Abstract
Purpose:
This work evaluates dosing frequency with intravitreal antivascular endothelial growth factor (anti-VEGF) agents over 2 years and visual acuity (VA) outcomes in neovascular age-related macular degeneration (nAMD).
Methods:
This retrospective analysis assesses electronic medical record data (Vestrum Health treatment and outcomes database) of newly diagnosed nAMD in patients who were initiated on intravitreal anti-VEGF treatment at US clinical sites. Eyes were divided into 2 injection frequency subcohorts (≤ 6 or > 6 injections/y); treatment frequency and change in mean VA (Early Treatment Diabetic Retinopathy Study letters) were evaluated.
Results:
Overall, 8127 of 213 824 eyes met inclusion criteria in year 1 and 4968 in year 2. During year 1, 77% of the eyes received more than 6 injections (n = 6287), the majority of which received injections at the same frequency during year 2. Mean VA gain from baseline at year 1 was lower in the ≤ 6 than > 6 injections/y subcohort (2.2 vs 6.5, P < .001). Decrease in mean VA from the end of year 1 to year 2 was significantly greater for eyes administered 6 or fewer injections in year 2 than those that received more frequent injections, irrespective of the frequency of injections in the first year.
Conclusions:
In routine clinical practice, most eyes with nAMD that completed at least 1 year of follow-up were treated with more than 6 injections of anti-VEGF agents during the first year of treatment, resulting in better VA gains than eyes treated less frequently during the same period.
Keywords: antivascular endothelial growth factor, bevacizumab, intravitreal aflibercept, neovascular age-related macular degeneration, ranibizumab, real-world outcomes, treatment frequency, visual acuity
Introduction
Age-related macular degeneration (AMD) is the leading cause of irreversible blindness in adults older than 50 years. 1 Over the past decade, intravitreal antivascular endothelial growth factor (anti-VEGF) agents have become the standard of care for neovascular AMD (nAMD). Multiple large trials have repeatedly demonstrated the benefit of intravitreal anti-VEGF therapy with either fixed dosing (monthly or bimonthly) or variable dosing with monthly monitoring. 2 -5
The dosing regimens evaluated in the clinical trial setting have not been completely mirrored in routine clinical practice, where variable dosing regimens have flourished in light of multiple factors related to disease, cost, and physician preference. 6 However, emerging evidence suggests that discontinuous—which leads to less frequent—dosing of anti-VEGF agents in patients with nAMD in routine clinical practice may lead to suboptimal outcomes. 7 Based on this hypothesis, this study was designed to assess the association between dosing frequency of intravitreal anti-VEGF agents, irrespective of the anti-VEGF used, and visual outcomes in patients with nAMD in routine clinical practice.
Methods
Data Source
Deidentified electronic medical records of patients with AMD in the Vestrum Health treatment and outcomes database (Vestrum Health, Naperville, Illinois) were analyzed. These records were collected from 251 retina specialists at 54 private clinics in the United States and included information about demographics, procedures performed, diseases diagnosed, medications prescribed, and treatment outcomes. Data were extracted from the database using structured query language queries. Institutional review board approval was not sought because this is not generally required for studies such as this in which data collection is in the form of historical, deidentified patient electronic health records and does not affect or influence patients’ treatment.
Study Population
The study population consisted of patients who were diagnosed with nAMD and administered their first (index) anti-VEGF injection (bevacizumab, ranibizumab, or aflibercept) between January 1, 2012, and April 30, 2015. Eyes were included in the study if they had a visual acuity (VA) reading on the index date, at month 12, and at least once during each quarter of the study period. VA at month 12 was identified as the reading closest to 12 months following the index date, between months 11 and 12. Eyes were excluded if there was a break from treatment for longer than 11 months at any point in the 24 months following the index date and if sex information was not recorded. Only VA readings from accepted measurements were used (distance-corrected, near corrected, or pinhole). To ensure comparable results, all VA measurements for an individual patient were required to use the same methodology; VA was calculated using the formula: Early Treatment Diabetic Retinopathy Study letters = 85 – (50 × logarithm of the minimum angle of resolution).
Observation Period
All patients were observed for 12 to 24 months following the index injection. The observation period began January 1, 2012, and ended April 30, 2017, inclusive of all eyes.
Cohorts
For data analysis, eyes were divided into 2 cohorts based on prior evidence 8 : year 1 cohort (eyes that were treated for ≥ 1 year) and year 2 cohort (eyes that were treated for 2 years). Each of these cohorts was further divided into 2 subcohorts based on whether 6 or fewer injections or more than 6 injections were administered per year (hereafter referred to as the ≤ 6-injections and > 6-injections subcohorts, respectively).
Statistical Methods
Descriptive statistics were calculated for the year 1 and year 2 cohorts to identify changes in injection frequency and VA over time. Paired t tests were used to determine whether the changes in VA over time were significant. Independent t tests assuming unequal variance were used to determine whether the differences in VA change between cohorts were significant. Calculations were performed using Microsoft Excel, and P less than .05 was considered statistically significant.
Results
Patient Disposition
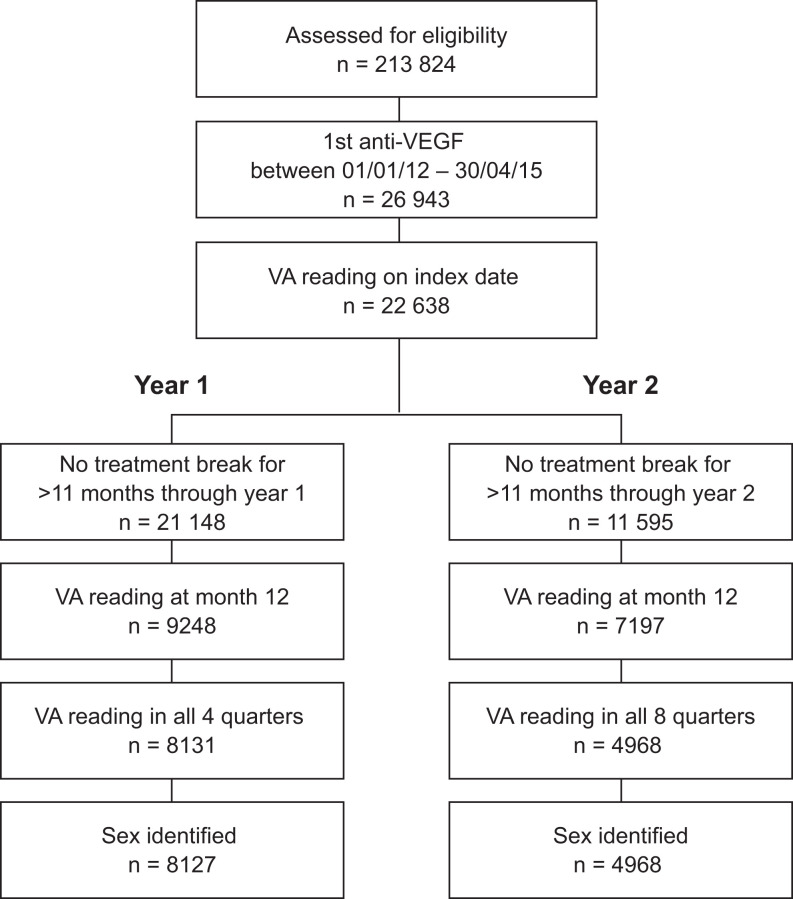
A total of 213 824 patients with AMD were assessed for eligibility (Figure 1). Of these, 22 638 received their first anti-VEGF injection between January 1, 2012, and April 30, 2015, and had a VA reading the same day as the index injection. After excluding patients who did not have all required quarterly VA readings or sex identification and those who had treatment breaks longer than 11 months during follow-up, 8127 and 4968 patients were included in the year 1 and year 2 cohorts, respectively. Of note, 62% (n = 5077) of patients in the year 1 cohort qualified for inclusion in the year 2 cohort, but 15% (n = 1223) did not have any visits in year 2.
Figure 1.
Patient disposition. VA indicates visual acuity; VEGF, vascular endothelial growth factor.
Demographic and Baseline Characteristics
In the year 1 cohort, the mean age was 80 years, and 64% of patients were female. The mean VA at baseline was 50 letters (Table S1). Generally, baseline demographics were similar in the ≤ 6-injections and > 6-injections subcohorts; however, the ≤ 6-injections subcohort had a lower mean VA (46 vs 51 letters), a higher proportion of eyes with a VA of less than 35 letters (24% vs 15%), and a lower proportion of eyes with a VA between 65 and 85 letters (39% vs 49%) than the > 6-injections subcohort.
Year 1 Outcomes
For eyes that were treated for 1 year or more, those in the ≤ 6-injections subcohort received a mean of 4.5 injections (range, 1-6 injections) in the first year, and eyes in the > 6-injections subcohort received a mean of 9.1 injections (range, 7-17 injections) in the first year (Table 1).
In the ≤ 6-injections subcohort, mean VA increased by 4.3 letters from 45.5 letters at baseline to 49.8 letters in the first quarter of year 1. Mean VA then decreased slightly to 47.7 letters at the end of year 1 but remained significantly higher than at baseline (P < .001) (Figure 2A). The mean VA gain of 4.3 letters in the first quarter coincided with the highest quarterly mean number of injections (2.4; see Table 1). Overall, after 1 year of treatment, the mean VA gain from baseline was 2.2 letters in the ≤ 6-injections subcohort.
Figure 2.
Visual acuity (VA) outcomes over 2 years. (A) Mean Early Treatment Diabetic Retinopathy Study (ETDRS) letters through year 1 in the ≤ 6-injections and > 6-injections subcohorts. Horizontal dotted lines indicate baseline (BSL) ETDRS. (B) Mean ETDRS letters at the start of year 2 (light gray bars) and end of year 2 (dark gray bars) by injection frequency in eyes that received 6 or fewer injections in year 1. (C) Mean ETDRS letters at the start of year 2 (light gray bars) and end of year 2 (dark gray bars) by injection frequency in eyes that received more than 6 injections in year 1. *P less than .001 for the gain in mean ETDRS letters from BSL in the ≤ 6-injections vs > 6-injections subcohort. †P equals .03 for the gain in mean ETDRS letters from BSL in the ≤ 6-injections vs > 6-injections subcohort. Q indicates quarter.
Table 1.
Number of Antivascular Endothelial Growth Factor Injections Administered During Year 1 in the Year 1 Cohort.
| Cohort | No. of injections | ||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Year 1 | |
| All eyes (n = 8127) | |||||
| Mean injections | 2.9 | 1.8 | 1.6 | 1.7 | 8.1 |
| Range | 1-6 | 0-5 | 0-6 | 0-6 | 1-17 |
| ≤ 6 injections (n = 1840) | |||||
| Mean injections | 2.4 | 0.8 | 0.6 | 0.7 | 4.5 |
| Range | 1-5 | 0-3 | 0-3 | 0-4 | 1-6 |
| > 6 injections (n = 6287) | |||||
| Mean injections | 3.1 | 2.1 | 1.9 | 2.0 | 9.1 |
| Range | 1-6 | 0-5 | 0-6 | 0-6 | 7-17 |
Abbreviation: Q, quarter.
In the > 6-injections subcohort, mean VA increased by 5.9 letters from 51.1 at baseline to 57.0 letters in the first quarter of year 1. Mean VA then remained relatively stable and was significantly higher (P < .001) at the end of year 1 than at baseline (see Figure 2A). After 1 year of treatment, the mean VA gain from baseline was 6.5 letters in the > 6-injections subcohort. The mean VA gain was significantly greater in the > 6-injections subcohort than in the ≤ 6-injections subcohort at year 1 (+6.5 vs +2.2, respectively; P < .001).
Year 2 Outcomes
In the year 2 cohort, 67.8% (415 of 613) of eyes that received 6 or fewer injections in year 1 continued to receive 6 or fewer injections in year 2. Eyes in this subcohort were administered a mean of 5.2 and 4.4 injections in years 1 and 2, respectively. These eyes started year 2 with a mean VA of 56.4 letters and ended year 2 with a mean of 52.5 Early Treatment Diabetic Retinopathy Study letters, corresponding to a mean VA loss of 3.9 letters. Eyes that received 6 or fewer injections in year 1 but more than 6 injections in year 2 received a mean of 5.3 and 8.2 injections in years 1 and 2, respectively. These eyes started year 2 with a VA of 60.8 letters and ended year 2 with a mean VA of 60.3 letters, corresponding to a mean VA loss of 0.5 letters. The mean VA loss for eyes that remained in the ≤ 6-injections subcohort for both years 1 and 2 was significantly greater than that of eyes that switched to the > 6-injections subcohort in year 2 (–3.9 vs –0.5, respectively; P = .01; Figure 2B).
A total of 67.8% (2954 of 4355) of eyes in the year 2 cohort that received more than 6 injections in year 1 continued to receive more than 6 injections in year 2. Eyes in this subcohort were administered a mean of 9.7 and 9.1 injections in years 1 and 2, respectively. These eyes started year 2 with a mean VA of 61.6 letters and ended year 2 with a mean VA of 59.4 letters, corresponding to a mean VA loss of 2.2 letters. Eyes that received more than 6 injections in year 1 but 6 or fewer injections in year 2 received a mean of 8.5 and 5.0 injections in years 1 and 2, respectively. These eyes started year 2 with a mean VA of 57.5 letters and ended year 2 with a mean VA of 54.2, corresponding to a mean VA loss of 3.3 letters. The mean VA loss for eyes that remained in the > 6-injections subcohort for both years 1 and 2 was significantly less than that of eyes that switched to the ≤ 6-injections subcohort in year 2 (–2.2 vs –3.3, respectively; P = .03; Figure 2C).
Annual Trend in Treatment Frequency
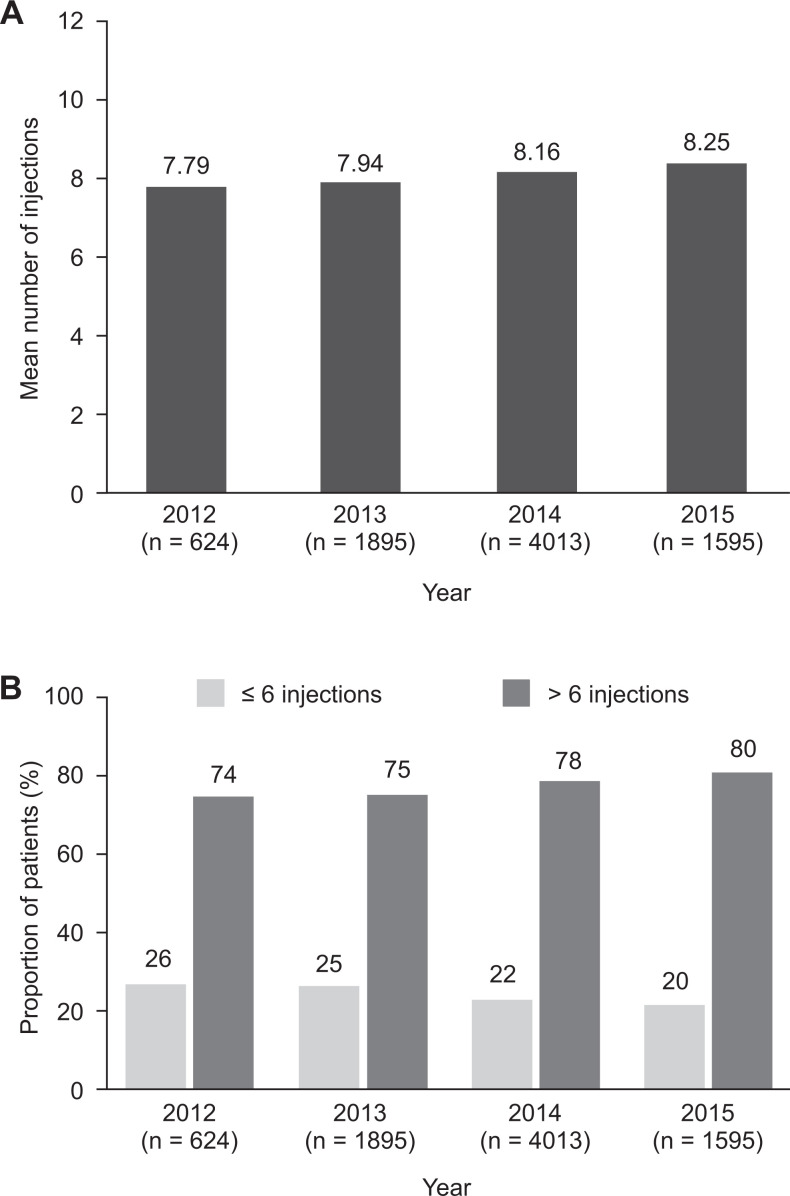
There was a trend toward a numerical increase in the mean number of anti-VEGF injections received during year 1 over calendar years 2012–2015 (Figure 3A). In addition, a numerically greater proportion of patients received more than 6 injections during year 1 in calendar year 2015 (80%) than in calendar year 2012 (74%) (Figure 3B).
Figure 3.
Change over time in injection frequency during the first year of treatment in the year 1 cohort. (A) Annual mean number of injections per patient in each calendar year of the analysis. (B) Proportion of patients in each calendar year that received 6 or fewer or more than 6 injections during the first year of treatment.
Conclusions
Careful treatment of patients with nAMD in clinical practice is important to improve vision and subsequently maintain the visual gains initially achieved with anti-VEGF therapy. Our study demonstrates that most eyes with at least 1 year of follow-up across multiple community-based retina clinics were treated with more than 6 injections during the first year, resulting in VA gains over time comparable to those seen in randomized clinical trials. The mean change in VA over time in our study followed a pattern commonly observed in clinical trials—an initial gain during the first 3 months followed by maintenance of vision. This was achieved by eyes receiving on average 3 initial monthly doses followed by dosing approximately every 6 weeks (mean, ∼2 injections/quarter). To our knowledge, this is the first study to demonstrate comparable VA change over time between routine clinical practice and randomized clinical trials across a large cohort of eyes with nAMD with similar numbers of injections.
Conversely, eyes that received less frequent dosing over the first year started with fewer than 3 initial monthly doses on average, which subsequently decreased to less than 1 injection per quarter for the remainder of the year, resulting in expected suboptimal visual gains. Interestingly, these patients presented with lower mean VA at baseline than those in the subcohort that received more frequent injections in the first year (∼20/126 vs 20/100). It is commonly believed that patients with lower VA, which would be indicative of more severe disease, tend to require more frequent injections, not less frequent injections. Although it is not possible to draw definitive conclusions regarding the reasons for this unexpected finding, physician and patient factors both could have been involved. For example, some patients could have had atrophy at baseline that worsened over the course of the first year of treatment; this could have decreased the impetus for physicians to continue frequent injections. Conversely, patients with good visual potential at baseline may have been treated more aggressively to maintain and further optimize visual function and to avoid loss of vision.
Although some patients shifted from one treatment-frequency subcohort to another between the first and second years, the majority (∼68%) remained in the same treatment frequency subcohort for both year 1 and year 2. This may indicate that physicians tend to avoid switching treatment frequency unless major changes in functional or anatomic outcomes are observed, but additional study into physician practices is warranted.
However, eyes with less frequent dosing during the second year had significantly greater decrease in vision from the end of the first year compared with those eyes that received more than 6 doses, irrespective of the dosing frequency in the first year. This suggests that frequent dosing in year 2 is needed to maintain the visual improvements gained in year 1. However, because no additional visual improvement was seen when treatment frequency was increased in the second year of follow-up, this implies that delayed intensification of dosing may preclude eyes from having optimal visual outcomes. Together, these data highlight the importance of optimal treatment frequency throughout the course of care in the preservation of visual improvements in patients undergoing treatment of nAMD.
Of note, we observed nominal, but steady, linear increases in the mean number of anti-VEGF injections administered in year 1 during the study period (2012-2015). Our observations are consistent with other studies that have reported an increase in the mean number of injections performed annually in routine clinical practice. 9,10 This suggests that retina specialists continue to adopt treatment paradigms for nAMD that more closely reflect dosing regimens from large clinical trials. Long-term data supporting the benefit of frequent and consistent treatment on maintaining vision may have influenced this growing trend.
The relationship between treatment frequency and visual outcomes observed in the present analysis is supported by other large, real-world studies of anti-VEGF use in patients with nAMD. 8,11 -13 Most recently, Ciulla et al 7 concluded that VA outcomes in clinical practices in the United States are suboptimal compared with those observed in randomized clinical trials. However, they focused on the relationship between vision change and duration of follow-up, whereas our study specifically evaluated 2 subcohorts based on injection frequency to explore whether the results from clinical trials are replicated in clinical practice. Our findings are similar to the analysis by Peden and colleagues of patients with nAMD demonstrating that with fixed and frequent injections, VA can be maintained over an extended period of time. 13
There are inherent limitations to this retrospective database analysis. Data were analyzed using only descriptive statistics; no adjustments were made for possible heterogeneity across subcohorts, which could have biased our findings. In addition, we were unable to explore the patient-specific factors (eg, clinical course, fixed vs variable dosing interval) that may have influenced treatment frequency in individual patients. Because our objective was to obtain a true understanding of the association between dosing frequency and visual outcomes in clinical practice, we specifically excluded eyes for which complete vision data, as we previously defined, were not available, hence limiting the size of our sample population.
In conclusion, the present analysis provides clinically relevant information on the importance of dosing frequency of anti-VEGF agents in attaining and sustaining visual gains over a 2-year period in patients with nAMD. These real-world data support the findings of more structured, prospective, randomized controlled clinical trials, wherein frequent and consistent dosing resulted in more favorable visual outcomes. The present study recapitulates this observation using data gleaned from routine clinical practice in diverse settings. Additional study is needed to further understand the physician- and patient-specific factors that influence the frequency of anti-VEGF injections in routine clinical practice and their impact on visual outcomes.
Supplemental Material
Supplement for Visual Acuity Outcomes in Patients Receiving Frequent Treatment of Neovascular Age-Related Macular Degeneration in Clinical Practice by Andrew A. Moshfeghi, John D. Pitcher, Genevieve Lucas, Nick Boucher and Namrata Saroj in Journal of VitreoRetinal Diseases
Footnotes
Authors’ Notes: The results of this study were presented at the 2018 American Society of Retina Specialists Annual Meeting, July 20 to 25, 2018, in Vancouver, British Columbia, Canada. Qualified researchers may request access to study documents (including the clinical study report, study protocol with any amendments, blank case report form, and statistical analysis plan) that support the methods and findings reported in this manuscript. Individual anonymized participant data will be considered for sharing once the product and indication have been approved by major health authorities (Food and Drug Administration, European Medicines Agency, Pharmaceuticals and Medical Devices Agency, etc) if there is legal authority to share the data, and there is not a reasonable likelihood of participant reidentification. Submit requests to https://vivli.org/.
Ethical Approval: Deidentified electronic medical records of patients with AMD in the Vestrum Health treatment and outcomes database constitute a limited data set in which all patient identifiers have been completely removed and site and clinician data have been pseudoanonymized. On this basis, formal ethics approval is not required, and formal ethics approval was not obtained.
Statement of Informed Consent: Informed consent was not required for this study because deidentified electronic medical records of patients with AMD in the Vestrum Health treatment and outcomes database constitute a limited data set.
The author(s) declare the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Andrew A. Moshfeghi has served as a consultant to Allegro, Allergan, Alimera, Graybug, Regeneron Pharmaceuticals, Inc, Genentech, Clearside, Bausch, EyePoint, and Novartis and as a speaker for Allergan. He has also received research support from Allegro, Novartis, Genentech, and Regeneron Pharmaceuticals, Inc and holds equity interest in Pr3vent, OptiSTENT, and Visunex. John D. Pitcher has served as a consultant to Allergan, Genentech, Novartis, Regeneron Pharmaceuticals, Inc, and REGENXBIO and as a speaker for Genentech, Novartis, and Regeneron Pharmaceuticals, Inc. He has also received research support from Alcon. Genevieve Lucas was a salaried employee of Vestrum Health at the time that these analyses were undertaken. Nick Boucher is a salaried employee of Vestrum Health. Namrata Saroj was a salaried employee of Regeneron Pharmaceuticals, Inc at the time that these analyses were undertaken and is currently a paid consultant for Regeneron Pharmaceuticals, Inc. Dr Saroj also serves as a consultant for Aerie, Allegro, Apellis, Adverum, and SamaCare and is an equity owner in Allegro, Pr3vent, and SamaCare.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by an unrestricted grant from Research to Prevent Blindness (New York, New York) to the Department of Ophthalmology, University of Southern California Roski Eye Institute, Keck School of Medicine, Los Angeles, California; and by Regeneron Pharmaceuticals, Inc. Medical writing support was provided by Melissa Purves, PhD, of Prime (Knutsford, UK) according to Good Publication Practice guidelines and was funded by Regeneron Pharmaceuticals, Inc. Regeneron Pharmaceuticals, Inc participated in the design and conduct of the study, analysis of the data, and preparation of the manuscript.
Supplemental Material: Supplemental material is available online with this article.
References
- 1. Pennington KL, DeAngelis MM. Epidemiology of age-related macular degeneration (AMD): associations with cardiovascular disease phenotypes and lipid factors. Eye Vis (Lond). 2016;3:34. doi:10.1186/s40662-016-0063-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rosenfeld PJ, Brown DM, Heier JS, et al. ; MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–1431. doi:10.1056/NEJMoa054481 [DOI] [PubMed] [Google Scholar]
- 3. Brown DM, Kaiser PK, Michels M, et al. ; ANCHOR Study Group. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432–1444. doi:10.1056/NEJMoa062655 [DOI] [PubMed] [Google Scholar]
- 4. Heier JS, Brown DM, Chong V, et al. ; VIEW 1 and VIEW 2 Study Groups. Intravitreal aflibercept (VEGF Trap-Eye) in wet age-related macular degeneration. Ophthalmology. 2012;119(12):2537–2548. doi:10.1016/j.ophtha.2012.09.006 [DOI] [PubMed] [Google Scholar]
- 5. CATT Research Group; Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364(20):1897–1908. doi:10.1056/NEJMoa1102673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haller JA. Current anti-vascular endothelial growth factor dosing regimens: benefits and burden. Ophthalmology. 2013;120(5 suppl):S3–S7. doi:10.1016/j.ophtha.2013.01.057 [DOI] [PubMed] [Google Scholar]
- 7. Ciulla T, Huang F, Westby K, Williams DF, Zaveri S, Patel SC. Real-world outcomes of anti-vascular endothelial growth factor therapy in neovascular age-related macular degeneration in the United States. Ophthalmol Retina. 2018;2(7):645–653. doi:10.1016/j.oret.2018.01.006 [DOI] [PubMed] [Google Scholar]
- 8. Holz FG, Tadayoni R, Beatty S, et al. Key drivers of visual acuity gains in neovascular age-related macular degeneration in real life: findings from the AURA study. Br J Ophthalmol. 2016;100(12):1623–1628. doi:10.1136/bjophthalmol-2015-308166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kataja M, Hujanen P, Huhtala H, Kaarniranta K, Tuulonen A, Uusitalo-Jarvinen H. Outcome of anti-vascular endothelial growth factor therapy for neovascular age-related macular degeneration in real-life setting. Br J Ophthalmol. 2018;102(7):959–965. doi:10.1136/bjophthalmol-2017-311055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wecker T, Grundel B, Reichl S, et al. Anti-VEGF injection frequency correlates with visual acuity outcomes in pro re nata neovascular AMD treatment. Sci Rep. 2019;9(1):3301. doi:10.1038/s41598-019-38934-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eldem B, Lai TYY, Ngah NF, et al. An analysis of ranibizumab treatment and visual outcomes in real-world settings: the UNCOVER study. Graefes Arch Clin Exp Ophthalmol. 2018;256(5):963–973. doi:10.1007/s00417-017-3890-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Holz FS, Souied E, Tuli R, Lacey S, Macfadden W, LS Group. Effectiveness of ranibizumab for the treatment of neovascular age-related macular degeneration: twelve-month results from the final analysis of the real-world LUMINOUS study. Presented at: Free Paper Session 14: AMD IV, the 17th EURETINA Congress, September 8, 2017; Barcelona, Spain. [Google Scholar]
- 13. Peden MC, Suñer IJ, Hammer ME, Grizzard WS. Long-term outcomes in eyes receiving fixed-interval dosing of anti-vascular endothelial growth factor agents for wet age-related macular degeneration. Ophthalmology. 2015;122(4):803–808. doi:10.1016/j.ophtha.2014.11.018 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement for Visual Acuity Outcomes in Patients Receiving Frequent Treatment of Neovascular Age-Related Macular Degeneration in Clinical Practice by Andrew A. Moshfeghi, John D. Pitcher, Genevieve Lucas, Nick Boucher and Namrata Saroj in Journal of VitreoRetinal Diseases