Abstract
Purpose:
This article reports 12-month outcomes of patients with diabetic macular edema previously treated with other antivascular endothelial growth factor agents and transitioned to fixed dosing of intravitreal aflibercept (IAI).
Methods:
This prospective, single-arm study enrolled patients to receive IAI 2 mg (0.05 mL) every 4 weeks until optical coherence tomography demonstrated no fluid. Patients then received fixed dosing of IAI 2 mg once every 8 weeks through 12 months. Primary outcome was mean absolute change from baseline central subfield thickness (CST) at 12 months measured by optical coherence tomography.
Results:
Twenty eyes were enrolled. At baseline, best-corrected visual acuity was 70.0 letters, mean CST was 419.7 µm ± 92.0, superficial capillary perfusion density (CPD) was 46.0 ± 4.2%, and deep CPD was 50.8 ± 4.3%. At 12 months, the mean CST improved to 287.2 µm ± 80.2 (P < .001), superficial CPD decreased to 43.6 ± 4.8% (P = .04), and deep CPD decreased to 47.6 ± 4.8% (P = .05).
Conclusions:
Patients who switched to fixed dosing of IAI demonstrated significant anatomic improvements in CST at 12 months. CPD values decreased significantly both in superficial and deep layers without significant changes in vision.
Keywords: aflibercept, anti-VEGF, capillary perfusion density, diabetic macular edema, optical coherence tomography angiography, switching
Introduction
Diabetic macular edema (DME) is the main cause of vision loss in diabetic retinopathy (DR) and is a leading cause of visual loss worldwide. 1 Vascular endothelial growth factor (VEGF) is a stimulus for DME, and VEGF levels have been shown to correlate with the severity of macular edema (ME). 2,3 Therefore, anti-VEGF therapy is typically standard of care in treating patients with DME. 4 Several randomized clinical trials have evaluated the use of anti-VEGF therapy, either alone or in conjunction with focal/grid laser treatment, and have shown efficacy of anti-VEGF agents in treating center-involved DME. 5 -12 Currently, there are 3 anti-VEGF agents commonly used to treat DME both on- and off-label. These include bevacizumab (Avastin, Genentech), ranibizumab (Lucentis, Genentech), and aflibercept (Eylea, Regeneron Pharmaceuticals).
Multiple studies have demonstrated significant improvements both in best-corrected visual acuity (BCVA) and anatomical outcomes in macular thickness with anti-VEGF treatment. 5 -12 Despite the anatomical and visual gains, there are reports of persistent DME regardless of continuous treatment. The Diabetic Retinopathy Clinical Research Network Protocol I revealed that approximately 40% of patients receiving monthly ranibizumab had persistent DME through 24 weeks. 12 A recent post hoc analysis of the Diabetic Retinopathy Clinical Research network Protocol T concluded that persistent DME was more likely with bevacizumab (65.6% of eyes) than with aflibercept (31.6%) or ranibizumab (41.5%) at 2 years. 13 Therefore, choice of anti-VEGF agent in DME may affect outcomes.
There is currently a lack of well-designed, prospective, clinical trial data evaluating the effect of switching anti-VEGF agents on DME outcomes, and further, how fixed dosing every 8 weeks after resolution of fluid on optical coherence tomography (OCT) affects visual acuity (VA), central subfield thickness (CST), and macular perfusion values over time in this patient population. The SWAP-TWO Study’s 6-month preplanned interim analysis demonstrated significant anatomic improvements in patients with prior anti-VEGF therapy who were switched to intravitreal aflibercept (IAI) through 6 months. 14
The purpose of this study is to report the 12-month primary outcome of patients with DME previously treated with other anti-VEGF agents who are switched to IAI therapy on a dosage regimen.
Methods
The study design for the SWAP-TWO clinical trial has been previously stated. 14 In short, the SWAP-TWO Study is a prospective, interventional, single-arm study performed at the Cole Eye Institute in Cleveland, Ohio.
Participants
This prospective study enrolled 20 eyes of 20 patients between December 2015 and August 2017 that demonstrated foveal involving retinal edema secondary to DR. Inclusion and exclusion criteria are outlined in the previously published 6-month results analysis. Briefly, inclusion criteria specified that participants have spectral-domain OCT (SD-OCT), CST greater than or equal to 325 µm, Early Treatment Diabetic Retinopathy Study (ETDRS) BCVA of 80 (20/25) to 20 (20/400), and history of previous intravitreal treatment with bevacizumab or ranibizumab with at least 4 previous injections in the last 6 months in the study eye. No patients had steroid injections in the 1 year prior to enrollment.
Pertinent exclusion criteria included patients who had any prior or concomitant therapy with another investigational agent to treat DME in the study eye, prior history of IAI, history of vitrectomy or panretinal photocoagulation in the study eye within 3 months, or previous intravitreal anti-VEGF therapy in the study eye within 30 days of enrollment. Epiretinal membranes were not considered an exclusion.
Only 1 eye per participant was enrolled in the study. If a nonstudy eye required treatment of DME at study entry or during participation in the study, the fellow eye could receive any anti-VEGF agent including IAI at the investigators’ discretion, but it was not considered an additional study eye. The frequency and course of fellow-eye treatment was based on investigators’ discretion.
Visits and Assessments
Patients received 2 mg (0.05 mL) of IAI administered every 4 weeks until SD-OCT demonstrated no evidence of intraretinal fluid. Patients were then transitioned to an every-8-weeks dosage. No evidence of intraretinal fluid was defined by the following OCT parameters: a lack of any subretinal fluid; CST of less than 320 µm; extrafoveal cystoid ME (fluid present but not involving the fovea); or foveal cystoid ME with a normal foveal depression present. If at any point during the fixed every-8-weeks dosing regimen patients experienced a loss of 15 or more ETDRS letters or an increase in 75 µm of CST as compared with the best previous measurement, they could receive additional treatment with IAI. IAI was supplied by Regeneron Pharmaceuticals and was administered using standard aseptic techniques.
Study visits were scheduled every 28 ± 7 days. Electronic ETDRS chart (M&S Systems) and protocol VA measurements were recorded at each visit, which consisted of BCVA testing and a forced-choice paradigm. 15 A comprehensive eye examination and SD-OCT scanning were performed at each visit. Fast macular thickness maps as well as high-definition 6.0 mm linear scans centered on the fovea by using the Cirrus SD-OCT (Zeiss) and a 3 × 3-mm (9-mm2) en face retinal map for vascular analysis that applied Avanti RTVue XR (Optovue, Inc) with projection artifact removal and automated segmentation. Ultra-widefield fluorescein angiography and OCT angiography (OCTA) were performed at baseline, 6 months, and 12 months.
Outcome Measures
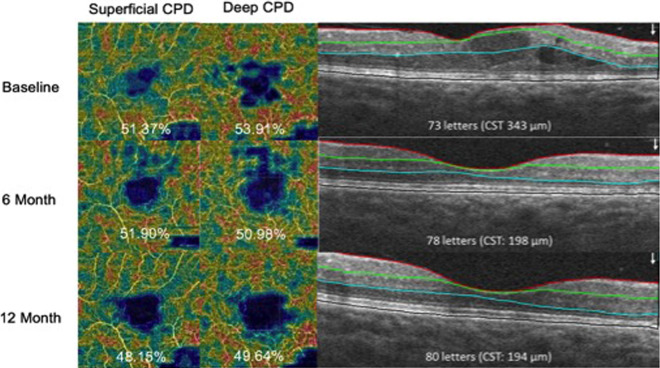
The primary outcome of the study was the mean absolute change from baseline CST at month 12 as measured by SD-OCT (defined as the average thickness within the central 1-mm subfield). Secondary outcomes included the mean change from baseline in ETDRS BCVA scores, OCTA capillary perfusion density (CPD) changes (Figure 1), DR severity changes as determined by clinical examination, and adverse events (AEs) at months 6, 12, and 24.
Figure 1.
Representative patient who had a decrease in superficial and deep capillary perfusion density (CPD) from baseline to 12 months. CST, central subfield thickness.
Safety Analyses
Safety assessments of both ocular and systemic events were conducted at each study visit. The AEs were categorized according to severity (mild, moderate, or severe) and possible or known relationship to the study drug.
Statistical Methods
The study variables were summarized using standard descriptive statistics and normality of measures using the Shapiro-Wilk test, and distributed comparisons with and between groups were performed using 2-sided paired t tests. Where appropriate, sensitivity analyses using nonparametric Wilcoxon signed-rank tests were also performed. Analyses were performed using SAS software, v9.2. A significance level of .05 was assumed for all tests. Missing data were accounted for by using a linear mixed-effects model.
Results
Twenty eyes from 20 patients were included in the study, and all completed 12 months of follow-up. The mean age was 63.7 years (range, 45-78 years), and the study included 13 women (65%). The average time from DME diagnosis to the first study injection in the cohort was 1.37 years (standard deviation = 0.98; range, 0.3-3.9 years). The average number of anti-VEGF treatments in the 6 months prior to enrollment was 4.25 injections (range, 4-6 injections) with an average washout time of 44.5 days (± 21.2). Prior to enrollment, 95% of patients were injected with bevacizumab, whereas 5% were treated with ranibizumab. None of the patients received more than 1 type of anti-VEGF medication prior to study enrollment. Additionally, 5 patients had previous focal laser treatment and 2 had a fovea involving ERM. Table 1 highlights the participants’ demographic information.
Table 1.
Demographics and Ocular Characteristics.
| Baseline | 6 Mo | 12 Mo | P a | P b | |
|---|---|---|---|---|---|
| Demographics | |||||
| Eyes | 20 | ||||
| Right eyes | 11 | ||||
| Left eyes | 9 | ||||
| Average age at screening, y | 63.7 | ||||
| Age range, y | 45-78 | ||||
| Male | 7 | ||||
| Female | 13 | ||||
| Average No. of prior injections | 4.25 | ||||
| ETDRS scores, average (range) | |||||
| Study eye | 69.95 (60-81) | 71.5 (54-83) | 74 (56-85) | .38 | .10 |
| Fellow eye | 73.65 (37-85) | 75.4 (36-88) | 76.3 (47-90) | ||
| Diabetic retinopathy severity | |||||
| Mild | 0 (0%) | 0 (0%) | 2 (10%) | ||
| Moderate | 9 (45%) | 9 (45%) | 7 (35%) | ||
| Severe | 5 (25%) | 5 (25%) | 5 (25%) | ||
| PDR | 6 (30%) | 6 (30%) | 6 (30%) | ||
| OCT values, average (range) | |||||
| Study eye | |||||
| CST, µm | 419.7 (328-585) | 303.7 (198-485) | 287.2 (176-472) | < .001 | < .001 |
| Cube volume, mm3 | 11.5 (9.1-13.9) | 10.6 (8.4-13.2) | 9.6 (8.3-14.2) | < .001 | < .001 |
| Cube average thickness, µm | 320.7 (253-386) | 297.2 (234-368) | 287.8 (229-394) | < .001 | < .001 |
| Fellow eye | |||||
| CST, µm | 300.4 (181-432) | 287.4 (188-546) | 265.8 (178-534) | ||
| Cube volume, mm3 | 10.8 (8.8-12.8) | 10.4 (7.2-12.6) | 10.5 (8.9-14.5) | ||
| Average thickness, µm | 300.2 (246-354) | 290.3 (200-350) | 290.3 (246-404) | ||
| Injection frequency | |||||
| Q4-wk injections | 20 | 13 | 5 | ||
| Q8-wk injections | 0 | 7 | 15 |
Abbreviations: CST, central subfield thickness; ETDRS, Early Treatment Diabetic Retinopathy Study; OCT, optical coherence tomography; PDR, proliferative diabetic retinopathy; Q4-wk, every 4 weeks; Q8-wk, every 8 weeks.
aP value between baseline and 6 months.
bP value between baseline and 12 months.
DR severity was judged clinically at the baseline fundus examination, with 0 eyes classified as mild DR, 9 eyes (45%) classified as moderate nonproliferative DR (NPDR), 5 (25%) as severe NPDR, and 6 (30%) as nonactive proliferative DR (PDR). All 6 nonactive PDR patients had full panretinal photocoagulation at baseline.
Anatomic Outcomes
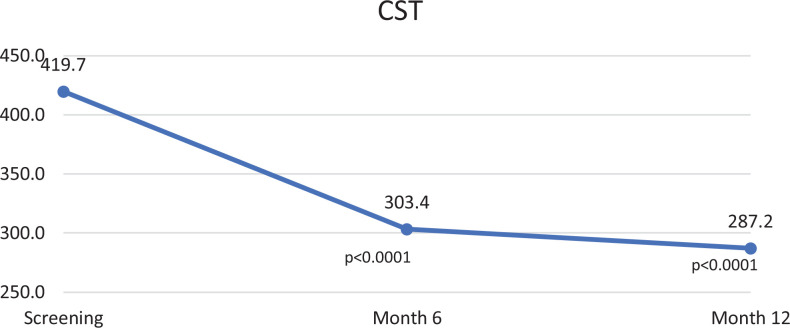
The changes in study variables from baseline to 12 months are summarized in Table 1. At baseline, CST was 419.7 ± 92.0 µm, cube volume was 11.5 ± 1.4 mm3, and cube average thickness was 320.7 ± 38.6 µm. The baseline average CST of the patients who were switched to an every-8-weeks injection regimen within the first 12 months (412.7 µm) was slightly smaller than the baseline average CST of all participants (419.7 µm; range, 328-584 µm), but this was not statistically significant (P = .83). At month 12, CST decreased by 31.6% to 287.2 ± 80.2 µm (P < .001), cube volume decreased by 9.6% to 10.4 ± 1.3 mm3 (P < .001), and cube average thickness decreased by 10.3% to 287.8 ± 35.4 µm (P < .001). The change in CST was statistically significant at both the 6- and 12-month interim analyses (Figure 2). Of note, both patients with a fovea involving ERM responded well to therapy and were transitioned to the every-8-weeks IAI group by 6 months.
Figure 2.
Change in central subfield thickness (CST) at each interim analysis.
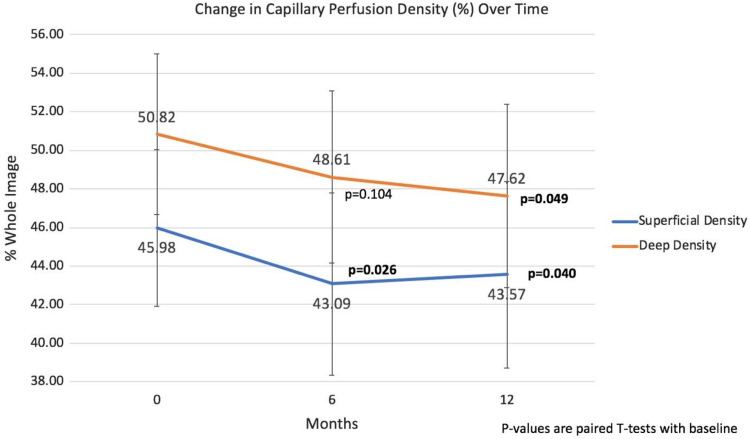
The average signal strength of all acquired OCTA images was 58.5 ± 9.0 at baseline, 57.0 ± 9.4 at month 6, and 56.9 ± 11.2 at month 12. The foveal avascular zone (FAZ) area did not demonstrate statistically significant changes from the baseline measurement of 0.29 mm3 ± 0.12 to 0.33 mm3 ± 0.15 at 12 months (P = .10). Furthermore, baseline superficial CPD was 46.0 ± 4.2% and deep CPD was 50.8 ± 4.3%. Both the superficial and deep CPD studies revealed statistically significant decreases in their values (Figure 3). The superficial CPD had decreased by 5.2% from a baseline of 46.0 ± 4.2% to 43.6 ± 4.8% at 12 months (P = .04), and the deep CPD had decreased by 6.3% from 50.8 ± 4.3% at baseline to 47.6 ± 4.8% by 12 months (P = .05).
Figure 3.
Change in capillary perfusion density through 12 months.
Visual Acuity
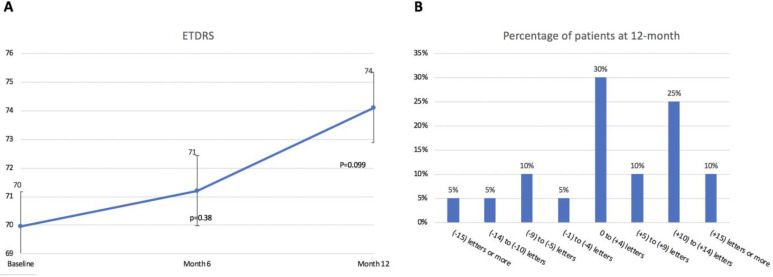
Figure 4A summarizes the BCVA measurements at baseline, 6 months, and 12 months. At baseline, BCVA was 70.0 letters and increased by +3.7 letters at month 12 but failed to reach statistical significance (P = .10). Additionally, by month 12, 6 patients (30%) gained +1 to +4 letters and 5 patients (25%) gained +10 to +14 letters. Figure 4B summarizes the VA changes through 12 months.
Figure 4.
(A) Change in best-corrected visual acuity (VA) at each interim analysis. (B) VA changes through 12 months. ETDRS, Early Treatment Diabetic Retinopathy Study.
At baseline, 65% (n = 13) of patients had 20/40 or better BCVA, whereas 35% of patients were 20/50 or worse. By 12 months, 85% (n = 17) of patients were 20/40 or better and 15% were 20/50 or worse. No patients were 20/200 or worse at baseline or 12 months.
In patients in the 20/40 or better group at baseline (average ETDRS 74.5 letters; Snellen equivalent, 20/32), there was a nonstatistically significant change seen at 6 months (average ETDRS 73.8 letters; Snellen, between 20/40 and 20/32, P = .76) and 12 months (average ETDRS 73.7 letters; Snellen, between 20/40 and 20/32, P = .75). In contrast, the 20/50 or worse group at baseline (average ETDRS 61.6 letters; Snellen, 20/63) had statistically significant improvements at 6 months (average ETDRS 67.3 letters; Snellen, 20/50, P = .04) and 12 months (average ETDRS 73.6 letters; Snellen, between 20/40 and 20/32, P < .001).
Treatment Frequency
The mean number of visits during the 12-month study was 9.5 (7-12) with a mean number of IAI treatments of 9.2 injections (range, 7-12 injections). By month 12, 15 patients (75%) met the study’s criteria for no intraretinal fluid and were transitioned to the every-8-weeks IAI, increasing from 7 patients at the 6-month interval. Patients underwent an average of 5.2 injections before switching to the every-8-weeks regimen. Five eyes (25%) still required injections every 4 weeks as compared with the 6-month time point of 13 patients (Table 1). All patients who were transitioned to the every-8-weeks treatment regimen remained in that cohort, as none needed to return to an every-4-weeks dosage. Through month 12, 13 fellow eyes received an average of 3.6 injections (range, 0-12 injections).
Evaluation by DR Severity
When analyzed by clinical DR severity, there was no statistically significant difference in baseline CST between the NPDR (401.6 µm) and PDR (461.8 µm) groups (P = .23). However, both groups with baseline NPDR and PDR showed a statistically significant decrease in CST at 12 months. Patients with baseline NPDR decreased by 101.9 µm (P < .001), and patients with baseline PDR decreased by 204.0 (P < .002).
No statistically significant change was observed in the FAZ area when eyes were evaluated based on DR severity. The baseline NPDR group (n = 14) had a FAZ area of 0.28 mm3 at baseline that increased to 0.31 mm3 at 12 months (P = .57). For the baseline PDR group (n = 6), the FAZ area had increased from a baseline of 0.34 mm3 to 0.38 mm3 at 12 months (P = .56).
Safety
There were 3 serious AEs during the 12-month follow-up, all of which occurred during the first 6 months of the study and were previously reported. 14 One patient suffered from dehydration, hypertension, and hyperglycemia at 2 months, another had bilateral leg cellulitis at month 6, and 1 patient developed chest pain and underwent cardiac catheterization at month 6.
Conclusions
The 12-month results’ primary analysis demonstrate continued significant anatomical improvements after switching from treatment with other anti-VEGF to a fixed dosage of IAI after a monthly loading phase. Compared with the 6-month findings, the 12-month results revealed additional reduction in the CST compared with baseline (27.6% reduction at 6 months vs 31.6% reduction at 12 months). On average, treatment frequency also decreased over time.
Across the entire cohort, there was no statistically significant improvement in BCVA. Interestingly, patients who initially had better vision (baseline BCVA of 20/40 or better) did not show a significant change in vision at months 6 and 12; however, patients with worse baseline vision (BCVA 20/50 or worse) showed statistically significant vision improvements at 6 and 12 months. We hypothesize that a ceiling effect may have limited the potential for improvement in the better VA group at baseline.
Several studies have evaluated the effects of switching anti-VEGF agents on DME outcomes. 16 -22 Prior studies showed that anatomic outcomes improved after switching, but they varied in their conclusions regarding VA, 18 with some reporting no improvements in VA 22 and others finding significant improvement. 16 -21 Our study demonstrated a statistically significant decrease in CST, but the BCVA improvement was not significant at 12 months across the entire cohort.
In this study, OCTA analysis revealed no significant difference in FAZ area through 12 months of treatment after switching to IAI. However, CPD measurements demonstrated a statistically significant decrease both in the superficial and deep layers. The implication of this has been investigated by prior studies. Samara et al discovered that there was a statistically significant direct correlation and found that decreases in the superficial and deep vascular CPD values were associated with lower VA. 23 Our study demonstrated an improvement of +3.7 letters in ETDRS BCVA from baseline through 12 months; however, these findings were not statistically significant. Studies by Agemy and colleagues 24 and Mastropasqua et al 25 concluded that CPD values in both the superficial and deep plexuses decrease as DR severity worsens. Another study by AttaAllah and colleagues evaluated CPD values in patients with diabetes with and without ME and compared them with controls. 26 Their study found that eyes with DME had significantly lower superficial and deep CPD values compared with controls. In addition, eyes with NPDR and DME had significantly reduced deep CPD values when compared with eyes with NPDR but without DME.
In this prospective study, statistically significant decreases in the superficial and deep CPD values occurred in clinical patients receiving fixed dosage of IAI treatment through 12 months. Prior studies have been retrospective 24 or observational analyses 25,27 and have not evaluated changes in CPD with fixed dosage of anti-VEGF agents. Decreases in the superficial and deep CPD values are somewhat counterintuitive in the setting of continued anti-VEGF therapy. Lower CPD values have been demonstrated in patients with diabetes but without DR. This is most likely because of capillary dropout within the microvascular network of the foveal region that may not be clinically evident at these early stages. With that in mind, it is possible that patients undergoing fixed dosage of anti-VEGF may continue to accumulate damage to these small capillary networks over time. It is also possible that CPD values in the setting of DME are less accurate than those obtained once the DME has resolved and could influence potential CPD changes over time in which the presence of intraretinal cystic changes produce artifact, affecting quantitative assessments. Further, DR severity may play a role in how CPD values change with anti-VEGF therapy.
This study was not adequately powered to evaluate specific changes in CPD values between or within different levels of DR severity. Further studies evaluating OCTA CPD analysis in the setting of fixed anti-VEGF dosage are needed to determine how DME and DR severity score may influence these changes.
Strengths of this study include the prospective nature evaluating switching anti-VEGF therapy to a fixed dosage and uniform extension criteria. In addition, this study evaluated changes in OCTA metrics prospectively in each treatment cohort. There are several limitations of this prospective study, including a relatively small sample size of 20 patients and a lack of a standardized treatment regimen with other anti-VEGF drugs prior to study entry. Further, 95% of patients were treated with bevacizumab before enrollment vs only 5% with ranibizumab, which could induce selection bias. Some selection bias may have been incurred through investigators’ determination of study-eye enrollment when both eyes were affected by the disease. Certainly, analyzing follow-up through 24 months will strengthen the results of this study.
The interim analysis of the SWAP-TWO Study offers useful clinical information regarding switching anti-VEGF agents to a fixed IAI dosage after a loading phase that continued anatomical improvements over 12 months. Further analysis at the 24-month primary outcome will yield durability and sustainability effects of this dosing regimen.
Footnotes
Authors’ Note: This paper was presented as a poster at the Association for Research in Vision and Ophthalmology Annual Meeting, April 29, 2019, in Vancouver, Canada.
Ethical Approval: This study received approval from the Cleveland Clinic Institutional Review Board and was conducted in accordance with the Declaration of Helsinki. The collection and evaluation of all protected patient health information were performed in a Health Insurance Portability and Accountability Act (HIPAA)–compliant manner.
Statement of Informed Consent: Informed consent was obtained prior to performing the procedure, including permission for publication of all photographs and images included herein.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by an unrestricted research grant from Regeneron Pharmaceuticals Inc.
References
- 1. Sabanayagam C, Yip W, Ting DS, Tan G, Wong TY. Ten emerging trends in the epidemiology of diabetic retinopathy. Ophthalmic Epidemiol. 2016;23(4):209–222. doi:10.1080/09286586.2016.1193618 [DOI] [PubMed] [Google Scholar]
- 2. Nguyen QD, Tatlipinar S, Shah SM, et al. Vascular endothelial growth factor is a critical stimulus for diabetic macular edema. Am J Ophthalmol. 2006;142(6):961–969. doi:10.1016/j.ajo.2006.06.068 [DOI] [PubMed] [Google Scholar]
- 3. Funatsu H, Yamashita H, Noma H, Mimura T, Yamashita T, Hori S. Increased levels of vascular endothelial growth factor and interleukin-6 in aqueous humor of diabetics with macular edema. Am J Ophthalmol. 2002;133(1):70–77. doi:10.1016/s0002-9394(01)01269-7 [DOI] [PubMed] [Google Scholar]
- 4. Solomon SD, Chew E, Duh EJ, et al. Diabetic retinopathy: a position statement by the American Diabetic Association. Diabetes Care. 2017;30(3):412–418. doi:10.2337/dc16-2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mitchell P, Bandello F, Schmidt Erfurth, et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118(4):615–625. doi:10.1016/j.ophtha.2011.01.031 [DOI] [PubMed] [Google Scholar]
- 6. Nguyen QD, Shah SM, Khwaja AA, et al. Two-year outcomes of ranibizumab for edema of the macula in diabetes (READ-2) study. Ophthalmology. 2010;117(11):2146–2151. doi:10.1016/j.ophtha.2010.08.016 [DOI] [PubMed] [Google Scholar]
- 7. Nguyen QD, Brown DM, Marcus DM. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119(4):789–801. doi:10.1016/j.ophtha.2011.12.039 [DOI] [PubMed] [Google Scholar]
- 8. Michaelides M, Kaines A, Hamilton RD, et al. A prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT study) 12-month data: report 2. Ophthalmology. 2010;117(6):1078–1086. doi:10.1016/j.ophtha.2010.03.045 [DOI] [PubMed] [Google Scholar]
- 9. Korobelnik JF, Do DV, Schmidt-Erfurth U, et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014;121(11):2247–2254. doi:10.1016/j.ophtha.2014.05.006 [DOI] [PubMed] [Google Scholar]
- 10. Wells JA, Glassman AR, Ayala AR, et al. Diabetic Retinopathy Clinical Research Network. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372(13):1193–1203. doi:10.1056/NEJMoa1414264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schmidt-Erfurth U, Lang GE, Holz FG, et al. Three-year outcomes of individualized ranibizumab treatment in patients with diabetic macular edema: the RESTORE extension study. Ophthalmology. 2014;121(5):1045–1053. doi:10.1016/j.ophtha.2013.11.041 [DOI] [PubMed] [Google Scholar]
- 12. Elman MJ, Bressler NM, Qin H, et al. Diabetic Retinopathy Clinical Research Network. Expanded 2-year follow-up of ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2011;118(4):609–614. doi:10.1016/j.ophtha.2010.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bressler NM, Beaulieu WT, Glassman AR, et al. Persistent macular thickening following intravitreous aflibercept, bevacizumab, or ranibizumab for central-involved diabetic macular edema with vision impairment: a secondary analysis of a randomized clinical trial. JAMA Ophthalmol. 2018;126(3):257–269. doi:10.1001/jamaophthalmol.2017.6565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Babiuch A, Conti T, Conti F, et al. Diabetic macular edema treated with intravitreal aflibercept injection after treatment with other anti-VEGF agents (SWAP-TWO Study): 6-month interim analysis. Int J Retina Vitreous. 2019;5:17. doi:10.1186/s40942-019-0167-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Beck RW, Moke PS, Turpin AH, et al. A computerized method of visual acuity testing: adaptation of the Early Treatment of Diabetic Retinopathy Study testing protocol. Am J Ophthalmol. 2003;135(2):194–205. doi:10.1016/s0002-9394(02)01825-1 [DOI] [PubMed] [Google Scholar]
- 16. Bahrami B, Hong T, Schlub TE, Chang AA. Aflibercept for persistent diabetic macular edema: forty-eight-week outcomes. Retina. 2018;39(1):61–68. doi:10.1097/IAE.0000000000002253 [DOI] [PubMed] [Google Scholar]
- 17. Laiginhas R, Silva MI, Rosas V, et al. Aflibercept in diabetic macular edema refractory to previous bevacizumab: outcomes and predictors of success. Graefes Arch Clin Exp Ophthalmol. 2018;256(1):83–89. doi:10.1007/s00417-017-3836-1 [DOI] [PubMed] [Google Scholar]
- 18. Banaee T, Ashraf M, Conti FF, Singh RP. Switching anti-VEGF drugs in the treatment of diabetic macular edema. Ophthalmic Surg Lasers Imaging Retina. 2017;48(9):748–754. doi:10.3928/23258160-20170829-10 [DOI] [PubMed] [Google Scholar]
- 19. Ashraf M, Souka AA, ElKayal H. Short-term effects of early switching to ranibizumab or aflibercept in diabetic macular edema cases with non-response to bevacizumab. Ophthalmic Surg Lasers Imaging Retina. 2017;48(3):230–236. doi:10.3928/23258160-20170301-06 [DOI] [PubMed] [Google Scholar]
- 20. Shah CP, Heier JS. Aflibercept for diabetic macular edema in eyes previously treated with ranibizumab and/or bevacizumab may further improve macular thickness. Ophthalmic Surg Lasers Imaging Retina. 2016;47(9):836–839. doi:10.3928/23258160-20160901-06 [DOI] [PubMed] [Google Scholar]
- 21. Lim LS, Ng WY, Mathur R, et al. Conversion to aflibercept for diabetic macular edema unresponsive to ranibizumab or bevacizumab. Clin Ophthalmol. 2015;9:1715–1718. doi:10.2147/OPTH.S81523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee JH, Lee WK, Kim SE. Short-term outcomes of switching to ranibizumab therapy for diabetic macular edema in patients with persistent fluid after bevacizumab therapy. J Ocul Pharmacol Ther. 2016;32(10):659–664. doi:10.1089/jop.2016.0074 [DOI] [PubMed] [Google Scholar]
- 23. Samara WA, Shahlaee A, Adam MK, et al. Quantification of diabetic macular ischemia using optical coherence tomography angiography and its relationship with visual acuity. Ophthalmology. 2017;124(2):235–244. doi:10.1016/j.ophtha.2016.10.008 [DOI] [PubMed] [Google Scholar]
- 24. Agemy SA, Scripsema NK, Shah CM, et al. Retinal vascular perfusion density mapping using optical coherence tomography angiography in normal and diabetic retinopathy patients. Retina. 2015;35(11):2353–2363. doi:10.1097/IAE.0000000000000862 [DOI] [PubMed] [Google Scholar]
- 25. Mastropasqua R, Toto L, Mastropasqua A, et al. Foveal avascular zone area and parafoveal vessel density measurements in different stages of diabetic retinopathy by optical coherence tomography angiography. Int J Ophthalmol. 2017;10(10):1545–1551. doi:10.18240/ijo.2017.10.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. AttaAllah HR, Mohamed AAM, Ali MA. Macular vessel density in diabetic retinopathy: quantitative assessment using optical coherence tomography angiography. Int Ophthalmol. 2019;39(8):1845–1859. doi:10.1007/s10792-018-1013-0 [DOI] [PubMed] [Google Scholar]
- 27. Wilkinson CP, Ferris FL III, Klein RE, et al. Global Diabetic Retinopathy Project Group. Proposed international clinical diabetic retinopathy and diabetic retinopathy macular edema disease severity scales. Ophthalmology. 2003;110(9):1677–1682. doi:10.1016/S0161-6420(03)00475-5 [DOI] [PubMed] [Google Scholar]