Abstract
Previous studies showed that the epidermal growth factor receptor (EGFR) can be transactivated by platelet-derived growth factor (PDGF) stimulation and that EGFR transactivation is required for PDGF-stimulated cell migration. To investigate the mechanism for cross talk between the PDGF β receptor (PDGFβR) and the EGFR, we stimulated rat aortic vascular smooth muscle cells (VSMC) with 20 ng of PDGF/ml. Transactivation of the EGFR, defined by receptor tyrosine phosphorylation, occurred with the same time course as PDGFβR activation. Basal formation of PDGFβR-EGFR heterodimers was shown by coimmunoprecipitation studies, and interestingly, disruption of this receptor heterodimer abolished EGFR transactivation. Breakdown of the heterodimer was observed when VSMC were pretreated with antioxidants or with a Src family kinase inhibitor. Disruption of heterodimers decreased ERK1 and ERK2 activation by PDGF. Although PDGF-induced PDGFβR activation was abolished after pretreatment with 1 μM AG1295 (a specific PDGF receptor kinase inhibitor), EGFR transactivation was still observed, indicating that PDGFβR kinase activity is not required. In conclusion, our data demonstrate that the PDGFβR and the EGFR form PDGFβR-EGFR heterodimers basally, and we suggest that heterodimers represent a novel signaling complex which plays an important role in PDGF signal transduction.
The traditional view of growth factor receptors and hormone receptors is that a specific ligand directly recognizes a highly selective binding site on its cognate receptor. For example, upon binding of platelet-derived growth factor (PDGF) to its specific tyrosine kinase receptors (PDGF α and β receptors [PDGFβR]), receptor dimerization and autophosphorylation occur. Tyrosine phosphorylation leads to binding and activation of signal transduction molecules containing Src homology 2 or phosphotyrosine binding domains, and consequently, many signaling pathways are initiated, leading to an integrated cell response.
There is accumulating evidence that the epidermal growth factor receptor (EGFR) may be transactivated (defined by receptor tyrosine phosphorylation) by ligands, which specifically bind to other membrane receptors (32). For example, G protein-coupled receptor ligands, including angiotensin II, carbachol, thrombin, endothelin, tetradecanoyl-phorbol-13-acetate, and lysophosphatidic acid, activate the EGFR (11, 12, 16, 27). In fact, many G protein-coupled receptors activate mitogen-activated protein kinases through transactivation of the EGFR (11, 15, 35). Yamauchi et al. demonstrated that growth hormone was able to transactivate the EGFR (41, 42). The EGFR has been shown by several investigators to be transactivated in response to PDGF (10, 13, 37, 38). In fact, Li et al. showed that PDGF-stimulated migration of murine fibroblasts was dependent upon EGFR expression and tyrosine phosphorylation (25). These observations suggest that cell responses induced by these ligands involve signaling pathways downstream of transactivated receptor tyrosine kinases.
While the biological importance of cross talk among different receptors has been gradually elucidated, the mechanism for transactivation is not understood. In the present study, we investigated PDGF-induced EGFR transactivation and derived three key findings. First, PDGFβR-EGFR heterodimers exist in unstimulated cells. Second, heterodimer formation is abolished by treatment with antioxidants and Src family kinase inhibitors. Finally, PDGF-induced EGFR transactivation does not depend on PDGFβR kinase activity. The physiologic importance of this pathway was demonstrated by the findings that disruption of PDGFβR-EGFR heterodimers both abolished EGFR transactivation and significantly inhibited PDGF-mediated ERK1 and ERK2 (ERK1/2) activation. These data suggest a general role for cross talk between tyrosine kinase-coupled receptors, at the level of the receptors themselves, in signal transduction.
MATERIALS AND METHODS
Reagents.
Reagents and other supplies were obtained from the following sources. Cell culture media were from GIBCO-BRL (Gaithersburg, Md. US). Protein A/G PLUS-agarose was from Santa Cruz Biotechnology (Santa Cruz, Calif.). Rabbit polyclonal anti-human PDGFβR (immunogen; C-terminal domain, amino acids 1013 to 1025), sheep polyclonal anti-human EGFR (immunogen; cytoplastic domain), mouse anti-Src (GD11), rabbit anti-phospho-Src (Y416), mouse monoclonal anti-phosphotyrosine antibody (4G10), normal sheep immunoglobulin G (IgG), and normal rabbit IgG were from Upstate Biotechnology (Lake Placid, N.Y.). Mouse monoclonal anti-human EGFR (immunogen; C-terminal domain, amino acids 996 to 1022) was from Transduction Laboratory (San Diego, Calif.). Rabbit polyclonal anti-human PDGFβR (immunogen; kinase domain, amino acids 699 to 798) were from Pharmingen (San Diego, Calif.). Rabbit polyclonal anti-phospho-specific ERK1/2 antibody was from New England Biolabs (Beverly, Mass.). PDGFβR tyrosine kinase inhibitor (AG1295), EGFR kinase inhibitor (AG1478), Src family kinase inhibitor (PP2), and JAK2 kinase inhibitor (AG490) were from Calbiochem (La Jolla, Calif.). Recombinant human PDGF-BB, N-acetyl-l-cysteine (NAC), and 4,5-dyhydroxy-1,3-benzenedisulfonic acid (Tiron) and a chemical cross-linker, 1-ethyl-3-(-3-dimethylamino propyl)carbodiimide (EDAC), were from Sigma (St. Louis, Mo.). Recombinant human epidermal growth factor (EGF) was from Clonetics (San Diego, Calif.). Pervanadate was freshly prepared by mixing 80 μl of 1 M sodium orthovanadate, 70 μl of phosphate-buffered saline, and 10 μl of 30% H2O2.
Cell culture.
Rat aortic vascular smooth muscle cells (VSMC) were isolated from the thoracic aortas of 200- to 250-g male Sprague-Dawley rats and maintained in Dulbecco modified Eagle medium supplemented with 10% serum as described previously (22). The growth of VSMC from passages 8 to 14 at 70 to 80% confluence was arrested by incubation in Dulbecco modified Eagle medium without serum for 48 h before use. A431 cells were cultured and serum-starved under the same conditions as those used for VSMC.
Immunoprecipitation and immunoblot analyses.
The immunoprecipitation and immunoblot analyses were performed following previously described methods (30). The anti-PDGFβR C-terminal domain and the anti-EGFR cytoplasmic domain were used in all experiments except those illustrated in Fig. 1D, where other domains were used as well. Growth-arrested cells were stimulated with PDGF-BB or EGF as indicated for each experiment. Cells were lysed in Triton–NP-40 lysis buffer (0.5% NP-40, 10 mM Tris [pH 7.5], 2.5 mM KCl, 150 mM NaCl, 20 mM β-glycerol phosphate, 50 mM NaF, 1 mM Na3VO4, 10-μg of aprotinin/ml, 10 μg of leupeptin/ml, 1 mM dithiothreitol), scraped off the dish, and centrifuged. Lysates containing equal amounts (1 mg) of protein were incubated with antibodies overnight at 4°C. After incubation with protein A/G PLUS-agarose for 2 h, precipitates were washed four times with the lysis buffer and then resuspended in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer. After being heated at 100°C for 5 min, samples were separated by SDS-PAGE (6 to 8% polyacrylamide) and transferred to nitrocellulose membranes. After incubation in blocking solution (5% bovine serum albumin, phosphate buffered saline [pH 7.5], 0.1% Tween 20), membranes were incubated with primary antibodies (1:1,000 dilution in blocking solution) for 2 h at room temperature. After the membranes were washed six times (5 min each) with washing buffer (phosphate-buffered saline [pH 7.5], 0.1% Tween 20), the blots were incubated with the appropriate secondary antibodies (1:5,000 dilution in blocking solution) for 1 h at room temperature. The membranes were washed six times, and proteins were detected by the ECL system (Amersham Inc., Buckinghamshire, England).
FIG. 1.
PDGF transactivates EGFR. Serum-starved VSMC were stimulated with 20 ng of PDGF-BB/ml for the times indicated. (A) Cell lysates were immunoprecipitated with anti-EGFR and immunoblotted with antiphosphotyrosine (4G10) (upper panel) and reprobed with anti-EGFR antibody (lower panel). The EGFR is shown as a single band at 170 kDa. (B) Relative phosphorylation of PDGFβR and EGFR. (C) The PDGFβR was coimmunoprecipitated with anti-EGFR. The PDGFβR and the EGFR were identified at 180 and 170 kDa, respectively. A band shown at 165 kDa (lanes 5 to 8) is nonspecific since it is not identified by 4G10. (D) Coprecipitation of EGFR and PDGFβR detected by diverse antibodies. Unstimulated cell lysates were immunoprecipitated with the anti-PDGFβR C-terminal domain (lane 1), the anti-PDGFβR kinase domain (lane 2), the anti-EGFR C-terminal domain (lane 3), and the anti-EGFR cytoplasmic domain (lane 4). Normal sheep IgG was used as a negative control for anti-EGFR (lane 5). Immunoblotting was performed with the anti-PDGFβR kinase domain. (E) Coprecipitation of PDGFβR by EGR antibody. Serum-starved VSMC were stimulated with 20 ng of PDGF-BB/ml for 5 min and treated with a cross-linker as described in Materials and Methods. Cell lysates were immunoprecipitated with the anti-EGFR C-terminal domain (lane 1), the anti-EGFR cytoplasmic domain (lane 2), the anti-PDGFβR C-terminal domain (lane 3), and the anti-PDGFβR kinase domain (lane 4). Normal rabbit IgG was used as a negative control for anti-PDGFβR. Immunoblotting was performed with the anti-EGFR C-terminal domain. (F) Serum-starved A431 cells were stimulated with 20 ng of PDGF-BB/ml or 10 ng of EGF/ml for 5 min. IP, immunoprecipitation, IB, immunoblotted.
Receptor dimer stabilization.
Receptor dimers were stabilized according to the procedure described by van der Vliet et al. (36) (see Fig. 1E). Shortly after agonist treatment, cells were incubated with 10 mM EDAC for 40 min at 37°C. Cells were then lysed and used for immunoprecipitation and immunoblot.
Measurement of ERK1/2 phosphorylation.
To determine ERK1/2 phosphorylation, 20 μg of whole-cell lysates was mixed with SDS-PAGE sample buffer, denatured at 100°C for 5 min, and loaded onto each lane of a 10% polyacrylamide gel for SDS-PAGE. Immunoblotting was performed as described above.
Statistical analysis.
Phosphorylation levels were measured by densitometry of autoradiograms using NIH Image 1.59 software. Results are presented as the mean ± standard error of the mean from at least three separate experiments. Significant differences (P < 0.01) were determined by Student's t test.
RESULTS
PDGF-induced EGFR transactivation and association between the EGFR and the PDGFβR.
Rat aortic VSMC express both PDGFβR and EGFR (Fig. 1). To measure the effect of PDGF on EGFR phosphorylation, VSMC were exposed to 20 ng of PDGF-BB/ml for 1 to 20 min. After the preparation of cell lysates, the EGFR was immunoprecipitated and immunoblotting for phosphotyrosine was performed. Transactivation of the EGFR as measured by the level of phosphotyrosine peaked (at a sixfold increase) at 10 min after exposure to PDGF (Fig. 1A and B). The time course for EGFR tyrosine phosphorylation was similar to that for PDGF induced PDGFβR tyrosine phosphorylation (Fig. 1B).
To determine the nature of EGFR-PDGFβR interactions, coimmunoprecipitation studies were performed. As shown in Fig. 1C, the molecular masses of EGFR and PDGFβR were 170 and 180 kDa, respectively. PDGF increased tyrosine phosphorylation by EGFR, peaking at 10 min (lanes 1 and 2). A 165-kDa band observed by anti-PDGFβR immunoblotting (lanes 5 to 8) is neither EGFR nor PDGFβR, based on immunoblots with two PDGFβR antibodies. There was no increase in EGFR expression by PDGF (lanes 3 and 4). The PDGFβR was coimmunoprecipitated with the EGFR under both basal and PDGF-stimulated conditions (lanes 5 and 6). There was no change in the amount of coimmunoprecipitated PDGFβR by PDGF stimulation. Expression of PDGFβR did not change (lanes 7 and 8). These data suggest that there was direct binding between PDGFβR and EGFR, resulting in the formation of a heterodimeric receptor complex.
To exclude the possibility of nonspecific binding of PDGFβR to the anti-EGFR antibody, we performed the immunoprecipitation and immunoblotting experiment using several other receptor antibodies raised against different portions of the EGFR and the PDGFβR. In Fig. 1D, lanes 1 and 2 show PDGFβR immunoprecipitated by different anti-PDGFβR antibodies. PDGFβR was detected in both samples that immunoprecipitated with two different anti-EGFR antibodies (lanes 3 and 4). No PDGFβR was detected when normal sheep IgG was used for immunoprecipitation (lane 5). Although EGFR was barely detected in PDGFβR-immunoprecipitated cell lysates, the presence of EGFR became clear after stabilization of intermolecular associations using the cross-linker EDAC (Fig. 1E). These results demonstrate the presence of PDGFβR-EGFR heterodimers under basal unstimulated conditions. The number of PDGFβR expressed on VSMC has been reported to be two to seven times greater than that reported for EGFR (20). Because of this disproportion in receptor expression, it seems likely that anti-PDGFβR predominantly binds to the PDGFβR that is free from the EGFR (EGFR-unbound PDGFβR). Coimmunoprecipitation of the EGFR by anti-PDGFβR was successfully detected only when the PDGFβR-EGFR complex was stabilized by a cross-linker treatment.
In A431 cells, which overexpress EGFR but lack PDGFβR, PDGF failed to activate the EGFR (Fig. 1F), confirming that PDGF does not stimulate the EGFR directly.
The PDGFβR-EGFR complex formation is not regulated by PTPases.
One mechanism for PDGF-induced EGFR transactivation may be via protein tyrosine phosphatase (PTPase) inhibition which takes place upon addition of PDGF, thereby increasing EGFR phosphorylation. To study this possibility, we treated VSMC with pervanadate, which is an irreversible inhibitor of all PTPases and which functions on intact cells because of its ability to permeate cells (21). EGFR phosphorylation increased in response to 100 μM pervanadate (Fig. 2, upper panel). However, phosphorylation of PDGFβR coimmunoprecipitated by anti-EGFR antibody decreased over 20 min in response to pervanadate (Fig. 2, lower panel). These data suggest that EGFR phosphorylation by pervanadate occurs independently of PDGFβR-EGFR complex formation. Conversely, because pervanadate stimulates dissociation of PDGFβR-EGFR complexes, we believe it is unlikely that PTPases play a significant role in PDGF-induced EGFR transactivation.
FIG. 2.
EGFR phosphorylation by pervanadate. Serum-starved VSMC were treated with 100 μM pervanadate for the times indicated. Cell lysates were immunoprecipitated with anti-EGFR and analyzed by immunoblotting, probed with 4G10 (upper panel), and reprobed with anti-EGFR (middle panel) and anti-PDGFβR (lower panel). IP, immuno precipitated; IB, immunoblotted.
EGFR transactivation does not require PDGFβR kinase activity.
To investigate the importance of PDGFβR kinase activity in PDGF-induced EGFR transactivation, we stimulated VSMC with PDGF following pretreatment with AG1295, a potent and specific inhibitor of PDGF receptor kinase (23) (Fig. 3). Although PDGFβR activation was completely abolished after AG1295 pretreatment (Fig. 3A), transactivation of EGFR was still observed (Fig. 3B). In contrast, pretreatment of VSMC with AG1478, a potent and specific EGFR kinase inhibitor (31), did not decrease PDGFβR activation but abolished EGFR transactivation by PDGF (compare Fig. 3A and B). These data suggest that PDGF-induced EGFR transactivation requires the activity of EGFR kinase but not that of PDGFβR kinase under these experimental conditions.
FIG. 3.
PDGF-induced EGFR transactivation after pretreatment with AG1295 or AG1478. Serum-starved VSMC were stimulated with 20 ng of PDGF-BB/ml for 5 min after preincubation with 10 μM AG1295 or 10 μM AG1478 for 30 min. Cell lysates were immunoprecipitated with anti-PDGFβR (A) or anti-EGFR (B) and analyzed by immunoblotting with 4G10 (upper panel) or anti-PDGFβR and anti-EGFR (lower panel). The PDGFβR and the EGFR were identified at 180 and 170 kDa, respectively. IP, immunoprecipitated; IB, immunoblotted.
EGFR transactivation is blocked by antioxidants.
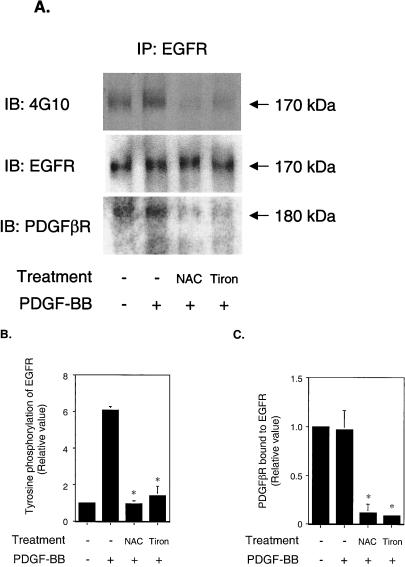
It is well known that reactive oxygen species (ROS) are generated upon activation of PDGFβR (7, 14, 33) and EGFR (4). It has been reported that ROS cause ligand-independent activation of EGFR (34). To determine the role of ROS in PDGF-induced EGFR transactivation, we pretreated VSMC with antioxidants, 10 mM NAC (a free radical scavenger), or 10 mM Tiron (a nonenzymatic superoxide scavenger), followed by PDGF stimulation. The PDGFβR was activated by PDGF with or without antioxidant pretreatment (data not shown). In contrast, EGFR transactivation was remarkably decreased in cells pretreated with antioxidants (Fig. 4A, upper panel, and B). Antioxidants had no effect on EGFR expression (Fig. 4A, middle panel). Significantly, the PDGFβR that coimmunoprecipitated with EGFR was decreased in antioxidant-treated cells (Fig. 4A, lower panel, and C). These data suggest that treatment of VSMC with antioxidants disrupts the PDGFβR-EGFR complex. We propose that EGFR transactivation was inhibited because disruption of PDGFβR-EGFR heterodimers prevented EGFR from interacting with the molecules required for its transactivation. Taken together, ROS appear to play an important role in PDGF-induced EGFR transactivation via control of receptor complex stability.
FIG. 4.
Effect of antioxidants on EGFR transactivation. Serum-starved VSMC were stimulated with 20 ng of PDGF-BB/ml for 5 min after pretreatment with 10 mM NAC or 10 mM Tiron for 60 min. (A) Cell lysates were immunoprecipitated with anti-EGFR and analyzed by immunoblotting, probed with 4G10 (upper panel), and reprobed with anti-EGFR (middle panel) or anti-PDGFβR (lower panel). IP, immunoprecipitated. IB, immunoblotted. (B) Relative tyrosine phosphorylation level of EGFR. Both NAC and Tiron significantly inhibited EGFR transactivation by PDGF. ∗, P < 0.001. (C) PDGFβR bound to EGFR. PDGFβR coimmunoprecipitated with EGFR significantly decreased after antioxidant treatment. ∗, P < 0.001.
Role of Src family kinase activity in transactivation.
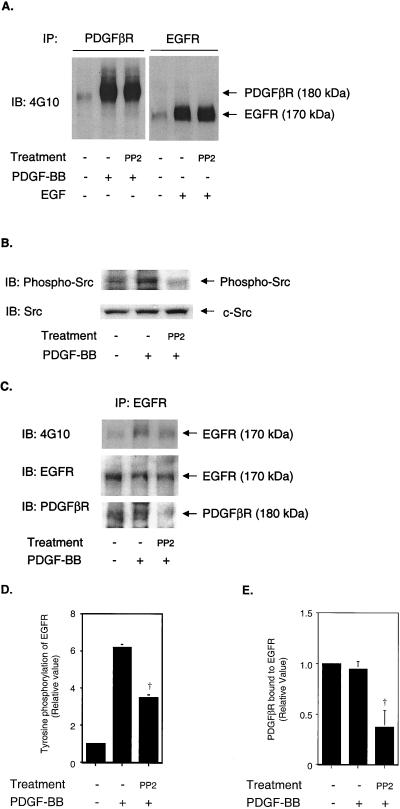
Because redox-sensitive pathways have been reported to be mediated by Src family kinases (1, 3, 29), we investigated the involvement of Src family kinases in PDGF-induced EGFR transactivation. VSMC were treated with 1 μM 4-amino-5-(4-chlorophenyl)-7- (t-butyl)pyrazolo[3,4-d]pyrimidine (PP2), which specifically inhibits Src family kinases (17). It has been suggested that PP2 may inhibit not only Src kinase activity but also PDGFR kinase activity at concentrations of >10 μM in NIH 3T3 and 527 cells (5, 6). However, Waltenberger et al. showed that PDGFR kinase activity was barely inhibited by 1 μM PP1 in human coronary artery smooth muscle cells (39). We also confirmed that 1 μM PP2 did not inhibit PDGFR kinase activity in VSMC (Fig. 5A) and believe that PP2 acts as a specific Src kinase inhibitor at this low concentration (Fig. 5B). PDGF-induced EGFR transactivation was significantly decreased (Fig. 5C and D). The decrease in transactivation caused by 1 μM PP2 correlated with a decrease in the association of PDGFβR with EGFR (Fig. 5A and E). These data suggest that Src family kinases are involved in EGFR transactivation through supporting the formation of PDGFβR-EGFR heterodimers. Although JAK2 kinase activity has been reported to be required for EGFR transactivation by growth hormone (41, 42), AG490, a specific JAK2 kinase inhibitor, did not prevent EGFR transactivation by PDGF (data not shown).
FIG. 5.
Effect of Src family kinase inhibitor on EGFR transactivation. Serum-starved VSMC were pretreated with 1 μM PP2 for 30 min, followed by stimulation with 20 ng of PDGF-BB/mi or 10 ng of EGF/ml for 5 min. (A) Cell lysates were immunoprecipitated with anti-PDGFβR (left panel) or anti-EGFR (right panel) and analyzed by immunoblotting with 4G10 as the probe. (B) Cell lysates were immunoblotted with anti-phospho-Src (Y416) and reprobed with anti-Src. (C) Cell lysates were immunoprecipitated with anti-EGFR, analyzed by immunoblotting with 4G10 as the probe (upper panel), and reprobed with anti-EGFR (middle panel) or anti-PDGFβR (lower panel) antibody. IP, immunoprecipitated. IB, immunoblotted. (D) Relative tyrosine phosphorylation level of EGFR. PP2 significantly inhibited EGFR transactivation by PDGF. †, P < 0.005. (E) PDGFβR bound to EGFR. The PDGFβR coimmunoprecipitated with the EGFR significantly decreased after antioxidant treatment. †, P < 0.005.
Effect of transactivation on ERK1/2 activity.
Since PDGF-induced EGFR transactivation has been reported to be required for cell migration (25), we investigated the effect of EGFR transactivation on ERK1/2 activity as an effector downstream of both the PDGFβR and the EGFR. The PDGFR kinase inhibitor AG1295 and the EGFR kinase inhibitor AG1478 inhibited PDGF-induced ERK1/2 activation by 70 and 30%, respectively (Fig. 6A). ERK1/2 activity which was not inhibited by AG1295 or AG1478 is presumed to represent signaling downstream of the PDGFβR or the EGFR, respectively. Antioxidants reduced PDGF-induced ERK1/2 activation by ∼50%, and PP2 caused nearly complete inhibition of ERK1/2 activity (Fig. 6B). The stronger inhibition of ERK1/2 activity than of EGFR transactivation by PP2 is reasonable, given the importance of Src family kinase activity in signaling downstream of the PDGFβR itself (9, 19). These data demonstrate an important role for EGFR transactivation in PDGF-induced ERK1/2 activity.
FIG. 6.
Effect of receptor kinase inhibitors and antioxidants on ERK1/2 activity. Serum-starved VSMC were stimulated with 20 ng of PDGF-BB/ml after pretreatment with 1 μM AG1295 or 10 μM AG1478 for 30 min (A) or with 10 mM NAC or Tiron for 60 min or 1 μM PP2 for 30 min (B). Twenty micrograms of cell lysate was loaded onto each lane and analyzed by immunoblotting with anti-phospho-ERK1/2 antibody as the probe. Lower panels show the relative phosphorylation levels of ERK1/2. Each agent significantly inhibited ERK1/2 phosphorylation induced by PDGF stimulation. ∗, P < 0.001; †, P < 0.005; ‡, P < 0.01.
DISCUSSION
The major findings of the present study are that PDGFβR-EGFR heterodimers exist basally in cells that express both receptors and that these receptors play an important role in PDGF-mediated signal transduction. Of interest is that EGFR transactivation was not dependent on PDGFβR kinase activity but heterodimer formation and signal transduction could be abolished by treatment with antioxidants or Src family kinase inhibitors. Based on these results, we propose a model for PDGFβR-mediated EGFR transactivation based on four findings (Fig. 7). First, PDGFβR-EGFR heterodimers exist in the absence of a ligand. Second, these dimers can be disrupted by treatment with the Src kinase inhibitor PP2, suggesting that phosphorylation of one of the receptors (or of a protein that links the receptors) by a Src kinase is required to maintain heterodimer formation. Inhibition by antioxidants may be due to inhibition of Src activity, which, as we and others have shown, is regulated by ROS (2, 3). Third, it is in response to PDGF binding to its receptor that PDGFβR dimers form. We propose that this process also brings EGFR dimers together. Transactivation of EGFR occurs via a process that is independent of PDGFβR kinase activity but is dependent on EGFR kinase activity. Finally, EGFR is an important component in PDGFβR-mediated signal transduction that involves the ERK1/2 pathway.
FIG. 7.
Model of basal PDGFβR-EGFR heterodimer formation and signal transduction. PDGFβR and EGFR form a receptor complex (heterodimer) under basal cell conditions. The formation of the receptor complex may provide a scaffold for other molecules required for the transactivation. The interaction between the two receptors is regulated by ROS and Src family kinases. Note that we believe that the PDGFR dimers are physically closer than the EGFR dimers in three dimensions.
Our data indicate that PDGFβR-EGFR heterodimers exist basally and that their activation is simultaneous. This conclusion is based on the fact that we were able to coprecipitate a significant proportion of the EGFR complexed to the PDGFβR in growth-arrested cells (Fig. 1). We cannot conclude whether binding between the PDGFβR and the EGFR is direct or is mediated by a linker protein. Basal receptor heterodimers are necessary for PDGF-induced EGFR activation (Fig. 7). Simultaneous activation was demonstrated by the time course which showed that PDGF-induced EGFR transactivation peaked at 5 to 10 min which is similar to the time course of PDGFβR activation. This time course is different from that of EGF-induced EGFR activation and angiotensin II-induced EGFR transactivation, which peaked at 1 to 3 min (18; data not shown).
We demonstrated an important role for ROS and Src in PDGF-mediated EGFR transaction. We previously reported that angiotensin II-induced PDGFβR transactivation was inhibited by pretreatment with Tiron and NAC (18), and another group found that EGFR transactivation by angiotensin II was inhibited by NAC (40). Because NAC and Tiron decreased association between the PDGFβR and the EGFR, we propose that antioxidants block transactivation by destabilizing heterodimers. A likely mechanism for antioxidant-mediated inhibition was via Src family kinases, since a similar effect was observed with the Src inhibitor PP2. Src family kinases have been reported to mediate signal transduction induced by ROS (1, 3, 29). However, our data suggest the presence of another ROS-dependent mechanism for heterodimer stabilization, since inhibition of EGFR transactivation by PP2 was not as effective as inhibition by antioxidants.
As suggested by other investigators (8, 11, 26, 28), the EGFR is well suited for its role in transactivation relative to other growth factor receptors by virtue of the fact that it does not require binding of its ligand for tyrosine kinase activity and dimerization. Kwatra et al. showed that the ligand binding domain of EGFR was not required for receptor dimerization (24), suggesting that the EGF receptor can form dimers and autophosphorylate in a manner different than that which occurs upon EGF ligand stimulation. The requirement for EGFR kinase activity, but not PDGFβR kinase activity, would appear to support this hypothesis. However, EGFR homodimerization by PDGF was difficult to detect when we attempted this experiment using a covalent cross-linker (data not shown). These findings suggest that EGFR homodimers are not very stable when formed in response to PDGFβR. In particular, we propose that PDGF binding to PDGFβR brings PDGFβR close together. In contrast, the EGFR are “on the outside” (Fig. 7), and while able to autophosphorylate, they are not as stable as when receptor dimerization occurs in response to EGF.
Our data indicate that EGFR transactivation contributes importantly to PDGFβR signal transduction. ERK1/2 activation by PDGF was inhibited by 30% after treatment with the EGFR kinase inhibitor AG1478. This finding correlates well with the effect of antioxidant pretreatment, which completely inhibited heterodimer formation and reduced PDGF-stimulated ERK1/2 activation by 50%. Recently, PDGF-induced EGFR transactivation was shown to play a critical role in cell migration. Specifically, Li et al. reported that B82L cells deficient in EGFR function (EGFR expressing a kinase-inactive EGFR or a carboxyl-truncated EGFR) exhibit little PDGF-stimulated migration (25). Our data support the functional importance of EGF transactivation by demonstrating cooperative ERK1/2 activation by PDGFβR and EGFR. In summary, our finding that PDGF-induced EGFR transactivation requires a heterodimeric complex of PDGFβR and EGFR provides a physical basis for PDGFβR-EGFR cross talk.
ACKNOWLEDGMENTS
This study was supported by grants HL49192 and HL59975 from the NHLBI to B.C.B., Banyu Fellowship in Lipid Metabolism and Atherosclerosis to Y.H. a Japan Heart Foundation & Bayer Yakuhin Research Grant Abroad to K.Y., and grant HA2868/1-1 from the Deutsche Forschungsgemeinschaft to J.H.
We thank Jun-ichi Abe for helpful discussions.
REFERENCES
- 1.Abe J, Berk B C. Fyn and JAK2 mediate Ras activation by reactive oxygen species. J Biol Chem. 1999;274:21003–21010. doi: 10.1074/jbc.274.30.21003. [DOI] [PubMed] [Google Scholar]
- 2.Abe J, Okuda M, Huang Q, Yoshizumi M, Berk B C. Reactive oxygen species activate p90 ribosomal S6 kinase via Fyn and Ras. J Biol Chem. 2000;275:1739–1748. doi: 10.1074/jbc.275.3.1739. [DOI] [PubMed] [Google Scholar]
- 3.Abe J, Takahashi M, Ishida M, Lee J D, Berk B C. c-Src is required for oxidative stress-mediated activation of big mitogen-activated protein kinase. 1. J Biol Chem. 1997;272:20389–20394. doi: 10.1074/jbc.272.33.20389. [DOI] [PubMed] [Google Scholar]
- 4.Bae Y S, Kang S W, Seo M S, Baines I C, Tekle E, Chock P B, Rhee S G. Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J Biol Chem. 1997;272:217–221. [PubMed] [Google Scholar]
- 5.Blake R A, Broome M A, Liu X, Wu J, Gishizky M, Sun L, Courtneidge S A. SU6656, a selective Src family kinase inhibitor, used to probe growth factor signaling. Mol Cell Biol. 2000;20:9018–9027. doi: 10.1128/mcb.20.23.9018-9027.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blake R A, Garcia-Paramio P, Parker P J, Courtneidge S A. Src promotes PKCdelta degradation. Cell Growth Differ. 1999;10:231–241. [PubMed] [Google Scholar]
- 7.Brar S S, Kennedy T P, Whorton A R, Murphy T M, Chitano P, Hoidal J R. Requirement for reactive oxygen species in serum-induced and platelet-derived growth factor-induced growth of airway smooth muscle. J Biol Chem. 1999;274:20017–20026. doi: 10.1074/jbc.274.28.20017. [DOI] [PubMed] [Google Scholar]
- 8.Burgaud J L, Baserga R. Intracellular transactivation of the insulin-like growth factor I receptor by an epidermal growth factor receptor. Exp Cell Res. 1996;223:412–419. doi: 10.1006/excr.1996.0096. [DOI] [PubMed] [Google Scholar]
- 9.Claesson W-L. Platelet-derived growth factor receptor signals. J Biol Chem. 1994;269:32023–32026. [PubMed] [Google Scholar]
- 10.Countaway J L, Girones N, Davis R J. Reconstitution of epidermal growth factor receptor transmodulation by platelet-derived growth factor in Chinese hamster ovary cells. J Biol Chem. 1989;264:13642–13647. [PubMed] [Google Scholar]
- 11.Daub H, Wallasch C, Lankenau A, Herrlich A, Ullrich A. Signal characteristics of G protein-transactivated EGF receptor. EMBO J. 1997;16:7032–7044. doi: 10.1093/emboj/16.23.7032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daub H, Weiss F U, Wallasch C, Ullrich A. Role of transactivation of the EGF receptor in signalling by G-protein-coupled receptors. Nature. 1996;379:557–560. doi: 10.1038/379557a0. [DOI] [PubMed] [Google Scholar]
- 13.Decker S J, Harris P. Effects of platelet-derived growth factor on phosphorylation of the epidermal growth factor receptor in human skin fibroblasts. J Biol Chem. 1989;264:9204–9209. [PubMed] [Google Scholar]
- 14.Griendling K K, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 15.Gutkind J S. The pathways connecting G protein-coupled receptors to the nucleus through divergent mitogen-activated protein kinase cascades. J Biol Chem. 1998;273:1839–1842. doi: 10.1074/jbc.273.4.1839. [DOI] [PubMed] [Google Scholar]
- 16.Hackel P O, Zwick E, Prenzel N, Ullrich A. Epidermal growth factor receptors: critical mediators of multiple receptor pathways. Curr Opin Cell Biol. 1999;11:184–189. doi: 10.1016/s0955-0674(99)80024-6. [DOI] [PubMed] [Google Scholar]
- 17.Hanke J H, Gardner J P, Dow R L, Changelian P S, Brisette W H, Weringer E J, Pollok B A, Connelly P A. Discovery of a novel, potent, and src family-selective tyrosine kinase inhibitor. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 18.Heeneman S, Haendeler J, Saito Y, Ishida M, Berk B C. Angiotensin II induces transactivation of two different populations of the PDGFβ-receptor: key role for the adaptor protein Shc. J Biol Chem. 2000;275:15926–15932. doi: 10.1074/jbc.M909616199. [DOI] [PubMed] [Google Scholar]
- 19.Heldin C H, Ostman A, Ronnstrand L. Signal transduction via platelet-derived growth factor receptors. Biochim Biophys Acta. 1998;1378:F79–F113. doi: 10.1016/s0304-419x(98)00015-8. [DOI] [PubMed] [Google Scholar]
- 20.Hicks C, Sleigh M J, Chesterman C N. Quantitation of platelet derived growth factor receptors on cells from human arterial segments. J Immunol Methods. 1994;170:83–92. doi: 10.1016/0022-1759(94)90248-8. [DOI] [PubMed] [Google Scholar]
- 21.Huyer G, Liu S, Kelly J, Moffat J, Payette P, Kennedy B, Tsaprailis G, Gresser M J, Ramachandran C. Mechanism of inhibition of protein-tyrosine phosphatases by vanadate and pervanadate. J Biol Chem. 1997;272:843–851. doi: 10.1074/jbc.272.2.843. [DOI] [PubMed] [Google Scholar]
- 22.Ishida T, Ishida M, Suero J, Takahashi M, Berk B C. Agonist-stimulated cytoskeletal reorganization and signal transduction at focal adhesions in vascular smooth muscle cells require c-Src. J Clin Invest. 1999;103:789–797. doi: 10.1172/JCI4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kovalenko M, Gazit A, Bohmer A, Rorsman C, Ronnstrand L, Heldin C H, Waltenberger J, Bohmer F D, Levitzki A. Selective platelet-derived growth factor receptor kinase blockers reverse sis-transformation. Cancer Res. 1994;54:6106–6114. [PubMed] [Google Scholar]
- 24.Kwatra M M, Bigner D D, Cohn J A. The ligand binding domain of the epidermal growth factor receptor is not required for receptor dimerization. Biochim Biophys Acta. 1992;1134:178–181. doi: 10.1016/0167-4889(92)90042-a. [DOI] [PubMed] [Google Scholar]
- 25.Li J, Kim Y N, Bertics P J. Platelet-derived growth factor-stimulated migration of murine fibroblasts is associated with epidermal growth factor receptor expression and tyrosine phosphorylation. J Biol Chem. 2000;275:2951–2958. doi: 10.1074/jbc.275.4.2951. [DOI] [PubMed] [Google Scholar]
- 26.Li X, Lee J W, Graves I M, Earp H S. Angiotensin II stimulates ERK via two pathways in epithelial cells: protein kinase C suppresses a G-protein coupled receptor-EGF receptor transactivation pathway. EMBO J. 1998;17:2574–2583. doi: 10.1093/emboj/17.9.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luttrell L M, Daaka Y, Lefkowitz R J. Regulation of tyrosine kinase cascades by G-protein-coupled receptors. Curr Opin Cell Biol. 1999;11:177–183. doi: 10.1016/s0955-0674(99)80023-4. [DOI] [PubMed] [Google Scholar]
- 28.Moriguchi Y, Matsubara H, Mori Y, Murasawa S, Masaki H, Maruyama K, Tsutsumi Y, Shibasaki Y, Tanaka Y, Nakajima T, Oda K, Iwasaka T. Angiotensin II-induced transactivation of epidermal growth factor receptor regulates fibronectin and transforming growth factor-beta synthesis via transcriptional and posttranscriptional mechanisms. Circ Res. 1999;84:1073–1084. doi: 10.1161/01.res.84.9.1073. [DOI] [PubMed] [Google Scholar]
- 29.Mukhin Y V, Garnovskaya M N, Collinsworth G, Grewal J S, Pendergrass D, Nagai T, Pinckney S, Greene E L, Raymond J R. 5-Hydroxytryptamine1A receptor/Gibetagamma stimulates mitogen-activated protein kinase via NAD(P)H oxidase and reactive oxygen species upstream of src in chinese hamster ovary fibroblasts. Biochem J. 2000;347:61–67. [PMC free article] [PubMed] [Google Scholar]
- 30.Okuda M, Takahashi M, Suero J, Murry C E, Traub O, Kawakatsu H, Berk B C. Shear stress stimulation of p130(cas) tyrosine phosphorylation requires calcium-dependent c-Src activation. J Biol Chem. 1999;274:26803–26809. doi: 10.1074/jbc.274.38.26803. [DOI] [PubMed] [Google Scholar]
- 31.Osherov N, Levitzki A. Epidermal-growth-factor-dependent activation of the src-family kinases. Eur J Biochem. 1994;225:1047–1053. doi: 10.1111/j.1432-1033.1994.1047b.x. [DOI] [PubMed] [Google Scholar]
- 32.Saito Y, Berk B C. Transactivation: a novel signaling pathway from angiotensin II to tyrosine kinase receptors. J Mol Cell Cardiol. 2001;33:3–7. doi: 10.1006/jmcc.2000.1272. [DOI] [PubMed] [Google Scholar]
- 33.Sundaresan M, Yu Z X, Ferrans V J, Irani K, Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270:296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- 34.Takeyama K, Dabbagh K, Jeong Shim J, Dao-Pick T, Ueki I F, Nadel J A. Oxidative stress causes mucin synthesis via transactivation of epidermal growth factor receptor: role of neutrophils. J Immunol. 2000;164:1546–1552. doi: 10.4049/jimmunol.164.3.1546. [DOI] [PubMed] [Google Scholar]
- 35.van Biesen T, Luttrell L M, Hawes B E, Lefkowitz R J. Mitogenic signaling via G protein-coupled receptors. Endocr Rev. 1996;17:698–714. doi: 10.1210/edrv-17-6-698. [DOI] [PubMed] [Google Scholar]
- 36.van der Vliet A, Hristova M, Cross C E, Eiserich J P, Goldkorn T. Peroxynitrite induces covalent dimerization of epidermal growth factor receptors in A431 epidermoid carcinoma cells. J Biol Chem. 1998;273:31860–31866. doi: 10.1074/jbc.273.48.31860. [DOI] [PubMed] [Google Scholar]
- 37.Walker F, Burgess A W. Reconstitution of the high affinity epidermal growth factor receptor on cell-free membranes after transmodulation by platelet-derived growth factor. J Biol Chem. 1991;266:2746–2752. [PubMed] [Google Scholar]
- 38.Walker F, deBlaquiere J, Burgess A W. Translocation of pp60c-src from the plasma membrane to the cytosol after stimulation by platelet-derived growth factor. J Biol Chem. 1993;268:19552–19558. [PubMed] [Google Scholar]
- 39.Waltenberger J, Uecker A, Kroll J, Frank H, Mayr U, Bjorge J D, Fujita D, Gazit A, Hombach V, Levitzki A, Bohmer F D. A dual inhibitor of platelet-derived growth factor-beta receptor and Src kinase activity potently interferes with motogenic and mitogenic responses to PDGF in vascular smooth muscle cells. Circ Res. 1999;85:12–22. doi: 10.1161/01.res.85.1.12. [DOI] [PubMed] [Google Scholar]
- 40.Wang D, Yu X, Cohen R A, Brecher P. Distinct effects of N-acetylcysteine and nitric oxide on angiotensin II-induced epidermal growth factor receptor phosphorylation and intracellular Ca(2+) levels. J Biol Chem. 2000;275:12223–12230. doi: 10.1074/jbc.275.16.12223. [DOI] [PubMed] [Google Scholar]
- 41.Yamauchi T, Ueki K, Tobe K, Tamemoto H, Sekine N, Wada M, Honjo M, Takahashi M, Takahashi T, Hirai H, Tsushima T, Akanuma Y, Fujita T, Komuro I, Yazaki Y, Kadowaki T. Growth hormone-induced tyrosine phosphorylation of EGF receptor as an essential element leading to MAP kinase activation and gene expression. Endocr J. 1998;45(Suppl.):S27–S31. doi: 10.1507/endocrj.45.suppl_s27. [DOI] [PubMed] [Google Scholar]
- 42.Yamauchi T, Ueki K, Tobe K, Tamemoto H, Sekine N, Wada M, Honjo M, Takahashi M, Takahashi T, Hirai H, Tushima T, Akanuma Y, Fujita T, Komuro I, Yazaki Y, Kadowaki T. Tyrosine phosphorylation of the EGF receptor by the kinase Jak2 is induced by growth hormone. Nature. 1997;390:91–96. doi: 10.1038/36369. [DOI] [PubMed] [Google Scholar]