Abstract
Medication during pregnancy is widespread, but there are few reports on its fetal safety. Recent studies suggest that medication during pregnancy can affect fetal morphological and functional development through multiple pathways, multiple organs, and multiple targets. Its mechanisms involve direct ways such as oxidative stress, epigenetic modification, and metabolic activation, and it may also be indirectly caused by placental dysfunction. Further studies have found that medication during pregnancy may also indirectly lead to multi-organ developmental programming, functional homeostasis changes, and susceptibility to related diseases in offspring by inducing fetal intrauterine exposure to too high or too low levels of maternal-derived glucocorticoids. The organ developmental toxicity and programming alterations caused by medication during pregnancy may also have gender differences and multi-generational genetic effects mediated by abnormal epigenetic modification. Combined with the latest research results of our laboratory, this paper reviews the latest research progress on the developmental toxicity and functional programming alterations of multiple organs in offspring induced by medication during pregnancy, which can provide a theoretical and experimental basis for rational medication during pregnancy and effective prevention and treatment of drug-related multiple fetal-originated diseases.
Key words: Medication, Pregnancy, Multiple organs, Developmental toxicity, Developmental programming, Maternal-derived glucocorticoids, Gender differences, Multi-generational genetic effects
Graphical abstract
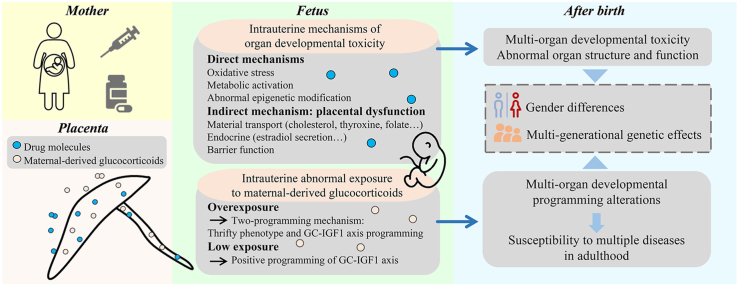
This review summarizes the intrauterine mechanisms, gender differences, and multi-generational genetic effects of offspring's multi-organ developmental toxicity, programming alterations and susceptibility to multiple diseases induced by medication during pregnancy.
1. Introduction
It is well known that medication during pregnancy is prevalent. Clinical studies in many countries have shown that more than 80% of pregnant women have used drugs during pregnancy1, 2, 3, mainly including antiemetics, antibiotics, and analgesics4. Since the storm in 1960s caused by the deformity of offspring due to the use of thalidomide during pregnancy, the fetal safety of medication during pregnancy has attracted more and more attention. Epidemiological investigations have shown that various drugs, such as anticoagulants, antiepileptics, and antihistamines, have fetal developmental toxicity, leading to miscarriage, birth defects, and other adverse pregnancy outcomes5, 6, 7. Many experimental studies have found that medication during pregnancy can result in multi-organ developmental toxicity and programming alterations in offspring, manifested as organ structure and function abnormalities and enhanced susceptibility to various adult chronic diseases in offspring. The “Developmental Origins of Health and Disease (DOHaD)” theory has been expanded in many fields since it was creatively opened up8, and the “DOHaD” theory is the best analysis of offspring's organ developmental programming, functional homeostasis changes, and susceptibility to related adult diseases owing to medication during pregnancy. Given the “double-edged sword” effect of medication during pregnancy on the mother and fetus, clinicians must meticulously weigh the risks and benefits of prenatal drug use and formulate a reasonable medication plan for pregnant women. This paper comprehensively reviews the intrauterine mechanisms, gender differences, and multi-generational genetic effects of offspring's multi-organ developmental toxicity and functional programming alterations caused by medication during pregnancy, which has important theoretical and practical significance for deeply analyzing “DOHaD” theory, guiding appropriate drug use during pregnancy, and effectually preventing and treating drug-related diseases of fetal origin.
2. Multi-organ developmental toxicity in offspring induced by medication during pregnancy and the mechanisms
Organ developmental toxicity refers to the structural and functional impairment of offspring's organs caused by exposure to adverse environments during pregnancy. During pregnancy, the adverse environment includes environmental factors (physical, chemical and biological factors) and maternal factors (maternal diseases, malnutrition and stress). Among them, drugs are one of the environmental inducing factors fully demonstrated to cause organ development toxicity.
2.1. Characteristics of multi-organ developmental toxicity in offspring induced by medication during pregnancy
Intrauterine organ development is vulnerable to adverse environments such as prenatal drug use, and many different kinds of drugs have been shown to damage the development of the same organ (Table 19, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56). The liver is the crucial organ for the metabolism of many exogenous compounds, including drugs. Studies have confirmed that hepatic development is susceptible to many kinds of drugs, including antibiotics9, 10, 11, 12, 13, 14, synthetic glucocorticoids15,16, antithyroid drugs17, hypoglycemic agents18, non-steroidal anti-inflammatory drugs (NSAIDs)19, antipsychotics20, antipyretic-analgesic drugs21, traditional Chinese medicine (or its ingredients)22, 23, 24, 25, 26, 27 and so on. Hippocampal, bone, and gonadal development are also vulnerable to a variety of drugs. Anesthetics28, 29, 30, 31, 32, antipsychotics33, 34, 35, 36, and synthetic glucocorticoids37 have been shown to affect hippocampal development; synthetic glucocorticoids38,39, antibiotics12,40, anticancer drugs41,42, antimalarials43, antiviral drugs44, NSAIDs45, and synthetic estrogen46 have been shown to affect bone development and chondrogenesis; NSAIDs47, 48, 49, antipyretic-analgesic drugs50,51, synthetic glucocorticoids52,53, synthetic estrogens54,55, and hypoglycemic agents56 can affect testicular or ovarian development. It is not difficult to find that studies on drug-induced organ developmental toxicity are mainly based on animal models. However, some findings in humans have been reported. For example, the use of phenobarbital, clindamycin, and tenofovir during pregnancy has been shown to respectively cause reduced hepatic functional maturity, bone deformities and lower bone mineral content in human offspring20,40,44. It is worth noting that thanks to mature in vitro culture and xenotransplantation techniques of human testis and ovaries, studies have proved that ibuprofen, acetaminophen and metformin can affect the development of human gonadal function48, 49, 50, 51,56.
Table 1.
Developmental toxicity of multiple drugs in the same organ.
| Organ | Drug type | Drug name | Dosage regimen | Developmental toxicity manifestation | Ref. |
|---|---|---|---|---|---|
| Liver | Antibiotic | Ciprofloxacin | 40 mg/kg·day, GD7–17, i.p. | Abnormal blood vessels and nuclear pyknosis in fetal rat liver | 9 |
| Erythromycin | 14.2 mg/kg·day, GD14–19, i.p. | Focal hepatic necrosis in offspring mice | 10 | ||
| Azithromycin | 150 mg/kg·day, GD9–18, i.p. | Vacuolation of fetal mouse hepatocytes and abnormal glucose and lipid metabolism | 11 | ||
| Doxycycline | 5 mg/kg·day, GD6–14, i.g. | Increased necrosis of fetal rat hepatocytes | 12 | ||
| Triclosan | 0.55 μmol/L, 6–120 hpf | Increased apoptosis of hepatocytes in zebrafish larvae | 13 | ||
| Isoniazid | 4 mmol/L, 6–72 hpf | Loose cell-to-cell contacts and large vacuoles in hepatocytes of zebrafish embryos | 14 | ||
| Synthetic glucocorticoids | Dexamethasone | 0.2 mg/kg·day, GD9–21, s.c. | Decreased proliferation of fetal rat hepatocytes | 15 | |
| Betamethasone | 0.1 mg/day, GD14, s.c. | Liver growth retardation in mice | 16 | ||
| Antithyroid drug | Methimazole | 4.5 mg/kg·day, GD9–18, i.g. | Abnormal structure and glucose and lipid metabolism of fetal mouse liver | 17 | |
| Hypoglycemic agent | Metformin | 250 mg/kg·day, GD0–18, p.o. | Abnormal differentiation and glucose metabolism of fetal mouse liver | 18 | |
| NSAID | Indomethacin | 13.97 μmol/L, 24–168 hpf | Suppressed hepatogenesis of zebrafish embryo | 19 | |
| Antipsychotic | Phenobarbital | 一 | Reduced functional maturity of human offspring liver | 20 | |
| Antipyretic-analgesic drug | Acetaminophen | 250 mg/kg·day, GD12, i.p. | Declined hematopoietic stem cell frequency in fetal mouse liver | 21 | |
| Traditional Chinese medicine | Monocrotaline | 20 mg/kg·day, GD9–20, i.g. | Decreased liver weight and liver index of fetal rats | 22 | |
| Retrorsine | 20 mg/kg·day, GD9–20, i.g. | Reduced hepatocyte numbers and potentiated hepatic apoptosis in fetal rats | 23 | ||
| Triptolide | 0.8 μmol/L, 72–120 hpf | Hepatocytes necrosis and decreased liver volume of zebrafish embryos | 24 | ||
| Xiaoaiping | 0.4 mg/mL, 6–120 hpf | Loose cell-to-cell contacts of zebrafish embryo hepatocytes | 25 | ||
| Psoralen | 3.54 μmol/L, 4–96 hpf | Reduced liver area of zebrafish embryos | 26 | ||
| Aconitine | 7.27 μmol/L, 4–96 hpf | Liver growth retardation in zebrafish | 27 | ||
| Hippocampus | Anesthetic | Isoflurane | 3%, GD14, inhale | Abnormal hippocampal structure and dysfunction of memory in offspring rats | 28 |
| Ketamine | 40–60 mg/kg·h for 2 h, GD14, i.v. | Hippocampal neuron injury, memory impairment, and mental disorder in offspring rats | 29 | ||
| Sevoflurane | 2.5% for 2 h, GD15–17, inhale | Inhibition of fetal rat hippocampus neurogenesis and impaired memory in offspring | 30 | ||
| Propofol | 20 mg/kg·h for 4 h, GD18, i.v. | Impaired learning and memory in offspring rats | 31 | ||
| Cocaine | 40 mg/kg·day, GD8–18, s.c. | Cognitive impairment in offspring mice | 32 | ||
| Antipsychotic | Quetiapine | 55 mg/kg·day, GD6–21, p.o. | Neuronal cell deficit and enhanced apoptotic neurodegeneration in fetal rat hippocampus | 33 | |
| Haloperidol | 10 mL/kg·day, GD6–16, i.p. | Abnormal hippocampal structure and impaired memory in offspring rats | 34 | ||
| Risperidone | 10 mL/kg·day, GD6–16, i.p. | Abnormal hippocampal structure and impaired memory in offspring rats | 34 | ||
| Phenytoin | 50 mg/kg·day, GD0–20, i.g. | Impaired learning in offspring rats | 35 | ||
| Carbamazepine (CBZ) | 3.5 g CBZ/kg in fodder, GD0–20, p.o. | Decrease of hippocampal neurons in newborn mice | 36 | ||
| Synthetic glucocorticoids | Dexamethasone | 0.2 mg/kg·day, GD9–20, s.c. | Decreased proliferation and increased apoptosis of fetal rat hippocampal cells and cognitive impairment | 37 | |
| Bone/Cartilage | Synthetic glucocorticoids | Dexamethasone | 0.8 mg/kg·day, GD12–14, s.c. | Bone and cartilage growth retardation in mice | 38,39 |
| Antibiotic | Doxycycline | 5 mg/kg·day, GD6–14, i.g. | Shortness in the ulna and radius bones of fetal rats | 12 | |
| Clindamycin | 一 | Skeletal malformations in human offspring | 40 | ||
| Anticancer drug | 5-Fluoro-2′-deoxyuridine | 55 mg/kg·day, GD11, s.c. | Thoracic and lumbar vertebra and sternum variations of fetal rats | 41 | |
| 6-Mercaptopurine-riboside | 7 mg/kg·day, GD11, s.c. | Thoracic and lumbar vertebra and sternum variations of fetal rats | 41 | ||
| Cyclophosphamide | 一 | Long bone hypoplasia of human offspring | 42 | ||
| Antimalarial | Artesunate | 15 mg/kg·day, GD9–11, i.g. | Fetal rat limb deformity | 43 | |
| Antiviral agent | Tenofovir | 一 | Lower bone mineral content in human newborn | 44 | |
| NSAID | Indomethacin | 10−5 mol/L for 24 h | Proliferation suppression and increased cell death of fetal rat chondrocytes | 45 | |
| Ketorolac | 10−5 mol/L for 24 h | Proliferation suppression and increased cell death of fetal rat chondrocytes | 45 | ||
| Diclofenac | 10−5 mol/L for 24 h | Proliferation suppression and increased cell death of fetal rat chondrocytes | 45 | ||
| Piroxicam | 10−5 mol/L for 24 h | Proliferation suppression and increased cell death of fetal rat chondrocytes | 45 | ||
| Synthetic estrogen | Diethylstilbestrol | 10 μg/kg·day, GD9–16, s.c. | Shorter femurs in female offspring mice | 46 | |
| Gonad | NSAID | Indomethacin | 10 μmol/L for 24 h | Decreased testosterone secretion in fetal rat testes | 47 |
| Ibuprofen | 10−5 mol/L for 72 h/10 μmol/L for 7 days | Decreased testosterone production in human fetal testes/Increased cell apoptosis and necrosis in human fetal ovaries | 48,49 | ||
| Antipyretic-analgesic drug | Acetaminophen | 60 mg/kg·day for 7 days, p.o./10 μmol/L for 7 days | Decreased testosterone production in human fetal testis xenografts/Reduced germ cells in human fetal ovaries | 50,51 | |
| Synthetic glucocorticoids | Dexamethasone | 0.2 mg/kg·day, GD9–20, s.c./0.5 mg/kg·day, GD16–18, s.c. | Abnormal morphology and decreased testosterone production of offspring rats/Decreased volume and increased germ cell apoptosis of fetal rat ovaries | 52,53 | |
| Synthetic estrogen | Diethylstilbestrol | 一/100 μg/kg·day, GD9–16, s.c. | Cryptorchidism in human offspring/Ovarian malformation in offspring mice | 54,55 | |
| Hypoglycemic agent | Metformin | 500 μmol/L for 72 h | Reduced testosterone secretion in human fetal testes | 56 |
GD, gestational day; i.p., intraperitoneal injection; i.g., intragastrically; hpf, hours post-fertilization; s.c., subcutaneous injection; p.o., orally; NSAID, non-steroidal anti-inflammatory drug; i.v., intravenous injection.
Studies have also found that a single drug may simultaneously impair the development of multiple organs, such as dexamethasone20,37, 38, 39,52,53,57, 58, 59, betamethasone16,60,61, diethylstilbestrol54,55,62,63, metformin18,56,64, acetaminophen21,50,51, indomethacin19,45,47, diclofenac45,65, phenobarbital20,66, Xiaoaiping25, and psoralen26 (Table 216,18, 19, 20, 21,25,26,37, 38, 39,45,47,50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66). Notably, the developmental toxicity of multiple organs caused by drugs used during pregnancy is often triggered through multiple targets. For example, in utero, dexamethasone can respectively up-regulate hepatic forkhead protein O1, inhibit hippocampal cAMP responsive element binding protein/brain-derived neurotrophic factor/tropomyosin receptor tyrosine B pathway, inhibit cartilage transforming growth factor β signaling pathway, down-regulate testicular steroidogenic acute regulatory protein, and inhibit adrenal cytochrome P450 cholesterol side chain cleavage enzyme, which affects the development of the corresponding organs15,37,38,52,59. Therefore, the developmental toxicity of drugs often has multi-organ and multi-target effects.
Table 2.
Multi-organ developmental toxicities of a single drug.
| Drug name | Organ | Dosage regimen | Developmental toxicity manifestation | Ref. |
|---|---|---|---|---|
| Dexamethasone | Liver | 一 | Reduced functional maturity of human offspring liver | 20 |
| Hippocampus | 0.2 mg/kg·day, GD9–20, s.c. | Decreased proliferation and increased apoptosis of fetal rat hippocampal cells and cognitive impairment | 37 | |
| Bone/Cartilage | 0.8 mg/kg·day, GD12–14, s.c. | Bone and cartilage growth retardation in mice | 38,39 | |
| Testis | 0.2 mg/kg·day, GD9–20, s.c. | Abnormal morphology and decreased testosterone production of male offspring rats | 52 | |
| Ovary | 0.5 mg/kg·day, GD16–18, s.c. | Decreased volume and increased germ cell apoptosis of fetal rat ovaries | 53 | |
| Kidney | 125 μg/kg·day, GD20–23, s.c. | Reduced nephron number in fetal spiny mice | 57 | |
| Heart | 0.1 mg/kg·day, GD12–15, s.c. | Impaired cardiac diastolic function in fetal rats | 58 | |
| Adrenal gland | 8 mg/kg·day, GD12–18, s.c. | Decreased synthesis of cortisol in fetal mouse adrenal | 59 | |
| Betamethasone | Liver | 0.1 mg/day, GD14, s.c. | Liver growth retardation in mice | 16 |
| Kidney | 0.17 mg/kg·day, GD80–81, i.m. | Decrease of nephrons and glomerular filtration rate of sheep offspring | 60 | |
| Adrenal gland | 一 | Decreased adrenal volume in human infants | 61 | |
| Diethylstilbestrol | Bone | 10 μg/kg·day, GD11–14, s.c. | Reduced bone mineral content in male offspring mice | 62 |
| Testis | 一 | Cryptorchidism and epididymal cyst in human male offspring | 54 | |
| Ovary | 100 μg/kg·day, GD9–16, s.c. | Ovarian malformation in female offspring mice | 55 | |
| Prostate | 0.02 μg/kg·day, GD13–18, s.c. | Prostate enlargement in offspring mice | 63 | |
| Metformin | Liver | 250 mg/kg·day, GD0–18, p.o. | Abnormal differentiation and glucose metabolism of fetal mouse liver | 18 |
| Testis | 500 μmol/L for 72 h | Reduced testosterone secretion in human fetal testes | 56 | |
| Pancreas | 100 μmol/L for 35 days | Abnormal pancreatic differentiation from human embryonic stem cells | 64 | |
| Acetaminophen | Liver | 250 mg/kg·day, GD12, i.p. | Declined hematopoietic stem cell frequency in fetal mouse liver | 21 |
| Testis | 60 mg/kg·day for 7 days, p.o. | Decreased testosterone production in human fetal testes | 50 | |
| Ovary | 10 μmol/L for 7 days | Reduced germ cells in human fetal ovaries | 51 | |
| Airway | 250 mg/kg·day, GD12, i.p. | Airway inflammation in mouse offspring | 21 | |
| Indomethacin | Liver | 13.97 μmol/L, 24–168 hpf | Decreased proliferation of zebrafish embryo hepatocytes | 19 |
| Cartilage | 10−5 mol/L for 24 h | Proliferation suppression and increased cell death of fetal rat chondrocytes | 45 | |
| Testis | 10 μmol/L for 24 h | Decreased testosterone secretion in fetal rat testes | 47 | |
| Diclofenac | Cartilage | 10−5 mol/L for 24 h | Proliferation suppression and increased cell death of fetal rat chondrocytes | 45 |
| Artery | 10 mg/kg, once, i.v. | Ductus arteriosus constriction and pulmonary hypertension in fetal rats | 65 | |
| Phenobarbital | Liver | 一 | Reduced functional maturity of human offspring liver | 20 |
| Adrenal gland | 250 μmol/L for 24 h | Decreased synthesis of cortisol, aldosterone, and progesterone in human fetal adrenal cortical cells | 66 | |
| Xiaoaiping | Liver | 0.4 mg/mL, 6–120 hpf | Loose cell-to-cell contacts of zebrafish embryo hepatocytes | 25 |
| Heart | 0.8 mg/mL, 6–120 hpf | Thinner heart walls and pericardial edema in zebrafish embryos | 25 | |
| Psoralen | Liver | 3.54 μmol/L, 4–96 hpf | Reduced liver area of zebrafish embryos | 26 |
| Heart | 3.54 μmol/L, 4–72 hpf | Increased pericardial area of zebrafish embryos | 26 |
GD, gestational day; s.c., subcutaneous injection; i.m., intramuscular injection; p.o., orally; i.p., intraperitoneal injection; hpf, hours post-fertilization; i.v., intravenous injection.
Sensitivity to drug-induced organ developmental toxicity on offspring differs in different gestational trimesters. The first trimester is the pivotal period for organogenesis67, so medication during this period often causes severe damage to offspring's organ development, such as deformity. For example, epidemiological studies have shown that exposure to cyclophosphamide and macrolides in early pregnancy significantly increases the risk of bone and digestive malformation in offspring, respectively, while the risk of teratogenesis is low when they are used in late pregnancy42,68. It is worth noting that sensitivity to drug-induced organ developmental toxicity may also be different in the second and third trimesters. For instance, the use of dexamethasone in the second trimester of pregnancy can more seriously affect the development of long bone and articular cartilage in offspring mice than in the third trimester38,39. Conversely, aspirin use in the third trimester can cause kidney damage in offspring, while its use in the second trimester is relatively safe69. Therefore, it is vital to explore the “toxic time window” of drugs on the intrauterine development of different organs for choosing proper medication timing during pregnancy.
2.2. Direct mechanisms of multi-organ developmental toxicity in offspring induced by medication during pregnancy
Drug molecules in maternal blood can be transferred across the placenta through passive diffusion, carrier-mediated transport, and endocytosis pathways. To some extent, almost all drugs can enter the fetal circulation across the placenta in one of these ways70 and then directly act on various fetal organs and affect their development. There are diverse direct mechanisms of organ development toxicity of drugs, mainly including oxidative stress injury, abnormal epigenetic modification, and metabolic activation.
2.2.1. Oxidative stress injury
There are both oxidation and antioxidant systems in the body. The oxidation system principally includes reactive oxygen species (ROS) and other active substances, while the antioxidant system includes superoxide dismutase (SOD), glutathione peroxidase, catalase, and reduced glutathione71. Oxidative stress refers to the pathological process where ROS production and the antioxidant defense in the body are out of balance under adverse conditions, which results in oxidative damage to tissues and cells. Exogenous substances can increase ROS production in the fetus and interfere with the key signals of organogenesis, thereby affecting the development of fetal organs72. The organ development toxicity of some drugs is closely related to oxidative stress. For example, the use of erythromycin during pregnancy can significantly increase the level of malondialdehyde (MDA) and decrease the level of reduced glutathione in offspring mouse liver, which suggests the occurrence of oxidative stress injury10; the use of ketamine during pregnancy can increase the autophagy rate and apoptosis rate of the fetal mouse hippocampus, which may be associated with the inhibition of antioxidant defense and excessive accumulation of ROS73. Zebrafish is a popular alternative animal model for drug toxicology research, and the organ development toxicity of many drugs mediated by oxidative stress has been proved in this model. The pathological lesion of zebrafish embryonic hepatic tissue caused by exposure to triclosan, Xiaoaiping, psoralen, and isoniazid is accompanied by increased production of ROS and MDA and decreased SOD activity13,14,25,26. In zebrafish embryos exposed to aconitine, the level of ROS increases in a dose-dependent manner, while the activity of SOD decreases, which leads to malformations and abnormal cardiac and hepatic structures of zebrafish offspring27. Exposure to acetyl-11-keto-beta-boswellic acid, a Chinese herbal medicine component, increases MDA levels in a dose-dependent manner and reduces catalase and glutathione peroxidase activities in zebrafish larvae, leading to their decreased heart rate and cardiac structural abnormalities such as pericardial edema74. In summary, oxidative stress injury is an important mechanism for the organ development toxicity of many drugs, mainly including Chinese herbal medicines such as Xiaoaiping, psoralen, aconitine, and acetyl-11-keto-beta-boswellic acid.
2.2.2. Abnormal epigenetic modification
Epigenetics refers to changes in phenotype without a change in DNA sequence. During intrauterine physiological development, epigenetic modification is vital for cell differentiation and organ development75. However, medication during pregnancy may lead to abnormal epigenetic modification in the fetus and thus damage fetal organ development.
DNA methylation and histone acetylation are important forms of epigenetic modification, and their level changes can mediate the impairment of organ function in offspring induced by medication during pregnancy. For example, prenatal cocaine exposure can promote the expression of DNA methyltransferase, increase the methylation levels of insulin-like growth factor 2 promoter region, and inhibit its expression in the offspring mouse hippocampus, which results in cognitive defects in offspring mice32. The organ development toxicity of dexamethasone is related to abnormal histone modification. Prenatal dexamethasone exposure (PDE) can enhance the expression of histone deacetylase (HDAC) 2 and reduce the histone H3 lysine 14 acetylation (H3K14ac) level of brain-derived neurotrophic factor and its expression in the fetal rat hippocampus, which weakens the memory ability of offspring rats76. PDE can also increase the expression of HDAC7 and then reduce the histone H3 lysine 9 acetylation (H3K9ac) level of the promoter region of steroidogenic acute regulatory protein and its expression in fetal rat testes, which impairs testosterone production in offspring rats52. In addition, prenatal betamethasone exposure can lead to abnormal DNA methylation and H3K9ac in the promoter region of specific genes in the hippocampus of fetal guinea pigs and affect the expression of these genes77, which may damage the hippocampal function of offspring guinea pigs.
Non-coding RNA is widely involved in embryonic development, and medication during pregnancy can also affect fetal post-transcriptional gene expression and organ function by targeting specific non-coding RNA. For example, exposure to metformin during pregnancy can promote the expression of H19 long-chain non-coding RNA and cause the hypomethylation and high expression of hepatocyte nuclear factor 4 α in fetal mice, leading to the activation of the gluconeogenetic program and abnormal glucose metabolism function of fetal mouse liver78. PDE can promote the expression of miRNA-134-5p and inhibit the expression of sex determining region Y-box 2 in the fetal rat hippocampus, which contributes to depressive behavior in offspring rats79. Furthermore, the hepatotoxic effect of triptolide on zebrafish larvae is accompanied by a decrease in the expression of miRNA-12224.
In brief, medication during pregnancy can lead to changes in DNA methylation and histone acetylation levels of particular gene promoter regions and changes in specific non-coding RNA in offspring organs, which affect the development of the corresponding organs.
2.2.3. Metabolic activation
Metabolic activation refers to the biotransformation process of transforming drugs or other foreign substances from non-toxic or low toxic substances to highly toxic metabolites. The metabolic activation of drugs requires specific enzymes, and cytochrome P450 (CYP) plays a significant role in fetal drug metabolism. For instance, CYP3A7 is the primary enzyme for glyburide and oxycodone metabolism in the fetal liver80,81. Drugs are transformed into corresponding active metabolites by CYP in the fetus, which can act on organ tissues and cause organ toxicity. Cyclophosphamide is metabolized into 4-hydroxycyclophosphamide, phosphoramide mustard, and acrolein by CYP, and these metabolites have been proven toxic to bone development, which can cause hypoplasia of radius, ulna, and tibia42. Both monocrotaline and retrorsine are pyrrolizidine alkaloids found in Chinese herbal medicine, and the dihydropyrrole transformed from pyrrolizidine alkaloids by CYP can result in hepatocyte injury in mice82. Further studies have found that exposure to monocrotaline and retrorsine during pregnancy can promote the expression of hepatic CYP3A in female fetal rats by activating the pregnane X receptor, which increases the production of toxic metabolites and causes hepatotoxicity22,23. Moreover, the expression of CYP and other metabolism-related genes is abnormal in zebrafish larvae exposed to indomethacin, and their weight, tail length, deformity rate and edema rate are noticeably correlated with these gene expressions, which suggests that metabolic activation plays an essential role in the developmental toxicity of indomethacin19. However, the relationship between the developmental toxicity of indomethacin to specific organs and metabolic activation remains to be further explored.
Additionally, single nucleotide variation and copy number variation of CYP genes are the main factors leading to the interindividual differences in drug toxicity83. Genetic variations in CYP can alter fetal drug exposure levels84, which may be the inducement of interindividual variability in drug-induced developmental toxicity. For example, a clinical study has found that the risk of intrauterine growth retardation (IUGR) due to prenatal alcohol exposure is associated with CYP genetic polymorphisms85. There is also interindividual variability in the organ development toxicity of offspring caused by medication during pregnancy86. Therefore, CYP genetic polymorphisms are potentially a critical factor of interindividual variability in drug-induced organ developmental toxicity.
In conclusion, the drug metabolic activation in the fetus primarily mediated by CYP can affect the fetus's particular organ development, and interindividual differences in drug-induced organ developmental toxicity may be related to CYP genetic polymorphisms.
2.3. Placental mechanism of multi-organ developmental toxicity in offspring induced by medication during pregnancy (the indirect mechanism)
As a special organ for the communication between the fetus and the mother, the placenta has the functions of material transport, endocrine, and barrier, which are essential for fetal survival and development87. The impaired placental function can lead to IUGR88, and IUGR individuals exhibit multiple organ dysplasia89. It has been shown that some drugs may indirectly induce multi-organ developmental toxicity by affecting the functional development of the placenta.
The placenta participates in exchanging hormones, nutrients, and metabolic wastes between the mother and the fetus. Studies have found that various drugs with organ development toxicity can affect placental transport function. For instance, dexamethasone can inhibit the expression of placental cholesterol transporters by down-regulating liver X receptor α in the rat placenta90; acetaminophen can inhibit the expression of thyroxine transporter and transferrin in the rat placenta, and can also affect the placental permeability of sucrose91; metformin can inhibit the uptake of folate and glucose in human placental cells92. Additionally, phenytoin and carbamazepine can also affect the folate uptake in human placental cells93, and they can simultaneously affect the hippocampal development that requires folate35,36,94.
The placenta is a highly active endocrine organ that secretes various hormones regulating fetal development. Using drugs during pregnancy may affect the endocrine function of the placenta. For example, estradiol is crucial for the development of fetal reproductive organs, and acetaminophen can inhibit estradiol secretion in human placental cells, which suggests that prenatal acetaminophen exposure may affect gonad development by inhibiting placental estradiol secretion95.
The barrier function of the placenta creates an independent developmental environment for the fetus, and efflux is an integral part of this function. Breast cancer resistance protein and multidrug resistance protein (MDR) are efflux transporters highly expressed in the placenta96, and their expression can be affected by drugs. For example, acetaminophen can inhibit the expression of breast cancer resistance protein in rat placenta97; valproic acid can reduce the expression of MDR1b in rat placenta but can increase that of MDR1a98.
In summary, some drugs can affect placental material transport, endocrine, and barrier functions, and abnormal placental function may be an important factor for these drugs to affect fetal organ development.
3. Maternal-derived glucocorticoids and multi-organ developmental programming of offspring induced by medication during pregnancy
The permanent changes of fetal structure and function to adapt to the changes of the uterine environment are called early life programming. The change of intrauterine maternal-derived glucocorticoid level is an essential factor to induce this programming99,100. Appropriate glucocorticoid level in utero is of paramount importance for fetal development101. However, the adverse environment during pregnancy can cause the fetus to be exposed to abnormal levels of maternal-derived glucocorticoids, leading to abnormal fetal development102. As a kind of prenatal adverse environment, medication during pregnancy may indirectly change the multi-organ developmental programming of offspring through intrauterine maternal-derived glucocorticoids.
3.1. Abnormal maternal-derived glucocorticoid exposure induced by medication during pregnancy and the mechanisms
It is known that glucocorticoids in fetal circulation are mainly derived from the mother. The maternal glucocorticoid level during pregnancy is much higher than that of the fetus, which suggests that there must be a glucocorticoid barrier between the mother and the fetus103. The change in the maternal glucocorticoid level or the destruction of the placental glucocorticoid barrier can lead to abnormal maternal-derived glucocorticoid levels in the fetus. A clinical study has found that cord blood cortisol concentrations in newborns of appropriate gestational age range from 4.7 to 15.4 μg/dL. When the fetal blood cortisol level is significantly higher and lower than this range, it respectively represents “overexposure” and “low exposure” to maternal-derived glucocorticoids. Fetal physiological glucocorticoid levels are different in different species104. Some drugs used during pregnancy have been shown to expose the fetus to too high or too low concentrations of maternal-derived glucocorticoids in utero.
Drugs can cause fetal overexposure to maternal-derived glucocorticoids by destroying the placental glucocorticoid barrier. 11β-hydroxysteroid dehydrogenase 2 (11β-HSD2) is one of the critical factors that constitute the placental glucocorticoid barrier. 11β-HSD2 is expressed in the syncytial layer of the placental villi, which is capable of converting biologically active cortisol into inactive cortisone so as to protect the fetus from the influence of the high maternal-derived glucocorticoid level. Adverse conditions during pregnancy such as hypoxia and nutritional restriction can affect the expression of placental 11β-HSD2105, and the inhibitory effect of many drugs on the expression of 11β-HSD2 has also been proved. Studies have found that carbenoxolone, nicotine, caffeine, triclosan, and diethylstilbestrol can inhibit the activity of 11β-HSD2 in the placenta, which destroys the placental glucocorticoid barrier and causes fetal overexposure to maternal-derived glucocorticoids in utero106, 107, 108, 109, 110. Moreover, bortezomib, licorice, itraconazole, and posaconazole are all inhibitors of 11β-HSD2111,112, which may also damage the placental glucocorticoid barrier. P-glycoprotein is also a critical component of the placental glucocorticoid barrier. As an efflux transporter highly expressed in the placenta, P-glycoprotein can reverse the concentration gradient to expel glucocorticoids from the fetus back to the mother, thereby reducing the level of maternal-derived glucocorticoids in the fetus113. Cannabidiol, ethanol, and opioid drugs methadone and buprenorphine can inhibit the efflux function of P-glycoprotein in the placenta, thus destroying the placental glucocorticoid barrier114, 115, 116. Additionally, mefloquine, primaquine, and antidepressants fluoxetine, clomipramine, paroxetine, and venlafaxine can also inhibit P-glycoprotein117, 118, 119, which may induce fetal overexposure to maternal-derived glucocorticoids.
In addition, drugs can also lead to fetal low exposure to maternal-derived glucocorticoids by reducing the level of glucocorticoids in the mother. A study has found that the use of dexamethasone during pregnancy can significantly reduce the level of cortisol in neonatal cord blood; the animal experiment has confirmed that PDE can inhibit maternal adrenal steroid synthesis, which decreases the glucocorticoid level in the mother and the level of maternal-derived glucocorticoids in the fetus120. In addition, prenatal metyrapone exposure can also inhibit maternal steroid synthesis and thus reduce fetal maternal-derived glucocorticoid level with decreased placental glucocorticoid receptor (GR) expression121.
3.2. Overexposure to maternal-derived glucocorticoids and multi-organ developmental programming alterations in offspring
Prenatal exposure to drugs such as caffeine and nicotine and the drug solvent ethanol can indirectly change the developmental programming of offspring's multiple organs (such as adrenal glands, liver, hippocampus, and bones) through high maternal-derived glucocorticoids.
3.2.1. Adrenal developmental programming alterations
As an organ for synthesizing and secreting steroids, the functional development of the adrenal gland is of significant importance for individual health. Prenatal exposure to excessively high levels of glucocorticoids can disrupt the homeostasis of offspring's adrenal function after birth, leading to offspring getting prone to diseases in adolescence and adulthood122. Medication during pregnancy is an important inducement of fetal adrenal overexposure to maternal-derived glucocorticoids. For example, prenatal nicotine exposure (PNE) can induce fetal intrauterine overexposure to maternal-derived glucocorticoids, which can inhibit the expression of adrenal steroid synthase in fetal rats107. The abnormal adrenal function of offspring rats caused by prenatal caffeine exposure (PCE) and prenatal ethanol exposure (PEE) involves “two programming”. On the one hand, intrauterine overexposure to maternal-derived glucocorticoids induced by PCE and PEE can inhibit the expression of adrenal steroid synthase in fetal rats, and this effect can continue after birth; on the other hand, maternal-derived glucocorticoids with a high concentration in utero can down-regulate insulin-like growth factor 1 (IGF1) and inhibit steroidogenesis in fetal rat adrenal glands, while the level of IGF1 increases in offspring rats separated from intrauterine high glucocorticoid environment after birth, which can induce their catch-up growth and adrenal steroidogenesis to tend to be normal108,123. A further study has shown that the adrenal steroid secretion in the PCE offspring rats is enhanced under chronic stress after birth, and thus the level of glucocorticoids in blood increases, which weakens the adrenal steroidogenic function by inhibiting the IGF1 signaling pathway in a negative-feedback way124. In summary, intrauterine overexposure to maternal-derived glucocorticoids caused by medication during pregnancy can affect the developmental programming of offspring's adrenal steroidogenesis function.
3.2.2. Hepatic developmental programming alterations
The development of hepatic lipid metabolism function is vital to maintain lipid homeostasis. Abnormal hepatic triglyceride and cholesterol metabolism can respectively lead to non-alcoholic fatty liver disease (NAFLD) and hypercholesterolemia. Intrauterine overexposure to maternal-derived glucocorticoids induced by medication during pregnancy can result in alterations in hepatic lipid metabolic function programming in offspring and susceptibilities to related metabolic diseases. PCE and PEE can cause offspring rats to be susceptible to NAFLD, closely related to the “two-programming” mechanism. Intrauterine overexposure to maternal-derived glucocorticoids induced by PCE can up-regulate GR/CCAAT enhancer binding protein α signal and thus down-regulate the expression of silent information regulator 1 in the fetal rat liver, which enhances the triglyceride synthesis; intrauterine overexposure to maternal-derived glucocorticoids induced by PEE can increase hepatic lipid synthesis and reduce lipid output in fetal rats. The changes mentioned above in hepatic triglyceride metabolism can continue after birth, which is the “first programming”125,126. PCE and PEE can also affect the development of the “glucocorticoid–insulin-like growth factor 1 (GC–IGF1) axis” in the offspring rat liver. After offspring rats are separated from the intrauterine high glucocorticoid environment after birth, the up-regulation of IGF1 can aggravate the lipid metabolic disorder under the condition of high-fat diet, which is the “second programming”126,127. PNE can promote the expression of phosphatidylinositol 3-kinase in offspring mouse liver, resulting in lipid metabolic disorder, insulin resistance, and NAFLD128, while glucocorticoids can induce phosphatidylinositol 3-kinase-related insulin resistance through GR129, which suggests that NAFLD in the offspring mice may be related to intrauterine overexposure to maternal-derived glucocorticoids induced by PNE. Furthermore, PNE can also induce susceptibility to hypercholesterolemia in postnatal offspring rats through “two programming”. On the one hand, intrauterine overexposure to maternal-derived glucocorticoids induced by PNE inhibits reverse cholesterol transport in the fetal rat liver, and this effect can continue after birth. On the other hand, PNE can induce the “GC–IGF1 axis” developmental programming, and the up-regulation of IGF1 in postnatal offspring rats with low serum glucocorticoid levels can promote cholesterol synthesis130. In conclusion, intrauterine overexposure to maternal-derived glucocorticoids caused by medication during pregnancy can affect the developmental programming of hepatic lipid metabolism in offspring and make the offspring susceptible to NAFLD and hypercholesterolemia after birth.
3.2.3. Hippocampal developmental programming alterations
Hippocampus is a central organ that regulates cognition, emotions, and other high-level neurological and spiritual activities, whose dysfunction can lead to memory and emotional disorders. Intrauterine overexposure to maternal-derived glucocorticoids induced by medication during pregnancy can lead to hippocampal developmental programming alterations and induce related neuropsychiatric diseases in offspring after birth. An epidemiological study has shown that after pregnant women consume a large amount of licorice, a kind of 11β-HSD2 inhibitors, their offspring may be exposed to more glucocorticoids in utero and show a decline in cognitive abilities such as memory after birth131, which suggests that prenatal licorice exposure may impair hippocampal functional development by inducing intrauterine overexposure to maternal-derived glucocorticoids. In addition, high maternal-derived glucocorticoids can attenuate the negative regulation of offspring's hippocampus on the hypothalamus–pituitary–adrenal (HPA) axis. Nerve fibers from the hippocampus participate in the input of glutamate signals in the hypothalamic paraventricular nucleus-projecting area and form a glutamate-γ-aminobutyric acid synaptic connection in this area, which negatively regulates the neuronal activity of the hypothalamic paraventricular nucleus132. Intrauterine overexposure to maternal-derived glucocorticoids induced by PEE can up-regulate hippocampal glutamate decarboxylase 67 in offspring rats and promote the transformation of glutamate to glutamate-γ-aminobutyric acid, which weakens the hippocampal negative regulation on the HPA axis and increases the potential hypothalamic excitability. This change can continue after birth and lead to the hypersensitivity of offspring rats' HPA axis133. A further study has found that the susceptibility to depression in offspring rats caused by PEE is related to the hypersensitivity of the HPA axis mediated by intrauterine maternal-derived glucocorticoids134. Moreover, prenatal opiate l-α-acetylmethadol exposure can enhance the HPA axis reactivity to stressors and increase the anxiety behavior of adult offspring rats135. Meanwhile, some opiates have been shown to inhibit the efflux function of placental P-glycoprotein116, which suggests that high maternal-derived glucocorticoids may be involved in offspring's hypersensitivity of the HPA axis induced by prenatal opiate exposure. In brief, intrauterine overexposure to maternal-derived glucocorticoids caused by medication during pregnancy can affect the developmental programming of offspring's hippocampal cognitive function and induce offspring to suffer from mental illnesses related to hypersensitivity of the HPA axis.
3.2.4. Bone and cartilage developmental programming alterations
Bone and cartilage are the peripheral organs that support and protect the body. Intrauterine overexposure to maternal-derived glucocorticoids caused by medication during pregnancy can affect bone and cartilage development. For instance, high maternal-derived glucocorticoids in utero induced by PCE can activate the local renin angiotensin system in fetal rat bones, resulting in bone growth retardation and adult bone loss in offspring rats136; intrauterine overexposure to maternal-derived glucocorticoids caused by PNE can inhibit endochondral ossification in long bones of fetal rats137. Further studies have found that bone and cartilage diseases have fetal developmental origin related to maternal-derived glucocorticoids. In utero, overexposure to maternal-derived glucocorticoids induced by PCE can inhibit IGF1/glucose transporter 1 signal and hinder the cartilage matrix synthesis in articular cartilages of fetal rats; after birth, the up-regulation of IGF1/glucose transporter 1 separated from the high glucocorticoid environment can enhance the cartilage matrix degradation. The intrauterine and postnatal effects make adult offspring rats susceptible to osteoarthritis138. Meanwhile, osteoarthritis is associated with cholesterol accumulation in the cartilage139, and the susceptibility to osteoarthritis in offspring rats induced by PNE involves cholesterol-related “two programming”. On the one hand, intrauterine overexposure to maternal-derived glucocorticoids down-regulates the expression of fetal cartilage IGF1 and leads to the obstruction of cholesterol outflow from articular cartilages, which can continue after birth; on the other hand, hypercholesterolemia mediated by the hepatic “GC–IGF1 axis” programming increases the source of cholesterol in articular cartilages140. In addition, intrauterine overexposure to maternal-derived glucocorticoids caused by PCE can decrease the expression of IGF1 in fetal rat osteoblasts, thereby inhibiting osteoblast differentiation. However, the up-regulation of IGF1 in offspring rats after birth is not enough to completely recover the osteogenic differentiation disorder, which contributes to their susceptibility to osteoporosis141. In conclusion, intrauterine overexposure to maternal-derived glucocorticoids induced by medication during pregnancy can inhibit the development of bone and cartilage in offspring and cause them to be susceptible to osteoarthritis and osteoporosis.
3.2.5. Developmental programming alterations of other organs
Intrauterine overexposure to maternal-derived glucocorticoids induced by medication during pregnancy can also affect the development of offspring's gonads, kidneys, and pancreas.
The intrauterine period is critical for gonadal development. High maternal-derived glucocorticoids in utero induced by medication during pregnancy can lead to testicular and ovarian developmental programming alterations, mainly manifested as testosterone and estradiol production inhibition. Intrauterine overexposure to maternal-derived glucocorticoids induced by PEE can inhibit the expression of steroidogenic factor 1 in offspring rat testis by activating GR and promote the expression of HDAC2, thereby reducing the H3K14ac level of 3β-hydroxysteroid dehydrogenase and its expression. The low expression of 3β-hydroxysteroid dehydrogenase can continue after birth, contributing to decreased testosterone production in offspring rats142. The developmental programming alterations of testicular and ovarian functions in offspring rats induced by PCE are related to the “GC–IGF1 axis”. In utero, intrauterine overexposure to maternal-derived glucocorticoids induced by PCE can down-regulate IGF1 in the testis/ovary of offspring rats, resulting in the inhibition of the testosterone/estradiol production; after birth, the production of testosterone/estradiol increases in offspring rats with enhanced expression of IGF1 in testis/ovary; in adulthood, the blood glucocorticoid level of offspring rats rises under chronic stress, and the production of testosterone/estradiol is suppressed again143,144.
Renal and pancreatic developmental programming is also susceptible to the high level of maternal-derived glucocorticoids. Intrauterine overexposure to maternal-derived glucocorticoids induced by PCE can result in low expression programming of angiotensin type two receptor and alterations in the “GC–IGF1 axis” programming in offspring rat kidneys, which respectively lead to the susceptibility of male and female offspring to glomerulosclerosis145. Alterations in the “GC–IGF1 axis” programming caused by PEE can lead to pancreas dysplasia and impaired insulin biosynthesis in offspring rats before and after birth, and offspring rats show age-characteristic changes in insulin sensitivity after birth, namely, insulin hypersensitivity in early life and insulin resistance in late life146.
3.3. Multi-organ “two-programming” mechanism mediated by overexposure to maternal-derived glucocorticoids
The destruction of the placental glucocorticoid barrier and intrauterine high maternal-derived glucocorticoids induced by medication during pregnancy can affect the development of multiple fetal organs in utero, and multi-organ functional abnormalities of offspring before and after birth are often related to the “two-programming” mechanism mediated by high maternal-derived glucocorticoids.
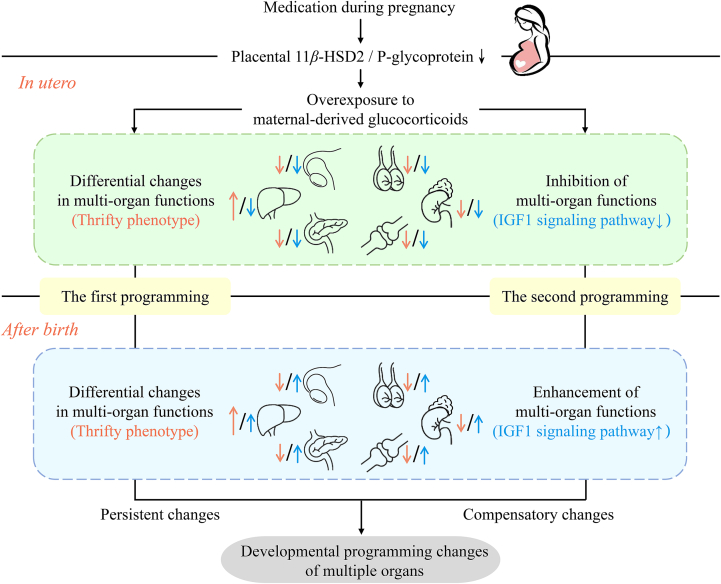
The “first programming” is “thrifty phenotype” programming. That is, intrauterine overexposure to maternal-derived glucocorticoids caused by medication during pregnancy can enhance the function of key organs and weaken that of secondary organs in fetuses, and this differential change can last until after birth. Studies have confirmed that intrauterine high maternal-derived glucocorticoids can inhibit adrenal steroidogenesis, osteoblast differentiation, pancreatic insulin production, and gonadal testosterone/estradiol production in offspring before and after birth108,141,142,144,146, while can promote hepatic triglyceride synthesis125. These differential and persistent changes attest to the hypothesis of “thrifty phenotype” development. Based on this hypothesis, to maintain survival and development in an adverse intrauterine environment, the fetus will change its metabolic process, redistribute limited energy, and limit the energy consumption of secondary organs, which ensures the development of key organs. The impact of this adaptive change on organ function will last for a long time or even be permanent147. Therefore, medication during pregnancy creates an adverse environment of intrauterine high maternal-derived glucocorticoids, in which the development of multiple organs shows plasticity.
The “second programming” is “GC–IGF1 axis” programming. That is, intrauterine overexposure to maternal-derived glucocorticoids induced by medication during pregnancy can cause glucocorticoid-dependent changes in IGF1 expression and multi-organ function of offspring before and after birth. IGF1 is known to play an essential role in the development of multiple organs148, 149, 150, and it is also a critical factor in inducing catch-up growth of IUGR offspring after birth151. After intrauterine overexposure to maternal-derived glucocorticoids, the glucocorticoid and IGF1 levels in offspring show reverse changes. In utero, the overexposure to maternal-derived glucocorticoids caused by medication during pregnancy can down-regulate IGF1 in fetal organs, thereby inhibiting the function of corresponding organs. However, after birth, the expression of IGF1 increases in offspring organs separated from the intrauterine high glucocorticoid environment, which enhances the function of corresponding organs108,123. Under the “GC–IGF1 axis” developmental programming, the offspring's organ functions show compensatory developmental changes before and after birth.
In summary, “thrifty phenotype” and “GC–IGF1 axis” jointly shape the multi-organ developmental programming mediated by intrauterine overexposure to maternal-derived glucocorticoids induced by medication during pregnancy (Fig. 1). The “thrifty phenotype” programming has organ characteristics, which is persistent and can last for a lifetime, while the “GC–IGF1 axis” programming is multi-organ commonality and shows compensatory changes.
Figure 1.
“Two-programming” mechanism mediated by overexposure to maternal-derived glucocorticoids. Drugs can cause fetal overexposure to maternal-derived glucocorticoids by inhibiting the placental 11β-hydroxysteroid dehydrogenase 2 (11β-HSD2) or P-glycoprotein. “Thrifty phenotype” and “glucocorticoid–insulin-like growth factor 1 (GC–IGF1) axis” jointly shape the multi-organ developmental programming mediated by intrauterine overexposure to maternal-derived glucocorticoids induced by medication during pregnancy. The “thrifty phenotype” programming has organ characteristics, which is persistent and can last for a lifetime, while the “GC–IGF1 axis” programming is multi-organ commonality and shows compensatory changes.
3.4. Low exposure to maternal-derived glucocorticoids and multi-organ developmental programming alterations in offspring
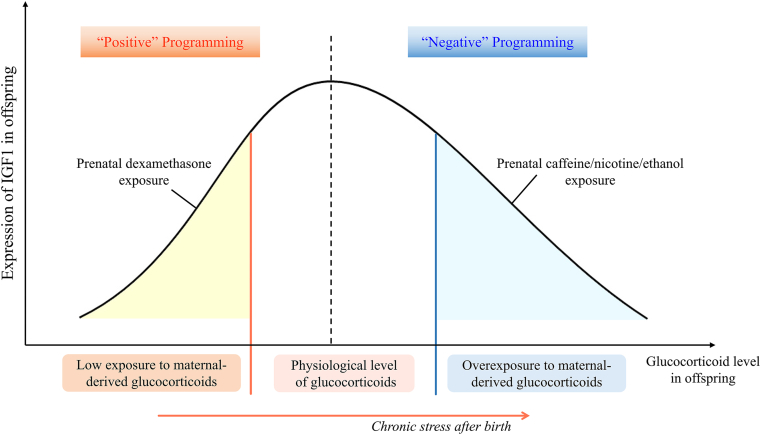
There are few studies on the alterations in multi-organ developmental programming mediated by intrauterine low exposure to maternal-derived glucocorticoids induced by medication during pregnancy. At present, only synthetic glucocorticoid drugs have related research reports. A clinical study has found that PDE can lead to neonatal subclinical adrenal insufficiency152. The latest study reveals the relationship between fetal adrenal dysfunction and intrauterine low exposure to maternal-derived glucocorticoids induced by PDE. Low maternal-derived glucocorticoid level in utero caused by PDE can increase the adrenal expression of silent information regulator 3 by down-regulating miRNA-370-3p, which leads to the histone H3 lysine 27 acetylation level and expression of IGF1 decrease, thus weakening the function of adrenal steroidogenesis in offspring rats120. According to another clinical study, long-term use of methylprednisolone (a synthetic glucocorticoid drug) during pregnancy can also inhibit the adrenal steroidogenesis of neonates153, and it is worth exploring whether its mechanism is also related to low exposure to maternal-derived glucocorticoids. Additionally, PDE can result in abnormal testicular function in offspring by inducing intrauterine low exposure to maternal-derived glucocorticoids. It has been found that the low glucocorticoid environment induced by PDE inhibits the miRNA-124-3p expression and thus up-regulates HDAC5, which reduces the H3K14ac level of IGF1 in fetal rat testis and its expression. The above effect can continue after birth and reduce testosterone synthesis in offspring rats. Under chronic stress after birth, the level of blood glucocorticoid in PDE offspring rats increases, so the expression of IGF1 and testosterone synthesis are also enhanced154. The above-mentioned studies suggest that after intrauterine low exposure to maternal-derived glucocorticoids, glucocorticoids and IGF1 in offspring change in the same direction (“positive” programming of GC–IGF1 axis), which is different from the context of intrauterine overexposure to maternal-derived glucocorticoids where glucocorticoids and IGF1 in offspring change in reverse direction (“negative” programming of GC–IGF1 axis) (Fig. 2).
Figure 2.
The “positive” and “negative” programming of glucocorticoid-insulin-like growth factor 1 (GC–IGF1) axis in offspring induced by intrauterine abnormal exposure to maternal-derived glucocorticoids: After intrauterine low exposure to maternal-derived glucocorticoids, glucocorticoids and IGF1 in offspring change in the same direction (“positive” programming of GC–IGF1 axis), which is different from the context of intrauterine overexposure to maternal-derived glucocorticoids where glucocorticoids and IGF1 in offspring change in reverse direction (“negative” programming of GC–IGF1 axis).
4. Gender differences in organ development toxicity and programming alterations caused by medication during pregnancy
Gender is an important variable in the field of “DOHaD.” Studies have found gender differences in the incidence of adverse pregnancy outcomes and the severity of chronic diseases in offspring after adverse prenatal exposure155,156. Medication during pregnancy may also sex-specifically affect the development and functional homeostasis of multiple organs in offspring. For example, the fetal hepatic toxicity induced by prenatal monocrotaline exposure is more significant in female offspring rats22. Prenatal betamethasone exposure can sex-specifically enhance the susceptibility of male offspring sheep kidneys to angiotensin II-induced oxidative stress, which is manifested by increased urinary excretion of 8-isoprostane and protein in male offspring157. The use of psychostimulant drugs during pregnancy impairs cognitive function more obviously in male offspring. For example, prenatal cocaine exposure can more significantly affect the memory ability of male offspring158. The hippocampus is the key organ for memory function, suggesting that cocaine use during pregnancy may gender-specifically affect the development of hippocampal function in offspring. Interestingly, PDE can induce hypercholesterolemia in both male and female adult offspring rats, but their hepatic metabolic programming mechanisms are different. In the male offspring, the mechanism is associated with the sustained decrease in hepatic low-density lipoprotein receptor expression159, and in the females, with the sustained high expression of hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase (to be published).
In addition, intrauterine overexposure to maternal-derived glucocorticoids induced by medication during pregnancy may also sex-specifically affect the offspring's multi-organ developmental programming. Female offspring rats of PCE show a significant increase in hepatic IGF1 expression and obviously aggravated NAFLD when fed a high-fat diet postnatally, compared to male offspring127. Intrauterine overexposure to maternal-derived glucocorticoids induced by PNE can sex-specifically cause cartilage dysplasia in female offspring rats, manifested as a more significant susceptibility to osteoarthritis in adult female offspring rats140.
The idea that the gender differences in fetal development are related to the sex dimorphism of placental development is long-standing160. The adverse environment during pregnancy can damage the structure and function of the placenta with gender differences and thus has different effects on female and male offspring161,162. Sex chromosomes, sex hormones, and GR subtypes are crucial in forming this placental sex dimorphism163. It has been found that PDE can affect the branching morphogenesis and glycogen metabolism of the placenta with gender differences164,165. Additionally, female and male guinea pigs exposed to betamethasone during pregnancy have different expression patterns of GR subtypes166. Hence, the gender differences in organ development toxicity and programming alterations caused by medication during pregnancy may be related to placental sex dimorphism.
5. Multi-generational genetic effects of organ development toxicity and programming alterations caused by medication during pregnancy
The multi-generational genetic effect means that after an individual is exposed to the adverse environment in the sensitive developmental period, its offspring of different generations show a consistent abnormal phenotype. The intrauterine period is the sensitive period of individual development. The effect of medication during pregnancy on organ development and functional homeostasis may have multi-generational genetic effects. The effect of PDE on the phenotype of offspring of multiple generations has been reported. A study has found that PDE can program the expression of corticotropin-releasing hormone (CRH) and CRH receptor type 1 in rat hippocampus across two generations and induce depression-like behavior in two generations, which is related to the hypomethylation of CRH receptor type 1 promoter167. Another study has found that PDE can also change the DNA methylation pattern of the guinea pig hippocampus across three generations through paternal lineage168. PDE can up-regulate the histone H3 lysine 27 acetylation level of angiotensin converting enzyme in F1 and F2 generation rats, resulting in low peak bone mass in the F1 and F2 generations169. Moreover, the combined use of acetaminophen and ibuprofen during pregnancy can inhibit the follicular activation in offspring rats after birth and cause low ovarian function and accelerated ovarian aging of the F2 generation170.
In addition, intrauterine overexposure to maternal-derived glucocorticoids caused by medication during pregnancy may also have multi-generational genetic effects on the offspring's organ development and functional homeostasis. Intrauterine high maternal-derived glucocorticoids induced by PEE can change the histone acetylation level of IGF1 and the “GC–IGF1 axis” programming in the offspring rat liver, which leads to heritable changes in hepatic lipid metabolism171. It can also change the H3K9ac level of cytochrome P450 cholesterol side chain cleavage enzyme and its expression, thereby triggering the intergenerational effect of abnormal steroidogenesis function in offspring rat adrenal glands172. Intrauterine high maternal-derived glucocorticoids induced by PNE can down-regulate the histone acetylation levels of transforming growth factor β receptor 1 and type II collagen a1 chain and their expression, causing heritable changes in articular cartilage morphology of offspring rats173. Interestingly, PCE can reduce adrenal steroidogenesis of F1 and F3 offspring rats but increase that of F2 offspring rats, which is associated with the “contemporary” epigenetic programming of H19/let-7 axis caused by intrauterine different maternal-derived glucocorticoid levels174.
In summary, the multi-generational genetic effects of organ development toxicity and programming alterations caused by medication during pregnancy are closely related to epigenetic modifications such as DNA methylation and histone acetylation. These abnormal epigenetic modifications can be passed to offspring through gametes and affect offspring's gene regulation and phenotype175.
6. Conclusions and prospects
In recent years, the “DOHaD” theory has become a hot research field, and the research on the safety of medication during pregnancy has been continuously deepened in this field. In conclusion, medication during pregnancy can affect fetal morphological and functional development through multiple pathways, multiple organs, and multiple targets. Furthermore, medication during pregnancy may also indirectly cause multi-organ developmental programming, functional homeostasis changes, and susceptibility to related diseases in offspring by inducing fetal intrauterine abnormal exposure to maternal-derived glucocorticoids. Meanwhile, the alterations of multi-organ developmental programming and functional homeostasis caused by medication during pregnancy may also have gender differences and multi-generational genetic effects. This paper systematically expounds on the research progress of offspring's multi-organ developmental toxicity and programming alterations caused by medication during pregnancy, which has guiding significance for rational prenatal drug use.
Although many drugs have been proved to have organ development toxicity, their long-term effects on offspring after birth have not been fully explored. Meanwhile, studies on the relationship between maternal-derived glucocorticoid levels in utero and the multi-organ developmental programming alterations are still limited. On the one hand, some drugs have been shown to affect the placental glucocorticoid barrier, but their effects on offspring's multi-organ development remain to be explored; on the other hand, the intrauterine low glucocorticoid effects of certain drugs used during pregnancy and the multi-organ developmental abnormalities mediated by them are still a research direction to be extensively explored. More importantly, in the context of drug abuse during pregnancy, it is urgent to facilitate the transformation of basic research results about multi-organ dysplasia caused by medication during pregnancy into the clinical application, so as to find effective methods for prevention and treatment of drug-related multiple fetal-originated diseases.
Acknowledgments
This work was supported by grants from the National Key Research and Development Program of China (No. 2020YFA0803900), the National Natural Science Foundation of China (Nos. 82030111 and 81673524), the Major Technological Innovation Projects of Hubei Province (Nos. 2019ACA140 and 2020BCA071), Hubei Province's Outstanding Medical Academic Leader program, and Medical Science Advancement Program (Basic Medical Sciences) of Wuhan University (No. TFJC2018001).
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Liaobin Chen, Email: liaobinchen@163.com.
Hui Wang, Email: wanghui19@whu.edu.cn.
Author contributions
Hui Wang and Liaobin Chen conceived and supervised the project. Zhengjie Lu summed up the literature and wrote the whole passage. Yu Guo, Dan Xu and Hao Xiao were in charge of checking and revision. Yongguo Dai and Kexin Liu were involved in drawing the figures.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.de Waard M., Blomjous B.S., Hol M.L.F., Sie S.D., Corpeleijn W.E., van Goudoever J.H.B., et al. Medication use during pregnancy and lactation in a Dutch population. J Hum Lactation. 2019;35:154–164. doi: 10.1177/0890334418775630. [DOI] [PubMed] [Google Scholar]
- 2.Ventura M., Maraschini A., D'Aloja P., Kirchmayer U., Lega I., Davoli M., et al. Drug prescribing during pregnancy in a central region of Italy, 2008–2012. BMC Publ Health. 2018;18:623. doi: 10.1186/s12889-018-5545-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lutz B.H., Miranda V.I.A., Silveira M.P.T., Dal Pizzol T.D.S., Mengue S.S., da Silveira M.F., et al. Medication use among pregnant women from the 2015 Pelotas (Brazil) birth cohort study. Int J Environ Res Publ Health. 2020;17:989. doi: 10.3390/ijerph17030989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haas D.M., Marsh D.J., Dang D.T., Parker C.B., Wing D.A., Simhan H.N., et al. Prescription and other medication use in pregnancy. Obstet Gynecol. 2018;131:789–798. doi: 10.1097/AOG.0000000000002579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beyer-Westendorf J., Tittl L., Bistervels I., Middeldorp S., Schaefer C., Paulus W., et al. Safety of direct oral anticoagulant exposure during pregnancy: a retrospective cohort study. Lancet Haematol. 2020;7:e884–e891. doi: 10.1016/S2352-3026(20)30327-6. [DOI] [PubMed] [Google Scholar]
- 6.Wen X., Hartzema A., Delaney J.A., Brumback B., Liu X., Egerman R., et al. Combining adverse pregnancy and perinatal outcomes for women exposed to antiepileptic drugs during pregnancy, using a latent trait model. BMC Pregnancy Childbirth. 2017;17:10. doi: 10.1186/s12884-016-1190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Etwel F., Faught L.H., Rieder M.J., Koren G. The risk of adverse pregnancy outcome after first trimester exposure to H1 antihistamines: a systematic review and meta-analysis. Drug Saf. 2017;40:121–132. doi: 10.1007/s40264-016-0479-9. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki K. The developing world of DOHaD. J Dev Orig Health Dis. 2018;9:266–269. doi: 10.1017/S2040174417000691. [DOI] [PubMed] [Google Scholar]
- 9.Dogan Z., Elbe H., Taslidere E., Soysal H., Cetin A., Demirtas S. Effects of ciprofloxacin on fetal rat liver during pregnancy and protective effects of quercetin. Biotech Histochem. 2017;92:481–486. doi: 10.1080/10520295.2017.1356469. [DOI] [PubMed] [Google Scholar]
- 10.Singh P., Singh L., Mondal S.C., Kumar S., Singh I.N. Erythromycin-induced genotoxicity and hepatotoxicity in mice pups treated during prenatal and postnatal period. Fundam Clin Pharmacol. 2014;28:519–529. doi: 10.1111/fcp.12055. [DOI] [PubMed] [Google Scholar]
- 11.Liu K., Wang G., Li L., Chen G., Gong X., Zhang Q., et al. GR-C/EBPalpha–IGF1 axis mediated azithromycin-induced liver developmental toxicity in fetal mice. Biochem Pharmacol. 2020;180 doi: 10.1016/j.bcp.2020.114130. [DOI] [PubMed] [Google Scholar]
- 12.Abd-Allah E.R., Abd El-Rahman H.A. Influence of doxycycline administration on rat embryonic development during organogenesis. J Biochem Mol Toxicol. 2021;35 doi: 10.1002/jbt.22613. [DOI] [PubMed] [Google Scholar]
- 13.Liu M., Ai W., Sun L., Fang F., Wang X., Chen S., et al. Triclosan-induced liver injury in zebrafish (Danio rerio) via regulating MAPK/p53 signaling pathway. Comp Biochem Physiol C Toxicol Pharmacol. 2019;222:108–117. doi: 10.1016/j.cbpc.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 14.Zou Y., Zhang Y., Han L., He Q., Hou H., Han J., et al. Oxidative stress-mediated developmental toxicity induced by isoniazide in zebrafish embryos and larvae. J Appl Toxicol. 2017;37:842–852. doi: 10.1002/jat.3432. [DOI] [PubMed] [Google Scholar]
- 15.Zhang D., Liu K., Hu W., Lu X., Li L., Zhang Q., et al. Prenatal dexamethasone exposure caused fetal rats liver dysplasia by inhibiting autophagy-mediated cell proliferation. Toxicology. 2021;449 doi: 10.1016/j.tox.2020.152664. [DOI] [PubMed] [Google Scholar]
- 16.Sienko A.E., Stewart J.D., Gonzalez C.L., Christensen H.D., Lerner M., Rayburn W.F. Placebo-controlled, blinded comparison of antenatal betamethasone on mouse liver development. Drug Chem Toxicol. 2001;24:49–61. doi: 10.1081/dct-100103085. [DOI] [PubMed] [Google Scholar]
- 17.Wang G., He B., Hu W., Liu K., Gong X., Kou H., et al. Low-expressional IGF1 mediated methimazole-induced liver developmental toxicity in fetal mice. Toxicology. 2018;408:70–79. doi: 10.1016/j.tox.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Deng J., Mueller M., Geng T., Shen Y., Liu Y., Hou P., et al. H19 lncRNA alters methylation and expression of Hnf4alpha in the liver of metformin-exposed fetuses. Cell Death Dis. 2017;8:e3175. doi: 10.1038/cddis.2017.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y., Junaid M., Wang Y., Tang Y.M., Bian W.P., Xiong W.X., et al. New toxicogenetic insights and ranking of the selected pharmaceuticals belong to the three different classes: a toxicity estimation to confirmation approach. Aquat Toxicol. 2018;201:151–161. doi: 10.1016/j.aquatox.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 20.Watkins J.B., Szczepanik P., Gould J.B., Klein P., Lester R. Bile salt metabolism in the human premature infant. Preliminary observations of pool size and synthesis rate following prenatal administration of dexamethasone and phenobarbital. Gastroenterology. 1975;69:706–713. [PubMed] [Google Scholar]
- 21.Karimi K., Kessler T., Thiele K., Ramisch K., Erhardt A., Huebener P., et al. Prenatal acetaminophen induces liver toxicity in dams, reduces fetal liver stem cells, and increases airway inflammation in adult offspring. J Hepatol. 2015;62:1085–1091. doi: 10.1016/j.jhep.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 22.Xiang E., Guo Q., Dai Y.G., Sun X.X., Liu J., Fan C.P., et al. Female-specific activation of pregnane X receptor mediates sex difference in fetal hepatotoxicity by prenatal monocrotaline exposure. Toxicol Appl Pharmacol. 2020;406 doi: 10.1016/j.taap.2020.115137. [DOI] [PubMed] [Google Scholar]
- 23.Dai Y., Luo J., Xiang E., Guo Q., He Z., Gong Z., et al. Prenatal exposure to retrorsine induces developmental toxicity and hepatotoxicity of fetal rats in a sex-dependent manner: the role of pregnane X receptor activation. J Agric Food Chem. 2021;69:3219–3231. doi: 10.1021/acs.jafc.0c06748. [DOI] [PubMed] [Google Scholar]
- 24.Vliegenthart A.D.B., Wei C., Buckley C., Berends C., de Potter C.M.J., Schneemann S., et al. Characterization of triptolide-induced hepatotoxicity by imaging and transcriptomics in a novel zebrafish model. Toxicol Sci. 2017;159:380–391. doi: 10.1093/toxsci/kfx144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J., Zhang Y., Liu K., He Q., Sun C., Han J., et al. Xiaoaiping induces developmental toxicity in zebrafish embryos through activation of er stress, apoptosis and the Wnt pathway. Front Pharmacol. 2018;9:1250. doi: 10.3389/fphar.2018.01250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xia Q., Wei L., Zhang Y., Kong H., Shi Y., Wang X., et al. Psoralen induces developmental toxicity in zebrafish embryos/larvae through oxidative stress, apoptosis, and energy metabolism disorder. Front Pharmacol. 2018;9:1457. doi: 10.3389/fphar.2018.01457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xia Q., Gao S., Rapael Gnanamuthu S.R., Zhuang K., Song Z., Zhang Y., et al. Involvement of Nrf2–HO-1/JNK–Erk signaling pathways in aconitine-induced developmental toxicity, oxidative stress, and ROS-mitochondrial apoptosis in zebrafish embryos. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.642480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kong F.J., Ma L.L., Hu W.W., Wang W.N., Lu H.S., Chen S.P. Fetal exposure to high isoflurane concentration induces postnatal memory and learning deficits in rats. Biochem Pharmacol. 2012;84:558–563. doi: 10.1016/j.bcp.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 29.Zhao T., Li Y., Wei W., Savage S., Zhou L., Ma D. Ketamine administered to pregnant rats in the second trimester causes long-lasting behavioral disorders in offspring. Neurobiol Dis. 2014;68:145–155. doi: 10.1016/j.nbd.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 30.Fang F., Song R., Ling X., Peng M., Xue Z., Cang J. Multiple sevoflurane anesthesia in pregnant mice inhibits neurogenesis of fetal hippocampus via repressing transcription factor Pax6. Life Sci. 2017;175:16–22. doi: 10.1016/j.lfs.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Zhong L., Luo F., Zhao W., Feng Y., Wu L., Lin J., et al. Propofol exposure during late stages of pregnancy impairs learning and memory in rat offspring via the BDNF–TrkB signalling pathway. J Cell Mol Med. 2016;20:1920–1931. doi: 10.1111/jcmm.12884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao Q., Hou J., Chen B., Shao X., Zhu R., Bu Q., et al. Prenatal cocaine exposure impairs cognitive function of progeny via insulin growth factor II epigenetic regulation. Neurobiol Dis. 2015;82:54–65. doi: 10.1016/j.nbd.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 33.Singh K.P., Tripathi N. Prenatal exposure to a novel antipsychotic quetiapine: impact on neuro-architecture, apoptotic neurodegeneration in fetal hippocampus and cognitive impairment in young rats. Int J Dev Neurosci. 2015;42:59–67. doi: 10.1016/j.ijdevneu.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 34.Wang H., Li J.T., Zhang Y., Liu R., Wang X.D., Si T.M., et al. Prenatal exposure to antipsychotics disrupts the plasticity of dentate neurons and memory in adult male mice. Int J Neuropsychopharmacol. 2019;22:71–82. doi: 10.1093/ijnp/pyy073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mowery T.M., McDowell A.L., Garraghty P.E. Chronic developmental exposure to phenytoin has long-term behavioral consequences. Int J Dev Neurosci. 2008;26:401–407. doi: 10.1016/j.ijdevneu.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 36.Aberg E., Holst S., Neagu A., Ogren S.O., Lavebratt C. Prenatal exposure to carbamazepine reduces hippocampal and cortical neuronal cell population in new-born and young mice without detectable effects on learning and memory. PLoS One. 2013;8 doi: 10.1371/journal.pone.0080497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dong W., Xu D., Hu Z., He X., Guo Z., Jiao Z., et al. Low-functional programming of the CREB/BDNF/TrkB pathway mediates cognitive impairment in male offspring after prenatal dexamethasone exposure. Toxicol Lett. 2018;283:1–12. doi: 10.1016/j.toxlet.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 38.Chen Z., Zhao Z., Li Y., Zhang X., Li B., Chen L., et al. Course-, dose-, and stage-dependent toxic effects of prenatal dexamethasone exposure on fetal articular cartilage development. Toxicol Lett. 2018;286:1–9. doi: 10.1016/j.toxlet.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 39.Chen Z., Zhao X., Li Y., Zhang R., Nie Z., Cheng X., et al. Course-, dose-, and stage-dependent toxic effects of prenatal dexamethasone exposure on long bone development in fetal mice. Toxicol Appl Pharmacol. 2018;351:12–20. doi: 10.1016/j.taap.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 40.Muanda F.T., Sheehy O., Berard A. Use of antibiotics during pregnancy and the risk of major congenital malformations: a population based cohort study. Br J Clin Pharmacol. 2017;83:2557–2571. doi: 10.1111/bcp.13364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chahoud I., Paumgartten F.J. Dose–response relationships of rat fetal skeleton variations: relevance for risk assessment. Environ Res. 2009;109:922–929. doi: 10.1016/j.envres.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 42.Rengasamy P. Congenital malformations attributed to prenatal exposure to cyclophosphamide. Anti Cancer Agents Med Chem. 2017;17:1211–1227. doi: 10.2174/1871520616666161206150421. [DOI] [PubMed] [Google Scholar]
- 43.Boareto A.C., Muller J.C., de Araujo S.L., Lourenco A.C., Lourenco E.L., Gomes C., et al. Study on the developmental toxicity of combined artesunate and mefloquine antimalarial drugs on rats. Reprod Toxicol. 2012;34:658–664. doi: 10.1016/j.reprotox.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 44.Siberry G.K., Jacobson D.L., Kalkwarf H.J., Wu J.W., DiMeglio L.A., Yogev R., et al. Lower newborn bone mineral content associated with maternal use of tenofovir disoproxil fumarate during pregnancy. Clin Infect Dis. 2015;61:996–1003. doi: 10.1093/cid/civ437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang J.K., Wu S.C., Wang G.J., Cho M.H., Ho M.L. Effects of non-steroidal anti-inflammatory drugs on cell proliferation and death in cultured epiphyseal-articular chondrocytes of fetal rats. Toxicology. 2006;228:111–123. doi: 10.1016/j.tox.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 46.Migliaccio S., Newbold R.R., Bullock B.C., Jefferson W.J., Sutton F.G., Jr., McLachlan J.A., et al. Alterations of maternal estrogen levels during gestation affect the skeleton of female offspring. Endocrinology. 1996;137:2118–2125. doi: 10.1210/endo.137.5.8612556. [DOI] [PubMed] [Google Scholar]
- 47.Kristensen D.M., Lesne L., Le Fol V., Desdoits-Lethimonier C., Dejucq-Rainsford N., Leffers H., et al. Paracetamol (acetaminophen), aspirin (acetylsalicylic acid) and indomethacin are anti-androgenic in the rat foetal testis. Int J Androl. 2012;35:377–384. doi: 10.1111/j.1365-2605.2012.01282.x. [DOI] [PubMed] [Google Scholar]
- 48.Ben Maamar M., Lesne L., Hennig K., Desdoits-Lethimonier C., Kilcoyne K.R., Coiffec I., et al. Ibuprofen results in alterations of human fetal testis development. Sci Rep. 2017;7 doi: 10.1038/srep44184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leverrier-Penna S., Mitchell R.T., Becker E., Lecante L., Ben Maamar M., Homer N., et al. Ibuprofen is deleterious for the development of first trimester human fetal ovary. ex vivo. Hum Reprod. 2018;33:482–493. doi: 10.1093/humrep/dex383. [DOI] [PubMed] [Google Scholar]
- 50.van den Driesche S., Macdonald J., Anderson R.A., Johnston Z.C., Chetty T., Smith L.B., et al. Prolonged exposure to acetaminophen reduces testosterone production by the human fetal testis in a xenograft model. Sci Transl Med. 2015;7:288ra80. doi: 10.1126/scitranslmed.aaa4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hurtado-Gonzalez P., Anderson R.A., Macdonald J., van den Driesche S., Kilcoyne K., Jorgensen A., et al. Effects of exposure to acetaminophen and ibuprofen on fetal germ cell development in both sexes in rodent and human using multiple experimental systems. Environ Health Perspect. 2018;126 doi: 10.1289/EHP2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu M., Chen B., Pei L., Zhang Q., Zou Y., Xiao H., et al. Decreased H3K9ac level of StAR mediated testicular dysplasia induced by prenatal dexamethasone exposure in male offspring rats. Toxicology. 2018;408:1–10. doi: 10.1016/j.tox.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 53.Ristic N., Nestorovic N., Manojlovic-Stojanoski M., Trifunovic S., Ajdzanovic V., Filipovic B., et al. Adverse effect of dexamethasone on development of the fetal rat ovary. Fundam Clin Pharmacol. 2019;33:199–207. doi: 10.1111/fcp.12415. [DOI] [PubMed] [Google Scholar]
- 54.Palmer J.R., Herbst A.L., Noller K.L., Boggs D.A., Troisi R., Titus-Ernstoff L., et al. Urogenital abnormalities in men exposed to diethylstilbestrol in utero: a cohort study. Environ Health. 2009;8:37. doi: 10.1186/1476-069X-8-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Newbold R.R., Bullock B.C., Mc Lachlan J.A. Exposure to diethylstilbestrol during pregnancy permanently alters the ovary and oviduct. Biol Reprod. 1983;28:735–744. doi: 10.1095/biolreprod28.3.735. [DOI] [PubMed] [Google Scholar]
- 56.Tartarin P., Moison D., Guibert E., Dupont J., Habert R., Rouiller-Fabre V., et al. Metformin exposure affects human and mouse fetal testicular cells. Hum Reprod. 2012;27:3304–3314. doi: 10.1093/humrep/des264. [DOI] [PubMed] [Google Scholar]
- 57.Dickinson H., Walker D.W., Wintour E.M., Moritz K. Maternal dexamethasone treatment at midgestation reduces nephron number and alters renal gene expression in the fetal spiny mouse. Am J Physiol Regul Integr Comp Physiol. 2007;292:R453–R461. doi: 10.1152/ajpregu.00481.2006. [DOI] [PubMed] [Google Scholar]
- 58.Agnew E.J., Garcia-Burgos A., Richardson R.V., Manos H., Thomson A.J.W., Sooy K., et al. Antenatal dexamethasone treatment transiently alters diastolic function in the mouse fetal heart. J Endocrinol. 2019;241:279–292. doi: 10.1530/JOE-18-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu D., Chen M., Pan X.L., Xia L.P., Wang H. Dexamethasone induces fetal developmental toxicity through affecting the placental glucocorticoid barrier and depressing fetal adrenal function. Environ Toxicol Pharmacol. 2011;32:356–363. doi: 10.1016/j.etap.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 60.Zhang J., Massmann G.A., Rose J.C., Figueroa J.P. Differential effects of clinical doses of antenatal betamethasone on nephron endowment and glomerular filtration rate in adult sheep. Reprod Sci. 2010;17:186–195. doi: 10.1177/1933719109351098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Karsli T., Strickland D., Livingston J., Wu Q., Mhanna M.J., Shekhawat P.S. Assessment of neonatal adrenal size using high resolution 2D ultrasound and its correlation with birth demographics and clinical outcomes. J Matern Fetal Neonatal Med. 2019;32:377–383. doi: 10.1080/14767058.2017.1378340. [DOI] [PubMed] [Google Scholar]
- 62.Rowas S.A., Haddad R., Gawri R., Al Ma'awi A.A., Chalifour L.E., Antoniou J., et al. Effect of in utero exposure to diethylstilbestrol on lumbar and femoral bone, articular cartilage, and the intervertebral disc in male and female adult mice progeny with and without swimming exercise. Arthritis Res Ther. 2012;14:R17. doi: 10.1186/ar3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.vom Saal F.S., Timms B.G., Montano M.M., Palanza P., Thayer K.A., Nagel S.C., et al. Prostate enlargement in mice due to fetal exposure to low doses of estradiol or diethylstilbestrol and opposite effects at high doses. Proc Natl Acad Sci U S A. 1997;94:2056–2061. doi: 10.1073/pnas.94.5.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nguyen L., Lim L.Y., Ding S.S.L., Amirruddin N.S., Hoon S., Chan S.Y., et al. Metformin perturbs pancreatic differentiation from human embryonic stem cells. Diabetes. 2021;70:1689–1702. doi: 10.2337/db20-0722. [DOI] [PubMed] [Google Scholar]
- 65.Shintaku K., Hori S., Satoh H., Tsukimori K., Nakano H., Fujii T., et al. Prediction and evaluation of fetal toxicity induced by NSAIDs using transplacental kinetic parameters obtained from human placental perfusion studies. Br J Clin Pharmacol. 2012;73:248–256. doi: 10.1111/j.1365-2125.2011.03921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang H., Huang M., Peng R.X., Le J. Influences of 3-methylcholanthrene, phenobarbital and dexamethasone on xenobiotic metabolizing-related cytochrome P450 enzymes and steroidogenesis in human fetal adrenal cortical cells. Acta Pharmacol Sin. 2006;27:1093–1096. doi: 10.1111/j.1745-7254.2006.00358.x. [DOI] [PubMed] [Google Scholar]
- 67.Huybrechts K.F., Hernandez-Diaz S., Patorno E., Desai R.J., Mogun H., Dejene S.Z., et al. Antipsychotic use in pregnancy and the risk for congenital malformations. JAMA Psychiatr. 2016;73:938–946. doi: 10.1001/jamapsychiatry.2016.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mallah N., Tohidinik H.R., Etminan M., Figueiras A., Takkouche B. Prenatal exposure to macrolides and risk of congenital malformations: a meta-analysis. Drug Saf. 2020;43:211–221. doi: 10.1007/s40264-019-00884-5. [DOI] [PubMed] [Google Scholar]
- 69.Bloor M., Paech M. Nonsteroidal anti-inflammatory drugs during pregnancy and the initiation of lactation. Anesth Analg. 2013;116:1063–1075. doi: 10.1213/ANE.0b013e31828a4b54. [DOI] [PubMed] [Google Scholar]
- 70.Tetro N., Moushaev S., Rubinchik-Stern M., Eyal S. The placental barrier: the gate and the fate in drug distribution. Pharm Res (N Y) 2018;35:71. doi: 10.1007/s11095-017-2286-0. [DOI] [PubMed] [Google Scholar]
- 71.Ruano C.S.M., Miralles F., Mehats C., Vaiman D. The impact of oxidative stress of environmental origin on the onset of placental diseases. Antioxidants. 2022;11:106. doi: 10.3390/antiox11010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Laforgia N., Di Mauro A., Favia Guarnieri G., Varvara D., De Cosmo L., Panza R., et al. The role of oxidative stress in the pathomechanism of congenital malformations. Oxid Med Cell Longev. 2018;2018 doi: 10.1155/2018/7404082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li X., Li Y., Zhao J., Li L., Wang Y., Zhang Y., et al. Administration of ketamine causes autophagy and apoptosis in the rat fetal hippocampus and in PC12 cells. Front Cell Neurosci. 2018;12:21. doi: 10.3389/fncel.2018.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Han L., Xia Q., Zhang L., Zhang X., Li X., Zhang S., et al. Induction of developmental toxicity and cardiotoxicity in zebrafish embryos/larvae by acetyl-11-keto-beta-boswellic acid (AKBA) through oxidative stress. Drug Chem Toxicol. 2022;45:143–150. doi: 10.1080/01480545.2019.1663865. [DOI] [PubMed] [Google Scholar]
- 75.Bellanti J.A. Epigenetic studies and pediatric research. Pediatr Res. 2020;87:378–384. doi: 10.1038/s41390-019-0644-9. [DOI] [PubMed] [Google Scholar]
- 76.Huang S., Dong W., Jiao Z., Liu J., Li K., Wang H., et al. Prenatal dexamethasone exposure induced alterations in neurobehavior and hippocampal glutamatergic system balance in female rat offspring. Toxicol Sci. 2019;171:369–384. doi: 10.1093/toxsci/kfz163. [DOI] [PubMed] [Google Scholar]
- 77.Crudo A., Suderman M., Moisiadis V.G., Petropoulos S., Kostaki A., Hallett M., et al. Glucocorticoid programming of the fetal male hippocampal epigenome. Endocrinology. 2013;154:1168–1180. doi: 10.1210/en.2012-1980. [DOI] [PubMed] [Google Scholar]
- 78.Deng J., Mueller M., Geng T., Shen Y., Liu Y., Hou P., et al. Correction: H19 lncRNA alters methylation and expression of Hnf4alpha in the liver of metformin-exposed fetuses. Cell Death Dis. 2019;10:592. doi: 10.1038/s41419-019-1812-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jiang T., Hu S., Dai S., Yi Y., Wang T., Li X., et al. Programming changes of hippocampal miR-134-5p/SOX2 signal mediate the susceptibility to depression in prenatal dexamethasone-exposed female offspring. Cell Biol Toxicol. 2021;38:69–86. doi: 10.1007/s10565-021-09590-4. [DOI] [PubMed] [Google Scholar]
- 80.Shuster D.L., Risler L.J., Prasad B., Calamia J.C., Voellinger J.L., Kelly E.J., et al. Identification of CYP3A7 for glyburide metabolism in human fetal livers. Biochem Pharmacol. 2014;92:690–700. doi: 10.1016/j.bcp.2014.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shum S., Isoherranen N. Human fetal liver metabolism of oxycodone is mediated by CYP3A7. AAPS J. 2021;23:24. doi: 10.1208/s12248-020-00537-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hessel-Pras S., Braeuning A., Guenther G., Adawy A., Enge A.M., Ebmeyer J., et al. The pyrrolizidine alkaloid senecionine induces CYP-dependent destruction of sinusoidal endothelial cells and cholestasis in mice. Arch Toxicol. 2020;94:219–229. doi: 10.1007/s00204-019-02582-8. [DOI] [PubMed] [Google Scholar]
- 83.Neyshaburinezhad N., Ghasim H., Rouini M., Daali Y., Ardakani Y.H. Frequency of important CYP450 enzyme gene polymorphisms in the iranian population in comparison with other major populations: a comprehensive review of the human data. J Personalized Med. 2021;11:804. doi: 10.3390/jpm11080804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hilli J., Heikkinen T., Rontu R., Lehtimaki T., Kishida I., Aklillu E., et al. MAO-A and COMT genotypes as possible regulators of perinatal serotonergic symptoms after in utero exposure to SSRIs. Eur Neuropsychopharmacol. 2009;19:363–370. doi: 10.1016/j.euroneuro.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 85.Delpisheh A., Topping J., Reyad M., Tang A., Brabin B.J. Prenatal alcohol exposure, CYP17 gene polymorphisms and fetal growth restriction. Eur J Obstet Gynecol Reprod Biol. 2008;138:49–53. doi: 10.1016/j.ejogrb.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 86.Paley B., O'Connor M.J. Behavioral interventions for children and adolescents with fetal alcohol spectrum disorders. Alcohol Res Health. 2011;34:64–75. [PMC free article] [PubMed] [Google Scholar]
- 87.Burton G.J., Fowden A.L., Thornburg K.L. Placental origins of chronic disease. Physiol Rev. 2016;96:1509–1565. doi: 10.1152/physrev.00029.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zou Z., Forbes K., Harris L.K., Heazell A.E.P. The potential role of the E SRRG pathway in placental dysfunction. Reproduction. 2021;161:R45–R60. doi: 10.1530/REP-20-0272. [DOI] [PubMed] [Google Scholar]
- 89.Lynegaard J.C., Hansen C.F., Kristensen A.R., Amdi C. Body composition and organ development of intra-uterine growth restricted pigs at weaning. Animal. 2020;14:322–329. doi: 10.1017/S175173111900171X. [DOI] [PubMed] [Google Scholar]
- 90.Huang W., Zhou J., Zhang G., Zhang Y., Wang H. Decreased H3K9 acetylation level of LXRalpha mediated dexamethasone-induced placental cholesterol transport dysfunction. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864 doi: 10.1016/j.bbalip.2019.158524. [DOI] [PubMed] [Google Scholar]
- 91.Koehn L.M., Huang Y., Habgood M.D., Kysenius K., Crouch P.J., Dziegielewska K.M., et al. Effects of paracetamol (acetaminophen) on gene expression and permeability properties of the rat placenta and fetal brain. F1000Res. 2020;9:573. doi: 10.12688/f1000research.24119.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Correia-Branco A., Keating E., Martel F. Involvement of mTOR, JNK and PI3K in the negative effect of ethanol and metformin on the human first-trimester extravillous trophoblast HTR-8/SVneo cell line. Eur J Pharmacol. 2018;833:16–24. doi: 10.1016/j.ejphar.2018.05.038. [DOI] [PubMed] [Google Scholar]
- 93.Rubinchik-Stern M., Shmuel M., Eyal S. Antiepileptic drugs alter the expression of placental carriers: an in vitro study in a human placental cell line. Epilepsia. 2015;56:1023–1032. doi: 10.1111/epi.13037. [DOI] [PubMed] [Google Scholar]
- 94.Wang X., Li W., Li S., Yan J., Wilson J.X., Huang G. Maternal folic acid supplementation during pregnancy improves neurobehavioral development in rat offspring. Mol Neurobiol. 2018;55:2676–2684. doi: 10.1007/s12035-017-0534-2. [DOI] [PubMed] [Google Scholar]
- 95.Addo K.A., Palakodety N., Fry R.C. Acetaminophen modulates the expression of steroidogenesis-associated genes and estradiol levels in human placental JEG-3 cells. Toxicol Sci. 2021;179:44–52. doi: 10.1093/toxsci/kfaa160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Han L.W., Gao C., Mao Q. An update on expression and function of P-gp/ABCB1 and BCRP/ABCG2 in the placenta and fetus. Expet Opin Drug Metabol Toxicol. 2018;14:817–829. doi: 10.1080/17425255.2018.1499726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Blazquez A.G., Briz O., Gonzalez-Sanchez E., Perez M.J., Ghanem C.I., Marin J.J. The effect of acetaminophen on the expression of BCRP in trophoblast cells impairs the placental barrier to bile acids during maternal cholestasis. Toxicol Appl Pharmacol. 2014;277:77–85. doi: 10.1016/j.taap.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 98.Jinno N., Furugen A., Kurosawa Y., Kanno Y., Narumi K., Kobayashi M., et al. Effects of single and repetitive valproic acid administration on the gene expression of placental transporters in pregnant rats: an analysis by gestational period. Reprod Toxicol. 2020;96:47–56. doi: 10.1016/j.reprotox.2020.04.077. [DOI] [PubMed] [Google Scholar]
- 99.Moisiadis V.G., Matthews S.G. Glucocorticoids and fetal programming part 1: outcomes. Nat Rev Endocrinol. 2014;10:391–402. doi: 10.1038/nrendo.2014.73. [DOI] [PubMed] [Google Scholar]
- 100.Moisiadis V.G., Matthews S.G. Glucocorticoids and fetal programming part 2: mechanisms. Nat Rev Endocrinol. 2014;10:403–411. doi: 10.1038/nrendo.2014.74. [DOI] [PubMed] [Google Scholar]
- 101.Liggins G.C. The role of cortisol in preparing the fetus for birth. Reprod Fertil Dev. 1994;6:141–150. doi: 10.1071/rd9940141. [DOI] [PubMed] [Google Scholar]
- 102.Apaydin D.C., Jaramillo P.A.M., Corradi L., Cosco F., Rathjen F.G., Kammertoens T., et al. Early-life stress regulates cardiac development through an IL-4-glucocorticoid signaling balance. Cell Rep. 2020;33 doi: 10.1016/j.celrep.2020.108404. [DOI] [PubMed] [Google Scholar]
- 103.Chen L., Guilmette J., Luo Z.C., Cloutier A., Wang W.J., Yang M.N., et al. Placental 11beta-HSD2 and cardiometabolic health indicators in infancy. Diabetes Care. 2019;42:964–971. doi: 10.2337/dc18-2041. [DOI] [PubMed] [Google Scholar]
- 104.Mericq V., Medina P., Kakarieka E., Marquez L., Johnson M.C., Iniguez G. Differences in expression and activity of 11beta-hydroxysteroid dehydrogenase type 1 and 2 in human placentas of term pregnancies according to birth weight and gender. Eur J Endocrinol. 2009;161:419–425. doi: 10.1530/EJE-09-0308. [DOI] [PubMed] [Google Scholar]
- 105.Zhu P., Wang W., Zuo R., Sun K. Mechanisms for establishment of the placental glucocorticoid barrier, a guard for life. Cell Mol Life Sci. 2019;76:13–26. doi: 10.1007/s00018-018-2918-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Saegusa H., Nakagawa Y., Liu Y.J., Ohzeki T. Influence of placental 11beta-hydroxysteroid dehydrogenase (11beta-HSD) inhibition on glucose metabolism and 11beta-HSD regulation in adult offspring of rats. Metabolism. 1999;48:1584–1588. doi: 10.1016/s0026-0495(99)90249-4. [DOI] [PubMed] [Google Scholar]
- 107.Chen M., Wang T., Liao Z.X., Pan X.L., Feng Y.H., Wang H. Nicotine-induced prenatal overexposure to maternal glucocorticoid and intrauterine growth retardation in rat. Exp Toxicol Pathol. 2007;59:245–251. doi: 10.1016/j.etp.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 108.He Z., Zhu C., Huang H., Liu L., Wang L., Chen L., et al. Prenatal caffeine exposure-induced adrenal developmental abnormality in male offspring rats and its possible intrauterine programming mechanisms. Toxicol Res (Camb) 2016;5:388–398. doi: 10.1039/c5tx00265f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang N., Wang W., Li W., Liu C., Chen Y., Yang Q., et al. Inhibition of 11beta-HSD2 expression by triclosan via induction of apoptosis in human placental syncytiotrophoblasts. J Clin Endocrinol Metab. 2015;100:E542–E549. doi: 10.1210/jc.2014-4376. [DOI] [PubMed] [Google Scholar]
- 110.Wang Y., Dong Y., Fang Y., Lv Y., Zhu Q., Li X., et al. Diethylstilbestrol inhibits human and rat 11beta-hydroxysteroid dehydrogenase 2. Endocr Connect. 2019;8:1061–1069. doi: 10.1530/EC-19-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhou C., Ye F., Wu H., Ye H., Chen Q. Recent advances in the study of 11beta-hydroxysteroid dehydrogenase type 2 (11beta-HSD2) inhibitors. Environ Toxicol Pharmacol. 2017;52:47–53. doi: 10.1016/j.etap.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 112.Beck K.R., Bachler M., Vuorinen A., Wagner S., Akram M., Griesser U., et al. Inhibition of 11beta-hydroxysteroid dehydrogenase 2 by the fungicides itraconazole and posaconazole. Biochem Pharmacol. 2017;130:93–103. doi: 10.1016/j.bcp.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mark P.J., Waddell B.J. P-glycoprotein restricts access of cortisol and dexamethasone to the glucocorticoid receptor in placental BeWo cells. Endocrinology. 2006;147:5147–5152. doi: 10.1210/en.2006-0633. [DOI] [PubMed] [Google Scholar]
- 114.Feinshtein V., Erez O., Ben-Zvi Z., Erez N., Eshkoli T., Sheizaf B., et al. Cannabidiol changes P-gp and BCRP expression in trophoblast cell lines. PeerJ. 2013;1:e153. doi: 10.7717/peerj.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li Y., Yan Y.E., Wang H. Enhancement of placental antioxidative function and P-gp expression by sodium ferulate mediated its protective effect on rat IUGR induced by prenatal tobacco/alcohol exposure. Environ Toxicol Pharmacol. 2011;32:465–471. doi: 10.1016/j.etap.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 116.Hemauer S.J., Patrikeeva S.L., Nanovskaya T.N., Hankins G.D., Ahmed M.S. Opiates inhibit paclitaxel uptake by P-glycoprotein in preparations of human placental inside-out vesicles. Biochem Pharmacol. 2009;78:1272–1278. doi: 10.1016/j.bcp.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kim J.H., Choi A.R., Kim Y.K., Yoon S. Co-treatment with the anti-malarial drugs mefloquine and primaquine highly sensitizes drug-resistant cancer cells by increasing P-gp inhibition. Biochem Biophys Res Commun. 2013;441:655–660. doi: 10.1016/j.bbrc.2013.10.095. [DOI] [PubMed] [Google Scholar]
- 118.Schrickx J.A., Fink-Gremmels J. Inhibition of P-glycoprotein by psychotherapeutic drugs in a canine cell model. J Vet Pharmacol Therapeut. 2014;37:515–517. doi: 10.1111/jvp.12111. [DOI] [PubMed] [Google Scholar]
- 119.Magalhaes P., Alves G., Fortuna A., Llerena A., Falcao A. Real-world clinical characterization of subjects with depression treated with antidepressant drugs focused on (non-)genetic factors, pharmacokinetics, and clinical outcomes: GnG-PK/PD-AD study. Exp Clin Psychopharmacol. 2020;28:202–215. doi: 10.1037/pha0000294. [DOI] [PubMed] [Google Scholar]
- 120.Chen Y., Xia X., Fang M., Chen G., Cao J., Qu H., et al. Maternally derived low glucocorticoid mediates adrenal developmental programming alteration in offspring induced by dexamethasone. Sci Total Environ. 2021;797 doi: 10.1016/j.scitotenv.2021.149084. [DOI] [PubMed] [Google Scholar]
- 121.Klemcke H.G., Vallet J.L., Christenson R.K. Lack of effect of metyrapone and exogenous cortisol on early porcine conceptus development. Exp Physiol. 2006;91:521–530. doi: 10.1113/expphysiol.2005.033134. [DOI] [PubMed] [Google Scholar]
- 122.Busada J.T., Cidlowski J.A. Mechanisms of glucocorticoid action during development. Curr Top Dev Biol. 2017;125:147–170. doi: 10.1016/bs.ctdb.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 123.Huang H., He Z., Zhu C., Liu L., Kou H., Shen L., et al. Prenatal ethanol exposure-induced adrenal developmental abnormality of male offspring rats and its possible intrauterine programming mechanisms. Toxicol Appl Pharmacol. 2015;288:84–94. doi: 10.1016/j.taap.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 124.He Z., Lv F., Ding Y., Huang H., Liu L., Zhu C., et al. High-fat diet and chronic stress aggravate adrenal function abnormality induced by prenatal caffeine exposure in male offspring rats. Sci Rep. 2017;7 doi: 10.1038/s41598-017-14881-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hu S., Xia L., Luo H., Xu Y., Yu H., Xu D., et al. Prenatal caffeine exposure increases the susceptibility to non-alcoholic fatty liver disease in female offspring rats via activation of GR–C/EBPalpha–SIRT1 pathway. Toxicology. 2019;417:23–34. doi: 10.1016/j.tox.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 126.Shen L., Liu Z., Gong J., Zhang L., Wang L., Magdalou J., et al. Prenatal ethanol exposure programs an increased susceptibility of non-alcoholic fatty liver disease in female adult offspring rats. Toxicol Appl Pharmacol. 2014;274:263–273. doi: 10.1016/j.taap.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 127.Wang L., Shen L., Ping J., Zhang L., Liu Z., Wu Y., et al. Intrauterine metabolic programming alteration increased susceptibility to non-alcoholic adult fatty liver disease in prenatal caffeine-exposed rat offspring. Toxicol Lett. 2014;224:311–318. doi: 10.1016/j.toxlet.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 128.Huang S.J., Chen S.Q., Lin Y., Yang H.Y., Ran J., Yan F.F., et al. Maternal nicotine exposure aggravates metabolic associated fatty liver disease via PI3K/Akt signaling in adult offspring mice. Liver Int. 2021;41:1867–1878. doi: 10.1111/liv.14902. [DOI] [PubMed] [Google Scholar]
- 129.Meng Z., Yu Y., Zhang Y., Yang X., Lv X., Guan F., et al. Highly bioavailable berberine formulation improves glucocorticoid receptor-mediated insulin resistance via reduction in association of the glucocorticoid receptor with phosphatidylinositol-3-kinase. Int J Biol Sci. 2020;16:2527–2541. doi: 10.7150/ijbs.39508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhou J., Zhu C., Luo H., Shen L., Gong J., Wu Y., et al. Two intrauterine programming mechanisms of adult hypercholesterolemia induced by prenatal nicotine exposure in male offspring rats. FASEB J. 2019;33:1110–1123. doi: 10.1096/fj.201800172R. [DOI] [PubMed] [Google Scholar]
- 131.Raikkonen K., Martikainen S., Pesonen A.K., Lahti J., Heinonen K., Pyhala R., et al. Maternal licorice consumption during pregnancy and pubertal, cognitive, and psychiatric outcomes in children. Am J Epidemiol. 2017;185:317–328. doi: 10.1093/aje/kww172. [DOI] [PubMed] [Google Scholar]
- 132.Herman J.P., Tasker J.G., Ziegler D.R., Cullinan W.E. Local circuit regulation of paraventricular nucleus stress integration: glutamate–GABA connections. Pharmacol Biochem Behav. 2002;71:457–468. doi: 10.1016/s0091-3057(01)00681-5. [DOI] [PubMed] [Google Scholar]
- 133.Lu J., Jiao Z., Yu Y., Zhang C., He X., Li Q., et al. Programming for increased expression of hippocampal GAD67 mediated the hypersensitivity of the hypothalamic–pituitary–adrenal axis in male offspring rats with prenatal ethanol exposure. Cell Death Dis. 2018;9:659. doi: 10.1038/s41419-018-0663-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yu Y., Xu D., Cheng S., Zhang L., Shi Z., Qin J., et al. Prenatal ethanol exposure enhances the susceptibility to depressive behavior of adult offspring rats fed a highfat diet by affecting BDNFassociated pathway. Int J Mol Med. 2020;45:365–374. doi: 10.3892/ijmm.2019.4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hamilton K.L., Harris A.C., Gewirtz J.C., Sparber S.B., Schrott L.M. HPA axis dysregulation following prenatal opiate exposure and postnatal withdrawal. Neurotoxicol Teratol. 2005;27:95–103. doi: 10.1016/j.ntt.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 136.Wen Y., Shangguan Y., Pan Z., Hu H., Magdalou J., Chen L., et al. Activation of local bone RAS by maternal excessive glucocorticoid participated in the fetal programing of adult osteopenia induced by prenatal caffeine exposure. Toxicol Appl Pharmacol. 2019;363:1–10. doi: 10.1016/j.taap.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 137.Hu H., Zhao X., Ma J., Shangguan Y., Pan Z., Chen L., et al. Prenatal nicotine exposure retards osteoclastogenesis and endochondral ossification in fetal long bones in rats. Toxicol Lett. 2018;295:249–255. doi: 10.1016/j.toxlet.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 138.Qing-Xian L., Lin-Long W., Yi-Zhong W., Liang L., Hui H., Liao-Bin C., et al. Programming changes in GLUT1 mediated the accumulation of AGEs and matrix degradation in the articular cartilage of female adult rats after prenatal caffeine exposure. Pharmacol Res. 2020;151 doi: 10.1016/j.phrs.2019.104555. [DOI] [PubMed] [Google Scholar]
- 139.Tsezou A., Iliopoulos D., Malizos K.N., Simopoulou T. Impaired expression of genes regulating cholesterol efflux in human osteoarthritic chondrocytes. J Orthop Res. 2010;28:1033–1039. doi: 10.1002/jor.21084. [DOI] [PubMed] [Google Scholar]
- 140.Tie K., Tan Y., Deng Y., Li J., Ni Q., Magdalou J., et al. Prenatal nicotine exposure induces poor articular cartilage quality in female adult offspring fed a high-fat diet and the intrauterine programming mechanisms. Reprod Toxicol. 2016;60:11–20. doi: 10.1016/j.reprotox.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 141.Shangguan Y., Wen Y., Tan Y., Qin J., Jiang H., Magdalou J., et al. Intrauterine programming of glucocorticoid-insulin-like growth factor-1 axis-mediated developmental origin of osteoporosis susceptibility in female offspring rats with prenatal caffeine exposure. Am J Pathol. 2018;188:2863–2876. doi: 10.1016/j.ajpath.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 142.Liu M., Zhang Q., Pei L., Zou Y., Chen G., Wang H. Corticosterone rather than ethanol epigenetic programmed testicular dysplasia caused by prenatal ethanol exposure in male offspring rats. Epigenetics. 2019;14:245–259. doi: 10.1080/15592294.2019.1581595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pei L.G., Zhang Q., Yuan C., Liu M., Zou Y.F., Lv F., et al. The GC–IGF1 axis-mediated testicular dysplasia caused by prenatal caffeine exposure. J Endocrinol. 2019;242:M17–M32. doi: 10.1530/JOE-18-0684. [DOI] [PubMed] [Google Scholar]
- 144.Lv F., Fan G., Wan Y., Chen Y., Ni Y., Huang J., et al. Intrauterine endogenous high glucocorticoids program ovarian dysfunction in female offspring secondary to prenatal caffeine exposure. Sci Total Environ. 2021;789 doi: 10.1016/j.scitotenv.2021.147691. [DOI] [PubMed] [Google Scholar]
- 145.Chen H., Zhu Y., Zhao X., He H., Luo J., Ao Y., et al. Prenatal ethanol exposure increased the susceptibility of adult offspring rats to glomerulosclerosis. Toxicol Lett. 2020;321:44–53. doi: 10.1016/j.toxlet.2019.11.026. [DOI] [PubMed] [Google Scholar]
- 146.Xiao D., Kou H., Gui S., Ji Z., Guo Y., Wu Y., et al. Age-characteristic changes of glucose metabolism, pancreatic morphology and function in male offspring rats induced by prenatal ethanol exposure. Front Endocrinol. 2019;10:34. doi: 10.3389/fendo.2019.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Vaag A.A., Grunnet L.G., Arora G.P., Brons C. The thrifty phenotype hypothesis revisited. Diabetologia. 2012;55:2085–2088. doi: 10.1007/s00125-012-2589-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Agrogiannis G.D., Sifakis S., Patsouris E.S., Konstantinidou A.E. Insulin-like growth factors in embryonic and fetal growth and skeletal development (Review) Mol Med Rep. 2014;10:579–584. doi: 10.3892/mmr.2014.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Netchine I., Azzi S., Le Bouc Y., Savage M.O. IGF1 molecular anomalies demonstrate its critical role in fetal, postnatal growth and brain development. Best Pract Res Clin Endocrinol Metabol. 2011;25:181–190. doi: 10.1016/j.beem.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 150.Magner N.L., Jung Y., Wu J., Nolta J.A., Zern M.A., Zhou P. Insulin and IGFs enhance hepatocyte differentiation from human embryonic stem cells via the PI3K/AKT pathway. Stem Cell. 2013;31:2095–2103. doi: 10.1002/stem.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tosh D.N., Fu Q., Callaway C.W., McKnight R.A., McMillen I.C., Ross M.G., et al. Epigenetics of programmed obesity: alteration in IUGR rat hepatic IGF1 mRNA expression and histone structure in rapid vs. delayed postnatal catch-up growth. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1023–G1029. doi: 10.1152/ajpgi.00052.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Grajwer L.A., Lilien L.D., Pildes R.S. Neonatal subclinical adrenal insufficiency. Result of maternal steroid therapy. JAMA. 1977;238:1279–1280. [PubMed] [Google Scholar]
- 153.Kurtoglu S., Sarici D., Akin M.A., Daar G., Korkmaz L., Memur S. Fetal adrenal suppression due to maternal corticosteroid use: case report. J Clin Res Pediatr Endocrinol. 2011;3:160–162. doi: 10.4274/jcrpe.v3i3.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Liu M., Liu Y., Pei L.G., Zhang Q., Xiao H., Chen Y.W., et al. Prenatal dexamethasone exposure programs the decreased testosterone synthesis in offspring rats by low level of endogenous glucocorticoids. Acta Pharmacol Sin. 2022;43:1461–1472. doi: 10.1038/s41401-021-00789-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rosa M.J., Nentin F., Bosquet Enlow M., Hacker M.R., Pollas N., Coull B., et al. Sex-specific associations between prenatal negative life events and birth outcomes. Stress. 2019;22:647–653. doi: 10.1080/10253890.2019.1608944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Goldstein J.M., Hale T., Foster S.L., Tobet S.A., Handa R.J. Sex differences in major depression and comorbidity of cardiometabolic disorders: impact of prenatal stress and immune exposures. Neuropsychopharmacology. 2019;44:59–70. doi: 10.1038/s41386-018-0146-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bi J., Contag S.A., Chen K., Su Y., Figueroa J.P., Chappell M.C., et al. Sex-specific effect of antenatal betamethasone exposure on renal oxidative stress induced by angiotensins in adult sheep. Am J Physiol Ren Physiol. 2014;307:F1013–F1022. doi: 10.1152/ajprenal.00354.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Traccis F., Frau R., Melis M. Gender differences in the outcome of offspring prenatally exposed to drugs of abuse. Front Behav Neurosci. 2020;14:72. doi: 10.3389/fnbeh.2020.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Li L., Hu W., Liu K., Zhang D., Liu M., Li X., et al. miR-148a/LDLR mediates hypercholesterolemia induced by prenatal dexamethasone exposure in male offspring rats. Toxicol Appl Pharmacol. 2020;395 doi: 10.1016/j.taap.2020.114979. [DOI] [PubMed] [Google Scholar]
- 160.Kalisch-Smith J.I., Simmons D.G., Pantaleon M., Moritz K.M. Sex differences in rat placental development: from pre-implantation to late gestation. Biol Sex Differ. 2017;8:17. doi: 10.1186/s13293-017-0138-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Giesbrecht G.F., Rash J.A., Edwards H.E., Wynne-Edwards K.E. Full-term deliveries without antecedent labor reveal sex differences in umbilical cord glucocorticoid concentrations. Psychoneuroendocrinology. 2016;74:121–125. doi: 10.1016/j.psyneuen.2016.08.030. [DOI] [PubMed] [Google Scholar]
- 162.Nugent B.M., O'Donnell C.M., Epperson C.N., Bale T.L. Placental H3K27me3 establishes female resilience to prenatal insults. Nat Commun. 2018;9:2555. doi: 10.1038/s41467-018-04992-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yu P., Chen Y., Ge C., Wang H. Sexual dimorphism in placental development and its contribution to health and diseases. Crit Rev Toxicol. 2021;51:555–570. doi: 10.1080/10408444.2021.1977237. [DOI] [PubMed] [Google Scholar]
- 164.O'Connell B.A., Moritz K.M., Walker D.W., Dickinson H. Synthetic glucocorticoid dexamethasone inhibits branching morphogenesis in the spiny mouse placenta. Biol Reprod. 2013;88:26. doi: 10.1095/biolreprod.112.100644. [DOI] [PubMed] [Google Scholar]
- 165.O'Connell B.A., Moritz K.M., Walker D.W., Dickinson H. Treatment of pregnant spiny mice at mid gestation with a synthetic glucocorticoid has sex-dependent effects on placental glycogen stores. Placenta. 2013;34:932–940. doi: 10.1016/j.placenta.2013.06.310. [DOI] [PubMed] [Google Scholar]
- 166.Saif Z., Dyson R.M., Palliser H.K., Wright I.M., Lu N., Clifton V.L. Identification of eight different isoforms of the glucocorticoid receptor in Guinea pig placenta: relationship to preterm delivery, sex and betamethasone exposure. PLoS One. 2016;11 doi: 10.1371/journal.pone.0148226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Xu Y.J., Sheng H., Wu T.W., Bao Q.Y., Zheng Y., Zhang Y.M., et al. CRH/CRHR1 mediates prenatal synthetic glucocorticoid programming of depression-like behavior across 2 generations. FASEB J. 2018;32:4258–4269. doi: 10.1096/fj.201700948RR. [DOI] [PubMed] [Google Scholar]
- 168.Constantinof A., Boureau L., Moisiadis V.G., Kostaki A., Szyf M., Matthews S.G. Prenatal glucocorticoid exposure results in changes in gene transcription and DNA methylation in the female juvenile Guinea pig hippocampus across three generations. Sci Rep. 2019;9 doi: 10.1038/s41598-019-54456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Xiao H., Wen Y., Pan Z., Shangguan Y., Qin J., Tan Y., et al. Increased H3K27ac level of ACE mediates the intergenerational effect of low peak bone mass induced by prenatal dexamethasone exposure in male offspring rats. Cell Death Dis. 2018;9:638. doi: 10.1038/s41419-018-0701-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Podratz P.L., Merlo E., de Araujo J.F.P., Ayub J.G.M., Pereira A.F.Z., Freitas-Lima L.C., et al. Disruption of fertility, placenta, pregnancy outcome, and multigenerational inheritance of hepatic steatosis by organotin exposure from contaminated seafood in rats. Sci Total Environ. 2020;723 doi: 10.1016/j.scitotenv.2020.138000. [DOI] [PubMed] [Google Scholar]
- 171.Hu W., Yuan C., Luo H., Hu S., Shen L., Chen L., et al. Glucocorticoid-insulin-like growth factor 1 (GC-IGF1) axis programming mediated hepatic lipid-metabolic in offspring caused by prenatal ethanol exposure. Toxicol Lett. 2020;331:167–177. doi: 10.1016/j.toxlet.2020.06.008. [DOI] [PubMed] [Google Scholar]
- 172.Cao J., Chen Y., Xia X., Qu H., Ao Y., Wang H. Intergenerational genetic programming mechanism and sex differences of the adrenal corticosterone synthesis dysfunction in offspring induced by prenatal ethanol exposure. Toxicol Lett. 2021;351:78–88. doi: 10.1016/j.toxlet.2021.08.007. [DOI] [PubMed] [Google Scholar]
- 173.Xie Z., Zhao Z., Yang X., Pei L., Luo H., Ni Q., et al. Prenatal nicotine exposure intergenerationally programs imperfect articular cartilage via histone deacetylation through maternal lineage. Toxicol Appl Pharmacol. 2018;352:107–118. doi: 10.1016/j.taap.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 174.He Z., Zhang J., Chen G., Cao J., Chen Y., Ai C., et al. H19/let-7 axis mediates caffeine exposure during pregnancy induced adrenal dysfunction and its multi-generation inheritance. Sci Total Environ. 2021;792 doi: 10.1016/j.scitotenv.2021.148440. [DOI] [PubMed] [Google Scholar]
- 175.Skvortsova K., Iovino N., Bogdanovic O. Functions and mechanisms of epigenetic inheritance in animals. Nat Rev Mol Cell Biol. 2018;19:774–790. doi: 10.1038/s41580-018-0074-2. [DOI] [PubMed] [Google Scholar]