Abstract
Peptide–drug conjugates (PDCs) are the next generation of targeted therapeutics drug after antibody–drug conjugates (ADCs), with the core benefits of enhanced cellular permeability and improved drug selectivity. Two drugs are now approved for market by US Food and Drug Administration (FDA), and in the last two years, the pharmaceutical companies have been developing PDCs as targeted therapeutic candidates for cancer, coronavirus disease 2019 (COVID-19), metabolic diseases, and so on. The therapeutic benefits of PDCs are significant, but poor stability, low bioactivity, long research and development time, and slow clinical development process as therapeutic agents of PDC, how can we design PDCs more effectively and what is the future direction of PDCs? This review summarises the components and functions of PDCs for therapeutic, from drug target screening and PDC design improvement strategies to clinical applications to improve the permeability, targeting, and stability of the various components of PDCs. This holds great promise for the future of PDCs, such as bicyclic peptide‒toxin coupling or supramolecular nanostructures for peptide-conjugated drugs. The mode of drug delivery is determined according to the PDC design and current clinical trials are summarised. The way is shown for future PDC development.
Key words: Peptide, Drug, Linker, Targeted therapy, Stability, Permeability
Graphical abstract
This review summarizes the function of each component of the peptide–drug conjugate (PDC) and design factors and the desired mechanism of action.
1. Introduction
Peptides exert multifunction during human life1, such as repairing cells, improving cell metabolism, preventing the cell from degeneration, etc. Peptides have biologically active as well as have an excellent capacity for targeted transport2,3. This feature can apply not only in oncology but also in targeted therapeutics for coronavirus disease 2019 (COVID-19), diabetes, rheumatism, and rheumatoid arthritis4,5. Peptide–drug conjugates (PDCs) with equivalent potency have a much broader application than antibody–drug conjugates (ADCs) now6. In addition to cancer treatment, PDCs can be applied to many other diseases such as COVID-197. We examine the advantages and disadvantages of peptides used in PDCs to drive targeted therapy, as well as how specific tumor microenvironments can aid in the design of PDCs8. The stability, permeability, and targeting of PDCs in the body transport could be improved by chemical modifications and formulas rarely reports9, 10, 11, which is valuable for the research and development of novel drugs, providing a technical basis for the development and clinical application of PDCs12. As an anticancer drug delivery modality, PDC has the advantage of covalently modifying a ligand peptide that can target the specific cell surface receptors or biomarkers at the tumor site, exert a long-lasting function and expand the effect of time, thereby conferring an overall desirable pharmacokinetic profile. A sufficient amount of drug is delivered to the cancer site while minimizing contact with healthy tissue.
In this review, we provide an overview of the overall and each part of PDCs function for therapeutics, summarise the targets, and the way of improving PDC drug bioavailability. We also discuss changes in clinical delivery and the future direction of PDCs based on PDC design, and the latest developments in current clinical trials.
2. Targeted tactics of therapy
Worldwide, cancer is the second leading cause of death and a major public health problem13. Patients were given one or more of the following treatments depending on their stage and tumor type: surgery, radiotherapy, or chemotherapy14. In the past three years since the end of 2019, the COVID-19 epidemic has swept the world and new drug developments have emerged15. Drug therapy has different degrees of poisonous and side effects, some severe poisonous and side reactions are direct causes for limiting the dose or use of drugs. Chemotherapy inhibits cell mitosis rapidly, with serious side effects16. Even if the neoplasm is successfully eradicated, healthy tissues may keep affected by chemotherapy lasting damage17.
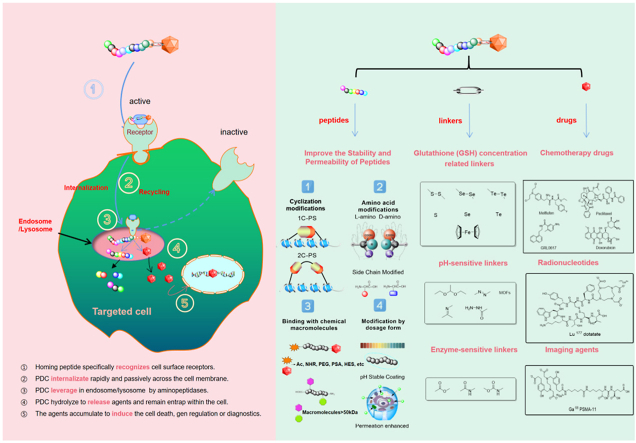
Fortunately, drug-targeted therapies can direct the characteristics of tumor cells (including cell pH, cell GSH content, cell morphology, and enzymes) to improve patient prognosis and reduce toxicity18. In cancer therapy, there are three targeted therapeutic modalities used to enhance the non-specific and antitumor activity of cancer treatment. Firstly, Targeted therapeutic agents can inhibit the expression of the protein, such as protein kinases or enzymes19; another approach is to fuse a potent molecular structure (e.g., ADC, phalloidin, or CAR-T) to an overexpressed protein on the surface of tumor cells and collaboratively inhibit tumor cell division while delivering a cytotoxic payload or stimulating a tumor-directed immune response20; the third approach is the application of PDC, which drives the accumulation of toxic payloads in tumor stem cells. The biggest challenge with transporting proteins and peptides in vivo is their instability. We should take consider molecular size, molecular charge, protein internal structure, solvent effect, lipid membrane packing and hydration, stability, affinity toward receptor and so on. Turning proteins and peptides into nanoparticles are prominent for refining delivery capacity. In ADC, the idiosyncrasy monoclonal antibody (mAb) targeting antigens expressed on cancer cells can be applied to the transportation of a cytotoxic substance, while reducing injuries to the normal cell, having better therapeutic effects, and enhancing the drug metabolism. However, mAb can cause immunogenicity. The targeting mechanism of PDC is related to many factors21. The function of receptors will determine the mechanism of action22. Similar to ADC, firstly, the homing peptide target the surface of cell membranes. triggering receptor-mediated drug endocytosis and internalization23. Then intracellular lysis leads to the release of cytotoxin24. In another situation, PDCs are not bound by receptors when lysis occurs extracellularly and then the cytotoxin internalizes in endosomes25. The homing peptides play a crucial role in the mechanism of action26,27. PDC and ADC are conceptually similar and have immensely different structures and properties. Antibodies have higher specificity and longer half-life time, whereas homing peptides have a better drug loading potency and tissue penetration capability. Moreover, the versatile linear or cyclic peptides are easier to change. The comparison of PDCs and ADCs is shown in Table 1.
Table 1.
The comparison of PDC and ADC.
| Property | PDC | ADC |
|---|---|---|
| Molecular weight | The weight of the molecule (2–20 kDa) is small, which makes it easier to penetrate the tumor stroma and enter the tumor cells | Large molecular weight (∼160 kDa) limits passive transport through epithelial cell membranes |
| Pharmacokinetic | Rapidly eliminated by the kidneys, making it less toxic to the bone marrow and liver | Non-specific uptake by the liver and reticuloendothelial system results in dose-limiting toxicity to the liver and bone marrow |
| Cost | It can be expressed in situ or chemically synthesized for simple production and easy scale-up | Relatively difficult to manufacture and costly to produce and qualify |
| Cytotoxic payload | Targeted formulations can be coupled with a variety of clinically proven cytotoxic molecules such as adriamycin, paclitaxel, camptothecin, cisplatin, and so on | Cytotoxic molecules are limited to a very few highly toxic candidates such as MMAE (monomethyl auristatin E), and DM-1 (mertansine) |
You et al.28 have reported that the PDC binding with doxorubicin (DOX), which is targeting metastatic breast cancer is cleaved by MMP-2 (matrix metallopeptidase 2) before internalization. Different pathways are involved after PDC enters the tumor environment. Adriamycin can diffuse to the membrane of tumor cells and exert its biological activity29. It is critical to consider the target receptor and influencing factors when designing PDC to ensure that the hypothetical mechanism can be realized30.
3. General consideration factor of PDC and clinical trials of PDCs
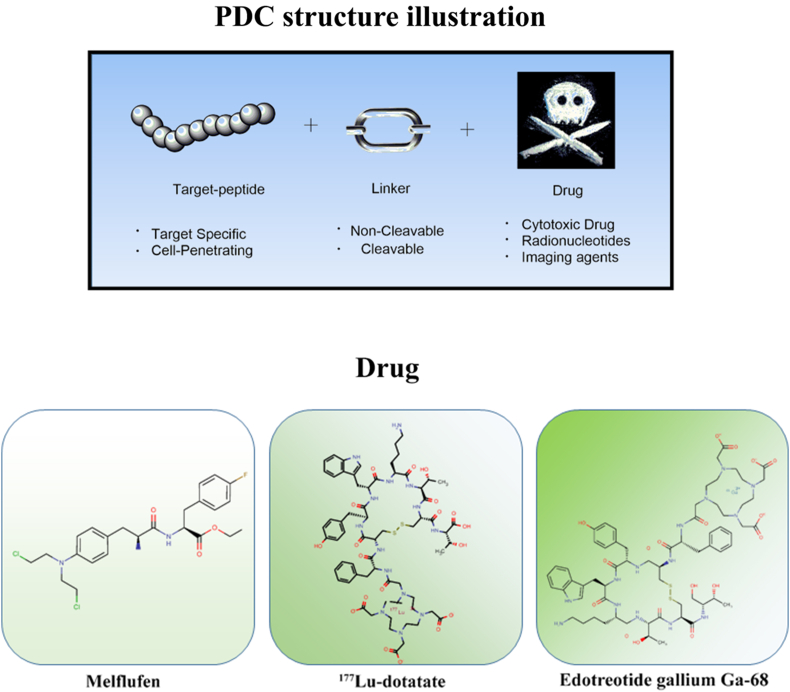
PDC is a targeted therapeutic whose function is similar to ADCs31. It is the connection between different types of peptides with drugs. PDC consists of three important components: homing peptides, linkers, and cytotoxic drugs32 (Fig. 1). All three parts synergistically deliver chemotherapeutic agents by targeting receptors on tumor cells, expanding their therapeutic effect to accommodate peptide therapies6.
Figure 1.
A schematic of a peptide–drug conjugate structure, consisting of a target peptide, linker, and drug, with different payloads.
The peptide is an important part of PDC. Bicyclic toxin peptides (BTPs), dendritic peptides, and self-assembly augment peptides have been shown as drug delivery systems. Bicyclic toxin peptides are typically composed of 9–20 amino acids and have three Cys residues33. These cys residues connect with small molecules to constrain the peptide in a rigid conformation34. The bicyclic ring can combine with transporter protein for transporting drug molecules. The larger molecular footprint allows for targeted protein interactions. It combines the advantages of the pharmacology of a biological drug and the pharmacokinetics of a small molecule drug, without immunogenicity. These PDC offer several advantages, such as tumor deeper penetration, less immunogenicity, and a faster renal clearance35. The drug is linked to the BTP, which ensures conformational stability.
In 2021, Bicycle Therapeutics (Nasdaq: BCYC) announced three investigational BTC (bicyclic peptide‒toxin coupled) drugs: BT1718, BT5528, and BT800936, 37, 38. All in phase I/II clinical development. BT1718 is a novel bicyclic peptide anticancer drug targeting membrane type I matrix metalloproteinase to release its toxic payload DM1. BT5528 has shown preliminary anti-tumor activity as a drug targeting EphA239. EphA2 (Ephrin type-A receptor 2) is a transmembrane tyrosine kinase receptor (RTK) that regulates cell migration, proliferation, and differentiation in the nervous and vascular systems40. EphA2 is overexpressed in a variety of refractory tumors, including breast, colon, uroepithelial, lung, cervical, and ovarian cancers41. BT8009, as a nectin-4 targeting drug, has demonstrated anti-tumor activity. Nectin-4 (nectin cell adhesion molecule 4), is a type I membrane protein that is overexpressed in the majority of the tumor, including uroepithelial, breast, pancreatic, and triple-negative breast cancers (TNBC), which could influence cell proliferation, differentiation, migration, and invasion42.
Dendritic polymers have been applied in many scientific fields: pharmaceuticals, nanotechnology, and biomedicine. they are characterized by their highly branched structure, which consists of several parts, including a core that exposes functional groups, a daughter, and a shell, among others. Dendritic polymers have several key properties unique, such as thermal properties, viscosity, and properties for encapsulation, which are different from linear peptides. Peptide dendritic polymers can be divided into two categories: covalent type and non-covalent type. Covalent peptide dendrimers are based on natural or non-natural amino acids; non-covalent peptide dendrimers are linked to multiple frameworks43.
The success of dendrimer peptide drug conjugates has been reported in selectively delivering DOX to cancer target sites44. PEG is a good biocompatible material in vivo because of its good solubility, less toxicity, less immunogenicity, high flexibility, and high protein fusibility. The PK properties of the dendrimer peptides were enhanced and the half-life extend by incorporating PEG into the dendrimer. The designed PEG dendrimer peptide conjugated with DOX uses a tetrapeptide (GFLG) as a linker by “click” chemistry. Overexpression of histone protease B cleaves the linker when cytokinesis, resulting in the release of the drug44. This study determined that the use of dendrimer nanoparticles in combination with DOX in mice with breast cancer in comparison with DOX, significantly reduced tumor size and the weight of the tumor decreased throughout the study, indicating that the modification of nanoparticles works in vivo.
The research of Falciani et al. demonstrated how dendrimers peptide is effective for the treatment of colorectal cancer by controlling the delivery of gemcitabine (GEM)45. Carboxymethyl chitosan/poly (amidomethylamine) (CMCht/PAMAM) dendrimer nanoparticles can form specific delivery vehicles through a short peptide conjugation with gemcitabine. YIGSR peptide conjugated dendrimer peptides are selective for HCT-116 cells with LR (Leptin receptor)46. Gemcitabine was released intracellularly which led to cell death47.
Wang et al.48 design a PDC composed of three modules: RGD targeting motif, assembly motif, and CPT molecule. This PDC binds cellular receptors and the assembly principle strategy improves PDC cell-permeability efficiency and increases intracellular drug bioavailability and therapeutic effect. and raises the maximum tolerated drug dose. which is of great significance in the future.
In the clinical trials of PDCs, two therapeutic PDCs are currently approved on the market: 177Lu-dotatate (lutathera) and melflufen. Others are in various stages of R&D (Table 2). The first PDC approved by the US Food and Drug Administration (FDA), 177Lu-dotatate, is used to treat gastrointestinal, pancreatic, and neuroendocrine tumors49. Growth hormone is an affinity peptide that can bind to the cytotoxic radiotherapeutic agent via the amide bond49. Melflufen is used for the early treatment of refractory multiple myeloma (MM)50,51, which can rapidly release alkylating agents to tumor by aminopeptidase in the cell50. However, in October 2021, The company oncopeptides AB announced the decision of withdrawing melflufen from the US market due to the failure of the phase III clinical trial because unsuccessfully reducing the risk of death in people with relapsed refractory multiple myeloma (HR = 1.104). Cybrexa’s CBX-12 was developed based on the alphalex™ technology platform. The alphalex technology is a drug delivery platform that allows the entry of the anti-cancer drug into the body without binding to cell surface antigens and directly into cancerous cells. The treatment process involves receiving the drug into the body, adjusting the pH environment in the body, loosening the cellular structure in a low pH environment, and delivering the drug into the cells. Alphalex is a therapeutic solution for patients who take drugs periodically and prevents cellular resistance52. The technology consists of pHLIP® peptides, linkers, and small molecule drugs53. pHLIP® peptides were first developed by Yale University and the University of Rhode Island. In April 2021, CBX-12 has received IND approval for the treatment of advanced solid tumors in phase I clinical trials. SNG1005 is in phase III clinical in China. It is the fastest progressing PDC program in China. With the core technology platform, Coherent Biopharma has developed a series of anti-tumor conjugated drugs. Among them, CBP-1008, a dual-targeted-ligand conjugation, is the most recent and is currently in phase Ia. Many PDC products are still in the preclinical stage. PDCs targeting GPCRs is currently seeking for an angel round funding to support the clinical development of Tye-1001 and Tye-1002. The primary product, peptide-coupled drugs targeting CXCR4, MB1707, is expected to initiate clinical trials in China and USA. While another product, MB010, is expected to expand therapeutic options in combination with the PD-L1 drug this year. In May 2022, innovative drug TH1902 announced phase 1b clinical trial in solid tumors.
Table 2.
Various types of peptide–drug conjugates in clinical trials and approved for marketing.
| Name | TTP | Payload | Linker | Indication | Development phase | Clinical trials registry | Ref. |
|---|---|---|---|---|---|---|---|
| ANG1005 | Angiopep-2 | Paclitaxel | Succinic acid | Leptomeningeal metastases | Phase III | NCT03613181 (2021) | 135,136 |
| Glioma Glioblastoma brain tumor, recurrent |
Phase II | NCT00539344 (2014) | 137 | ||||
| Breast cancer brain metastases | Phase II | NCT02755987 (2016) | 138 | ||||
| NCT01967810 (2020) | |||||||
| Advanced solid tumors with and without bain metastases | Phase I | NCT02048059 (2020) | 139 | ||||
| NCT00539383 (2016) | |||||||
| GRN1005 | Angiopep-2 | Paclitaxel | Succinic acid | Breast cancer brain metastases; non-small cell lung cancer (nsclc) with brain metastases | Phase II | NCT01480583 (2016) | 89,140 |
| NCT01497665 (2019) | |||||||
| NCT01679743 (2019) | |||||||
| BT1718 | MT1-MMP binder | DM1 | Disulfide | Advanced solid tumours non-small cell lung cancer non-small cell lung sarcoma oesophageal cancer |
Phase I/II | NCT03486730 (2020) | 38 |
| BT5528 | EphA2 binder | MMAE | Amide | Solid tumours EphA2-positive NSCLC |
Phase I | NCT04180371 (2021) | 36,39,141 |
| BT8009 | Nectin-4 binder | MMAE | Amide | Solid tumors | Phase I | NCT04561362 (2021) | 142,143 |
| TH1902 | TH19P01 | Docetaxel | Succinic acid | Solid tumors | Phase I | NCT04706962 (2021) | 144 |
| TH1904 | TH19P01 | Doxorubicin | Succinic acid | Solid tumors | None | None | |
| G-202 (mipsagargin) | DγEγEγEγE | Thapsigargin | Amide | Solid tumors | Phase II | NCT02381236 (2017) | 145 |
| NCT02067156 (2017) | |||||||
| NCT02607553 (2017) | |||||||
| NCT02876003 (2017) | |||||||
| NCT01777594 (2016) | |||||||
| NCT01056029 (2015) | |||||||
| NGR015 (NGR-hTNF) | CNGRCG (1,5 SS) | hTNF | Amide | Malignant pleural mesothelioma | Phase III | NCT01098266 (2019) | 146 |
| tTF-NGR | GNGRAHA | tTF | Amide | Malignant solid tumors lymphomas | Phase I | NCT02902237 (2020) | 147 |
| PEN-221 | fCYwKTCC (2,7 SS) | DM-1 | Disulfide | Neuroendocrine tumors carcinoma, small cell lung | Phase I/II | NCT02936323 (2021) | 148,149 |
| Zoptarelin doxorubicin | d-Lys6-LHRH | Doxorubicin | Amide | Pre-treated advanced/metastatic recurrent endometrial cancer | Phase III | NCT01767155 (2020) | 150 |
| CBP-1008 | CB-20BK | MMAE | Amide | Advanced solid tumor | Phase I | NCT04740398 (2022) | 151 |
| CBP-1018 | LDC10B | MMAE | Amide | Lung tumor | Phase I | NCT04928612 (2022) | 151 |
| SOR-C13 | folate | MMAE | Amide | Advanced malignant solid neoplasm | Phase I | NCT03784677 (2021) | 152 |
| NCT01578564 (2016) | |||||||
| Melflufen (delisted) | Flufenamide | Melphalan | AcOH | Multiple myeloma | Approved for marketing | NCT04534322 (2021) | 153,154 |
| 177Lu-dotatate (Lutathera) | Tyr-3-octreotate | 177Lu | DOTA | Neuroendocrine tumors | Approved for marketing | NCT03325816 (2021) | 155,156 |
| NCT01456078 (2019) | |||||||
| NCT01578239 (2017) | |||||||
| NCT02125474 (2019) | |||||||
| 177Lu-PSMA-617 | Glu-urea-R | 177Lu | DOTA | Prostate cancer | Phase I | NCT05079698 (2021) | 157 |
| [18F]AlF-NOTA-octreotide | octreotide | 18F | NOTA | PET or GEP-NETs; Neuroendocrine tumors | Phase I/II/III | NCT03511768 (2018) | 158,159 |
| NCT04552847 (2020) | |||||||
| NCT03883776 (2020) | |||||||
| [18F]Fluciclatide | RGD | 18F | PEG | PET imaging | Phase II | NCT00918281 (2014) | 160,161 |
| NCT00565721 (2014) | |||||||
| [18F]RGD-K5 | cyclo (RGDfK) | 18F | NOTA | PET imaging | Phase II | NCT00988936 (2012) | 162, 163, 164 |
| NCT02490891 (2018) | |||||||
| NCT03364270 (2020) | |||||||
| 68Ga-NODAGA-E [cyclo (RGDyK)]2 | E [cyclo (RGDyK)]2 | 68Ga | NODAGA | PET imaging | Phase II | NCT03445884 (2020) | 165,166 |
| NCT03271281 (2021) | |||||||
| 68Ga-NOTA-BBN-RGD | cyclo (RGDyK) and BBN | 68Ga | NOTA | PET/CT and PET imaging | Phase I | NCT02749019 (2016) | 167,168 |
| NCT02747290 (2016) | |||||||
| 90Y-DOTATOC | 3Tyr-octreotate | 90Y | DOTA | PRRT | Phase II | NCT03273712 (2019) | 169 |
| 99mTc-3PRGD2 | 3Tyr-octreotate | 99mTc | 3PRGD2 | Breast cancer SPECT/CT scan |
Phase I | NCT02742168 (2020) | 170,171 |
| NCT02615067 (2019) | |||||||
| 111In-DTPA-octreotide | 3Tyr-octreotate | 111In | DTPA | Brain and central nervous system Tumors PET imaging |
Phase I | NCT00002947 (2014) | 172 |
These PDCs mentioned above are therapeutic agents, however, through the coupled of radionucleotides, PDCs can also act as diagnostic agents49. It is critical to understand the role of each component when designing a PDC. The considered design includes the mechanism of action and alternative approaches which can ameliorate current limitations54.
4. Peptides in PDCs
The choice of peptide affects the efficiency of drug endocytosis in PDCs, once the target has been selected, the selection of a suitable peptide for the PDC is also important and has a significant impact on efficacy, pharmacokinetic/pharmacodynamic profile, and therapeutic indices. The ideal peptide for PDCs should have a strong target binding affinity, high stability, low immunogenicity, efficient internalization, and a long plasma half-life.
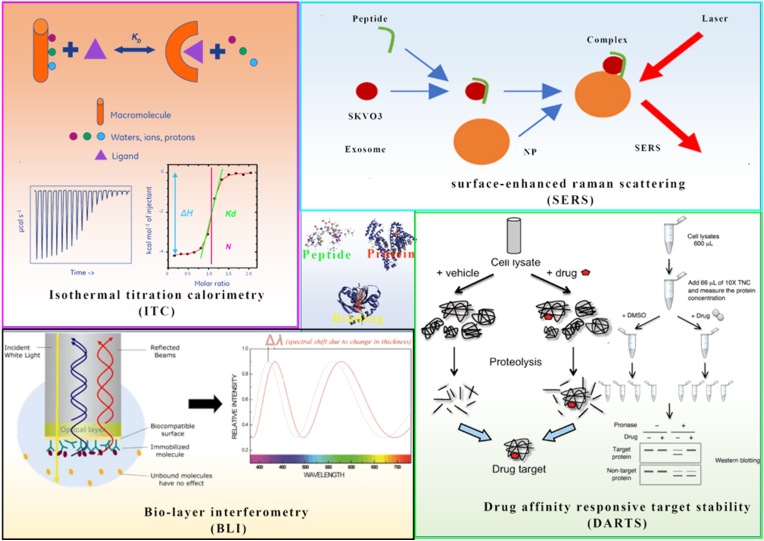
Homing peptides are specific target overexpressed protein receptors in tumor tissue55. It direct transmitted the loading drug to the target cell, limiting chemotherapeutic agent delivery off-target56 (Table 2). These homing peptides have previously been reported to have a high binding affinity for target sites in nanomolar concentrations57. To determine their binding affinity, a variety of techniques can be used, including surface-enhanced raman scattering (SERS)58, bio-layer interferometry (BLI)59, isothermal titration calorimetry (ITC), and drug affinity responsive target stability (DARTS) (Fig. 2).
Figure 2.
Homing peptide and target binding affinity assay technique.
The homing peptide’s secondary structure influences their binding affinity significantly. As a result, it is critical that linkers improve homing peptide binding affinity by stabilizing the secondary structure60. When the linker binds to the chemotherapeutic drug, the secondary structure of the homing peptide must be maintained. A homing peptide database by Kapoor et al.61 details a variety of homing peptides for many targets (https://webs.iiitd.edu.in/raghava/tumorhope/).
In addition to their targeting properties, some peptides also act as cell-penetrating peptides (CPPs), which are summarized on the following website (http://crdd.osdd.net/raghava/cppsite/)62,63. These peptides exhibit some properties such as hydrophobicity, amphiphilicity, and a negative charge that facilitates across cell membranes64. The cell-penetrating peptide not only transmits drugs to the targeted tissue but also allows cell internalization. CPPs with positive charge have disadvantages, such as the unstable of target selectivity, resulting in non-specific cellular uptake. Therefore, anionic CPPs are often used in PDC to improve the specificity of tumor cells65.
Peptides are ideal carrier molecules as they have the same ability as monoclonal antibodies. They have a high affinity for receptors overexpressed on the surface of tumor cells without exhibiting the disadvantages of mAb. However, the binding of the payload to the peptide molecule is particularly critical as the spatial structure of the payload affects receptor binding and selectivity, thus interfering with receptor recognition. Therefore, an understanding of peptide‒receptor interactions is required for rational drug selection. Amino acids such as lysine, cysteine, glutamate, and serine that are not involved in receptor recognition can be used directly for payload binding on the side chain or unwanted sites can be replaced by these amino acids to introduce possible modification sites. Many peptides also allow for simple N-terminus modifications as their N-terminus is not involved in receptor recognition. Ideally, peptide carriers contain multiple modified side chains so that multiple payloads can be incorporated into each peptide molecule, allowing for increased concentrations of drug at the tumor site and thus enhanced therapeutic efficacy.
AI and peptide libraries could be used in innovative peptide screening, which offering unique advantages in activity, druggability, and safety, deciding different routes of administration and clinical outcomes in various molecular forms for a better clinical use. Peptide libraries are constructed using overlapping or non-overlapping peptides with target antigen fragments, called peptide library methods or array-based epitope mapping, from which fragments with high affinity for the target antibody are selected to analyze the conformation of the antigen and antibody binding site66,67. Peptide discovery can also use AI autonomous algorithms to find peptide molecules with high activity and specificity for specific targets and to design and optimize their structure and sequence for the characteristics of the drug to obtain better cell penetration, structural stability, and longer half-life, thus greatly improving its drug-forming properties68. We can establish a mature AI biologic drug discovery tool and platform with deep integration of efficient predictive algorithms, experimental data, and experience for peptides of expert, recombinant proteins, small proteins, antibodies, and other biologic drug discovery areas.
4.1. The pharmacokinetics of peptides
It is critical to study pharmacokinetics (PK) and pharmacodynamics (PD) when designing drugs69. Traditionally, peptides and small molecules have significantly different pharmacokinetic properties70. One limitation of these peptides is their low bioavailability and drug intake, unfortunately, these properties make oral administration of peptides impossible. However, oral administration is one of the most convenient methods, by which the patient is more likely to comply with medication71 (Fig. 3).
Figure 3.
The properties of the peptide in vivo and in vitro with ADME.
Peptides have a shorter half-life and circulating than biomacromolecule, which needs frequency dosing, and caused the slow pace of research in both ADC and PDC72. The half-life of hydrophilic peptides is determined by several soluble enzymes in the blood and on the cell membrane. Exopeptidase is among the most predominant soluble enzymes. which is related to peptide catabolism and plasma chemical instability. Exopeptidases are classified into two types: aminopeptidases and carboxypeptidases, which respectively target the N- and C-terminal73.
The rapid renal clearance rate and short half-life hinder the study of peptides in vivo, and the factors affect their properties of drug forming. The glomerular capillaries in the kidney are approximately 8 nm. Peptides molecular-weight less than 25 kDa are filtered out from the glomerulus and are not reabsorbed by the tubules70. Formulations can have an ameliorative effect in this context and have been successfully developed in the new drug semaglutide, FDA recently approved the oral peptide N-[8-(2-hydroxy benzoyl)aminocaprylic acid] (SNAC) as an adjunct to albiglutide for the treatment of type 2 diabetes71. SNAC acts as a buffer in the stomach and reduces the activity of proteolytic enzymes. Although semaglutide is approved for use by FDA, there is still a long road for the delivery of oral peptides before clinical application74.
Several approaches improve the ADME properties of peptides, such as increasing cell permeability, enhancing chemical stability, the resistance of protein hydrolysis, and reducing renal clearance, leading to a prolongation of the circulating half-life75. Half-life extension is beneficial for economic rationality and patient compliance. Based on this feature, there can be a large scope for dose adjustment for PDC administration.
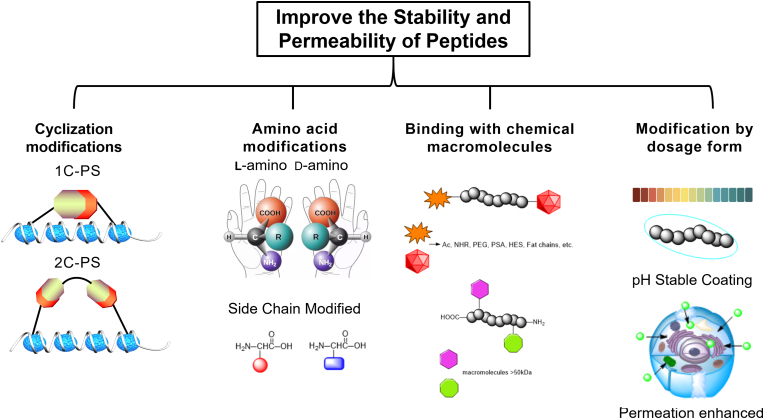
4.2. Improvement of peptide stability and cell permeability
4.2.1. Cyclization modification
Cyclization reactions are widely used in peptide synthesis and include head-to-tail cyclization, head-to-tail cyclization, side-chain cyclization, and side-chain and side-chain cyclization76. Peptide anastomosis is often used to determine the secondary structure of peptides, such as α-helix and β-sheet, which can improve the binding affinity of peptides to the target and increase their ADME77.
Peptide stapling (PS) has two subtypes: single-component PS (1C-PS) and two-component PS (2C-PS)78. In 1C-PS, usually depending on the higher-order hierarchical structure, the presence of intramolecular bonding between non-natural amino acid side chains can be cyclized. The first example of 1C-PS is the closed-loop complexation (RCM) of Blackwell and Grubbs using O-allyl serine residues79. The first stapling peptide, ALRN-6924, has entered clinical trials and completed phase I. Recently, the focus on stapling peptides has shifted from 1C-PS to 2C-PS. 2C-PS performs late modifications to the binding peptide when needed, and 2C-PS developed by Christian Heinis and Greg Winter has been widely used in bicyclic peptide therapy33.
The bicyclic structure is formed by the cross-linking between three cysteine residues and three functionalized linker groups. Its emphasis on the advantage of a low “blood-to-tumor ratio”, thus, by increasing a few times the dose of PDC, the molar dose of the chemotherapeutic drug can reach similar levels; at the same time, due to the targeted of the peptide, the chemotherapeutic drugs can reach the tumour site precisely, it is promising to improve the therapeutic effect and reducing toxic side effects.
4.2.2. Amino acid modifications
One way to increase peptide stability is to use d-amino acids instead of l-amino acids. Amino acid sequence substrate recognition and binding affinity are reduced for proteolytic enzymes70,73. The octreotide, a peptide for the treatment of gastrointestinal tumors80, two amino acid sites are replaced by the d-amino acids, The final half-life of octreotide has increased from several minutes to 1.5 h, resulting in the improvement of PK properties81. Although this example highlights the benefit of d-amino acid substitution, in some cases, d-amino acid peptides have a shorter half-life than the l-amino acid peptides70. In some cases, a strategy for targeted conjugation through the incorporation of unnatural amino acids (UAAs) to explore the effects of epidermal growth factor receptors (EGFR). It is shown that protein stability and targeting delivery is enhanced by tuning EGFR ligands and aggregating these ligands82. At the same time, a chemical approach was explored based on highly efficient conserved sites of the protein, the use of epitope mimicry to achieve fully synthetic peptides, which mimic amino acid sequences that inhibit their biological activity when bound to antibodies83,84. They also have a high bioavailability, and stablility in vivo. It is important to consider these types of modifications may affect the higher-order structure of the peptide and intramolecular or intermolecular interactions.
4.2.3. The modification of binding with chemical macromolecules
The charge of the peptide is related to renal clearance. Negatively charged peptides have longer half-lives than positively charged peptides. The presence of anionic charges on the glomerular inner membrane limits the filtration of anionic compounds in urine. The binding of peptides which has a larger molecule’s weight (>450 kDa) can increase peptide lipid solubility. In addition, binding with albumin could enhance the site-blocking, preventing peptides from being filtered out through the kidney with longer periods of blood circulation. Typically, peptide chains catabolize the active site by exonucleases in the N- or C- terminus. Changes in the N- and C-terminal lead to decreased renal clearance22. There are several ways to increase hydrolytic resistance by modifying N- or C- termini. Such as cyclic amide binds the C- and N-termini of the peptide together to prevent enzymatic degradation, enhance enzymes hydrolysis resistance73 and prolong the half-life. Furthermore, N-methylation of the amide bond can also enhance metabolic resistance85.
Polyethylene glycol (PEG) is a candidate for peptide modification: inexpensive, high bioavailability, bioaccessibility, and non-immunogenic. HM-3 peptide has a short half-life and requires twice-daily dosing, which makes it need to extend the half-life to prolong the intracellular drug retention time. mPEG-Ald (methoxy-poly (ethylene glycol)-aldehyde) is the preferred PEG-modified linker to the N-terminal. With this modification, the half-life of the HM-3 peptide has been extended by 5.86-fold86,87. Another PEGylated peptide is Bayer’s PEG-adrenomedullin (PEG-ADM), which is used to treat patients who have acute respiratory distress syndrome (ARDS) with less ability to breathe88.
Other widely used macromolecules for peptide modification are polysialic acid (PSA) and hydroxyethyl starch (HES)89. Modifying the fat chains can also extend the half-life of peptides90. Glucagon-like peptide (GLP-1) receptor agonists have been used to control blood glucose levels in patients with type 2 diabetes. For example, exenatide is administered twice daily with a half-life of 30 min91. Liraglutide is an analog of human GLP-1 which has a fatty acid chain that is attached to the peptide backbone to bind albumin. It exhibits a significant improvement of GLP-1 and increased intravenous half-life of 8–10 h, administered once daily92. The semaglutide is also an agonist of GLP-1 with a γGlu-2xOEG linker to the C18 fatty acid chain. Studies in animal minipigs demonstrate that the semaglutide contributed a significant improvement with a half-life of 46.1 h, allowing for weekly dosing, compared to earlier GLP-1 agonists93.
4.2.4. Modification by dosage form
Intracellular protein delivery systems generally rely on the fusion of genetic proteins with membrane-penetrating tags and protein-encapsulating carriers based on cationic liposomes, polymers, and inorganic nanomaterials94. Several approaches have been reported to enhance the oral bioavailability of peptide therapeutics through dosage forms, such as penetration enhancers and acid-stable coatings95,96. Penetration enhancers can transport peptides across epithelial cells by interfering with cell-to-cell junctions and adhesion proteins. Another way for improved oral utilization of drugs using acid-stabilized coatings which are pH-sensitive and able to remain intact at low pH levels in the stomach. Conversely, when they are transported to the colorectal, the pH increases, causing coating ruptures and the releasing of the inclusions. Citric acid can help to neutralize the optimal synergies with multiple gastrointestinal peptidases at alkaline pH to delay peptidase-induced degradation.
The dosage form also increases the bioavailability of intravenous polypeptides. For example, in the FDA-approved Sandostatin LAR, the octreotide is encapsulated in a dextrose-poly (lactide) star polymer (Glu-PLGA)97, making polypeptides into hemi-solvent form. The use of hemi-solvents showed varying degrees of PLGA solubility due to the L:G ratio. The lactic acid selectivity of this hemi-solvent allows for the deconstruction and analysis of complex PLGA formulations. Hemi-solvents can be used as quality control and reverse engineering tools for pharmaceuticals containing PLGA polymer mixtures. Thus, improving the overall chemical and enzymatic stability of peptides through these approaches may reveal new therapeutics, including targeted therapies such as peptide–drug conjugates98. The four ways of modifying PDC stability and cell penetration in this section are shown in Fig. 4.
Figure 4.
Different methods used to improve the stability and permeability of peptides.
5. Linker in PDCs
The choice of the linker is one of the key factors in the design of a PDC and needs to take into account the microenvironment in which the PDC is located so as not to interfere with the binding affinity and drug efficacy of the peptide to its receptor. There are different types of linkers used in PDCs depending on their length, stability, release mechanism, functional groups, hydrophilicity/hydrophobicity, and other characteristics99. Linkers used in PDCs must exhibit stability to prevent premature and nonspecific drug release. The linker is one of the keys to drug stability and treatment window. Firstly, the linker needs to have a certain level of stability so that it can ensure the integrity of the PDC during circulation before it reaches the tumor cells, avoiding the early release of toxin drugs leading to off-target toxicity and affecting the treatment window of the PDC. And after entering the target cells, the linker has to ensure the effective release of the toxin drug to play its killing effect.
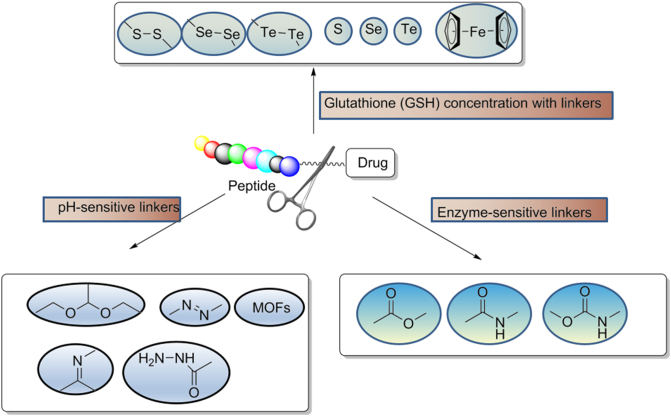
Linkers can be cleavable or non-cleavable. Cleavable linkers can be cleaved enzymatically or chemically. Among these, the chemically cleavable linkers include several chemical motifs, such as a pH-sensitive linker, ‒S‒S‒ linker, and cleave the linker by exogenous stimuli100 (Fig. 5). Usually, endogenous stimuli are easier to control and have the advantage that in vivo biological activity should not affect the cleavage rate of the linker, and the cleavage effect persists when the concentration of the endogenous trigger substrate is low. Detailed reviews on various aspects of cleavable linkers were published by Alas et al.99 and Liang101 in 2021 and 201899,101. The non-cleavable linkers cannot be activated by external stimuli. The non-cleavable linkers are worked after peptide metabolism release the payload. Although cleavable linkers are better in developing targeted therapeutic agents, non-cleavable linkers are more stable in metabolic cycling in vivo. The choice of cleavable or non-cleavable linker depends on the design of the targeted therapeutic agent and the needs of the mode of action102.
Figure 5.
The chemical structures of different linker chemistries are shown with the plausible method of release of the drug from PDC after cellular uptake or under in vivo conditions.
5.1. Non-cleavable linkers
Chemical components such as succinimidyl thioethers, oximes, and triazoles have been used in non-cleavable linkers to bind chemotherapeutic drugs to target peptides103, 104, 105. However, there are chemical groups present in the linker that link not only the drug, but also the peptide, non-cleavable functional groups existing in the middle of the linker as well. After PDC administration, the most unstable site is cleaved first and becomes a mixture including unmodified drugs, drugs with partial linkers, or linkers with amino acids. The advantage of non-cleavable linkers is that these may not cleave in the plasma as other linker chemistries do and release some drugs prematurely in the plasma before reaching the target cancer site.
5.2. GSH concentration-sensitive linkers
GSH concentration in the tumor medium is 4 times that of normal cells106. Disulfide bond linkers are very stable in the blood and have tumor specificity. Thus, GSH concentrations are also tumor-dependent. The colorectal cancer has GSH levels in the range of 90 nmol/mg compared to normal GSH levels of 10–20 nmol/mg106. Tumor cells cause oxidative stress, which results in high GSH levels and promotes payload release. Glutathione cleaves a variety of chemical bonds, including disulfides, thioethers/selenium/tellurium, diesel/disulfides, ferrocene, and metal thiols107,108.
5.3. pH-Sensitive linkers
The pH value of healthy human tissue and cells is 7.4, whereas around 6.8 for the tumor microenvironment. Lysosomes in tumor cells are acidic organelles which pH values ranging from 4.7 to 5.0109. Intracellular hypoxia generates energy via anaerobic glycolysis and produces lactic acid, which causes pH value to decrease. Numerous chemical bonds are pH-sensitive, including acetals, hemiacetals, imines, hydrazines, and various metal–organic frameworks (MOFs). In general, they are stable in the normal body environment but hydrolyzed at an acidic pH value110.
The chemically cleavable linkers rely on lysosomal pH. The N-acyl hydrazine linker induces drug release directly. This linker could cleave in lysosomes and is successfully applied in Mylotarg (gemtuzumab ozogamicin, Pfizer) approved by FDA111. It has shown good stability in vivo and in vitro in mice at pH = 4.5–7.4112. Hydrazone bond is also pH sensitive. Structurally stable at neutral pH in the bloodstream, while it is hydrolyzed to release payload in acidic cellular compartments such as lysosomes (pH 4.8) or late endosomes (pH 5.5–6.2). However, the junction is not stable during circulation, is slowly hydrolyzed in the blood, and shed toxin drugs. So its application is currently limited mainly to hematologic tumors. The linker technique has been applied successfully as mentioned above. However, the linkers field has shifted from acid-cleaved to enzyme-cleaved113 (Table 3).
Table 3.
pH of each part of the tumor microenvironment and its corresponding pH-sensitive structure, pH-sensitive carrier function.
| Tumor microenvironment pH | pH-Sensitive groups | pH-Sensitive structure | pH-Sensitive carrier function | ||
|---|---|---|---|---|---|
| Extracellular tumors | pH 6.5–7.2 |
|
|
|
|
| Intracellular tumors | Endosome: pH 5.0–6.5 Lysosomes: pH 4.5–5.0 |
|
|
|
|
5.4. Enzyme-sensitive linkers
Proteases are involved in many biological functions in the human body. Their dysregulation leads to cancer, hematological disorders, and neurodegenerative diseases. The proteases family which was found in cancers is matrix metalloproteinases (MMPs), a class of lysosomal proteases114. Elevated levels of MMPs have been observed in the tumor microenvironment in a variety of cancers. Therefore, MMPs are an ideal stimulator to initiate chemotherapeutic drug release in targeted therapies115. There are limitations to the application of MMPs, so designing PDC targeting selective is challenging. Other proteases trigger targeted releases, such as histone B and cardamom proteases. They lead to cancer progression, recolonization, and invasion116. And the proteases, especially histones, are overexpressed in specific cancers. For example, histones L, K, and D overexpress in breast cancer, while histone E overexpresses in pancreatic and gastric tumors. These proteases are not only overexpressed in cancer, but also overexpressed in a variety of other diseases, such as Alzheimer's disease and atherosclerosis. The acidic environment prevents disease irreversible denaturation, which facilitates the activation of drug release.
Enzyme-cleaved linkers are a common choice applied to ADCs and PDCs, which the drug selectively released at the target protein site. Dipeptides are widely a practical substrate for enzyme cleaved linkers: such as Val–Ala and Val–Cit show good stability in circulation. Cleavage of the dipeptide occurs in the presence of histone or carboxylesterase 1 (CES-1) in mouse plasma117. The cleavage subsequently triggered the drug release is also through para-aminobenzoate (PABC)118. However, the cleaving of linkers in mouse plasma caused by hydrolysis has led to preclinical problems in vivo studies. The efficiency of in vivo studies was further compromised.
The latest advance in cleavable linkers is aryl sulfate linkers cleaved by lysosomal sulfatases119. After cleaving, the aryl sulfate linker will release the unmodified drug by 1,6-elimination. This linker has high stability and hydrophilicity in human and mouse plasma120 (Table 4).
Table 4.
Types of enzymes aberrantly expressed in tumor tissues and their specific cleavage substrates, corresponding vector functions.
| Types of enzyme | High expression site | Specific cleavage substrate | Carrier design and function |
|---|---|---|---|
| Matrix metalloproteinases (MMP-2 and MMP-9 also known as gelatinase class) | Tumor extracellular microenvironment | GPLGIAGQ XGPLGV.PVGLIG |
|
| Prostate-specific antigen (PSA) | The extracellular microenvironment of prostate cancer | MuHSSKLQL.AcOmASKLQSL AcHypSSChgQSSP |
|
| Tissue proteinase B (CaB) | Tumor cell endosomes | GFLG |
|
| Phospholipase A2 (PLA2) | Tumor extracellular microenvironment or site of inflammation | DPPC, DSPE, DSPC, POPC, PLA, PCL, etc. |
|
| α-Amylase | The extracellular microenvironment of the tumor or necrotic tumor area | Catalyzes the formation of small glucose molecules from polysaccharides. For example β-cyclodextrin, glycolic acid, etc. | the enzymatic action of drug release |
| Lysyl oxidase (LOX) | Highly expressed in the tumor extracellular matrix (ECM) | Antibody | Targeting LOX in the extracellular matrix of tumors, thereby altering the structure of ECM and inhibiting tumor growth and invasion |
| β-Glucuronidase | Necrotizing tumor area | Glucuronide | The enzymatic action of drug release |
| Fibroblast activation protein (FAP) | Cell-associated fibroblasts (CAF) surface | Hydrolysis of the N-terminal closed pro-X bond | Targeting CAF, killing the microenvironment on which tumor cells live, and thus killing tumor cells |
6. Drugs in PDCs
Toxin drugs are integral to tumor-killing. After the PDC enters the cell, the toxin drug is the primary agent that ultimately causes the death of the target cell; therefore, the toxicity and physicochemical properties of the toxin drug can directly affect the ability of the drug to kill the tumor and therefore the efficacy. Cytotoxins for coupling must have four requirements as followed: a clear mechanism of action, a small molecular weight, high cytotoxicity, and retained anti-tumor activity after chemical coupling to the peptide121.
Each drug often presents its limitation, such as undesirable PK properties. However, the non-selectivity of the drug is the biggest drawback, causing serious side effects. The peptides allow for specific targeting and therefore broaden the therapeutic field. As the chemotherapeutic drug attaches to the peptide, it is often required an increased dose for delivery to compromise the cytotoxic dose decreasing. There are many criteria to determine the cytotoxicity of PDCs, such as circulatory stability in vivo, high efficiency of drug effect, and the presence of viable ligand sites to connect to the linker. The chemotherapeutic agent of choice typically has a low IC50, usually in the nanomolar range. Chemotherapeutic agents in PDC include adriamycin, paclitaxel, Zoloft Melphalan, etc.98, also includes radionuclides, such as the 177Lu-dotatate49.
Drugs in the PDC are not only therapeutic agents but also imaging agents, such as In-DTPA-octreotide (octreoscan), the first FDA-approved PDC containing a radioactive nucleotide, which is primarily used for diagnosis. The radioactive nucleotides allow imaging of tumors by various techniques to determine the precise location of the tumor because homing peptides bind specifically to receptors on the target, and ectopic targeting is very rare. PET imaging is possible when the binding is labeled with positron-emitting radioisotopes, such as gallium (68Ga), copper (64Cu), and fluorine (18F)122. Single-photon emission computed tomography (SPECT) imaging can also be used for tumor visualization when using gamma-emitting radioisotopes, such as iodine (123I) and Tech99m (99mTc)123, 124, 125. Bifunctional chelators are often used to imaging agent: including 1,4,7,10 tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) and diethylenetriaminepentaacetic acid (DTPA)125. The virus inhibitor, GRL0617, can also be linked to peptides to treat COVID-19, which provides promising opportunities for antiviral drug design7.
7. PDC stability
Similar to peptides, the main disadvantage of PDC is the undesirable circulatory stability and rapid renal clearance. PDC should be stable within the circulation to prevent pre-release chemotherapy and systemic exposure. Nanoparticles have been investigated to enhance PDC stability now. One way to overcome poor cycling stability is to combine PDCs with gold nanoparticles (AuNPs). Overall stability can be improved due to their desirable physicochemical properties, safety, relative ease of synthesis, and long-circulating half-life. Kalimuthu et al. have reported an increase of 90-fold in PDC circulating half-life when PDC developed for the treatment of murine lymphoma cells were coupled with PEG-coated AuNP to produce selective PEG-AuNP-PDC126. Zhang and his colleagues127 have recently demonstrated that nanoparticles improve the stability of pre-drug PDCs with a bifunctional approach. The design principle is based on a non-invasive anti-tumor therapy via near-infrared light called photothermal therapy (PTT), The enhancement of the PK properties of PDC by nanomaterials is an ongoing area of research and makes PDC great potential for clinical trials128.
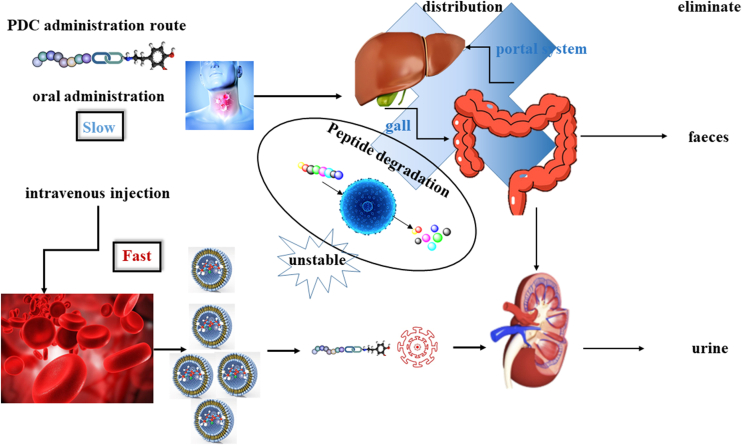
8. PDC administration route
PDC is not oral and must be given to patients via intravenous injection, which is similar to ADCs. The delivery system for the peptides and proteins requires more research. Recently, Liu et al.129 have published a novel method for delivering peptides by the oral route using the peptides self-assembly method to create pectin-dihydroartemisinin/hydrooxycampothecin nanoparticles (PDC-H-NPs). The PDC-H-NPs were combined with a hydrophilic pectin and anticancer drugs dihydroartemisinin or hydroxycamptothecin, which could increase drug loading, improve water solubility, and achieve drug release (Fig. 6)130. Ezhilarasan et al. found the route of administration, size, pharmacokinetic properties, immune clearance and so on hamper nanomedicines' clinical application. they focus on the benefits, avenues and challenges of nanoparticle-based oral systems for lung cancer treatment131. Casazza et al.132 focus on the characterization of PhAc-ALGP-Dox, a targeted tetrapeptide prodrug with a novel dual-step mechanism, designed to circumvent Dox-related toxicities and coupling to the phosphonoacetyl (PhAc)-capped tetrapeptide forms a cell-impermeable, inactive compound, PhAc-ALGP-Dox. Cardoso et al.133 address advances in the principles of dosage form selection, employing lipid-based nanoparticles and cell-produced exosomes as drug delivery systems. In the future, oral administration may be a new strategy for using PDC candidates in clinical trials134.
Figure 6.
The relationship between the PDC delivery route and drug stability in vivo.
9. Conclusions
PDC is a combination of the peptide and chemotherapeutic agent, combining the selectivity of a peptide with the lethality of a chemotherapeutic agent. By modifying the amino acid sequence of the peptide, PDC can change the hydrophobic and ionizing properties of the conjugate, solving the problems of poor water solubility and untimely metabolism, while promoting cell permeability and avoiding the high wear and tear of small molecule drugs in clinical development due to their poor physical–chemical properties. In addition, PDCs with lower molecular weight can be more easily purified, which is essential in pharmacokinetics.
Peptides are currently deficient in clinical compared to small molecule and biologics, however, they exhibit excellent versatility. Peptide conjugated drugs can enhance tumor cell permeability, reduce immunogenicity, and cut down the cost. Although peptides have a smaller molecular weight and have rapid renal clearance, these problems have been solved by several methods such as chemical modification and physical techniques (cyclization, binding peptides, dosage forms). These well-established methods have been shown to slow down the rate of renal clearance and therefore, significantly give support to clinical studies. PDCs are at the forefront of research and are promising for the future, as witnessed by the two PDCs on the market. PDCs with rational design and precise targets can have an impact on the target therapeutic market. Peptides already exist in clinical trials in the form of BTC to treat a variety of cancers. Combining materials science through nanoparticles with peptides is an effective strategy to address the stability issues encountered with peptides. This review highlights the potential of peptides as a diverse tool for targeted drug delivery. Despite that PDCs can achieve enhanced efficacy, increased circulation time, and lowered toxicity, the synthetic technology for a peptide with better stability used in PDCs is needed to overcome. In the future, dual-function PDC technology is set to shine. There is still a long way to go in trying to achieve a bifunctional combination by focusing drugs on tumor tissue through tumor-specific targets (TSA) and another target as an endocytic target, and we expect pharmaceutical companies to make a breakthrough. Research and development is a very complex discipline that opens a window and closes a door for you at the same time.
PDC drugs have strong tumor penetration, low production cost and no immunogenicity. Compared with other conjugated drugs, PDC drugs have a broader industrial base and clinical value. China is experiencing the era of biosimilars, PD-1/L1, and ADC drugs are piling up. With the resolution of related technical issues and breakthroughs in supporting technologies, PDC drugs will also become a hot spot for R&D and a crowded track for investment in the next decade. More innovative biopharmaceutical companies will join in to enable the rapid development of PDC drugs for the benefit of more patients.
In conclusion, As an emerging research field in the fight against cancer, PDC has its advantages in comparison with ADC, but there are still many “hard bones” to overcome. Fortunately, with the experience of ADC, PDC research may have some shortcuts and fewer detours. Meanwhile, as the technology becomes more innovative, it is believed that PDC research will gradually be clinically validated, thus driving the development of the field and bringing more choices to therapy.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (NSFC, No. 82073884, China), NSFC-Liaoning joint fund key program (No. U20A20413, China), Postdoctoral Research Startup Project (No. 3110211220, China), and Postdoctoral Research Startup Project (No. 3110210640, China).
Footnotes
Peer review under the responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Xinli Liu, Email: cinlylau@hotmail.com.
Zhaojin Yu, Email: yuzhaojin19830813@163.com.
Minjie Wei, Email: mjwei@cmu.edu.cn.
Author contributions
Chen Fu, Zhaojin Yu, and Minjie Wei conceived, designed, and revised the manuscript; Chen Fu, Yuxi Miao wrote and revised the manuscript; Lifeng Yu and Xinli Liu revised the manuscript and discussed interpretation. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted, and agree to be accountable for all aspects of the work.
Conflict of interest
The authors declare no conflicts of interest.
References
- 1.Tsomaia N. Peptide therapeutics: targeting the undruggable space. Eur J Med Chem. 2015;94:459–470. doi: 10.1016/j.ejmech.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 2.Chatzisideri T., Leonidis G., Sarli V. Cancer-targeted delivery systems based on peptides. Mol Pharm. 2018;10:2201–2226. doi: 10.4155/fmc-2018-0174. [DOI] [PubMed] [Google Scholar]
- 3.Gronewold A., Horn M., Neundorf I. Design and biological characterization of novel cell-penetrating peptides preferentially targeting cell nuclei and subnuclear regions. Beilstein J Org Chem. 2018;14:1378–1388. doi: 10.3762/bjoc.14.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zaazouee M.S., Hamdallah A., Helmy S.K., Hasabo E.A., Sayed A.K., Gbreel M.I., et al. Semaglutide for the treatment of type 2 diabetes mellitus: a systematic review and network meta-analysis of safety and efficacy outcomes. Diabetes Metabol Syndr. 2022;16 doi: 10.1016/j.dsx.2022.102511. [DOI] [PubMed] [Google Scholar]
- 5.Eberle R.J., Gering I., Tusche M., Ostermann P.N., Muller L., Adams O., et al. Design of d-amino acids SARS-CoV-2 main protease inhibitors using the cationic peptide from rattlesnake venom as a scaffold. Pharmaceuticals. 2022;15:540. doi: 10.3390/ph15050540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma L., Wang C., He Z., Cheng B., Zheng L., Huang K. Peptide‒drug conjugate: a novel drug design approach. Curr Med Chem. 2017;24:3373–3396. doi: 10.2174/0929867324666170404142840. [DOI] [PubMed] [Google Scholar]
- 7.Liu N., Zhang Y., Lei Y., Wang R., Zhan M., Liu J., et al. Design and evaluation of a novel peptide‒drug conjugate covalently targeting SARS-CoV-2 papain-like protease. J Med Chem. 2022;65:876–884. doi: 10.1021/acs.jmedchem.1c02022. [DOI] [PubMed] [Google Scholar]
- 8.Vhora I., Patil S., Bhatt P., Misra A. Protein- and peptide‒drug conjugates: an emerging drug delivery technology. Adv Protein Chem Struct Biol. 2015;98:1–55. doi: 10.1016/bs.apcsb.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Zhou X., Smith Q.R., Liu X. Brain penetrating peptides and peptide‒drug conjugates to overcome the blood‒brain barrier and target CNS diseases. Int J Mol Sci. 2021;13:e1695. doi: 10.1002/wnan.1695. [DOI] [PubMed] [Google Scholar]
- 10.Wu C., Cheng Z., Lu D., Liu K., Cheng Y., Wang P., et al. Novel N-methylated cyclodepsipeptide prodrugs for targeted cancer therapy. Molecules. 2021;64:991–1000. doi: 10.1021/acs.jmedchem.0c01387. [DOI] [PubMed] [Google Scholar]
- 11.Vrettos E.I., Tzakos A.G. Construction of peptide‒drug conjugates for selective targeting of malignant tumor cells. Bioanalysis. 2021;2207:327–338. doi: 10.1007/978-1-0716-0920-0_23. [DOI] [PubMed] [Google Scholar]
- 12.Lindberg J., Nilvebrant J., Nygren P.Å., Lehmann F. Progress and future directions with peptide–drug conjugates for targeted cancer therapy. Molecules. 2021;26:6042. doi: 10.3390/molecules26196042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siegel R.L., Miller K.D., Fuchs H., Jemal A. Cancer statistics, 2022. CA A Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 14.Wessels D., Lusche D.F., Voss E., Soll D.R. 3D and 4D tumorigenesis model for the quantitative analysis of cancer cell behavior and screening for anticancer drugs. Methods Mol Biol. 2022;2364:299–318. doi: 10.1007/978-1-0716-1661-1_14. [DOI] [PubMed] [Google Scholar]
- 15.Jin T., Liu M. Letter to the editor: comment on GLP-1-based drugs and COVID-19 treatment. Acta Pharm Sin B. 2020;10:1249–1250. doi: 10.1016/j.apsb.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan B.S., Dawson A.H., Buckley N.A. What can clinicians learn from therapeutic studies about the treatment of acute oral methotrexate poisoning?. Clin Toxicol. 2017;55:88–96. doi: 10.1080/15563650.2016.1271126. [DOI] [PubMed] [Google Scholar]
- 17.Fares J., Fares M.Y., Khachfe H.H., Salhab H.A., Fares Y. Molecular principles of metastasis: a hallmark of cancer revisited. Signal Transduct Targeted Ther. 2020;5:28. doi: 10.1038/s41392-020-0134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He Q., Chen J., Yan J., Cai S., Xiong H., Liu Y., et al. Tumor microenvironment responsive drug delivery systems. Asian J Pharm Sci. 2020;15:416–448. doi: 10.1016/j.ajps.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou F., Zhu H., Fu C. Clinical therapeutic development against cancers resistant to targeted therapies. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.816896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin Y., Edalatian Zakeri S., Bahal R., Wiemer A.J. New technologies bloom together for bettering cancer drug conjugates. Pharmacol Rev. 2022;74:680–711. doi: 10.1124/pharmrev.121.000499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vrettos E.I., Karampelas T., Sayyad N., Kougioumtzi A., Syed N., Crook T., et al. Development of programmable gemcitabine-GnRH pro-drugs bearing linker controllable “click” oxime bond tethers and preclinical evaluation against prostate cancer. Eur J Med Chem. 2021;211 doi: 10.1016/j.ejmech.2020.113018. [DOI] [PubMed] [Google Scholar]
- 22.Huizi W., Jiaguo H. Optimization of protein and peptide drugs based on the mechanisms of kidney clearance. Protein Pept Lett. 2018;25:514–521. doi: 10.2174/0929866525666180530122835. [DOI] [PubMed] [Google Scholar]
- 23.Wang J., Chen M., Li S., Ye R.D. Targeted delivery of a ligand‒drug conjugate via formyl peptide receptor 1 through cholesterol-dependent endocytosis. Mol Pharm. 2019;16:2636–2647. doi: 10.1021/acs.molpharmaceut.9b00188. [DOI] [PubMed] [Google Scholar]
- 24.Kim H., Hwang D., Choi M. Antibody-assisted delivery of a peptide‒drug conjugate for targeted cancer therapy. Anal Chem. 2019;16:165–172. doi: 10.1021/acs.molpharmaceut.8b00924. [DOI] [PubMed] [Google Scholar]
- 25.Sangtani A., Petryayeva E., Susumu K. Nanoparticle-peptide‒drug bioconjugates for unassisted defeat of multidrug resistance in a model cancer cell line. Bioconjugate Chem. 2019;30:525–530. doi: 10.1021/acs.bioconjchem.8b00755. [DOI] [PubMed] [Google Scholar]
- 26.Kozaki I., Shimizu K., Honda H. Disulfide linked hetero dimeric peptide arrays for screening functional peptides inside cells. J Biosci Bioeng. 2020;129:613–618. doi: 10.1016/j.jbiosc.2019.11.012. [DOI] [PubMed] [Google Scholar]
- 27.Deng X., Mai R., Zhang C., Yu D., Ren Y., Li G., et al. Discovery of novel cell-penetrating and tumor-targeting peptide‒drug conjugate (PDC) for programmable delivery of paclitaxel and cancer treatment. Eur J Med Chem. 2020;213 doi: 10.1016/j.ejmech.2020.113050. [DOI] [PubMed] [Google Scholar]
- 28.You X., Guo W., Wang L., Hou Y., Zhang H., Pan Y., et al. Subcellular distribution of RAD23B controls XPC degradation and DNA damage repair in response to chemotherapy drugs. Cell Signal. 2017;36:108–116. doi: 10.1016/j.cellsig.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 29.Li S., Zhao H., Fan Y., Zhao G., Wang R., Wen F., et al. Design, synthesis, and in vitro antitumor activity of a transferrin receptor-targeted peptide‒doxorubicin conjugate. Chem Biol Drug Des. 2020;95:58–65. doi: 10.1111/cbdd.13613. [DOI] [PubMed] [Google Scholar]
- 30.Jerath G., Goyal R., Trivedi V., Santhoshkumar T.R., Ramakrishnan V. Syndiotactic peptides for targeted delivery. Acta Biomater. 2019;87:130–139. doi: 10.1016/j.actbio.2019.01.036. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y., Cheetham A.G., Angacian G., Su H., Xie L., Cui H. Peptide‒drug conjugates as effective prodrug strategies for targeted delivery. Adv Drug Deliv Rev. 2017;110–111:112–126. doi: 10.1016/j.addr.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li S., Roberts R.W. A novel strategy for in vitro selection of peptide‒drug conjugates. Chem Biol. 2003;10:233–239. doi: 10.1016/s1074-5521(03)00047-4. [DOI] [PubMed] [Google Scholar]
- 33.Heinis C., Rutherford T., Freund S., Winter G. Phage-encoded combinatorial chemical libraries based on bicyclic peptides. Nat Chem Biol. 2009;5:502. doi: 10.1038/nchembio.184. [DOI] [PubMed] [Google Scholar]
- 34.Redko B., Ragozin E., Andreii B., Helena T., Amnon A., Talia S.Z., et al. Synthesis, drug release, and biological evaluation of new anticancer drug-bioconjugates containing somatostatin backbone cyclic analog as a targeting moiety. Biopolymers. 2015;104:743–752. doi: 10.1002/bip.22694. [DOI] [PubMed] [Google Scholar]
- 35.Weiss G.A., Chamberlin R. Bridging the synthetic and biopolymer worlds with peptide‒drug conjugates. Chem Biol. 2003;10:201–202. doi: 10.1016/s1074-5521(03)00056-5. [DOI] [PubMed] [Google Scholar]
- 36.Bennett G., Brown A., Mudd G., Huxley P., Van Rietschoten K., Pavan S., et al. MMAE delivery using the bicycle toxin conjugate BT5528. Mol Cancer Therapeut. 2020;19:1385–1394. doi: 10.1158/1535-7163.MCT-19-1092. [DOI] [PubMed] [Google Scholar]
- 37.Rigby M., Beswick P., Mudd G., Van Rietschoten K., Chen L.H., Watcham S.M., et al. BT8009: a bicyclic peptide toxin conjugate targeting Nectin-4 (PVRL4) displays efficacy in preclinical tumor models. Cancer Res. 2019;79:13. [Google Scholar]
- 38.Gowland C., Berry P., Errington J., Jeffrey P., Bennett G., Godfrey L., et al. Development of a LC‒MS/MS method for the quantification of toxic payload DM1 cleaved from BT1718 in a phase I study. Bioanalysis. 2021;13:101–113. doi: 10.4155/bio-2020-0256. [DOI] [PubMed] [Google Scholar]
- 39.Bicyclic peptide makes targeting EphA2 possible. Cancer Discov. 2021;11:2951–2952. doi: 10.1158/2159-8290.CD-NB2021-0393. [DOI] [PubMed] [Google Scholar]
- 40.Garcia-Monclus S., Lopez-Alemany R., Almacellas-Rabaiget O., Herrero-Martin D., Huertas-Martinez J., Lagares-Tena L., et al. EphA2 receptor is a key player in the metastatic onset of ewing sarcoma. Int J Cancer. 2018;143:1188–1201. doi: 10.1002/ijc.31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barile E., Wang S., Das S.K., Noberini R., Dahl R., Stebbins J.L., et al. Design, synthesis and bioevaluation of an EphA2 receptor-based targeted delivery system. ChemMedChem. 2014;9:1403–1412. doi: 10.1002/cmdc.201400067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rigby M., Bennett G., Chen L.H., Mudd G., Beswick P., Harrison H., et al. BT8009, a bicycle toxin conjugate targeting Nectin-4, shows target selectivity, and efficacy in preclinical large and small tumor models. Mol Cancer Therapeut. 2019;18:151–156. [Google Scholar]
- 43.Delyanee M., Akbari S., Solouk A. Amine-terminated dendritic polymers as promising nanoplatform for diagnostic and therapeutic agents' modification: a review. Eur J Med Chem. 2021;221 doi: 10.1016/j.ejmech.2021.113572. [DOI] [PubMed] [Google Scholar]
- 44.Xu L., Wang Y., Zhu C., Ren S., Shao Y., Wu L., et al. Morphological transformation enhances tumor retention by regulating the self-assembly of doxorubicin‒peptide conjugates. Theranostics. 2020;10:8162–8178. doi: 10.7150/thno.45088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Falciani C., Brunetti J., Pagliuca C., Menichetti S., Vitellozzi L., Lelli B., et al. Design and in vitro evaluation of branched peptide conjugates: turning nonspecific cytotoxic drugs into tumor-selective agents. ChemMedChem. 2010;5:567–574. doi: 10.1002/cmdc.200900527. [DOI] [PubMed] [Google Scholar]
- 46.Hawryłkiewicz A., Ptaszyńska N. Gemcitabine peptide-based conjugates and their application in targeted tumor therapy. Molecules. 2021;26:364. doi: 10.3390/molecules26020364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pethő L., Kasza G. Amphiphilic drug-peptide-polymer conjugates based on poly(ethylene glycol) and hyperbranched polyglycerol for epidermal growth factor receptor targeting: the effect of conjugate aggregation on in vitro activity. Soft Matter. 2020;16:5759–5769. doi: 10.1039/d0sm00428f. [DOI] [PubMed] [Google Scholar]
- 48.Wang M.D., Hou D.Y., Lv G.T., Li R.X., Hu X.J., Wang Z.J., et al. Targeted in situ self-assembly augments peptide drug conjugate cell-entry efficiency. Biomaterials. 2021;278 doi: 10.1016/j.biomaterials.2021.121139. [DOI] [PubMed] [Google Scholar]
- 49.Ortega C., Wong R.K., Schaefferkoetter J., Veit-Haibach P., Myrehaug S., Juergens R., et al. Quantitative 68Ga-DOTATATE PET/CT parameters for the prediction of therapy response in patients with progressive metastatic neuroendocrine tumors treated with 177Lu-DOTATATE. J Nucl Med. 2021;62:1406–1414. doi: 10.2967/jnumed.120.256727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hajek R., Hassoun H., Rodríguez-Otero P., Paner A., Schjesvold F.H., Gullbo J., et al. Melflufen: a peptide‒drug conjugate for the treatment of multiple myeloma. Expet Opin Invest Drugs. 2020;9:3120. doi: 10.1080/13543784.2020.1808884. [DOI] [PubMed] [Google Scholar]
- 51.Flanagan K., Kumari R., Miettinen J.J., Haney S.L., Varney M.L., Williams J.T., et al. The peptide‒drug conjugate melflufen modulates the unfolded protein response of multiple myeloma and amyloidogenic plasma cells and induces cell death. Hemasphere. 2022;6:e687. doi: 10.1097/HS9.0000000000000687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bindra R., Sundaram R.K., Aiello R.J., Marshall D., Bourassa P., Csengery J., et al. Unlocking PARP inhibitor efficacy for HRD-negative cancers using the alphalex tumor targeting platform. J Clin Oncol. 2019;37:32. [Google Scholar]
- 53.Yu M., Chen Y., Wang Z., Ding X. pHLIP(Var7)-P1AP suppresses tumor cell proliferation in MDA-MB-231 triple-negative breast cancer by targeting protease activated receptor 1. Breast Cancer Res Treat. 2020;180:379–384. doi: 10.1007/s10549-020-05560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Langer M., Kratz F., Rothen-Rutishauser B., Wunderli-Allenspach H., Beck-Sickinger A.G. Novel peptide conjugates for tumor-specific chemotherapy. J Med Chem. 2001;44:1341–1348. doi: 10.1021/jm001065f. [DOI] [PubMed] [Google Scholar]
- 55.Ulapane K.R., Kopec B.M., Moral M.E.G., et al. Peptides and drug delivery. Adv Exp Med Biol. 2017;1030:167–184. doi: 10.1007/978-3-319-66095-0_8. [DOI] [PubMed] [Google Scholar]
- 56.Laakkonen P., Vuorinen K. Homing peptides as targeted delivery vehicles. Integr Biol. 2010;2:326–337. doi: 10.1039/c0ib00013b. [DOI] [PubMed] [Google Scholar]
- 57.King A., Ndifon C., Lui S., Widdows K., Kotamraju V.R., Agemy L., et al. Tumor-homing peptides as tools for targeted delivery of payloads to the placenta. Sci Adv. 2016;2 doi: 10.1126/sciadv.1600349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zheng X.S., Zong C., Wang X., Ren B. Cell-penetrating peptide conjugated sers nanosensor for in situ intracellular pH imaging of single living cells during cell cycle. Anal Chem. 2019;91:8383–8389. doi: 10.1021/acs.analchem.9b01191. [DOI] [PubMed] [Google Scholar]
- 59.Rhea K. Determining the binding kinetics of peptide macrocycles using bio-layer interferometry (BLI) Methods Mol Biol. 2022;2371:355–372. doi: 10.1007/978-1-0716-1689-5_19. [DOI] [PubMed] [Google Scholar]
- 60.Lingasamy P., Teesalu T. Homing peptides for cancer therapy. Adv Exp Med Biol. 2021;1295:29–48. doi: 10.1007/978-3-030-58174-9_2. [DOI] [PubMed] [Google Scholar]
- 61.Kapoor P., Singh H., Gautam A., Chaudhary K., Kumar R., Raghava G.P. TumorHoPe: a database of tumor homing peptides. PLoS One. 2012;7 doi: 10.1371/journal.pone.0035187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu Z., Wu K. Peptides homing to tumor vasculature: imaging and therapeutics for cancer. Recent Pat Anti-Cancer Drug Discov. 2008;3:202–208. doi: 10.2174/157489208786242250. [DOI] [PubMed] [Google Scholar]
- 63.Agrawal P., Bhalla S., Usmani S.S., Singh S., Chaudhary K., Raghava G.P.S., et al. CPPsite 2.0: a repository of experimentally validated cell-penetrating peptides. Nucleic Acids Res. 2016;44:D1098–D1103. doi: 10.1093/nar/gkv1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xue Y., Ye X., Wei L., Zhang X., Sakurai T., Wei L. Better performance with transformer: CPPFormer in precise prediction of cell-penetrating peptides. Curr Med Chem. 2021;29:881–893. doi: 10.2174/0929867328666210920103140. [DOI] [PubMed] [Google Scholar]
- 65.Shi N.Q., Gao W., Xiang B., Qi X.R. Enhancing cellular uptake of activable cell-penetrating peptide‒doxorubicin conjugate by enzymatic cleavage. Int J Nanomed. 2012;7:1613–1621. doi: 10.2147/IJN.S30104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yu X., Conyne M., Lake M.R., Walter K.A., Min J. In silico high throughput mutagenesis and screening of signal peptides to mitigate N-terminal heterogeneity of recombinant monoclonal antibodies. mAbs. 2022;14 doi: 10.1080/19420862.2022.2044977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Binette V., Mousseau N., Tuffery P. A generalized attraction-repulsion potential and revisited fragment library improves PEP-FOLD peptide structure prediction. J Chem Theor Comput. 2022;18:2720–2736. doi: 10.1021/acs.jctc.1c01293. [DOI] [PubMed] [Google Scholar]
- 68.Speck-Planche A., Kleandrova V.V., Scotti M.T. In silico drug repurposing for anti-inflammatory therapy: virtual search for dual inhibitors of caspase-1 and TNF-alpha. Biomolecules. 2021;11:1832. doi: 10.3390/biom11121832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mustafa N.H., Sekar M., Fuloria S., Begum M.Y., Gan S.H., Rani N.N.I.M., et al. Chemistry, biosynthesis and pharmacology of sarsasapogenin: a potential natural steroid molecule for new drug design, development and therapy. Molecules. 2022;27:2032. doi: 10.3390/molecules27062032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Drucker D.J. Advances in oral peptide therapeutics. Nat Rev Drug Discov. 2020;19:277–289. doi: 10.1038/s41573-019-0053-0. [DOI] [PubMed] [Google Scholar]
- 71.Zhong P., Zeng H., Huang M., He G., Chen Z. Efficacy and safety of subcutaneous and oral semaglutide administration in patients with type 2 diabetes: a meta-analysis. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.695182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Singh A.P., Shah D.K. A "dual" cell-level systems PK-PD model to characterize the bystander effect of ADC. J Pharmacol Sci. 2019;108:2465–2475. doi: 10.1016/j.xphs.2019.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Werle M., Bernkop-Schnürch A. Strategies to improve plasma half life time of peptide and protein drugs. Amino Acids. 2006;30:351–367. doi: 10.1007/s00726-005-0289-3. [DOI] [PubMed] [Google Scholar]
- 74.Meier J.J., Gallwitz B., Giorgino F. Reviews and novel clinical perspectives on semaglutide: a GLP-1 receptor agonist with both injectable and oral formulations. Front Endocrinol. 2021;12 doi: 10.3389/fendo.2021.760153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang B., Xie N., Li B. Influence of peptide characteristics on their stability, intestinal transport, and in vitro bioavailability: a review. J Food Biochem. 2019;43 doi: 10.1111/jfbc.12571. [DOI] [PubMed] [Google Scholar]
- 76.Hamamoto T., Sisido M., Ohtsuki T., Taki M. Synthesis of a cyclic peptide/protein using the NEXT-A reaction followed by cyclization. Chem Commun. 2011;47:9116–9118. doi: 10.1039/c1cc12196k. [DOI] [PubMed] [Google Scholar]
- 77.Choi S.H., Jeong W.J., Choi S.J., Lim Y.B. Highly efficient and fast pre-activation cyclization of the long peptide: succinimidyl ester-amine reaction revisited. Bioorg Med Chem Lett. 2015;25:5335–5338. doi: 10.1016/j.bmcl.2015.09.038. [DOI] [PubMed] [Google Scholar]
- 78.Lau Y.H., de Andrade P., Wu Y., Spring D.R. Peptide stapling techniques based on different macrocyclisation chemistries. Chem Soc Rev. 2015;44:91–102. doi: 10.1039/c4cs00246f. [DOI] [PubMed] [Google Scholar]
- 79.Blackwell H.E., Grubbs R.H. Highly efficient synthesis of covalently cross-linked peptide helices by ring-closing metathesis. Angew Chem, Int Ed. 1998;37:3281–3284. doi: 10.1002/(SICI)1521-3773(19981217)37:23<3281::AID-ANIE3281>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 80.Mas E., Borrelli O., Broekaert I., Martin de-Carpi J., Dolinsek J., Miele E., et al. Drugs in focus: octreotide use in children with gastrointestinal disorders. J Pediatr Gastroenterol Nutr. 2021;74:1–6. doi: 10.1097/MPG.0000000000003294. [DOI] [PubMed] [Google Scholar]
- 81.Harris A.G. Somatostatin and somatostatin analogues: pharmacokinetics and pharmacodynamic effects. Gut. 1994;35:S1. doi: 10.1136/gut.35.3_suppl.s1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lieser R.M., Chen W., Sullivan M.O. Controlled epidermal growth factor receptor ligand display on cancer suicide enzymes via unnatural amino acid engineering for enhanced intracellular delivery in breast cancer cells. Bioconjugate Chem. 2019;30:432–442. doi: 10.1021/acs.bioconjchem.8b00783. [DOI] [PubMed] [Google Scholar]
- 83.Meuleman T.J., Cowton V.M., Patel A.H., Liskamp R.M.J. Design and synthesis of HCV-E2 glycoprotein epitope mimics in molecular construction of potential synthetic vaccines. Viruses. 2021;13:326. doi: 10.3390/v13020326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Karoyan P., Vieillard V., Gomez-Morales L., Odile E., Guihot A., Luyt C.E., et al. Human ACE2 peptide-mimics block SARS-CoV-2 pulmonary cells infection. Commun Biol. 2021;4:197. doi: 10.1038/s42003-021-01736-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Räder A.F.B., Reichart F., Weinmüller M., et al. Improving oral bioavailability of cyclic peptides by N-methylation. Bioorg Med Chem. 2018;26:2766–2773. doi: 10.1016/j.bmc.2017.08.031. [DOI] [PubMed] [Google Scholar]
- 86.Zhou K., Zheng X., Xu H.-M., Zhang J., Chen Y., Xi T., et al. Studies of poly(ethylene glycol) modification of HM-3 polypeptides. Bioconjugate Chem. 2009;20:932–936. doi: 10.1021/bc900070r. [DOI] [PubMed] [Google Scholar]
- 87.Mayolo-Deloisa K., Gonzalez-Gonzalez M., Simental-Martinez J., Rito-Palomares M. Aldehyde PEGylation of laccase from Trametes versicolor in route to increase its stability: effect on enzymatic activity. J Mol Recogn. 2015;28:173–179. doi: 10.1002/jmr.2405. [DOI] [PubMed] [Google Scholar]
- 88.Nagata S., Yamasaki M., Kitamura K. Anti-inflammatory effects of PEGylated human adrenomedullin in a mouse DSS-induced colitis model. Drug Dev Res. 2017;78:129–134. doi: 10.1002/ddr.21383. [DOI] [PubMed] [Google Scholar]
- 89.Kurzrock R., Gabrail N., Chandhasin C., Moulder S., Smith C., Brenner A., et al. Safety, pharmacokinetics, and activity of GRN1005, a novel conjugate of angiopep-2, a peptide facilitating brain penetration, and paclitaxel, in patients with advanced solid tumors. Mol Cancer Therapeut. 2012;11:308–316. doi: 10.1158/1535-7163.MCT-11-0566. [DOI] [PubMed] [Google Scholar]
- 90.Wang J., Wu D., Shen W.C. Structure‒activity relationship of reversibly lipidized peptides: studies of fatty acid-desmopressin conjugates. Pharm Res (N Y) 2002;19:609–614. doi: 10.1023/a:1015397811161. [DOI] [PubMed] [Google Scholar]
- 91.Issar T., Kwai N.C.G., Poynten A.M., Arnold R., Milner K.L., Krishnan A.V. Effect of exenatide on peripheral nerve excitability in type 2 diabetes. Clin Neurophysiol. 2021;132:2532–2539. doi: 10.1016/j.clinph.2021.05.033. [DOI] [PubMed] [Google Scholar]
- 92.Lau J., Bloch P., Schäffer L., Pettersson I., Spetzler J., Kofoed J., et al. Discovery of the once-weekly glucagon-like peptide-1 (GLP-1) analogue semaglutide. J Med Chem. 2015;58:7370–7380. doi: 10.1021/acs.jmedchem.5b00726. [DOI] [PubMed] [Google Scholar]
- 93.Yamada Y., Yabe D., Hertz C.L., Horio H., Nakamura J., Nielsen A.M., et al. Efficacy and safety of oral semaglutide by baseline age in Japanese patients with type 2 diabetes: a subgroup analysis of the PIONEER 9 and 10 Japan trials. Diabetes Obes Metabol. 2021;24:321–326. doi: 10.1111/dom.14571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yu S., Yang H., Li T., Pan H., Ren S., Luo G., et al. Efficient intracellular delivery of proteins by a multifunctional chimaeric peptide in vitro and in vivo. Nat Commun. 2021;12:5131. doi: 10.1038/s41467-021-25448-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Huber R., Kohl B., Sachs G., Senn-Bilfinger J., Simon W.A., Sturm E. Review article: the continuing development of proton pump inhibitors with particular reference to pantoprazole. Aliment Pharmacol Ther. 1995;9:363–378. doi: 10.1111/j.1365-2036.1995.tb00394.x. [DOI] [PubMed] [Google Scholar]
- 96.Franchi-Gazzola R., Visigalli R., Dall'Asta V., Sala R., Woo S.K., Kwon H.M., et al. Amino acid depletion activates TonEBP and sodium-coupled inositol transport. Am J Physiol Cell Physiol. 2001;280:C1465–C1474. doi: 10.1152/ajpcell.2001.280.6.C1465. [DOI] [PubMed] [Google Scholar]
- 97.Skidmore S., Hadar J., Garner J., Park H., Park K., Wang Y., et al. Complex sameness: separation of mixed poly(lactide-co-glycolide)s based on the lactide:glycolide ratio. J Control Release. 2019;300:174–184. doi: 10.1016/j.jconrel.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 98.Vrettos E.I., Mező G., Tzakos A.G. On the design principles of peptide‒drug conjugates for targeted drug delivery to the malignant tumor site. Beilstein J Org Chem. 2018;14:930–954. doi: 10.3762/bjoc.14.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Alas M., Saghaeidehkordi A., Kaur K. Peptide‒drug conjugates with different linkers for cancer therapy. J Med Chem. 2021;64:216–232. doi: 10.1021/acs.jmedchem.0c01530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kufka R., Rennert R., Kaluđerović G.N. Synthesis of a tubugi-1-toxin conjugate by a modulizable disulfide linker system with a neuropeptide Y analogue showing selectivity for hY1R-overexpressing tumor cells. Beilstein J Org Chem. 2019;15:96–105. doi: 10.3762/bjoc.15.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liang Y., Li S., Wang X., Zhang Y., Sun Y., Wang Y., et al. A comparative study of the antitumor efficacy of peptide‒doxorubicin conjugates with different linkers. J Med Chem. 2018;275:129–141. doi: 10.1016/j.jconrel.2018.01.033. [DOI] [PubMed] [Google Scholar]
- 102.Böhme D., Beck-Sickinger A.G. Controlling toxicity of peptide‒drug conjugates by different chemical linker structures. ChemMedChem. 2015;10:804–814. doi: 10.1002/cmdc.201402514. [DOI] [PubMed] [Google Scholar]
- 103.Dokus L.E., Lajko E., Randelovic I., Mezo D., Schlosser G., Kohidai L., et al. Phage display-based homing peptide‒daunomycin conjugates for selective drug targeting to PANC-1 pancreatic cancer. Pharmaceutics. 2020;12:576. doi: 10.3390/pharmaceutics12060576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Deeks E.D. Polatuzumab Vedotin: first global approval. Drugs. 2019;79:1467–1475. doi: 10.1007/s40265-019-01175-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Feni L., Parente S., Robert C., Gazzola S., Arosio D., Piarulli U., et al. Kiss and run: promoting effective and targeted cellular uptake of a drug delivery vehicle composed of an integrin-targeting diketopiperazine peptidomimetic and a cell-penetrating peptide. Bioconjugate Chem. 2019;30:2011–2022. doi: 10.1021/acs.bioconjchem.9b00292. [DOI] [PubMed] [Google Scholar]
- 106.Han J., Fu J., Yang Q., Zhou F., Chen X., Li C., et al. Rational design and biological evaluation of gemfibrozil modified Xenopus GLP-1 derivatives as long-acting hypoglycemic agents. Nano Lett. 2020;198 doi: 10.1016/j.ejmech.2020.112389. [DOI] [PubMed] [Google Scholar]
- 107.Song Q., Chuan X., Chen B., He B., Zhang H., Dai W., et al. A smart tumor targeting peptide‒drug conjugate, pHLIP-SS-DOX: synthesis and cellular uptake on MCF-7 and MCF-7/Adr cells. Drug Deliv. 2016;23:1734–1746. doi: 10.3109/10717544.2015.1028601. [DOI] [PubMed] [Google Scholar]
- 108.Lin X., Wang L., Zhao L., Zhu Z., Chen T., Chen S., et al. Curcumin micelles suppress gastric tumor cell growth by upregulating ROS generation, disrupting redox equilibrium and affecting mitochondrial bioenergetics. Food Funct. 2020;11:4146–4159. doi: 10.1039/d0fo00260g. [DOI] [PubMed] [Google Scholar]
- 109.Mizuma T., Koyanagi A., Awazu S. Intestinal transport and metabolism of glucose-conjugated kyotorphin and cyclic kyotorphin: metabolic degradation is crucial to intestinal absorption of peptide drugs. Biochim Biophys Acta. 2000;1475:90–98. doi: 10.1016/s0304-4165(00)00051-9. [DOI] [PubMed] [Google Scholar]
- 110.Anantharaju P.G., Gowda P.C., Vimalambike M.G., Madhunapantula S.V. An overview on the role of dietary phenolics for the treatment of cancers. Nutr J. 2016;15:99. doi: 10.1186/s12937-016-0217-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Raabe M., Heck A.J., Fuhrer S., Schauenburg D., Pieszka M., Wang T., et al. Assembly of pH-responsive antibody‒drug-inspired conjugates. Macromol Biosci. 2022;22 doi: 10.1002/mabi.202100299. [DOI] [PubMed] [Google Scholar]
- 112.Jen E.Y., Ko C.-W., Lee J.E., Del Valle P.L., Aydanian A., Jewell C., et al. FDA approval: gemtuzumab ozogamicin for the treatment of adults with newly diagnosed CD33-positive acute myeloid leukemia. Clin Cancer Res. 2018;24:3242–3246. doi: 10.1158/1078-0432.CCR-17-3179. [DOI] [PubMed] [Google Scholar]
- 113.Wu H., Li J., Zhang Q., Yan X., Guo L., Gao X., et al. A novel small odorranalectin-bearing cubosomes: preparation, brain delivery and pharmacodynamic study on amyloid-β25-35-treated rats following intranasal administration. Eur J Pharm Biopharm. 2012;80:368–378. doi: 10.1016/j.ejpb.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 114.Hua D., Tang L., Wang W., Tang S., Yu L., Zhou X., et al. Improved antiglioblastoma activity and BBB permeability by conjugation of paclitaxel to a cell-penetrative MMP-2-cleavable peptide. Adv Sci. 2020;8 doi: 10.1002/advs.202001960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hua D. Improved antiglioblastoma activity and BBB permeability by conjugation of paclitaxel to a cell-penetrative MMP-2-cleavable peptide. J Med Chem. 2021;8 doi: 10.1002/advs.202001960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nagumo Y., Kandori S., Tanuma K., Nitta S., Chihara I., Shiga M., et al. PLD1 promotes tumor invasion by regulation of MMP-13 expression via NF-κB signaling in bladder cancer. Cancer Lett. 2021;511:15–25. doi: 10.1016/j.canlet.2021.04.014. [DOI] [PubMed] [Google Scholar]
- 117.Bernkop-Schnürch A., Kirchmayer R., Kratzel M. Synthesis, development and in vitro evaluation of drug delivery systems with protective effect against degradation by pepsin. J Drug Target. 1999;7:55–63. doi: 10.3109/10611869909085492. [DOI] [PubMed] [Google Scholar]
- 118.Kim S.H., Moreira R. Biological evaluation of naproxen-dehydrodipeptide conjugates with self-hydrogelation capacity as dual LOX/COX inhibitors. Toxicol Res. 2020;12:122. doi: 10.3390/pharmaceutics12020122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dünnhaupt S., Barthelmes J., Iqbal J., Perera G., Thurner C.C., Friedl H., et al. In vivo evaluation of an oral drug delivery system for peptides based on S-protected thiolated chitosan. J Control Release. 2012;160:477–485. doi: 10.1016/j.jconrel.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 120.Kreutzfeldt J., Rozeboom B., Dey N., De P. The trastuzumab era: current and upcoming targeted HER2+ breast cancer therapies. Am J Cancer Res. 2020;10:1045–1067. [PMC free article] [PubMed] [Google Scholar]
- 121.Zhu Y.S., Tang K., Lv J. Peptide‒drug conjugate-based novel molecular drug delivery system in cancer. Trends Pharmacol Sci. 2021;42:857–869. doi: 10.1016/j.tips.2021.07.001. [DOI] [PubMed] [Google Scholar]
- 122.Dagar S., Sekosan M., Lee B.S., Rubinstein I., Onyüksel H. VIP receptors as molecular targets of breast cancer: implications for targeted imaging and drug delivery. J Control Release. 2001;74:129–134. doi: 10.1016/s0168-3659(01)00326-1. [DOI] [PubMed] [Google Scholar]
- 123.Borbély A. Structural characterization of daunomycin‒peptide conjugates by various tandem mass spectrometric techniques. Chem Soc Rev. 2021;22:1648. doi: 10.3390/ijms22041648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pethő L., Mező G., Schlosser G. Overcharging effect in electrospray ionization mass spectra of daunomycin‒tuftsin bioconjugates. Molecules. 2019;24:2981. doi: 10.3390/molecules24162981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vats K., Satpati D. 99mTc-labeled NGR-chlorambucil conjugate, 99mTc-HYNIC-CLB-c(NGR) for targeted chemotherapy and molecular imaging. J Label Compd Radiopharm. 2017;60:431–438. doi: 10.1002/jlcr.3522. [DOI] [PubMed] [Google Scholar]
- 126.Kalimuthu K., Lubin B.C., Bazylevich A., Gellerman G., Shpilberg O., Luboshits G., et al. Gold nanoparticles stabilize peptide‒drug-conjugates for sustained targeted drug delivery to cancer cells. J Nanobiotechnol. 2018;16:34. doi: 10.1186/s12951-018-0362-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang Q., Zhang P., Jian S., Li J., Li F., Sun X., et al. Drug-bearing peptide-based nanospheres for the inhibition of metastasis and growth of cancer. Mol Pharm. 2020;17:3165–3176. doi: 10.1021/acs.molpharmaceut.0c00118. [DOI] [PubMed] [Google Scholar]
- 128.Bumbaca B., Li Z., Shah D.K. Pharmacokinetics of protein and peptide conjugates. Bioconjugate Chem. 2019;34:42–54. doi: 10.1016/j.dmpk.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liu Y., Zheng D., Ma Y., Dai J., Li C., Xiao S., et al. Self-assembled nanoparticles platform based on pectin-dihydroartemisinin conjugates for codelivery of anticancer drugs. ACS Biomater Sci Eng. 2018;4:1641–1650. doi: 10.1021/acsbiomaterials.7b00842. [DOI] [PubMed] [Google Scholar]
- 130.Gao C., Bhattarai P., Chen M., Zhang N., Hameed S., Yue X., et al. Amphiphilic drug conjugates as nanomedicines for combined cancer therapy. Mol Pharm. 2018;29:3967–3981. doi: 10.1021/acs.bioconjchem.8b00692. [DOI] [PubMed] [Google Scholar]
- 131.Ezhilarasan D., Lakshmi T., Mallineni S.K. Nano-based targeted drug delivery for lung cancer: therapeutic avenues and challenges. Nanomedicine (Lond) 2022;37 doi: 10.2217/nnm-2021-0364. [DOI] [PubMed] [Google Scholar]
- 132.Casazza A., Van Helleputte L., Van Renterghem B., Pokreisz P., De Geest N., De Petrini M., et al. PhAc-ALGP-Dox, a novel anticancer prodrug with targeted activation and improved therapeutic index. Mol Cancer Therapeut. 2022;21:568–581. doi: 10.1158/1535-7163.MCT-21-0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cardoso A.M., Guedes J.R., Cardoso A.L., Morais C., Cunha P., Viegas A.T., et al. Recent trends in nanotechnology toward CNS diseases: lipid-based nanoparticles and exosomes for targeted therapeutic delivery. Int Rev Neurobiol. 2016;130:1–40. doi: 10.1016/bs.irn.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 134.Cominetti M.M.D., Russell D.A., Searcey M., Qian C., Wang J., Qian Y., et al. Tumor-cell-surface adherable peptide‒drug conjugate prodrug nanoparticles inhibit tumor metastasis and augment treatment efficacy. Bioconjugate Chem. 2020;20:4153–4161. doi: 10.1021/acs.nanolett.0c00152. [DOI] [PubMed] [Google Scholar]
- 135.Regina A., Demeule M., Che C., Lavallee I., Poirier J., Gabathuler R., et al. Antitumour activity of ANG1005, a conjugate between paclitaxel and the new brain delivery vector angiopep-2. Br J Pharmacol. 2008;155:185–197. doi: 10.1038/bjp.2008.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kumthekar P., Tang S.C., Brenner A.J., Kesari S., Piccioni D.E., Anders C., et al. ANG1005, a brain-penetrating peptide‒drug conjugate, shows activity in patients with breast cancer with leptomeningeal carcinomatosis and recurrent brain metastases. Clin Cancer Res. 2020;26:2789–2799. doi: 10.1158/1078-0432.CCR-19-3258. [DOI] [PubMed] [Google Scholar]
- 137.Thomas F.C., Taskar K., Rudraraju V., Goda S., Thorsheim H.R., Gaasch J.A., et al. Uptake of ANG1005, a novel paclitaxel derivative, through the blood‒brain barrier into brain and experimental brain metastases of breast cancer. Pharm Res (N Y) 2009;26:2486–2494. doi: 10.1007/s11095-009-9964-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.O'Sullivan C.C., Lindenberg M., Bryla C., Patronas N., Peer C.J., Amiri-Kordestani L., et al. ANG1005 for breast cancer brain metastases: correlation between 18F-FLT-PET after first cycle and MRI in response assessment. Breast Cancer Res Treat. 2016;160:51–59. doi: 10.1007/s10549-016-3972-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Li F., Tang S.C. Targeting metastatic breast cancer with ANG1005, a novel peptide–paclitaxel conjugate that crosses the blood‒brain-barrier (BBB) Genes Dis. 2017;4:1–3. doi: 10.1016/j.gendis.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Drappatz J., Brenner A., Wong E.T., Eichler A., Schiff D., Groves M.D., et al. Phase I study of GRN1005 in recurrent malignant glioma. Clin Cancer Res. 2013;19:1567–1576. doi: 10.1158/1078-0432.CCR-12-2481. [DOI] [PubMed] [Google Scholar]
- 141.Mäde V., Els-Heindl S., Beck-Sickinger A.G. Automated solid-phase peptide synthesis to obtain therapeutic peptides. Beilstein J Org Chem. 2014;10:1197–1212. doi: 10.3762/bjoc.10.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mckean M., Bendell J.C., Petrylak D.P., Powles T.B., Sonpavde G.P., Dickson A., et al. BT8009-100 phase I/II study of the safety, pharmacokinetics, & preliminary clinical activity of BT8009 in patients with Nectin-4 expressing advanced malignancies. Ann Oncol. 2020;31:S500–S501. [Google Scholar]
- 143.Campbell C.T., Smale R., Rigby M., Watson S., Dickson A., Gelb T., et al. A multi tumor survey of Nectin-4 expression to guide BT8009 indication selection. Cancer Res. 2021;81:1197. [Google Scholar]
- 144.Demeule M., Charfi C., Currie J.C., Larocque A., Zgheib A., Kozelko S., et al. TH1902, a new docetaxel‒peptide conjugate for the treatment of sortilin-positive triple-negative breast cancer. Cancer Sci. 2021;112:4317–4334. doi: 10.1111/cas.15086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mahalingam D., Peguero J., Cen P., Arora S.P., Sarantopoulos J., Rowe J., et al. A phase II, multicenter, single-arm study of mipsagargin (G-202) as a second-line therapy following sorafenib for adult patients with progressive advanced hepatocellular carcinoma. Cancers. 2019;11:833. doi: 10.3390/cancers11060833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gregorc V., Gaafar R.M., Favaretto A., Grossi F., Jassem J., Polychronis A., et al. NGR-hTNF in combination with best investigator choice in previously treated malignant pleural mesothelioma (NGR015): a randomised, double-blind, placebo-controlled phase 3. Lancet Oncol. 2018;19:799–811. doi: 10.1016/S1470-2045(18)30193-1. [DOI] [PubMed] [Google Scholar]
- 147.Schliemann C., Gerwing M., Heinzow H., Harrach S., Schwoppe C., Wildgruber M., et al. First-in-class CD13-targeted tissue factor tTF-NGR in patients with recurrent or refractory malignant tumors: results of a phase I dose-escalation study. Cancers. 2020;12:1488. doi: 10.3390/cancers12061488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.White B.H., Whalen K., Kriksciukaite K., Alargova R., Au Yeung T., Bazinet P., et al. Discovery of an SSTR2-targeting maytansinoid conjugate (PEN-221) with potent activity in vitro and in vivo. J Med Chem. 2019;62:2708–2719. doi: 10.1021/acs.jmedchem.8b02036. [DOI] [PubMed] [Google Scholar]
- 149.Whalen K.A., White B.H., Quinn J.M., Kriksciukaite K., Alargova R., Au Yeung TP., et al. Targeting the somatostatin receptor 2 with the miniaturized drug conjugate, PEN-221: a potent and novel therapeutic for the treatment of small cell lung cancer. Mol Cancer Therapeut. 2019;18:1926–1936. doi: 10.1158/1535-7163.MCT-19-0022. [DOI] [PubMed] [Google Scholar]
- 150.Miller D.S., Scambia G., Bondarenko I., Westermann A.M., Oaknin A., Oza A.M., et al. ZoptEC: phase III randomized controlled study comparing zoptarelin with doxorubicin as second line therapy for locally advanced, recurrent, or metastatic endometrial cancer ( NCT01767155) J Clin Oncol. 2018;36:5503. [Google Scholar]
- 151.Gong J.F., Hu X.C., Zhang J., Du Y.Q., Huang R., Teng Y., et al. Phase Ia study of CBP-1008, a bi-specific ligand drug conjugate targeting FR alpha and TRPV6, in patients with advanced solid tumors. J Clin Oncol. 2021;39:3077. [Google Scholar]
- 152.Rice C., Lutes T., Davey M., Lloyd V., MacCormack T.J., Stewart J.M., et al. The central role of NFAT signalling in the mechanism of action of the TRPV6 oncochannel inhibitor and clinical candidate SOR-C13. Cancer Res. 2018;78:1936. [Google Scholar]
- 153.Schjesvold F., Robak P., Pour L., Aschan J., Sonneveld P. OCEAN: a randomized phase III study of melflufen + dexamethasone to treat relapsed refractory multiple myeloma. Sci Adv. 2020;16:631–641. doi: 10.2217/fon-2020-0024. [DOI] [PubMed] [Google Scholar]
- 154.Oriol A., Larocca A., Leleu X. Melflufen for relapsed and refractory multiple myeloma. Expet Opin Invest Drugs. 2020;29:1069–1078. doi: 10.1080/13543784.2020.1808884. [DOI] [PubMed] [Google Scholar]
- 155.Hennrich U., Kopka K. Lutathera®: the first FDA- and EMA-approved radiopharmaceutical for peptide receptor radionuclide therapy. Pharmaceuticals. 2019;12:114. doi: 10.3390/ph12030114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kim C., Liu S.V., Subramaniam D.S., Torres T., Loda M., Esposito G., et al. Phase I study of the 177Lu-DOTA(0)-Tyr(3)-Octreotate (lutathera) in combination with nivolumab in patients with neuroendocrine tumors of the lung. J Immunother Cancer. 2020;8 doi: 10.1136/jitc-2020-000980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kind F., Michalski K., Yousefzadeh-Nowshahr E., Meyer P.T., Mix M., Ruf J. Bone marrow impairment during early [177Lu]PSMA-617 radioligand therapy: haematotoxicity or tumour progression? EJNMMI Res. 2022;12:20. doi: 10.1186/s13550-022-00891-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tshibangu T., Cawthorne C., Serdons K., Pauwels E., Gsell W., Bormans G., et al. Automated GMP compliant production of [18F]AlF-NOTA-octreotide. EJNMMI Radiopharm Chem. 2020;5:4. doi: 10.1186/s41181-019-0084-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Long T., Yang N., Zhou M., Chen D., Li Y., Li J., et al. Clinical application of 18F-AlF-NOTA-Octreotide PET/CT in combination with 18F-FDG PET/CT for imaging neuroendocrine neoplasms. Clin Nucl Med. 2019;44:452–458. doi: 10.1097/RLU.0000000000002578. [DOI] [PubMed] [Google Scholar]
- 160.Mena E., Owenius R., Turkbey B., Sherry R., Bratslavsky G., Macholl S., et al. [18F]fluciclatide in the in vivo evaluation of human melanoma and renal tumors expressing αvβ3 and αvβ5 integrins. Eur J Nucl Med Mol Imag. 2014;41:1879–1888. doi: 10.1007/s00259-014-2791-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kenny L.M., Tomasi G., Turkheimer F., Larkin J., Gore M., Brock C.S., et al. Preliminary clinical assessment of the relationship between tumor αvβ3 integrin and perfusion in patients studied with [18F]fluciclatide kinetics and [15O]H2O PET. EJNMMI Res. 2014;4:30. doi: 10.1186/s13550-014-0030-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tonnelet D., Bohn M.D.P., Becker S., Decazes P., Camus V., Thureau S., et al. Angiogenesis imaging study using interim [18F] RGD-K5 PET/CT in patients with lymphoma undergoing chemotherapy: preliminary evidence. EJNMMI Res. 2021;11:37. doi: 10.1186/s13550-021-00776-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Doss M., Kolb H.C., Zhang J.J., Belanger M.J., Stubbs J.B., Stabin M.G., et al. Biodistribution and radiation dosimetry of the integrin marker 18F-RGD-K5 determined from whole-body PET/CT in monkeys and humans. J Nucl Med. 2012;53:787–795. doi: 10.2967/jnumed.111.088955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chen S.H., Wang H.M., Lin C.Y., Chang J.T., Hsieh C.H., Liao C.T., et al. RGD-K5 PET/CT in patients with advanced head and neck cancer treated with concurrent chemoradiotherapy: results from a pilot study. Eur J Nucl Med Mol Imag. 2016;43:1621–1629. doi: 10.1007/s00259-016-3345-1. [DOI] [PubMed] [Google Scholar]
- 165.Clemmensen A., Hansen A.E., Holst P., Schoier C., Bisgaard S., Johannesen H.H., et al. [68Ga]Ga-NODAGA-E[(cRGDyK)](2)PET and hyperpolarized [1-13C] pyruvate MRSI (hyperPET) in canine cancer patients: simultaneous imaging of angiogenesis and the Warburg effect. Eur J Nucl Med Mol Imag. 2021;48:395–405. doi: 10.1007/s00259-020-04881-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bentsen S., Clemmensen A., Loft M., Flethoj M., Debes K.P., Ludvigsen T.P., et al. [68Ga]Ga-NODAGA-E[(cRGDyK)]2 angiogenesis PET/MR in a porcine model of chronic myocardial infarction. Diagnostics. 2021;11:1807. doi: 10.3390/diagnostics11101807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Iagaru A. Dual-integrin alphavbeta3- and gastrin-releasing peptide receptor-targeting PET radiotracer (68Ga-BBN-RGD) J Nucl Med. 2017;58:1706. doi: 10.2967/jnumed.117.191478. [DOI] [PubMed] [Google Scholar]
- 168.Zhang J., Mao F., Niu G., Peng L., Lang L., Li F., et al. 68Ga-BBN-RGD PET/CT for GRPR and integrin alphavbeta3 imaging in patients with breast cancer. Theranostics. 2018;8:1121–1130. doi: 10.7150/thno.22601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Menda Y., Madsen M.T., O'Dorisio T.M., Sunderland J.J., Watkins G.L., Dillon J.S., et al. 90Y-DOTATOC dosimetry-based personalized peptide receptor radionuclide therapy. J Nucl Med. 2018;59:1692–1698. doi: 10.2967/jnumed.117.202903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhang Z., Zhao X., Ding C., Wang J., Zhang J., Wang F. 99mTc-3PRGD2 SPECT/CT imaging for monitoring early response of EGFR-TKIs therapy in patients with advanced-stage lung adenocarcinoma. Cancer Biother Radiopharm. 2016;31:238–245. doi: 10.1089/cbr.2016.2052. [DOI] [PubMed] [Google Scholar]
- 171.Chen Q., Xie Q., Zhao M., Chen B., Gao S., Zhang H., et al. Diagnostic value of 99mTc-3PRGD2 scintimammography for differentiation of malignant from benign breast lesions: comparison of visual and semi-quantitative analysis. Hellenic J Nucl Med. 2015;18:193–198. doi: 10.1967/s002449910302. [DOI] [PubMed] [Google Scholar]
- 172.Pool S.E., Kam B.L., Koning G.A., Konijnenberg M., Ten Hagen T.L., Breeman W.A., et al. [111In-DTPA]octreotide tumor uptake in GEPNET liver metastases after intra-arterial administration: an overview of preclinical and clinical observations and implications for tumor radiation dose after peptide radionuclide therapy. Cancer Biother Radiopharm. 2014;29:179–187. doi: 10.1089/cbr.2013.1552. [DOI] [PubMed] [Google Scholar]