Abstract
The treatment for endometrial cancer is rapidly evolving with the development of molecular analysis and novel strategies. Surgical resection, cytotoxic chemotherapy, endocrine or hormonal treatment, and radiation have been the staples of treatment for decades. However, precision based approaches for tumours are rapidly becoming a part of these strategies. Biomarker driven treatments are now a part of primary and recurrent treatment algorithms. This review aims to describe the current state of molecular analysis and treatment for endometrial cancer as well as to elucidate potential approaches for the near future.
Keywords: molecular medicine, medical oncology, genetics
Introduction
Endometrial cancer is the most common gynaecological malignancy in the US with an estimated 66 000 new cases (2022 estimates) annually.1 Globally, it is the sixth most common cancer, with 417 000 new cases and 97 000 deaths in 2020.2
Evidence reporting
Despite recent advances and novel treatments, survival for endometrial cancer has decreased in the past 10 years and represents one of the few cancers with an impaired survival.1 Uterine cancer is one of the few cancers with an increase in overall mortality, with a rise of 1.7% over the past 10 years.3 Common risk factors for endometrial cancer include obesity, states of unopposed oestrogen (eg, obesity, polycystic ovary syndrome), early menarche or late menopause, and hereditary cancer syndromes including Lynch and Cowden’s syndrome. Treatment for endometrial cancer has historically been related to the known histopathological risk factors of the tumour that might indicate better or worse recurrence risk. However, over the past decade, molecular analysis and molecular driven treatments have become an important aspect of patient directed care in endometrial cancer. This review summarises the known current molecular aberrations of endometrial cancer and associated treatment options. It highlights the current clinical trial landscape and potential therapeutic shifts based on molecular analysis. This review is aimed towards patients and healthcare providers.
Uterine histopathological status
Uterine factors, histopathological features, and cancer stage continue to be important factors of risk stratification in endometrial cancer. Several studies have examined the importance of numerous factors and have shown that grade, depth of invasion, presence or absence of lymphovascular space invasion, tumour size, and lower uterine segment involvement are all prognostic factors for survival and recurrence.4–7 These tenets help stratify patients into low, intermediate, high intermediate, and high risk criteria for adjuvant treatment.6 Today, patients at low risk are recommended for observation alone while those at high intermediate risk and high risk (advanced stage, serous or clear cell histology, grade 3 with deep invasion) are advised for more aggressive adjuvant treatments. The importance of chemotherapy with consideration of radiation for adjuvant treatment in the high risk population has been evaluated in several studies with recent reports from the GOG 258 and PORTEC-3 trials8 9 establishing current standards of care based on histopathology and stage. The current standard cytotoxic treatment in advanced and recurrent endometrial cancer is carboplatin and paclitaxel.10 Second line options for chemotherapy are limited in endometrial cancer, with poor response rates of about 15%.11
Molecular classification
Newer risk stratification models aimed at improving treatment algorithms for patients with endometrial cancer are centred around molecular classification. The Cancer Genome Atlas was the first to evaluate a large number of cancers through whole genome sequencing, including endometrial cancer.12 The Cancer Genome Atlas identified four subgroups of endometrial cancer with distinct genetic profiles: DNA polymerase ε (POLE, ultramutated), microsatellite instability (MSI, hypermutated), copy number low, and copy number high. Critically, the Cancer Genome Atlas showed that clinical outcomes were closely tied to these molecular subgroups. The initial Cancer Genome Atlas used whole genome sequencing for evaluation, which is not clinically or economically feasible on a large population base scale. However, multiple groups have since validated an equivalent molecular analysis using more feasible techniques of immunohistochemistry and Sanger or next generation sequencing analysis.13–15 Specifically, the ProMisE (proactive molecular risk classifier for endometrial cancer) technique has been proven to be a reliable method to identify tumours into the four subclassifications of the Cancer Genome Atlas (figure 1).15 Based on the knowledge gained from this classification system, current guidelines from the National Comprehensive Cancer Network recommend universal testing for mismatch repair (MMR) or MSI status and acknowledge potential benefit to further molecular analysis of tumour protein TP53 and POLE status.16
Figure 1.
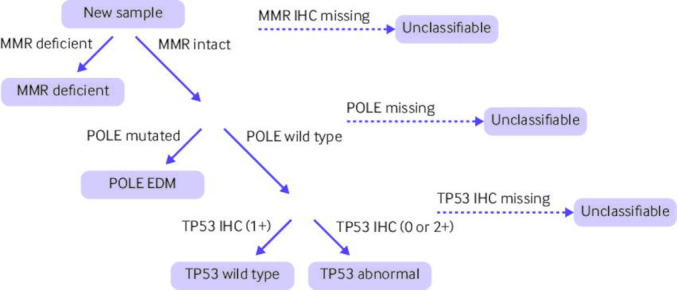
Steps of molecular classification using the ProMisE (proactive molecular risk classifier for endometrial cancer) technique to classify endometrial cancers using immunohistochemistry and Sanger sequencing techniques. MMR=mismatch repair; POLE=polymerase ε; IHC=immunochemistry; EDM=exonuclease domain mutations; TP53=tumour protein p53. Figure adapted with permission15
Sources and selection criteria
We obtained data for ongoing clinical trials by searching ClinicalTrials.gov and using the terms "uterine cancer," "endometrial cancer," or "molecular classification." We excluded single site clinical trials and trials that had a focus on in vitro analysis. We prioritised phase 2 and 3 clinical trials. For the molecular analysis sections, we performed a PubMed and Medline search from 2010 to April 2022 using the molecular analysis terms associated and listed in each subgroup heading. Systematic and biomarker specific reviews were prioritised. We excluded publications not published in English and excluded editorials and other non-interventional evaluations.
Molecular analysis effects on treatment
Polymerase ε
The novel subgroup of endometrial cancers defined in the Cancer Genome Atlas by mutations in POLE and by a considerably high burden of somatic mutations (>100 mutations per megabase) captured clinical interest initially after showing no recurrences within the Cancer Genome Atlas cohort (n=17).12 Subsequent reports from multiple additional cohorts have identified that recurrences do occur in POLE mutated tumours at low frequencies.15 17–21 The POLE enzyme is responsible for high fidelity replication; therefore, mutations within the exonuclease domain region lead to high mutation burden, increased neo-antigens, and improved immune surveillance—likely in part to explain the improved outcomes that have been reported.22 About 5-10% of endometrial cancers have a POLE mutation and these occur more frequently in endometrioid histology and at a younger age. The histopathological findings in POLE mutated tumours tend to have more aggressive features including higher grade, depth of invasion, as well as lymph vascular space invasion. Consequently, many POLE mutated tumours that have been reported have received adjuvant treatment.15 17–21
It remains unclear whether the improved outcomes observed in POLE mutated endometrial cancers are associated with an improved response to treatment or improved immune surveillance that would have prevented recurrence regardless of treatment. The improved outcomes and biological rationale for these observations as well as the available retrospective data, although limited, have led to the treatment concept of de-escalation treatment for POLE mutated endometrial cancer. McAlpine et al reported an individual patient data meta-analysis of patients with endometrial cancer and POLE mutations that included 294 pathogenic POLE mutated tumours. The authors concluded that adjuvant treatment did not appear to benefit endometrial cancer and that recurrences had a high sustained salvage rate. Eleven patients had recurrences in their report, and about 60% of patients received adjuvant treatment.23 In the PORTEC-3 phase 3 randomised trial, researchers recently reported molecular subgroup data on 410 high risk patients receiving adjuvant radiotherapy with and without chemotherapy.24 In the POLE mutated classification the authors reported only one recurrence among the 51 patients with endometrial cancer. Thus, clear data indicate that POLE mutated tumours have improved outcomes; however, the contribution of adjuvant treatment remains unknown. De-escalation with foregoing traditional, pathology based risk factors in favour of molecular subtyping, including POLE, is currently being evaluated in multiple clinical trials including PORTEC-4a (NCT03469674), TAPER (NCT04705649), and the TransPORTEC RAINBO study. These trials will hopefully provide guidance for the safety of de-escalation treatment.
Other important clinical factors should be considered when integrating POLE testing into clinical decision making. Firstly, POLE testing currently can only be accomplished by sequencing strategies (next generation sequencing or targeted Sanger sequencing), and in-house testing strategies have yet to be widely adopted. Secondly, only select mutations have been associated with the ultramutated phenotype. Eleven well defined pathogenic POLE mutations have been described, with P286R and V411L being the most common hot spot mutations (ie, a mutation that occurs considerably more frequently than expected from background frequency). All pathogenic mutations are located within the exonuclease domain region (exons 9, 11, 13, and 14), and mutations that are non-pathogenic have worse outcomes than pathogenic mutations (hazard ratio 3.42; P<0.01).23 25 Finally, POLE mutations might also coincide with other molecular features such as TP53 mutations or defects in mismatch repair genes. In these circumstances with so-called multiple classifiers, a pathogenic POLE mutation status can be considered the driver for molecular classification and thus would be the first step in a pragmatic hierarchal approach.25 26
If recurrence does occur, POLE mutated tumours have high salvage rates.23 Furthermore, their exceptionally high burden of somatic tumour mutations make them excellent candidates for immunotherapy if cytotoxic treatment is unsuccessful. The efficacy of immunotherapy strategies in POLE mutated tumours has been reported, and use of immunotherapy should be considered in people who have relapsed endometrial cancer with POLE mutations.27–29
Mismatch repair deficiency or proficiency and microsatellite instability
The MMR pathway is a multiprotein pathway through which cells recognise and repair DNA damage. The MMR system comprises a series of specific DNA mismatch repair enzymes and are usually dependent on four key genes; mutL homologue 1 (MLH1), post-meiotic segregation increased 2 (PMS2), mutS homologue 2 (MSH2), and mutS 6 (MSH6).30 Mutations in any of the genes can be hereditary (commonly known as Lynch syndrome) or somatic, and loss of protein expression can also be due to epigenetic silencing. Defects in the MMR pathway result in the accumulation of hundreds to thousands of mutations throughout the genome and are therefore oncogenic. MSI refers to the cellular phenotype of hypermutability (ie, MSI-H) that results from MMR deficiency (dMMR). As such, the terms dMMR and MSI-H are often used interchangeably, although the dMMR refers to the genotype and MSI-H refers to the resulting phenotype.31
About 20-30% of endometrial cancers are caused by dMMR or MSI-H.12 32 Of these, about 3-5% are hereditary (Lynch syndrome) while the remainder are somatic (double somatic mutation or epigenetic silencing of the MLH1 gene).31 MMR status has been shown to have both prognostic and therapeutic implications for patients with endometrial cancer.
Endometrial tumours with mismatch deficiency
MMR deficiency can be oncogenic, but also creates neoantigens that can make the tumours susceptible to immunotherapies. Immunotherapy checkpoint inhibition of programed death receptor 1 (PD-1) is the prime example of immunotherapy, where the body’s immune system is used against tumour cells. dMMR/MSI-H is an effective biomarker for PD-1 blockade treatment with strong correlation to effectiveness in endometrial cancer.33 Initial studies across tumour types have shown that dMMR tumours are often susceptible to PD-1 blockade. The first large scale, prospective study on this topic enrolled patients with any MSI-H solid tumour, including endometrial cancer, who had failed previous treatment. Of the 15 patients with endometrial carcinoma enrolled in the study, the objective response rate was 52% and the disease control rate was 73%, including three complete responses and five partial responses.34
Based on this trial, the US Food and Drug Administration approved pembrolizumab as treatment for any unresectable or metastatic dMMR/MSI-H solid tumours (site agnostic) that have progressed after previous treatment or do not have alternative treatments available.16 35 Subsequent work enrolling a larger number of MSI-H endometrial cancers has been similarly favourable. Keynote-158, a prospective, open label, phase 2 trial enrolled 49 patients with MSI-H endometrial cancer who had failed previous treatment. All participants received single agent pembrolizumab. The study found a 57% objective response rate and median progression-free survival rate 26 months in the recurrent setting.36 More recent long term follow-up of this trial reported on 79 patients with an objective response rate of 48% (14% complete response and 24% partial response) with a duration of response of ≥3 years for 68% of patients.37
Dostarlimab is a second PD-1 inhibitor with recent accelerated FDA approval for patients with dMMR/MSI-H recurrent endometrial cancer. In the GARNET trial, researchers evaluated 71 patients and observed a 42.3% objective response rate with 12.7% complete response and 29.6% partial response; 93% of patients had a duration of response of at least six months.38 The use of PD-1 and programmed death ligand 1 (PD-L1) blockade has become standard of care for patients with dMMR/MSI-H recurrent endometrial cancer who have received previous cytotoxic treatment.
Endometrial tumours with mismatch repair proficiency
Although promising for dMMR/MSI-H tumours, single agent PD-1 blockade is substantially less effective for tumours that are MMR proficient (pMMR) or microsatellite stable (MSS). In a phase 1 trial of single agent pembrolizumab in PD-L1 positive endometrial cancer, of which 18 of 19 tumours were MSS, the objective response rate was 13%, and progression-free survival was 1.8 months.39 Similarly, in a phase 2 study of durvalumab (PD-L1 inhibitor), the pMMR cohort (36 patients) had only a 3% objective tumour response rate and a substantially lower progression-free survival than the dMMR cohort (35 patients).40
Despite the poor results of single agent immunotherapy, the landmark evaluation of combination lenvatinib plus pembrolizumab has changed the landscape for treatment in MMR/MSS recurrent endometrial cancer. In this circumstance, the absence of a molecular marker (MSI-H or dMMR) would triage to this combination treatment. Initial evaluation of this combination was a single arm, phase 2 trial of lenvatinib (an oral multikinase inhibitor of vascular endothelial growth factor receptors 1-3, FGFR 1-4, platelet derived growth factor receptor α, RET, and KIT) in combination with pembrolizumab. Enrolment was limited to patients who had received up to two lines of previous treatment but included patients with both MSS and MSI-H tumours. Of the 94 patients with MSS tumours included in analysis, the objective response rate was 37% and median progression-free survival was 7.4 months. The objective response rate was 64% with median progression-free survival 18.9 months among the 11 patients with MSI-H tumours. Toxicity occurred with 67% of patients having grade 3 or 4 treatment related toxicity—most commonly hypertension (31%), fatigue (7%), and diarrhoea (7%).41
The follow-up confirmatory trial was Keynote 775, which evaluated the combination versus physicians' treatment of choice chemotherapy. Of the 827 patients, 697 had pMMR tumours. In the pMMR population, progression-free survival was 6.6 versus 3.8 months (hazard ratio 0.60; 95% confidence interval 0.50 to 0.72; P<0.001) in favour of lenvatinib and pembrolizumab. Similarly, overall survival improved in patients with pMMR tumours receiving the drug combination compared with physician's choice of chemotherapy (17.4 v 12.0 months (0.68; 0.56 to 0.84); P<0.001). The objective response rate was 30.3% versus 15.1%.11 Arguably, this combination of lenvatinib and pembrolizumab has become the standard of care for patients with recurrent pMMR endometrial cancers who have previously received carboplatin and paclitaxel treatment.
Copy number low (or no specific molecular profile)
Of the four defined molecular classes in the Cancer Genome Atlas, the so-called copy number low or endometrioid-like group of endometrial cancers is the most common. In the clinical testing strategies that have been developed to parallel the Cancer Genome Atlas findings, the copy number low group is the default grouping for tumours without a POLE mutation, abnormal TP53, or dMMR/MSI-H (figure 1). This grouping has also been termed as having no specific molecular profile. The prognosis of this molecular class has been intermediate, and this large molecular grouping should have future opportunity for further stratification and therapeutic planning.
The grouping with no specific molecular profile is composed mostly of lower grade tumours with positive oestrogen/progesterone receptors and low tumour mutational burdens when compared with the POLE and dMMR/MSI-H groupings. The copy number low group includes frequent mutations in PTEN, CTNNB1, PIK3CA, ARID1A, and KRAS. Further risk stratification of this molecular group has been proposed and is being incorporated in clinical trial designs, including CTNNB1 and L1CAM. Mutations in CTNNB1 are associated with poorer prognosis within this molecular grouping42–44; in PORTEC-4a, CTNNB1 can be used as a triage for therapeutic decision making between vaginal brachytherapy and observation in endometrial cancer at high intermediate risk.45 Furthermore, CTNNB1 might also provide therapeutic guidance beyond risk as well. In GOG86P, a randomized phase II study of 349 patients, CTNNB1 mutations were evaluated and identified in 26% of patients; the patients with CTNNB1 mutated tumours had the largest benefit of bevacizumab, suggesting opportunities for therapeutic decision making based on molecular features beyond the main molecular classes.46 Similarly, in the evaluation of everolimus and letrozole in a phase 2 trial of 38 patients,47 Slomovitz et al described four patients with CTNNB1 mutations responded to everolimus and letrozole treatment, suggesting that this approach can be important for this more aggressive tumour molecular feature. Additionally, novel therapeutic strategies have been reported, including inhibition of the Wnt/β catenin pathway in tumours with CTNNB1 mutations, and are promising strategies for future therapeutic development.48
The inclusion in the molecular class with no specific molecular profile also suggests other therapeutic strategies that are not as reliant on the above mentioned molecular findings. For instance, tumours in the copy number low group have a high proportion of hormone receptor positivity. This factor, along with the associated histological lower grade tumours associated with the molecular class, make hormonal or endocrine treatment an appealing option. This treatment is well tolerated and modestly effective in low grade tumours in the recurrent or metastatic setting. Hormonal or endocrine therapeutic strategies can also leverage molecular findings within this molecular class (eg, frequent mutations observed in phosphatidylinositol-3-kinase (PI3K)/protein kinase B (Akt)/ mammalian target rapamycin (mTOR) pathway). Slomovitz et al reported their results of a phase 2 trial of 38 patients demonstrating the efficacy of adding the mTOR inhibitor everolimus to endocrine treatment with letrozole with a response rate of 32%.47
Another novel combination with hormone therapy that has made recent advances is the cyclin dependent kinase (CDK) 4/6 inhibitors. The PALEO trial combined palbociclib with letrozole in a randomised, double blind, placebo controlled phase 2 trial of 77 patients, and found the combination improved progression-free survival to 8.3 months versus 3.0 months when compared with letrozole with placebo (hazard ratio 0.56; 95% confidence interval 0.32 to 0.98; P=0.041).49 Similar results were found with the combination of abemaciclib and letrozole showing a 30% partial response and 75% clinical benefit rate in a phase 2 study of 30 patients.50 With the low response rates observed in high grade and non-endometrioid tumours, our understanding of molecular markers could continue to complement development of hormonal or endocrine treatment strategies. The incorporation of hormonal or endocrine treatment within this group is being examined in the TransPORTEC RAINBO trial, in which patients will be randomised to adjuvant chemoradiation versus radiation with hormonal or endocrine treatment for the group with no specific molecular profile.
The group with no specific molecular profile is the most heterogenous; therefore, improved understanding of additional molecular and pathological characteristics to guide treatment will continue to be an area of interest. While this group will include a large proportion of low grade, early stage, endometrial cancers likely to be cured from surgery alone, a percentage of women who have tumours with no specific molecular profile and with higher risk histological features will require more intensive treatments.
Copy number high
The molecular classification of copy number high or serous-like endometrial cancers from the Cancer Genome Atlas was determined by extensive molecular characterisation, including evaluation of >1.3 million single nucleotide polymorphisms to determine copy number status. People with tumours in the molecular class of copy number high had the poorest progression-free survival of the four Cancer Genome Atlas groups, and there has been interest in clinically accessible identification of this molecular group to allow for improved treatment strategies. In the Cancer Genome Atlas, the majority (46/60) of these tumours were histologically serous or had mixed histology as expected by TP53 mutations. However, 14 endometrioid histology cases were identified in this subgroup and probably identify a previously unrecognised population for which clinically meaningful interventions can be implemented.12 24 Understanding that the methods of the Cancer Genome Atlas were not clinically feasible, researchers have evaluated surrogate markers for the molecular class. An observation of the copy number high group was that TP53 mutations were frequent and with this in mind, several groups have evaluated TP53 status either through sequencing or with immunohistochemistry.15 21 In a recent analysis of the GOG-86P cohort, including 213 patients from the phase 2 trial, the concordance of TP53 by next generation sequencing and immunohistochemistry was 88%. This concordance was improved to 92% when multiple classifiers of POLE and dMMR were removed.51
A possible clinical challenge with increasing assessment of TP53 status is the discordant findings of low grade histology with TP53 abnormalities or high grade histology with normal TP53 status. The relative rarity of low grade histology tumours that also harbour TP53 abnormalities suggests that we are unlikely to be able to definitively resolve this discrepancy; however, TP53 normal tumours continue to be reported in high risk endometrial cancers. In a recent cohort study of 367 patients, Leon-Castillo and colleagues reported the prognostic relevance of molecular classification in high grade endometrial cancer for patients staged by lymphadenectomy and without adjuvant treatment.52 In the 367 patients with high risk cases, 44% were classified as having p53 abnormal tumours, and 16.6% were classified as having tumours with no specific molecular profile, which were TP53 wild type. The outcomes between these two groups were the same, with a five year recurrence rate of 41.5% for the TP53 abnormal group and 37.9% for the group with no specific molecular profile. These data suggest that molecular testing will likely continue to be complementary to traditional clinical-pathological features in prognostic and treatment counselling and that TP53 status cannot be evaluated independent of other features.
The mounting evidence suggests that TP53 alterations, regardless of method of detection, elicit a poorer prognosis.12 15 21 53 54 Escalation of treatment or targeted strategies for those tumours with a TP53 alterations could provide an opportunity to improve outcomes in this high risk group. Data from the PORTEC-3 phase 3 trial once again provided insight into treatment effect by molecular class. In PORTEC-3, TP53 status as determined by immunohistochemistry had the poorest outcomes among endometrial cancers already deemed as high risk.24 Furthermore, of the four molecular classes, those in the TP53 abnormal group were the only group that derived a benefit from the addition of chemotherapy to radiation. These findings suggest that increased intensity of treatment (or at least cytotoxic treatment) could have a clinically meaningful benefit even in early stage disease. In PORTEC-3, 58 of the 93 individuals with TP53 abnormal tumours had early stage disease, and in an exploratory subanalysis, the benefit of adding chemotherapy to radiation remained significant (P<0.001) for recurrence-free survival in these patients with early stage tumours.
While intensity of treatment or addition of cytotoxic chemotherapy (as was done in PORTEC-324) provides an opportunity to potentially improve outcomes, more targeted approaches to treatment could provide additional improvements in survival. TP53 as a biomarker for treatment was evaluated in the GOG86P study.55 This exploratory study of the phase 2 GOG86P study evaluation of 349 patients by Leslie et al reported that chemotherapy with bevacizumab could enhance progression-free survival and overall survival for patients with mutated TP53 tumours. This hypothesis generating evaluation provides evidence that molecular findings such as TP53 might not only provide insight in prognosis but also be used for therapeutic decision making.
The success of poly-ADP ribose polymerase (PARP) inhibitors in ovarian cancer has led to the examination of a possible role of this class of treatment in endometrial cancer. While several ongoing clinical trials are being undertaken, TP53 status has been suggested as a biomarker for PARP inhibitor use. There are currently limited data to suggest the prevalence of homologous recombination deficiency (HRD) in endometrial cancer. While some studies have reported rates of HRD in the 46-53% range, only 15% of the Cancer Genome Atlas cohort was determined to have an HRD-like phenotype.56–58 Furthermore, the definition of HRD in endometrial cancer has yet to be defined, and it is likely to be different than the scores more commonly used in ovarian cancer.
Furthermore, TP53 mutated tumours might signal further molecular findings of clinical importance. For instance, human epidermal growth factor receptor 2 (HER2)/neu amplification is highly associated with the TP53 abnormal subgroup regardless of histology; thus, those patients with high risk tumours that have TP53 abnormalities and HER2/neu amplification could benefit from the addition of trastuzumab even in non-serous histology.59 Given the US National Comprehensive Cancer Network's recommendations for trastuzumab in serous carcinomas based on the work by Fader et al, continued investigation of biomarker driven therapeutics will likely extend beyond the histological diagnosis.16 60
Human epidermal growth factor receptor 2/neu
Human epidermal growth factor receptor 2 (HER2/neu) is a receptor tyrosine protein kinase (erbB-2) that is encoded by the ERBB2 gene and is overexpressed in roughly 20-60% of uterine serous carcinomas, 30-60% of uterine clear cell carcinomas, and offers a therapeutic target.61 Trastuzumab is a humanised monoclonal antibody that targets HER2/neu, with demonstrated efficacy in other tumours with HER2/neu overexpression including breast and gastric cancers.61
Initial evaluation in a phase 2 trial of 286 patients of single agent trastuzumab in advanced and recurrent endometrial cancer overexpressing HER2/neu showed no progression-free or overall survival benefit.62 However, trastuzumab in combination with standard-of-care carboplatin and paclitaxel in uterine serous carcinomas with HER2/neu overexpression has demonstrated efficacy. A randomised, phase 2 trial published in 2020 evaluated six cycles of carboplatin and paclitaxel with or without trastuzumab maintenance, in patients with newly diagnosed stage III or IV or recurrent uterine serous carcinomas overexpressing HER2/neu. Of the 58 patients in the evaluation, progression-free survival was 8.0 months in the control arm versus 12.9 months for those also receiving trastuzumab. Of the 48 patients treated in the primary setting, progression-free survival was 9.3 months versus 17.7 months in favour of the arm with trastuzumab. Overall survival also improved to 29.6 months from 24.2 months (hazard ratio 0.58; 90% confidence interval 0.34 to 0.99; P=0.046). Toxicity was similar with and without trastuzumab.63
As described above, studies so far have demonstrated efficacy in targeting HER2/neu as a biomarker in advanced and recurrent disease. Erickson et al have described the potential importance of this biomarker in early stage disease prognosis. They showed that HER2/neu positivity is associated with worse progression-free survival and overall survival in stage I uterine serous carcinomas, in a multicentre cohort study of 169 patients.64 A confirmatory phase 3 trial evaluating chemotherapy plus HER2 targeting agents for patients with early stage disease as well as non-serous histologies with HER2/neu overexpression and amplification is in development as GY026 (NCT05256225).
Future of molecular analysis
The Cancer Genome Atlas subclassifications have clear clinical implications. However, as knowledge of molecular classification expands, additional markers with potential therapeutic implications are elucidated. These approaches could include PIK3/AKT/mTOR pathway alterations; homologous recombination deficiency (HRD) genes; and L1CAM, ARID1A, and CCNE1 (cyclin E1) amplifications.
The PI3K/AKT/mTOR pathway is the most commonly altered pathway in oestrogen derived endometrial cancer.65 The pathway is oncogenic via promotion of cell growth and proliferation, reduction in apoptosis, and increase in angiogenesis.66 This pathway is regulated by PTEN, a tumour suppressor gene that is mutated in about 30-60% of early stage endometrial carcinomas.67 Tumours with dysregulation of this pathway might be candidates for selective mTOR inhibition such as everolimus and temsirolimus. Phase 2 studies have investigated the role of everolimus in patients with recurrent endometrial cancer refractory to previous cytotoxic agents. Single agent everolimus showed clinical benefit response, defined as confirmed complete or partial response or prolonged stable disease, in 22% of patients at 20 weeks of treatment.68 Newer agents targeting this pathway are also in evaluation and include novel agents such as capivasertib, an oral AKT inhibitor.
CCNE1 amplification is prevalent in many gynaecological malignancies, including 40% of uterine carcinosarcomas and 7.5% of other uterine histological subtypes.69 70 CCNE1 is oncogenic via its effect on cyclin E1, through which it dysregulates the cell cycle. Wee1 kinase inactivates cyclin E1 to control DNA replication, and therefore inhibition of Wee1 is a therapeutic target for CCNE1 mutant tumours.71 A recent phase 2 study of 34 patients examined the use of an oral Wee1 inhibitor, adavosertib, in patients with recurrent uterine serous cancer. The objective response rate was 30% with a tolerable side effect profile.72 Further evaluation of this agent, and other Wee1 inhibitors are ongoing.
The AT rich interacting domain containing protein 1A (ARID1A) is oncogenic via its effects on chromatin remodelling in both ovarian and endometrial cancers. It is thought be an early mutation in both endometriosis related ovarian cancers, and in the progression from endometrial hyperplasia to invasive cancer. For this reason it has theoretical appeal for early diagnosis, but not yet demonstrated prospectively.73 Loss of ARID1A has been associated with activation of the PI3K/AKT pathway, and ARID1A has been identified in up to 47% of low grade endometrioid endometrial carcinomas, 60% of high grade endometrioid adenocarcinomas, and 11% of serous adenocarcinomas.73 Clinically, evaluation of tazemetostat, a small molecule inhibitor of EZH2 (enhancer of zeste 2 polycomb repressive complex 2 subunit), is under evaluation for its activity in ARID1A mutated ovarian and endometrial cancers in the GY014 study (NCT03348631). ARID1A has been previously demonstrated as a viable biomarker for EZH2 inhibition, having appeared to be enhanced in ARID1A mutated tumours.74–76
Other molecular markers have known prognostic implications that could eventually lead to targeted treatments. For example, L1 cell adhesion molecule (L1CAM) overexpression identifies patients at increased risk of recurrence, particularly in patients with low and intermediate risk endometrial cancer.77 L1CAM blockade has shown modest benefit in mouse models, although its usefulness in human endometrial cancers has yet to be established.78 79
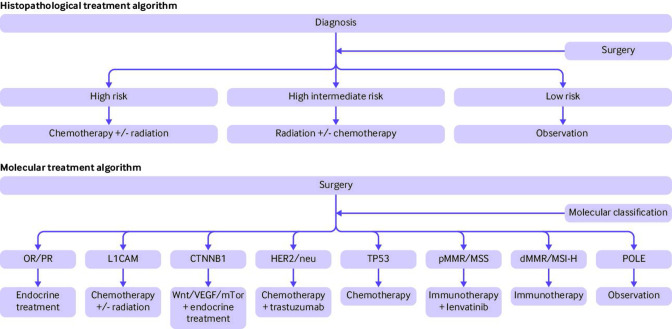
Combining the ProMisE molecular algorithm with additional molecular markers provides the potential for a new range of treatment options. Figure 2 demonstrates the variation from the historical histopathological treatment algorithm to a molecularly driven treatment algorithm. This model is created from the conglomeration of existing data and potential outcomes from future trials, but is not a validated treatment algorithm. It is portrayed to present the movement into a molecularly driven treatment model. Future clinical trials will help elucidate if molecular based treatments should replace histopathological based treatments, or if the two models should be combined.
Figure 2.
Examples of potential treating algorithms for primary endometrial cancer. The two treatment methods highlight the variation in treatment strategies. The histopathological algorithm relies solely on cancer stage and histopathological features, and the molecular algorithm highlights the multitude of tumour focused treatment strategies. Future studies will help determine whether one approach is superior to the other, or whether a combined analysis is more prudent. OR/PR=oestrogen/progesterone receptors: L1CAM=L1 cell adhesion molecule; Wnt=wingless related integration site; VEGF=vascular endothelial growth factor; mTor=mammalian target of rapamycin; HER2=human epidermal growth factor receptor 2; TP53=tumour protein 53; pMMR=mismatch repair proficient tumours; MSS=microsatellite stable; dMMR=mismatch repair deficient tumours; MSI-H=microsatellite instability, hypermutated; POLE=DNA polymerase ε
Future treatments
Treatment for primary adjuvant and recurrent endometrial cancer is in an era of rapid advancements. Additional practice changing regimens have already been conducted, with treatments such as checkpoint inhibition using single agent immunotherapy, and combination strategies. By far, immunotherapy has resulted in the greatest shift in treatment over the past decade. In addition to responding to a highly effective drug class, MMR genes also have a specific biomarker. Current clinical trials require inclusive molecular analysis as we shift to care tailored to tumours and patients.
Future treatments, based on open clinical trials, are largely reliant on molecular analysis (table 1). Immunotherapy has a large role in these investigations in trying to optimise the timing of this treatment, as well as determining the role of using the treatment over again. Furthermore, there are currently no approved maintenance treatments in endometrial cancer. As newer agents are developed, maintenance treatment is seeing early clinical successes. Finally, as newer drugs are in development, we are building on the range of available treatment options for recurrent or advanced disease.
Table 1.
Ongoing clinical trials in endometrial cancer
| Trial identifier | Disease characteristics in patient population | Treatment arms | Trial No |
| Primary adjuvant treatment | |||
| GY020 | Stage I/II dMMR endometrioid tumours | Local radiation Local radiation and pembrolizumab |
NCT04214067 |
| RUBY | Advanced or recurrent disease | Carboplatin and paclitaxel, and dostarlimab → dostarlimab every 6 weeks up to 3 years Carboplatin and paclitaxel, and placebo → placebo |
NCT03981796 |
| GY018 | Advanced or recurrent disease | Carboplatin and paclitaxel, and pembrolizumab → pembrolizumab every 3 weeks up to 5 years Carboplatin and paclitaxel, and placebo → placebo |
NCT03914612 |
| AtTEnd | Advanced or recurrent disease | Carboplatin and paclitaxel, and atezolizumab → atezolizumab every 3 weeks to disease progression Carboplatin and paclitaxel, and placebo → placebo |
NCT03603184 |
| GOG3053/KEYNOTE-B21/ENGOT-en11 | Non-endometrioid stage I/II with myometrial invasion TP53 aberrant tumours Stage III/IV |
Carboplatin and paclitaxel, and pembrolizumab → pembrolizumab, six cycles Carboplatin and paclitaxel, and placebo → placebo |
NCT04634877 |
| LEAP001 | Advanced or recurrent disease | Carboplatin and paclitaxel Lenvatinib and pembrolizumab |
NCT03884101 |
| GOG3064 | Advanced or recurrent disease with dMMR tumours | Carboplatin and paclitaxel Pembrolizumab |
NCT05173987 |
| Maintenance treatment | |||
| DUO-E | Advanced or recurrent disease | Carboplatin and paclitaxel, and placebo → placebo Carboplatin and paclitaxel, and durvalumab → durvalumab every 4 weeks and placebo Carboplatin and paclitaxel, and durvalumab → durvaulmab every 4 weeks and olaparib |
NCT04269200 |
| PARPI maintenance | Advanced or recurrent disease | Rucaparib until disease progression Placebo |
NCT03617679 |
| SIENDO | Stage IV or first relapse | Selinexor until disease progression Placebo |
NCT03555422 |
| Recurrent disease | |||
| GY012 | Recurrent | Cediranib Olaparib Olaparib and cediranib Olaparib and capivasertib Olaparib and durvalumab Cediranib and durvalumab |
NCT03660826 |
| GOG3038/PODIUM/ENGOT-en12 | Advanced or recurrent disease | No previous treatment involving checkpoint inhibitors MSI-H: retifanliimab; dMMR or POLE: retifanlimab Previous treatment involving checkpoint inhibitors Unselected: retifanlimab and epacadostat Fibroblast growth factor receptor 1/2/3 mutation: retifanlimab and pemgatinib |
NCT04463771 |
| ADAGIO | Recurrent or persistent uterine serous carcinomas | Adavosertib | NCT04590248 |
| GOG3039 | Advanced or recurrent | Abemaciclib and letrazole | NCT04393285 |
| Solely molecularly driven treatments | |||
| PORTEC-4a | Stage I/II, high intermediate risk | Favourable disease: observation Intermediate: vaginal brachytherapy Unfavourable: external beam radiation |
NCT03469674 |
| TAPER | Stage I/II, high intermediate risk, POLE and p53 wild type | No treatment | NCT04705649 |
| RAINBO | Stage I-IV | TP53 Chemotherapy Chemotherapy and olaparib dMMR Radiation Radiation and durvalumab No specific molecular profile Chemoradiation Radiation and megestrol acetate POLE mutation Observation |
NCT05255653 |
Table does not include all ongoing trials, because more than 900 clinical trials for endometrial cancer were listed on ClinicalTrials.gov at time of publication. Arrows indicate the second stage of the trial. dMMR=mismatch repair deficient tumours; MSI-H=microsatellite instability, hypermutated; POLE=DNA polymerase ε; TP53=tumour protein p53; PARPI=poly-ADP ribose polymerase inhibitors.
Primary adjuvant treatment
Checkpoint inhibition has already shown efficacy in recurrent endometrial cancer and is now being evaluated as primary adjuvant treatment. The GY020 trial of 168 planned patients (NCT04214067) evaluates the addition of one year of pembrolizumab to standard-of-care radiotherapy in high intermediate risk dMMR tumours. This trial is randomised 2:1 for the pembrolizumab arm versus radiation alone with a primary endpoint of three year recurrence-free survival. The trial looks at the distant recurrence risk of patients at high intermediate risk in addition to the potential synergistic and abscopal effects of pembrolizumab and radiotherapy.80
The use of checkpoint inhibitors in primary treatment for advanced disease is also currently being thoroughly evaluated. Three major clinical trials are evaluating this approach: RUBY (NCT03981796), GY018 (NCT03914612), and AtTend (NCT 03603184). Each trial uses the addition of dostarlimab (PD-L1 inhibitor), pembrolizumab (PD-1 inhibitor), or atezolizumab (PD-L1 inhibitor), respectively, to carboplatin and paclitaxel treatment. The trials vary in the duration of treatment. Each trial is placebo controlled and dostarlimab is extended three years in RUBY after combination with carboplatin and paclitaxel versus 14 cycles of Q6 week dosing pembrolizumab in GY018, and atezolizumab is continued until progression of disease in AtTend. Stratification of these trials vary slightly as well, however, all are based around MMR/MSI-H status and include MSS tumours. This combination strategy is also being expanded to all high risk patients who would typically receive chemotherapy in the GOG3053/KEYNOTE-B21/ENGOT-en11 trial (NCT04634877). This trial models the combination of pembrolizumab with carboplatin and paclitaxel, extending pembrolizumab treatment for six cycles beyond chemotherapy, but includes all high risk patients, including those with carcinosarcoma or early stage non-endometrioid cancer, or any TP53 aberrant tumour.
Two ongoing clinical trials are evaluating the use of immunotherapy in adjuvant treatment compared with standard chemotherapy. The LEAP-001 (NCT03884101) and GOG3064 (NCT05173987) trials aim to eliminate the use of chemotherapy in primary adjuvant therapy. LEAP-001 is a 1:1 randomisation of carboplatin and paclitaxel to a combination of lenvatinib and pembrolizumab in stage III/IV or recurrent endometrial carcinomas. The eligibility criteria for this trial is selective for patients with worse disease after surgical resection, because it requires measurable or radiographically apparent disease. Furthermore, this trial will also evaluate the tolerability comparison of these regimens by patient reported outcomes. Tolerability will probably be a key component of this trial because the toxicity profiles of these regimens is quite different. The duration of treatment also differs—lenvatinib/pembrolizumab is used for two years while carboplatin/paclitaxel is used for the standard six cycles (18 weeks). GOG3064 has a similar evaluation, but eligibility limits this analysis to only patients with dMMR tumours. This trial evaluates single agent pembrolizumab versus carboplatin and paclitaxel with similar durations of treatment. This trial also has a built-in crossover for patients who progress on chemotherapy to receive pembrolizumab on trial. If the results of these trials are positive, chemotherapy could become a second line or later line treatment option for endometrial cancer.
Maintenance treatment
Mutations in homologous recombination genes, including BRCA1 and BRCA2, identify ovarian cancer patients ho are likely to benefit from poly-ADP ribose polymerase inhibitors (PARPIs). Somatic BRCA gene mutations are known to be present in roughly 15% of patients with endometrial cancer, although the therapeutic implications of those mutations are yet to be demonstrated.81 Preclinical data indicate that endometrial cancers deficient in PTEN are more sensitive to PARPIs than those proficient in PTEN.82 The trials DUO-E (NCT04269200) and RUBY part 2 (NCT03981796) build off of the expected positive results of adding immunotherapy to primary chemotherapy with the addition of PARPI maintenance. DUO-E is a three arm study of patients with advanced or recurrent endometrial cancer who are naive to chemotherapy. The first arm is carboplatin and paclitaxel with placebo maintenance. The second arm adds durvalumab (PD-1 inhibitor) to the chemotherapy and extends durvalumab treatment until disease progression. The third arm adds olaparib to durvalumab maintenance, evaluating the combination that is placebo controlled in arm 2. Similarly, the RUBY part 2 trial has added the placebo controlled evaluation of maintenance with niraparib and dostarlimab (PD-1 inhibitor). Single agent PARPI maintenance is also being evaluated in several trials, including the placebo controlled trial with rucaparib in NCT03617679. In each of these trials, BRCA/HRD status is being evaluated, but this factor will be a similarly effective biomarker as seen in ovarian cancer. The rational for treatment of a larger potential population is more reliant on a PTEN mutation rather than BRCA/HRD status.
Selinexor is an oral nuclear export protein specific to inhibiting exportin 1 (XPO1). This inhibitor effectively blocks the transport of key cancer cell growth proteins from the nucleus to the cytoplasm, which can lead to cell cycle arrest and apoptosis. The SIENDO clinical trial is a 2:1 placebo controlled trial of 263 patients with oral Selinexor as maintenance treatment. Recently, this trial announced that it has met its primary endpoint of a significant improvement in progression-free survival of 5.7 months compared with 3.7 months for placebo (hazard ratio 0.70, P=0.0486). Subgroup analysis of the patients with TP53 wildtype showed an improvement in progression-free survival of 13.7 months with Selinexor treatment compared with 3.7 months for placebo (hazard ratio 0.375; 95% confidence interval 0.21 to 0.67; P<0.001.83 As the data from this trial matures, this approach could become the first approved maintenance treatment in endometrial cancer.
Recurrent disease
The evaluation of novel therapeutics dominates the evaluation of recurrent disease. GY012 (NCT03660826) is a platform trial, with target accrual of 168 patients, with six arms evaluating a multitude of treatments. The advantage of this trial is a true side-by-side comparison of these novel agents, rather than an attempt for cross trial analysis. The first three arms of this trial have completed enrolment and evaluate cediranib monotherapy as the reference arm, olaparib monotherapy and combination olaparib plus cediranib. Cediranib is an orally available tyrosine kinase inhibitor of vascular endothelial growth factor and therefore has anti-angiogenic properties. The combination with PARP inhibition is believed to be effective based on the increased HRD properties induced by cediranib.84 Recent evaluation of this combination in platinum sensitive ovarian cancer indicated that the combination shows clinical activity but failed to improve progression-free survival compared with chemotherapy and had reduced patient reported outcomes.85 The three additional arms to this trial are the combinations of olaparib and capivasertib (an oral pan-AKT inhibitor targeting the PI3K/AKT/mTOR pathway), olaparib and durvalumab, and finally cediranib with durvalumab.
GOG 3038/PODIUM/ENGOT-en12 (NCT04463771) is an umbrella study using the biomarkers of MMR as well as FGFR gene mutations to evaluate retifanlimab (PD-1 inhibitor), retifanlimab combined with epacadostat (indoleamine-2,3-dioxygenase (IDO) 1 inhibitor), and retifanlimab combined with pemigatinib (an inhibitor to FGFR 1-3). Epacadostat inhibits IDO1 by competitively blocking it, without interfering with IDO2 or tryptophan 2,3-dioxygenase (TDO).86 Epacadostat has anti-tumour activity in some models, although is most effective when combined with other immunotherapy agents.86 87 Pemigatinib inhibited FGFR 1-3 phosphorylation and signalling and decreased cell viability in cancer cell lines with activating FGFR amplifications and fusions that resulted in constitutive activation of FGFR signalling.88
Another promising molecular target is Wee1 inhibition. Liu et al reported on the promising results of a phase 2 trial using single agent adavosertib in recurrent uterine serous carcinomas.72 In 34 patients, researchers found an objective response rate of 29.4% (95% confidence interval 15.1% to 47.5%) with a median progression-free survival of 6.1 months and median duration of response of 9 months. Adavosertib (AZD1775) is a highly potent inhibitor of Wee1 kinase, which is a key regulator of the G2/M and S phase checkpoints. Additionally, Wee1 inhibition can increase replication stress by inducing aberrant firing of replication origins and depletion of the nucleotide pool. Preclinical studies suggest that Wee1 inhibition results in anti-cancer activity, both as monotherapy in certain biomarker selected populations (eg, CCNE1 or MYC amplification), or in combination with chemotherapy or radiation.72 89 90 This indication has led to the ADAGIO trial (NCT04590248), of approximately 120 expected patients, which evaluates single agent adavosertib in recurrent or persistent uterine serous carcinomas with at least one previous chemotherapy regimen.
GOG3039 (NCT04393285), with an accrual goal of 50 patients, evaluates a hormonal treatment combination of letrozole (aromatase inhibitor) with abemaciclib. Abemaciclib is an inhibitor of CDK4 and CDK6. These kinases are activated on binding to D cyclins, and induce a cell cycle arrest via the G1 to S cell cycle checkpoint. The cyclin D/CDK complex is downstream of oestrogen signalling, which is a potential synergistic activity when combined with aromatase inhibitors.
Guidelines
Three major international guidelines committees (National Comprehensive Cancer Network, European Society for Medical Oncology, European Society of Gynaecological Oncology/European Society Radiation Oncology/European Society of Pathology)16 91 92 each give recommendations on molecular classification of endometrial carcinoma. Consensus exists for classification of endometrial cancer into the four Cancer Genome Atlas subclassifications of POLE, dMMR, copy number high, and copy number low (no specific molecular profile) based on next generation sequencing and immunohistochemistry analysis. There is also uniformity in that these are currently diagnostic only. Posible treatments avaialable based on these biomarkers and available data are outlined in this manuscript. Full analysis might not be feasible at all locations for patient care and tumour analysis but should continue to be recommended for possible therapeutic benefit.
Conclusion
The future treatments for endometrial cancer are very promising. The development of molecular analysis combined with novel agents and new drug classes have revolutionised precision medicine for patients with endometrial cancer. Uterine risk factors have been used for decades to stratify risk and recommended treatments. Whether molecular analysis will replace or supplement this risk stratification model is unknown. However, our ability to treat patients with more options is clearly improving based on this knowledge.
Questions for future research.
Should molecular analysis be performed on all patients wtih endometrial cancer in the upfront or recurrent setting or both?
Should all future trials on endometrial cancer incorporate biomarker driven molecular evaluation?
Is molecular classification of endometrial cancer into the four known subclassifications an adequate evaluation?
Patient involvement.
Patients and the public were not involved in the design, conduct, reporting, or dissemination plans of this research.
Footnotes
Contributors: All authors contributed to the intellectual content, literature search, preparation, editing, and critical review of the manuscript. BC prepared the manuscript and performed final editing. CG, DS, and SG contributed substantially to the writing of the manuscript and review. BC is the guarantor of this article.
Funding: This work was supported in part by the University of Colorado Cancer Center’s Shared Resource, funded by National Cancer Institute grant P30CA046934.
Competing interests: We have read and understood the BMJ policy on declaration of interests and declare the following interests: BC has held positions on advisory boards for Immunogen, Merck, AstraZeneca, Novocure, and GSK, and declares research funding from Clovis; DS has a family member who is employed by Invitae with equity holding.
Provenance and peer review: Commissioned; externally peer reviewed.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7–34. 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 2. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–49. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 3. Giaquinto AN, Broaddus RR, Jemal A, et al. The changing landscape of gynecologic cancer mortality in the United States. Obstet Gynecol 2022;139:440–2. 10.1097/AOG.0000000000004676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Creasman WT, Morrow CP, Bundy BN, et al. Surgical pathologic spread patterns of endometrial cancer. A gynecologic Oncology Group study. Cancer 1987;60:2035–41. [DOI] [PubMed] [Google Scholar]
- 5. Guntupalli SR, Zighelboim I, Kizer NT, et al. Lymphovascular space invasion is an independent risk factor for nodal disease and poor outcomes in endometrioid endometrial cancer. Gynecol Oncol 2012;124:31–5. 10.1016/j.ygyno.2011.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Keys HM, Roberts JA, Brunetto VL, et al. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a gynecologic Oncology Group study. Gynecol Oncol 2004;92:744–51. 10.1016/j.ygyno.2003.11.048 [DOI] [PubMed] [Google Scholar]
- 7. Kizer NT, Gao F, Guntupalli S, et al. Lower uterine segment involvement is associated with poor outcomes in early-stage endometrioid endometrial carcinoma. Ann Surg Oncol 2011;18:1419–24. 10.1245/s10434-010-1454-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de Boer SM, Powell ME, Mileshkin L, et al. Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): final results of an international, open-label, multicentre, randomised, phase 3 trial. Lancet Oncol 2018;19:295–309. 10.1016/S1470-2045(18)30079-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Matei D, Filiaci V, Randall ME, et al. Adjuvant chemotherapy plus radiation for locally advanced endometrial cancer. N Engl J Med 2019;380:2317–26. 10.1056/NEJMoa1813181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Miller DS, Filiaci VL, Mannel RS, et al. Carboplatin and paclitaxel for advanced endometrial cancer: final overall survival and adverse event analysis of a phase III trial (NRG Oncology/GOG0209). J Clin Oncol 2020;38:3841–50. 10.1200/JCO.20.01076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Makker V, Colombo N, Casado Herráez A, et al. Lenvatinib plus pembrolizumab for advanced endometrial cancer. N Engl J Med 2022;386:437–48. 10.1056/NEJMoa2108330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cancer Genome Atlas Research Network, Kandoth C, Schultz N, et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013;497:67–73. 10.1038/nature12113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stelloo E, Bosse T, Nout RA, et al. Refining prognosis and identifying targetable pathways for high-risk endometrial cancer; a TransPORTEC initiative. Mod Pathol 2015;28:836–44. 10.1038/modpathol.2015.43 [DOI] [PubMed] [Google Scholar]
- 14. Talhouk A, McAlpine JN. New classification of endometrial cancers: the development and potential applications of genomic-based classification in research and clinical care. Gynecol Oncol Res Pract 2016;3:14. 10.1186/s40661-016-0035-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Talhouk A, McConechy MK, Leung S, et al. Confirmation of ProMisE: a simple, genomics-based clinical classifier for endometrial cancer. Cancer 2017;123:802–13. 10.1002/cncr.30496 [DOI] [PubMed] [Google Scholar]
- 16. Abu-Rustum NR, Yashar CM, Bradley K, et al. NCCN Guidelines® insights: uterine neoplasms, version 3.2021. J Natl Compr Canc Netw 2021;19:888–95. 10.6004/jnccn.2021.0038 [DOI] [PubMed] [Google Scholar]
- 17. Church DN, Stelloo E, Nout RA, et al. Prognostic significance of pole proofreading mutations in endometrial cancer. J Natl Cancer Inst 2015;107:402. 10.1093/jnci/dju402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Billingsley CC, Cohn DE, Mutch DG, et al. Polymerase ɛ (pole) mutations in endometrial cancer: clinical outcomes and implications for Lynch syndrome testing. Cancer 2015;121:386–94. 10.1002/cncr.29046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cosgrove CM, Tritchler DL, Cohn DE, et al. An NRG Oncology/GOG study of molecular classification for risk prediction in endometrioid endometrial cancer. Gynecol Oncol 2018;148:174–80. 10.1016/j.ygyno.2017.10.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McConechy MK, Talhouk A, Leung S, et al. Endometrial carcinomas with pole exonuclease domain mutations have a favorable prognosis. Clin Cancer Res 2016;22:2865–73. 10.1158/1078-0432.CCR-15-2233 [DOI] [PubMed] [Google Scholar]
- 21. Stelloo E, Nout RA, Osse EM, et al. Improved risk assessment by integrating molecular and clinicopathological factors in early-stage endometrial Cancer-Combined analysis of the PORTEC cohorts. Clin Cancer Res 2016;22:4215–24. 10.1158/1078-0432.CCR-15-2878 [DOI] [PubMed] [Google Scholar]
- 22. van Gool IC, Eggink FA, Freeman-Mills L, et al. Pole proofreading mutations elicit an antitumor immune response in endometrial cancer. Clin Cancer Res 2015;21:3347–55. 10.1158/1078-0432.CCR-15-0057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McAlpine JN, Chiu DS, Nout RA, et al. Evaluation of treatment effects in patients with endometrial cancer and pole mutations: an individual patient data meta-analysis. Cancer 2021;127:2409–22. 10.1002/cncr.33516 [DOI] [PubMed] [Google Scholar]
- 24. León-Castillo A, de Boer SM, Powell ME, et al. Molecular classification of the PORTEC-3 trial for high-risk endometrial cancer: impact on prognosis and benefit from adjuvant therapy. J Clin Oncol 2020;38:3388–97. 10.1200/JCO.20.00549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. León-Castillo A, Britton H, McConechy MK, et al. Interpretation of somatic pole mutations in endometrial carcinoma. J Pathol 2020;250:323–35. 10.1002/path.5372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. León-Castillo A, Gilvazquez E, Nout R, et al. Clinicopathological and molecular characterisation of 'multiple-classifier' endometrial carcinomas. J Pathol 2020;250:312–22. 10.1002/path.5373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dong D, Lei H, Liu D, et al. Pole and mismatch repair status, checkpoint proteins and tumor-infiltrating lymphocytes in combination, and tumor differentiation: identify endometrial cancers for immunotherapy. Front Oncol 2021;11:640018. 10.3389/fonc.2021.640018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mehnert JM, Panda A, Zhong H, et al. Immune activation and response to pembrolizumab in POLE-mutant endometrial cancer. J Clin Invest 2016;126:2334–40. 10.1172/JCI84940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang F, Zhao Q, Wang Y-N, et al. Evaluation of pole and POLD1 mutations as biomarkers for immunotherapy outcomes across multiple cancer types. JAMA Oncol 2019;5:1504–6. 10.1001/jamaoncol.2019.2963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhao P, Li L, Jiang X, et al. Mismatch repair deficiency/microsatellite instability-high as a predictor for anti-PD-1/PD-L1 immunotherapy efficacy. J Hematol Oncol 2019;12:54. 10.1186/s13045-019-0738-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ryan NAJ, Glaire MA, Blake D, et al. The proportion of endometrial cancers associated with Lynch syndrome: a systematic review of the literature and meta-analysis. Genet Med 2019;21:2167–80. 10.1038/s41436-019-0536-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cortes-Ciriano I, Lee S, Park W-Y, et al. A molecular portrait of microsatellite instability across multiple cancers. Nat Commun 2017;8:15180. 10.1038/ncomms15180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yamashita H, Nakayama K, Ishikawa M, et al. Microsatellite instability is a biomarker for immune checkpoint inhibitors in endometrial cancer. Oncotarget 2018;9:5652–64. 10.18632/oncotarget.23790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Le DT, Uram JN, Wang H, et al. Pd-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015;372:2509–20. 10.1056/NEJMoa1500596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Marabelle A, Le DT, Ascierto PA, et al. Efficacy of pembrolizumab in patients with Noncolorectal high microsatellite Instability/Mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol 2020;38:1. 10.1200/JCO.19.02105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357:409–13. 10.1126/science.aan6733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. O'Malley D, Bariani GM, Cassier PA, et al. 795MO pembrolizumab (pembro) in patients (PTS) with microsatellite instability-high (MSI-H) advanced endometrial cancer (EC): updated results from KEYNOTE-158. Annals of Oncology 2021;32:S730–1. 10.1016/j.annonc.2021.08.1237 [DOI] [Google Scholar]
- 38. Oaknin A, Tinker AV, Gilbert L, et al. Clinical activity and safety of the Anti-Programmed death 1 monoclonal antibody Dostarlimab for patients with recurrent or advanced mismatch repair-deficient endometrial cancer: a nonrandomized phase 1 clinical trial. JAMA Oncol 2020;6:1766–72. 10.1001/jamaoncol.2020.4515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ott PA, Bang Y-J, Berton-Rigaud D, et al. Safety and antitumor activity of pembrolizumab in advanced programmed death ligand 1-positive endometrial cancer: results from the KEYNOTE-028 study. J Clin Oncol 2017;35:2535–41. 10.1200/JCO.2017.72.5952 [DOI] [PubMed] [Google Scholar]
- 40. Antill Y, Kok P-S, Robledo K, et al. Clinical activity of durvalumab for patients with advanced mismatch repair-deficient and repair-proficient endometrial cancer. A nonrandomized phase 2 clinical trial. J Immunother Cancer 2021;9:e002255. 10.1136/jitc-2020-002255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Makker V, Taylor MH, Aghajanian C, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer. J Clin Oncol 2020;38:2981. 10.1200/JCO.19.02627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kurnit KC, Kim GN, Fellman BM, et al. Ctnnb1 (beta-catenin) mutation identifies low grade, early stage endometrial cancer patients at increased risk of recurrence. Mod Pathol 2017;30:1032–41. 10.1038/modpathol.2017.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liu Y, Patel L, Mills GB, et al. Clinical significance of CTNNB1 mutation and Wnt pathway activation in endometrioid endometrial carcinoma. J Natl Cancer Inst 2014;106:dju245. 10.1093/jnci/dju245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Travaglino A, Raffone A, Raimondo D, et al. Prognostic significance of CTNNB1 mutation in early stage endometrial carcinoma: a systematic review and meta-analysis. Arch Gynecol Obstet 2022;306:423–31. 10.1007/s00404-021-06385-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. van den Heerik ASVM, Horeweg N, Nout RA, et al. PORTEC-4a: international randomized trial of molecular profile-based adjuvant treatment for women with high-intermediate risk endometrial cancer. Int J Gynecol Cancer 2020;30:2002–7. 10.1136/ijgc-2020-001929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Aghajanian C, Filiaci V, Dizon DS, et al. A phase II study of frontline paclitaxel/carboplatin/bevacizumab, paclitaxel/carboplatin/temsirolimus, or ixabepilone/carboplatin/bevacizumab in advanced/recurrent endometrial cancer. Gynecol Oncol 2018;150:274–81. 10.1016/j.ygyno.2018.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Slomovitz BM, Jiang Y, Yates MS, et al. Phase II study of everolimus and letrozole in patients with recurrent endometrial carcinoma. J Clin Oncol 2015;33:930–6. 10.1200/JCO.2014.58.3401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Moroney MR, Woodruff E, Qamar L, et al. Inhibiting Wnt/beta-catenin in CTNNB1-mutated endometrial cancer. Mol Carcinog 2021;60:511–23. 10.1002/mc.23308 [DOI] [PubMed] [Google Scholar]
- 49. Mirza MR, Bjørge L, Marmé F, et al. LBA28 a randomised double-blind placebo-controlled phase II trial of palbociclib combined with letrozole (L) in patients (PTS) with oestrogen receptor-positive (ER+) advanced/recurrent endometrial cancer (EC): NSGO-PALEO / ENGOT-EN3 trial. Annals of Oncology 2020;31:S1160–S60. 10.1016/j.annonc.2020.08.2258 [DOI] [Google Scholar]
- 50. Konstantinopoulos PA, Lee EK, Xiong N, et al. Phase 2, two-stage study of letrozole and abemaciclib in estrogen receptor (ER) positive recurrent or metastatic endometrial cancer (EC). Presented at: 2022 SGO Annual Meeting on Women’s Cancer; March 18-21, Phoenix, AZ, 2022. [Google Scholar]
- 51. Thiel KW, Devor EJ, Filiaci VL, et al. TP53 Sequencing and p53 Immunohistochemistry Predict Outcomes When Bevacizumab Is Added to Frontline Chemotherapy in Endometrial Cancer: An NRG Oncology/Gynecologic Oncology Group Study. J Clin Oncol 2022:JCO2102506. 10.1200/JCO.21.02506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Leon-Castillo A, Horeweg N, Peters EEM, et al. Prognostic relevance of the molecular classification in high-grade endometrial cancer for patients staged by lymphadenectomy and without adjuvant treatment. Gynecol Oncol 2022;164:577–86. 10.1016/j.ygyno.2022.01.007 [DOI] [PubMed] [Google Scholar]
- 53. Bosse T, Nout RA, McAlpine JN, et al. Molecular classification of grade 3 endometrioid endometrial cancers identifies distinct prognostic subgroups. Am J Surg Pathol 2018;42:561–8. 10.1097/PAS.0000000000001020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Brett MA, Atenafu EG, Singh N, et al. Equivalent survival of p53 mutated endometrial endometrioid carcinoma grade 3 and endometrial serous carcinoma. Int J Gynecol Pathol 2021;40:116–23. 10.1097/PGP.0000000000000674 [DOI] [PubMed] [Google Scholar]
- 55. Leslie KK, Filiaci VL, Mallen AR, et al. Mutated p53 portends improvement in outcomes when bevacizumab is combined with chemotherapy in advanced/recurrent endometrial cancer: an NRG oncology study. Gynecol Oncol 2021;161:113–21. 10.1016/j.ygyno.2021.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ashley CW, Da Cruz Paula A, Kumar R, et al. Analysis of mutational signatures in primary and metastatic endometrial cancer reveals distinct patterns of DNA repair defects and shifts during tumor progression. Gynecol Oncol 2019;152:11–19. 10.1016/j.ygyno.2018.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. de Jonge MM, Auguste A, van Wijk LM, et al. Frequent homologous recombination deficiency in high-grade endometrial carcinomas. Clin Cancer Res 2019;25:1087–97. 10.1158/1078-0432.CCR-18-1443 [DOI] [PubMed] [Google Scholar]
- 58. Jönsson J-M, Bååth M, Björnheden I, et al. Homologous recombination repair mechanisms in serous endometrial cancer. Cancers 2021;13:254. 10.3390/cancers13020254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Vermij L, Horeweg N, Leon-Castillo A, et al. Her2 status in high-risk endometrial cancers (PORTEC-3): relationship with Histotype, molecular classification, and clinical outcomes. Cancers 2020;13:44. 10.3390/cancers13010044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fader AN, Roque DM, Siegel E, et al. Randomized phase II trial of carboplatin-paclitaxel versus Carboplatin-Paclitaxel-Trastuzumab in uterine serous carcinomas that overexpress human epidermal growth factor receptor 2/neu. J Clin Oncol 2018;36:2044–51. 10.1200/JCO.2017.76.5966 [DOI] [PubMed] [Google Scholar]
- 61. Saito A, Yoshida H, Nishikawa T, et al. Human epidermal growth factor receptor 2 targeted therapy in endometrial cancer: clinical and pathological perspectives. World J Clin Oncol 2021;12:868. 10.5306/wjco.v12.i10.868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fleming GF, Sill MW, Darcy KM, et al. Phase II trial of trastuzumab in women with advanced or recurrent, HER2-positive endometrial carcinoma: a gynecologic Oncology Group study. Gynecol Oncol 2010;116:15–20. 10.1016/j.ygyno.2009.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fader AN, Roque DM, Siegel E, et al. Randomized phase II trial of carboplatin-paclitaxel compared with Carboplatin-Paclitaxel-Trastuzumab in advanced (stage III-IV) or recurrent uterine serous carcinomas that overexpress HER2/neu (NCT01367002): updated overall survival analysis. Clin Cancer Res 2020;26:3928–35. 10.1158/1078-0432.CCR-20-0953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Erickson BK, Najjar O, Damast S, et al. Human epidermal growth factor 2 (HER2) in early stage uterine serous carcinoma: a multi-institutional cohort study. Gynecol Oncol 2020;159:17–22. 10.1016/j.ygyno.2020.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Naumann RW. The role of the phosphatidylinositol 3-kinase (PI3K) pathway in the development and treatment of uterine cancer. Gynecol Oncol 2011;123:411–20. 10.1016/j.ygyno.2011.08.002 [DOI] [PubMed] [Google Scholar]
- 66. Yeramian A, Moreno-Bueno G, Dolcet X, et al. Endometrial carcinoma: molecular alterations involved in tumor development and progression. Oncogene 2013;32:403–13. 10.1038/onc.2012.76 [DOI] [PubMed] [Google Scholar]
- 67. Mutter GL, Lin MC, Fitzgerald JT, et al. Altered PTEN expression as a diagnostic marker for the earliest endometrial precancers. J Natl Cancer Inst 2000;92:924–30. 10.1093/jnci/92.11.924 [DOI] [PubMed] [Google Scholar]
- 68. Slomovitz BM, Lu KH, Johnston T, et al. A phase 2 study of the oral mammalian target of rapamycin inhibitor, everolimus, in patients with recurrent endometrial carcinoma. Cancer 2010;116:5415–9. 10.1002/cncr.25515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cerami E, Gao J, Dogrusoz U. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. AACR, 2012: 401–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 2013;6:pl1. 10.1126/scisignal.2004088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Brown JS, O'Carrigan B, Jackson SP, et al. Targeting DNA repair in cancer: beyond PARP inhibitors. Cancer Discov 2017;7:20–37. 10.1158/2159-8290.CD-16-0860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Liu JF, Xiong N, Campos SM, et al. Phase II study of the Wee1 inhibitor adavosertib in recurrent uterine serous carcinoma. J Clin Oncol 2021;39:1531–9. 10.1200/JCO.20.03167 [DOI] [PubMed] [Google Scholar]
- 73. Takeda T, Banno K, Okawa R, et al. ARID1A gene mutation in ovarian and endometrial cancers (review). Oncol Rep 2016;35:607–13. 10.3892/or.2015.4421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bitler BG, Aird KM, Zhang R. Epigenetic synthetic lethality in ovarian clear cell carcinoma: EZH2 and ARID1A mutations. Mol Cell Oncol 2016;3:e1032476. 10.1080/23723556.2015.1032476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Bitler BG, Fatkhutdinov N, Zhang R. Potential therapeutic targets in ARID1A-mutated cancers. Expert Opin Ther Targets 2015;19:1419–22. 10.1517/14728222.2015.1062879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bitler BG, Aird KM, Garipov A, et al. Synthetic lethality by targeting EZH2 methyltransferase activity in ARID1A-mutated cancers. Nat Med 2015;21:231–8. 10.1038/nm.3799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Van Gool IC, Stelloo E, Nout RA, et al. Prognostic significance of L1CAM expression and its association with mutant p53 expression in high-risk endometrial cancer. Mod Pathol 2016;29:174–81. 10.1038/modpathol.2015.147 [DOI] [PubMed] [Google Scholar]
- 78. Doberstein K, Harter PN, Haberkorn U, et al. Antibody therapy to human L1CAM in a transgenic mouse model blocks local tumor growth but induces EMT. Int J Cancer 2015;136:E326–39. 10.1002/ijc.29222 [DOI] [PubMed] [Google Scholar]
- 79. Arlt MJE, Novak-Hofer I, Gast D, et al. Efficient inhibition of intra-peritoneal tumor growth and dissemination of human ovarian carcinoma cells in nude mice by anti-L1-cell adhesion molecule monoclonal antibody treatment. Cancer Res 2006;66:936–43. 10.1158/0008-5472.CAN-05-1818 [DOI] [PubMed] [Google Scholar]
- 80. Vatner RE, Cooper BT, Vanpouille-Box C, et al. Combinations of immunotherapy and radiation in cancer therapy. Front Oncol 2014;4:325. 10.3389/fonc.2014.00325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Fehniger J, Levine DA, Pothuri B. Brca 1/2 somatic mutations in patients with advanced or recurrent endometrial cancer. Gynecol Oncol 2020;158:e16. 10.1016/j.ygyno.2020.04.037 [DOI] [Google Scholar]
- 82. Dedes KJ, Wetterskog D, Mendes-Pereira AM, et al. Pten deficiency in endometrioid endometrial adenocarcinomas predicts sensitivity to PARP inhibitors. Sci Transl Med 2010;2:53ra75. 10.1126/scitranslmed.3001538 [DOI] [PubMed] [Google Scholar]
- 83. Makker V, Perez-Fidalgo JA, Bergamini A, et al. Randomized phase III study of maintenance selinexor versus placebo in endometrial cancer (ENGOT-EN5/GOG-3055/SIENDO): impact of subgroup analysis and molecular classification. JCO 2022;40:5511–11. 10.1200/JCO.2022.40.16_suppl.5511 [DOI] [Google Scholar]
- 84. Kaplan AR, Gueble SE, Liu Y, et al. Cediranib suppresses homology-directed DNA repair through down-regulation of BRCA1/2 and Rad51. Sci Transl Med 2019;11:eaav4508. 10.1126/scitranslmed.aav4508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Liu JF, Brady MF, Matulonis UA, et al. Olaparib with or without cediranib versus platinum-based chemotherapy in recurrent platinum-sensitive ovarian cancer (NRG-GY004): a randomized, open-label, phase III trial. J Clin Oncol 2022;40:JCO2102011. 10.1200/JCO.21.02011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Liu X, Shin N, Koblish HK, et al. Selective inhibition of IDO1 effectively regulates mediators of antitumor immunity. Blood 2010;115:3520–30. 10.1182/blood-2009-09-246124 [DOI] [PubMed] [Google Scholar]
- 87. Jochems C, Fantini M, Fernando RI, et al. The IDO1 selective inhibitor epacadostat enhances dendritic cell immunogenicity and lytic ability of tumor antigen-specific T cells. Oncotarget 2016;7:37762–72. 10.18632/oncotarget.9326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Wu L, Zhang C, He C, et al. Discovery of Pemigatinib: a potent and selective fibroblast growth factor receptor (FGFR) inhibitor. J Med Chem 2021;64:10666–79. 10.1021/acs.jmedchem.1c00713 [DOI] [PubMed] [Google Scholar]
- 89. Takebe N, Naqash AR, O'Sullivan Coyne G, et al. Safety, antitumor activity, and biomarker analysis in a phase I trial of the once-daily Wee1 inhibitor Adavosertib (AZD1775) in patients with advanced solid tumors. Clin Cancer Res 2021;27:3834–44. 10.1158/1078-0432.CCR-21-0329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Zhao S, Choi M, Overton JD, et al. Landscape of somatic single-nucleotide and copy-number mutations in uterine serous carcinoma. Proc Natl Acad Sci U S A 2013;110:2916–21. 10.1073/pnas.1222577110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Concin N, Matias-Guiu X, Vergote I, et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Radiother Oncol 2021;154:327–53. 10.1016/j.radonc.2020.11.018 [DOI] [PubMed] [Google Scholar]
- 92. Oaknin A, Bosse TJ, Creutzberg CL, et al. Endometrial cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol 2022;33:860–77. 10.1016/j.annonc.2022.05.009 [DOI] [PubMed] [Google Scholar]