Abstract
Purpose:
This work subclassifies retinoblastoma vitreous seeds and evaluates the efficacy, regression patterns, and adverse effects of combination intravitreal melphalan and topotecan chemotherapy for resistant and recurrent vitreous seeds.
Methods:
A retrospective review of medical records was conducted of patients with retinoblastoma and resistant or recurrent vitreous seeds who were treated with intravitreal melphalan and topotecan injections from August 2014 to July 2018. Main outcome measures included regression pattern, time for regression, time for recurrence of seeds, treatment outcomes, and ocular toxicity.
Results:
Nineteen eyes received 138 intravitreal injections over 74 treatment sessions (mean, 7.26 injections per eye); vitreous seeds regressed in 18 eyes. Of cloud vitreous seeds, curvilinear (n = 2) and sphero-linear (n = 2) subtypes were observed. During regression, some sphere seeds showed an intermediary streak-like pattern and took longer to regress (mean, 11.13 ± 14.05 months and 11.67 ± 8.62 injections) than those without the intermediary streak-like pattern (mean, 3.55 ± 2.57 months and 4.2 ± 1.87 injections). Mean follow-up was 34.87 ± 21.09 months (median, 35 months; range, 11-96 months). Anterior segment toxicity was seen in 10 (53%) eyes and posterior segment toxicity in 5 (26%) eyes. Kaplan-Meier survival estimates for globe salvage at 2 years was 94% and 73% at 5 years. Kaplan-Meier survival for vitreous seed–free status was 94% at 2 years and 65% at 5 years.
Conclusions:
An expanded vitreous seed classification system that further subcategorizes hitherto unrecognized vitreous seed morphology is needed. An intermediate streaking process results in a prolonged regression time for sphere vitreous seeds.
Keywords: eye, intravitreal chemotherapy, regression, retinoblastoma, tumor, vitreous seeds
Introduction
With recent leaps in retinoblastoma (RB) care resulting in increased life survival, increasing attention is now being focused on ocular globe salvage and vision optimization. 1 Munier has described vitreous seeds classification, and hitherto forsaken eyes have been salvaged with intravitreal chemotherapy (IvitC). 2 Vitreous seeding of the tumor remains one of the most common causes of treatment failure because these seeds result from clonal proliferation of tumor cells that are able to survive in an avascular environment and tend to be chemo-resistant. 3,4
IvitC has emerged as an effective treatment against these resistant and recurrent vitreous seeds. 5 -12 Francis et al reported on the influence of vitreous seeds’ classification and response to intravitreal melphalan in a cohort of patients with RB treated predominantly with intra-arterial chemotherapy. 13 Berry and colleagues similarly reported on the outcomes of intravitreal melphalan in a cohort of patients with RB treated predominantly with intravenous chemotherapy. 14 Histopathologically, dust vitreous seeds are made up of scattered macrophages and necrotic cells around a single viable tumor cell, spheres are composed of spherical clusters of viable cells, and clouds are mainly made up of necrotic material. 15
We report on the hitherto unrecognized presentation subtypes of vitreous seeds, regression patterns, and treatment outcomes in our series of 138 intravitreal injections using combination melphalan and topotecan, treated over 4 years. An intermediary phenomenon of vitreous seed streaking in regression from spheres with a potential prognosticating value is also reported.
Methods
A retrospective review was conducted of patients who received IvitC for RB from August 2014 to July 2018. Prior institutional review board approval was obtained. This study adhered to the tenets of the Declaration of Helsinki. Patients previously treated with intravenous chemotherapy or intra-arterial chemotherapy who had persistently active or recurrent vitreous seeds were included in the study. Melphalan (20 µ), topotecan (20 µ), or both were injected intravitreally based on the physician’s discretion, which was decided on the basis of the vitreous seed load in each eye. Written informed consent was obtained from parents or guardians for treatment and the use of data for research purposes.
Indirect ophthalmoscopy was performed under general anesthesia to evaluate the status of the disease. Details regarding the main tumor and vitreous seeds were noted. Intravitreal injections were performed under sterile conditions. Intravitreal melphalan, topotecan, or both were injected 1 to 3 mm from the limbus through a biplanar pars plana entry with a 30-gauge needle. The injection site was chosen in the quadrant opposite of the vitreous seeds’ location, in a quadrant free of seeds. Care was taken to avoid injection of the drug in the retrohyaloid space, wherever applicable. Triple freeze-thaw cryotherapy was performed at the injection site during withdrawal of the needle. The globe was shaken with forceps for 10 to 20 seconds to enhance drug dispersal through the vitreous cavity. Following injection, topical povidone iodine 5% (Betadine solution, Purdue Products LP) was instilled in the conjunctival fornix and the eye was patched for 2 hours. Topical antibiotic-steroid combination drops (Betnesol-N, UCB Pharma Ltd), which were to be applied 4 times daily and tapered, were prescribed postoperatively for 1 week.
Patients were examined after 3 to 4 weeks. Injection was repeated if seeding activity persisted. This was continued until there were no signs of activity. The seeds were considered regressed if their appearance resembled one of the regression patterns as given by Munier—type 0: not visible, type 1: calcific, type 2: amorphous, type 3: combination of types 1 and 2. 2 RetCam (Natus Medical Inc.) images were obtained at each visit. B-scan ultrasonography and ultrasound biomicroscopy were performed when necessary.
Patient data included age, sex, laterality, hereditary status, symptom duration, and duration of follow-up. Tumor details included the International Classification of Retinoblastoma, vitreous seed classification, and details regarding vitreous and subretinal seeds. Treatment details included prior treatment, number of intravitreal injections, the clock-hour position of injection, time interval between the injections, and other treatment given. Outcomes evaluated included regression pattern of seeds and the main tumor, time for regression, and details regarding toxicity.
Statistical analysis was performed using SPSS software, version 14. A 1-way analysis of variance test was used to compare the time for regression and the number of IvitC injections required for regression among the types of vitreous seeds. Mann-Whitney U test was used to compare the time for regression and the number of IvitC injections required for regression in eyes with spheres with or without intermediary streak formation. For ocular survival, enucleation was considered an adverse event. For disease-free survival, seed recurrence was considered an event. Ocular survival and disease-free survival were estimated using the Kaplan-Meier method.
Results
Nineteen eyes of 19 patients (male: 11, female: 8) were included in the study. Mean age at RB diagnosis was 23.42 ± 21.58 months (median, 15; range, 2.5-94 months). Baseline characteristics summarizing the tumor characteristics of 19 patients with IvitC are shown in Table 1. Twelve of 13 patients with bilateral RB had their fellow eye enucleated. Macular involvement by the main tumor was seen in 4 (21%) eyes. At presentation, visual acuity was fixation and following light in 12 (63%) eyes, only light fixation in 3 (16%) eyes, and neither fixing nor following light in 4 (21%) eyes.
Table 1.
Baseline Characteristics of the 19 Patients With Vitreous Seeds.
| Feature | No. (%) |
|---|---|
| Laterality of disease (n = 19 patients) | |
| Unilateral | 6 (32) |
| Bilateral | 13 (68) |
| Inheritance pattern (n = 19 patients) | |
| Sporadic | 18 (95) |
| Familial | 1 (5) |
| ICRB at diagnosis (n = 19 eyes) | |
| Group A | 0 (0) |
| Group B | 0 (0) |
| Group C | 6 (32) |
| Group D | 10 (52) |
| Group E | 3 (16) |
| Growth pattern of solid tumor | |
| Endophytic | 12 (63) |
| Exophytic | 4 (21) |
| Mixed | 3 (16) |
| Vitreous seeds | |
| None | 0 (0) |
| 1 quadrant | 2 (11) |
| 2 quadrants | 6 (32) |
| 3 quadrants | 2 (11) |
| 4 quadrants | 9 (47) |
| Predominant vitreous seed type (n = 19 eyes) | |
| Clouds | 5 (26.3) |
| Spheres | 9 (47.4) |
| Dust | 5 (26.3) |
Abbreviation: ICRB, International Classification of Retinoblastoma.
These 19 eyes received 138 intravitreal injections with 72 melphalan (median, 3; range, 1-17) and 66 topotecan (median, 2; range, 0-17) injections over 4.6 ± 5.7 months (median, 2.8 months). Mean age at first intravitreal injection was 34.87 ± 21.09 months (median, 35 months; range, 11-96 months). Mean interval between prior systemic treatment and first IvitC injection was 5.41 ± 4.94 months (median, 4.43 months; range, 0.7 to 11.66 months). Mean injection interval was 44.9 ± 44.9 days (median, 34 days; range, 9-252 days). Vitreous seeds were classified as spheres (n = 13), clouds (n = 6), and dust (n = 16) and the predominant vitreous seed type was identified in each eye (sphere, n = 9 eyes; cloud, n = 5 eyes; and dust, n = 5 eyes). Of cloud vitreous seeds, sphero-linear (n = 2; Figure 1) and curvilinear (n = 2; Figure 2) subtypes were observed. The clinical and treatment details of each patient are listed in Table 2. A single-eyed patient presented with concomitant anterior chamber (sphere) and vitreous (dust) seeds and received bicameral melphalan (10 μg) while the primary tumor was treated with intra-arterial chemotherapy (Figure 3).
Figure 1.
(A) Fundus photograph at presentation reveals group D retinoblastoma with diffuse cloud and sphero-linear seed (arrow). (B) After 2, (C) 3, and (D) 4 cycles of intra-arterial chemotherapy, main tumor regression is noted with persisting vitreous seeds. (E) After 3 intravitreal injections of melphalan and topotecan, there is amorphous regression (arrow). (F) After 5 intravitreal injections of melphalan and topotecan, there is complete regression of the sphero-linear seed.
Figure 2.
(A) Right fundus photograph at presentation reveals group C tumor with curvilinear seed and (B) another tumor inferiorly. (C) After 6 cycles of intravenous chemotherapy and focal treatment, residual vitreous seeds persist. (D) After 1 injection of intravitreal melphalan and topotecan, seeds show amorphous regression. (E) After 3 injections of intravitreal melphalan and topotecan, seeds have disappeared with grade I retinal toxicity and (F) grade III retinopathy is noticed temporal to the regressed tumor (indicated with oblique arrows).
Table 2.
Tumor Characteristics of 19 Patients With Intravitreal Chemotherapy.
| No. |
Age at diag, mo | Sex | Laterality | ICRB of main tumor |
Type of VS | Predominant type of VS | No. of quad | No. of melph inj | No. of topo inj | Intermediate VS type | Final VS pattern | Duration of treatment, mo | Time for regression, mo | Other treatment during/after IvitC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 12 | F | B | D | Sphere: active Dust: active |
Sphere | 2 | 3 | 2 | Dust: type III | Sphere: type 0 Dust: type II |
2.77 | 4.80 | None |
| 2 | 10 | F | B | E | Sphere: active Dust: active |
Sphere | 3 | 2 | 2 | Sphere: type II Dust: type I |
Sphere: type III Dust: type I |
1.53 | 2.43 | Focal |
| 3 | 2.5 | M | B | C | Dust: active | Dust | 4 | 1 | 1 | NA | Dust: type I | 0 | 1.93 | Focal |
| 4 | 8.5 | M | B | D | Sphere: active Dust: active |
Sphere | 4 | 2 | 1 | NA | Sphere: type I Dust: type 0 |
1 | 2.10 | Focal |
| 5 | 12 | F | U | D | Sphere: active Cloud: active Dust: active |
Cloud | 4 | 3 | 1 | NA | Sphere: type 0 Cloud: type 0 Dust: type I |
8 | 9.63 | Focal |
| 6 | 36 | M | B | D | Sphere: active Cloud: active Dust: active |
Cloud | 4 | 4 | 3 | NA | Sphere: type I Cloud: type I Dust: type I |
3.43 | 4.33 | Focal |
| 7 | 18 | F | B | C | Cloud: active Dust: active |
Cloud | 4 | 17 | 17 | Cloud: regressed f/b reactivation Dust: regressed f/b reactivation |
No response, enuc |
15.4 | NA | Focal, EBRT, enuc |
| 8 | 20 | M | U | D | Sphere: active Cloud: active Dust: active |
Cloud | 4 | 11 | 9 | Sphere: linear streak→type III Cloud: type III Dust: type III |
Relapse of main tumor, enuc |
21.87 | 23.27 | Focal, enuc |
| 9 | 9 | M | B | D | Sphere: active Dust: active |
Sphere | 2 | 2 | 2 | Sphere: type II→linear streak Dust: type II |
Sphere: type 0 Dust: type 0 |
0.87 | 1.63 | Focal |
| 10 | 7 | M | B | C | Sphere: active | Sphere | 1 | 1 | 0 | Sphere: type II | 0.00 | 0.97 | Focal | |
| 11 | 12 | M | B | D | Sphere: active Dust: active |
Sphere | 4 | 4 | 6 | Sphere: linear streak, type II Dust: type 0 |
Sphere: type 0 Dust: type 0 |
15.63 | 17.03 | Focal |
| 12 | 24 | M | B | D | Sphere: active, linear streak | Sphere | 2 | 2 | 2 | Sphere: type II | Sphere: type 0 | 1.17 | 2.33 | Focal, plaque brachy |
| 13 | 48 | M | U | D | Cloud: type II Dust: type II |
Cloud | 2 | 7 | 7 | Cloud: type III Dust: type 0 |
Cloud: type III Dust: type 0 |
6.27 | 6.27 | Focal |
| 14 | 42.9 | F | B | C | Dust: active | Dust | 1 | 1 | 1 | Dust: type 0 | 0.00 | 1.63 | Focal | |
| 15 | 30 | F | U | C | Sphere: active Dust: active |
Sphere | 2 | 3 | 3 | Sphere: type 0 Dust: type 0 |
Enuc | 2.70 | 2.70 | Focal, enuc |
| 16 | 15 | F | U | D | Dust: active | Dust | 4 | 2 | 1 | Dust: type I | 3.50 | 4.67 | Focal | |
| 17 | 94 | F | B | E | Dust: type I | Dust | 3 | 3 | 3 | Cloud: active (reactivation) Dust: type I |
Cloud: type II Dust: type 0 |
3.07 | 4.23 | None |
| 18 | 8 | M | B | C | Sphere: active | Sphere | 1 | 3 | 3 | NA | Sphere: type 0 | 2.73 | 5.07 | Focal |
| 19 | 36 | M | U | E | Sphere: active Dust: active |
Dust | 4 | 1 | 1 | NA | Sphere: type 0 Dust: type 0 |
0.00 | 1.17 | Focal |
Abbreviations: B, bilateral; Diag, diagnosis; EBRT, external beam radiation therapy; enuc, enucleation; F, female; f/b, followed by; ICRB, International Classification of Intraocular Retinoblastoma; inj, injection; IvitC, intravitreal chemotherapy; melph, melphalan; M, male; NA, not applicable; quad, quadtrant; topo, topotecan; U, unilateral; VS, vitreous seeds.
Figure 3.
At presentation, external photograph shows (A) multiple creamy retinoblastoma seeds in the anterior chamber, while (B) fundus examination reveals recurrent retinoblastoma in the inferotemporal periphery. Following a first cycle of intra-arterial chemotherapy (IAC), an external photograph shows (C) partial regression of retinoblastoma seeds in the anterior chamber, while (D) fundus examination reveals complete regression of the main retinoblastoma tumor. Following a second cycle of IAC, an external photograph shows (E) further regression of retinoblastoma seeds, while (F) fundus examination remains stable. Following a third cycle of IAC and 3 consecutive sessions of a bicameral injection of melphalan (10 µg × 2), an external photograph shows (G) complete regression of retinoblastoma seeds, while (H) fundus examination remains stable.
Mean number of injections per seed type were 15.8 ± 11.9 for clouds, 4.22 ± 2.73 for spheres, and 3.2 ± 1.6 for dust. Mean number of intravitreal injections for eyes that also received intra-arterial chemotherapy (n = 10) was 6.1 ± 5.76 (melphalan, 3.10 ± 2.92; topotecan, 3.00 ± 2.91) and for those eyes without intra-arterial chemotherapy (n = 9) was 8.56 ± 10.25 (melphalan, 4.56 ± 5.0; topotecan, 4.00 ± 5.27) (P = .661). Mean follow-up was 34.97 ± 17.77 months (median, 35.0; range, 5.5-66.70 months). Three eyes underwent enucleation, 2 for recurrence of primary tumor and 1 because of failure of the primary tumor to regress with systemic chemotherapy. Two of these eyes had cloud as the predominant seed type.
Overall, vitreous seeds resolved in 18 of 19 eyes with a mean of 7.26 ± 8.05 injections. An intermediary stage of streaks during regression of sphere seeds was seen in 3 of 13 eyes (Figure 4). Mean time to regression of spheres with intermediate streaks was 11.13 ± 14.05 months requiring mean 11.67 ± 8.62 injections, and for those without intermediary streaks was 3.55 ± 2.57 months (P = .573) requiring mean 4.2 ± 1.87 injections (P = .112). Sphere seeds with intermediate streaks further disappeared completely (type 0 regression), whereas the ones without an intermediate streaks pattern showed different types of regression patterns (type 0 = 5, type 1 = 1, type 2 = 2, type 3 = 2). Vitreous seeds’ regression patterns according to Munier’s classification are shown in Table 3, and treatment outcomes are shown in Table 4.
Figure 4.
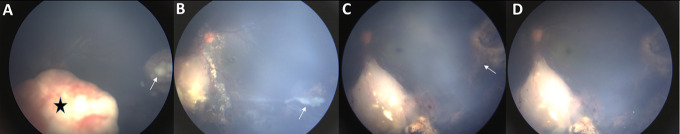
(A) Fundus photograph shows group D tumor with disc overhang (star) and sphere overlying a peripheral tumor (arrow). Following 6 cycles of intravenous chemotherapy, this eye was treated with combination melphalan and topotecan intravitreal injections. (B) Regression pattern reveled an intermediate streak, (C) leading to an amorphous residue and (D) eventual complete regression after 10 injections.
Table 3.
The Regression Pattern of Vitreous Seeds Following Chemotherapy.
| Vitreous seed type | Regression type |
|---|---|
| Dust | Type 0: 50% (n = 8) Type 1: 31% (n = 5) Type 2: 6% (n = 1) Type 3: 6% (n = 1) No regression: 6% (n = 1) |
| Spheres | Type 0: 54% (n = 7) Type 1: 15% (n = 2) Type 2: 15% (n = 2) Type 3: 15% (n = 2) |
| Cloud | Type 0: 17% (n = 1) Type 1: 17% (n = 1) Type 2: 17% (n = 1) Type 3: 33% (n = 2) No regression: 17% (n = 1) |
Table 4.
Treatment Outcomes in 19 Eyes Following Intravitreal Chemotherapy.
| Feature | No. (%) |
|---|---|
| Vitreous seed response | |
| Complete regression | 18 (95) |
| Partial regression | 1 (5) |
| No regression | 0 (0) |
| Time to regression of vitreous seeds (mean ± SD), mo | |
| Overall | 5.8 ± 6.7 |
| Clouds | 11.9 ± 10.5 |
| Spheres | 3.2 ± 1.6 |
| Dusts | 2.7 ± 1.6 |
| Vitreous seed recurrence | 3 (16) |
| Follow-up, mean ± SD (median, range), mo | 34.9 ± 17.8 (35.0, 5.5-66.7) |
| Vitreous seed–free period, mean ± SD (median, range), mo | 19.7 ± 15.8 (11.2, 0-45.6) |
| Intervention free interval, mean ± SD (median, range), mo | 20.9 ± 12.4 (12.4, 2.1-47.5) |
| Time to onset of toxicity, mean ± SD (median, range), mo | |
| Anterior segment | 4.9 ± 3.6 (3.7, 1.6-11.6) |
| Posterior segment | 8.1 ± 3.4 (9.6, 3.9-12.6) |
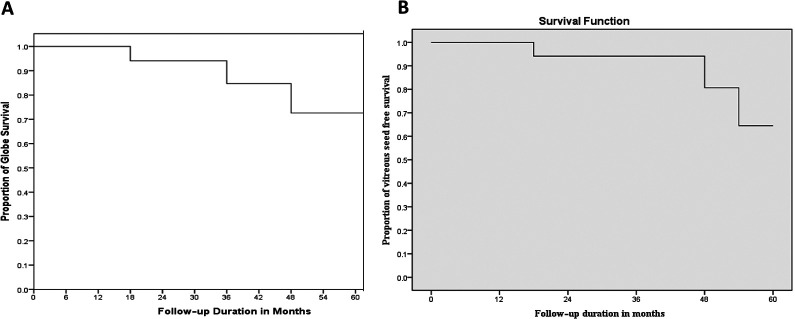
Toxicity was noted in 10 (53%) eyes and involved the anterior (n = 10; 53%) and posterior segment (n = 5; 26%). This included anterior uveitis (n = 1), iris atrophy (n = 3), iris heterochromia (n = 1), posterior synechiae (n = 3), posterior subcapsular cataract (n = 5), band-shaped keratopathy (n = 2), vascular sheathing (n = 1; grade III retinopathy), sclerosed vessel (n = 2; grade III retinopathy), hemorrhagic maculopathy (n = 1; grade V retinopathy; Figure 5), optic atrophy (n = 1; grade V retinopathy; see Figure 5) and subretinal fibrosis (n = 1; grade III retinopathy). None of the patients encountered glaucoma, endophthalmitis, extraocular extension, metastasis, or death. Kaplan-Meier survival estimates for globe salvage at 2 years was 94% and 73% for 5 years. Kaplan-Meier survival for vitreous seed–free status was 94% at 2 years and 65% for 5 years (Figure 6).
Figure 5.
At baseline, (A) a fundus photograph shows a large cloud overlying the main tumor in the inferotemporal quadrant, with spheres and dust (arrowheads) in all 4 quadrants. (B; arrowhead) Vitreous seeds persisted after 4 cycles of intravenous chemotherapy and were treated with combination melphalan and topotecan intravitreal injection. One month later, (C) injections were repeated and vitreous seeds (D; arrowhead) showed complete regression. However, 7 months later, fine dust was noticed and (E) intravitreal melphalan injection was repeated. (F) At next follow-up 1 month later, submacular hemorrhage, grade V retinopathy, and optic disc pallor were observed.
Figure 6.
(A) Kaplan-Meier survival curve showing globe salvage rates. (B) Kaplan-Meier survival curve showing vitreous seed–free rates over 5 years.
Conclusions
Discussion
IvitC has reemerged as a valuable treatment option for vitreous seeds in recent years thanks to safety-enhanced techniques. 16 We encountered some peculiar presenting features, regression patterns with intermediary stages, and comparable treatment outcomes in our series. The most commonly presenting vitreous seed type was dust. This is similar to that reported by Berry et al. 14 However, unlike that observed by Barry and colleagues, we also found sphere vitreous seeds in 13 (68%) eyes at presentation. The most common type of seeds in eyes undergoing enucleation in our series was cloud, unlike that reported by Francis et al and Berry et al, who found dust to be the most common type. 13,14
We also noted interesting patterns of vitreous seeds’ presentation in our patients. Among eyes with cloud vitreous seeds, 2 morphological patterns appeared: sphero-linear (cases 8 and 10; see Figure 1) and curvilinear (cases 7 and 18; see Figure 2). These patterns and subtypes of cloud vitreous seeds appear to be more than mere wisps of the main body of the cloud. It is hypothesized that the sphero-linear pattern of vitreous seeds is likely related to the arrangement of vitreous fibers, whereas curvilinear is probably not as influenced by a single particular factor. Ocular movements, superior or inferior seed location, and role of gravity may be other influencing factors. It was observed that sphero-linear seeds had type 0 regression, whereas curvilinear seeds and linear dust had amorphous regression. It was also observed that a tumor cyst could rupture and liberate secondary seeds into the vitreous cavity. 17 These findings have not been reported previously and, if detected in a larger group of patients in multiple centers, can provide useful input for an expanded version of the current classification of vitreous seeds.
In our series, the mean number of injections for complete regression was 5, which is similar to that reported by Shields et al. 18 A vitreous seed regression pattern was observed as type 0 (n = 16), type 1 (n = 8), type 2 (n = 4), or type 3 (n = 5). In the study by Francis and colleagues, dust on regression was not visible (type 0 regression). 13 However, we did observe type 1 (cases 3, 5, 6, 8, and 16) and type 2 (cases 1, 2, 8, and 9) regression patterns with dust vitreous seeds. Spheres on regression went through an intermediary stage of streak formation. The spheres with the intermediary stage of streak formation took a longer time for regression and ultimately disappeared. Three eyes had a recurrence of seeds, but these eyes did not have any common seed type. Type of systemic chemotherapy did not affect the number of injections given. We have previously reported about the complications following IvitC in detail. 19 It is postulated that melphalan may be preferentially taken up by pigmented tissues. 20 In our experience, retinal pigment and iris pigment toxicity appears to be cumulative in some cases (unpublished data). Only a few reports of hemorrhagic retinal toxicity following intravitreal melphalan has been reported to date. 21 -23
In general, there are some safety concerns with IvitC that warrant a review of the current recommendations. First, this procedure should be performed under the direction of an expert pediatric ocular oncologist. This is not a procedure for the retina specialist, even as an expert in intravitreal injection therapy. Second, eyes with seeds in the anterior chamber or seeds located in the anterior vitreous cavity should have an evaluation with ultrasound biomicroscopy to potentially identify zonular involvement. Third, a safety-enhanced technique must be used. 24
Our study has several limitations including those related to the retrospective nature of this study. Also, longer follow-up will provide more information regarding lasting response and any unforeseen long-term complications and allow us to ascertain long-term visual outcomes. However, certain presenting patterns based on seed morphology that do not fit into the existing classification criteria prompt us to propose certain subtypes of seed categories. Recognition of certain regression patterns such as intermediary streaking that show a longer time to regression can help estimate the number of treatment sessions in such eyes. Larger, multicenter studies with longer follow-up will help us understand these aspects better.
Summary
An expanded vitreous seed classification system that further subcategorizes hitherto unrecognized vitreous seed morphology is needed. An intermediate streaking process results in a prolonged regression time for sphere vitreous seeds. Recognition of certain regression patterns such as intermediary streaking that show a longer time to regression can help us estimate the number of treatment sessions in such eyes. Larger, multicenter studies with longer follow-up will help us understand these aspects better.
Acknowledgments
The authors thank Mr Viswanathan Natarajan, MSc (Biostatistics), for statistical analysis of the data.
Footnotes
Ethical Approval: Ethical approval for this study was obtained from the institutional review board, Vision Research Foundation (approval code 546-2016-P).
Statement of Informed Consent: Written informed consent was obtained from all participants before the study.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Pukhraj Rishi, MD  https://orcid.org/0000-0001-5615-7734
https://orcid.org/0000-0001-5615-7734
References
- 1. Eagle RC, Jr. The pathology of ocular cancer. Eye (Lond). 2013;27(2):128–136. doi:10.1038/eye.2012.237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Munier FL. Classification and management of seeds in retinoblastoma. Ellsworth Lecture Ghent August 24th 2013. Ophthalmic Genet. 2014;35(4):193–207. doi:10.3109/13816810.2014.973045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Amemiya T, Yoshida H, Ishigooka H. Vitreous seeds in retinoblastoma, clinical significance and ultrastructure. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1979;211(3):205–213. doi:10.1007/BF02387425 [DOI] [PubMed] [Google Scholar]
- 4. Günalp I, Yalçindağ N, Unal E, et al. Causes of chemoreduction failure in retinoblastoma and analysis of associated factors leading to eventual treatment with external beam radiotherapy and enucleation. Ophthalmology. 2004;111(10):1917–1924. doi:10.1016/j.ophtha.2004.04.016 [DOI] [PubMed] [Google Scholar]
- 5. Munier FL, Gaillard MC, Balmer A, et al. Intravitreal chemotherapy for vitreous disease in retinoblastoma revisited: from prohibition to conditional indications. Br J Ophthalmol. 2012;96(8):1078–1083. doi:10.1136/bjophthalmol-2011-301450 [DOI] [PubMed] [Google Scholar]
- 6. Ghassemi F, Shields CL. Intravitreal melphalan for refractory or recurrent vitreous seeding from retinoblastoma. Arch Ophthalmol. 2012;130(10):1268–1271. doi:10.1001/archophthalmol.2012.1983 [DOI] [PubMed] [Google Scholar]
- 7. Munier FL, Gaillard MC, Balmer A, Beck-Popovic M. Intravitreal chemotherapy for vitreous seeding in retinoblastoma: recent advances and perspectives. Saudi J Ophthalmol. 2013;27(3):147–150. doi:10.1016/j.sjopt.2013.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shields CL, Manjandavida FP, Arepalli S, Kaliki S, Lally SE, Shields JA. Intravitreal melphalan for persistent or recurrent retinoblastoma vitreous seeds: preliminary results. JAMA Ophthalmol. 2014;132(3):319–325. doi:10.1001/jamaophthalmol.2013.7666 [DOI] [PubMed] [Google Scholar]
- 9. Ghassemi F, Shields CL, Ghadimi H, Khodabandeh A, Roohipoor R. Combined intravitreal melphalan and topotecan for refractory or recurrent vitreous seeding from retinoblastoma. JAMA Ophthalmol. 2014;132(8):936–941. doi:10.1001/jamaophthalmol.2014.414 [DOI] [PubMed] [Google Scholar]
- 10. Tuncer S, Balci Ö, Tanyildiz B, Kebudi R, Shields CL. Intravitreal lower-dose (20 µg) melphalan for persistent or recurrent retinoblastoma vitreous seeds. Ophthalmic Surg Lasers Imaging Retina. 2015;46(9):942–948. doi:10.3928/23258160-20151008-07 [DOI] [PubMed] [Google Scholar]
- 11. Rao R, Honavar SG, Sharma V, Reddy VAP. Intravitreal topotecan in the management of refractory and recurrent vitreous seeds in retinoblastoma. Br J Ophthalmol. 2018;102(4):490–495. doi:10.1136/bjophthalmol-2017-310641 [DOI] [PubMed] [Google Scholar]
- 12. Kiratli H, Koç İ, Varan A, Akyüz C. Intravitreal chemotherapy in the management of vitreous disease in retinoblastoma. Eur J Ophthalmol. 2017;27(4):423–427. doi:10.5301/ejo.5000921 [DOI] [PubMed] [Google Scholar]
- 13. Francis JH, Abramson DH, Gaillard MC, Marr BP, Beck-Popovic M, Munier FL. The classification of vitreous seeds in retinoblastoma and response to intravitreal melphalan. Ophthalmology. 2015;122(6):1173–1179. doi:10.1016/j.ophtha.2015.01.017 [DOI] [PubMed] [Google Scholar]
- 14. Berry JL, Bechtold M, Shah S, et al. Not all seeds are created equal: seed classification is predictive of outcomes in retinoblastoma. Ophthalmology. 2017;124(12):1817–1825. doi:10.1016/j.ophtha.2017.05.034 [DOI] [PubMed] [Google Scholar]
- 15. Amram AL, Rico G, Kim JW, et al. Vitreous seeds in retinoblastoma: clinicopathologic classification and correlation. Ophthalmology. 2017;124(10):1540–1547. doi:10.1016/j.ophtha.2017.04.015 [DOI] [PubMed] [Google Scholar]
- 16. Munier FL, Soliman S, Moulin A, Gaillard MC, Balmer A, Beck-Popovic M. Profiling safety of intravitreal injections for retinoblastoma using an anti-reflux procedure and sterilization of the needle track. Br J Ophthalmol. 2012;96(8):1084–1087. doi:10.1136/bjophthalmol-2011-301016 [DOI] [PubMed] [Google Scholar]
- 17. Rishi P, Sharma U, Sharma T. Cavitary retinoblastoma: clinical observations. Eye (Lond). 2020;34(4):704–710. doi:10.1038/s41433-019-0581-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shields CL, Douglass AM, Beggache M, Say EAT, Shields JA. Intravitreous chemotherapy for active vitreous seeding from retinoblastoma outcomes after 192 consecutive injections. The 2015 Howard Naquin Lecture. Retina. 2016;36(6):1184–1190. doi:10.1097/IAE.0000000000000903 [DOI] [PubMed] [Google Scholar]
- 19. Rishi P, Sharma T, Agarwal V, et al. Complications of intravitreal chemotherapy in eyes with retinoblastoma. Ophthalmol Retina. 2017;1(5):448–450. doi:10.1016/j.oret.2017.03.006 [DOI] [PubMed] [Google Scholar]
- 20. Schaiquevich P, Buitrago E, Taich P, et al. Pharmacokinetic analysis of melphalan after superselective ophthalmic artery infusion in preclinical models and retinoblastoma patients. Invest Ophthalmol Vis Sci. 2012;53(7):4205–4212. doi:10.1167/iovs.12-9501 [DOI] [PubMed] [Google Scholar]
- 21. Aziz HA, Kim JW, Munier FL, Berry JL. Acute hemorrhagic retinopathy following intravitreal melphalan injection for retinoblastoma: a report of two cases and technical modifications to enhance the prevention of retinal toxicity. Ocul Oncol Pathol. 2017;3(1):34–40. doi:10.1159/000448718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xue K, Ren H, Meng F, Zhang R, Qian J. Ocular toxicity of intravitreal melphalan for retinoblastoma in Chinese patients. BMC Ophthalmol. 2019;19(1):61. doi:10.1186/s12886-019-1059-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Berry JL, Lee R, Patel L, et al. Posterior vitreous detachment and the associated risk of retinal toxicity with intravitreal melphalan treatment for retinoblastoma. Ocul Oncol Pathol. 2019;5(4):238–244. doi:10.1159/000493687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Smith SJ, Smith BD. Evaluating the risk of extraocular tumour spread following intravitreal injection therapy for retinoblastoma: a systematic review. Br J Ophthalmol. 2013;97(10):1231–1236. doi:10.1136/bjophthalmol-2013-303188 [DOI] [PubMed] [Google Scholar]