Abstract
Introduction
The broad and sustained efficacy of apremilast for psoriasis has been demonstrated in randomized and real-world observational studies. Data from Central and Eastern Europe (CEE) are lacking. Moreover, apremilast use in this region is limited by country-specific reimbursement criteria. This is the first study to report data on the real-world use of apremilast in the region.
Methods
APPRECIATE (NCT02740218) was an observational, retrospective, cross-sectional study assessing psoriasis patients 6 (± 1) months after apremilast treatment initiation. The study aimed to describe the characteristics of patients with psoriasis receiving apremilast, estimate treatment outcomes, including Psoriasis Area Severity Index (PASI), Body Surface Area (BSA), and Dermatology Life Quality Index (DLQI), and assess dermatologists' and patients' perspectives on treatment using questionnaires including the Patient Benefit Index (PBI). Adverse event reports were taken from the medical records.
Results
Fifty patients (Croatia: 25; Czech Republic: 20; Slovenia: 5) were enrolled. In patients continuing apremilast at 6 (± 1) months, mean (± SD) PASI score was reduced from 16.2 ± 8.7 points at treatment initiation to 3.1 ± 5.2 at 6 (± 1) months; BSA from 11.9% ± 10.3% to 0.8% ± 0.9%; DLQI from 13.7 ± 7.4 points to 1.6 ± 3.2. PASI 75 was reached by 81% of patients. Physicians reported that the overall treatment success fulfilled their expectations in more than two thirds of patients (68%). At least three-quarters of patients reported apremilast had a quite or very high benefit on the needs they identified as being most important. Apremilast was well tolerated; no serious or fatal adverse events were identified.
Conclusion
Apremilast was effective in reducing skin involvement and improving quality of life in CEE patients having severe disease. Treatment satisfaction among physicians and patients was very high. These data add to the growing body of evidence showing consistent effectiveness of apremilast across the continuum of psoriasis disease severity and manifestations.
Trial registration
ClinicalTrials.gov identifier, NCT02740218.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-023-02468-3.
Keywords: Apremilast, Psoriasis drug therapy, Real-world data, Health-related quality of life, Psoriasis severity scores
Key Summary Points
| Why carry out this study? |
| While the broad and sustained efficacy of apremilast for the treatment of psoriasis has been demonstrated in randomized and real-world observational studies, its use in clinical practice varies according to country-specific reimbursement criteria |
| A better understanding of the clinical value of apremilast in different countries and patient populations could potentially improve the care and outcomes of psoriasis patients |
| The APPRECIATE study assessed the clinical value of apremilast for the treatment of psoriasis across multiple European countries from the perspectives of the patient and their treating physician |
| What was learned from the study? |
| Compared with the overall study population, patients from Central and Eastern Europe had more severe psoriasis, a higher rate of prior systemic therapy, and highly impaired quality of life |
| Despite these differences, patients from Central and Eastern Europe experienced substantial improvements in disease signs and symptoms and reported apremilast met or exceeded their expectations, demonstrating the clinical value of apremilast in this region |
Introduction
Psoriasis is a chronic inflammatory disease [1] with a broad range of clinical manifestations, including inflamed and scaly skin lesions across any area of skin, and is commonly associated with a number of comorbidities. In Central and Eastern Europe (CEE), the prevalence of psoriasis is reported to be 1.45% (95% CI: 0.49–4.08), with little variation across individual countries. The prevalence is 1.36% (0.4–4.48) in Croatia, 1.45% (0.4–4.08) in the Czech Republic, and 1.47% (0.45–4.68) in Slovenia [2].
Psoriasis is a life-long disease that places substantial burden on patients’ quality of life even in those with limited skin involvement. Certain areas and symptoms of psoriasis can have a disproportionate impact on quality of life, such as manifestations of disease in highly visible or sensitive locations, such as the scalp, nails, palms and/or soles, and genital areas [3–5]. Psoriasis is often associated with itch, which is particularly burdensome for many patients as it can lead to sleep disturbances and fatigue and social embarrassment when in public. Moreover, the visible disfigurations caused by psoriasis carry a similar psychological burden as depression [1, 6, 7] and have a significant impact on health-related quality of life (HRQoL) [8].
Patient-perceived disease severity, however, is often not represented by standard clinical scores, which can lead to misalignment between a patient and their treating physician regarding their disease severity and treatment goals [9, 10]. For example, limited skin involvement as measured by body surface area (BSA) may be perceived as moderate or severe disease by the patient, particularly if highly visible or sensitive locations are affected, while their physician may consider this to be mild disease [11, 12]. A better understanding of patient-relevant treatment goals and expectations is thus needed to overcome patient-physician disparity [13]. As outlined by the EuroGuiDerm Guideline on the treatment of plaque psoriasis, involvement of visible or sensitive areas (such as scalp, nails, genital, palms and/or soles) may upgrade disease severity from mild to moderate to severe; consequently, systemic therapy can be considered, even in patients with limited skin involvement [14, 15]. The use of ‘upgrade criteria’ in relation to the treatment goals has been shown to significantly increase patient satisfaction with care [16, 17].
Apremilast is an oral non-biologic (small molecule) phosphodiesterase-4 inhibitor [18] approved in Europe for the treatment of moderate-to-severe chronic plaque psoriasis and psoriatic arthritis as well as the treatment of oral ulcers associated with Behçet's disease [18]. In clinical trials [19–22] and real-world observational studies [23–28], apremilast has shown broad and sustained efficacy and has an established long-term safety profile. Data from CEE are, however, lacking. Moreover, apremilast use in this region is limited by country-specific reimbursement criteria. In Croatia, patients may receive apremilast if Psoriasis Area Severity Index (PASI) or Dermatology Life Quality Index (DLQI) is > 15 or if BSA exceeds 15% and if they did not respond or cannot tolerate or have contraindications to at least two different previously implemented systemic drugs including psoralen and ultraviolet A light (PUVA), retinoids, cyclosporine, and methotrexate, as recommended by specialist dermato-venerologists. In Czech Republic, adult patients with PASI > 10 are eligible if they have previously undergone one of the following conventional systemic treatments: acitretin, cyclosporine, methotrexate, or phototherapy (PUVA or narrow-band ultraviolet B [NBUVB] light) and are not suitable for treatment with methotrexate mainly because of contraindications.
The multinational Apremilast Clinical Treatment Experience in Psoriasis (APPRECIATE) study was conducted to assess the effectiveness of apremilast in the real-world setting, and data from Northwestern Europe (Austria, Germany, Ireland, Sweden, Switzerland, and the UK) [23, 24], Austria [29], and Spain [25] have been published. This is the first report on the real-world use of apremilast in the CEE region. The present cohort included patients from Croatia, Czech Republic, and Slovenia.
Methods
The detailed methods of the APPRECIATE study have been described by Augustin et al. [23] and Klein et al. [24] and are briefly summarized below. Supplemental Figure S1 provides an overview of the study design and outcome measures.
Study Design and Objectives
The multinational, retrospective, cross-sectional APPRECIATE study included psoriasis patients treated with apremilast in real-world clinical practice. A physician’s appointment conducted 6 (± 1) months after apremilast initiation served as enrolment and the ‘study visit.’ The study objectives were to (1) describe patient and disease characteristics at apremilast initiation; (2) assess the outcome of apremilast treatment at 6 (± 1) months; (3) assess patient and physician satisfaction with apremilast at 6 (± 1) months.
Eligibility Criteria
The APPRECIATE study included consenting adults with physician-diagnosed chronic plaque psoriasis who were initiated with apremilast in routine clinical practice and according to country specific reimbursement criteria 6 month prior to study participation (Supplemental Table S1); study participation was possible regardless of whether apremilast treatment was ongoing or discontinued at the time of the study visit. Patients who were participating in any clinical trial were excluded from the study.
Outcome Measures
At the study visit, i.e., 6 (± 1) months after apremilast initiation, the following variables describing apremilast treatment initiation were retrospectively collected from medical records: patient demographics, disease characteristics, co-morbidities, prior psoriasis treatments, time since psoriasis diagnosis, clinical manifestations of psoriasis, presence of psoriatic arthritis, other significant disease history, and reasons for apremilast initiation.
The following apremilast outcomes were also collected at the study visit: apremilast status (ongoing or discontinued; if discontinued, reasons for discontinuation), change in disease severity (PASI [30], Physician Global Assessment [PGA] [30], BSA [30]), change in DLQI (31), and adverse events reported during apremilast therapy as documented in the medical records. Satisfaction with apremilast at 6 (± 1) months among patients and their treating physicians was also assessed using study-specific questionnaires. In addition, patients completed the Patient Benefit Index (PBI) [32], consisting of the Patient Needs Questionnaire (PNQ) and the Patient Benefit Questionnaire (PBQ), and the Treatment Satisfaction Questionnaire for Medication (TSQM-9) [33].
Table S2 shows an overview of the disease scores and questionnaires used.
Compliance with Ethics Guidelines
This study was performed in accordance with the 1964 Declaration of Helsinki and its later amendments. The study protocol was approved by the institutional review boards and/or national central ethics committees of each participating country, as per local requirements. In the Czech Republic, the State Institute of Drug Control (SUKL) approved APPRECIATE under the approval number 2002110009. In Croatia, the Central Ethics Committee approved the study under the class UP/1-530-07/20-08/06, number 381-13-02/340-20-03. In Slovenia, the Commission of the Republic of Slovenia for Medical Ethics approved the study under approval number 0120-221/2020/5. All patients provided informed consent prior to any data collection. APPRECIATE was registered in ClinicalTrials.gov (NCT02740218).
Statistical Methods
All analyses were descriptive in nature. Categorical variables were summarized as number, percentage, and 95% confidence intervals (CIs). Continuous variables were summarized as mean, standard deviation (SD), 95% CI, median, first and third quartiles (Q1, Q3), and range. Data were analysed with no imputation for missing data.
Results
The CEE cohort (n = 50, full analysis set [FAS]) included 25 patients from Croatia (5 study centers), 20 from the Czech Republic (5 study centers), and 5 from Slovenia (1 study center).
Patient and Disease Characteristics
Table 1 shows patient and disease characteristics of the CEE cohort at apremilast initiation.
Table 1.
Patient demographics and disease characteristics
| All patients (N = 50) | |
|---|---|
| Sex, n (%) | |
| Male | 28 (56) |
| Female | 22 (44) |
| Age at apremilast initiation, years | |
| Mean (SD) | 53.5 (14.8) |
| Median (Q1, Q3) | 54.0 (40.0, 68.0) |
| Age category, n (%) | |
| < 35 years | 7 (14) |
| 35–65 years | 28 (56) |
| > 65 years | 15 (30) |
| BMI (kg/m2) | |
| Mean (SD) | 30.5 (6.5) |
| Median (Q1, Q3) | 30.0 (26.7, 33.9) |
| Psoriasis disease severity and HRQoL scores at apremilast initiation | |
| PASI (points), n = 47a | |
| Mean (SD) | 16.1 (7.5) |
| Median (Q1, Q3) | 15.2 (11.5, 19.8) |
| PGA (points), n = 28a | |
| Mean (SD) | 3.0 (0.5) |
| Median (Q1, Q3) | 3.0 (3.0, 3.0) |
| PGA categories [n (%)], n = 28a | |
| Missing | 22 (44) |
| Clear | 0 (0) |
| Almost clear | 0 (0) |
| Moderate | 4 (8) |
| Severe | 20 (40) |
| Very severe | 4 (8) |
| BSA (%), n = 30a | |
| Mean (SD) | 13.8 (10.0) |
| Median (Q1, Q3) | 12.0 (5.0, 20.0) |
| DLQI (points), n = 46a | |
| Mean (SD) | 15.9 (6.4) |
| Median (Q1, Q3) | 17.0 (11.0, 20.0) |
| Psoriasis locations, n (%) | |
| Any visible psoriasis manifestation (nail, scalp, palmoplantar)b | 46 (92) |
| Scalp psoriasis | 34 (68) |
| Nail psoriasis | 25 (50) |
| Genital psoriasis | 11 (22) |
| Palmoplantar psoriasis | 10 (20) |
| Nongenital inverse psoriasis | 10 (20) |
| Palmoplantar pustulosis | 3 (6) |
| Psoriasis symptoms, n (%) | |
| Pruritus | 34 (68) |
| Fatigue | 8 (16) |
| Prior treatments for psoriasis, n (%) | |
| Any prior treatment for psoriasis | 49 (98) |
| Conventional systemic | 49 (98) |
| Phototherapy | 24 (48) |
| Biologics | 3 (6) |
| Others | 3 (6) |
| Comorbidities (> 5% of patients), n (%) | |
| Psoriatic arthritis | 17 (34) |
| Metabolic syndrome | 10 (20) |
| Hypertension | 6 (12) |
| Diabetes mellitus | 3 (6) |
| Obesity | 3 (6) |
BSA body surface area, DLQI Dermatology Life Quality Score, HRQoL health-related quality of life, PASI Psoriasis Area Severity Index, PGA Physician Global Assessment, SD standard deviation
aNumber of patients with scores recorded
bPatients with more than one manifestation are only counted once
Mean (± SD) age of patients in the CEE cohort was 53.5 ± 14.81 years and all patients were white. Mean (± SD) body mass index (BMI) was 30.5 ± 6.48 kg/m2.
At apremilast initiation, mean (± SD) time since psoriasis diagnosis was 18.7 ± 11.99 years. PASI, BSA, and DLQI scores indicated that patients on average had severe psoriasis, i.e., PASI > 10 or BSA > 10, and DLQI > 10, as per EuroGuiDerm Guideline definition (15). Table 1 shows mean (± SD) and median (Q1, Q3) levels of PASI, affected BSA, DLQI, and PGA; however, PGA was recorded in only 28 (56%) patients.
Most patients (92%, n = 46) had visible psoriatic lesions as detailed in Table 1. Two-thirds (68%, n = 34) reported pruritus symptoms, and 16% (n = 8) reported fatigue. Most patients (98%, n = 49) had received prior conventional systemic therapy for psoriasis, approximately half (48%, n = 24) had received prior phototherapy, and few patients had received prior biologics (6%, n = 3).
Overall, 70% of patients (n = 35) had comorbidities as shown in Table 1, the most common being psoriatic arthritis (34%, n = 17) and metabolic syndrome (20%, n = 10).
Apremilast Use
At 6 (± 1) months (i.e., the study visit), 62% of patients (n = 31) were continuing apremilast treatment and 38% (n = 19) had discontinued. Mean (± SD) treatment duration was 163.5 ± 49.2 days overall, 184.2 ± 20.8 days in patients continuing apremilast, and 127.8 ± 62.7 days in patients who had discontinued apremilast. Reasons for apremilast discontinuation were lack of efficacy (24%, n = 12), safety or tolerability issues (10%, n = 5), or other reasons (4%, n = 2; Table 2).
Table 2.
Apremilast use
| All patients (N = 50) | |
|---|---|
| Time between psoriasis diagnosis and apremilast initiation, years | |
| Mean (SD) | 18.7 (12.0) |
| Median (Q1, Q3) | 17.5 (9.0, 25.0) |
| Reason for apremilast initiation, n (%) | |
| Previous therapy ineffective | 34 (68) |
| Intolerant to previous therapy | 13 (26) |
| Contraindications to conventional therapies | 2 (4) |
| Delaying use of biologics | 1 (2) |
| Patient choice | 0 (0) |
| Other | 0 (0) |
| Apremilast treatment status at 6 (± 1) months, n (%) | |
| Ongoing | 31 (62) |
| Discontinued | 19 (38) |
| Reason for apremilast discontinuation, n (%) | |
| Lack of efficacy | 12 (24) |
| Safety/tolerability | 5 (10) |
| Other | 2 (4) |
SD standard deviation
Effectiveness of Apremilast
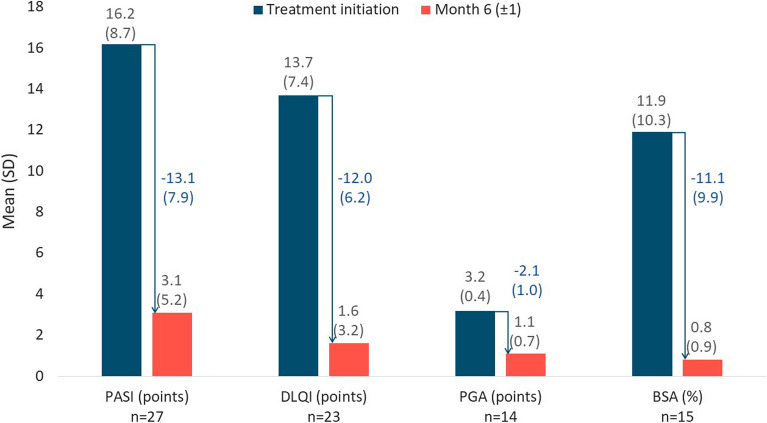
Figure 1 summarizes treatment effectiveness in patients continuing apremilast at 6 (± 1) months who had non-missing scores at apremilast initiation and 6 (± 1) months. PASI score (n = 27) was reduced by a mean (± SD) of 83% ± 23% to 3.1 ± 5.2 points. Most patients with non-missing data achieved a PASI 50 and a PASI 75: 24/27 (89%; 95% CI: 71%-98%) and 22/27 (81%; 95% CI: 62%-94%), respectively. BSA (n = 15) was reduced by a mean (± SD) of 11.1% ± 9.9% to 0.8% ± 0.9%. PGA (n = 14) was reduced by a mean (± SD) of 2.1 ± 1.0 points to 1.1 ± 0.7 points. DLQI (n = 23) was reduced by a mean (± SD) of 12.0 ± 6.2 points to 1.6 ± 3.2 points, and all patients with a DLQI ≥ 5 at apremilast initiation (n = 21, 100%) achieved a clinically meaningful improvement, i.e., a reduction in DLQI score of at least 5 points.
Fig. 1.
Psoriasis disease severity and HRQoL scores at treatment initiation and at month 6 (± 1) and absolute change in patients with ongoing apremilast therapy. HRQoL health-related quality of life, SD standard deviation. Mean (SD) values are calculated based on the number of patients with assessments at baseline and month 6 (± 1) visit (n). The instruments used were Psoriasis Area and Severity Index (PASI) [30], Dermatology Life Quality Index (DLQI) [31], Physician Global Assessment (PGA) [30], and body surface area (BSA) [30]
Physician Assessment of Apremilast Effectiveness
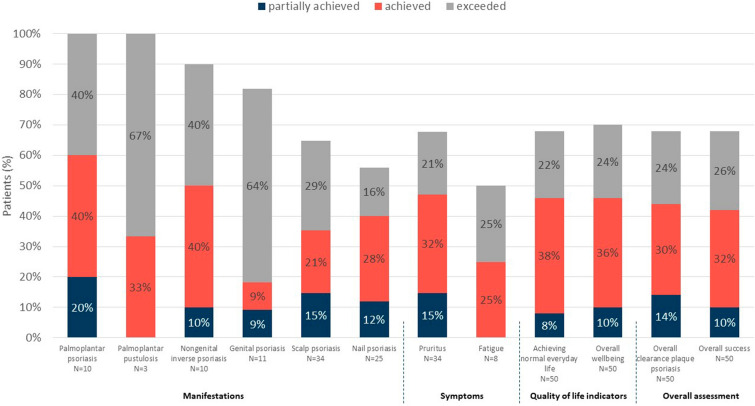
Physician satisfaction with apremilast at 6 (± 1) months, reported for various manifestations and symptoms, is summarised in Fig. 2. For most of the reported manifestations and symptoms, physicians reported that apremilast fulfilled their expectations (partially achieved, achieved or exceeded their expectations) in at least 65% of patients that experienced these manifestations and symptoms at apremilast initiation. For nail psoriasis, expectations were fulfilled in 56% and for fatigue in 50% of patients, respectively. Apremilast met physicians' expectations for overall clearance of plaque psoriasis, overall patient wellbeing and achievement of normal everyday life in over two-thirds of patients [34/50 (68%), 35/50 (70%), and 34/50 (68%), respectively]. Physicians reported that the overall treatment success fulfilled their expectations in more than two thirds of patients (68%; Fig. 2), with 10% (5/50) reporting partial achievement of expectations, 32% (16/50) reporting achievement of expectations, and 26% (13/50) reporting their expectations were exceeded.
Fig. 2.
Partial achievement of, achievement of, or exceeding physicians’ expectations. Percentages show the proportions of patients partially achieving, achieving, or exceeding dermatologists’ expectations in any of the indicated areas. Physicians’ expectations were assessed using a study-specific questionnaire
Treatment Needs and Satisfaction
Patients’ and physicians’ satisfaction with apremilast treatment, expressed as “agree” or “strongly agree” on a given statement, is summarised in Fig. 3.
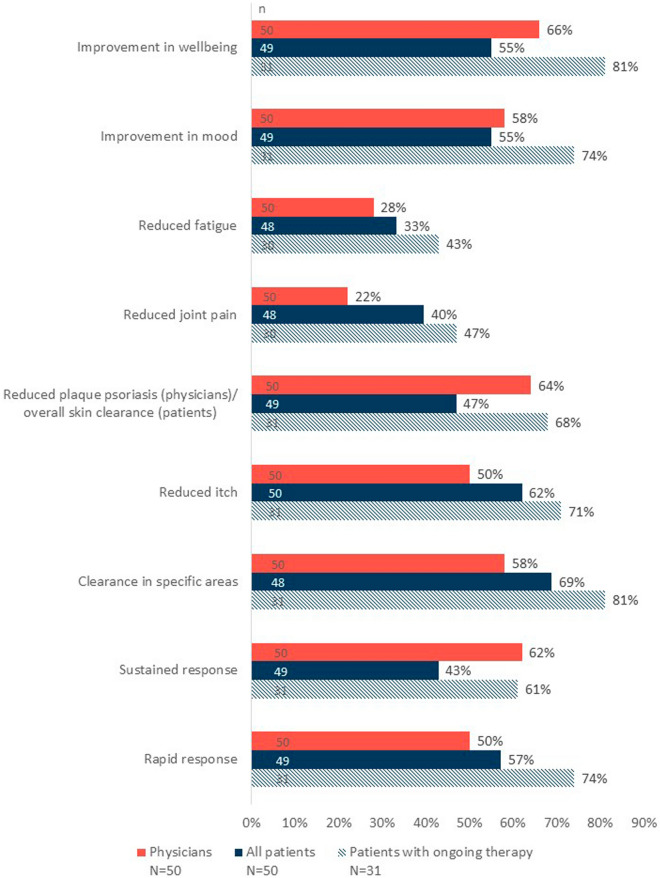
Fig. 3.
Treatment effect of apremilast on symptoms assessed by patients and physicians. Percentages represent individuals responding “agree” or “strongly agree” and are based on non-missing responses (n). Specific areas of psoriasis were the scalp, hands, soles of feet, nails, or genitals. Physicians’ and patients’ estimates of apremilast treatment effect were assessed using a study-specific questionnaire
Physicians agreed or strongly agreed that apremilast notably reduced plaque psoriasis and improved overall wellbeing in two-thirds [33/50 (66%)] of patients, provided a sustained response in 62% (31/50) of their patients, led to clearance in specific areas and improved mood in 58% (29/50) of patients, and provided a rapid response and reduced itch in half [25/50 (50%)] of patients (Fig. 3).
Over two-thirds of all patients reported clearance in their affected specific location(s) of psoriasis [33 of 48 (69%) reported “agree” or “strongly agree”]. More than half of all patients reported a rapid response to apremilast, reduced itch, and improved mood and wellbeing [28/49 (57%), 31/50 (62%), 27/49 (55%), and 27/49 (55%), respectively]. Among patients continuing apremilast at 6 (± 1) months, for most treatment effect measures, over two-thirds of patients achieved improvements in those areas. Importantly, 81% (25/31) reported improvement in wellbeing and clearance in their affected specific location(s) of psoriasis; 74% (23/31) reported a rapid response and an improvement in their mood (Fig. 2).
As measured by the TSQM-9 questionnaire, patient’s satisfaction with apremilast effectiveness and convenience and their global satisfaction with apremilast were high, especially in patients with ongoing apremilast treatment. On the 100-point treatment effectiveness scale, patients’ mean (± SD) score was 61.3 ± 27.42 overall and 73.7 ± 23.66 for patients with ongoing apremilast treatment. Patients’ mean (± SD) convenience score was 80.1 ± 14.20 overall and 84.6 ± 12.06 for patients with ongoing apremilast treatment. Patients’ mean (± SD) global satisfaction score was 60.0 ± 27.91 overall and 74.2 ± 20.09 for patients with ongoing apremilast treatment (Fig. S2).
In patients continuing apremilast treatment at 6 (± 1) months, the highest ranked patient needs in the PNQ were the nine questions comprising the treatment dimensions of “reducing impairments due to therapy,” “having confidence in healing,” and “other,” which includes quick skin improvement and regaining disease control (Fig. S3A). Assessing apremilast treatment satisfaction with the PBQ, over two-thirds of patients reported a quite or very high benefit of apremilast treatment for almost all questions. For the dimensions ranked as having the highest need as per the PNQ, at least three-quarters of patients reported a quite or very high benefit for seven out of the nine questions comprising the above-mentioned dimensions (Fig. S3B). The mean (± SD) global PBI was 2.5 ± 1.26 overall and 3.1 ± 0.97 in patients with continuing apremilast treatment.
Safety of Apremilast
Of the 50 patients in the CEE cohort, 11 reported a total of 22 apremilast-related adverse events; none were serious or fatal. Treatment-related adverse events leading to apremilast discontinuation were reported in three patients. The most frequent treatment-related adverse events (> 5% of patients; Table 3) were nausea (4 patients, 8%) and headache (3 patients, 6%).
Table 3.
Apremilast-related adverse events by system organ class and preferred term
| System organ class preferred term | All patients (N = 50) |
|---|---|
| Gastrointestinal disorders, n (%) | 7 (14) |
| Nausea | 4 (8) |
| Diarrhea | 2 (4) |
| Abdominal pain | 1 (2) |
| Frequent bowel movement | 1 (2) |
| Gastrointestinal disorder | 1 (2) |
| Nervous system disorders, n (%) | 4 (8) |
| Headache | 3 (6) |
| Tremor | 1 (2) |
| Cardiac disorders, n (%) | 2 (4) |
| Tachycardia | 2 (4) |
| General disorders and administration site conditions, n (%) | 2 (4) |
| Fatigue | 1 (2) |
| Pyrexia | 1 (2) |
| Psychiatric disorders, n (%) | 1 (2) |
| Insomnia | 1 (2) |
| Metabolism and nutrition disorders, n (%) | 1 (2) |
| Decreased appetite | 1 (2) |
| Musculoskeletal and connective tissue disorders, n (%) | 1 (2) |
| Back pain | 1 (2) |
| Respiratory, thoracic and mediastinal disorders, n (%) | 1 (2) |
| Cough | 1 (2) |
| Skin and subcutaneous tissue disorders, n (%) | 1 (2) |
| Hyperhidrosis | 1 (2) |
Discussion
Data on the effectiveness and tolerability of apremilast and physician and patient satisfaction with treatment in real-world clinical practice in CEE countries are currently lacking. Psoriasis registries in the region that were originally set up for biologics in the Czech Republic and Slovenia contain limited data on apremilast [34]. The present study, conducted in 50 patients from Croatia, Czech Republic, and Slovenia, is the first in the region to assess apremilast's effectiveness in lowering psoriasis disease burden in the real-world setting. In addition, our study assessed physician and patient satisfaction with apremilast treatment, including the extent to which apremilast met the treatment goals that patients identified ‘very important.’
Although data from the APPRECIATE family of studies have been previously reported [23, 25, 29], it remains important to collect data from different European regions. In CEE, apremilast use is limited by country-specific reimbursement criteria, which are reflected in our cohort. Patients enrolled in our study had more severe disease at apremilast initiation than patients in Northwestern Europe (N = 480) [23] and Spain (N = 80) [25]. For example, mean PASI (± SD) scores were 16.1 ± 7.5 points in the CEE cohort versus 12.5 ± 8.4 points in Northwestern Europe and 8.3 ± 5.3 points in Spain. Mean (± SD) DLQI was also higher in CEE compared to other regions: CEE, 15.9 ± 6.4 points; Northwestern Europe, 13.4 ± 7.5 points; Spain, 8.9 ± 6.6 points [23, 25].
Patients in the CEE cohort who continued apremilast at 6 (± 1) months had a similar benefit from apremilast treatment as patients in other regions despite their greater disease severity at treatment initiation, including objective clinical measures and patient-reported outcomes. In the CEE cohort, the mean (± SD) PASI score at 6 (± 1) months was 3.1 ± 5.2 points and mean (± SD) affected BSA was 0.8% ± 0.9%; in the Spanish cohort [25], mean PASI was 3.4 (95% CI: 2.1–4.7) points and BSA was 2.6% (95% CI: 1.6–3.7%). For PGA and DLQI, the scores after 6 (± 1) months of apremilast treatment were also similar in both cohorts [25].
Psoriasis of the scalp, face, palmoplantar region, genitals, nails, and intertriginous areas is often underdiagnosed [35], and these are commonly considered difficult-to-treat manifestations of psoriasis [36]. A large US prevalence study of psoriasis phenotypes reported 52–55% scalp, 14–16% palmoplantar, and 23–27% nail [37]; among patients prescribed apremilast in the APPRECIATE studies, the prevalence of these manifestations was higher. The proportion of patients with scalp psoriasis was 68% in CEE and in Northwestern Europe and 43% in Spain [23, 25]. Palmoplantar psoriasis was similar across all cohorts (CEE, 20%; Northwestern Europe, 24%; Spain, 25%); nail psoriasis was present in 50%, 38%, and 34%, respectively [23, 25]. Pruritus was present in two-thirds of patients in the CEE and Northwestern European cohorts (both 68%) and 58% of patients in Spain [23, 25]. In the CEE cohort, 92% of patients had psoriasis at visible locations, with 81% in Northwestern Europe [23].
Prior treatments differed between the regions. Almost all CEE patients had a prior systemic treatment (98%), in line with the approved label recommending apremilast as second-line therapy. Prior therapy was mostly conventional systemic therapies (98%) and phototherapy (48%); the use of biologics was rare (6%). In Northwestern Europe, the use of conventional systemic therapies was lower (68%), and phototherapy (56%) and biologics (15%) were used more frequently [23]. Prior biologic use was highest in Spain (19%), where conventional systemic therapies were frequently used (79%) and phototherapy was only used in one-third of patients (33%) [25]. In the CEE region, treatment prescriptions are often carefully controlled, e.g., in Croatia, hospital drug committees decide on the extension of prescriptions based on objective outcome measures (see “Limitations” below).
In our CEE cohort, treatment satisfaction among physicians and patients was very high, which is aligned with the data from Northwestern Europe and Spain [23–25]. Dermatologists indicated that the overall success of apremilast treatment exceeded their expectations in one-quarter of treated patients in the present cohort compared with 23% in the Northwestern European cohort [23] and 50% in Spain [25]. Expectations were partly achieved or achieved in 10% and 32% in the CEE cohort and 22% and 31% in the Northwestern European cohort [23], respectively (data not published for Spain). Global satisfaction in all patients reached 60% in the CEE cohort, 58% in the Northwestern European cohort [23], and 52% in Spain [25]. In patients continuing apremilast at 6 (± 1) months, the respective rates were 74%, 70%, and 71%. This suggests that patients who were satisfied with the treatment outcomes tended to stay on apremilast therapy.
The findings of the APPRECIATE CEE cohort add to the growing body of real-world evidence showing improvement in objective psoriasis outcome scores under apremilast treatment and high satisfaction among physicians and patients. Several longitudinal studies, such as OTELO from Belgium [26], LAPIS PSO from Germany [38], and APPRAISAL from Greece [28], have also assessed apremilast in routine clinical practice for the treatment of psoriasis. OTELO observed 122 patients with plaque psoriasis for up to 18 months and assessed similar outcomes to APPRECIATE: the PBI (primary outcome), PGA, DLQI, PASI, and BSA (secondary outcomes). OTELO confirmed the fulfilment of dermatologist and patient treatment expectations. LAPIS PSO [38] and APPRAISAL [28] assessed improvement in DLQI score as their primary outcome measure with standard disease severity scores as secondary outcomes. Both studies demonstrated sustained improvement in patients’ HRQoL and improvements in disease severity. In a Finish registry analysis of clinical practice [39], patients with psoriasis or psoriatic arthritis tended to use apremilast mainly between conventional systemics and biologics. Apremilast persistence was high (14 months in patients with psoriasis; 11 months in patients with psoriatic arthritis) and increased with age [39]. In Russian studies reporting a pharmacogenomic model [40] and immunological predictors of response [41] in 34 patients with moderate-to-severe and severe plaque psoriasis receiving apremilast for 26 weeks, 14 patients (41%) achieved a PASI 75. The authors attribute this response rate to the severe psoriasis, long disease duration, and multiple prior therapies observed in more than half (53%) of the patients. In addition, this study was interventional and did not collect patient-reported outcomes, which are the focus of the current study.
The data from our CEE cohort have some limitations. The sample size was very small because of some unexpected occurrences during the conduct of the study: Due to the COVID-19 pandemic, patients were reluctant to and often advised not to visit the hospitals for the study visit. Additionally, there was a gap in Czech apremilast reimbursement from August 2020 till November 2021. The drug could not be prescribed in routine clinical practice during that time. The reimbursement for apremilast was granted again in December 2021. Only 17 patients (34%) of the cohort had psoriatic arthritis with nine patients continuing apremilast at 6 (± 1) months. Due to this small sample size, no meaningful conclusions could be drawn on the effectiveness of apremilast in psoriatic arthritis. The Belgian APOLO study [27] focused specifically on apremilast use and real-world effectiveness in this setting and reported improvement of signs and symptoms of psoriatic arthritis as well as a benefit in HRQoL. In the present study, the discontinuation rate was high (38% discontinued; 24% discontinued due to lack of efficacy) and the results for ongoing patients may be affected by a degree of selection bias. This high rate of discontinuation is attributed to structural mechanisms inherent to the local process followed for the prolongation of prescriptions. Specifically, in Croatia, every patient is reviewed on a 4-monthly basis by a hospital committee and prescriptions are extended (or not) based on benefit assessment using PASI (improvement of at least 50% in 4 months) and DLQI (achievement of DLQI value < 5). However, PASI 75 alone is not an optimal measure of treatment benefit; treatment satisfaction and clinical benefits from the patients’ perspective should also be considered [27]. Global satisfaction in the CEE cohort was 60% among all patients and 74% among those continuing apremilast at 6 (± 1) months, reaching similar proportions in the Northwestern European [23] and Spanish [25] cohorts. In patients continuing apremilast at 6 (± 1) months, the respective rates were 74%, 70%, and 71% [23, 25]. Generalizability of our data is therefore limited.
Conclusions
In our cohort of patients from CEE participating in the APPRECIATE study, dermatologists and patients were highly satisfied with apremilast's effectiveness, and patients reported improvements in their quality of life and across a range of symptoms. Compared to other regions participating in APPRECIATE, patients in the CEE cohort had a similar treatment benefit, including objective clinical measures and patient reported outcomes, despite their greater disease severity. Our data add to the growing body of evidence regarding the consistent effectiveness of apremilast across the spectrum of psoriasis patients with various degrees of disease severity and manifestations.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the participating patients, physicians, and study personnel. Kantar Health GmbH, Munich, Germany, acted as the clinical research organization, sponsored by Celgene.
Funding
This study was sponsored by Celgene; Amgen Inc. acquired the worldwide rights to Otezla® (apremilast) on November 21, 2019. Amgen GmbH, Vienna, Austria, funded the journal’s Rapid Service and Open Access Fees.
Medical Writing and Editorial Assistance
Margit Hemetsberger of Hemetsberger Medical Services, Vienna, Austria, received a fee from Amgen GmbH for medical writing and editing of this manuscript. Claire Desborough of Amgen, Uxbridge, UK, provided editorial assistance. Medical writing and editorial assistance were funded by Amgen.
Author Contributions
Petra Cetkovská, Iva Dediol, Marija Šola, Martina Kojanová, Katarina Trčko, Antoanela Čarija, Romana Čeović, Daniela Ledić-Drvar, and Marija Kaštelan were involved in patient enrollment and data acquisition for the CEE study cohort. Myriam Cordey Missoup was involved in the design of the study. All authors substantially contributed to the interpretation of the study results. All authors were involved in drafting of the manuscript, provided critical revisions for important intellectual content, approved the final version submitted for publication, and agreed to be accountable for all aspects of the work.
List of Investigators
Czech Republic: David Stuchlík (Pardubice), Marie Policarová (Jihlava), Martina Kojanová (Prague), Jorga Fialová (Prague), Petr Arenberger (Prague), Spyridon Gkalpakiotis (Prague), Petra Cetkovská (Pilsen), Adisa Duranović (Pilsen); Croatia: Marija Kaštelan (Rijeka), Antoanela Čarija (Split), Marija Šola (Osijek), Romana Čeović (Zagreb), Daniela Ledić-Dvar (Zagreb), Iva Dediol (Zagreb); Slovenia: Katarina Trčko (Maribor).
Disclosures
Petra Cetkovská served as consultant, speaker, or investigator for Abbvie, Almirall, Amgen, Eli-Lilly, Janssen, Leo Pharma, Novartis, Pfizer, Sanofi, and UCB. Iva Dediol has no conflict of interest to disclose. Marija Šola served as consultant, speaker, or investigator for Abbvie, Amgen, Eli-Lilly, Janssen, Novartis and Sandoz. Martina Kojanová served as consultant, speaker, or investigator for Abbvie, Amgen, Eli-Lilly, Janssen, Leo Pharma, Novartis, Pfizer, Sanofi, and UCB. Katarina Trčko served as consultant, speaker, or investigator for Amgen, Abbvie, Eli-Lilly, Janssen and Novartis. Antoanela Čarija has served as an investigator, speaker and/or advisor for Abbott, AbbVie, Amgen, Celgene, Eli Lilly, Fresnius Kabi, Janssen, LEO Pharma, Mylan, Novartis, Pfizer, Sandoz Biopharmaceuticals. Romana Čeović served as an advisor/received speaker’s honoraria for AbbVie, Amgen, Boehringer Ingelheim, Celgene, Eli Lilly, Jansen, LEO Pharma, Medis, Novartis, Sandoz and Takeda. Daniela Ledić-Drvar served as consultant, speaker, or investigator for Amgen, Abbvie, Eli-Lilly, Janssen and Novartis. Marija Kaštelan served as consultant, speaker, or investigator for Abbvie, Amgen, Eli-Lilly, Janssen, Leo Pharma, Novartis and Pfizer. Myriam Cordey Missoup, Khalid Mamun, and Andina Hrabar are employees of Amgen and own Amgen stock.
Compliance with Ethics Guidelines
This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. The study protocol was approved by the institutional review boards and/or national central ethics committees of each participating country, as per local requirements. In the Czech Republic, the State Institute of Drug Control (SUKL) approved APPRECIATE under the approval number 2002110009. In Croatia, the Central Ethic Committee approved the study under the class UP/1-530-07/20-08/06, number 381-13-02/340-20-03. In Slovenia, the Commission of the Republic of Slovenia for Medical Ethics approved the study under approval number 0120-221/2020/5. All patients provided informed consent prior to any data collection. APPRECIATE was registered in ClinicalTrials.gov (NCT02740218).
Data Availability
Qualified researchers may request data from Amgen clinical studies. Complete data are available at the following: https://wwwext.amgen.com/about/how-we-operate/policies-practices-and-disclosures/ethical-research/clinical-data-transparency-practices/clinical-trial-data-sharing-request.
References
- 1.Boehncke WH, Schön MP. Psoriasis. Lancet. 2015;386(9997):983–994. doi: 10.1016/s0140-6736(14)61909-7. [DOI] [PubMed] [Google Scholar]
- 2.International Federation of Psoriasis Associations (IFPA), International League of Dermatological Societies (ILDS), International Psoriasis Council (IPC). Global Psoriasis Atlas. 2021. https://www.globalpsoriasisatlas.org/en/statistics/prevalence-data.
- 3.Balp MM, Khalil S, Tian H, Gabriel S, Vietri J, Zuberbier T. Burden of chronic urticaria relative to psoriasis in five European countries. J Eur Acad Dermatol Venereol. 2018;32(2):282–290. doi: 10.1111/jdv.14584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amatya B, Wennersten G, Nordlind K. Patients' perspective of pruritus in chronic plaque psoriasis: a questionnaire-based study. J Eur Acad Dermatol Venereol. 2008;22(7):822–826. doi: 10.1111/j.1468-3083.2008.02591.x. [DOI] [PubMed] [Google Scholar]
- 5.Globe D, Bayliss MS, Harrison DJ. The impact of itch symptoms in psoriasis: results from physician interviews and patient focus groups. Health Qual Life Outcomes. 2009;7:62. doi: 10.1186/1477-7525-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rapp SR, Feldman SR, Exum ML, Fleischer AB, Jr, Reboussin DM. Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol. 1999;41(3 Pt 1):401–407. doi: 10.1016/s0190-9622(99)70112-x. [DOI] [PubMed] [Google Scholar]
- 7.Lebwohl MG, Bachelez H, Barker J, Girolomoni G, Kavanaugh A, Langley RG, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70(5):871–81.e1-30. doi: 10.1016/j.jaad.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 8.Feldman SR, Goffe B, Rice G, Mitchell M, Kaur M, Robertson D, et al. The challenge of managing psoriasis: unmet medical needs and stakeholder perspectives. Am Health Drug Benefits. 2016;9(9):504–513. [PMC free article] [PubMed] [Google Scholar]
- 9.Langley RG, Krueger GG, Griffiths CE. Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis. 2005;64(Suppl 2):ii18–23. doi: 10.1136/ard.2004.033217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Armstrong A, Jarvis S, Boehncke WH, Rajagopalan M, Fernandez-Penas P, Romiti R, et al. Patient perceptions of clear/almost clear skin in moderate-to-severe plaque psoriasis: results of the Clear About Psoriasis worldwide survey. J Eur Acad Dermatol Venereol. 2018;32(12):2200–2207. doi: 10.1111/jdv.15065. [DOI] [PubMed] [Google Scholar]
- 11.Reich K, Gisondi P, Stein Gold L, van de Kerkhof P, Langley RGP, Carle, Puig L, et al., editors. Differences in patient and dermatologist perspectives on psoriasis treatment: results from the UPLIFT survey. In: 30th European Academy of Dermatology and Venereology (EADV) congress; 2021 (virtual).
- 12.Kavanaugh A, Helliwell P, Ritchlin CT. Psoriatic arthritis and burden of disease: patient perspectives from the population-based multinational assessment of psoriasis and psoriatic arthritis (MAPP) survey. Rheumatol Ther. 2016;3(1):91–102. doi: 10.1007/s40744-016-0029-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zozaya N, Villoro R, Abdalla F, Alfonso Zamora S, BaleaFilgueiras J, Carrascosa Carrillo JM, et al. Unmet needs in the management of moderate-to-severe psoriasis in Spain: a multidimensional evaluation. Acta Dermato-Venereol. 2022;102:adv00678. doi: 10.2340/actadv.v102.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nast A, Smith C, Spuls PI, Avila Valle G, Bata-Csorgo Z, Boonen H, et al. EuroGuiDerm guideline on the systemic treatment of psoriasis vulgaris—Part 2: specific clinical and comorbid situations. J Eur Acad Dermatol Venereol. 2021;35(2):281–317. doi: 10.1111/jdv.16926. [DOI] [PubMed] [Google Scholar]
- 15.Nast A, Smith C, Spuls PI, Avila Valle G, Bata-Csörgö Z, Boonen H, et al. EuroGuiDerm guideline on the systemic treatment of psoriasis vulgaris—Part 1: treatment and monitoring recommendations. J Eur Acad Dermatol Venereol. 2020;34(11):2461–2498. doi: 10.1111/jdv.16915. [DOI] [PubMed] [Google Scholar]
- 16.Mrowietz U, Augustin M. Using the upgrade criteria of the European Psoriasis Consensus is best practice care according to the people-centred healthcare concept of the World Health Organization. Br J Dermatol. 2022;187:1007–8. 10.1111/bjd.21827. [DOI] [PubMed]
- 17.Strober B, Ryan C, van de Kerkhof P, van der Walt J, Kimball AB, Barker J, et al. Recategorization of psoriasis severity: Delphi consensus from the International Psoriasis Council. J Am Acad Dermatol. 2020;82(1):117–122. doi: 10.1016/j.jaad.2019.08.026. [DOI] [PubMed] [Google Scholar]
- 18.European Medicines Agency. Otezla summary of medicinal product characteristics London: European Medicines Agency; 2021. https://www.ema.europa.eu/en/documents/product-information/otezla-epar-product-information_en.pdf. Accessed 04 Oct 2021.
- 19.Papp K, Reich K, Leonardi CL, Kircik L, Chimenti S, Langley RG, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1) J Am Acad Dermatol. 2015;73(1):37–49. doi: 10.1016/j.jaad.2015.03.049. [DOI] [PubMed] [Google Scholar]
- 20.Paul C, Cather J, Gooderham M, Poulin Y, Mrowietz U, Ferrandiz C, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2) Br J Dermatol. 2015;173(6):1387–1399. doi: 10.1111/bjd.14164. [DOI] [PubMed] [Google Scholar]
- 21.Reich K, Gooderham M, Green L, Bewley A, Zhang Z, Khanskaya I, et al. The efficacy and safety of apremilast, etanercept and placebo in patients with moderate-to-severe plaque psoriasis: 52-week results from a phase IIIb, randomized, placebo-controlled trial (LIBERATE) J Eur Acad Dermatol Venereol. 2017;31(3):507–517. doi: 10.1111/jdv.14015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stein Gold L, Bagel J, Lebwohl M, Jackson JM, Chen R, Goncalves J, et al. Efficacy and safety of apremilast in systemic- and biologic-naive patients with moderate plaque psoriasis: 52-week results of UNVEIL. J Drugs Dermatol. 2018;17(2):221–228. [PubMed] [Google Scholar]
- 23.Augustin M, Kleyn CE, Conrad C, Sator PG, Stahle M, Eyerich K, et al. Characteristics and outcomes of patients treated with Apremilast in the real world: results from the APPRECIATE study. J Eur Acad Dermatol Venereol. 2021;35(1):123–134. doi: 10.1111/jdv.16431. [DOI] [PubMed] [Google Scholar]
- 24.Klein TM, Blome C, Kleyn CE, Conrad C, Sator PG, Stahle M, et al. Real-world experience of patient-relevant benefits and treatment satisfaction with Apremilast in patients with psoriasis: an analysis of the APPRECIATE study. Dermatol Ther (Heidelb) 2022;12(1):81–95. doi: 10.1007/s13555-021-00628-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herranz P, Trasobares L, Mateu A, Martínez E, Ruiz-Villaverde R, Baniandrés O, et al. Characterization and outcomes in patients treated with Apremilast in routine clinical practice in Spain: results from the APPRECIATE study. Actas Dermo-Sifiliografic. 2021 doi: 10.1016/j.ad.2021.05.010. [DOI] [PubMed] [Google Scholar]
- 26.Ghislain PD, Lambert J, Hoai XL, Hillary T, Roquet-Gravy PP, de la Brassinne M, et al. Real-life effectiveness of Apremilast for the treatment of psoriasis in Belgium: results from the observational OTELO study. Adv Ther. 2022;39(2):1068–1080. doi: 10.1007/s12325-021-01981-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Vlam K, Toukap AN, Kaiser MJ, Vanhoof J, Remans P, Van den Berghe M, et al. Real-world efficacy and safety of Apremilast in Belgian patients with psoriatic arthritis: results from the prospective observational APOLO study. Adv Ther. 2022;39(2):1055–1067. doi: 10.1007/s12325-021-02016-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ioannides D, Antonakopoulos N, Georgiou S, Chasapi V, Katsantonis I, Drosos A, et al. Effectiveness and safety of apremilast in biologic-naïve patients with moderate psoriasis treated in routine clinical practice in Greece: the APRAISAL study. J Eur Acad Dermatol Venereol. 2021;35(9):1838–1848. doi: 10.1111/jdv.17392. [DOI] [PubMed] [Google Scholar]
- 29.Jonak C, Göttfried I, Perl-Convalexius S, Gruber B, Schütz-Bergmayr M, Vujic I, et al. Characteristics and outcomes of patients with psoriasis treated with apremilast in the real-world in Austria—results the APPRECIATE study. Ther Adv Chronic Dis. 2023;14:20406223231152785. doi: 10.1177/20406223231152785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chalmers RJ. Assessing psoriasis severity and outcomes for clinical trials and routine clinical practice. Dermatol Clin. 2015;33(1):57–71. doi: 10.1016/j.det.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 31.Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210–216. doi: 10.1111/j.1365-2230.1994.tb01167.x. [DOI] [PubMed] [Google Scholar]
- 32.Feuerhahn J, Blome C, Radtke M, Augustin M. Validation of the patient benefit index for the assessment of patient-relevant benefit in the treatment of psoriasis. Arch Dermatol Res. 2012;304(6):433–441. doi: 10.1007/s00403-012-1256-y. [DOI] [PubMed] [Google Scholar]
- 33.Atkinson MJ, Kumar R, Cappelleri JC, Hass SL. Hierarchical construct validity of the treatment satisfaction questionnaire for medication (TSQM version II) among outpatient pharmacy consumers. Value Health. 2005;8(Suppl 1):S9–S24. doi: 10.1111/j.1524-4733.2005.00066.x. [DOI] [PubMed] [Google Scholar]
- 34.Amin M, Lee EB, Bhutani T, Wu JJ. Review of European registries for psoriasis. J Dermatol Treat. 2019;30(3):227–236. doi: 10.1080/09546634.2018.1506084. [DOI] [PubMed] [Google Scholar]
- 35.Merola JF, Qureshi A, Husni ME. Underdiagnosed and undertreated psoriasis: nuances of treating psoriasis affecting the scalp, face, intertriginous areas, genitals, hands, feet, and nails. Dermatol Ther. 2018;31(3):e12589. doi: 10.1111/dth.12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aldredge LM, Higham RC. Manifestations and management of difficult-to-treat psoriasis. J Dermatol Nurses' Assoc. 2018;10(4):189. doi: 10.1097/JDN.0000000000000418. [DOI] [Google Scholar]
- 37.Merola JF, Li T, Li WQ, Cho E, Qureshi AA. Prevalence of psoriasis phenotypes among men and women in the USA. Clin Exp Dermatol. 2016;41(5):486–489. doi: 10.1111/ced.12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reich K, Korge B, Magnolo N, Manasterski M, Schwichtenberg U, Staubach-Renz P, et al. Quality-of-life outcomes, effectiveness and tolerability of apremilast in patients with plaque psoriasis and routine German dermatology care: results from LAPIS-PSO. Dermatol Ther (Heidelb) 2022;12(1):203–221. doi: 10.1007/s13555-021-00658-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koskivirta I, Ruotsalainen J, Kurki S, Lakkakorpi P, Salminen-Mankonen H, Pirilä L, et al. Real-world registry-based study on apremilast use in psoriasis and psoriatic arthritis in Finland. Scand J Rheumatol. 2023 doi: 10.1080/03009742.2022.2151109. [DOI] [PubMed] [Google Scholar]
- 40.Verbenko DA, Karamova AE, Artamonova OG, Deryabin DG, Rakitko A, Chernitsov A, et al. Apremilast pharmacogenomics in Russian patients with moderate-to-severe and severe psoriasis. J Pers Med. 2021;11(1):20. doi: 10.3390/jpm11010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kubanov AA, Artamonova OG, Karamova AE, Vasileva EL, Deryabin DG. Cytokine levels of skin lesions in moderate and severe psoriasis as predictors for the type 4 fosphodiesterase inhibitor (apremilast) therapy effectivness. Ann RAMS. 2020;75(5):500–507. doi: 10.15690/vramn1361. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Qualified researchers may request data from Amgen clinical studies. Complete data are available at the following: https://wwwext.amgen.com/about/how-we-operate/policies-practices-and-disclosures/ethical-research/clinical-data-transparency-practices/clinical-trial-data-sharing-request.