Abstract
Purpose:
This report describes a patient with bilateral, sequential central retinal artery occlusions (CRAOs) due to infective endocarditis (IE).
Methods:
A case report is presented.
Results:
A 35-year-old man with IE who recently completed a course of intravenous antibiotic therapy presented with sudden right-eye vision loss. Examination revealed hand motion vision, a cherry-red spot in the macula in the right eye, and an embolus in the inferotemporal arcade of the left eye. The diagnosis of right-eye CRAO secondary to IE was made, with the presumed source being his dental caries. The patient was admitted with plans for aortic valve replacement and dental extraction. During his hospitalization, the patient suffered from a CRAO in his left eye, resulting in bilateral loss of vision.
Conclusions:
IE can have severe embolic complications; prompt diagnosis and treatment medically and surgically are necessary to reduce further morbidity and mortality.
Keywords: central retinal artery occlusion, infective endocarditis, septic emboli, vegetation
Introduction
Case Report
A 35-year-old Hispanic man presented to the emergency department for evaluation of acute, painless vision loss that occurred 2 days prior. His medical history included infective endocarditis (IE) with Streptococcus mitis and S oralis bacteremia complicated by aortic valve regurgitation, which was diagnosed 1 month prior at an outside hospital and for which he completed 4 weeks of intravenous antibiotics. The patient admitted to alcohol consumption but denied cigarette smoking or illicit drug use. Review of systems was positive for dental pain due to multiple dental caries.
At initial presentation, his best-corrected visual acuity was hand motion vision in the right eye and 20/20 in the left eye. The patient had a relative afferent pupillary defect in the right eye. Funduscopic examination showed a cherry-red spot in the macula, attenuated vessels, a few scattered cotton-wool spots, and intraretinal hemorrhages, as well as an inactive chorioretinal scar of unknown etiology superior to the nerve in the right eye (Figure 1A). The asymptomatic left eye showed an embolus along the inferotemporal arcade. Intraocular pressures (IOPs), extraocular motility, and slit lamp examination findings were normal.
Figure 1.
(A) Fundus photograph of the right eye showing a cherry-red spot in the macula along with scattered cotton-wool spots, intraretinal hemorrhages, and attenuated vessels, as well as a chorioretinal scar superior to the nerve. (B) Fundus photograph of the left eye with a cherry-red spot and retinal whitening in the macula.
Methods
Optical coherence tomography (OCT) showed intense inner retinal hyperreflectivity and thickening in the right eye, consistent with ischemia of the inner retina due to central retinal artery occlusion (CRAO; Figure 2A). OCT observations were normal in the left eye (Figure 2B).
Figure 2.
(A) Optical coherence tomography of the right eye with intense inner retinal hyperreflectivity and thickening consistent with central retinal artery occlusion. (B) Normal optical coherence tomography findings of the left eye.
The patient was admitted to the hospital. Blood culture results were negative, and the patient remained afebrile. Transthoracic echocardiography showed severe aortic insufficiency with aortic valve vegetation, and aortic valve replacement was planned. Magnetic resonance imaging of the brain showed multiple, subacute-to-chronic embolic infarcts. Computed tomography of the chest, abdomen, and pelvis showed multiple splenic infarcts and a large gastroduodenal artery aneurysm, thought to be mycotic. Records obtained from the patient’s initial hospitalization noted vegetation measuring 2.98 cm on the aortic valve with severe aortic regurgitation on transesophageal echocardiogram. The patient underwent embolization of the mycotic gastrointestinal aneurysm with interventional radiology. The oral surgery department was consulted, and multiple dental caries without acute infection were noted. Because the dental caries were the suspected source of endocarditis, dental extraction with prophylactic antibiotics was planned.
One week after admission, the night before the scheduled dental extraction, the patient complained of acute vision loss in the left eye, which had previously been 20/20. Examination revealed no light perception vision in both eyes with fundus findings of a cherry-red spot and retinal opacification in the macula of the left eye (Figure 1B) and stable appearance of the right eye (see Figure 1A).
Ocular massage and anterior chamber paracentesis were performed in the left eye immediately; however, the vision did not improve. Fluorescein angiography (FA) showed a severe delay in filling of the retinal circulation of the left eye but intact choroidal circulation, consistent with a CRAO (Figure 3B). OCT of the left eye (Figure 4B) showed diffuse inner retinal hyperreflectivity, also consistent with a CRAO. FA of the right eye (Figure 3A) showed trace late staining of some retinal vessels in the macula. OCT of the right eye (Figure 4A) showed persistent inner retinal hyperreflectivity with less thickening than the previous OCT, consistent with prior CRAO.
Figure 3.
(A) Fluorescein angiography of the right eye (at 2 minutes and 40 seconds) showing staining of the chorioretinal scar and (B) the left eye (at 1 minute and 25 seconds) showing severe delay in filling and nonperfusion of the retinal circulation.
Figure 4.
Optical coherence tomography of the (A) right eye with inner retinal hyperreflectivity, consistent with previous central retinal artery occlusion and (B) the left eye with diffuse inner retinal hyperreflectivity, consistent with acute central retinal artery occlusion.
Results
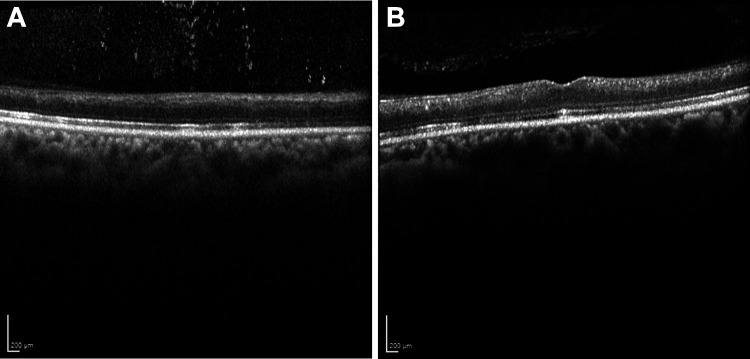
The patient underwent dental extraction as planned the following day and subsequently underwent aortic valve replacement. The patient was discharged, and low-vision resources were provided. One month after discharge, his vision improved to light perception in the right eye and counting fingers in the left eye; however, 6 months later, his vision had deteriorated to no light perception again in the right eye and remained at counting fingers in the left eye. No neovascularization of the iris, disc, or retina was noted. OCT showed marked retinal atrophy in both eyes (Figure 5).
Figure 5.
Optical coherence tomography of the (A) right and (B) left eye showing marked retinal atrophy in both eyes.
Conclusions
IE is a bacterial infection of the heart valve with systemic manifestations such as fever, chills, fatigue, or night sweats. Risk factors for IE include intravenous drug use, mitral valve prolapse, rheumatic heart disease, prosthetic heart valve, chronic hemodialysis, and poor oral hygiene. 1 Incidence ranges from 1.4 to 6.2 per 100 000, and prognosis is poor with considerable morbidity and mortality. 2 Systemic thromboembolization is an important complication of IE caused by fragmentation of vegetations and occurs in about 22% to 50% of cases. 3 Ophthalmic manifestations include retinal hemorrhages, cotton-wool spots, Roth spots, CRAO, branch retinal artery occlusion, cilioretinal artery occlusion, and ophthalmic artery occlusion. 4 -7 Most ocular embolic events have been reported to occur within the first 2 to 4 weeks of antimicrobial therapy. 8,9
CRAO is a rare but blinding ocular emergency, with an incidence of about 1 per 100 000 per year. 10 Embolism is the most common cause of CRAO, typically arising from the carotid artery, with the heart accounting for less than 10% of cases. 4,11 CRAO of embolic origin in patients suffering from IE is a very uncommon but potentially blinding complication. Other rare sources of emboli include fat or air secondary to trauma, talc emboli due to intravenous drug use, and synthetic emboli from iatrogenic procedures. Bilateral CRAOs have been reported in the setting of granulomatosis with polyangiitis, giant cell arteritis, homocystinuria, sickle cell disease, Henoch-Schönlein purpura, atherosclerosis, mitral valve prolapse, and head trauma. 12 -16 To our knowledge, there is only 1 other reported case in the literature of bilateral CRAO from endocarditis. 17
Approximately 5% of patients with what appears to be a CRAO actually have an ophthalmic artery occlusion. Because ophthalmic artery occlusion interrupts both the retinal and posterior ciliary circulations, the visual prognosis is usually worse, often with vision of light perception or no light perception. A cherry-red spot typically occurs in CRAOs because of a contrast between the normal orange reflex at the foveola that is supplied by the intact choroidal vasculature and the surrounding opaque retina in the posterior pole, where the nerve fiber and ganglion cell layers are the thickest, that is supplied by the central retinal artery. However, a cherry-red spot is not pathognomonic for CRAO; some degree of cherry-red spot is seen in around 60% of cases of ophthalmic artery occlusion. It is presumed that the choroidal hypoperfusion may have improved or been insufficient to produce an infarction of the outer retina and retinal pigment epithelium in these cases. 7
Because this patient’s vision was no light perception at one point, the diagnosis of ophthalmic artery occlusion (or multiple emboli in both the central retinal and posterior ciliary arteries) was considered. However, FA and OCT were consistent with inner retinal ischemia and a relatively intact outer retinal and choroidal circulation.
The proportion of patients with IE undergoing valve surgery is approximately 50%. 18 Decisions about valve replacement depend on the infecting organism, vegetation size, presence of perivalvular infection, presence of heart failure, age, noncardiac comorbidities, and presence of embolism. In addition, the optimal timing of valve surgery is not well agreed on in the literature. 19 In our patient, the initial treatment plan was to complete a 4-week course of antibiotics followed by dental extraction within 1 week of discharge and then perform aortic valve replacement.
According to the European Society of Cardiology and American Heart Association (AHA)/American College of Cardiology (ACC) guidelines, prevention of embolic complications is an indication for surgery in patients with IE. The European Society of Cardiology guideline mentions urgent surgery in patients with IE for isolated vegetation greater than 15 mm, and the AHA/ACC guideline recommends early surgery for large mobile vegetation on a native valve. 20 During his initial admission, our patient met the indication, with vegetation mass measuring 2.98 cm on his native aortic valve confirmed on transesophageal echocardiogram. The AHA/ACC guideline defines early surgery as between the initial hospitalization and completion of a full course of antibiotics. The benefit of surgery is reportedly greatest early in the treatment course to prevent the increased risk of embolic events within the first 2 to 4 weeks of antimicrobial therapy. There is a possibility that had the patient undergone valve repair during his initial admission, before completing antibiotics, that embolization could have been prevented and his vision preserved.
Although treatment of CRAO can be attempted by reducing the IOP via a combination of ocular massage, IOP-lowering medications, or anterior chamber paracentesis to increase retinal perfusion pressure, there are no proven treatments to reverse vision loss from CRAO. 4,21 Therefore, prompt recognition and treatment of IE are critical to prevent devastating embolic complications such as CRAO and to further reduce morbidity and mortality.
Footnotes
Ethical Approval: This case report was conducted in accordance with the Declaration of Helsinki. The collection and evaluation of all protected patient health information was performed in a Health Insurance Portability and Accountability Act (HIPAA)–compliant manner.
Statement of Informed Consent: Written consent to publish this case has not been obtained. This report does not contain any personal identifying information.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Tahira Scholle, MD  https://orcid.org/0000-0002-8500-1269
https://orcid.org/0000-0002-8500-1269
References
- 1. Wang A, Gaca JG, Chu VH. Management considerations in infective endocarditis: a review. JAMA. 2018;320(1):72–83. doi:10.1001/jama.2018.7596 [DOI] [PubMed] [Google Scholar]
- 2. Tleyjeh IM, Abdel-Latif A, Rahbi H, et al. A systematic review of population-based studies of infective endocarditis. Chest. 2007;132(3):1025–1035. doi:10.1378/chest.06-2048 [DOI] [PubMed] [Google Scholar]
- 3. Bayer AS, Bolger AF, Taubert KA, et al. Diagnosis and management of infective endocarditis and its complications. Circulation. 1998;98(25):2936–2948. doi:10.1161/01.cir.98.25.2936 [DOI] [PubMed] [Google Scholar]
- 4. Ziakas NG, Kotsidis S, Ziakas A. Central retinal artery occlusion due to infective endocarditis. Int Ophthalmol. 2014;34(2):315–319. doi:10.1007/s10792-013-9779-6 [DOI] [PubMed] [Google Scholar]
- 5. Manor RS, Sachs W. Visible retinal emboli in a case of subacute bacterial endocarditis. Ophthalmologica. 1973;166(1):10–15. doi:10.1159/000306791 [DOI] [PubMed] [Google Scholar]
- 6. Schocket S, Braver D. Cilioretinal artery occlusion in a patient with suspected subacute bacterial endocarditis. South Med J. 1970;63(1):1–4. doi:10.1097/00007611-197001000-00001 [DOI] [PubMed] [Google Scholar]
- 7. Wathek C, Kharrat O, Maalej A, Nafaa MF, Rannen R, Gabsi S. Ophthalmic artery occlusion as a complication of infectious endocarditis. J Fr Ophtalmol. 2014;37(10):e161–e163. doi:10.1016/j.jfo.2014.03.013 [DOI] [PubMed] [Google Scholar]
- 8. Garvey GJ, Neu HC. Infective endocarditis—an evolving disease. A review of endocarditis at the Columbia-Presbyterian Medical Center, 1968-1973. Medicine (Baltimore). 1978;57(2):105–127. doi:10.1097/00005792-197803000-00001 [PubMed] [Google Scholar]
- 9. Loughrey PB, Armstrong D, Lockhart CJ. Classical eye signs in bacterial endocarditis. QJM. 2015;108(11):909–910. doi:10.1093/qjmed/hcv055 [DOI] [PubMed] [Google Scholar]
- 10. Rumelt S, Brown GC. Update on treatment of retinal arterial occlusions. Curr Opin Ophthalmol. 2003;14(3):139–141. doi:10.1097/00055735-200306000-00005 [DOI] [PubMed] [Google Scholar]
- 11. Babikian V, Wijman CA, Koleini B, Malik SN, Goyal N, Matjucha IC. Retinal ischemia and embolism. Etiologies and outcomes based on a prospective study. Cerebrovasc Dis. 2001;12(2):108–113. doi:10.1159/000047689 [DOI] [PubMed] [Google Scholar]
- 12. Gold D. Retinal arterial occlusion. Trans Sect Ophthalmol Am Acad Ophthalmol Otolaryngol. 1977;83(3 Pt 1):OP392–OP408. [PubMed] [Google Scholar]
- 13. Narang S, Kochhar S, Gupta S, Gupta H, Bansal R, Sood S. Bilateral simultaneous central retinal artery occlusion following head injury. Int Ophthalmol. 2007;27(6):387–390. doi:10.1007/s10792-007-9094-1 [DOI] [PubMed] [Google Scholar]
- 14. Costello F, Gilberg S, Karsh J, Burns B, Leonard B. Bilateral simultaneous central retinal artery occlusions in Wegener granulomatosis. J Neuroophthalmol. 2005;25(1):29–32. doi:10.1097/00041327-200503000-00008 [DOI] [PubMed] [Google Scholar]
- 15. Riley AF, Aburn NS. Recovery of vision after bilateral arteritic central retinal artery occlusion. Clin Exp Ophthalmol. 2004;32(2):226–228. doi:10.1111/j.1442-9071.2004.00789.x [DOI] [PubMed] [Google Scholar]
- 16. Nakamura H, Sakaue H, Yoshida H, Kamizuru H, Fukuda H. A case of bilateral continuous central retinal artery occlusion [in Japanese]. Nippon Ganka Gakkai Zasshi. 1999;103(4):327–331. [PubMed] [Google Scholar]
- 17. Reese LT, Shafer D. Retinal embolization from endocarditis. Ann Ophthalmol. 1978;10(12):1655–1657. [PubMed] [Google Scholar]
- 18. Duval X, Delahaye F, Alla F, et al. ; AEPEI Study Group. Temporal trends in infective endocarditis in the context of prophylaxis guideline modifications: three successive population-based surveys. J Am Coll Cardiol. 2012;59(22):1968–1976. doi:10.1016/j.jacc.2012.02.029 [DOI] [PubMed] [Google Scholar]
- 19. Baddour LM, Wilson WR, Bayer AS; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132(15):1435–1486. doi:10.1161/CIR.0000000000000296 [DOI] [PubMed] [Google Scholar]
- 20. Kang DH. Timing of surgery in infective endocarditis. Heart. 2015;101(22):1786–1791. doi:10.1136/heartjnl-2015-307878 [DOI] [PubMed] [Google Scholar]
- 21. Youn TS, Lavin P, Patrylo M, et al. Current treatment of central retinal artery occlusion: a national survey. J Neurol. 2018;265(2):330–335. doi:10.1007/s00415-017-8702-x [DOI] [PubMed] [Google Scholar]