Abstract
Objective
To investigate the clinical outcomes of myocarditis associated with mRNA vaccines against the SARS-CoV-2 virus compared with other types of myocarditis.
Design
Population based cohort study.
Setting
Nationwide register data from four Nordic countries (Denmark, Finland, Norway, and Sweden), from 1 January 2018 to the latest date of follow-up in 2022.
Participants
The Nordic myocarditis cohort; 7292 individuals aged ≥12 years who had an incident diagnosis of myocarditis as a main or secondary diagnosis, in a population of 23 million individuals in Denmark, Finland, Norway, and Sweden.
Main outcome measures
Heart failure, or death from any cause within 90 days of admission to hospital for new onset myocarditis, and hospital readmission within 90 days of discharge to hospital for new onset myocarditis. Clinical outcomes of myocarditis associated with SARS-CoV-2 mRNA vaccination, covid-19 disease, and conventional myocarditis were compared.
Results
In 2018-22, 7292 patients were admitted to hospital with new onset myocarditis, with 530 (7.3%) categorised as having myocarditis associated with SARS-CoV-2 mRNA vaccination, 109 (1.5%) with myocarditis associated with covid-19 disease, and 6653 (91.2%) with conventional myocarditis. At the 90 day follow-up, 62, nine, and 988 patients had been readmitted to hospital in each group (vaccination, covid-19, and conventional myocarditis groups, respectively), corresponding to a relative risk of readmission of 0.79 (95% confidence interval 0.62 to 1.00) and 0.55 (0.30 to 1.04) for the vaccination type and covid-19 type myocarditis groups, respectively, compared with the conventional myocarditis group. At the 90 day follow-up, 27, 18, and 616 patients had a diagnosis of heart failure or died in the vaccination type, covid-19 type, and conventional myocarditis groups, respectively. The relative risk of heart failure within 90 days was 0.56 (95% confidence interval 0.37 to 0.85) and 1.48 (0.86 to 2.54) for myocarditis associated with vaccination and covid-19 disease, respectively, compared with conventional myocarditis; the relative risk of death was 0.48 (0.21 to 1.09) and 2.35 (1.06 to 5.19), respectively. Among patients aged 12-39 years with no predisposing comorbidities, the relative risk of heart failure or death was markedly higher for myocarditis associated with covid-19 disease than for myocarditis associated with vaccination (relative risk 5.78, 1.84 to 18.20).
Conclusions
Compared with myocarditis associated with covid-19 disease and conventional myocarditis, myocarditis after vaccination with SARS-CoV-2 mRNA vaccines was associated with better clinical outcomes within 90 days of admission to hospital.
Keywords: COVID-19, Epidemiology, Heart failure
WHAT IS ALREADY KNOWN ON THIS TOPIC
Myocarditis is a known adverse event associated with mRNA vaccines against the SARS-CoV-2 virus, but the clinical outcomes are not well described at the population level
How the clinical outcomes of myocarditis associated with vaccination compare with outcomes after myocarditis associated with covid-19 infection and conventional myocarditis is unclear
WHAT THIS STUDY ADDS
In a population based study covering 23 million individuals, myocarditis after SARS-CoV-2 mRNA vaccination was associated with a lower risk of heart failure within 90 days of admission to hospital compared with myocarditis associated with covid-19 disease and conventional myocarditis
Among younger individuals with no predisposing comorbidities, myocarditis related to covid-19 disease was associated with a markedly higher risk of heart failure or death within 90 days of admission to hospital compared with myocarditis associated with vaccination
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE, OR POLICY
These findings suggest that the clinical outcomes of myocarditis associated with SARS-CoV-2 mRNA vaccination are less severe than the outcomes of other types of myocarditis, which is relevant for doctors, their patients, and the public when considering vaccination policy
Introduction
Myocarditis is a rare adverse event after vaccination with the two mRNA vaccines, tozinameran (BNT162b2) and elasomeran (mRNA-1273), against the SARS-CoV-2 virus. The risk seems to be highest in younger age groups, in men, and after the second dose of vaccine,1–10 with the number of excess patients with myocarditis per 100 000 men aged 16-24 years after a second dose of a SARS-CoV-2 mRNA vaccine estimated at 5.6 per 100 000 for tozinameran and 18.4 per 100 000 for elasomeran. How the clinical outcomes of myocarditis after vaccination with SARS-CoV-2 mRNA vaccines compare with outcomes after other types of myocarditis, such as myocarditis associated with covid-19 disease and myocarditis not related to covid-19 or vaccination (ie, conventional myocarditis) is unclear.
The clinical outcomes of myocarditis range from no sequelae to chronic heart failure or death.11 Hence a large scale evaluation of the clinical outcomes of these two new types of myocarditis compared with conventional myocarditis is needed to better evaluate the risks and benefits of vaccination, especially in younger individuals. Based on nationwide data on all incident admissions to hospital for myocarditis in 2018-22, sourced from a population of 23 million individuals in Denmark, Finland, Norway, and Sweden, we compared the clinical outcomes of myocarditis associated with vaccination, myocarditis associated with covid-19 disease, and conventional myocarditis, with respect to readmission to hospital, heart failure, and death.
Methods
Nordic myocarditis cohort
We conducted a population based multi-country study of nationwide register data from four Nordic countries (Denmark, Finland, Norway, and Sweden), as defined in a prespecified common protocol (online supplemental material 1). Our Nordic myocarditis cohort included all individuals aged ≥12 years who had an incident diagnosis of myocarditis as a main or secondary diagnosis (defined by ICD-10 (international classification of diseases and related health problems, 10th revision) codes I40.0, I40.1, I40.8, I40.9, I41.1, I41.8, or I51.4) at discharge (alive or dead) from inpatient hospital care, from 1 January 2018 to the latest date of follow-up in 2022, which was specific for each country (online supplemental table S1).
bmjmed-2022-000373supp001.pdf (236.7KB, pdf)
bmjmed-2022-000373supp002.pdf (375.5KB, pdf)
In Denmark, inpatient hospital care was defined as a hospital stay of ≥24 hours because duration of hospital contacts coding has replaced inpatient hospital care coding in current Danish patient registries. Patients with a pre-existing diagnosis of myocarditis (defined by ICD-10 codes) or heart failure (ICD-10 codes defined in online supplemental table S2) were excluded from the study. Online supplemental table S1 describes the washout periods for pre-existing diagnoses in the different countries. Only patients with a potential 90 days of follow-up in the register data were included in the study. Information on clinical diagnoses and length of hospital stay was sourced from national registries (online supplemental table S3).
Individuals admitted to hospital for myocarditis within 28 days of vaccination with a SARS-CoV-2 mRNA vaccine (any dose) were categorised as having myocarditis associated with vaccination; individuals admitted to hospital for myocarditis within 28 days of a positive polymerase chain reaction (PCR) test result for the SARS-CoV-2 virus were categorised as having myocarditis associated with covid-19 disease. The remaining patients who were admitted to hospital for myocarditis were categorised as conventional myocarditis. If an individual had received an mRNA vaccine and had a positive PCR test result for SARS-CoV-2 infection within 28 days, the latest exposure defined the type of myocarditis. Information on mRNA vaccinations for the SARS-CoV-2 virus were obtained from national vaccination registries in each country, and information on positive PCR test results were taken from national infectious disease surveillance registries (online supplemental table S3).
Length of stay after admission to hospital for new onset myocarditis was calculated as day of discharge minus day of admission + 1. If a patient with incident myocarditis had a subsequent admission to hospital within 24 hours of discharge, the subsequent admission to hospital was counted as part of the original admission to hospital for new onset myocarditis.
Cohort stratification
To evaluate the role of predisposing comorbidity, we included information on pre-existing diagnoses (in the two years before admission to hospital for new onset myocarditis) of malignancy, cardiovascular disease, or autoimmune diseases (ICD-10 codes defined in online supplemental table S2), from main or secondary diagnoses recorded in inpatient or specialist outpatient care. Also, we recorded whether the admission for new onset myocarditis was on or after 1 January 2020, to separate patients with myocarditis occurring before or during the covid-19 pandemic.
Outcomes
The clinical outcomes investigated were a new onset diagnosis of heart failure within 90 days of admission to hospital for new onset myocarditis; death from any cause within 90 days of admission to hospital for new onset myocarditis; and readmission to inpatient hospital care for any cause within 90 days of discharge from hospital for new onset myocarditis. If new onset heart failure was diagnosed in the same admission period as new onset myocarditis, new onset heart failure was categorised as occurring on the day after admission to hospital for myocarditis.
Statistical analysis
Cumulative 90 day relative risks of heart failure and death by type of myocarditis type for all four countries were estimated, with conventional myocarditis as the reference category. In subgroup analyses, we used a combined outcome of heart failure or death within 90 days of myocarditis, to preserve the statistical power to describe differences between the myocarditis groups. Subgroup analyses were performed by age group (12-39 years and ≥40 years), sex, and in those admitted to hospital for new onset myocarditis on or after 1 January 2020 (reference was those admitted to hospital for new onset myocarditis before the pandemic). To investigate outcomes in healthy younger individuals with myocarditis, we conducted another analysis in those aged <40 years with no pre-existing diagnoses of malignancy, cardiovascular disease, or autoimmune disease. Similarly, subgroup analyses of cumulative 90 day relative risks of readmission after discharge from hospital for new onset myocarditis were performed by age group, sex, and in those admitted to hospital for new onset myocarditis on or after 1 January 2020 (reference was those admitted to hospital for new onset myocarditis before the pandemic), in these younger individuals with no pre-existing diagnoses of malignancy, cardiovascular disease, or autoimmune disease.
Cumulative incidences of heart failure and death by country for conventional myocarditis and myocarditis associated with vaccination, as a function of time from admission to hospital for myocarditis, were estimated with the Kaplan-Meier estimator (calculated as 1−Kaplan-Meier estimates). Cumulative incidence of readmission by country was also estimated with the Kaplan-Meier estimator but with time from discharge from hospital as the underlying time scale. Also, Kaplan-Meier estimates in multiples of 10 days of follow-up, by country, were combined with a random effects meta-analysis implemented with the mixmeta package12 of R.13 As input to the meta-analyses, log odds of estimates specific to each country were used as estimates, and the difference between log odds of confidence limits specific to each country were divided by 2×1.96 as standard errors. If at least one of the country estimates was zero, then the sum of events divided by the total number of patients with myocarditis was used to obtain results combined across countries. Cumulative incidences by country were not estimated for myocarditis associated with covid-19 disease because of limited statistical power.
Patient and public involvement
No patients or members of the public were directly involved in the design, analysis, or writing up of the study, owing to insufficient funds and time. The study was conducted by national public health institutions in Denmark, Finland, Norway, and Sweden, who have a legal obligation to investigate potential health hazards to the public.
Results
Characteristics of the Nordic myocarditis cohort
Our cohort included 7292 patients with a diagnosis of myocarditis; 530 (7.3%) were categorised as having myocarditis associated with vaccination, 109 (1.5%) were associated with covid-19 disease, and 6653 (91.2%) had conventional myocarditis (table 1). Patients were predominantly men (5304 (72.7%) overall) and 3715 (50.9%) were aged <40 years. Online supplemental table S4 shows median (interquartile range) age by type of myocarditis, age group, and country. Eighty five (16.0%), 54 (49.5%), and 1893 (28.5%) patients were admitted to hospital for ≥7 days in the vaccination type, covid-19 type, and conventional myocarditis groups, respectively. Only 11 of 639 patients with non-conventional myocarditis had received a SARS-CoV-2 mRNA vaccine and had covid-19 disease within 28 days of admission to hospital for myocarditis.
Table 1.
Characteristics of 7292 individuals with new onset myocarditis (myocarditis associated with SARS-CoV-2 mRNA vaccination, myocarditis associated with covid-19 disease, and conventional myocarditis), in Denmark, Finland, Norway, and Sweden, 2018-22 (Nordic myocarditis cohort)
| Characteristics | Type of myocarditis | ||
| Vaccination | Covid-19 | Conventional | |
| Total No of patients | 530 (100.0) | 109 (100.0) | 6653 (100.0) |
| No of patients by country: | |||
| Denmark | 98 (18.5) | 8 (7.3) | 695 (10.4) |
| Finland | 140 (26.4) | 25 (22.9) | 2059 (30.9) |
| Norway | 109 (20.6) | 18 (16.5) | 1161 (17.5) |
| Sweden | 183 (34.5) | 58 (53.2) | 2738 (41.2) |
| Time period: | |||
| 2018-19 | 0 | 0 | 3820 (57.4) |
| 2020-22 | 530 (100.0) | 109 (100) | 2833 (42.6) |
| Age group (years): | |||
| 12-24 | 202 (38.1) | 19 (17.4) | 1620 (24.3) |
| 25-39 | 138 (26.0) | 29 (26.6) | 1707 (25.7) |
| ≥40 | 190 (35.8) | 61 (56.0) | 3326 (50.0) |
| Sex: | |||
| Women | 117 (22.1) | 33 (30.3) | 1838 (27.6) |
| Men | 413 (77.9) | 76 (69.7) | 4815 (72.4) |
| Length of initial admission to hospital (days): | |||
| ≤3 | 154 (29.1) | 21 (19.3) | 2069 (31.1) |
| 4-6 | 291 (54.9) | 34 (31.2) | 2691 (40.4) |
| ≥7 | 85 (16.0) | 54 (49.5) | 1893 (28.5) |
| Predisposing comorbidity: | |||
| Any* | 71 (13.4) | 12 (11.0) | 1100 (16.5) |
| None | 459 (86.6) | 97 (89.0) | 5553 (83.5) |
Values are numbers (percentages).
*Diagnosis of malignancy, cardiovascular disease, or autoimmune disease before admission to hospital for new onset myocarditis.
Absolute and relative risk of heart failure and death
At 90 days of follow-up for new onset myocarditis, heart failure was diagnosed in 22 (4.5 %), 12 (11.0 %), and 496 (7.5 %) patients with myocarditis associated with vaccination, myocarditis associated with covid-19 disease, and conventional myocarditis, respectively. We found that patients with myocarditis after vaccination had a significantly decreased risk of heart failure at 90 days after admission to hospital for myocarditis (relative risk 0.56, 95% confidence interval 0.37 to 0.85, P=0.006) compared with those with conventional myocarditis (table 2). Conversely, we found a non-significant increased risk of heart failure at 90 days in patients with myocarditis associated with covid-19 disease (1.48, 0.86 to 2.54) compared with conventional myocarditis. Death was rare during the 90 days of follow-up, with six (1.1%), six (5.5%), and 156 (2.3%) patients dying of any cause within 90 days of admission to hospital in the vaccination, covid-19, and conventional myocarditis groups, respectively. The relative risk of death over 90 days of follow-up was 0.48 (0.21 to 1.09) for patients with myocarditis associated with vaccination and 2.35 (1.06 to 5.19) for patients with myocarditis associated with covid-19 disease, compared with conventional myocarditis (table 2).
Table 2.
Relative risk of incident heart failure or death within 90 days of follow-up from admission to hospital for new onset myocarditis in individuals with myocarditis associated with SARS-CoV-2 mRNA vaccination, myocarditis associated with covid-19 disease, and conventional myocarditis (Nordic myocarditis cohort)
| Type of myocarditis | Diagnosis of heart failure (No of patients) | Death from any cause (No of patients) | Total No of patients | Relative risk (95% CI) of heart failure | Relative risk (95% CI) of death |
| Vaccination | 22 | 6 | 530 | 0.56 (0.37 to 0.85) | 0.48 (0.21 to 1.09) |
| Covid-19 | 12 | 6 | 109 | 1.48 (0.86 to 2.54) | 2.35 (1.06 to 5.19) |
| Conventional | 496 | 156 | 6653 | 1 (reference) | 1 (reference) |
CI=confidence interval.
For estimates of the risk of heart failure in individual countries, we found similar patterns for myocarditis associated with vaccination and conventional myocarditis across the Nordics countries (online supplemental figure S1); limited statistical power did not allow calculation of risk estimates for myocarditis associated with covid-19 disease. We found no indication of differences between the Nordic countries for the risk of death for patients with vaccination type myocarditis within 90 days of follow-up, although statistical power was limited because of few deaths (online supplemental figure S2). In combined meta-analyses, we found that estimates of risk for both heart failure and death were lower for patients with myocarditis associated with vaccination compared with conventional myocarditis for all time points during follow-up (figure 1).
Figure 1.
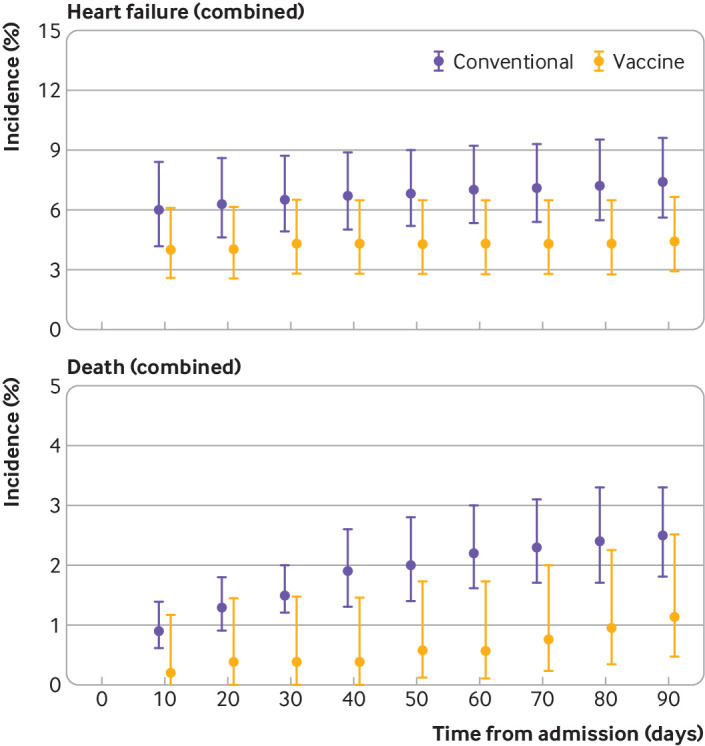
Cumulative incidences of heart failure and death, combined from all countries, during follow-up (at multiples of 10 days) in the Nordic myocarditis cohort, for patients with myocarditis associated with SARS-CoV-2 mRNA vaccination and patients with conventional myocarditis
Absolute and relative risk of readmission
At 90 days of follow-up from discharge for new onset myocarditis, 62 (11.7%), nine (8.3%), and 988 (14.9%) patients were readmitted to hospital in the vaccination type, covid-19 type, and conventional myocarditis groups, respectively. The relative risk of readmission between types of myocarditis within 90 days of discharge from hospital, with conventional myocarditis as the reference, was 0.79 (95% confidence interval 0.62 to 1.00) for patients with myocarditis associated with vaccination and 0.55 (0.30 to 1.04) for patients with myocarditis associated with covid-19 disease (table 3). Online supplemental figure S3 presents the risk of readmission in the vaccination and conventional myocarditis groups in 10 day periods for each country. In a combined Nordic analysis, we found that the estimated risk of readmission to hospital was numerically lower for patients with myocarditis associated with vaccination compared with those with conventional myocarditis at all periods during follow-up (online supplemental figure S4).
Table 3.
Relative risk of readmission to hospital for any cause within 90 days of follow-up from discharge for new onset myocarditis in individuals with myocarditis associated with SARS-CoV-2 mRNA vaccination, myocarditis associated with covid-19 disease, and conventional myocarditis (Nordic myocarditis cohort)
| Type of myocarditis | Readmission to hospital for any cause (No of patients) | Total No of patients | Relative risk (95% CI) of readmission |
| Vaccination | 62 | 530 | 0.79 (0.62 to 1.00) |
| Covid-19 | 9 | 109 | 0.55 (0.30 to 1.04) |
| Conventional | 988 | 6638 | 1 (reference) |
CI=confidence interval.
Subgroup analyses
In subgroup analyses, we found similar patterns to the main analyses in younger patients, older patients, and in men and women, with a lower risk of the combined outcome of heart failure and death by 90 days of follow-up for patients with myocarditis associated with vaccination compared with those with conventional myocarditis or myocarditis associated with covid-19 disease (online supplemental table S4). Among men and when the analysis was restricted to patients admitted on or after 1 January 2020, we found a significantly lower risk of heart failure or death for patients with myocarditis associated with SARS-CoV-2 mRNA vaccination compared with the two other types of myocarditis (P=0.005 and P=0.002, compared with convential myocarditis, respectively, and both P<0.001 compared with covid-19 type myocarditis, respectively; online supplemental table S5). We found no significant differences in the risk of readmission in younger patients and in women for the three myocarditis groups (online supplemental table S6), but a significantly reduced risk of readmission was found for men and those admitted on or after 1 January 2020 in the vaccination type myocarditis group compared with the conventional myocarditis group (P=0.03 and P=0.03, respectively). We also found a significantly reduced risk of readmission among older patients in the covid-19 type myocarditis group compared with the conventional myocarditis group (P=0.02, online supplemental table S6). Sensitivity analyses of patients with conventional myocarditis admitted to hospital before 1 January 2020 (ie, before the pandemic) as reference were comparable with the results of the main analyses (online supplemental table S7).
Finally, we performed an analysis restricted to patients aged 12-39 years with no pre-existing registered diagnoses of malignancy, heart disease, or autoimmune disease (table 4). We found that myocarditis after SARS-CoV-2 mRNA vaccination was associated with a non-significant reduced relative risk of heart failure or death at 90 days of follow-up compared with conventional myocarditis (relative risk 0.50, 95% confidence interval 0.22 to 1.12). In this subgroup, we also found that myocarditis after covid-19 disease was associated with an increased risk of heart failure or death within 90 days of admission (relative risk 5.78, 1.84 to 18.20) compared with myocarditis associated with vaccination. We found no differences in the risk of readmission between the types of myocarditis for patients aged <40 years with no diagnoses of malignancy, heart disease, or autoimmune disease (online supplemental table S6).
Table 4.
Relative risk of incident heart failure or death, as a combined outcome, within 90 days of follow-up from admission to hospital for new onset myocarditis in individuals aged 12-39 years, with no predisposing comorbidities, and with myocarditis associated with SARS-CoV-2 mRNA vaccination, myocarditis associated with covid-19 disease, or conventional myocarditis
| Type of myocarditis | Diagnosis of heart failure or death (No of patients) | Total No of individuals | Relative risk (95% CI) of diagnosis of heart failure or death |
| Vaccination | 6 | 326 | 0.50 (0.22 to 1.12) |
| Covid-19 | 5 | 47 | 2.87 (1.23 to 6.70) |
| Conventional | 114 | 3077 | 1 (ref.) |
CI=confidence interval.
Discussion
Principal findings
In a population based study of 23 million individuals in Denmark, Finland, Norway, and Sweden, we found that myocarditis after vaccination with SARS-CoV-2 mRNA vaccines was associated with a significantly lower risk of heart failure within 90 days of admission compared with conventional myocarditis and myocarditis after covid-19 disease (P=0.006 and P=0.005, respectively). Also, among younger patients with no potentially predisposing comorbidities for developing myocarditis, we found that myocarditis after covid-19 disease was associated with a substantially higher risk of heart failure or death at 90 days of follow-up compared with myocarditis after vaccination. Taken together, our findings suggested that the outcomes of myocarditis after vaccination were less severe than other types of myocarditis during the first 90 days after the onset of myocarditis.
Strengths and limitations
Our study was based on nationwide health registers in four Nordic countries, covering all patients with myocarditis admitted to hospital aged ≥12 years. Also, the register data on SARS-CoV-2 mRNA vaccination, PCR test results for SARS-CoV-2 infection, admissions to hospital for myocarditis, and outcomes after myocarditis were collected prospectively as part of routine clinical and administrative practices, thereby eliminating potential recall bias.
A limitation of our study was that we did not have information on paraclinical evaluations of the severity of myocarditis (eg, electrocardiography, echocardiography, or cardiac magnetic resonance imaging (MRI)). Our prespecified outcome of a diagnosis of heart failure by a hospital physician, however, has previously been associated with high validity in the non-geriatric population.14 Also, compared with only radiographic findings, a diagnosis of heart failure is likely to reflect clinically relevant impairment. A second limitation of the study was that patients with myocarditis not related to vaccination or covid-19 disease were combined into one category, with some incidences of myocarditis caused by drug treatment for an underlying condition (eg, myocarditis induced by cancer chemotherapy), which inherently could result in a higher risk of readmission to hospital, heart failure, and death. In our sensitivity analysis restricted to younger individuals without predisposing comorbidities, however, our findings were similar to the main analysis.
A third limitation of the study was the potential for misclassification of the cause of myocarditis for patients with myocarditis associated with vaccination and covid-19 disease. This potential bias is difficult to avoid in large scale studies, however, and most likely is non-differential. Furthermore, in our sensitivity analyses with those admitted to hospital for new onset myocarditis before the pandemic as reference, we found similar findings to our main analyses, suggesting no strong bias from misdiagnosed cases during the pandemic period. A fourth limitation was the slight heterogeneity in the definition of myocarditis, because for patients in Denmark, admission to hospital was defined as ≥24 hours because of a current lack of distinction between inpatents and outpatients in Danish registries. A fifth limitation was no examination of medical prescriptions before diagnosis, which could have indicated the cause of myocarditis for a small subset of patients. Finally, because of current regulations on data privacy, we could not adjust the combined Nordic cohort for individual level covariates, and therefore we conducted subgroups analyses.
Comparison with other studies
Our results are compatible with the findings of smaller cohort studies in individual clinical centres,15 16 which found that myocarditis associated with SARS-CoV-2 mRNA vaccination was associated mainly with mild clinical outcomes. Nevertheless, six patients with myocarditis after vaccination died within 90 days of admission to hospital. Establishing causality given the rarity of death is difficult (1.1% of patients with myocarditis associated with vaccination), however, because these deaths could have been from other causes or from conventional myocarditis occurring by chance within 28 days of vaccination. Schauer et al, in their study covering three to eight months after the first admission to hospital for myocarditis associated with vaccination, reported that resolution of cardiac MRI abnormalities were not complete in all patients.15 Because the clinical significance of these abnormalities is not yet known, continued surveillance of this patient group to detect possible developing cardiomuscular disease is warranted. The overall findings on outcomes of myocarditis associated with vaccination by us and others are reassuring, however, and should be considered when weighing the benefits and potential risks of mRNA vaccines against the SARS-CoV-2 virus at the individual and population levels.
Although our study consistently suggested that the outcomes of myocarditis after vaccination were less severe than for other types of myocarditis, we found only minimal differences in the relative risk of readmission to hospital within 90 days by type of myocarditis. This finding could reflect increased clinical interest in patients with myocarditis associated with vaccination, however, which could have resulted in increased rates of readmission for further clinical evaluation. Also, the higher risk of death among patients with myocarditis after covid-19 disease could potentially bias the risk of readmission downward for this patient group.
Compared with myocarditis associated with vaccination, myocarditis after covid-19 disease had substantially worse clinical outcomes, with a longer stay for the initial admission to hospital and increased risk of heart failure or death among younger individuals with no predisposing comorbidities. The difference in clinical outcomes for the two types of myocarditis could indicate differences in cause rather than a similar exposure to the SARS-CoV-2 spike protein, which is expressed during both mRNA vaccination and SARS-CoV-2 infection. The absolute risk of covid-19 myocarditis in the four Nordic countries was low during the study period,7 however, despite high testing rates for the SARS-CoV-2 virus and high seroprevalence of SARS-CoV-2 nucleocapsid antibodies.17
Our population based study provides new prognostic information on myocarditis associated with vaccination. The low cumulative incidence of heart failure or death by 90 days for patients developing myocarditis after vaccination is reassuring. We previously found that the incidence of myocarditis after a second dose of mRNA vaccine was higher than after a positive test result for SARS-CoV-2 infection among younger patients.7 In this study, however, our findings strongly suggested that the clinical outcomes were substantially worse for myocarditis associated with covid-19 disease. Among younger patients with no predisposing comorbidities, we found that the risk of heart failure or death within 90 days of new onset myocarditis was about six times higher for patients with myocarditis associated with covid-19 disease than for those with myocarditis after vaccination. Also, comparing the incidence of myocarditis after vaccination versus after covid-19 disease might not be meaningful when determining recommendations for the use of mRNA vaccines against the SARS-CoV-2 virus. Vaccination with mRNA vaccines has many well described beneficial properties, including protection against severe forms of covid-19 disease18 19 and death.18 20
Future studies of patients who developed myocarditis after SARS-CoV-2 mRNA vaccination should aim for an extended follow-up period of at least one year. Also, longitudinal evaluation of changes in paraclinical parameters, such as measurement of systolic and diastolic cardiac dysfunction, scarring on cardiac MRI, assessment of heart arrhythmia, and biological markers will be valuable for determining the natural history of myocarditis after vaccination with mRNA vaccines.
Conclusions
We found that myocarditis after vaccination with SARS-CoV-2 mRNA vaccines was associated with a lower risk of heart failure within 90 days of admission to hospital compared with conventional myocarditis and myocarditis after covid-19 disease. Less severe outcomes of myocarditis after vaccination were found in different subgroups, including younger patients with no predisposing comorbidities, and in both men and women. Our results suggested that the outcome of myocarditis associated with SARS-CoV-2 mRNA vaccination was less severe than for other types of myocarditis.
Acknowledgments
We thank chief statistician, Jan Wohlfahrt, for fruitful comments on the handling of the combined analyses of time point estimates.
Footnotes
Twitter: @anders_hviid
Contributors: AHu, HLG, PH, OK, RL, and AHu contributed to the concept and design of the study; AHu, HLG, PH, JVH, NP, NG, TH, JD, OK, and RL, contributed to the acquisition and analysis of the data; JVH, NP, NG, TH, and OK performed the statistical analysis; all authors contributed to critical revision of the manuscript for important intellectual content. The guarantor (AHu) accepts full responsibility for the finished work and the conduct of the study, had access to the data, and controlled the decision to publish. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. Transparency: The lead author (the guarantor) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Funding: AHu is supported by a postdoctoral research grant from the Lundbeck Foundation (grant No R322-2019-2800). The funder had no role in considering the study design or in the collection, analysis, interpretation of data, writing of the report, or decision to submit the article for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: no support from any organisation for the submitted work; AHu is supported by a postdoctoral research grant from the Lundbeck Foundation (grant No R322-2019-2800); AHv reports being a Scientific Advisory Board member for VAC4EU; LK reports speaker’s fees from Novo Nordisk, Novartis, AstraZeneca, Bayer, and Boehring, outside of the submitted work; PH reports being a member of an expert group, Future Rheumatology Advisory Board (2016 and 2017), funded by Pfizer; the Finnish Institute for Health and Welfare has a strategic policy of public private partnership, but none of the researchers of this report was involved with industry sponsored studies; OK reports participation in research projects funded by Novo Nordisk and LEO Pharma, all regulator mandated phase IV studies, all with funds paid to his institution (no personal fees), outside of the submitted work; HLG reports previous participation in research projects and clinical trials funded by Novo Nordisk, GSK, AstraZeneca, and Boheringer-Ingelheim, all related to diabetes and paid to her previous institution, Oslo University Hospital (no personal fees); HLG has received lecture fees and participated in advisory boards from several pharmaceutical companies before 2019 related to diabetes and metabolism; RL reports participation in a research project funded by Sanofi-Aventis, a regulator mandated phase IV study, with funds paid to his institution (no personal fees) (2011), outside of the submitted work; RL has received a lecture fee from Pfizer (2016), outside of the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data may be obtained from a third party and are not publicly available. Individual level data underlying the country specific analyses were only available within each Nordic country. The data do not belong to the authors and they are not permitted to share these data, except as presented in this manuscript.
Ethics approval
The cohort study was carried out according to the legal and ethical regulations in each participating country, as previously described in detail.21 Denmark: the study was conducted with administrative register data. According to Danish law, ethics approval is not required for such research. Finland: the study was a part of vaccine surveillance work, one of the duties of the Finnish Institute for Health and Welfare (THL). For this work, the institute is obliged to use all available data, including register data, to investigate potential harmful effects of the vaccines. Consent to participate was not applicable as this was a register based study. Norway: the study was approved by the Norwegian Regional Committee for Health Research Ethics South East (REK Sør-Øst A, reference 122745), and has conformed to the principles embodied in the Declaration of Helsinki. The emergency preparedness register was established according to the Health Preparedness Act §2-4. Consent to participate was not applicable as this was a register based study. Sweden: the study was approved by the Swedish Ethical Review Authority (2020-06859, 2021-02186) and has conformed to the principles embodied in the Declaration of Helsinki. Consent to participate was not applicable as this was a register based study. exempted this study. For all four participating countries (Denmark, Finland, Norway, and Sweden), consent to participate was not applicable in this register based study.
References
- 1. Montgomery J, Ryan M, Engler R, et al. Myocarditis following immunization with mRNA covid-19 vaccines in members of the US military. JAMA Cardiol 2021;6:1202. 10.1001/jamacardio.2021.2833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mevorach D, Anis E, Cedar N, et al. Myocarditis after BNT162b2 mRNA vaccine against covid-19 in Israel. N Engl J Med 2021;385:2140–9. 10.1056/NEJMoa2109730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Witberg G, Barda N, Hoss S, et al. Myocarditis after covid-19 vaccination in a large health care organization. N Engl J Med 2021;385:2132–9. 10.1056/NEJMoa2110737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Husby A, Hansen JV, Fosbøl E, et al. SARS-CoV-2 vaccination and myocarditis or myopericarditis: population based cohort study. BMJ 2021;375:e068665. 10.1136/bmj-2021-068665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Patone M, Mei XW, Handunnetthi L, et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with covid-19 vaccination or SARS-CoV-2 infection. Nat Med 2022;28:410–22. 10.1038/s41591-021-01630-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oster ME, Shay DK, Su JR, et al. Myocarditis cases reported after mRNA-based covid-19 vaccination in the US from December 2020 to August 2021. JAMA 2022;327:331–40. 10.1001/jama.2021.24110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Karlstad Øystein, Hovi P, Husby A, et al. SARS-CoV-2 vaccination and myocarditis in a Nordic cohort study of 23 million residents. JAMA Cardiol 2022;7:600. 10.1001/jamacardio.2022.0583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Buchan SA, Seo CY, Johnson C, et al. Epidemiology of myocarditis and pericarditis following mRNA vaccination by vaccine product, schedule, and interdose interval among adolescents and adults in Ontario, Canada. JAMA Netw Open 2022;5:e2218505. 10.1001/jamanetworkopen.2022.18505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Le Vu S, Bertrand M, Jabagi M-J, et al. Age and sex-specific risks of myocarditis and pericarditis following covid-19 messenger RNA vaccines. Nat Commun 2022;13:1–9. 10.1038/s41467-022-31401-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Park DY, An S, Kaur A, et al. Myocarditis after covid-19 mRNA vaccination: a systematic review of case reports and case series. Clin Cardiol 2022;45:691–700. 10.1002/clc.23828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kragholm KH, Lindgren FL, Zaremba T, et al. Mortality and ventricular arrhythmia after acute myocarditis: a nationwide registry-based follow-up study. Open Heart 2021;8:e001806. 10.1136/openhrt-2021-001806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sera F, Armstrong B, Blangiardo M, et al. An extended mixed-effects framework for meta-analysis. Stat Med 2019;38:5429–44. 10.1002/sim.8362 [DOI] [PubMed] [Google Scholar]
- 13. R . The R project for statistical computing. Available: https://www.r-project.org/ [Accessed 30 Sep 2022].
- 14. Sundbøll J, Adelborg K, Munch T, et al. Positive predictive value of cardiovascular diagnoses in the Danish national patient registry: a validation study. BMJ Open 2016;6:e012832. 10.1136/bmjopen-2016-012832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schauer J, Buddhe S, Gulhane A, et al. Persistent cardiac magnetic resonance imaging findings in a cohort of adolescents with post-coronavirus disease 2019 mRNA vaccine myopericarditis. J Pediatr 2022;245:233–7. 10.1016/j.jpeds.2022.03.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Patel T, Kelleman M, West Z, et al. Comparison of multisystem inflammatory syndrome in children–related myocarditis, classic viral myocarditis, and covid‐19 vaccine‐related myocarditis in children. J Am Heart Assoc 2022;11:24393. 10.1161/JAHA.121.024393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Erikstrup C, Laksafoss AD, Gladov J, et al. Seroprevalence and infection fatality rate of the SARS-CoV-2 omicron variant in Denmark: a nationwide serosurveillance study. Lancet Reg Health Eur 2022;21:100479. 10.1016/j.lanepe.2022.100479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Andrews N, Tessier E, Stowe J, et al. Duration of protection against mild and severe disease by covid-19 vaccines. N Engl J Med 2022;386:340–50. 10.1056/NEJMoa2115481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Powell AA, Kirsebom F, Stowe J, et al. Effectiveness of BNT162b2 against covid-19 in adolescents. Lancet Infect Dis 2022;22:581–3. 10.1016/S1473-3099(22)00177-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cohn BA, Cirillo PM, Murphy CC, et al. SARS-CoV-2 vaccine protection and deaths among US veterans during 2021. Science 2022;375:331–6. 10.1126/science.abm0620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ludvigsson JF, Håberg SE, Knudsen GP, et al. Ethical aspects of registry-based research in the Nordic countries. Clin Epidemiol 2015;7:491. 10.2147/CLEP.S90589 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjmed-2022-000373supp001.pdf (236.7KB, pdf)
bmjmed-2022-000373supp002.pdf (375.5KB, pdf)
Data Availability Statement
Data may be obtained from a third party and are not publicly available. Individual level data underlying the country specific analyses were only available within each Nordic country. The data do not belong to the authors and they are not permitted to share these data, except as presented in this manuscript.


