [DOI] [PubMed] [Google Scholar]
- 18. Gualano MR, Gili R, Scaioli Get al. General population’s knowledge and attitudes about antibiotics: a systematic review and meta-analysis. Pharmacoepidemiol Drug Saf 2015; 24: 2–10. 10.1002/pds.3716 [DOI] [PubMed] [Google Scholar]
- 19. WHO . WHO Competency Framework for Health Workers’ Education and Training on Antimicrobial Resistance. Geneva: World Health Organization, 2018; 28.. Https://apps.who.int/iris/bitstream/handle/10665/272766/WHO-HIS-HWF-AMR-2018.1-eng.pdf?ua=1. [Google Scholar]
- 20. Pearson M, Chandler C. Knowing antimicrobial resistance in practice: a multi-country qualitative study with human and animal healthcare professionals. Glob Health Action 2019; 12: 1599560. 10.1080/16549716.2019.1599560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Burckhardt CS, Anderson KL. The quality of life scale (QOLS): reliability, validity, and utilization. Health Qual Life Outcomes 2003; 1: 60. 10.1186/1477-7525-1-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Panc T, Mihalcea A, Panc I. Self-efficacy survey: a new assessment tool. Procedia Soc Behav Sci 2012; 33: 880–4. 10.1016/j.sbspro.2012.01.248 [DOI] [Google Scholar]
- 23. Busija L, Pausenberger E, Haines TPet al. Adult measures of general health and health-related quality of life: medical outcomes study short form 36-item (SF-36) and short form 12-item (SF-12) health surveys, Nottingham health profile (NHP), sickness impact profile (SIP), medical outcomes study short form 6D (SF-6D), health utilities index mark 3 (HUI3), quality of well-being scale (QWB), and assessment of quality of life (AQOL). Arthritis Care Res 2011; 63: S383–412. 10.1002/acr.20541 [DOI] [PubMed] [Google Scholar]
- 24. Button B. Quality of Well-Being (QWB) Scale. In: Michalos AC, ed. Encyclopedia of Quality of Life and Well-Being Research. Dordrecht: Springer Netherlands, 2014; 5314–20. [Google Scholar]
- 25. Vetter TR, Cubbin C. Psychometrics: trust, but verify. Anesth Anal 2019; 128: 176–81. 10.1213/ANE.0000000000003859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Boateng GO, Neilands TB, Frongillo EAet al. Best practices for developing and validating scales for health, social, and behavioral research: a primer. Front Public Health 2018; 6: 149. 10.3389/fpubh.2018.00149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McEwen SA, Collignon PJ. Antimicrobial resistance: a one health perspective. Microbiol Spectr 2018; 6: 1–26. 10.1128/microbiolspec.ARBA-0009-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pearson M, Doble A, Glogowski Ret al. Antibiotic Prescribing and Resistance: Views from LMIC Prescribing and Dispensing Professionals. Report to World Health Organisation AMR Secretariat, 2018. [Google Scholar]
- 29. Rasch G. Probabilistic Models for Some Intelligence and Attainment Tests. Copenhagen: Danish Institute for Education Research: University of Chicago Press; Expanded edition, 1980. [Google Scholar]
- 30. Chandler CI, Pearson M, Crocker Cet al. The health care professional antibiotic resistance awareness scale v1: report on development and testing. Research online platform: Antimicrobial Resistance Centre. London School of Hygiene & Tropical Medicine 2018. 10.17037/PUBS.04664614 [DOI]
- 31. Rodrigues A T, Ferreira M, Roque Fet al. Physicians’ attitudes and knowledge concerning antibiotic prescription and resistance: questionnaire development and reliability. BMC Infect Dis 2016; 16: 7. 10.1186/s12879-015-1332-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alumran A, Hou X-Y, Hurst C. Validity and reliability of instruments designed to measure factors influencing the overuse of antibiotics. J Infect Public Health 2012; 5: 221–32. 10.1016/j.jiph.2012.03.003 [DOI] [PubMed] [Google Scholar]
- 33. Denyer L, Chandler C. Anthropology’s Contribution to AMR Control. Investment and Society. 2018. http://resistancecontrol.info/wp-content/uploads/2018/05/104-08-chandler.pdf.
- 34. Lundborg C S, Tamhankar AJ. Understanding and changing human behaviour–antibiotic mainstreaming as an approach to facilitate modification of provider and consumer behaviour. Ups J Med Sci 2014; 119: 125–33. 10.3109/03009734.2014.905664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. ReAct-Group . TOOLBOX—RAISE AWARENESS: Behavioral change theories. https://www.reactgroup.org/toolbox/raise-awareness/behavioral-change-theories/#zp-ID-11340-280251-2DEEER3H.
- 36. García C, Llamocca LP, García K, et al. Knowledge, attitudes and practice survey about antimicrobial resistance and prescribing among physicians in a hospital setting in Lima, Peru. BMC Clin Pharmacol 2011; 11: 18. 10.1186/1472-6904-11-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Asante KP, Boamah EA, Abdulai MAet al. Knowledge of antibiotic resistance and antibiotic prescription practices among prescribers in the Brong Ahafo region of Ghana; a cross-sectional study. BMC Health Serv Res 2017; 17: 422. 10.1186/s12913-017-2365-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jamil LA, Aminu B, Magaji BA. Knowledge, belief and practice of interventions to contain antimicrobial resistance among physicians in Sokoto, northwestern Nigeria. Orient J Med 2015; 27: 71–8. [Google Scholar]
- 39. WHO . The WHO Essential Medicines List Antibiotic Book: improving antibiotic AWaReness. In: World Health Organization, ed. Improving Antibiotic AWaReness. Online: WHO, 2022; 420. https://cdn.who.int/media/docs/default-source/essential-medicines/eml-antibiotic-book-draft.pdf? sfvrsn=cb6cb7c2_6&download=true
- 40. WASHINHCF . Country Progress Tracker. https://washinhcf.org/country-progress-tracker/#country-progress-tracker
- 41. Gabbay J, May A. Evidence-based guidelines or collectively constructed “mindlines?” ethnographic study of knowledge management in primary care. BMJ 2004; 329: 1013. 10.1136/bmj.329.7473.1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Charani E, McKee M, Ahmad Ret al. Optimising antimicrobial use in humans–review of current evidence and an interdisciplinary consensus on key priorities for research. Lancet Reg Health Eur 2021; 7: 100161. 10.1016/j.lanepe.2021.100161 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.