Abstract
Objective
To determine the effect of covid-19 vaccination, given before and after acute infection with the SARS-CoV-2 virus, or after a diagnosis of long covid, on the rates and symptoms of long covid.
Design
Systematic review.
Data sources
PubMed, Embase, and Cochrane covid-19 trials, and Europe PubMed Central (Europe PMC) for preprints, from 1 January 2020 to 3 August 2022.
Eligibility criteria for selecting studies
Trials, cohort studies, and case-control studies reporting on patients with long covid and symptoms of long covid, with vaccination before and after infection with the SARS-CoV-2 virus, or after a diagnosis of long covid. Risk of bias was assessed with the ROBINS-I tool.
Results
1645 articles were screened but no randomised controlled trials were found. 16 observational studies from five countries (USA, UK, France, Italy, and the Netherlands) were identified that reported on 614 392 patients. The most common symptoms of long covid that were studied were fatigue, cough, loss of sense of smell, shortness of breath, loss of taste, headache, muscle ache, difficulty sleeping, difficulty concentrating, worry or anxiety, and memory loss or confusion. 12 studies reported data on vaccination before infection with the SARS-CoV-2 virus, and 10 showed a significant reduction in the incidence of long covid: the odds ratio of developing long covid with one dose of vaccine ranged from 0.22 to 1.03; with two doses, odds ratios were 0.25-1; with three doses, 0.16; and with any dose, 0.48-1.01. Five studies reported on vaccination after infection, with odds ratios of 0.38-0.91. The high heterogeneity between studies precluded any meaningful meta-analysis. The studies failed to adjust for potential confounders, such as other protective behaviours and missing data, thus increasing the risk of bias and decreasing the certainty of evidence to low.
Conclusions
Current studies suggest that covid-19 vaccines might have protective and therapeutic effects on long covid. More robust comparative observational studies and trials are needed, however, to clearly determine the effectiveness of vaccines in preventing and treating long covid.
Protocol registration
Open Science Framework https://osf.io/e8jdy.
Keywords: covid-19
WHAT IS ALREADY KNOWN ON THIS TOPIC
Long covid is a serious new public health problem, and how vaccination against covid-19 disease affects patients with long covid is unclear
WHAT THIS STUDY ADDS
No randomised controlled trials have assessed the effect of covid-19 vaccination on preventing or treating long covid
Data from 16 observational studies suggest that covid-19 vaccination could protect against long covid
Observational studies suggest that vaccination might help those with a diagnosis of long covid
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE, OR POLICY
More robust comparative observational studies and trials are needed to clearly determine the effectiveness of vaccines in preventing and treating long covid
Introduction
Long covid, also known as post-acute covid-19 sequalae or post-acute covid-19 syndrome, is recognised as a major concern after infection with the SARS-CoV-2 virus, and will likely cause substantial global morbidity for many years.1 2 With global numbers of infections of more than 500 million and a conservative prevalence of 20-30%, more than 100 million people could be currently affected by long covid worldwide.3–5
In October 2021, the World Health Organization defined long covid as symptoms occurring in people with a history of probable or confirmed SARS-CoV-2 infection, usually within three months, and lasting for at least two months, that cannot be explained by an alternative diagnosis.6 7 Many symptoms associated with long covid have been reported that can last for months, and the common symptoms include, but are not limited to, fatigue, cognitive dysfunction, head, body, and joint pains, and dyspnoea.8 9 Factors such as female sex, severe initial disease, and comorbid conditions seem to be associated with the risk of long covid.10
Interest in the effect of covid-19 vaccination on long covid has been growing.2 11 Recent observational studies give contradictory results, however, and have methodological flaws, which preclude firm conclusions on the effect of vaccination on long covid.12 13 The covid-19 vaccines could work on three levels to prevent or treat long covid: firstly, by preventing infection with the SARS-CoV-2 virus; secondly, by reducing the severity of the disease in people who have been vaccinated and are then infected with the virus; and thirdly, by benefiting people who already have long covid. Hence the aim of our study was to assess the effect of covid-19 vaccination, given before and after acute infection with the SARS-CoV-2 virus, and also after a diagnosis of long covid, on the rates and symptoms of long covid.
Methods
We conducted a systematic review with enhanced processes and automation tools.14 The systematic review is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.15 Our protocol was shared on the Open Science Framework (https://osf.io/e8jdy) on 2 March 2022.
We searched the PROSPERO and Open Science Framework databases to exclude similar reviews. We then searched PubMed, Embase, and Cochrane covid-19 trials for published studies, and Europe PubMed Central (Europe PMC) for preprints, from 1 January 2020 to 3 August 2022. A search string of medical subject headings terms and words was developed in PubMed and translated to run in other databases with the Polyglot search translator.16 Online supplemental file 1 shows the search strategies for all databases.
bmjmed-2022-000385supp001.pdf (308.2KB, pdf)
We also conducted forward and backward citation searches of the included studies. For registered studies, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform. Searches were run from inception to 3 August 2022 (appendix 1). We also checked the VIEW-hub database (www.view-hub.org), a collaboration between the International Vaccine Access Centre and Johns Hopkins Bloomberg School of Public Health. No publication type or language restrictions were applied. We also contacted authors of large vaccine trials for any unpublished data on long covid.
We included randomised controlled trials, cohort studies (retrospectively or prospectively assembled), interrupted time series, and case-control studies. We excluded case reports, case series, cross sectional studies, and modelling studies. We searched for studies that assessed vaccination status and the emergence of long covid (history of confirmed or probable covid-19 within the past three months and symptoms that lasted at least two months that could not be explained by an alternative diagnosis). Studies conducted in the community, primary care, and hospital settings were included.
Our inclusion criteria were people of all ages who were eligible to receive a covid-19 vaccine. The interventions were any dose of a covid-19 vaccine recognised by WHO (ie, BNT 162b2 (tozinameran, Pfizer-BioNTech), mRNA-1273 (elasomeran, Moderna), ChAdOx1 nCoV-19 (Oxford-AstraZeneca), and Ad26.COV2.S (Janssen or Johnson & Johnson)), before or after the first SARS-CoV-2 infection, or after a diagnois of long covid. Comparators were no vaccination, an active non-covid-19 vaccine control (eg, influenza vaccine), or placebo.
The primary outcomes were patients with a diagnosis of long covid, according to the WHO definition (ie, history of confirmed or probable covid-19 within the past three months and symptoms that lasted at least two months that could not be explained by an alternative diagnosis), and remission or resolution of long covid in patients who were vaccinated after a diagnosis of long covid. The secondary outcome was prevalence of individual symptoms of long covid, such as prolonged fatigue, shortness of breath, cognitive difficulties, and loss of sense of smell. We excluded protocols, studies that did not report long covid outcomes, and studies with uncertain vaccination status at the time of infection (figure 1).
Figure 1.
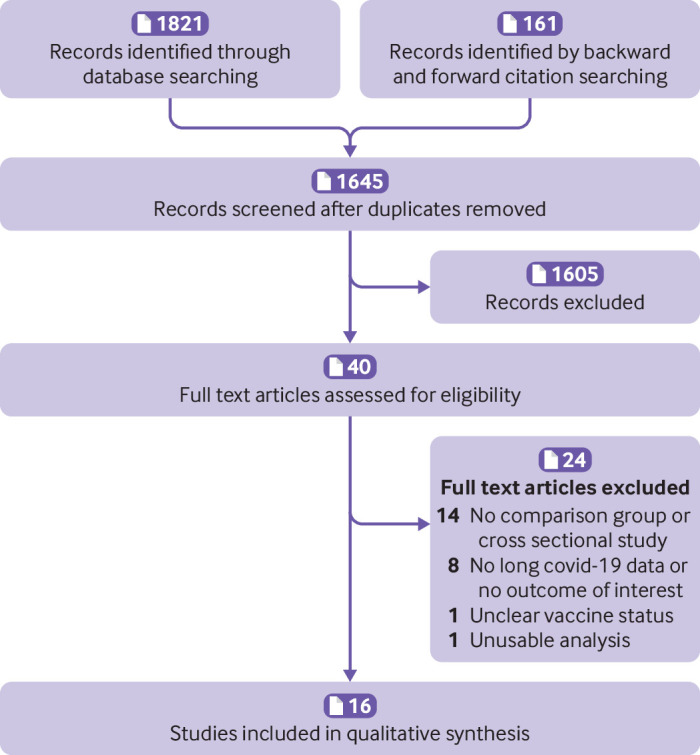
Screening and selection of studies
Study selection and screening
Two of the authors (OB and PS) independently screened the titles and abstracts, and full text articles were retrieved for potentially eligible articles. The full texts were then reviewed against the inclusion criteria. Discrepancies were resolved by referring to a third author (PG). Figure 1 summarises the screening process. Online supplemental file 2 lists the excluded articles and reasons for exclusion.
Data extraction
Two of the authors (OB and PS) extracted the data with Microsoft Excel. Study characteristics and outcomes extracted from each study were: methods (study authors, year, country, study design, length of follow-up, and setting); participants (number of participants, age, sex, and any co-comorbidities); interventions (type of intervention, dose, and frequency) and type of comparators (no treatment, other non-covid-19 vaccine, or placebo); and outcomes (patients with long covid (primary outcome) and prevalence of individual symptoms (secondary outcome)).
Assessment of risk of bias
Risk of bias was assessed with the ROBINS-I tool, which can assess both randomised and non-randomised studies on a common template.17 Two of the authors (OB and PS) independently assessed the risk of bias for each study.
Data analysis
We did not conduct meta-analyses because of the high heterogeneity of the data. For dichotomous outcomes, the effect of the intervention was calculated with odds ratios. For one study, we calculated the odds ratio from the reported mean differences.18 We used individual participants as the unit of analysis. When data were missing or unclear, the study investigators were contacted. We found no registered trials for vaccines and long covid. We could only present subgroups by dose of vaccine and timing of vaccine dose.
Patient and public involvement
Patients and the public were not involved in this review. Systematic reviews identify and analyse relevant primary studies to answer a specific research question, but they are not conducted on patients or public directly. We plan to disseminate our results through open access publication, our institute’s monthly newsletter, and preprint database update.
Results
Of 1645 titles and abstracts screened, 40 full text articles were assessed for inclusion (figure 1). We found no eligible randomised trials. The 16 eligible observational studies (including seven preprints) were based on data from five countries (USA (n=8), UK (n=4), the Netherlands (n=2), France (n=1), and Italy (n=1)) that included 614 392 patients19–34 (tables 1 and 2). Online supplemental file 2 lists the articles that were excluded and the reasons for exclusion.
Table 1.
Characteristics of included studies of vaccines given before infection
| Study, year, country | Study type and timeframe | Patient or data source | Intervention population | Intervention | Comparator | Outcomes of interest and length of follow-up |
| Al-Aly 2022, USA19 |
Retrospectively assembled cohort. 1 January-1 December 2021 |
US Veterans Health Administration electronic health databases | n=33 940 patients who were fully vaccinated ≥14 days before a positive covid-19 test result, who were still alive 30 days after a positive test result | One dose of Janssen, or two doses of Moderna or Pfizer | n=113 474 propensity score matched patients who were not vaccinated and alive 30 days after a positive covid-19 test result and no vaccination | Risk of ≥1 sequelae after the acute infection at 6 months |
| Antonelli 2022, UK20 |
Prospective, community based, nested, case-control study, 8 December 2020-4 July 2021 | Covid-19 symptom study app users | n=3071 adult app users with a positive covid-19 test result ≥14 days after their first vaccine or at least seven days after their second vaccine; had no positive test result before vaccination and who had used the app for ≥14 consecutive days after the test | First or second dose of Pfizer, Moderna, or AstraZeneca | n=3244 participants reporting a positive SARS-CoV-2 test result who were not vaccinated and who had used the app for ≥14 days after the test | Long duration (≥1 month) of symptoms after one dose. Most common symptoms |
| Ayoubkhani 2022, UK, preprint21 | Retrospectively assembled cohort, 26 April 2020-30 November 2021 | Covid-19 infection survey participants | n=3090 adult participants who tested positive for SARS-CoV-2 between 26 April 2020 and 30 November 2021; who had received two vaccines at least two weeks before infection | Two or more doses of AstraZeneca, Pfizer, or Moderna | n=3090 1:1 propensity score matched patients based on sociodemographic characteristics and time from infection to follow-up for long covid who were not vaccinated | Rates of long covid >3 months after infection |
| Azzolini 2022, Italy22 | Retrospective cohort, March 2020-April 2022 | Healthcare workers from nine hospitals | n=318 healthcare workers who received >1 dose of vaccine before SARS-CoV-2 infection | One, two, or three doses of Pfizer | n=421 healthcare workers who were not vaccinated before infection | Rates of long covid >1 month after infection |
| Ioannou 2022, USA23 |
Retrospectively assembled cohort. 1 Feb 2020-30 Apr 2021 |
US Veterans Health Administration electronic health databases | n=8357 people with 1-2 doses of vaccines with a positive SARS-CoV-2 test result between 1 February 2020 and 30 April 2021, who were still alive 3 months after infection, with no evidence of reinfection | One or two doses of Moderna or Pfizer | n=58 693 people who were not vaccinated at the time of infection, who were still alive three months after infection, with no evidence of reinfection | Rates and risk factors of long covid. Care at >3 months after infection |
| Mohr 2022, USA, preprint24 |
Prospective cohort, December 2020-August 2021 | Healthcare professionals in 12 states participating in vaccine effectiveness study (PREVENT trial) | n=180 healthcare professionals who had two doses of vaccines >14 days before covid-19 disease | Two doses of Moderna or Pfizer | n=239 healthcare professionals who had covid-19 with no previous vaccination | Presence of symptoms 1.5 months after onset of covid-19 disease |
| Pell 2022, UK, preprint25 |
Prospective cohort, April 2020-May 2021 | Long Covid in Scotland Study (Long CISS) | n=1154 adults with 1-4 doses of vaccine, who had a positive PCR test result | Not specified | n=32 127 adults who were not vaccinated | Confusion and difficulty concentrating at 6-18 months |
| Simon 2021, USA, preprint31 |
Retrospectively assembled cohort, February 2020-May 2021 | Arcadia data research dataset with >150 million patient records | n=2392 patients who had their first dose of vaccine before a diagnosis of covid-19 | One dose of Pfizer, Moderna, or Janssen | n=220 460 patients who were not vaccinated before covid-19 and 12 weeks after | Odds of having long covid at 3 months |
| Tannous 2022, USA, preprint26 |
Retrospectively assembled cohort, 3 March 2020-20 November 2021 | Houston Methodist Covid-19 Surveillance and Outcomes Registry (CURATOR) electronic health record database | n=3781 adult patients vaccinated >14 days before covid-19 disease | Two doses of mRNA vaccines or one dose of Janssen | n=49 458 patients who were not vaccinated | Odds of having long covid >1 month. Most common symptoms |
| Taquet 2021, USA27 |
Retrospectively assembled cohort, 1 Janurary-31 August 2021 |
TriNetX Analytics, a federated network of linked electronic health records, with 81 million patient records | n=9479 adults who received a covid-19 vaccine at least 2 weeks before SARS-CoV-2 infection | Pfizer, Moderna, or Janssen |
n=9479 propensity matched patients who had received the influenza vaccine at any time | Odds of having any long covid symptoms within 6 months |
| van der Maaden, Netherlands, preprint28 |
Prospective cohort, 19 May-13 December 2021 | Dutch prospective long covid study | n=3838 adult patients (aged <65), who were fully vaccinated three months after a positive SARS-CoV-2 test result | >1 dose of Janssen or >2 doses of mRNA vaccine | n=528 patients (<65 years) who were not vaccinated, three months after a positive SARS-CoV-2 test result | Odds of at least one significantly raised long covid symptom at >2 months |
| Zisis 2022, USA29 |
Retrospectively assembled cohort, 21 September 2020-14 December 2021 | TriNetX Analytics, a federated network of linked electronic health records, with 81 million patient records | n=25 225 adult patients who were vaccinated with a confirmed diagnosis of covid-19 | Not specified | n=25225 1:1 propensity score matched patients who were not vaccinated | New onset of long covid symptoms such as fatigue at 3 month follow-up |
PCR=polymerase chain reaction; PREVENT=Project PREVENT (PReventing Emerging Infections through Vaccine EffectiveNess Testing Project) trial.
Table 2.
Characteristics of included studies when vaccines were given after infection or after a diagnosis of long covid
| Study, year, country | Study type and timeframe | Patient or data source | Intervention population | Intervention | Comparator | Outcomes of interest and length of follow-up |
| Ayoubkhani 2022, UK30 | Interrupted time series, 3 February-5 September 2021 | Covid-19 infection survey participants | n=28 356 patients with long covid, who had received at least one vaccine after diagnosis | AstraZeneca, Pfizer, or Moderna | Self-controlled (symptoms before vaccine) | Long covid of any severity after first and second dose ≥3 months. 10 most common symptoms |
| Simon 2021, USA, preprint31 |
Retrospectively assembled cohort, February 2020-May 2021 | Arcadia data research dataset with >150 million patient records | n=17 796 patients with a diagnosis of covid-19, by PCR or ICD-10 code U07.1 at any time or B97.29 before May 2020, and who were vaccinated within 12 weeks after a diagnosis of covid-19 | One dose of Pfizer, Moderna, or Janssen | n=220 460 patients who were not vaccinated before covid-19 and 12 weeks after | Odds of having long covid at 3 months |
| Tran 2021, France, preprint32 |
Prospective cohort, December 2020-September 20218 | ComPaRe (cohort of patients with chronic diseases) | n=455 adults with a confirmed or suspected SARS-CoV-2 infection and at least one symptom attributable to long covid reported at baseline and persisting for >3 weeks | AstraZeneca, Pfizer, Moderna, or Janssen | n=455 1:1 propensity matched controls from the same cohort who were not vaccinated | Remission of all long covid symptoms by four months |
| Wisnivesky 2022, USA33 | Prospective cohort, 20 July 2020-August 2021 | Patients with covid-19 enrolled in prospective registry established at Mount Sinai Health System | n=324 adult patients with a history of laboratory confirmed covid-19, with one or more symptoms of long covid, treated at Mount Sinai and who were fully vaccinated | Pfizer, Moderna, or Janssen | n=129 patients from the same cohort who were not vaccinated | Fatigue at six months |
| Wynberg 2022, Netherlands34 |
Prospective cohort, 11 May 2020-1 November 2021 | RECoVERED study participants | n=36 patients with long covid, aged 16-85 years, with laboratory confirmed covid-19, Amsterdam residents, with >3 months of follow-up | AstraZeneca, Pfizer, Moderna, or Janssen | n=32 participants who were not vaccinated matched 1:1 on age, sex, obesity status, and time since illness onset to participants who were vaccinated | Recovery from long covid symptoms at ≥3 months since onset of illness |
PCR=polymerase chain reaction; ICD-10=international classification of diseases, 10th version.
Eleven studies assessed the effect of a vaccine given before infection with the SARS-CoV-2 virus19–29 (table 1); four studies assessed the effects of a vaccine after infection and after a diagnosis of long covid30 32–34 (table 2). One study provided data for both vaccination before and after infection and therefore was included in both tables.31 Five of the studies used data from three large medical databases,19 23 27 29 31 five studies used the covid-19 symptom study app user data or national covid-19 survey data,20 21 25 28 30 two studies involved healthcare workers and professionals,22 24 and four studies recruited patients who already had symptoms of long covid to prospectively follow for remission or recovery.30 32–34
All but one study was conducted and concluded by December 2021, and thus did not include data on the omicron variant of the SARS-CoV-2 virus.22 Only one study cited the current case definition of long covid by WHO.34 Five studies did not provide a clear definition of long covid but reported 3-6 months of follow-up outcomes.19 21 23 25 28 Five studies used symptoms lasting longer than 28 days since the onset of acute infection as the cut-off for long covid.20 22 26 29 30 Nine studies used self-reported symptoms as a diagnosis of long covid,20–22 24 28 30 32–34 five studies used ICD-10 (international classification of diseases, 10th revision) codes to determine organ-system symptoms related to long covid to establish the presence of long covid,19 23 27 29 31 one study used electronic health record data,26 and one study used a combination of patient self-report and ICD-10 codes.25
Secondary outcomes were reported in four studies.20 25 26 30 The most common symptoms of long covid were fatigue, cough, weakness and tiredness, loss of sense of smell, shortness of breath, loss of taste, headache, difficulty sleeping, difficulty concentrating, muscle ache, worry or anxiety, and memory loss or confusion.
Effect of vaccination on outcomes of long covid
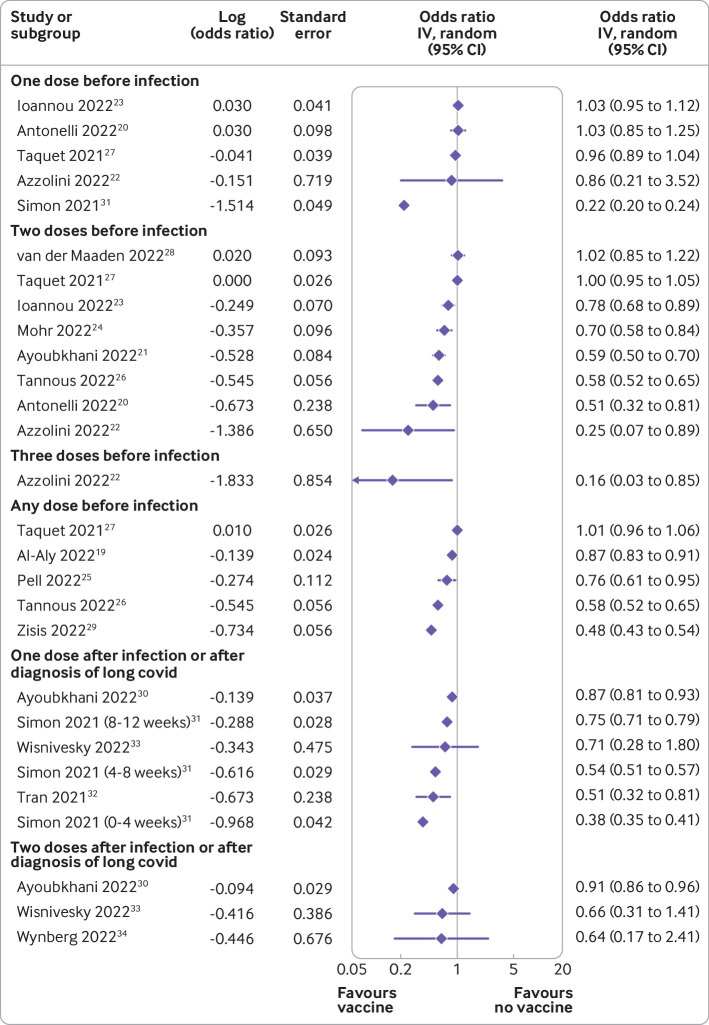
The high heterogeneity between studies precluded a meaningful meta-analysis. The forest plot of the outcomes of each study showed high heterogeneity (figure 2). Twelve studies reported data on vaccination before infection with the SARS-CoV-2 virus,19–29 31 of which 10 showed a significant reduction in the incidence of long covid.19–26 29 31 The odds ratio of developing long covid with one dose of vaccine before infection ranged from 0.22 to 1.03; for two doses, odds ratios were 0.25-1.02; and with any dose of vaccine before infection, the odds ratio was 0.48-1.01. One study reported the odds of having long covid at one month after infection with three doses of vaccine (odds ratio 0.16, 95% confidence interval 0.03 to 0.85).22 The five studies that reported data on vaccination after infection had odds ratios ranging from 0.38 to 0.91. Two studies that assessed remission32 and recovery34 from long covid reported the odds of not recovering when patients were vaccinated after infection as 0.51 (95% confidence interval 0.32 to 0.81) and 0.64 (0.17 to 2.33), respectively. Online supplemental file 3 shows all ratios and their explanations, along with timeframes.
Figure 2.
Forest plot of the effect of covid-19 vaccine doses on long covid. Only relevant outcomes from all reported outcomes in individual studies were chosen. The ratios have a range of time frames (tables 1 and 2, and online supplemental file 3). IV=inverse variance
Risk of bias in included studies
The risk of bias of the included studies was assessed by the ROBINS-I tool for non-randomised studies of interventions. The risk of bias of the individual studies was judged overall as moderate to critical. The primary sources of increased bias were domains that dealt with confounding, missing data, and measurement of outcomes. The main concerns arising from confounding were not accounting for vaccine hesitancy or severity of the original disease. Most of the studies did not report on how missing data were dealt with.
Bias in measurement of outcomes was rated moderate to critical in studies where the exposure (vaccination) and outcome measurements (symptoms of long covid) were collected together, or where participants were aware of their exposure at the time of the measurement and thus the reporting of the outcome could be potentially influenced by that knowledge. Another reason for the increased bias in outcome measurements was the unclear definition of long covid, particularly in studies that analysed data from electronic health record databases (table 3). Online supplemental file 4 provides further methodological details of the included studies.
Table 3.
Risk of bias in included studies assessed by the ROBINS-I tool
| Risk-of-bias domains | ||||||||
| Study, year | Confounding | Selection of participants | Classification of interventions | Deviations from intended intervention | Missing data | Measurement of outcomes | Overall risk of bias | Selection of reported result |
| Vaccination before SARS-CoV-2 infection | ||||||||
| Al-Aly 202219 | Low | Low | Low | Low | Low | Moderate | Low | Moderate |
| Antonelli 202220 | Moderate | Low | Low | Low | Serious | Serious | Low | Serious |
| Ayoubkhani 202221 | Critical | Low | Low | Low | Moderate | Low | Low | Critical |
| Azzolini 202222 | Moderate | Low | Moderate | Low | NI | Critical | Low | Critical |
| Ioannou 202223 | Moderate | Low | Low | Low | NI | Low | Moderate | Moderate |
| Mohr 202224 | Serious | Serious | Low | Low | Serious | Moderate | Moderate | Serious |
| Pell 202225 | Serious | Low | Serious | Low | Serious | Moderate | Moderate | Serious |
| Tannous 202226 | Serious | Low | Low | Low | Low | Serious | NI | Serious |
| Taquet 202127 | Moderate | Low | Low | Low | NI | Low | Low | Moderate |
| van der Maaden 202228 | Serious | Low | Moderate | Low | Low | Low | Low | Serious |
| Zisis 202229 | Serious | Critical | Serious | Low | NI | Serious | NI | Critical |
| Vaccination after SARS-CoV-2 infection or after diagnosis of long covid | ||||||||
| Ayoubkhani 202230 | Low | Low | Low | Low | Moderate | Low | Low | Moderate |
| Simon 202131 | Serious | Low | Low | Low | Moderate | Low | Low | Serious |
| Tran 202132 | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Wisnivesky 202233 | Serious | Serious | Serious | Low | Low | Low | Low | Serious |
| Wynberg 202234 | Serious | Moderate | Serious | Low | Low | Serious | Low | Serious |
NI=no information.
Discussion
Principal findings
We found no randomised controlled trials, but 16 observational studies provided outcomes on long covid. Six of the eight studies of two or more doses of vaccine given before infection with the SARS-CoV-2 virus found significant reductions in the rates of long covid. A similar result was less clear with only one dose of vaccine. Three of the five studies of vaccination after the infection showed significant reductions in patients with long covid, but none showed any harm of vaccination. Owing to insufficient data, we could not examine any dose-response association. All 16 studies were non-randomised, and most were assessed as having a moderate to critical risk of bias. Thus the evidence summarised here is of low certainty.
Strengths and weaknesses of the study
The strengths of our review were the search of multiple databases for published (including preprints) and unpublished articles, and public health reports. We critically assessed the risk of bias of the included studies to identify the main sources of bias.
Our study had several limitations. The greatest challenge in conducting this review was the validity of the diagnoses of long covid in the included studies. Most studies established a diagnosis of long covid based on the length of time symptoms were reported by participants or on data from electronic health records and ICD-10 codes, rather than from healthcare professionals, as anticipated. The studies also used different cut-off times for long covid; the shortest was 28 days. After infection with the virus, many symptoms, such as fatigue, routinely last more than a month.35 Although the WHO Delphi consensus on the definition of long covid was much needed, lack of awareness of the definition by health professionals might be hindering the diagnosis of long covid and therefore real world data on long covid.
Furthermore, we could not recalculate a common ratio for most of the studies and so we plotted relative risk ratio, odds ratio, and hazard ratio reported by the studies together as a close approximation.36 Also, we could not conduct a meta-analysis of the studies because of the high heterogeneity and lack of data on the types of vaccines, time between exposure and disease, and variants of the virus, highlighting the need for standardisation and validation studies of outcome measures for ongoing research on long covid.
Another limitation was that not many of our included studies reported on our secondary outcome, prevalence of individual symptoms of long covid. Several studies showed changes in symptoms after vaccination, but they were mostly cross sectional in design and thus establishing true causality was not possible; these studies were excluded. Furthermore, the characteristics and symptomatology of long covid are becoming well established with global data.1 5 37
Strengths and weaknesses in relation to other studies
One systematic review,38 one scoping review,39 and two government reports (by Public Health Ontario and UK Health Security agency) estimated the effect of vaccination on long covid.40 41 The government reports were rapid reviews and therefore a rigorous search or quality assessments on the reported studies was not done. All four studies included multiple cross sectional studies and only narratively explained the findings. Because of the lack of rigorous inclusion criteria, these reviews cannot be used to establish the effectiveness of vaccines in preventing long covid. Our review also includes more up-to-date evidence.
Meaning of the study
Vaccines against covid-19 disease have been found to prevent infection in patients, particularly for the earlier variants of the SARS-CoV-2 virus, and so would prevent long covid by preventing the initial infection. Less clear, although highly plausible, has been whether vaccines, by reducing the severity of symptoms of covid-19, reduce the prevalence of long covid after infection. The studies we identified were inconsistent, although the results showed a tendency towards vaccines reducing the prevalence of long covid. Vaccination after infection and in those with long covid has been more controversial, but the studies we identified are reassuringly consistent in being protective.
Unanswered questions and future research
A key finding of this review was the lack of high quality studies, particularly randomised trials, to determine the effect of vaccines on long covid. This finding has several implications for future research. Firstly, the best data on the effect of vaccines in patients with long covid after breakthrough infections (ie, infections that occur after vaccination) could have come from large clinical trials of vaccines. Our search for these data showed that trials on the efficacy of vaccines did not plan or collect suitable data for these outcome. Designing follow-up studies of breakthrough infections from ongoing vaccine trials to estimate rates of long covid is still possible.
Secondly, ongoing trials on the effectiveness of vaccines in children should include provisions for longer follow-up of patients who are infected with the virus after vaccination. Thirdly, the studies included in our review were conducted up to December 2021 and so do not include data on the omicron variant of the SARS-CoV-2 virus. Data from the UK Office for National Statistics found that the omicron variant of the virus caused the greatest number of patients with covid-19 and long covid in the UK.42 But a new analysis that compared the periods in the UK when the delta and omicron variants of the SARS-CoV-2 virus were the most prevalent, showed that during the omicron wave, the prevalence of long covid was about half that in previous waves, and patients infected with the omicron variant were less likely to have long covid even with more than six months between vaccination and infection (odds ratio 0.24-0.50).43
Mapping long covid data to the different subvariants of the SARS-CoV-2 virus will also help inform public health measures against the spread of the pandemic. In the meantime, researchers should use trial emulation techniques to better estimate the effect of vaccines on different age groups and variants. In our review, only one study explicitly emulated a target trial27 and less than half used propensity score matching when creating their comparator cohorts.19 21 29 32–34
Fourthly, the data from our included studies also suggested that covid-19 vaccines at least provide equipoise in terms of prevention and treatment of long covid, and thus trials on the effect of vaccination in patients after infection and after a diagnosis of long covid should be conducted as a priority. Although vaccine coverage might seem high in many western countries, several studies reported vaccine hesitancy in patients with long covid (>50%) because of fear of worsening symptoms and the belief that covid-19 vaccines were contraindicated in long covid.44 45 Finally, awareness of the case definition of long covid by medical professionals and management in parallel with the care needs of patients with long covid should be explored.
Conclusions
Covid-19 vaccines have saved millions of lives and prevented severe forms of the disease. The effect of the vaccines on preventing or treating long covid, however, was not conclusively established in this review. Many questions need to be answered as a priority, which will require agreed standards for outcomes, improved methods and analysis, better reporting, and application of these questions to current and future studies. This approach is particularly important for ongoing or new trials where consent should be obtained for follow-up of symptoms of long covid.
Acknowledgments
We thank the authors of the eligible papers for their replies to our queries. We also thank David Henry for his methodological expertise on the risk-of-bias assessment.
Footnotes
Twitter: @OyukaMDPhD
Contributors: PG conceived the study and co-designed the study with OB, PS, and KA. JC led the literature searches including backward and forward citation analysis. OB and PS conducted the parallel title, abstract, and full text screening. OB and PS did data extraction and analysis. All authors contributed to resolving disagreements throughout the study and to writing of the manuscript. PG is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. Transparency: The lead author (the guarantor) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial, or not-for-profit sectors.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No additional data available.
Ethics approval
Not applicable.
References
- 1. Michelen M, Manoharan L, Elkheir N, et al. Characterising long covid: a living systematic review. BMJ Glob Health 2021;6:e005427. 10.1136/bmjgh-2021-005427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ledford H. Do vaccines protect against long covid? What the data say. Nature 2021;599:546–8. 10.1038/d41586-021-03495-2 [DOI] [PubMed] [Google Scholar]
- 3. Chen C, Haupert SR, Zimmermann L, et al. Global prevalence of post-coronavirus disease 2019 (covid-19) condition or long covid: a meta-analysis and systematic review. J Infect Dis 2022;226:1593–607. 10.1093/infdis/jiac136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Whitaker M, Elliott J, Chadeau-Hyam M, et al. Persistent covid-19 symptoms in a community study of 606,434 people in England. Nat Commun 2022;13:1957. 10.1038/s41467-022-29521-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yoo SM, Liu TC, Motwani Y, et al. Factors associated with post-acute sequelae of SARS-CoV-2 (PASC) after diagnosis of symptomatic covid-19 in the inpatient and outpatient setting in a diverse cohort. J Gen Intern Med 2022;37:1988–95. 10.1007/s11606-022-07523-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Soriano JB, Murthy S, Marshall JC, et al. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis 2022;22:e102–7. 10.1016/S1473-3099(21)00703-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Soriano JB, Murthy S, Marshall JC, et al. A clinical case definition of post-covid-19 condition by a Delphi consensus. Lancet Infect Dis 2022;22:e102–7. 10.1016/S1473-3099(21)00703-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. More than 50 long-term effects of covid-19: a systematic review and meta-analysis. Sci Rep 2021;11:16144. 10.1038/s41598-021-95565-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hayes LD, Ingram J, Sculthorpe NF. More than 100 persistent symptoms of SARS-CoV-2 (long covid): a scoping review. Front Med 2021;8:750378. 10.3389/fmed.2021.750378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Han Q, Zheng B, Daines L, et al. Long-term sequelae of covid-19: a systematic review and meta-analysis of one-year follow-up studies on post-covid symptoms. Pathogens 2022;11:269. 10.3390/pathogens11020269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Venkatesan P. Do vaccines protect from long covid? Lancet Respir Med 2022;10:e30–e. 10.1016/S2213-2600(22)00020-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kuodi P, Gorelik Y, Zayyad H, et al. Association between vaccination status and reported incidence of post-acute covid-19 symptoms in Israel: a cross-sectional study of patients tested between March 2020 and November 2021. medRxiv 2022. 10.1038/s41541-022-00526-5 [DOI] [Google Scholar]
- 13. Peghin M, De Martino M, Palese A, et al. Post-covid-19 syndrome and humoral response association after 1 year in vaccinated and unvaccinated patients. Clin Microbiol Infect 2022;28:1140–8. 10.1016/j.cmi.2022.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Clark J, Glasziou P, Del Mar C, et al. A full systematic review was completed in 2 weeks using automation tools: a case study. J Clin Epidemiol 2020;121:81–90. 10.1016/j.jclinepi.2020.01.008 [DOI] [PubMed] [Google Scholar]
- 15. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–9. 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- 16. Clark JM, Sanders S, Carter M, et al. Improving the translation of search strategies using the Polyglot search translator: a randomized controlled trial. J Med Libr Assoc 2020;108:195–207. 10.5195/jmla.2020.834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schunemann JH, Vist EG, Higgins PJ, et al. Chapter 15: Interpreting results and drawing conclusions. In: Cochrane Handbook for Systematic Reviews of Interventions, version 63, 2022. [Google Scholar]
- 19. Al-Aly Z, Bowe B, Xie Y. Long covid after breakthrough SARS-CoV-2 infection. Nat Med 2022;28:1461–7. 10.1038/s41591-022-01840-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Antonelli M, Penfold RS, Merino J, et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the covid symptom study app: a prospective, community-based, nested, case-control study. Lancet Infect Dis 2022;22:43–55. 10.1016/S1473-3099(21)00460-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ayoubkhani D, Bosworth ML, King S, et al. Risk of long covid in people infected with SARS-CoV-2 after two doses of a covid-19 vaccine: community-based matched cohort study. Open Forum Infect Dis 2022. 10.1093/ofid/ofac464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Azzolini E, Levi R, Sarti R, et al. Association between BNT162b2 vaccination and long covid after infections not requiring hospitalization in health care workers. JAMA 2022;328:676. 10.1001/jama.2022.11691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ioannou GN, Baraff A, Fox A, et al. Rates and factors associated with documentation of diagnostic codes for long covid in the National Veterans Affairs health care system. JAMA Netw Open 2022;5:e2224359–undefined. 10.1001/jamanetworkopen.2022.24359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mohr NM, Plumb ID, Harland KK, et al. Presence of symptoms 6 weeks after covid-19 among vaccinated and unvaccinated U.S. healthcare personnel. medRxiv 2022. 10.1101/2022.02.16.22271092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pell J, Hastie C, Lowe D. Long-covid COVID in Scotland study: a nationwide, population cohort study. Research Square 2022. 10.21203/rs.3.rs-1656915/v1 [DOI] [Google Scholar]
- 26. Tannous J, Pan AP, Potter T, et al. Real world evidence of effectiveness of covid-19 vaccines and anti SARS-CoV-2 monoclonal antibodies against post-acute sequelae of SARS-CoV-2 infection. medRxiv 2022. 10.1101/2022.06.30.22277105 [DOI] [Google Scholar]
- 27. Taquet M, Dercon Q, Harrison PJ. Six-month sequelae of post-vaccination SARS-CoV-2 infection: a retrospective cohort study of 10,024 breakthrough infections. Brain Behav Immun 2022;103:154-162. 10.1016/j.bbi.2022.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van der Maaden T, Mutubuki EN, de Bruijn S, et al. Prevalence and severity of symptoms 3 months after infection with SARS-CoV-2 compared to test-negative and population controls in the Netherlands. J Infect Dis 2022. doi: 10.1093/infdis/jiac474. [Epub ahead of print: 07 Dec 2022]. [DOI] [PubMed] [Google Scholar]
- 29. Zisis SN, Durieux JC, Mouchati C, et al. The protective effect of coronavirus disease 2019 (COVID-19) vaccination on postacute sequelae of covid-19: a multicenter study from a large national health research network. Open Forum Infect Dis 2022;9:ofac228–undefined. 10.1093/ofid/ofac228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ayoubkhani D, Bermingham C, Pouwels KB, et al. Trajectory of long covid symptoms after covid-19 vaccination: community based cohort study. BMJ 2022;377:e069676. 10.1136/bmj-2021-069676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Simon MA, Luginbuhl RD, Parker R. Reduced incidence of long-covid symptoms related to administration of covid-19 vaccines both before COVID-19 diagnosis and up to 12 weeks after. medRxiv 2021. 10.1101/2021.11.17.21263608 [DOI] [Google Scholar]
- 32. Tran V-T, Perrodeau E, Saldanha J, et al. Efficacy of covid-19 vaccination on the symptoms of patients with long covid: a target trial emulation using data from the compare e-Cohort in France. SSRN Journal 2021. 10.2139/ssrn.3932953 [DOI] [Google Scholar]
- 33. Wisnivesky JP, Govindarajulu U, Bagiella E, et al. Association of vaccination with the persistence of post-covid symptoms. J Gen Intern Med 2022;37:1748–53. 10.1007/s11606-022-07465-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wynberg E, Han AX, Boyd A, et al. The effect of SARS-CoV-2 vaccination on post-acute sequelae of covid-19 (PASC): a prospective cohort study. Vaccine 2022;40:4424–31. 10.1016/j.vaccine.2022.05.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sandler CX, Wyller VBB, Moss-Morris R, et al. Long covid and post-infective fatigue syndrome: a review. Open Forum Infect Dis 2021;8:ofab440. 10.1093/ofid/ofab440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rothman JK, Greenland S. Modern epidemiology. Chapter 4. Lippincott-Raven: Measures of Effect and Measures of Association, 1998. [Google Scholar]
- 37. Xie Y, Bowe B, Al-Aly Z. Burdens of post-acute sequelae of covid-19 by severity of acute infection, demographics and health status. Nat Commun 2021;12:6571. 10.1038/s41467-021-26513-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Notarte KI, Catahay JA, Velasco JV, et al. Impact of covid-19 vaccination on the risk of developing long-covid and on existing long-covid symptoms: a systematic review. EClinicalMedicine 2022;53:101624. 10.1016/j.eclinm.2022.101624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mumtaz A, Sheikh AAE, Khan AM, et al. Covid-19 vaccine and long covid: a scoping review. Life 2022;12:1066. 10.3390/life12071066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ontario Agency for Health Protection and Promotion (Public Health Ontario) . Impact of vaccination on post-acute covid-19 syndrome (PACS) – what we know so far. Toronto, ON: Queen’s Printer for Ontario, 2022. [Google Scholar]
- 41. UK Health Security Agency . The effectiveness of vaccination against long covid: a rapid evidence briefing; 2022. https://ukhsa.koha-ptfs.co.uk/cgi-bin/koha/opac-retrieve-file.pl?id=fe4f10cd3cd509fe045ad4f72ae0dfff
- 42. Office for National Statistics . Prevalence of ongoing symptoms following coronavirus (covid-19) infection in the UK; 2022. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk/1june2022 [Accessed 01 Jun 2022].
- 43. Antonelli M, Pujol JC, Spector TD, et al. Risk of long covid associated with delta versus omicron variants of SARS-CoV-2. Lancet 2022;399:2263–4. 10.1016/S0140-6736(22)00941-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Scherlinger M, Pijnenburg L, Chatelus E, et al. Effect of SARS-CoV-2 vaccination on symptoms from post-acute sequelae of COVID-19: results from the nationwide VAXILONG study. Vaccines 2022;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Buonsenso D, Valentini P, Macchi M, et al. Caregivers' attitudes toward covid-19 vaccination in children and adolescents with a history of SARS-CoV-2 infection. Front Pediatr 2022;10:867968. 10.3389/fped.2022.867968 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjmed-2022-000385supp001.pdf (308.2KB, pdf)
Data Availability Statement
No additional data available.