Highlights
-
•
Triggered imaging is an effective method of assessing intrafraction motion for stereotactic spine patients with hardware.
-
•
The technology provides the ability to detect intrafraction motion exceeding 1 mm so treatment can be paused and reassessed.
-
•
In our cohort, the average intrafraction motion was 0.35 mm with 1 of 33 fractions exceeding the 1 mm motion tolerance.
Keywords: Intrafraction motion review, Triggered imaging, Advanced imaging, Spine, Stereotactic
Abstract
Background and purpose
Mitigation of intrafraction motion (IM) is valuable in stereotactic radiotherapy (SRT) radiotherapy where submillimeter accuracy is desired. The purpose of this study was to investigate the application of triggered kilovoltage (kV) imaging for spine SRT patients with hardware by correlating kV imaging with patient motion and summarizing implications of tolerance for IM based on calculated dose.
Materials and methods
Ten plans (33 fractions) were studied, correlating kV imaging during treatment with pre- and post-treatment cone beam computed tomography (CBCT). Images were taken at 20-degree gantry angle intervals during the arc-based treatment. The contour of the hardware with a 1 mm expansion was displayed at the treatment console to manually pause treatment delivery if the hardware was visually detected outside the contour. The treatment CBCTs were compared using retrospective image registration to assess the validity of contour-based method for pausing treatment. Finally, plans were generated to estimate dose volume objective differences in case of 1 mm deviation.
Results
When kV imaging during treatment was used with the 1 mm contour, 100 % of the post-treatment CBCTs reported consistent results. One patient in the cohort exhibited motion greater than 1 mm during treatment which allowed intervention and re-setup during treatment. The average translational motion was 0.35 mm. Treatment plan comparison at 1 mm deviation showed little differences in calculated dose for the target and cord.
Conclusions
Utilizing kV imaging during treatment is an effective method of assessing IM for SRT spine patients with hardware without increasing treatment time.
1. Introduction
Spinal metastasis occurs in 40–70 % of cancer patients where progression can lead to mechanical instability, cord compression, or functional compromise [1], [2], [3], [4], [5]. Surgery and radiation therapy are often used when indications such as instability or cord compression are present, with the combination of the two treatment approaches enhancing the overall quality of life for patients [6]. The use of stereotactic radiosurgery (SRT) has been widely adapted for radiation resistant histologies and bone metastasis [6], [7], [8], [9], [10]. SRT allows for a conformal dose distribution delivered with submillimeter accuracy, but this approach is technically challenging, especially when considering patient comfort and set up in the post-operative setting [11].
Patient motion during radiation therapy treatment is of concern due to the possibility of a geometric miss of the target volume and increased toxicity to organs at risk. There are now many technical strategies for mitigating the risk of intrafraction patient motion [12], [13], [14], [15]. Specialized treatment systems with planar kilovoltage (kV) imaging allow for localization and the ability to quickly acquire intrafraction imaging to verify positioning during treatment [16], [17]. Previous studies have shown effective implementation of triggered imaging for various disease sites, including prostate [18], [19], [20], [21], [22], [23], [24] and gastrointestinal tumors [25], [26]. In the case of patients with a spinal fixation device or hardware, the high contrast region can be tracked over the course of treatment.
Feasibility studies have been conducted on the detection of vertebral bodies and spinous processes in a phantom, and it was found that shifts in setup were detectable with visual inspection of contours overlayed on kV images captured during treatment delivery [27]. Detectability was proportional to the amount of the phantom’s misalignment and improved with the observers’ experience utilizing the technique.
The purpose of this study was to report clinical application of triggered kV imaging to monitor IM in spine SRT patients using spinal hardware as a landmark. Quantitative analysis reports correlation of kV imaging to overall patient motion assessed via pre- and post-treatment cone beam computed tomography (CBCT) images. The appropriateness of 1 mm tolerance for intrafraction motion (IM) was also evaluated through deviation in dose and volume endpoints due to motion.
2. Material and methods
2.1. Patient treatment
This study included patients treated with SRT for spine metastasis in the cervical and thoracic vertebral bodies. The patients were required to have a spinal fixation device (hardware) with treatment target volumes contained in the superior/anterior length of the hardware to be used as a surrogate for monitoring motion with triggered kV imaging during treatment. This study was approved by the The Ohio State University Institutional Review Board (ID 2019C0152).
Patients treated for cervical spine were immobilized using a thermoplastic mask, arms positioned at sides holding pegs, and a knee sponge. Patients treated for thoracic spine were immobilized using a wing board to reproducibly position the patient’s arms above their head, a patient specific bag conformed to the body, and an indexed knee sponge with a fixation device on the patient’s thighs.
Clinical plans were developed with 2-arc volumetric modulated arc therapy (VMAT) plans in Varian Eclipse (Version 15.6.05) using planning CTs with 1.25 mm slice thickness reconstructed with a metal artifact reduction protocol. Patient target volumes were determined using the spine radiosurgery consensus guidelines found in Cox et al. where the planning target volume (PTV) was generated from the clinical target volume without any additional margin [28]. The spinal cord volume was delineated using either the registered T2 MR imaging or a diagnostic myelogram study. A uniform 2 mm expansion was used to create the planning organ at risk volume (PRV) for the spinal cord.
Generated plans were normalized such that the prescribed dose (either 27 Gy in 3 fractions or 30 Gy in 5 fractions) covered 95 % of the PTV. Plans were optimized to meet the spinal cord dose constraints outlined in AAPM Task Group Report 101 guidelines [12] for 3 and 5 fraction dose regimens to the 2 mm PRV when feasible, but to always meet guidelines for the true cord volume. Conformity indices (ratio of prescription isodose volume to treatment volume) for the plans were between 0.98 and 1.03. The spinal hardware was contoured and isotropically expanded 1 mm as a high-resolution structure for display at the console and clinical decision making on whether to pause treatment due to IM.
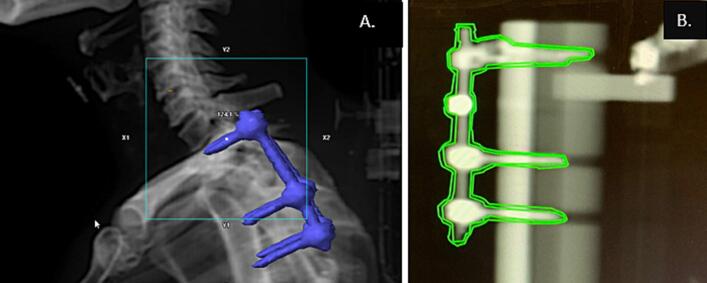
Prior to treatment, a CBCT image of the patient was taken for localization. The Intrafraction Motion Review (IMR) application on the Varian (Palo Alto, CA) TrueBeam® was used to acquire triggered kV imaging during the arc-based treatment at 20-degree gantry angle intervals. Using this imaging frequency, the approximate imaging dose to central axis is 0.03 Gy per fraction for a VMAT plan using two arcs. The projection of the hardware in the image relative to the gantry position and its 1 mm expanded contour were displayed at the treatment console (Fig. 1). The position of the hardware in the kV image relative to the 1 mm expansion contour of the hardware was used as a clinical decision-making threshold. If there was visual indication for motion greater than 1 mm, the treatment was paused, and the patient was reimaged using CBCT. At the end of treatment, a CBCT was acquired for patients as a surrogate to quantify the motion over the course of treatment. The post-treatment CBCT was registered to the pre-treatment CBCT retrospectively to estimate the shifts, based on which, calculated dose and volume endpoints including changes to coverage of the target volume and dose to the spinal cord could be assessed.
Fig. 1.
Example of patient with spinal fixation hardware (A) and example of contour of hardware and 1 mm expansion used for clinical decision-making during treatment (B).
2.2. Intrafraction motion assessment
Ten patient plans were studied in this cohort with triggered kV imaging during treatment. The size of the treatment field varied based on the extent of patient disease with patient plans including 1 to 5 vertebral bodies. The average length of the hardware was 16 cm with the maximum length of 28 cm and minimum length of 3 cm. A summary of the treatment volumes and hardware are found in Supplementary Table S1.
A total of 33 treatment fractions obtained a mid- or post-treatment CBCT for cumulative IM analysis. (Imaging for three fractions in the cohort were not obtained due to technical or clinical reasons.) CBCT imaging data during treatment was imported to Velocity Version 4.1 (Varian Medical Systems, Palo Alto, CA) where registrations were created to retrospectively assess the differences between the two time points, which corresponded to patient motion during the fraction. Details of the registration method can be found in Supplementary Materials Section B. The average translational motion and the vectoral length (r) from the components of the IM is calculated for each fraction.
2.3. Calculated dose analysis
Impact in terms of dose volume histogram (DVH) differences of a 1 mm shift was assessed by applying this shift to the original treatment plan to simulate the threshold for intervention during treatment. A group of six plans was generated for each patient’s plan using Varian Eclipse Plan Uncertainty Parameters calculation module. Translational shifts are applied to the patient data set in each axis in the both the positive and negative direction, and new plans were calculated using AcurosXB External Beam algorithm with 1 mm calculation resolution. Examples of the DVH deviation results from the plans are shown in Supplementary Material Section C. In this study, the worst-case scenario was considered to report the greatest difference within the group of plans for the coverage of the PTV and maximum dose to the spinal cord and PRV. The objectives analyzed included PTV maximum dose, PTV D95%, PTV minimum dose, cord maximum dose, and cord PRV maximum dose. Due to the small sample size in this cohort of 10 patient plans analyzed, a two-tailed paired t-test was used to compare these calculated dose objectives and the Shapiro Wilk test was used to verify normality of the data. The null hypothesis was that on average there are no differences in calculated dose between the original plan and 1 mm shift for the PTV and spinal cord using a two-tailed paired t-test with α = 0.05.
For this study, maximum dose to the PTV is defined as the nominal dose maximum reported by the treatment planning system, minimum dose to PTV is that to 99.99 %, and maximum cord dose to 0.03 cm3. For comparison of spinal cord constraints between 3 and 5 fraction plans, the maximum dose to the cord (0.03 cm3) was subtracted from the AAPM TG-101 planning objective for each patient’s plan where 30 Gy was used as the planning objective for 5 fractions and 21.9 Gy was used for 3 fraction radiotherapy treatments.
3. Results
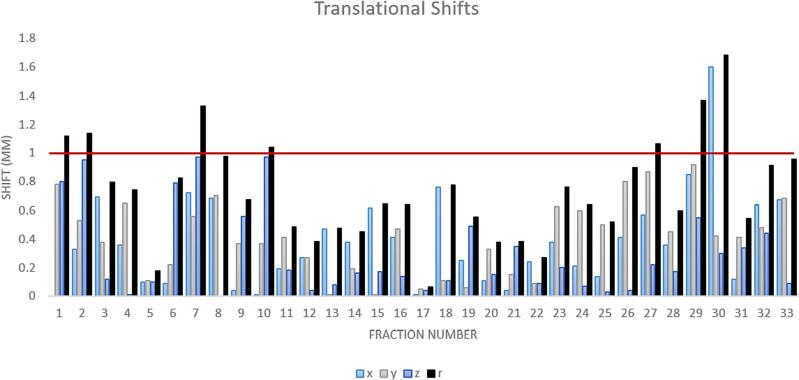
The addition of triggered kV imaging did not increase patient time on the table unless interruption due to motion was required. When the treatment team visually indicated the hardware was within the 1 mm contour, the post-treatment CBCT (upon registration with the pre-treatment CBCT) confirmed the patient was within the 1 mm clinical tolerance at the end of treatment shown in Fig. 2. The average translational IM of the patients in the cohort was 0.35 mm.
Fig. 2.
Translational shifts for each fraction calculated from pre- and post-CBCT. Vectoral length (r) from the components is calculated and displayed for reference. Difference during fraction 30 was identified with triggered imaging mid-treatment with a CBCT initiated due to the kV data suggesting intrafraction motion. Patient treatment was paused to realign patient before continuing. Red line indicates the 1 mm threshold for clinical intervention. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Of the 33 fractions, one was paused due to observed motion outside of the 1 mm tolerance represented in Fig. 1 in the x-axis exceeding the 1 mm threshold during fraction 30. The change was identified mid-treatment by the triggered imaging process allowing the treatment team to manage the patient motion.
The null hypothesis was rejected that on average there were no dosimetric differences between the original plan and 1 mm shift for PTV D95%, PTV Minimum Dose, spinal cord maximum dose, and spinal cord PRV maximum dose (<0.01) with exception of the PTV maximum dose (p =.90) using a two-tailed paired t-test with α = 0.05. While the differences were statistically significant, the clinical significance of the differences in the plan quality remains minimal in most cases. Additional data for each of these parameters is presented in more detail in Supplementary Material Section D.
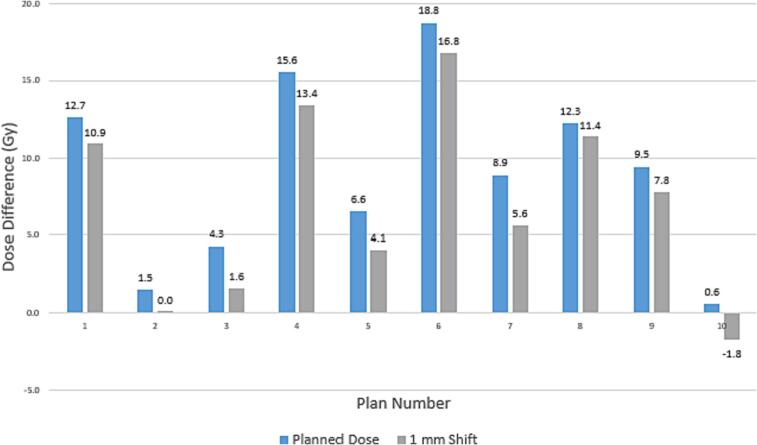
The dose volume deviations in spinal cord maximum dose due to 1 mm shifts are shown in Fig. 3, indicating the treatment planning is robust with shifts up to 1 mm in 9 of the 10 patients in this cohort. Within the cohort, only one of the ten clinical plans would have exceeded recommended planning objectives for the spinal cord. The average difference for the cord dose with a 1 mm shift was 2.06 ± 0.68 Gy. The average difference for the PRV cord dose with 2 mm margin with a 1 mm shift was 2.72 ± 1.31 Gy.
Fig. 3.
Difference in treatment planning dose to spinal cord compared to TG-101 recommendations by number of fractions. Positive numbers indicate the current planned treatment dose is within TG-101 recommendations and negative values represent plans that would exceed TG-101 recommendations.
4. Discussion
In this study, triggered imaging has been shown as a feasible motion assessment option for postoperative spine patients. One patient in the cohort exhibited motion greater than 1 mm during the retrospective study, which was identified by the clinical methodology. Without the use of triggered imaging, this deviation may not have been detected clinically. Other fractions when within tolerance showed consistency between kV imaging during treatment and post-CBCT. 1 mm was shown to be a reasonable tolerance to prevent significant dose deviations to the target and spinal cord using our current treatment planning techniques.
Motion of the spine has components which are both systematic and random. While studies have reported submillimeter median or mean positional differences over the course of treatment [29], [30], others have observed values in the 1–2 mm range [31], [32] with the largest shifts reported could be greater than 3 mm [29], [33]. Studies have shown that the motion is dependent on immobilization, anatomical location of the treated vertebrae [30], [32], and time spent on the treatment table [34], [35]. In our study, the average translational motion for the patients was 0.35 mm, but IM approaching 1 mm tolerance was observed in some cases. The addition of kV imaging during treatment added no additional time to treatment delivery if the patients did not indicate motion outside of tolerance.
kV images during treatment can be obtained using orthogonal imaging systems in the room (ceiling or floor) or with gantry-mounted kV imaging systems [36], [37], [38], [39], [40], [41]. Limitations to kV imaging for IM assessment include two-dimensional data, additional ionization dose to the patient, and lack of contrast in soft tissue. However, this technique can be helpful for determining IM for patients with hardware receiving radiation treatments with very high dose per fraction. With a gantry-based imaging solution, no additional imaging hardware is necessary making triggered kV imaging for IM assessment accessible to users of c-arm linacs.
Vendor systems can support automatically turning the beam off when specific criteria is met indicating IM [21]. The methods in this study using a clinically generated contour with triggered imaging are not currently a vendor-supported or automated process using a library of pre-defined fiducials for automated feedback to the clinical user for real-time decision making since a standard marker geometry is not used for tracking. Implementation requires training for the development of appropriate contours, interpretation of imaging at the console, manual pausing of treatment, and clinical decision-making at the treatment console.
Limitations of this study include small sample size due to the desire to minimize the addition of a post-treatment CBCT for patients in effort to reduce total treatment time and imaging dose. Due to the retrospective nature of the review, there was limited initial data to assess because once the clinical process was deemed safe and clinically acceptable, the clinical team agreed to discontinue validation imaging post-treatment. For this study, the registrations were conducted offline retrospectively in a separate software which is different from real-time clinical assessment at the treatment console. An assumption is that the CBCT imaging is a surrogate of patient motion during treatment, but these methods only represent two distinct time points in the treatment process for comparison. It is possible patient motion could occur during treatment or after treatment before the post-treatment CBCT that would not be represented in the data presented.
Some metastatic spine tumors are rapidly progressive, painful for patients, poor in prognosis, and difficult to treat [8]. Because of the potential discomfort of immobilizing patients with spine metastasis, methods for IM should be considered, especially in SRT cases where large doses with high gradients are being delivered. The use of triggered imaging is feasible for monitoring IM in SRT spine cases for patients with hardware. The calculated dose results have shown clinically acceptable differences when appropriate contours using 1 mm contour expansion from the hardware are displayed at the treatment console and the clinical team is trained to interpret the results when there are indications of patient motion during treatment.
This work showed triggered kV imaging as a powerful tool in IM monitoring for spine SRT patients with hardware. The proposed workflow was sensitive to motion >1 mm, which does not correlate to significant impact in calculated dose for patient treatment plan.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
We would like to thank Dr. Andrea Arnett and all involved in the clinical implementation of this technology in our department to continue to provide excellent patient care.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.phro.2023.100422.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Arguello F., Duerst R.E., McQueen K., Frantz C.N., Baggs R.B., Johnstone L. Pathogenesis of vertebral metastasis and epidural spinal cord compression. Cancer. 1990;65:98–106. doi: 10.1002/1097-0142(19900101)65:1<98::AID-CNCR2820650121>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 2.Pennington Z., Ahmed A.K., Molina C.A., Ehresman J., Laufer I., Sciubba D.M. Minimally invasive versus conventional spine surgery for vertebral metastases: a systematic review of the evidence. Ann Transl Med. 2018:6. doi: 10.21037/atm.2018.01.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blakaj D.M., Palmer J.D., Dibs K., Olausson A., Bourekas E.C., Boulter D., et al. Postoperative stereotactic body radiotherapy for spinal metastasis and predictors of local control. Neurosurgery. 2021;88:1021–1027. doi: 10.1093/neuros/nyaa587. [DOI] [PubMed] [Google Scholar]
- 4.Patchell R.A., Tibbs P.A., Regine W.F., Payne R., Saris S., Kryscio R.J., et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366:643–648. doi: 10.1016/S0140-6736(05)66954-1. [DOI] [PubMed] [Google Scholar]
- 5.Redmond K.J., Lo S.S., Fisher C., Sahgal A. Postoperative stereotactic body radiation therapy (SBRT) for spine metastases: a critical review to guide practice. Int J Radiat Oncol Biol Phys. 2016;95:1414–1428. doi: 10.1016/j.ijrobp.2016.03.027. [DOI] [PubMed] [Google Scholar]
- 6.Guckenberger M., Hawkins M., Flentje M., Sweeney R.A. Fractionated radiosurgery for painful spinal metastases: DOSIS-a phase II trial. BMC Cancer. 2012;12:1–9. doi: 10.1186/1471-2407-12-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sahgal A., Bilsky M., Chang E.L., Ma L., Yamada Y., Rhines L.D., et al. Stereotactic body radiotherapy for spinal metastases: current status, with a focus on its application in the postoperative patient: a review. J Neurosurg - Spine. 2011;14:151–166. doi: 10.3171/2010.9.SPINE091005. [DOI] [PubMed] [Google Scholar]
- 8.Sprave T., Verma V., Förster R., Schlampp I., Bruckner T., Bostel T., et al. Randomized phase II trial evaluating pain response in patients with spinal metastases following stereotactic body radiotherapy versus three-dimensional conformal radiotherapy. Radiother Oncol. 2018;128:274–282. doi: 10.1016/j.radonc.2018.04.030. [DOI] [PubMed] [Google Scholar]
- 9.Glicksman R.M., Tjong M.C., Neves-Junior W.F., Spratt D.E., Chua K.L., Mansouri A., et al. Stereotactic ablative radiotherapy for the management of spinal metastases: a review. JAMA Oncol. 2020;6:567–577. doi: 10.1001/jamaoncol.2019.5351. [DOI] [PubMed] [Google Scholar]
- 10.Huo M., Sahgal A., Pryor D., Redmond K., Lo S., Foote M. Stereotactic spine radiosurgery: review of safety and efficacy with respect to dose and fractionation. Surg Neurol Int. 2017:8. doi: 10.4103/2152-7806.200581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Redmond K.J., Lo S.S., Soltys S.G., Yamada Y., Barani I.J., Brown P.D., et al. Consensus guidelines for postoperative stereotactic body radiation therapy for spinal metastases: results of an international survey. J Neurosurg - Spine. 2017;26:299–306. doi: 10.3171/2016.8.SPINE16121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benedict S.H., Yenice K.M., Followill D., Galvin J.M., Hinson W., Kavanagh B., et al. Stereotactic body radiation therapy: the report of AAPM Task Group 101. Med Phys. 2010;37:4078–4101. doi: 10.1118/1.3438081. [DOI] [PubMed] [Google Scholar]
- 13.Matsuo Y., Onishi H., Nakagawa K., Nakamura M., Ariji T., Kumazaki Y., et al. Guidelines for respiratory motion management in radiation therapy. J Radiat Res. 2013;54:561–568. doi: 10.1093/jrr/rrs122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korreman S.S. Motion in radiotherapy: photon therapy. Phys Med Biol. 2012;57:R161. doi: 10.1088/0031-9155/57/23/R161. [DOI] [PubMed] [Google Scholar]
- 15.Keall P.J., Mageras G.S., Balter J.M., Emery R.S., Forster K.M., Jiang S.B., et al. The management of respiratory motion in radiation oncology report of AAPM Task Group 76 a. Med Phys. 2006;33:3874–3900. doi: 10.1118/1.2349696. [DOI] [PubMed] [Google Scholar]
- 16.Yamoah K., Zaorsky N.G., Siglin J., Shi W., Werner-Wasik M., Andrews D.W., et al. Spine stereotactic body radiation therapy residual setup errors and intra-fraction motion using the stereotactic x-ray image guidance verification system. Int J Med Phys Clin Eng Radiot Oncol. 2014;3:1. doi: 10.4236/ijmpcero.2014.31001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryu S.I., Chang S.D., Kim D.H., Murphy M.J., Le Q.-T., Martin D.P., et al. Image-guided hypo-fractionated stereotactic radiosurgery to spinal lesions. Neurosurgery. 2001;49:838–846. doi: 10.1097/00006123-200110000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Cetnar A., Ayan A.S., Graeper G., Weldon M., Woods K., Klamer B., et al. Prospective dual-surrogate validation study of periodic imaging during treatment for accurately monitoring intrafraction motion of prostate cancer patients. Radiother Oncol. 2021;157:40–46. doi: 10.1097/00006123-200110000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Chasseray M., Dissaux G., Lucia F., Boussion N., Goasduff G., Pradier O., et al. Kilovoltage intrafraction monitoring during normofractionated prostate cancer radiotherapy. Cancer Radiother. 2020;24:2 doi: 10.1016/j.canrad.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 20.Fenoglietto P., Llacer C., Riou O., Bedos L., Azria D. Online verification of intrafraction motion in VMAT stereotactic prostate treatment. Rep Pract Oncol Radiother. 2013;18 doi: 10.1016/j.rpor.2013.03.545. [DOI] [Google Scholar]
- 21.Kaur G., Lehmann J., Greer P., Simpson J. Assessment of the accuracy of truebeam intrafraction motion review (IMR) system for prostate treatment guidance. Australas Phys Eng Sci Med. 2019;42:585–598. doi: 10.1007/s13246-019-00760-7. [DOI] [PubMed] [Google Scholar]
- 22.Korpics M.C., Rokni M., Degnan M., Aydogan B., Liauw S.L., Redler G. Utilizing the TrueBeam Advanced Imaging Package to monitor intrafraction motion with periodic kV imaging and automatic marker detection during VMAT prostate treatments. J Appl Clin Med Phys. 2020;21:184–191. doi: 10.1002/acm2.12822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosario T., van der Weide L., Admiraal M., Piet M., Slotman B., Cuijpers J. Toward planning target volume margin reduction for the prostate using intrafraction motion correction with online kV imaging and automatic detection of implanted gold seeds. Pract Radiat Oncol. 2018;8:422–428. doi: 10.1016/j.prro.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Kisivan K., Antal G., Gulyban A., Glavak C., Laszlo Z., Kalincsak J., et al. Triggered imaging with auto beam hold and pre-/posttreatment CBCT during prostate SABR: analysis of time efficiency, target coverage, and normal volume changes. Pract Radiat Oncol. 2021;11:e210–e218. doi: 10.1016/j.prro.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 25.Zeng C., Xiong W., Li X., Reyngold M., Gewanter R.M., Cuaron J.J., et al. Intrafraction tumor motion during deep inspiration breath hold pancreatic cancer treatment. J Appl Clin Med Phys. 2019;20:37–43. doi: 10.1002/acm2.12577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yorke E., Xiong Y., Han Q., Zhang P., Mageras G., Lovelock M., et al. Kilovoltage imaging of implanted fiducials to monitor intrafraction motion with abdominal compression during stereotactic body radiation therapy for gastrointestinal tumors. Int J Radiat Oncol Biol Phys. 2016;95:1042–1049. doi: 10.1016/j.ijrobp.2015.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koo J., Nardella L., Degnan M., Andreozzi J., H-hM Y.u., Penagaricano J., et al. Triggered kV imaging during spine SBRT for intrafraction motion management. Technol Cancer Res Treat. 2021;20:15330. doi: 10.1177/15330338211063033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cox B.W., Spratt D.E., Lovelock M., Bilsky M.H., Lis E., Ryu S., et al. International Spine Radiosurgery Consortium consensus guidelines for target volume definition in spinal stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2012;83:e597–e605. doi: 10.1016/j.ijrobp.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 29.Tseng C.-L., Sussman M.S., Atenafu E.G., Letourneau D., Ma L., Soliman H., et al. Magnetic resonance imaging assessment of spinal cord and cauda equina motion in supine patients with spinal metastases planned for spine stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2015;91:995–1002. doi: 10.1016/j.ijrobp.2014.12.037. [DOI] [PubMed] [Google Scholar]
- 30.Stoiber E.M., Lechsel G., Giske K., Muenter M.W., Hoess A., Bendl R., et al. Quantitative assessment of image-guided radiotherapy for paraspinal tumors. Int J Radiat Oncol Biol Phys. 2009;75:933–940. doi: 10.1016/j.ijrobp.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 31.Li W., Sahgal A., Foote M., Millar B.-A., Jaffray D.A., Letourneau D. Impact of immobilization on intrafraction motion for spine stereotactic body radiotherapy using cone beam computed tomography. Int J Radiat Oncol Biol Phys. 2012;84:520–526. doi: 10.1016/j.ijrobp.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 32.Yenice K.M., Lovelock D.M., Hunt M.A., Lutz W.R., Fournier-Bidoz N., Hua C.-H., et al. CT image-guided intensity-modulated therapy for paraspinal tumors using stereotactic immobilization. Int J Radiat Oncol Biol Phys. 2003;55:583–593. doi: 10.1016/s0360-3016(02)03942-1. [DOI] [PubMed] [Google Scholar]
- 33.Agazaryan N., Tenn S.E., Desalles A.A., Selch M.T. Image-guided radiosurgery for spinal tumors: methods, accuracy and patient intrafraction motion. Phys Med Biol. 2008;53:1715. doi: 10.1088/0031-9155/53/6/015. [DOI] [PubMed] [Google Scholar]
- 34.Ma L., Sahgal A., Hossain S., Chuang C., Descovich M., Huang K., et al. Nonrandom intrafraction target motions and general strategy for correction of spine stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 2009;75:1261–1265. doi: 10.1016/j.ijrobp.2009.04.027. [DOI] [PubMed] [Google Scholar]
- 35.Hirai R, Ohkubo Y, Igari M, Kumazaki Y, Aoshika T, Ryuno Y, et al. Time dependence of intra-fractional motion in spinal stereotactic body radiotherapy. In Vivo 2021;35:2433-7. 10.21873/invivo.12521. [DOI] [PMC free article] [PubMed]
- 36.Rossi E., Fiorino C., Fodor A., Deantoni C., Mangili P., Di Muzio N.G., et al. Residual intra-fraction error in robotic spinal stereotactic body radiotherapy without immobilization devices. Phys Imaging Radiat Oncol. 2020;16:20–25. doi: 10.1016/j.phro.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keall P.J., Aun Ng J., O'Brien R., Colvill E., Huang C.Y., Rugaard Poulsen P., et al. The first clinical treatment with kilovoltage intrafraction monitoring (KIM): a real-time image guidance method. Med Phys. 2015;42:354–358. doi: 10.1118/1.4904023. [DOI] [PubMed] [Google Scholar]
- 38.Kotte A.N.T.J., Hofman P., Lagendijk J.J.W., van Vulpen M., van der Heide U.A. Intrafraction motion of the prostate during external-beam radiation therapy: analysis of 427 patients with implanted fiducial markers. Int J Radiat Oncol Biol Phys. 2007;69:419–425. doi: 10.1016/j.ijrobp.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 39.Stevens M.T.R., Parsons D.D., Robar J.L. Continuous monitoring of prostate position using stereoscopic and monoscopic kV image guidance. Med Phys. 2016;43:2558–2568. doi: 10.1118/1.4947295. [DOI] [PubMed] [Google Scholar]
- 40.Tsai J.-T., Lin J.-W., Chiu W.-T., Chu W.-C. Assessment of image-guided CyberKnife@ radiosurgery for metastatic spine tumors. J Neurooncol. 2009;94:119–127. doi: 10.1007/s11060-009-9814-7. [DOI] [PubMed] [Google Scholar]
- 41.Chang Z., Wang Z., Ma J., O’Daniel J.C., Kirkpatrick J., Yin F.-F. 6D image guidance for spinal non-invasive stereotactic body radiation therapy: Comparison between ExacTrac X-ray 6D with kilo-voltage cone-beam CT. Radiother and Oncol. 2010;95:116–121. doi: 10.1016/j.radonc.2009.12.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.