Abstract
Introduction
This systematic review and meta-analysis evaluates the overall effects of lifestyle interventions upon hepatic fat content and metabolism-related indicators among adults with metabolic associated fatty liver disease.
Methods
It was registered under PROSPERO (CRD42021251527). We searched PubMed, EMBASE, MEDLINE, Cochrane, CINAHL, Scopus, CNKI, Wan-fang, VIP, and CBM from the inception of each database to May 2021 for RCT studies of lifestyle interventions on hepatic fat content and metabolism-related indicators. We used Review Manager 5.3 for meta-analysis and used text and detailed tabular summaries when heterogeneity existed.
Results
Thirty-four RCT studies with 2652 participants were included. All participants were obesity, 8% of whom also had diabetes, and none was lean or normal weight. Through subgroup analysis, we found low carbohydrate diet, aerobic training and resistance training significantly improved the level of HFC, TG, HDL, HbA1c, and HOMA-IR. Moreover, low carbohydrate diet is more effective in improving HFC than low fat diet and resistance training is better than aerobic training in reduction in HFC and TG (SMD, -0.25, 95% CI, -0.45 to -0.06; SMD, 0.24, 95% CI, 0.03 to 0.44, respectively).
Discussion
Overall, this is the first review that systematically synthesizes studies focused on the effects of various lifestyle on adults with MAFLD. The data generated in this systematic review were more applicable to obesity MAFLD rather than lean or normal weight MAFLD.
Systematic Review Registration
https://www.crd.york.ac.uk/prospero/, identifier (CRD42021251527).
Keywords: MAFLD, diet, exercise, lifestyle, systematic review, meta-analysis
1. Introduction
Metabolic associated fatty liver disease (MAFLD) is a chronic liver disease characterized by the excessive accumulation of fat in liver cells and metabolic dysfunction (1, 2). MAFLD, formerly known as non-alcoholic fatty liver disease (NAFLD), is redefined in the guidelines of the Asia-Pacific Society of Liver Diseases in October 2020. The new definition of MAFLD is based on the presence of fatty liver as indicated by liver biopsy or imaging or blood biomarkers, along with one of three conditions: overweight/obesity, type 2 diabetes, and metabolic dysfunction. New definition attaches importance to the pathogenesis of MAFLD and makes change to end points of study, which differ from NAFLD that excludes alcohol consumption (3). MAFLD affects approximately one quarter of the global population, which not only causes liver inflammation, fibrosis, and malignant tumors, but also often merges with a variety of metabolic disorders, causing major diseases such as gout, type 2 diabetes, hypertension, and atherosclerosis and posing a major health and economic burden to all societies (1, 4–6). Therefore, an effective approach to address such a serious situation is urgently needed.
The high prevalence of MAFLD is fueled by the rapid rise in unhealthy lifestyle including sedentary behavior and unreasonable dietary structure (7). Currently, lifestyle interventions are the primary recommended therapy for MAFLD, especially in the absence of approved pharmaceutical agents (1, 8). However, lifestyle interventions for MAFLD patients vary widely among studies, which focus on diet and/or exercise, mainly encompassing Mediterranean diet, Dietary Approaches to Stop Hypertension, high-dietary-fiber diet, aerobic exercise, and resistance exercise (9–15). In addition, the effects of lifestyle interventions differ in the available studies. The effects regarding lifestyle interventions on MAFLD are reflected in the hepatic fat content (HFC) and metabolism-related indicators, such as body mass index (BMI), waist circumference (WC), blood pressure (BP), plasma triglycerides (TG), plasma high-density lipoprotein cholesterol (HDL), fasting blood glucose (FBG), HbA1c, homeostasis model assessment of insulin resistance score (HOMA-IR), and plasma high-sensitivity C-reactive protein (CRP) level, yet these indicators are not fully included in the respective studies (10, 12, 16–18).
Although reviews focus on NAFLD(19–23), to date, none have applied systematic approaches to examine the efficacy or effectiveness of various lifestyle interventions on MAFLD after the name changed and the new diagnostic criteria were redefined. Therefore, we conducted a systematic review and meta-analysis to integrate and evaluate the relevant evidence of various lifestyle interventions in adults with MAFLD and to provide reference for clinical care teams.
2. Methods
This study protocol was registered on PROSPERO (CRD42021251527). The study was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (24).
2.1. Eligibility criteria for this study
2.1.1. Types of participants
NAFLD adults who met the diagnostic criteria of MAFLD and adults diagnosed with MAFLD were included in this review. The diagnostic criteria for MAFLD were based on histological, imaging, or blood biomarker evidence of liver fat accumulation, combined with one of the following three conditions: overweight/obesity, type 2 diabetes, or lean/normal weight but presence of at least two metabolic risk abnormalities (2).
2.1.2. Types of interventions
Interventions included lifestyle interventions, such as diet, physical activity, sleeping, or a combination of two or three. Studies designed to prove the effectiveness of dietary supplements or herbal preparations were excluded.
2.1.3. Types of comparators
Comparators included no intervention, standard/usual care, or other lifestyle interventions.
2.1.4. Types of outcomes
The primary outcomes of interest were changes in HFC assessed by histological, imaging, or blood biomarkers. The secondary outcomes included BMI, WC, BP, plasma TG, plasma HDL, FBG, HbA1c, HOMA-IR, and plasma high-sensitivity CRP level.
2.1.5. Types of study
Only randomized clinical trials were included in this review.
2.2. Search strategy and study selection
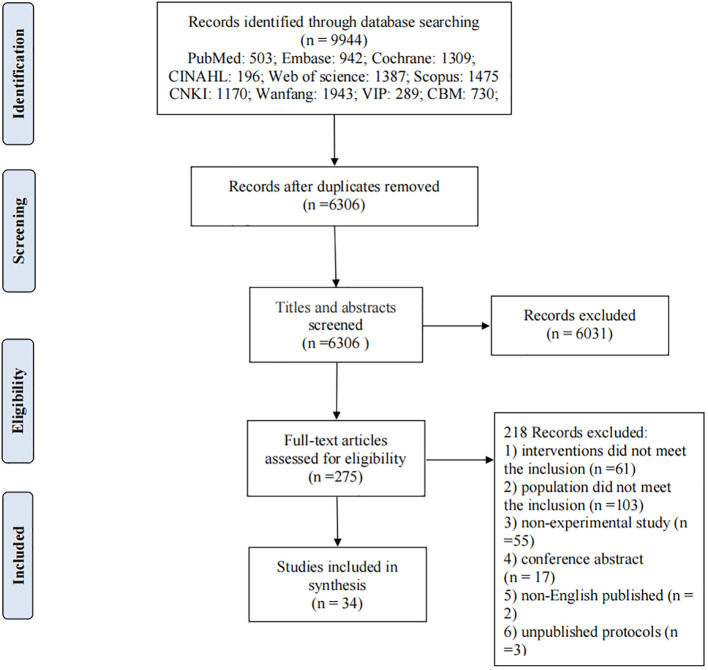
Searches were performed in English and Chinese databases, such as PubMed, EMBASE, MEDLINE, Cochrane, CINAHL and Scopus for English literature and CNKI, Wanfang, VIP, and CBM for Chinese literature. The search period was from the inception of each database to May 2021. The Medical Subject Headings and related free words were widely used to capture the literature, including non-alcoholic fatty liver disease, metabolic associated fatty liver disease, lifestyle, diet, exercise, sleep, and all synonyms of these keywords. The detailed search strategy of PubMed is shown in Table 1 . Other database search strategies are listed in the Appendix. Articles that satisfied the eligibility criteria and studies published in English or Chinese were included. The screening process included four steps and is shown in Figure 1 . First, all titles and abstracts retrieved were downloaded to the Endnote X9 library, and duplicate articles were removed. Second, articles were excluded based on titles and abstracts. Third, the full text of the article was reviewed to determine eligibility based on the inclusion criteria. Finally, the reference lists of the included articles and related reviews were screened for potentially relevant articles. The searching and screening processes were conducted by two independent researchers, and disagreements were resolved by a third researcher.
Table 1.
Search strategy of PubMed.
| Main Concept | Search Formula | Results |
|---|---|---|
| 1. MAFLD | Non-alcoholic Fatty Liver Disease[Mesh] OR Metabolic associated fatty liver disease OR MAFLD OR Metabolic-dysfunction-associated fatty liver disease OR Non alcoholic Fatty Liver Disease OR NAFLD OR Nonalcoholic Fatty Liver Disease OR Fatty Liver, Nonalcoholic OR Fatty Livers, Nonalcoholic OR Liver, Nonalcoholic Fatty OR Livers, Nonalcoholic Fatty OR Nonalcoholic Fatty Liver OR Nonalcoholic Fatty Livers OR Nonalcoholic Steatohepatitis OR Nonalcoholic Steatohepatitides OR Steatohepatitides, Nonalcoholic OR Steatohepatitis, Nonalcoholic | 34802 |
| 2. Lifestyle | Healthy Lifestyle[Mesh] OR Life Style[Mesh] OR Sedentary Behavior[Mesh] OR Life Styles[Title/Abstract] OR (Lifestyle[Title/Abstract] OR Lifestyles[Title/Abstract] OR Life Style Induced Illness[Title/Abstract] OR Lifestyle Factors[Title/Abstract] OR Factor, Lifestyle[Title/Abstract] OR Lifestyle Factor[Title/Abstract] OR Lifestyle, Healthy[Title/Abstract] OR Lifestyles, Healthy[Title/Abstract] OR Healthy Life Styles[Title/Abstract] OR Healthy Lifestyles[Title/Abstract] OR Healthy Life Style[Title/Abstract] OR Life Style, Healthy[Title/Abstract] OR Life Styles, Healthy[Title/Abstract] OR Behavior, Sedentary[Title/Abstract] OR Sedentary Behaviors[Title/Abstract] OR Sedentary Lifestyle[Title/Abstract] OR Lifestyle, Sedentary[Title/Abstract] OR Physical Inactivity[Title/Abstract] OR Inactivity, Physical[Title/Abstract] OR Lack of Physical Activity[Title/Abstract] OR Sedentary Time[Title/Abstract] OR Sedentary Times[Title/Abstract] OR Time, Sedentary[Title/Abstract] OR Exercise[Mesh] OR Exercises[Title/Abstract] OR Physical Activity[Title/Abstract] OR Activities, Physical[Title/Abstract] OR Activity, Physical[Title/Abstract] OR Physical Activities[Title/Abstract] OR Exercise, Physical[Title/Abstract] OR Exercises, Physical[Title/Abstract] OR Physical Exercise[Title/Abstract] OR Physical Exercises[Title/Abstract] OR Acute Exercise[Title/Abstract] OR Acute Exercises[Title/Abstract] OR Exercise, Acute[Title/Abstract] OR Exercises, Acute[Title/Abstract] OR Exercise, Isometric[Title/Abstract] OR Exercises, Isometric[Title/Abstract] OR Isometric Exercises[Title/Abstract] OR Isometric Exercise[Title/Abstract] OR Exercise, Aerobic[Title/Abstract] OR Aerobic Exercise[Title/Abstract] OR Aerobic Exercises[Title/Abstract] OR Exercises, Aerobic[Title/Abstract] OR Exercise Training[Title/Abstract] OR Exercise Trainings[Title/Abstract] OR Training, Exercise[Title/Abstract] OR Trainings, Exercise[Title/Abstract] OR Diet[Mesh] OR diets[Title/Abstract] OR Sleep[Mesh] OR Sleeping Habits[Title/Abstract] OR Sleep Habits[Title/Abstract] OR Habit, Sleep[Title/Abstract] OR Habits, Sleep[Title/Abstract] OR Sleep Habit[Title/Abstract] OR Sleeping Habit[Title/Abstract] OR (Habit, Sleeping[Title/Abstract] OR (Habits, Sleeping[Title/Abstract] | 887252 |
| 3. RCT | randomized controlled trial [Publication Type] OR randomized [Title/Abstract] OR placebo[Title/Abstract] | 888684 |
| #1 AND #2 AND #3 | 503 |
Figure 1.
PRISMA flow diagram.
2.3. Assessment of methodological quality
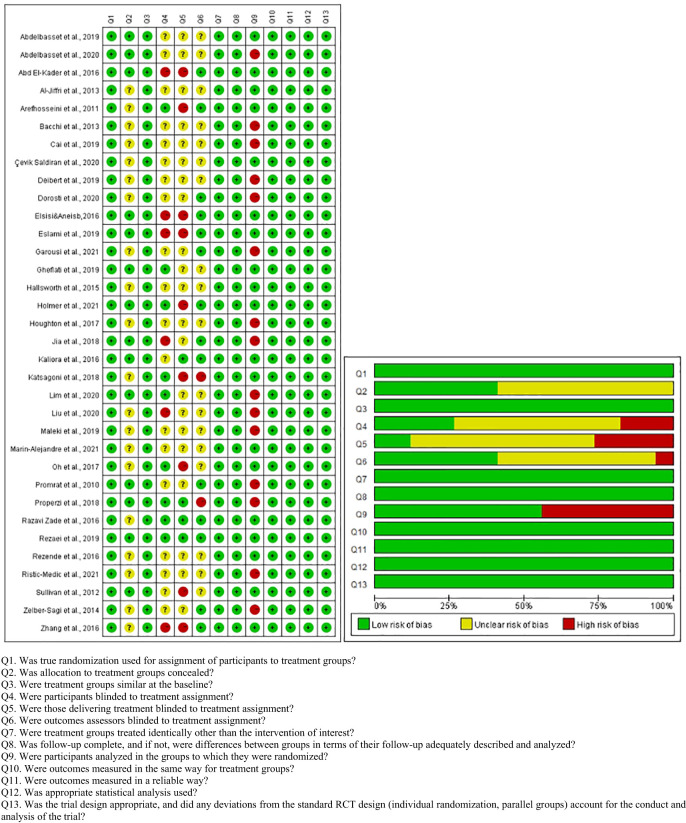
The quality of the included articles was assessed by two independent researchers using the Joanna Briggs Institute Critical Appraisal tools for Checklist for RCTs, which contain 13 entries ( Figure 2 ). Each entry was answered with yes, no, or unclear.
Figure 2.
Methodological quality of included studies.
2.4. Data extraction
All data were extracted by two independent researchers by using standardized forms, and disagreements were resolved by a third researcher. The standard form included authors and year of publication, country, study design, subject, diagnosis methods, sample size, the intervention of experimental and control groups, duration, outcomes, results, and conclusion. Among the articles included in the systematic review, those studies that provided intervention values of HFC, BMI, WC, BP, TG, HDL, FG, HbA1c, and HOMA-IR were included in the meta-analysis.
2.5. Data synthesis
ReviewManager 5.3 version was used for data consolidation, heterogeneity test, sensitivity analysis, forest map mapping, and subgroup analysis. First, clinical heterogeneity was determined, and if heterogeneity existed, meta-analysis was discarded, and the results were presented in the form of text and detailed tabular summaries. Secondly, statistical heterogeneity could be explored by chi-square test after excluding clinical heterogeneity. The results were presented as standardized mean difference (SMD) with 95% confidence intervals (CIs), and P<0.05 was considered statistically significant. A fixed-effects model was applied when the heterogeneity test indicated no significant difference (P>0.1 and I2<50%); otherwise, a random-effects model was used. Meta-analysis was abandoned, and descriptive methods were used when the source of heterogeneity could not be determined.
3. Results
3.1. Study selection and study characteristics
The PRISMA flow diagram summarized the selection process of the systematic review and meta-analysis (as shown in Figure 1 ). A total of 8469 studies were identified through search in nine databases. Duplicate references were identified and removed (N=3620). After examining the titles and abstracts, 252 full texts were further screened, and 34 studies met the inclusion criteria and were included in the systematic review and meta-analysis. The reasons for exclusion at this full-text level (N=218) are listed in Figure 1 .
The characteristics of the included studies are shown in Table 2 . The 34 included studies reported 2929 adults with MAFLD, and 27 of them reported information about participants lost at follow-up; the final total number was 2652. For diagnosis of MAFLD, 93% of them were based on imaging-detected liver fat accumulation combined with obesity; the remaining 7% were biopsy-proven liver fat accumulation combined with obesity. Moreover, all participants were obesity, and 8% of them had a diagnosis of type 2 diabetes. The sample size of the participants ranged from 18 to 461, and the age of participants ranged from 27 to 68 years. We classified these articles into three categories on the basis of the form of lifestyle interventions: diet interventions, exercise interventions, and diet combined with exercise interventions, which are detailed in Tables 3 – 5 , respectively.
Table 2.
Characteristics of the included articles.
| Reference | Clinical group | Country | N | Age | M/F | BMI | Intervention form | ||
|---|---|---|---|---|---|---|---|---|---|
| (25) | Obesity and biopsy-proven NASH | USA | 31/28 | 35–60 | 22/9 | 34(25~40) | Diet combined with exercise | ||
| (26) | Obesity and ultrasonography-proven NAFLD | Iran | 44 | 30–49 | 22/22 | 28.99 ± 2.72/29.21 ± 2.61 | Diet | ||
| (9) | Obesity and MRI-proven NAFLD | USA | 33/18 | 44–50 | 5/13 | 38.1 ± 4.6 | Exercise | ||
| (27) | Obesity, DM2, and biopsy-proven steatosis | Saudi Arabia | 100 | 35–55 | 100/0 | 32.11 ± 3.54/32.37 ± 3.92 | Diet combined with exercise | ||
| (28) | Obesity, DM2, and MRI scan-proven NAFLD | Italy | 32/31 | 53–58 | 22/9 | 30.5 ± 1.0/28.8 ± 1.1 | Exercise | ||
| (10) | Obesity and ultrasonography-proven NAFLD | Israel | 82/64 | 36–58 | 34/30 | 30.75 ± 4.52/31.3 ± 4.14 | Exercise | ||
| (29) | Obesity and fibrosis scan-proven NAFLD | UK | 29/23 | 44–64 | U | 31 ± 4/31 ± 5 | Exercise | ||
| (30) | Obesity and ultrasonography-proven NAFLD | Egypt | 32 | 33–56 | 15/17 | 33.99 ± 2.57/33.13 ± 2.11 | Exercise | ||
| (16) | Obesity and ultrasonography-proven NAFLD | Greece | 55/44 | 40–61 | 23/32 | 29.5 ± 4.3/28.2 ± 3.7 | Diet | ||
| (11) | Obesity and ultrasonography-proven NAFLD | Iran | 60/60 | 32–53 | 30/30 | 28.5 ± 3.2/28.3 ± 3.3 | Diet | ||
| (12) | Obesity and ultrasonography-proven NAFLD | China | 220/211/208 | 46–62 | 71/149 | 27.9 ± 2.7/28.1 ± 3.3/28.0 ± 2.7 | Exercise | ||
| (31) | Obesity and ultrasonography-proven NAFLD | Saudi Arabia | 100 | 45–57 | 70/30 | 32.35 ± 2.54/31.76 ± 2.92 | Diet combined with exercise | ||
| (32) | Obesity and fibrosis scan-proven NAFLD | Brazil | 44/40 | 48–63 | 0/40 | 34.1 ± 4.4/32.0 ± 5.0 | Exercise | ||
| (33) | Obesity and biopsy-proven NASH | Australia | 26/24 | 42–67 | U | 33 ± 7/33 ± 5 | Exercise | ||
| (34) | Obesity and fibrosis scan-proven NAFLD | Japan | 61/52 | 46–53 | 52/0 | 27.2 ± 0.9/28.4 ± 0.9/28.8 ± 1.1 | Exercise | ||
| (13) | Obesity and ultrasonography-proven NAFLD | Greece | 63/63 | 38–60 | 43/20 | 30.04/31.67/32.44 | Diet combined with exercise | ||
| (35) | Obesity and ultrasonography-proven NAFLD | China | 474/461 | 48–62 | 230/231 | 75.28 ± 10.18/74.94 ± 10.37/74.69 ± 10.3 | Exercise | ||
| (14) | Obesity and MRI scan-proven NAFLD | Australia | 51/50/50/49/48 | 38–62 | 25/26 | 30.2 ± 5.6/31.5 ± 4.1 | Diet | ||
| (36) | Obesity and ultrasonography-proven NAFLD | Iran | 70/64 | 36–56 | 19/45 | 30.90 ± 3.62/31.36 ± 3.74 | Diet | ||
| (37) | Obesity and ultrasonography-proven NAFLD | Iran | 66/54(66) | 32–60 | 29/37 | 30.6 ± 3.6/29.6 ± 3.9 | Diet | ||
| (38) | Obesity and ultrasonography-proven NAFLD | Iran | 66/62 | 36–56 | 19/43 | 30.85 ± 3.53/31.29 ± 3.77 | Diet | ||
| (39) | Obesity and ultrasonography-proven NAFLD | Iran | 60/54 | 31–49 | 12/48 | 32.77 ± 3.63/31.09 ± 3.24 | Diet | ||
| (40) | Obesity and MRI-proven NASH | Germany | 26/22 | 40–68 | 13/9 | 32.2 ± 3.4/32.4 ± 3.7 | Diet combined with exercise | ||
| (17) | Obesity and ultrasonography-proven NAFLD | China | 271/264 | 27–41 | 87/184 | 26.76 ± 1.59/26.12 ± 2.21/26.34 ± 2.73 | Diet | ||
| (41) | Obesity, DM2, and MRI scan-proven NAFLD | Saudi Arabia | 32/32 | 49–61 | 19/13 | 36.3 ± 4.5/35.9 ± 5.3 | Exercise | ||
| (15) | Obesity and ultrasonography-proven NAFLD | Iran | 112/94 | 34–52 | 39/55 | 32.5 ± 4.1/31.8 ± 4.4 | Diet | ||
| (18) | Obesity, DM2, and MRI scan-proven NAFLD | Saudi Arabia | 48/47 | 46–63 | 27/20 | 36.3 ± 4.5/36.7 ± 3.4/35.9 ± 5.3 | Exercise | ||
| (42) | Obesity and ultrasonography-proven NAFLD | Singapore | 108/101(108) | 35–57 | 68/40 | 30.1 ± 4.0/30.8 ± 4.8 | Diet combined with exercise | ||
| (43) | Obesity and fibrosis scan-proven NAFLD | China | 158/147 | 30–55 | 113/34 | 27.63 ± 3.46/28.39 ± 2.69/27.11 ± 2.73 | Exercise | ||
| (44) | Obesity and ultrasonography-proven NAFLD | Turkey | 32/31 | 36–54 | 12/19 | 32.74 ± 4.78/33.17 ± 4.91 | Exercise | ||
| (45) | Obesity and ultrasonography-proven NAFLD | Serbia | 27/24 | 28–39 | U | 30.43 ± 1.81/30.17 ± 2.28 | Diet | ||
| (46) | Obesity and imaging-proven NAFLD | Sweden | 74/64 | 47–67 | 33/41 | 32.1 ± 3.8/32.3 ± 2.7/32.9 ± 5.2 | Diet | ||
| (47) | Obesity and ultrasonography-proven NAFLD | Iran | 80/75 | 33–54 | 36/39 | 32.02 ± 4.57/30.06 ± 3.81 | Diet | ||
| (48) | Obesity and ultrasonography-proven NAFLD | Spain | 98/76/72/58 | 41–60 | 55/43 | 33.7 ± 4/33.3 ± 4 | Diet | ||
The subjects were included in studies and analysis. M/F, male/female; BMI, body mass index; NAFLD, non-alcoholic fatty liver disease; DM2, type-2 diabetes mellitus; MRI, magnetic resonance imaging; CT, computerized tomography; NASH, non-alcoholic steatohepatitis.
Table 3.
Diet interventions.
| Reference | Interventions | Interveners | Control | Duration | Outcomes | Measurer | Results | |
|---|---|---|---|---|---|---|---|---|
| (26) | Low carbohydrate diet: carbohydrate:fat:protein: 40:40:20 500 kcal less than energy requirement |
Dietitians | Low fat diets: carbohydrate:fat:protein: 55:25:20 500 kcal less than energy requirement |
6 weeks | HFC, BMI, TG, HDL | Same trained assessor | TG decreased 18.09% in low carbohydrate diet. HFC and BMI decreased in both groups. Low carbohydrate diet. is better than low fat diet in improving HFC |
|
| (14) | Low carbohydrate diet: carbohydrate:fat:protein:40:40:20 |
Dietitians | Low-fat diets: carbohydrate:fat:protein: 50:30:20 |
12 weeks | HFC, BMI, WC, SBP, DBP, TG, HDL, FBG, HbA1c, HOMA-IR | Unmentioned | TG and HbA1c decreased in low carbohydrate diet. HFC decreased in both groups. Low carbohydrate diet. is better than low fat diet in improving HFC |
|
| (45) | Low carbohydrate diet: carbohydrate:fat:protein: 50:30+:15, 600–800 kcal less than energy requirement |
Nutritionists | Low-fat diets: carbohydrate:fat:protein: 60:25:15 600–800 kcal less than energy requirement |
3 months | HFC, BMI, WC, TG, HDL, FBG, HOMA-IR, CRP | Unmentioned | HFC and TG decreased in low carbohydrate diet. HDL increased in low carbohydrate diet. WC, FBG, and HOMA-IR decreased in both groups. |
|
| (46) | Low carbohydrate diet: carbohydrate:fat:protein: 5–10:50–80:10–40 |
Dietitians | Low-fat diets: carbohydrate:fat:protein: 45–60:20:10–20 |
12 weeks | HFC, BMI, SBP, DBP, TG, HDL, HbA1c, HOMA-IR | Unmentioned | HFC decreased 7.2% in LCHF diet. HFC decreased 6.1% in 5:2 diet. |
|
| (48) | Low carbohydrate diet: carbohydrate:fat:protein: 40–45:30–35:25, 7 meals/day, 30% less than energy requirement |
Unmentioned | Low-fat diets: carbohydrate:fat:protein: 55:30:15, 3–5 meals/day, 30% less than energy requirement |
24 months | HFC, BMI, WC, SBP, DBP, TG, HDL, HbA1c, HOMA-IR | Unmentioned | HFC, WC, and HOMA-IR decreased in FLiO group. | |
| (16) | Currant replacing snacks of similar caloric content and dietary counseling | Dietitians | Dietary counseling | 24 weeks | HFC, BMI, WC, SBP, DBP, TG, HDL, HbA1c | Unmentioned | WC and FBG reduced in the currant arm. HFC, BMI, HbA1c, and CRP reduced in both arms. |
|
| (11) | Rich in fruits, vegetables, whole grains, and low-fat dairy products and low in saturated fats, cholesterol, refined grains, and sweets 350–700 kcal less than energy requirement |
Dietitians | Usual diets 350–700 kcal less than energy requirement |
8 weeks | HFC, BMI, WC, TG, HDL, FBG, HOMA-IR, CRP | Same trained examiner | BMI, WC, HOMA-IR, TG, and CRP decreased in DASH diet. | |
| (37) | Olive oil 20 g per day 500 kcal less than energy requirement |
Dietitians | Sunflower oil 20 g per day 500 kcal less than energy requirement |
12 weeks | HFC, BMI, WC, SBP, DBP, TG, HDL, FBG, HOMA-IR | Unmentioned | HFC and TG reduced in olive group. WC, SBP, and DBP reduced in both groups. |
|
| (36) | Soy milk instead of 1 serving of grains and fats 500 kcal less than energy requirement |
Unmentioned | Usual diets 500 kcal less than energy requirement |
8 weeks | HFC, BMI, WC, TG, HDL, CRP | Unmentioned | CRP decreased in soy milk group. | |
| (38) | Soy milk instead of 1 serving of grains and fats 500 kcal less than energy requirement |
Unmentioned | Usual diets 500 kcal less than energy requirement |
8 weeks | SBP, DBP, FBG, HOMA-IR | Unmentioned | DBP and HOMA-IR reduced in soy milk group. | |
| (39) | Purslane seeds 10 g/day before breakfast and dinner 500 kcal less than energy requirement |
Unmentioned | Usual diets 500 kcal less than energy requirement |
8 weeks | SBP, DBP, TG, HDL, FBG, HOMA-IR | Unmentioned | FBG and HOMA-IR reduced in purslane seed group | |
| (15) | At least half of cereal servings from whole-grain foods each day | Dietitians | Usual cereals | 12 weeks | HFC, SBP, DBP, TG, HDL, FBG, HOMA-IR | Same trained assessor | HFC, SBP, and DBP decreased in whole-grain foods | |
| (17) | A fast day with 25% of energy requirement and a feed day with ad libitum food | Unmentioned | Control: 80% of energy requirement | 12 weeks | HFC, BMI, WC, TG, HDL, FBG | Unmentioned | BMI and TG decreased in both ADF and TRF groups. | |
| An 8-h window for food intake and a 16-h window of fasting | ||||||||
| (47) | Protein restraining in meat products, 500 kcal less than energy requirement |
Unmentioned | Protein freely 500 kcal less than energy requirement |
3 months | HFC, BMI, WC, SBP, DBP, TG, HDL, FBG, HOMA-IR | Same trained assessor | HFC, BMI, WC, SBP, TG, FBG, and HOMA-IR decreased in LOV-D. | |
HFC, hepatic fat content; BMI, body mass index; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; TG, triglycerides; HDL, high density lipoprotein; FBG, fasting blood glucose; HbA1c, glycated hemoglobin; HOMA-IR, homoeostasis model of assessment-estimated insulin resistance; CRP, high-sensitivity C-reactive protein.
Table 5.
Diet combined with exercise interventions.
| Reference | Intervention | Control | Duration | Outcome Measures | Result |
|---|---|---|---|---|---|
| (25) | Diet: 1000–1500 kcal/day, 25% from fat Exercise: 10,000 steps/day, 200 min/week |
Usual lifestyle | 48 weeks | HFC, BMI, WC, TG, HDL, HbA1c, HOMA-IR | HFC reduced in intervention group. |
| (27) | Diet: 1200 kcal/day, Carbohydrate:fat:protein: 55:30–35:15 Exercise: 40 min×36 times, 65%–75% of maximum HR |
No intervention | 3 months | BMI, HOMA-IR | BMI and HOMA-IR reduced in intervention group. |
| (31) | Diet: 1200 kcal/day, carbohydrate:fat:protein: 55:30–35:15 Exercise: 40 min×3 times/week, 65%–75% of maximum HR |
No intervention | 3 months | BMI, TG, HDL, HOMA-IR | BMI, TG, and HOMA-IR reduced in intervention group. HDL increased in intervention group. |
| (13) | Diet: 1500 kcal/day for women, 1800 kcal/day for men, carbohydrate:fat:protein: 45:35:20, Exercise: 30min/day, optimal sleep |
Usual lifestyle | 6 months | HFC, BMI, TG, HDL, FBG, HOMA-IR | HFC decreased in both intervention groups. |
| Diet: 1500 kcal/day for women, 1800 kcal/day for men, carbohydrate:fat:protein: 45:35:20 | |||||
| (40) | Diet: first 6 weeks: 2 daily meals replace soy–yogurt–honey Following 18 weeks, 1 daily meal replace soy–yogurt–honey |
Usual lifestyle | 24 weeks | HFC, BMI, WC, HG, HDL, FBG, HbA1c | HFC reduced in intervention group. |
| (42) | Individualized caloric goal 10,000 steps/day |
Usual lifestyle | 6 months | BMI, WC, SBP, DBP | WC, SBP, and DBP reduced in intervention group. |
HFC, hepatic fat content; BMI, body mass index; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; TG, triglycerides; HDL, high-density lipoprotein; FBG, fasting blood glucose; HbA1c, glycated hemoglobin; HOMA-IR, homoeostasis model of assessment-estimated insulin resistance.
3.2. Methodological quality
The quality assessment details of each study are presented in Figure 2 . Twenty studies did not specify whether allocation concealment was performed, which may cause selection bias. Fifteen studies did not perform intention to treat analysis, potentially leading to follow-up bias. Participants, interveners, and outcome assessors were not fully blinded, probably causing performance bias.
3.3. Effects of interventions
3.3.1. Effects of diet interventions
The diet interventions were divided into two categories in this article, as shown in Table 3 : 1) the first five studies were reduction in the proportion of carbohydrates, and 2) the last nine studies were food serving replacement under isocaloric conditions. A meta-analysis of the diet interventions was deemed inappropriate due to the heterogeneity of the diet forms. We found that low carbohydrate diet significantly improved the level of HFC, TG, HDL, HbA1c, and HOMA-IR, but not BMI, BP, FBG, and CRP when compared to low fat diet. In addition, food serving replacement under isocaloric conditions was most significant in improving HOMA-IR in MAFLD, followed by HFC, WC, TG, FBG, BMI, BP, and CRP, but not HDL and HbA1c.
3.3.2. Effects of exercise interventions
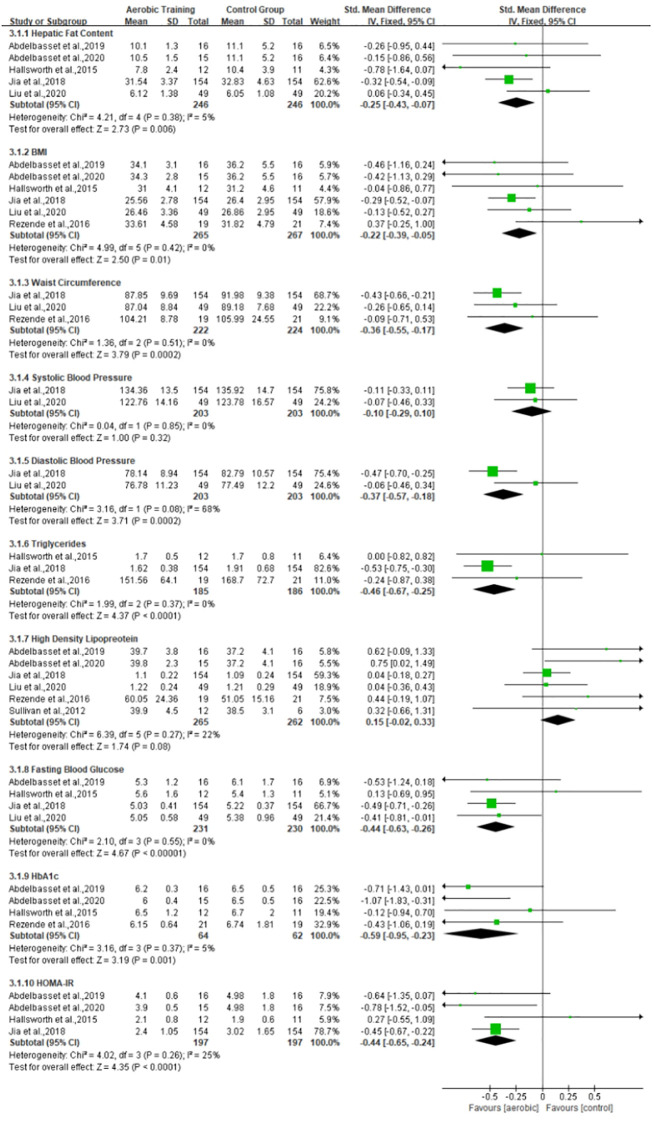
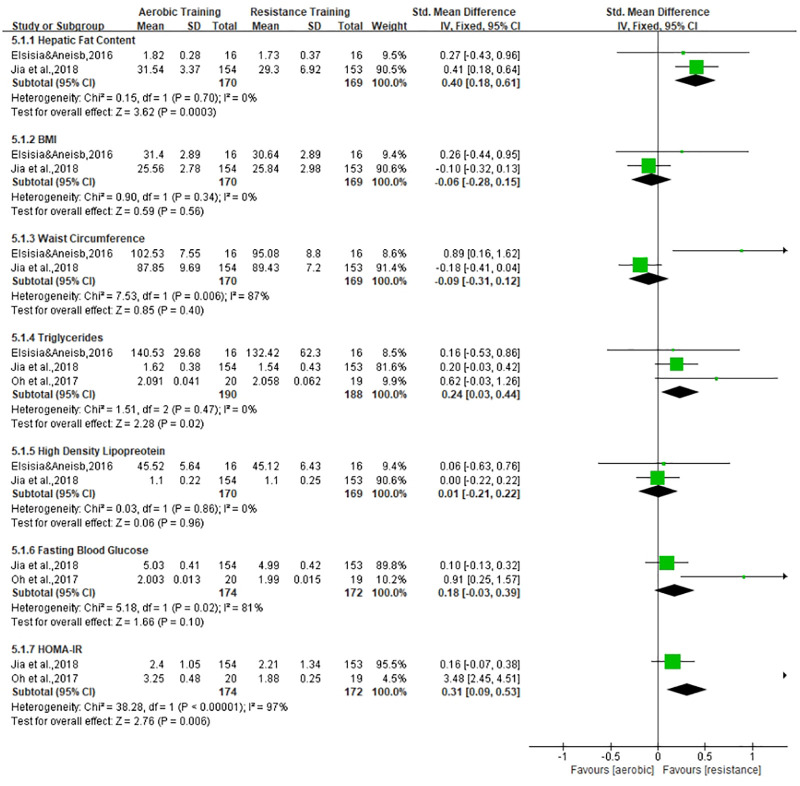
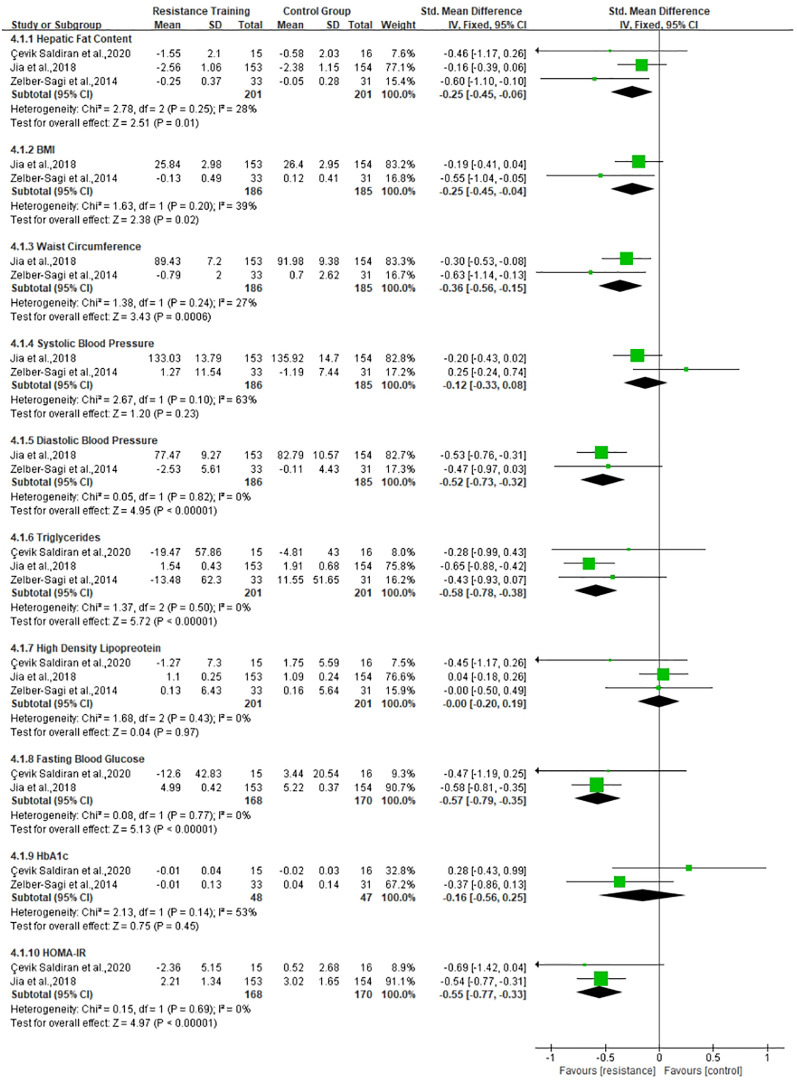
The exercise interventions were divided into aerobic training and resistance training, as shown in Table 4 . According to the different types of exercise, we will conduct meta-analysis in three subgroups: 1) the effects of aerobic training on MAFLD, 2) the effects of resistance exercise on MAFLD, and 3) the difference between aerobic and resistance exercise, which are shown in Figures 3 – 5 , respectively. Aerobic training was associated with the improvement of the indicator of MAFLD including HFC, BMI, WC, TG, FBG, HbA1c, and HOMA-IR (SMD, -0.25, 95% CI, -0.43 to -0.07; SMD, -0.22, 95% CI, -0.39 to -0.05; SMD, -0.36, 95% CI, -0.55 to -0.17; SMD, -0.46, 95% CI, -0.67 to -0.25; SMD, -0.44, 95% CI, -0.63 to -0.26; SMD, -0.59, 95% CI, -0.95 to -0.23; SMD, -0.44, 95% CI, -0.65 to -0.24, respectively). Resistance training was associated with the improvement of the indicator of MAFLD including HFC, BMI, WC, DBP, TG, FBG, and HOMA-IR (SMD, -0.25, 95% CI, -0.45 to -0.06; SMD, -0.25, 95% CI, -0.56 to -0.15; SMD, -0.36, 95% CI, -0.56 to -0.15; SMD, -0.52, 95% CI, -0.73 to -0.32; SMD, -0.58, 95% CI, -0.78 to -0.38; SMD, -0.57, 95% CI, -0.79 to -0.35; SMD, -0.55, 95% CI, -0.77 to -0.33, respectively). Resistance training was better than aerobic training in reduction in HFC and TG (SMD, -0.25, 95% CI, -0.45 to -0.06; SMD, 0.24, 95% CI, 0.03 to 0.44, respectively). Aerobic and resistance training had no effect on HDL and BP.
Table 4.
Exercise interventions.
| Reference | Types | Intervention | Control | Duration | Outcome | Result | |||
|---|---|---|---|---|---|---|---|---|---|
| Types | Intensity | Supervision | Types | Intensity | |||||
| (9) | Aerobic training | Aerobic training: 30–60 min×5 times/week |
45%–55% of VO2 peak | Under supervision: 1 time/week |
Daily activities | Unmentioned | 16 weeks | HFC, BMI, TG, HDL | HFC decreased in aerobic training group. |
| (28) | Both | Aerobic training: 60 min×3 times/week |
45%–55% of VO2 peak | Under supervision All |
Resistance training: 10repetitions×3×3times/week |
70%–80% 1RM | 4 months | HFC, BMI, TG, HDL, HbA1c | HFC and HbA1c decreased in both groups. |
| (10) | Resistance training | Resistance training: 8–12 repetitions×3×3 times/week |
Unmentioned | Under supervision ≥2 times |
Daily activities | Unmentioned | 3 months | HFC, BMI, WC, BP, TG, HDL, FBG, HbA1c | HFC, BMI, and WC decreased in resistance training group. |
| (29) | Aerobic training | Aerobic training: 30–40min×3 times/week |
Unmentioned | Under supervision first 2 sessions |
Daily activities | Unmentioned | 12 weeks | HFC, BMI, TG, FBG, HbA1c, HOMA-IR | HFC and BMI decreased in HIIT group. |
| (30) | Both | Resistance exercise: 10 repetitions×2–3×3 times/week |
70%–80% 1RM, RPE: 13–14 |
Under supervision Closely |
Aerobic training: 20–30 min×3times/week |
75% of max HR | 12 weeks | HFC, BMI, WC, TG, HDL | HFC and WC decreased in resistance training group. |
| (12) | Aerobic training | Aerobic training I: Moderate 6month; 30 min×5 times/week and vigorous 6months 30 min×5times/week×6months |
60%–80% of max HR for vigorous exercise; 45%–55% of max HR for moderate exercise |
Under supervision 1 time/week |
Daily activities | Unmentioned | 12 months | HFC, BMI, WC, BP, TG, HDL | WC and SBP reduced in aerobic training I group. HFC reduced in both aerobic training groups. |
| Aerobic training II: 30min×5times/week×12months |
45%–55% of max HR | ||||||||
| (32) | Aerobic training | Aerobic training: 30–50 min×2times/week |
10% below RCP | Under supervision | Daily activities | Unmentioned | 24 weeks | HFC, BMI, WC, TG, HDL,HbA1c,HOMA-IR | WC decreased and HDL increased in aerobic group. |
| (34) | Both | Aerobic trainingI: 3min×3vigorous +2 min×2 rests)×3 times/week | 80%–85% of VO2 peak | Unmentioned | Resistance training: 3times/week | 1RM | 12 weeks | HFC, TG, HOMA-IR | HFC reduced in both aerobic groups. |
| Aerobic training II: 40min×3times/week | 60%–65% of VO2 peak | ||||||||
| (33) | Both | Aerobic training and/or Resistance training:45–60 min×3 times/week | Aerobic training: RPE: 16–18; Resistance training: RPE: 14–16 | Under supervision | Daily activities | Unmentioned | 12 weeks | HFC, TG, HbA1c, HOMA-IR, CRP | HFC and TG reduced in interventions group. |
| (35) | Both | Aerobic training: 45 min×5 times/week, | 50%–70% of max HR | Unmentioned | Daily activities | Unmentioned | 6 months | HFC, BMI, WC, BP, TG,HLD,FBG,HOMA-IR | HFC, WC, DBP, TG, FBG, and HOMA-IR reduced in both interventions groups. |
| Resistance training:: 10–15 repetitions×3×3/week | Unmentioned | ||||||||
| (41) | Aerobic training | Aerobic training: 4 min×3vigorous+2 min ×2 rests)×3/week | 80%–85% of VO2 peak | Unmentioned | Daily activities | Unmentioned | 8 weeks | HFC, BMI, TG, FBG, HbA1c, HOMA-IR | HFC, FBG, and HbA1c decreased in the aerobic group. |
| (44) | Resistance training: | Resistance training:: 40 min×4 times/week | 60%–80% of max HR | Under supervision | Usual activities | Unmentioned | 8 weeks | HFC, TG, HDL, FBG, HbA1c, HOMA-IR | HOMA-IR decreased in resistance training group. |
| (43) | Aerobic training | Aerobic training 30–60 min×5 times/week |
60%–80% of max HR | Under supervision 1 time/week |
Daily activities | Unmentioned | 3 months | HFC, BMI, WC, BP, TG, FBG | HFC, WC, and TG reduced in aerobic training group. |
| (18) | Both | Aerobic training I:(4 min×3 vigorous+2 min×2rests)×3times/week | 80%–85% of VO2 peak | Under supervision | Daily activities | Unmentioned | 8 weeks | HFC, BMI, TG, HDL, HbA1c, HOMA-IR | HFC reduced in both intervention groups. |
| Aerobic training II:(4 min×3 ME at +2 min×2rests)×3 times/week | 60%–70% of max HR | ||||||||
HFC, hepatic fat content; BMI, body mass index; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; TG, triglycerides; HDL, high-density lipoprotein; FBG, fasting blood glucose; HbA1c, glycated hemoglobin; HOMA-IR, homoeostasis model of assessment-estimated insulin resistance; CRP, high-sensitivity C-reactive protein; RPE, rating of perceived exertion; CWT, circuit weight training; HR, heart rate; RCP, respiratory compensation point.
Figure 3.
Effect of aerobic training vs. control group on MAFLD.
Figure 5.
Effect of aerobic training vs. resistance training on MAFLD.
Figure 4.
Effect of resistance training vs. control group on MAFLD.
3.3.3. Effects of diet combined with exercise interventions
A meta-analysis of diet combined with exercise interventions was deemed inappropriate due to the heterogeneity of the intervention forms. As shown in Table 5 , diet combined with exercise interventions was most significant in improving HFC, followed by BMI, HOMA-IR, WC, BP, TG, and HDL. However, diet combined with exercise interventions was not significant in improving FBG and HbA1c.
4. Discussion
To our knowledge, this is the first systematic review and meta-analysis examining the effects of lifestyle interventions on adults with MAFLD after the name NAFLD changed to MAFLD and the diagnostic criteria redefined. We find that the intervention effects of low carbohydrate diet are significant on MAFLD. After comparing the effects of aerobic exercise and resistance exercise, resistance exercise is more effective than aerobic exercise in improving HFC and TG on MAFLD in our meta-analysis. We also find diet combined with exercise interventions are significant on MAFLD. However, there are insufficient evidence about the effects of lifestyle intervention on lean/normal weight (49)MAFLD and the effects of lifestyle intervention on certain metabolism-related indicators.
We find the intervention effects of low carbohydrate diet are significant on MAFLD. Our results suggest that low carbohydrate diet is more effective in improving HFC than low fat diet in adults with MAFLD. Although meta-analysis was abandoned for the different proportions of carbohydrates, all the five articles showed significant effects. However, the results of a meta-analysis about the effect of low carbohydrate diet on NAFLD showed no significant difference between low carbohydrate diet group and low-fat diet group (50). The possible reason for the discrepancy would be the changed definition and redefined criteria. The inclusion criteria of participants with MAFLD are based on the new diagnostic criteria, which excludes participants with NAFLD unrelated to metabolic dysfunction, therefore, the participants are more homogeneous. A recent randomized controlled trial on Type 2 Diabetes combined with NAFLD showed that low carbohydrate diet had greater clinically meaningful improvements in glycemic control and weight compared with low fat diet, which is in line with our results. The participants in this RCT meet the diagnostic criteria for MAFLD (49). However, the participants in this RCT are part of the MAFLD, which are not representative of the MAFLD as a whole. Furthermore, the RCT also showed the changes were not sustained 3 months after intervention. The Mechanisms maybe that carbohydrate, the main raw material for liver’s fat synthesis, reduced in diets would inhibit the fat accumulation in hepatocytes, while initiating lipolysis to reduce HFC (51). In a word, it deserves our attention that the results are different after MAFLD redefinition and more researches about comparing low carbohydrate diet with low fat diet are needed.
Our meta-analysis compares the intervention effects of aerobic and resistance training in adults with MAFLD. We find that resistance exercise is more effective than aerobic exercise in improving HFC and TG. This result is supported by a previous meta-analysis (52). Their results showed that resistance exercise leads to more effective outcomes in improving the metabolic syndrome and cardiovascular risk parameters compared to aerobic exercise. Resistance exercise was the most significant effective training method in ameliorating body fat while aerobic exercise was best in improving BMI significantly. The possible reason is that resistance exercise increases muscular strength, endurance, and muscle mass. Muscle mass engaged during exercise stimulates more IL-6 release that has a direct impact on glucose and lipid metabolism (53). However, a recent RCT showed that aerobic training and resistance training with dietary modification are equally effective for reducing HFC and improving underlying insulin resistance among patients with NAFLD (54). The possible reason for the discrepancy would be the different interventions. Hence, future research should distinguish aerobic and resistance exercise and focus on the advantages of resistance exercise in improving HFC and TG.
We also find diet combined with exercise interventions are significant on MAFLD. This finding is consistent with a published systematic review, which shows that a range of lifestyle interventions significantly improve NAFLD (19). Meanwhile, this finding is similar to two other systematic reviews in children with NAFLD, which reports that lifestyle interventions significantly improve NAFLD (22, 23). The results are also in line with a recent RCT study, which shows lifestyle intervention with diet and regular exercise improved functional fitness in middle-aged patients with NAFLD and Metabolic Syndrome (55). However, none studies have compared the differences of diet, exercise and diet combined exercise interventions on MAFLD. Hence, further research should consider diet combined exercise interventions, and comparing their effects from diet only or exercise only intervention on MAFLD.
Our results remind evidence about lean/normal weight MAFLD is insufficient. According to the new diagnostic criteria of MAFLD, MAFLD could be divided into three subgroups, including overweight/obesity, type 2 diabetes and lean/normal weight. We find that all the participants included in this article are obesity, 8% of them have diabetes together, but none is lean or normal weight, which makes the results more applicable to obesity MAFLD rather than lean or normal weight MAFLD. A retrospective cohort study shows improvement in histological liver steatosis on liver biopsy in non-obese patients with NAFLD treated with diet combined with exercise after a mean of 10 weeks (56). A Japanese meta-analysis showed lean NAFLD individuals makeup 20% of the NAFLD population, were older, and had higher mortality (57). A recent meta-analysis also found that a higher mortality in patients with lean NAFLD than those with non-lean NAFLD (58). The results remind that further research about the risk factors and effective interventions of lean NAFLD is warranted. Hence, we suggest that future research should focus not only on obesity MAFLD, but also on lean or normal weight MAFLD.
Our results also remind evidence about certain metabolism-related indicators is needed. NAFLD focus on glutathione aminotransferase, aspartate aminotransferase and fatty fibrosis, while MAFLD focus on HFC and metabolism-related indicators. The outcomes of interest in our study include HFC, WC, BP, TG, HDL, FBG, HbA1c, HOMA-IR, and CRP. However, not all metabolism-related indicators were analyzed in each included study, especially CRP. We could not analysis the effects of lifestyle interventions on CRP in MAFLD according to available data. These suggest that certain metabolism-related indicators deserve attention in future research.
4.1. Limitations
This review has several limitations. First, there is moderate heterogeneity among the analyzed studies, in part due to the differences in inclusion criteria and type and duration of lifestyle interventions. Second, not all interesting end points are reported in the included studies. For example, CRP is not reported in a majority of included studies, and the effect of lifestyle on CRP could not be evaluated. Third, selection bias, performance bias, and follow-up bias in some of the analyses could be another limitation. Therefore, the results should be taken with caution, and more studies on the effect of lifestyle interventions are required to reinforce the recommendations of lifestyle in the treatment and prevention of MAFLD.
4.2. Implications for practice
The recommendations for future research are provided as follows. First, the MAFLD subjects can be divided into overweight/obesity, type 2 diabetes, or lean/normal weight, and subjects with lean/normal weight MAFLD should be focused more because they are more likely to be overlooked and there is insufficient evidence from existing studies. Second, lifestyle interventions should include both diet and exercise and respect for preferences of MAFLD; for example, resistance training may be more feasible than aerobic training for MAFLD patients who are less fit or unable to tolerate aerobic training. Lastly, future studies should focus more on all metabolism-related indicators, especially CRP.
5. Conclusion
This systematic review and meta-analysis examine the effects of lifestyle interventions on adults MAFLD for the first time after the name changed. Low carbohydrate diet is more effective in improving HFC than low fat diet in adults with MAFLD. Resistance exercise is more effective than aerobic exercise in improving HFC and TG in adults MAFLD. Diet combined with exercise interventions are significant on MAFLD. However, our results more applicable to obesity MAFLD rather than lean or normal weight MAFLD.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
X-NC: Data Curation, Formal analysis, Writing - original draft. B-QZ: Data Curation, Validation, Investigation. NN: Data Curation, Validation. TP: Formal analysis. FX: Formal analysis. S-HH: Formal analysis. N-NC: Formal analysis. MS: Conceptualization; Methodology; Writing - review and editing. All authors contributed to the article and approved the submitted version.
Acknowledgments
We would like to thank Jacqueline Wah for their help in polishing our paper.
Funding Statement
This work was supported by the Hunan Provincial Innovation Foundation for Postgraduate (CX20210348), the Fundamental Research Funds for the Central Universities of Central South University (No. 2021zzts1008), the Fundamental Research Funds for the Central Universities of Central South University (No. 2021zzts1016). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2023.1081096/full#supplementary-material
References
- 1. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American association for the study of liver diseases. Hepatology (2018) 67(1):328–57. doi: 10.1002/hep.29367 [DOI] [PubMed] [Google Scholar]
- 2. Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol (2020) 73(1):202–9. doi: 10.1016/j.jhep.2020.03.039 [DOI] [PubMed] [Google Scholar]
- 3. Eslam M, Sarin SK, Wong VW, Fan JG, Kawaguchi T, Ahn SH, et al. The Asian pacific association for the study of the liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol Int (2020) 14(6):889–919. doi: 10.1007/s12072-020-10094-2 [DOI] [PubMed] [Google Scholar]
- 4. Fazel Y, Koenig AB, Sayiner M, Goodman ZD, Younossi ZM. Epidemiology and natural history of non-alcoholic fatty liver disease. Metabolism (2016) 65(8):1017–25. doi: 10.1016/j.metabol.2016.01.012 [DOI] [PubMed] [Google Scholar]
- 5. Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol (2018) 15(1):11–20. doi: 10.1038/nrgastro.2017.109 [DOI] [PubMed] [Google Scholar]
- 6. Younossi Z, Tacke F, Arrese M, Chander Sharma B, Mostafa I, Bugianesi E, et al. Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology (2019) 69(6):2672–82. doi: 10.1002/hep.30251 [DOI] [PubMed] [Google Scholar]
- 7. Inoue Y, Qin B, Poti J, Sokol R, Gordon-Larsen P. Epidemiology of obesity in adults: Latest trends. Curr Obes Rep (2018) 7(4):276–88. doi: 10.1007/s13679-018-0317-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. EASL-EASD-EASO . EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. Diabetologia (2016) 59(6):1121–40. doi: 10.1007/s00125-016-3902-y [DOI] [PubMed] [Google Scholar]
- 9. Sullivan S, Kirk EP, Mittendorfer B, Patterson BW, Klein S. Randomized trial of exercise effect on intrahepatic triglyceride content and lipid kinetics in nonalcoholic fatty liver disease. Hepatology (2012) 55(6):1738–45. doi: 10.1002/hep.25548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zelber-Sagi S, Buch A, Yeshua H, Vaisman N, Webb M, Harari G, et al. Effect of resistance training on non-alcoholic fatty-liver disease a randomized-clinical trial. World J Gastroenterol (2014) 20(15):4382–92. doi: 10.3748/wjg.v20.i15.4382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Razavi Zade M, Telkabadi MH, Bahmani F, Salehi B, Farshbaf S, Asemi Z. The effects of DASH diet on weight loss and metabolic status in adults with non-alcoholic fatty liver disease: a randomized clinical trial. Liver Int (2016) 36(4):563–71. doi: 10.1111/liv.12990 [DOI] [PubMed] [Google Scholar]
- 12. Zhang HJ, He J, Pan LL, Ma ZM, Han CK, Chen CS, et al. Effects of moderate and vigorous exercise on nonalcoholic fatty liver disease: A randomized clinical trial. JAMA Intern Med (2016) 176(8):1074–82. doi: 10.1001/jamainternmed.2016.3202 [DOI] [PubMed] [Google Scholar]
- 13. Katsagoni CN, Papatheodoridis GV, Ioannidou P, Deutsch M, Alexopoulou A, Papadopoulos N, et al. Improvements in clinical characteristics of patients with non-alcoholic fatty liver disease, after an intervention based on the Mediterranean lifestyle: a randomised controlled clinical trial. Br J Nutr (2018) 120(2):164–75. doi: 10.1017/S000711451800137X [DOI] [PubMed] [Google Scholar]
- 14. Properzi C, O’Sullivan TA, Sherriff JL, Ching HL, Jeffrey GP, Buckley RF, et al. Ad libitum Mediterranean and low-fat diets both significantly reduce hepatic steatosis: A randomized controlled trial. Hepatology (2018) 68(5):1741–54. doi: 10.1002/hep.30076 [DOI] [PubMed] [Google Scholar]
- 15. Dorosti M, Jafary Heidarloo A, Bakhshimoghaddam F, Alizadeh M. Whole-grain consumption and its effects on hepatic steatosis and liver enzymes in patients with non-alcoholic fatty liver disease: a randomised controlled clinical trial. Br J Nutr (2020) 123(3):328–36. doi: 10.1017/S0007114519002769 [DOI] [PubMed] [Google Scholar]
- 16. Kaliora AC, Kokkinos A, Diolintzi A, Stoupaki M, Gioxari A, Kanellos PT, et al. The effect of minimal dietary changes with raisins in NAFLD patients with non-significant fibrosis: a randomized controlled intervention. Food Funct (2016) 7(11):4533–44. doi: 10.1039/c6fo01040g [DOI] [PubMed] [Google Scholar]
- 17. Cai H, Qin YL, Shi ZY, Chen JH, Zeng MJ, Zhou W, et al. Effects of alternate-day fasting on body weight and dyslipidaemia in patients with non-alcoholic fatty liver disease: a randomised controlled trial. BMC Gastroenterol (2019) 19(1):219. doi: 10.1186/s12876-019-1132-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abdelbasset WK, Tantawy SA, Kamel DM, Alqahtani BA, Elnegamy TE, Soliman GS, et al. Effects of high-intensity interval and moderate-intensity continuous aerobic exercise on diabetic obese patients with nonalcoholic fatty liver disease: A comparative randomized controlled trial. Med (Baltimore) (2020) 99(10):e19471. doi: 10.1097/MD.0000000000019471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thoma C, Day CP, Trenell MI. Lifestyle interventions for the treatment of non-alcoholic fatty liver disease in adults: a systematic review. J Hepatol (2012) 56(1):255–66. doi: 10.1016/j.jhep.2011.06.010 [DOI] [PubMed] [Google Scholar]
- 20. Orci LA, Gariani K, Oldani G, Delaune V, Morel P, Toso C. Exercise-based interventions for nonalcoholic fatty liver disease: A meta-analysis and meta-regression. Clin Gastroenterol Hepatol (2016) 14(10):1398–411. doi: 10.1016/j.cgh.2016.04.036 [DOI] [PubMed] [Google Scholar]
- 21. Medrano M, Cadenas-Sanchez C, Alvarez-Bueno C, Cavero-Redondo I, Ruiz JR, Ortega FB, et al. Evidence-based exercise recommendations to reduce hepatic fat content in youth- a systematic review and meta-analysis. Prog Cardiovasc Dis (2018) 61(2):222–31. doi: 10.1016/j.pcad.2018.01.013 [DOI] [PubMed] [Google Scholar]
- 22. Utz-Melere M, Targa-Ferreira C, Lessa-Horta B, Epifanio M, Mouzaki M, Mattos AA. Non-alcoholic fatty liver disease in children and adolescents: Lifestyle change - a systematic review and meta-analysis. Ann Hepatol (2018) 17(3):345–54. doi: 10.5604/01.3001.0011.7380 [DOI] [PubMed] [Google Scholar]
- 23. Mann JP, Tang GY, Nobili V, Armstrong MJ. Evaluations of lifestyle, dietary, and pharmacologic treatments for pediatric nonalcoholic fatty liver disease: A systematic review. Clin Gastroenterol Hepatol (2019) 17(8):1457–1476 e1457. doi: 10.1016/j.cgh.2018.05.023 [DOI] [PubMed] [Google Scholar]
- 24. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (2009) 339:b2700. doi: 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Promrat K, Kleiner DE, Niemeier HM, Jackvony E, Kearns M, Wands JR, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology (2010) 51(1):121–9. doi: 10.1002/hep.23276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Arefhosseini SR, Ebrahimi-Mameghani M, Farsad Naeimi A, Khoshbaten M, Rashid J. Lifestyle modification through dietary intervention: Health promotion of patients with non-alcoholic fatty liver disease. Health Promot Perspect (2011) 1(2):147–54. doi: 10.5681/hpp.2011.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Al-Jiffri O, Al-Sharif FM, Abd El-Kader SM, Ashmawy EM. Weight reduction improves markers of hepatic function and insulin resistance in type-2 diabetic patients with non-alcoholic fatty liver. Afr Health Sci (2013) 13(3):667–72. doi: 10.4314/ahs.v13i3.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bacchi E, Negri C, Targher G, Faccioli N, Lanza M, Zoppini G, et al. Both resistance training and aerobic training reduce hepatic fat content in type 2 diabetic subjects with nonalcoholic fatty liver disease (the RAED2 randomized trial). Hepatology (2013) 58(4):1287–95. doi: 10.1002/hep.26393 [DOI] [PubMed] [Google Scholar]
- 29. Hallsworth K, Thoma C, Hollingsworth KG, Cassidy S, Anstee QM, Day CP, et al. Modified high-intensity interval training reduces liver fat and improves cardiac function in non-alcoholic fatty liver disease: a randomized controlled trial. Clin Sci (Lond) (2015) 129(12):1097–105. doi: 10.1042/CS20150308 [DOI] [PubMed] [Google Scholar]
- 30. Elsisia HF, Aneisb YM. High-intensity circuit weight training versus aerobic training in patients with nonalcoholic fatty liver disease. Bull Faculty Phys Ther (2016) 20(2):181–92. doi: 10.4103/1110-6611.174717 [DOI] [Google Scholar]
- 31. Abd El-Kader SM, Al-Shreef FM, Al-Jiffri OH. Biochemical parameters response to weight loss in patients with non-alcoholic steatohepatitis. Afr Health Sci (2016) 16(1):242–9. doi: 10.4314/ahs.v16i1.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rezende RE, Duarte SM, Stefano JT, Roschel H, Gualano B, de Sa Pinto AL, et al. Randomized clinical trial: benefits of aerobic physical activity for 24 weeks in postmenopausal women with nonalcoholic fatty liver disease. Menopause (2016) 23(8):876–83. doi: 10.1097/GME.0000000000000647 [DOI] [PubMed] [Google Scholar]
- 33. Houghton D, Thoma C, Hallsworth K, Cassidy S, Hardy T, Burt AD, et al. Exercise reduces liver lipids and visceral adiposity in patients with nonalcoholic steatohepatitis in a randomized controlled trial. Clin Gastroenterol Hepatol (2017) 15(1):96–102.e103. doi: 10.1016/j.cgh.2016.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Oh S, So R, Shida T, Matsuo T, Kim B, Akiyama K, et al. High-intensity aerobic exercise improves both hepatic fat content and stiffness in sedentary obese men with nonalcoholic fatty liver disease. Sci Rep (2017) 7:43029. doi: 10.1038/srep43029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jia GY, Han T, Gao L, Wang L, Wang SC, Yang L, et al. A randomized controlled study of aerobic and resistance exercise to improve nonalcoholic fatty liver disease. Chin J Liver Dis (2018) 26(01):34–41. doi: 10.3760/cma.j.issn.1007-3418.2018.01.009 [DOI] [PubMed] [Google Scholar]
- 36. Eslami O, Shidfar F, Maleki Z, Jazayeri S, Hosseini AF, Agah S, et al. Effect of soy milk on metabolic status of patients with nonalcoholic fatty liver disease: A randomized clinical trial. J Am Coll Nutr (2019) 38(1):51–8. doi: 10.1080/07315724.2018.1479990 [DOI] [PubMed] [Google Scholar]
- 37. Rezaei S, Akhlaghi M, Sasani MR, Barati Boldaji R. Olive oil lessened fatty liver severity independent of cardiometabolic correction in patients with non-alcoholic fatty liver disease: A randomized clinical trial. Nutrition (2019) 57:154–61. doi: 10.1016/j.nut.2018.02.021 [DOI] [PubMed] [Google Scholar]
- 38. Maleki Z, Jazayeri S, Eslami O, Shidfar F, Hosseini AF, Agah S, et al. Effect of soy milk consumption on glycemic status, blood pressure, fibrinogen and malondialdehyde in patients with non-alcoholic fatty liver disease: a randomized controlled trial. Complement Ther Med (2019) 44:44–50. doi: 10.1016/j.ctim.2019.02.020 [DOI] [PubMed] [Google Scholar]
- 39. Gheflati A, Adelnia E, Nadjarzadeh A. The clinical effects of purslane (Portulaca oleracea) seeds on metabolic profiles in patients with nonalcoholic fatty liver disease: A randomized controlled clinical trial. Phytother Res (2019) 33(5):1501–9. doi: 10.1002/ptr.6342 [DOI] [PubMed] [Google Scholar]
- 40. Deibert P, Lazaro A, Schaffner D, Berg A, Koenig D, Kreisel W, et al. Comprehensive lifestyle intervention vs soy protein-based meal regimen in non-alcoholic steatohepatitis. World J Gastroenterol (2019) 25(9):1116–31. doi: 10.3748/wjg.v25.i9.1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Abdelbasset WK, Tantawy SA, Kamel DM, Alqahtani BA, Soliman GS. A randomized controlled trial on the effectiveness of 8-week high-intensity interval exercise on intrahepatic triglycerides, visceral lipids, and health-related quality of life in diabetic obese patients with nonalcoholic fatty liver disease. Med (Baltimore) (2019) 98(12):e14918. doi: 10.1097/MD.0000000000014918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lim SL, Johal J, Ong KW, Han CY, Chan YH, Lee YM, et al. Lifestyle intervention enabled by mobile technology on weight loss in patients with nonalcoholic fatty liver disease: Randomized controlled trial. JMIR Mhealth Uhealth (2020) 8(4):e14802. doi: 10.2196/14802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liu YY, Liu YP, Liu YR, Sun P. A prospective study on the implementation of aerobic exercise intervention in patients with metabolism-related fatty liver disease. J Clin Hepatol (2020) 36(11):2467–72. doi: 10.3969/j.issn.1001-5256.2020.11.014 [DOI] [Google Scholar]
- 44. Çevik Saldiran T, Mutluay FK, Yağci İ., Yilmaz Y. Impact of aerobic training with and without whole-body vibration training on metabolic features and quality of life in non-alcoholic fatty liver disease patients. Ann Endocrinol (Paris) (2020) 81(5):493–9. doi: 10.1016/j.ando.2020.05.003 [DOI] [PubMed] [Google Scholar]
- 45. Ristic-Medic D, Kovacic M, Takic M, Arsic A, Petrovic S, Paunovic M, et al. Calorie-restricted Mediterranean and low-fat diets affect fatty acid status in individuals with nonalcoholic fatty liver disease. Nutrients (2020) 13(1):15. doi: 10.3390/nu13010015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Holmer M, Lindqvist C, Petersson S, Moshtaghi-Svensson J, Tillander V, Brismar TB, et al. Treatment of NAFLD with intermittent calorie restriction or low-carb high-fat diet - a randomised controlled trial. JHEP Rep (2021) 3(3):100256. doi: 10.1016/j.jhepr.2021.100256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Garousi N, Tamizifar B, Pourmasoumi M, Feizi A, Askari G, Clark CCT, et al. Effects of lacto-ovo-vegetarian diet vs. standard-weight-loss diet on obese and overweight adults with non-alcoholic fatty liver disease: a randomised clinical trial. Arch Physiol Biochem (2021) 9:1–9. doi: 10.1080/13813455.2021.1890128 [DOI] [PubMed] [Google Scholar]
- 48. Marin-Alejandre BA, Cantero I, Perez-Diaz-Del-Campo N, Monreal JI, Elorz M, Herrero JI, et al. Effects of two personalized dietary strategies during a 2-year intervention in subjects with nonalcoholic fatty liver disease: A randomized trial. Liver Int (2021) 41(7):1532–44. doi: 10.1111/liv.14818 [DOI] [PubMed] [Google Scholar]
- 49. Hansen CD, Gram-Kampmann EM, Hansen JK, Hugger MB, Madsen BS, Jensen JM, et al. Effect of calorie-unrestricted low-carbohydrate, high-fat diet versus high-carbohydrate, low-fat diet on type 2 diabetes and nonalcoholic fatty liver disease. Ann Internal Med (2023) 176(1):10–22. doi: 10.7326/P22-0022 [DOI] [PubMed] [Google Scholar]
- 50. Ahn J, Jun DW, Lee HY, Moon JH. Critical appraisal for low-carbohydrate diet in nonalcoholic fatty liver disease: Review and meta-analyses. Clin Nutr (2019) 38(5):2023–30. doi: 10.1016/j.clnu.2018.09.022 [DOI] [PubMed] [Google Scholar]
- 51. Brouns F. Overweight and diabetes prevention: is a low-carbohydrate-high-fat diet recommendable? Eur J Nutr (2018) 57(4):1301–12. doi: 10.1007/s00394-018-1636-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Liang M, Pan Y, Zhong T, Zeng Y, Cheng ASK. Effects of aerobic, resistance, and combined exercise on metabolic syndrome parameters and cardiovascular risk factors: a systematic review and network meta-analysis. Rev Cardiovasc Med (2021) 22(4):1523–33. doi: 10.31083/j.rcm2204156 [DOI] [PubMed] [Google Scholar]
- 53. Pedersen BK. Anti-inflammatory effects of exercise: role in diabetes and cardiovascular disease. Eur J Clin Invest (2017) 47(8):600–11. doi: 10.1111/eci.12781 [DOI] [PubMed] [Google Scholar]
- 54. Charatcharoenwitthaya P, Kuljiratitikal K, Aksornchanya O, Chaiyasoot K, Bandidniyamanon W, Charatcharoenwitthaya N. Moderate-intensity aerobic vs resistance exercise and dietary modification in patients with nonalcoholic fatty liver disease: A randomized clinical trial. Clin Trans Gastroenterol (2021) 12:e00316. doi: 10.14309/ctg.0000000000000316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mascaró CM, Bouzas C, Montemayor S, Casares M, Llompart I, Ugarriza L, et al. Effect of a six-month lifestyle intervention on the physical activity and fitness status of adults with NAFLD and metabolic syndrome. Nutrients (2022) 14(9):1813. doi: 10.3390/nu14091813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jin YJ, Kim KM, Hwang S, Lee SG, Ha TY, Song GW, et al. Exercise and diet modification in non-obese non-alcoholic fatty liver disease: analysis of biopsies of living liver donors. J Gastroenterol Hepatol (2012) 27(8):1341–7. doi: 10.1111/j.1440-1746.2012.07165.x [DOI] [PubMed] [Google Scholar]
- 57. Ito T, Ishigami M, Zou B, Tanaka T, Takahashi H, Kurosaki M, et al. The epidemiology of NAFLD and lean NAFLD in Japan: a meta-analysis with individual and forecasting analysis 1995-2040. Hepatol Int (2021) 15(2):366–79. doi: 10.1007/s12072-021-10143-4 [DOI] [PubMed] [Google Scholar]
- 58. Ha J, Yim SY, Karagozian R. Mortality and liver-related events in lean versus non-lean nonalcoholic fatty liver disease: A systematic review and meta-analysis. Clin Gastroenterol Hepatol (2022) 26:S1542-3565(22)01099-0. doi: 10.1016/j.cgh.2022.11.019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.