Summary
Aphelenchoides bicaudatus associated with grass in South Africa was identified morphologically and molecularly. This population is characterized by a body length of 409 – 529 μm, a stylet length of 9.5 – 13 μm, a post-vulval uterine sac of 45 – 50 μm, and the characteristic tail bifurcated at the end with one prong longer than the other. Molecular analyses based on the 18S and ITS rDNA data confirmed the primary morphological identification of the A. bicaudatus species. The obtained phylogenetic trees revealed a close positioning of the South African population to other representatives of A. bicaudatus with the maximum (1.00) posterior probability value. Principal component analysis (PCA) also indicated a variation within the populations of A. bicaudatus. This is the first report of A. bicaudatus from South Africa.
Keywords: Aphelenchoides, grass, morphology, PCA, phylogeny, rDNA, South Africa
Introduction
Grasslands are one of the most critical biomes in South Africa (Le Roux et al., 2011; Richardson et al., 2020), which cover the northern parts of the Western Cape Province. Grasslands constitute a significant component of the natural vegetation. The interface between grasslands and other biomes contributes substantially to their floristic and faunal diversity and to their important role in the agricultural economy, including livestock. The grasslands of South Africa are also home to most of the human population across the country (Le Roux et al., 2011; Richardson et al., 2020). Aphelenchoides Fischer, 1894 species is large and abundant genus with a worldwide distribution. They are found in a wide range of trophics such as fungal feeder in soil (e.g., A. pseudogoodeyi Oliveira, Subbotin, Alvarez-Ortega, Desaeger, Brito, Xavier, Freitas, Vau & Inserra, 2019), mushroom (e.g., A. composticola Franklin, 1957), plants (e.g., A. besseyi Christie, 1942), and insects (A. microstylus Kaisa, 2000) (Nickle, 1970; Handoo et al., 2020). Aphelenchoides bicaudatus first reported from Japan by Imamura (1931) from a paddy field. This species originally considered as a fungal feeder; however, it has been reported in association with several agricultural crops (Jen et al., 2012; Kim et al., 2016). Aphelenchoides bicaudatus has been reported from India (Das, 1960), Australia (Colbran, 1964), Venezuela (Loof, 1964), USA (Siddiqui and Taylor, 1967), Taiwan (Jen et al., 2012), South Korea (Kim et al, 2016), and Pakistan (Israr et al., 2017). To date, it has been reported in association with more than 200 plant species (Handoo et al., 2020). For instance, in South Korea, A. bicaudatus has been found with the leaves and shoot tips of chrysanthemum (Kim et al., 2016). However, A. bicaudatus reported from soil and mushroom (Mcleod, 1967). To date, A. arachidis Boss, 1977 and A. ritzemabosi (Schwartz, 1911) Steiner and Buhrer, 1932 have been reported from South Africa (Lesufi et al., 2015). However, A. bicaudatus not yet been reported from South Africa.
Therefore, the aims of the present work were 1) to study the morphology of A. bicaudatus, and 2) to study the molecular characters of A. bicaudatus using the 18S and ITS rDNA markers.
Materials and Methods
Nematode extraction, and processing
Specimens were collected at the Kirstenbosch National Botanical Garden in Cape Town (S: 33° 59’ 13.19”; E 18° 25’ 29.39”) and Magoebaskloof (S: 23°52’40.368”; E: 29°56’14.459”) from the rhizosphere of grass plants (Family: Poaceae; Pennisetum clandestinum). The specimens were extracted using the tray method (Shokoohi, 2021) and were fixed with a hot 4 % formaldehyde solution and transferred to anhydrous glycerin using the De Grisse (1969) method. The classification provided by Handoo et al. (2020) was used for the taxonomical study of Aphelenchoides. Pictures were taken with a Zeiss Axiolab (Germany) microscope at the Aquaculture Research Unit, equipped with a digital camera. Next, the pictures were used for line illustration. All samples were processed at the Aquaculture Research Unit of the University of Limpopo.
DNA extraction, PCR, and phylogenetic analysis
DNA extraction was done using the Chelex method (Straube & Juen, 2013). Five specimens of the analyzed species were hand-picked with a fine tip needle and transferred to a 1.5 ml Eppendorf tube containing 20 μl double distilled water. The nematodes in the tube were crushed with the tip of a fine needle and vortexed. Thirty microliters of 5 % Chelex® 50 and 2 μL of proteinase K were added to the microcentrifuge tube that contained the crushed nematodes and mixed. These separate microcentrifuge tubes with the nematode lysate were incubated at 56 °C for two hours and then incubated at 95 °C for 10 minutes to deactivate the proteinase K and finally spin for 2 min at 16000 rpm (Shokoohi, 2021). The supernatant was then extracted from the tube and stored at –20 °C. Following this step, the forward and reverse primers, 988F (5ʹ-CTCAAAGATTAAGCCATGC-3ʹ) and 1912R (5ʹ-TTTACGGTCAGAACTAGGG-3ʹ) for 18S rDNA (Holterman et al., 2006), and TW81 (5ʹ-GTTTCCGTAGGTGAACCTGC-3ʹ), and AB28 (5ʹ-ATATGCTTAAGTTCAGCGGGT-3ʹ) for ITS rDNA (Joyce et al., 1994) were used in the PCR reactions for partial amplification of the 18S and ITS rDNA region, respectively. PCR was conducted with eight μl of the DNA template, 12.5 μl of 2X PCR OneTaq® Quick-Load® 2X Master Mix with Standard Buffer (Inqaba Biotec, South Africa), one μl of each primer (10 pmol μl-1), and ddH2O for a final volume of 30 μl. The amplification was processed using an Eppendorf Mastercycler gradient (Eppendorf, Hamburg, Germany), with the following program: initial denaturation for 3 min at 94 °C, 37 cycles of denaturation for 45 s at 94°C; 54 °C and 53°C annealing temperatures for 18S rDNA and ITS rDNA for 30 s, respectively; extension for 45 s to 1 min at 72 °C, and finally an extension step of 6 min at 72 °C followed by a temperature on hold at 4 °C. After DNA amplification, four μl of product from each tube was loaded on a 1 % agarose gel in TBE buffer (40 mM Tris, 40 mM boric acid, and one mM EDTA) for evaluation of the DNA bands. The bands were stained with the SafeView™ Classic stain (Applied Biological Materials Inc. (abm); Canada) and visualized and photographed on a UV transilluminator. The PCR products for 18S rDNA and ITS rDNA were stored at –20 °C. Finally, the PCR products were purified and sequenced by Inqaba Biotech (South Africa). The obtained ribosomal DNA sequences were analyzed and edited with BioEdit (Hall, 1999) and aligned using CLUSTAL W (Thompson et al., 1994). Phylogenetic trees were generated using the Bayesian inference method as implemented in the program Mr Bayes 3.1.2 (Ronquist & Huelsenbeck, 2003). The HKY+Γ (gamma distribution of rate variation with a proportion of invariable sites) model was selected using jModeltest 2.1.10 (Guindon & Gascuel, 2003; Darriba et al., 2012). Analysis using the GTR+G+I model was initiated with a random starting tree and ran with the Markov chain Monte Carlo (MCMC) for 106 generations for 18S and ITS rDNA. The trees were visualized with the TreeView program (Page, 1996). Also, as outgroups, Bursaphelenchus xylophilus (MF669500, KX856336 for 18S rDNA, and ITS rDNA, respectively) were selected based on Handoo et al. (2020). The original partial 18S and ITS rDNA sequences of A. bicaudatus were deposited in GenBank under the accession numbers OM883916 and OM910735.
Statistical analysis
To evaluate the morphological variations between the populations of A. bicaudatus, principal component analyses (PCA) were conducted using XLSTAT software (Addinsoft, 2007). Various morphometric features obtained from fixed nematodes, including body length, a (body length/greatest body diameter), b (body length/ neck length), c (body length/tail length), cˈ (tail length/anal body diameter), V (% anterior end to vulva/body length), stylet length, and tail length were included in the PCA analyses (Table 2). The morphometric measurements for the different populations were taken from their original descriptions. The measures were normalized using XLSTAT software before their analysis (Addinsoft, 2007). The scores values were determined for each species based on each of the principal components, and the scores for the first two components were used to form a two-dimensional plot (PC1 and PC2) of each isolate based on the eigenvalues given by the software XLSTAT.
Table 2.
Morphological important characters of females of Aphelenchoides bicaudatus from various localities. All measurements are in pm and in form: mean ± SD (range), except for ratio. * = extracted from original drawings.
| Present study | Imamura, 1931 (Filipjev and Schuurmans Stekhoven (1941)) | Siddiqui and Taylor (1967) | Jen etal., 2012 | Kim et a/., 2016 | Israr et al., 2017 | |
|---|---|---|---|---|---|---|
| Population | South Africa | Japan | USA | Taiwan | South Korea | Pakistan |
| L(μm) | 455.3 ± 64.5 (409-529) | 430 (380 - 470) | 460 (410-550) | 499.12 ±67.95 (376-637) | 517.9 ±3.8 (513.6-522.6) | 360 |
| a | 29.1 ±2.0 (27.1-31.1) | 31.5 (31.3-31.7) | 28.0 (25-31) | 33.03 ± 2.42 (27.00-38.64) | 28.3 ± 0.5 (27.7-28.8) | 30.1 -32.7 |
| b | 4.6 ±0.5 (4.1-5.1) | 7.4 (6.8-8.4) | 8.2 (7.3-9.6) | 9.0 ±0.7 (7.5-10.0) | 7.3 ±0.0 (7.3-7.4) | 7.2-8.8 |
| c | 16.3 ±2.0 (14.1-17.8) | 10.6 (9.4-12.6) | 11.4(9.8-13.7) | 11.94 ± 0.93 (10.16-14.80) | 11.3 ±0.5 (10.7-11.9) | 11.3-12.0 |
| c' | 3.2 ±0.6 (2.6-3.6) | 4.4* | 4.7* | 5.41 ±0.56 (4.13-7.14) | 4.6 ±0.1 (4.4-4.8) | 2.9-3.7 |
| Tail (μm) | 28.0 ±3.6 (24-31) | 44* | 41 | 37-43 | 45.9 ±2.5 (43.1 -48.8) | 30-31 |
| V(%) | 68.8 ± 1.6 (67-71) | 70.4 (61.7-90.2) | 67.5 (65-70) | 68.53 ± 1.20 (64.90-71.83) | 66.0 ± 0.2 (65.7-66.4) | 66.8-67.2 |
| Stylet (μm) | 11.1 ±1.7(10-13) | 10* | 11.2(10-12) | 10.38 ±0.63 (9-12) | 11.2 ± 0.5 (10.4 - 11.7) | 10-11 |
Results
Aphelenchoides bicaudatus (Imamura, 1931) Filipjev & Schuurmans Stekhoven, 1941
Morphological characterization (Eight females in a good state of preservation)
Fig. 1.
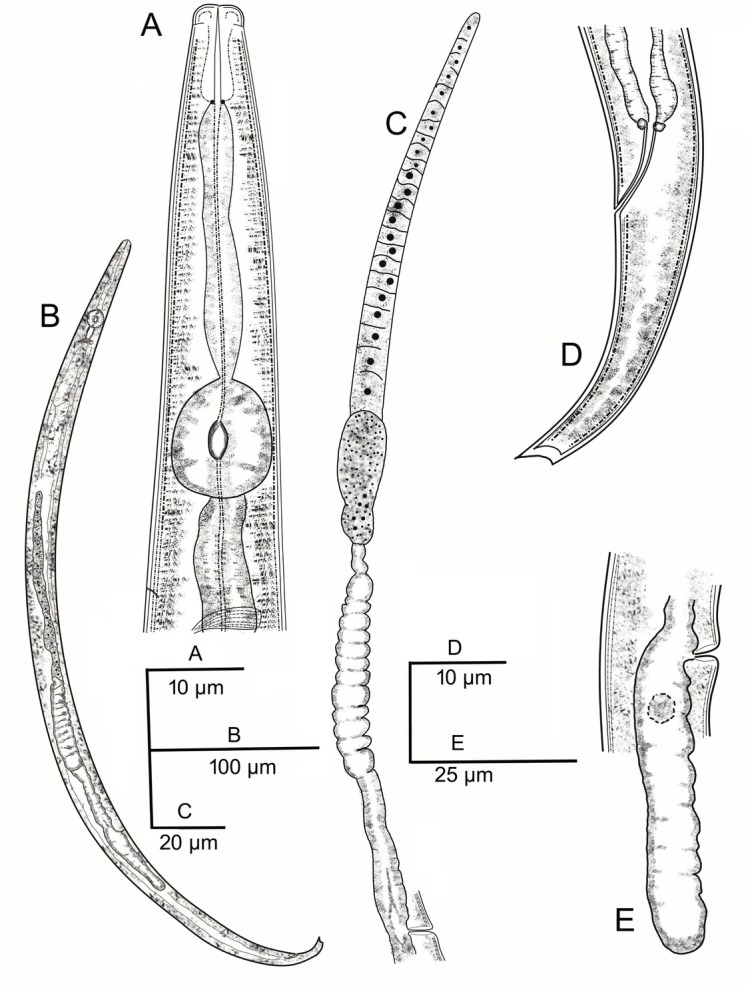
Aphelenchoides bicaudatus (Imamura, 1931) Filipjev and Schuurmans Stekhoven, 1941.
(A) anterior end; (B) entire female; (C) reproductive system; (D) female posterior end; (E) Post-vulval uterine sac.
Table 1.
Measurements of females of Aphelenchoides bicaudatus from South Africa. All measurements are in μm and in form: mean ± SD (range), except for ratio.
| Province | Western Cape | Limpopo |
|---|---|---|
| Locality | Cape Town | Magoebaskloof |
| n | 8 females | 4 females |
| L | 455.3 ± 64.5 (409 – 529) | 451.7 ± 56.7 (415 – 517) |
| a | 29.1 ± 2.0 (27.1 – 31.1) | 28.9 ± 0.7 (28.2 – 29.6) |
| b | 4.6 ± 0.5 (4.1 – 5.1) | 4.4 ± 0.3 (4.1 – 4.7) |
| M | 47.9 ± 3.4 (44.1 – 50.6) | 49.4 ± 3.6 (45.5 – 52.6) |
| c | 16.3 ± 2.0 (14.1 – 17.8) | 15.2 ± 1.3 (14.3 – 16.7) |
| c’ | 3.2 ± 0.6 (2.6 – 3.6) | 3.3 ± 0.3 (3.1 – 3.6) |
| V (%) | 68.8 ± 1.6 (67 – 71) | 68.7 ± 1.7 (67 – 70) |
| Lip region height | 2.8 ± 0.9 (2 – 4) | 3.1 ± 0.5 (3 – 4) |
| Lip region width | 5.7 ± 0.1 (5.6 – 5.8) | 5.4 ± 0.4 (5.1 – 5.8) |
| Stylet length | 11.1 ± 1.7 (10 – 13) | 9.6 ± 0.6 (9.5 – 10) |
| Conus | 4.7 ± 0.3 (4.5 – 5.0) | 4.8 ± 0.3 (4.5 – 5.0) |
| Mid of median bulb to anterior end. | 47.3 ± 3.2 (45 – 51) | 48.3 ± 2.6 (46 – 51) |
| Median bulb diameter | 9.0 ± 0.8 (8.5 – 10.0) | 9.2 ± 0.8 (8.0 – 10.0) |
| Median bulb length | 11.2 ± 0.7 (11 – 12) | 10.9 ± 1.0 (10 – 12) |
| Pharynx length | 88.3 ± 11.2 (76 – 98) | 93.2 ± 7.3 (86 – 100) |
| Neck | 99.0 ± 8.7 (89 – 104) | 103.3 ± 7.5 (95 – 110) |
| Nerve ring from anterior end | 71.7 ± 0.6 (71 – 76) | 73.0 ± 2.0 (71 – 75) |
| Excretory pore from anterior end | 61.3 ± 3.2 (60 – 65) | 60.3 ± 1.5 (59 – 62) |
| Body diameter at median bulb | 12.5 ± 1.8 (11 – 15) | 12.3 ± 1.5 (11 – 14) |
| Body diameter at mid body | 15.6 ± 1.5 (14 – 17) | 15.7 ± 2.1 (14 – 18) |
| Body diameter at anus | 8.8 ± 0.7 (8 – 9) | 9.1 ± 0.1 (8 – 10) |
| Anterior branch of reproductive system | 179.3 ± 18.0 (167 – 200) | 199.7 ± 53.2 (167 – 261) |
| Post-vulval uterine sac | 47.0 ± 2.6 (45 – 50) | 46.0 ± 1.0 (45 – 47) |
| Vagina length | 6.3 ± (6 – 7) | 6.7 ± 0.6 (6 – 7) |
| Rectum | 14.3 ± (12 – 16) | 14.7 ± 1.5 (13 – 16) |
| Tail length | 28.0 ± (24 – 31) | 29.7 ± 1.2 (29 – 31) |
Description
Female: Body slender, tapering slightly anteriorly, and more prominently toward posterior end. Body straight and tail region only slightly curved after heat relaxation. Cuticle annulated; 0.4 – 0.6 μm thick; annuli 0.5 – 0.7 μm wide. Lateral field with two incisures at mid-body region. Lip region rounded, offset, 5.6 – 5.8 μm wide and 2.0 – 3.7 μm high; no annules. Stylet weak, with small basal swellings. Procorpus wider anteriorly, gradually narrowing posteriorly, then widening at median bulb. Median bulb rounded to ovoid, occupying approximately 69 – 78 % of body width, and measuring 8.5 – 10.0 μm wide and 10.7 – 12.0 μm long. Cardia conoid and surrounded by intestinal tissue. Nerve ring about behind median bulb, at 68 – 82 % of the neck length. Excretory pore at isthmus level, at 57 – 73 % of the neck length. Vulva a transverse slit and slightly protruding, at 68 – 71 % of body length from anterior end. Anterior genital branch usually extending to region of pharyngeal gland lobe, 167 – 200 μm long. Post-vulval uterine sac 45 – 50 μm long, and extending for 32 – 38 % of distance from vulva to end of tail. Rectum prominent, straight, near ventral body wall, 12 – 16 μm long. Tail gradually tapering to terminus, which is unevenly bifurcate (Fig 1. D) with one prong longer than the other, 24 – 31 μm long.
Male: not found.
Other material examined: A population from Magoebaskloof, Limpopo Province, was recovered from the rhizosphere of a grass, which resembles the Cape Town population of A. bicaudatus.
Remarks: The South African population of A. bicaudatus resembles the previous populations studied from Japan (Imamura, 1931 (Filipjev & Schuurmans Stekhoven (1941), the USA (Siddiqui & Taylor, 1967), Taiwan (Jen et al., 2012), and South Korea (Kim et al., 2016). However, compared with the Japanese population, they differ in the lower range of the body length (409–529 vs 380–470 μm), b (4.1–5.1 vs 6.8–8.4), c (14.1–17.8 vs 9.4–12.6), and the upper range of V (67–71 vs 61.7–90.2). Compared with the American population, they differ in b (4.1–5.1 vs 7.3–9.6), c (14.1–17.8 vs 9.8–13.7), and tail length (24–31 vs 41 measurement extracted from original drawing). Compared with South Korean population, they differ in the lower range of body length (409–529 vs 514–523 μm), b (4.1–5.1 vs 7.3–7.4), c (14.1–17.8 vs 10.7–11.9), and tail length (24–31 vs 43–49 μm). Compared with the population from Taiwan, they differ in body length (409–529 vs 376–637 μm), b (4.1–5.1 vs 7.5–10.0), c (14.1–17.8 vs 10.16– 14.80), and c’ (2.6–3.6 vs 4.13–7.14). However, compared with a population from Pakistan (Israr et al., 2017), they differ body length (409–529 vs 360 μm), and b (4.1–5.1 vs 7.2–8.8).
DNA characters
The nBlast test of 18S rDNA showed 98 % similarity of the test population with the South Korean population of A. bicaudatus (KX345119), Taiwan (JN887884), and China (MH722388).
The phylogenetic analysis using 18S and ITS rDNA, placed the South African A. bicaudatus population in a clade together with other A. bicaudatus populations with the maximum posterior probability value (Figs. 2 and 3).
Fig. 2.
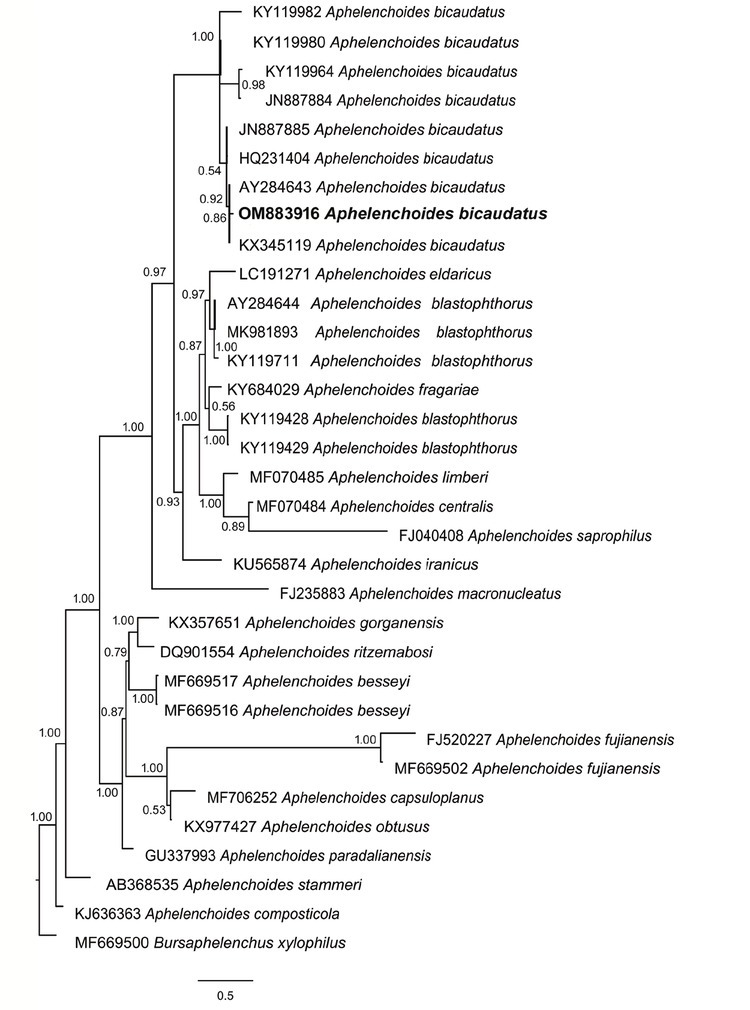
Phylogenetic position of Aphelenchoides bicaudatus from South Africa based on 18S rDNA.
Fig. 3.
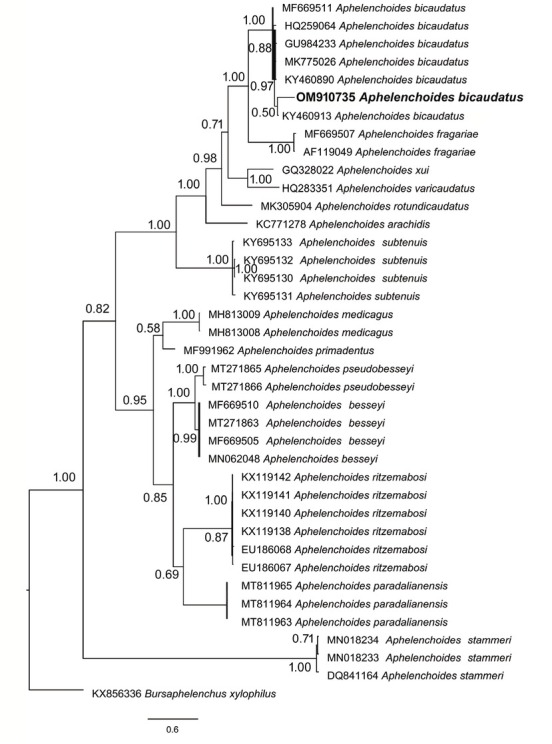
Phylogenetic position of Aphelenchoides bicaudatus from South Africa based on ITS rDNA.
Statistical analysis
An accumulated variability of 74.86 % was observed in female based PCA, specifically, 42.66 % in the PC1 and 32.20 % in the PC2 (Fig. 4). The variables b (r = 0.865), c (r = -0.862), c’ (r = 0.866), and tail length (r = 0.811) were responsible for the significant variability of the PC1. Regarding the PC2, body length (r = 0.714), a (r = -0.784), V (r = -0.668), and stylet length (r = 0.852) showed a significant correlation (Table 3). The PCA plot separated different populations of A. bicaudatus indicating a morphological variation between the populations (Fig. 4; Table 4). The result categorized the populations of A. bicaudatus into three groups, including 1) South Africa and Pakistan, 2) USA and South Korea, and 3) Taiwan and Japan.
Fig. 4.
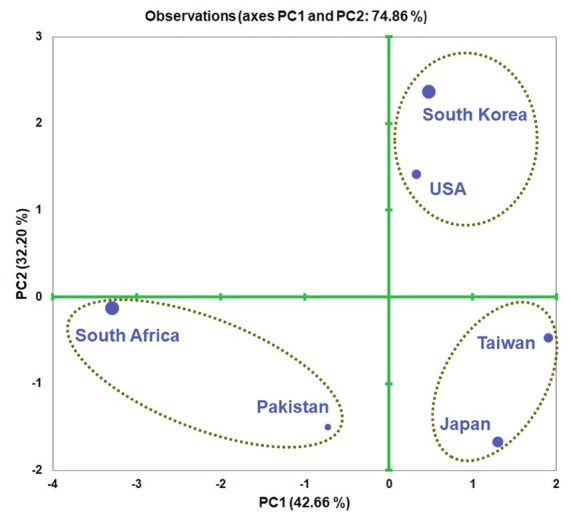
PCA plot of different populations of Aphelenchoides bicaudatus from various locations.
Table 3.
Loading factor of the variables of the different populations of Aphelenchoides bicaudatus.
| PC1 | PC2 | |
|---|---|---|
| L | 0.293 | 0.714 |
| a | 0.435 | -0.784 |
| b | 0.865 | -0.037 |
| c | -0.862 | -0.008 |
| c′ | 0.866 | 0.323 |
| Tail | 0.811 | 0.417 |
| V | 0.073 | -0.668 |
| Stylet | -0.483 | 0.852 |
Table 4.
Factor score of the variables of the different populations of Aphelenchoides bicaudatus.
| Observation | PC1 | PC2 |
|---|---|---|
| South Africa | -3.296 | -0.120 |
| Japan | 1.298 | -1.668 |
| USA | 0.332 | 1.408 |
| Taiwan | 1.912 | -0.479 |
| South Korea | 0.476 | 2.367 |
| Pakistan | -0.723 | -1.507 |
Discussion
The genus Aphelenchoides comprises a diverse group of species (Handoo et al., 2020). The morphological and molecular findings in the current study were in agreement with the mophometrics and phylogenies of Aphelenchoides species studied (Jen et al., 2012; Kim et al., 2016; Handoo et al., 2020). Among the species belong to the Aphelenchoides, two species, namely A. bicaudatus and A. hainanensis (Rahm, 1938) Goodey, 1951 having bifurcated tail. However, they distinguished by female body length (360 – 637 μm in A. bicaudatus vs 900 – 1300 μm in A. hainanensis), a (25.0 – 38.6 in A. bicaudatus vs 42.4 – 46.1 in A. hainanensis), and tail tip morphology (A. bicaudatus with bifurcated in female and conical in male vs A. hainanensis with bifurcated in both sexes). Therefore, the data of the present study confirm the South African species as A. bicaudatus.
Additionally, principal component analysis using morphometric features of species of A. bicaudatus, including the populations from South Africa, showed that A. bicaudatus has morphometric variation. The analyzed morphological characters allowed a clear separation between the populations of A. bicaudatus of this study.
The results indicated that there is intraspecific morphological variation across A. bicaudatus populations, which depends on the sampling locations. The populations of A. bicaudatus from South Africa and Pakistan stand close to each other than other populations. The populations from South Africa and Pakistan similar in tail length, a, c’, and V. However, they differ in body length, which indicating a variation between the two populations. PCA showed previously a useful tool to study the variation between the populations of the same species, as mentioned in Butlerius butleri (Shokoohi & Abolafia, 2021). Besides, the PCA also indicated a variation between the populations of Xiphinema hispanum complex group (Archidona-Yuste et al., 2020). The result of the present study is in agreement with the previous studies.
Three permanent microscope slides, containing the females of A. bicaudatus were deposited in the Aquaculture Research Unit of the University of Limpopo, South Africa. According to the literature, this is the first record of A. bicaudatus in South Africa. In conclusion, the morphological variation exists among the A. bicaudatus (e.g., body length, a, b, c, c’, vulval position, and tail length,) is due to the geographical location of the population. Besides, the ecological role of A. bicaudatus needs to be investigated in the grassland quality of South Africa.
References
- Addinsoft. XLSTAT, Analyse de données et statistique avec Ms Excel [XLSTAT, Data analysis and statistics with Ms Excel] Addinsoft, NY, USA. (In French): 2007. [Google Scholar]
- Archidona-Yuste A., Cai R., Cantalapiedra-Navarrete C., Carreira J.A., Rey A., Viñegla B., Liébanas G., Palomares-Rius J.E., Castillo P.. Morphostatic speciation within the dagger nematode Xiphinema hispanum-complex species (Nematoda: Longidoridae) Plants. 2020;9(12):1649. doi: 10.3390/plants9121649. DOI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos W.S.. Aphelenchoides arachidis n. sp. (Nematoda: Aphelenchoidea), an endoparasite of the testa of groundnuts in Nigeria. Z Pflanzenkr Pflanzenschutz. 1977;84:95–99. [Google Scholar]
- Christie J.R.. A description of Aphelenchoides besseyi n.sp., the summer- dwarf nematode of strawberries, with comments on the identity of Aphelenchoides subtenuis (cobb, 1929) and Aphelenchoides hodsoni goodey, 1935. Proc Helminth Soc Wash. 1942;9:82–84. [Google Scholar]
- Colbran R.C.. Studies of plant and soil nematodes. 7. Queensland records of the order Tylenchida and the genera Trichodorus and Xiphinema. Queensland J Agric Sci. 1964;21(1):77–123. [Google Scholar]
- Darriba D., Taboada G.L., Doallo R., Posada D.. jModel-Test 2: more models, new heuristics and parallel computing. Nat Methods. 2012;9:772. doi: 10.1038/nmeth.2109. DOI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das V. M.. Studies on the nematode parasites of plants in Hyderabad (Andhra Pradesh, India) Z Parasitenkd. 1960;19(6):553–605. doi: 10.1007/BF00260158. [DOI] [PubMed] [Google Scholar]
- De Grisse A.. Redescription ou modifications de quelques techniques utililisés dans l’étude des nématodes phytoparasitaires [Redescription or modifications of some techniques used in the study of plant parasitic nematodes] Meded. Rijksfac. Landb.Wet. Gent. 1969;34:351–369. (In French) [Google Scholar]
- Filipjev I.N., Stekhoven J.H.S. A manual of agricultural helminthology. Brill Archive; Leiden: 1941. pp. 1–878. [Google Scholar]
- Fischer M.. Über eine Clematis - krankheit [About a clematis disease] Bericht aus dem Physiolischen Laboratorium des Landwirthschaftlichen, Instituts der Universitat Halle [Report from the Physiological Laboratory of the Agricultural Institute of the University of Halle] 1894;11(3):1–11. (In German) [Google Scholar]
- Franklin MT.. Aphelenchoides composticola n.sp. and a. Aprophilus n.sp. from mushroom compost and rotting plant tissues. Nematologica. 1957;11:306–313. [Google Scholar]
- Goodey T. Soil and freshwater nematodes. London: Methuen and Co; 1951. [Google Scholar]
- Guindon S., Gascuel O.. A simple, fast and accurate method to estimate large phylogenies by maximum-likelihood. Syst Biol. 2003;52:696–704. doi: 10.1080/10635150390235520. DOI. [DOI] [PubMed] [Google Scholar]
- Hall T.A.. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Sym Series. 1999;41:95–98. [Google Scholar]
- Handoo Z., Kantor M., Carta L.. Taxonomy and Identification of Principal Foliar Nematode Species Aphelenchoides and Litylenchus. Plants. 2020;9:1490. doi: 10.3390/plants9111490. DOI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura S.. Nematodes in the paddy field, with notes on their population before and after irrigation. J. Coll. Agric. Imp. Univ. Tokyo. 1931;11:193–240. [Google Scholar]
- Israr M., Shahina F., Nasira K.. Description of Aphelenchoides turnipi n. sp. and redescription of A. siddiqii with notes on A. bicaudatus (Nematoda: Aphelenchoididae) from Pakistan. Pak J Nematol. 2017;35:3–12. [Google Scholar]
- Jen F.Y., Tsay T.T., Chen P.. Aphelenchoides bicaudatus from ornamental nurseries in Taiwan and its relationship with some agricultural crops. Plant Dis. 2012;96:1763–1766. doi: 10.1094/PDIS-03-12-0229-RE. DOI. [DOI] [PubMed] [Google Scholar]
- Joyce S.A., Reid A., Driver F., Curran J. Burnell A.M., Ehlers R-U, Masson J.P. Cost 812 biotechnology: genetics of entomopathogenic nematode-bacterium complexes; Proceedings of Symposium & Workshop. St. Patrick’s College, Maynooth, County Kildare, Ireland. Luxembourg: European Commission; 1994. Application of polymerase chain reaction (PCR) methods to identification of entomopathogenic nematodes; pp. 178–187. (Eds) [Google Scholar]
- Kaisa T.R.. Aphelenchoides microstylus n. sp. and Seinura onondagensis n. sp. (Nemata: Aphelenchina) from New York. J Nematol. 2000;32(4):396–402. [PMC free article] [PubMed] [Google Scholar]
- Kim J., Kim T., Park J.K.. First report of Aphelenchoides bicaudatus (Nematoda: Aphelenchoididae) from South Korea. Anim Syst Evol Divers. 2016;32:253. [Google Scholar]
- Le Roux X., Recous S., Attard E. Lemaire G., Hodgson J., Chabbi A. Grassland Productivity and Ecosystem Services. CAB International; 2011. Soil Microbial Diversity in Grasslands and its Importance for Grassland Functioning and Services. (Eds) [Google Scholar]
- Lesufi M.M., Swart A., Mc Donald A.H., Knoetze R., Tiedt L.R., Truter M.. Morphological and molecular studies on Aphelenchoides arachidis Bos, 1977 (Tylenchina: Aphelenchoididae) from groundnuts in South Africa. Nematology. 2015;17(4):433–445. doi: 10.1163/15685411-00002879. DOI. [DOI] [Google Scholar]
- Loof P.A.A.. Free-living and plant-parasitic nematodes from Venezuela. Nematologica. 1964;10(2):201–300. [Google Scholar]
- Mcleod R.. Aphelenchoides bicaudatus, a parasite of cultivated mushroom. Nature. 1967;214:1163–1164. doi: 10.1038/2141163a0. DOI. [DOI] [Google Scholar]
- Nickle W. R.. A taxonomic review of the genera of the Aphelenchoidea (Fuchs, 1937) Thorne, 1949 (Nematoda: Tylenchida) J Nematol. 1970;2:375. [PMC free article] [PubMed] [Google Scholar]
- Oliveira C.J., Subbotin S.A., Álvarez-Ortega S., Desaeger J., Brito J.A., Xavier K.V., Freitas L.G., Vau S., Inserra R.N.. Morphological and molecular identification of two Florida populations of foliar nematodes Aphelenchoides spp.) isolated from strawberry with the description of Aphelenchoides pseudogoodeyi sp. n. (Nematoda: Aphelenchoididae) and notes on their bionomics. Plant Dis. 2019;103(11):2825–2842. doi: 10.1094/pdis-04-19-0752-re. DOI. [DOI] [PubMed] [Google Scholar]
- Page R.D.M.. Treeview: an application to display phylogenetic trees on personal computers. Comput Appl Biosci. 1996;12:357–358. doi: 10.1093/bioinformatics/12.4.357. [DOI] [PubMed] [Google Scholar]
- Rahm G. F.. Freilebende und Saprophytische nematoden der insel hainan (mit besonderer berücksichtigung der bisher bekannt gewordenen nematoden nordchinas und Japans) [Free-living and saprophytic nematodes of the island of Hainan (with special consideration of the nematodes of northern China and Japan that have become known so far)] Ann Zool Jap. 1938;17:646–667. (In German) [Google Scholar]
- Richardson D.M., Foxcroft L.C., Latombe G., Le Maitre D.C., Rouget M., Wilson J.R. van Wilgen B., Measey J., Richardson D., Wilson J., Zengeya T. Biological Invasions in South Africa. Invading Nature-Springer Series in Invasion Ecology. vol 14. Springer; Cham: 2020. The biogeography of South African terrestrial plant invasions. (Eds) DOI. [DOI] [Google Scholar]
- Ronquist F., Huelsenbeck J.. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. DOI. [DOI] [PubMed] [Google Scholar]
- Shokoohi E.. First report of Bitylenchus ventrosignatus (Tobar Jiménez, 1969) Siddiqi, 1986 associated with wild grass in Botswana. J Nematol. 2021;53:1–9. doi: 10.21307/jofnem-2021-037. DOI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shokoohi E., Abolafia J.. Redescription of a predatory and cannibalistic nematode, Butlerius butleri Goodey, 1929 (Rhabditida: Diplogastridae), from South Africa, including its first SEM study. Nematology. 2021;23:969–986. doi: 10.1163/15685411-bja10089. DOI. [DOI] [Google Scholar]
- Siddiqui I.A., Taylor D.P.A.. Redescription of Aphelenchoides bicaudatus (Imamura, 1931) Filipjev & Schuurmans Stekhoven, 1941 (Nematoda: Aphelenchoididae), with a description of the previously undescribed male. Nematologica. 1967;13:581–585. [Google Scholar]
- Straube D., Juen A.. Storage and shipping of tissue samples for DNA analyses: A case study on earthworms. Eur J Soil Biol. 2013;57:13–18. doi: 10.1016/j.ejsobi.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J.D., Higgins D.G., Gibson T.J.. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nuc Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]


