Abstract
Background
The gastroesophageal reflux disease (GERD) worldwide prevalence is increasing maybe due to population aging and the obesity epidemic. Nissen fundoplication is the most common surgical procedure for GERD with a failure rate of approximately 20% which might require a redo surgery. The aim of this study was to evaluate the short- and long-term outcomes of robotic redo procedures after anti-reflux surgery failure including a narrative review.
Methods
We reviewed our 15-year experience from 2005 to 2020 including 317 procedures, 306 for primary, and 11 for revisional surgery.
Results
Patients included in the redo series underwent primary Nissen fundoplication with a mean age of 57.6 years (range, 43–71). All procedures were minimally invasive and no conversion to open surgery was registered. The meshes were used in five (45.45%) patients. The mean operative time was 147 min (range, 110–225) and the mean hospital stay was 3.2 days (range, 2–7). At a mean follow-up of 78 months (range, 18–192), one patient suffered for persistent dysphagia and one for delayed gastric emptying. We had two (18.19%) Clavien–Dindo grade IIIa complications, consisting of postoperative pneumothoraxes treated with chest drainage.
Conclusion
Redo anti-reflux surgery is indicated in selected patients and the robotic approach is safe when it is performed in specialized centers, considering its surgical technical difficulty.
Keywords: Gastro-esophageal reflux disease, Redo Nissen, Hiatal hernia, Robotic fundoplication, Anti-reflux surgery, Nissen fundoplication, Toupet fundoplication, Roux-en-Y gastric bypass
Introduction
Gastro-esophageal reflux disease (GERD) is a common disorder caused by the stomach contents reflux into the esophagus, linked to several symptoms and complications, and the worldwide prevalence (less 10% in 1990 versus 14% in 2017) is increasing maybe due to population aging and the obesity epidemic [1].
360° Nissen fundoplication is the most common surgical treatment of GERD, while 270° Toupet fundoplication represents a less performed alternative [2]. Nowadays, laparoscopy is considered the standard surgical approach [2].
Patient satisfaction rate after anti-reflux surgery reaches 85–90%, the failure rate is approximately 10–20% due to reflux symptoms persistence, recurrence or postoperative complications [3]. Persistent dysphagia lasting more than 3 months is the most common cause of re-intervention, fewer common indications are wrap disruption, slippage or telescoping, recurrent hiatal hernia, and gas bloat syndrome [4, 5]. Reoperation rate is about 5% after primary surgeries [6].
Concerning redo surgery, different surgical techniques have been reported including redo fundoplication (Nissen or more frequently partial 270° Toupet procedure), conversion to Roux-en-Y procedures, Collis gastroplasty with distal esophagectomy [7, 8]. Recently, Roux-en-Y conversion showed successful results especially in selected patients with high body mass index (BMI) [9].
Minimally invasive approach, which is the actual standard surgical approach for many complex surgical procedures [10–12], has also showed its benefit in the redo anti-reflux surgery. However, the conversion rate to open surgery is higher when compared to primary surgery, furthermore the robotic approach is described only in few cases [13].
The aim of our study is to evaluate the short- and long-term outcomes of robotic redo procedures after anti-reflux surgery failure also reviewing other literature experience.
Materials and methods
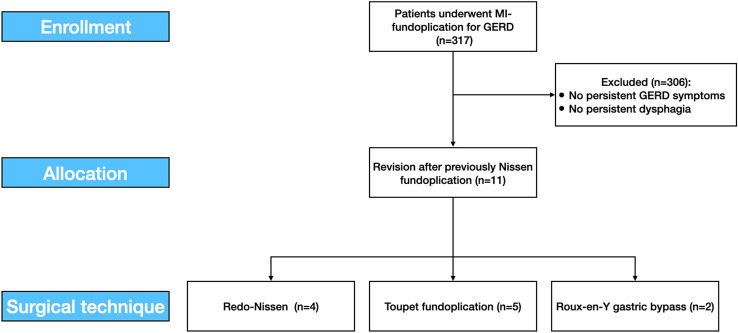
From September 2005 to September 2020, 11 redo procedures for primary surgery failures were performed out of 317 minimally invasive fundoplication for GERD associated or not with hiatal hernias were performed (Fig. 1). Indications for redo surgery and the most likely mechanisms of failure are listed in Table 1.
Fig. 1.
The study flowchart according to the STROBE statements. MI minimally invasive, GERD gastroesophageal reflux disease
Table 1.
Demographic data for patients undergoing revision anti-reflux surgery after failure of primary surgery between 2005 and 2020
| Primary surgery, n | 306 |
| Robotic-redo surgery, n (%) | 11 (3.6) |
| Sex, n (%) | |
| Female | 6 (54.55) |
| Male | 5 (45.45) |
| Age at reoperation, mean (range) | 57.6 (43–71) years |
| Body mass index > 30 kg/m2, (%) | 4 (36.4) |
| Previous surgery, n (%) | |
| Nissen fundoplication | 11 (100) |
| Time after primary surgery, mean (range) | 42 (7–108) months |
| Mechanism failurea, n | |
| Stomach herniation | 5 |
| Crural/Wrap too tight | 5 |
| Telescoping of valve | 2 |
| Wrap dehiscence | 1 |
| Upside down stomach/recurrent hiatal hernia | 1 |
| Causes leading to reoperation, n (%) | |
| Persistent dysphagia | 6 (54.55) |
| Persistent GERD symptoms | 5 (45.45) |
GERD gastroesophageal reflux disease
aEach patient has undergone more than one procedure during the same surgery
Patients with persistent GERD symptoms or persistent dysphagia lasting more than 3 months after primary surgery were evaluated through endoscopy with biopsy, barium swallow, esophageal manometry, and 24-h impedance-pH monitoring [14]. A chest-abdomen CT scan was performed in selected cases.
We reviewed the operative time, estimated blood loss, associate procedures, conversion to open surgery, intra-operative and post-operative complications according to Clavien–Dindo score [15], postoperative length of hospital stay.
Follow-up was planned at 30 and 90 days after surgery and once a year. During the COVID-19 pandemic, telemedicine has been used to perform a follow-up and prescribing therapies thanks to communication technologies.
This retrospective study was developed according to the Strengthening the Reporting of Observational Studies in Epidemiology [16] statement for cohort studies (Fig. 1).
An informed consent, for the scientific anonymous use of clinical data, was obtained from all patients. This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of the University of Molise (protocol number 10/21, approved date: 12 May 2021).
Redo fundoplication technique
A standard robotic 4–5 port technique is generally performed. The first steps were: adhesiolysis and wrap dissection using a blunt technique to identify the original anatomy may be challenging, giving its related risk of visceral perforation (stomach or esophagus).
When associated to hiatal hernia, the hernial sac was removed.
Surgical technique chosen for redo surgery depends on the cause of failure: sometimes a new floppy Nissen may be required, while most frequently a partial 270° posterior Toupet fundoplication represents the best choice, especially in case of postoperative dysphagia. Given the risk of recurrent hernias, the diaphragmatic crus usually need to be treated with a single non-absorbable suture, leaving enough free space around the esophagus.
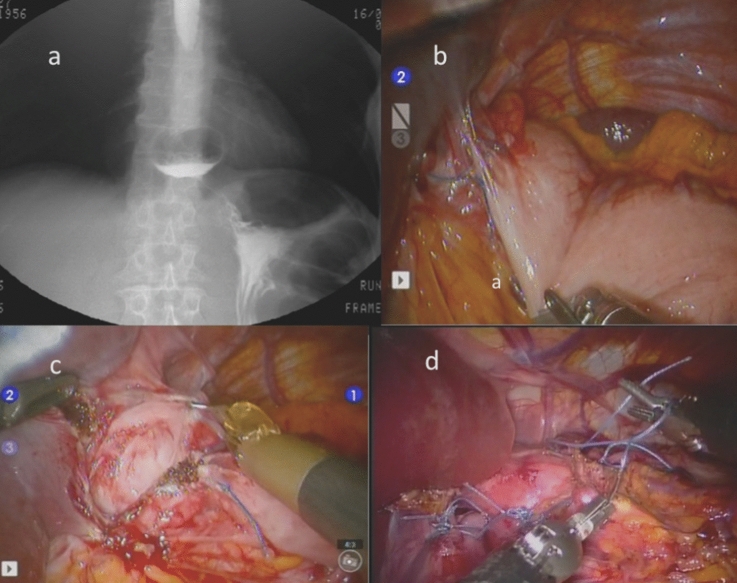
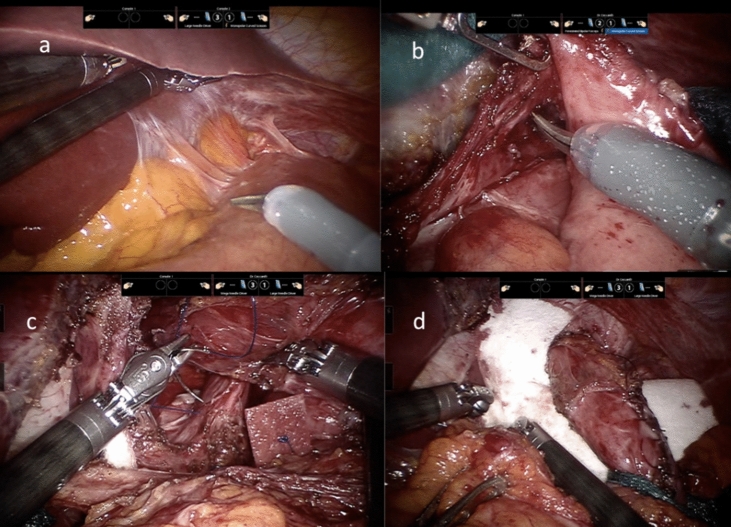
Stitches may be reinforced with pledgets and sometimes an absorbable tailored mesh is used as crural reinforcement and fixed to prevent early migration or displacement (Figs. 2 and 3).
Fig. 2.
a Case of slipped Nissen fundoplication (X ray swallow); b periesophageal adhesions; c dissection and anatomy restoration; d robotic partial Toupet 270° re-fundoplication
Fig. 3.
a Case of post-Nissen stomach herniation, periesophageal adhesions; b robotic gastric valve dissection; c hiatoplasty using pledgets; d absorbable tailored mash positioning
All procedures were performed by two surgeons experienced in laparoscopic, robotic, and upper GI surgery.
Results
The mean age of the patients (five males and six females) was 57.6 years (range, 43–71 years), and in four cases, the body mass index (BMI) was greater than 30 kg/m2. The mean time from the first operation was 42 months (range, 7–108 months). All patients had previously undergone Nissen fundoplication.
Indication to revision surgery was dysphagia and persistent GERD symptoms. The pathophysiological mechanisms of failure is summarized in Table 1. More than one mechanism was found in each patient.
All cases were treated with a robotic approach, in no case it was necessary to convert to open.
We performed four (36.36%) redo Nissen, five (45.45%) conversions to Toupet fundoplication, two (18.19%) Roux-en-Y gastric bypass. Additional procedures are listed in Table 2. The meshes were used in five (45.54%) cases.
Table 2.
Perioperative and postoperative data of patients who underwent revision surgery
| Type of approach, n (%) | |
| Robot | 11 (100) |
| Type of redo surgery, n (%) | |
| Redo Toupet fundoplication | 5 (45.45) |
| Redo Nissen fundoplication | 4 (36.36) |
| Roux-en-Y “short limb” | 2 (18.19) |
| Additional procedurea, n | |
| Hiatal correction/hiatoplasty | 8 |
| Mesh hiatoplasty (Bio-A) | 5 |
| Gastropexy | 3 |
| Gastrostomy | 2 |
| Conversion to open, n (%) | 0 (0) |
| Emergency, n (%) | 1 (9) |
| Operative time, mean (range) | 147 (110–225) min |
| Estimation of blood loss, mean (range) | 45 (30–180) ml |
| Hospital stay, mean (range) | 3.2 (2–7) days |
| Clavien–Dindo score ≥ III, n (%) | 2 cases of IIIab (18.19) |
| Persistent symptoms, n (%) | |
| Dysphagia | 1 (9) |
| Gastroplegia/delayed gastric emptying | 1 (9) |
| GERD | 0 () |
| Second or more reoperation, n (%) | 0 (0) |
GERD gastroesophageal reflux disease
aEach patient has undergone more than one procedure during the same surgery
bChest drainage placement
Mean operative time was 147 min (range, 110–225 min), mean estimated blood loss was 45 ml (range, 30–180 ml), mean hospital stay was 3.2 days (range, 2–7 days).
We had two (18.19%) Clavien–Dindo grade IIIa complications, consisting of postoperative pneumothoraxes treated with chest drainage.
At a mean follow-up of 78 months (range, 18–192 months), one patient suffered for persistent dysphagia and one for delayed gastric emptying.
Discussion
The results of this study show that the robotic redo fundoplication is effective alternative to treat patients affected by persistent GERD after previous Nissen fundoplication. Additionally, the redo fundoplication significantly improve the quality of life in these patients.
In our case series, we found that 9 out of 11 (81.82%) patients solved symptoms after surgery. We reported just one (9%) case of dysphagia and one (9%) case of gastroplegia. Patient selection, technique of repair, and meshes use may play an important role for a more effective treatment. In particular, we would underline patients presenting laparoscopic Nissen fundoplication failure should undergo to a careful clinical and instrumental evaluation based on esophageal manometry, 24-h pH study and upper gastrointestinal endoscopy before redo surgery indication.
Minimally invasive approaches
As describe by Granderath et al. [17], laparoscopic re-fundoplication performed by experienced surgeons is a safe procedure that leads to excellent functional results with a significant improvement in patients’ quality of life. Nevertheless, it is considered more cost-effective than open approach to perform reoperation in anti-reflux surgery [18] and linked with significantly higher morbidity rate when it was compared to primary interventions, considering the technical challenge.
Nowadays, robotic surgery for primary and redo anti-reflux procedures is widely used in many centers [19, 20]. Therefore, it is not clearly analyzed the benefit obtained though the robotic approach compared to other abdominal surgery techniques due to limited literature experiences.
Notably, Tolboom et al. compared conventional laparoscopic versus robot-assisted redo anti-reflux surgery, in a single institute series of 75 patients between 2008 and 2013 and observed that robotic technology when available can offer benefits in redo surgery both for gastroesophageal reflux disease and hiatal hernia [20]. However, Tolboom et al. pointed out the challenging scenario during redo surgery due to adhesions, altered anatomy, and trouble finding dissection planes [20]. These findings have led to increased conversion and complication rates representing by stomach or esophagus perforation of the stomach or esophagus. Therefore, robotic redo surgery could be an effective option thanks to better operative field visualization and adhesiolysis.
According to our findings, Elmously et al. in 2018 reported a retrospective review of 200 patients (162 primary anti-reflux surgery and 38 reoperative surgery), concluding that the robotic approach leads to very low morbidity, short length of stay, and excellent functional outcomes in patients undergoing reoperative surgery when compared to patients undergoing primary anti-reflux surgery [13]. Furthermore, Elmously et al. demonstrated that patients who underwent robotic redo surgery experienced complete or partial benefits from preoperative symptoms and more than 80% not require a daily PPI use [13].
New interventions for treatment GERD are the transoral approaches: their feasibility have also been explored. In 2013, Bell et al. reported a series of 11 cases, demonstrating that transoral fundoplication as a revision of failed tradition fundoplication is feasible and leads to an improvement in GERD symptoms avoiding the risks of the laparoscopic re-intervention [21].
Moreover, Testoni et al. performed transoral incisionless fundoplication reporting a significant improvement of symptoms and PPI consumption after 3 years of follow-up, but more than 30% of patients presented persistent esophagitis at 1 year [22].
Choice of re-intervention type
The identification of the type of re-intervention is a challenge and depends on many factors.
Notably, the main cause of re-intervention has been reported to be a persistent postoperative dysphagia related to the closed fundic wrap of an inadequate calibrated Nissen fundoplication or to a narrow hiatal crural repair [23].
In cases characterized by severe dysphagia, it is often preferred to perform a new fundoplication according to the Toupet technique (270°) [23].
In the case of a short esophagus, a new fundoplication with Collis gastroplasty is more indicated [24].
Partial fundoplication, especially if posterior, would seem to reduce some postoperative complications such as dysphagia and gas bloat [25]. Nevertheless, the partial fundoplication would be burdened by a higher rate of reflux recurrence, mainly when it was performed an anterior partial fundoplication [26].
Roux-en-Y gastric bypass (RYGB) should be considered in obese patients [9]. Other options are fundoplication takedown or hiatal closure only.
Many authors suggest using preoperative esophageal motility studies to choose the most appropriate fundoplication, reserving partial fundoplication for patients with ineffective esophageal motility [27].
Redo fundoplication versus Roux-en-Y gastric bypass
While dysphagia and gas bloat syndrome are considered the most frequent causes of redo surgery in patient affected by GERD symptoms recurrence, in literature are less reported the intrathoracic fundic wrap migration and the large paraesophageal type III hiatal hernias with one third of the stomach migrated into the chest cavity.
Moreover, young age, female gender, and chronic lung diseases are associated with a higher rate of reoperation after fundoplication [28].
After surgical failure, that can occur up to 30% of patients underwent fundoplication, all redo fundoplication techniques showed good long-term outcomes [29]. Nevertheless, it appears less suitable than primary anti-reflux surgery, while RYGB demonstrated to be a safety alternative to redo fundoplication [30].
As reported by Stefanidis et al. in a series of 25 patients, laparoscopic RYGB guarantee an excellent reflux control and quality of life but, considering high morbidity rate, it should be reserved for centers with bariatric centers [31].
Similar findings are described by Kim et al. [32] that analyzed 45 patients with a mean BMI of 33 kg/m2.
Despite that RYGB needs a longer operative time and duration of hospitalizations, a higher complications rate and rehospitalization within 30 days than redo fundoplication, minimally invasive RYGB leads to long-term resolution of symptoms in most cases, especially in patients affected by obesity, gastroparesis, and esophagitis [33].
In addition, after multiple fundoplications, it might be necessary to perform an esophagojejunostomy [34].
Sato et al.[35], in a consecutive series of 139 patients after laparoscopic short Nissen fundoplication with or without fundic mobilization and a mean follow-up period of 27 ± 21 months, analyzed the impact of short gastric vessel division on postoperative dysphagia and reported an acceptable long-term dysphagia, underlining the importance of an appropriate patient selection, of a previous identification of short esophagus, and of an adequate surgical technique.
Mesh
We performed in five (45.45%) cases redo fundoplication using Bio-A® meshes.
The use of biosynthetic prosthetic meshes is related to a very low infection and erosion rates, a good short-term and medium-term outcome as well as to an improved quality of life. On the other hand, biologic meshes have not showed to reduce hernia recurrence rates and the use of synthetic non-absorbable meshes at the hiatus still remains a controversial topic as mesh erosion into the esophageal lumen has been associated with catastrophic consequences [36].
A single-institution retrospective review published in 2021 by PR Armijo et al., involving 292 patients undergoing anti-reflux surgery with hiatal hernia repair between 2004 and 2016, demonstrated a recurrence rate of 39%; three different types of absorbable meshes were used (human tissue matrix, biosynthetic mesh, and porcine tissue matrix) and the authors concluded that the outcomes of the three mesh groups were similar, but there was a significant difference in mesh cost (about $1100, $550, and $1300, respectively) [37].
In 2018, MT Olson et al. analyzed long-term outcomes and surgical re-intervention rates of 399 patients after paraesophageal hernias repair [38] using a synthetic bio-absorbable mesh (Bio-A® mesh W. L. Gore & Associates) and examined BMI as a possible risk factor for recurrence[38]. After a mean follow-up of 44.7 ± 22.8 months, it was concluded that laparoscopic primary paraesophageal hernia repair with only Bio-A® mesh gave excellent long-term patient outcomes and acceptable symptomatic recurrence rate and BMI seemed to be not related to a higher recurrence rate [38].
In 2019, A Iossa et al. published a retrospective evaluation of 120 consecutive patients submitted to hiatal hernia repair with bio-absorbable synthetic mesh, divided into two groups according to BMI: 92 obese patients treated with reinforced hiatoplasty during bariatric surgery and 28 non-obese patients treated with reinforced hiatoplasty during anti-reflux surgery [39]. Following a mean follow-up of 41 months, the bio-absorbable mesh on the hiatus, in obese and non-obese patients, demonstrated a very low recurrence rate and negligible gastroesophageal disease [39].
Complications
Our complication rates are superimposable to other experiences: no complication during surgery and two (18.19%) patients’ complications were classified as Clavien–Dindo III [40, 41]. No mortality was reported, according to mortality rate described in other literature experiences [40, 42].
Neuhauser et al. reported a 30% of perioperative and postoperative complications following redo surgery which included stomach perforation, significant bleeding, esophageal mucosal perforation, gastrocutaneous fistula, small bowel enterotomy with consequent fistula, and tension pneumothorax [43].
Nevertheless, Mertens et al. reported an early postoperative complication rate of 10.6%. Four (2.6%) patients were affected by major complications [42].
With regard to the incidence of postoperative delayed gastric emptying after primary surgery, it appears to be low, but it increases after recurrent paraesophageal hernia repair, especially following the repair of each subsequent recurrence [44].
A single-institution retrospective review by MC Hamrick et al. analyzed the incidence of delayed gastric emptying associated with revisional laparoscopic paraesophageal hernia repair which suggested that delayed gastric emptying should be anticipated and patients were informed of the ramifications of this problem preoperatively [45].
As for the risk of postoperative gastroparesis, in 2019, Lu et al. published a multicenter study of revisional surgeries following fundoplication procedures, regarding a series of 5,656 patients who underwent primary fundoplication (62.1%) or primary paraesophageal hernia repair (37.9%); 3.8% patients among the first group and 4.4% among the second one, especially the ones with associated comorbidities (i.e., hypertension, obesity, and fluid and electrolyte disorders), were diagnosed with gastroparesis or were treated with specific pyloroplasty/pyloromyotomy [46].
Limitations
This study has several limitations.
The retrospective data collection and the poor number of patients are the major limitations. In addition, a longer follow-up is essential to evaluate the long success of the redo anti-reflux surgery in patients affected by multiple comorbidities and further prospective randomized studies are required to define the risk factors for failure of the redo operation after robotic approach.
Conclusion
Revision anti-reflux surgery may be indicated in selected cases after failure of previous anti-reflux surgery. Different procedures have been proposed based on the cause of the failure.
However, it is a challenging surgery with a higher complication rate than primary surgery. Preoperative clinical instrumental evaluation and patient selection are essential for the choice of surgical technique, as are the experience of the surgical team in this area.
Eligible techniques include redo fundoplication, gastric resections, the Collis–Nissen procedure, Roux-en-Y bypass, and others. In most cases, the minimally invasive approach can be used, considering that robotic surgery represents a safety and promising alternative to laparoscopy.
Author contributions
Conceptualization: GC, AR; methodology; GC, PA, AR; writing: GC, MV, MdR, MC; writing—review and editing: GC, MF, GS, PS, AR; supervision: GC, AR; formal analysis and investigation: all authors. The authors read and approved the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors have no conflict of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Graziano Ceccarelli, Email: g.cecca2003@libero.it.
Manuel Valeri, Email: valeri.manuel02@gmail.com.
Lavinia Amato, Email: lavinia.amato18@gmail.com.
Michele De Rosa, Email: morrisey1759@hotmail.it.
Fabio Rondelli, Email: rondellif@hotmail.com.
Micaela Cappuccio, Email: micaelacappuccio24@gmail.com.
Francesca Elvira Gambale, Email: elvira.gambale@gmail.com.
Mariarita Fantozzi, Email: mariaritafan@gmail.com.
Guido Sciaudone, Email: guido.sciaudone@unimol.it.
Pasquale Avella, Email: avella.p@libero.it.
Aldo Rocca, Email: aldo.rocca@unimol.it.
References
- 1.Sweis R, Fox M. The global burden of gastro-oesophageal reflux disease: more than just heartburn and regurgitation. Lancet Gastroenterol Hepatol. 2020;5(6):519–521. doi: 10.1016/S2468-1253(20)30002-9. [DOI] [PubMed] [Google Scholar]
- 2.Dallemagne B, et al. Laparoscopic Nissen fundoplication: preliminary report. Surg Laparosc Endosc. 1991;1(3):138–143. [PubMed] [Google Scholar]
- 3.Bais JE, et al. Surgical treatment for recurrent gastro-oesophageal reflux disease after failed antireflux surgery. Br J Surg. 2000;87(2):243–249. doi: 10.1046/j.1365-2168.2000.01299.x. [DOI] [PubMed] [Google Scholar]
- 4.Humphries LA, et al. Causes of dissatisfaction after laparoscopic fundoplication: the impact of new symptoms, recurrent symptoms, and the patient experience. Surg Endosc. 2013;27(5):1537–1545. doi: 10.1007/s00464-012-2611-y. [DOI] [PubMed] [Google Scholar]
- 5.Schlottmann F, et al. Outcomes of laparoscopic redo fundoplication in patients with failed antireflux surgery: a systematic review and meta-analysis. Ann Surg. 2021;274(1):78–85. doi: 10.1097/SLA.0000000000004639. [DOI] [PubMed] [Google Scholar]
- 6.Zhou T, et al. Reoperation rates after laparoscopic fundoplication. Surg Endosc. 2015;29(3):510–514. doi: 10.1007/s00464-014-3660-1. [DOI] [PubMed] [Google Scholar]
- 7.Watson MD, et al. Roux-en-Y gastric bypass following Nissen fundoplication: higher risk same reward. Obes Surg. 2017;27(9):2398–2403. doi: 10.1007/s11695-017-2643-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Celasin H, et al. Laparoscopic revision surgery for gastroesophageal reflux disease. Medicine (Baltimore) 2017;96(1):e5779. doi: 10.1097/MD.0000000000005779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grover BT, Kothari SN. Reoperative antireflux surgery. Surg Clin North Am. 2015;95(3):629–640. doi: 10.1016/j.suc.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 10.Ceccarelli G, et al. Minimally invasive approach to gastric GISTs: analysis of a multicenter robotic and laparoscopic experience with literature review. Cancers (Basel) 2021;13(17):4351. doi: 10.3390/cancers13174351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rocca A, et al. Robotic surgery for colorectal liver metastases resection: a systematic review. Int J Med Robot. 2021;17(6):e2330. doi: 10.1002/rcs.2330. [DOI] [PubMed] [Google Scholar]
- 12.Miskovic D, et al. Standardization of laparoscopic total mesorectal excision for rectal cancer: a structured international expert consensus. Ann Surg. 2015;261(4):716–722. doi: 10.1097/SLA.0000000000000823. [DOI] [PubMed] [Google Scholar]
- 13.Elmously A, et al. Robotic reoperative anti-reflux surgery: low perioperative morbidity and high symptom resolution. World J Surg. 2018;42(12):4014–4021. doi: 10.1007/s00268-018-4708-5. [DOI] [PubMed] [Google Scholar]
- 14.Yadlapati R, Gyawali CP, Pandolfino JE. Personalized approach to the evaluation and management of gastroesophageal reflux disease. Clin Gastroenterol Hepatol. 2022 doi: 10.1016/j.cgh.2022.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vandenbroucke JP, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Int J Surg. 2014;12(12):1500–1524. doi: 10.1016/j.ijsu.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 17.Granderath FA, et al. Is laparoscopic refundoplication feasible in patients with failed primary open antireflux surgery? Surg Endosc. 2002;16(3):381–385. doi: 10.1007/s00464-001-9102-x. [DOI] [PubMed] [Google Scholar]
- 18.Banki F, et al. Laparoscopic reoperative antireflux surgery is more cost-effective than open approach. J Am Coll Surg. 2017;225(2):235–242. doi: 10.1016/j.jamcollsurg.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 19.Luberice K, et al. Robotic Complex Fundoplication in Patients at High-Risk to Fail. Jsls. 2021;25(2):e2020.00111. doi: 10.4293/JSLS.2020.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tolboom RC, Draaisma WA, Broeders IA. Evaluation of conventional laparoscopic versus robot-assisted laparoscopic redo hiatal hernia and antireflux surgery: a cohort study. J Robot Surg. 2016;10(1):33–39. doi: 10.1007/s11701-016-0558-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bell RC, et al. Revision of failed traditional fundoplication using EsophyX transoral fundoplication. Surg Endosc. 2013;27(3):761–767. doi: 10.1007/s00464-012-2542-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Testoni SGG, et al. Transoral incisionless fundoplication with Medigus ultrasonic surgical endostapler (MUSE) for the treatment of gastro-esophageal reflux disease: outcomes up to 3 years. Surg Endosc. 2021;36(7):5023–5031. doi: 10.1007/s00464-021-08860-w. [DOI] [PubMed] [Google Scholar]
- 23.Al Hashmi AW, et al. A retrospective multicenter analysis on redo-laparoscopic anti-reflux surgery: conservative or conversion fundoplication? Surg Endosc. 2019;33(1):243–251. doi: 10.1007/s00464-018-6304-z. [DOI] [PubMed] [Google Scholar]
- 24.Swanstrom LL, Marcus DR, Galloway GQ. Laparoscopic Collis gastroplasty is the treatment of choice for the shortened esophagus. Am J Surg. 1996;171(5):477–481. doi: 10.1016/S0002-9610(96)00008-6. [DOI] [PubMed] [Google Scholar]
- 25.Kornmo TS, Ruud TE. Long-term results of laparoscopic Nissen fundoplication due to gastroesophageal reflux disease. A ten year follow-up in a low volume center. Scand J Surg. 2008;97(3):227–230. doi: 10.1177/145749690809700304. [DOI] [PubMed] [Google Scholar]
- 26.Baigrie RJ, et al. Randomized double-blind trial of laparoscopic Nissen fundoplication versus anterior partial fundoplication. Br J Surg. 2005;92(7):819–823. doi: 10.1002/bjs.4803. [DOI] [PubMed] [Google Scholar]
- 27.Booth MI, et al. Randomized clinical trial of laparoscopic total (Nissen) versus posterior partial (Toupet) fundoplication for gastro-oesophageal reflux disease based on preoperative oesophageal manometry. Br J Surg. 2008;95(1):57–63. doi: 10.1002/bjs.6047. [DOI] [PubMed] [Google Scholar]
- 28.Obeid NR, et al. Patterns of reoperation after failed fundoplication: an analysis of 9462 patients. Surg Endosc. 2018;32(1):345–350. doi: 10.1007/s00464-017-5682-y. [DOI] [PubMed] [Google Scholar]
- 29.Rudolph-Stringer V, et al. Randomized trial of laparoscopic Nissen versus anterior 180 degree partial fundoplication - late clinical outcomes at 15 to 20 years. Ann Surg. 2022;275(1):39–44. doi: 10.1097/SLA.0000000000004643. [DOI] [PubMed] [Google Scholar]
- 30.Giulini L, Razia D, Mittal SK. Redo fundoplication and early Roux-en-Y diversion for failed fundoplication: a 3-year single-center experience. Surg Endosc. 2021;36(5):3094–3099. doi: 10.1007/s00464-021-08610-y. [DOI] [PubMed] [Google Scholar]
- 31.Stefanidis D, et al. Laparoscopic fundoplication takedown with conversion to Roux-en-Y gastric bypass leads to excellent reflux control and quality of life after fundoplication failure. Surg Endosc. 2012;26(12):3521–3527. doi: 10.1007/s00464-012-2380-7. [DOI] [PubMed] [Google Scholar]
- 32.Kim M, et al. Minimally invasive Roux-en-Y gastric bypass for fundoplication failure offers excellent gastroesophageal reflux control. Am Surg. 2014;80(7):696–703. doi: 10.1177/000313481408000726. [DOI] [PubMed] [Google Scholar]
- 33.Coakley KM, et al. Roux-En-Y gastric bypass following failed fundoplication. Surg Endosc. 2018;32(8):3517–3524. doi: 10.1007/s00464-018-6072-9. [DOI] [PubMed] [Google Scholar]
- 34.Weber CE, et al. Roux-en-Y gastric bypass as a salvage procedure in complicated patients with failed fundoplication(s) Surg Endosc. 2019;33(3):738–744. doi: 10.1007/s00464-018-6337-3. [DOI] [PubMed] [Google Scholar]
- 35.Sato K, et al. Causes of long-term dysphagia after laparoscopic Nissen fundoplication. JSLS. 2002;6(1):35–40. [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriguez HA, Oelschlager BK. Secrets for successful laparoscopic antireflux surgery: mesh hiatoplasty. Ann Laparosc Endosc Surg. 2017;2:50. doi: 10.21037/ales.2017.02.16. [DOI] [Google Scholar]
- 37.Armijo PR, et al. Surgical and clinical outcomes comparison of mesh usage in laparoscopic hiatal hernia repair. Surg Endosc. 2021;35(6):2724–2730. doi: 10.1007/s00464-020-07703-4. [DOI] [PubMed] [Google Scholar]
- 38.Olson MT, et al. Primary paraesophageal hernia repair with Gore(R) Bio-A(R) tissue reinforcement: long-term outcomes and association of BMI and recurrence. Surg Endosc. 2018;32(11):4506–4516. doi: 10.1007/s00464-018-6200-6. [DOI] [PubMed] [Google Scholar]
- 39.Iossa A, Silecchia G. Mid-term safety profile evaluation of Bio-A absorbable synthetic mesh as cruroplasty reinforcement. Surg Endosc. 2019;33(11):3783–3789. doi: 10.1007/s00464-019-06676-3. [DOI] [PubMed] [Google Scholar]
- 40.Awais O, et al. Reoperative antireflux surgery for failed fundoplication: an analysis of outcomes in 275 patients. Ann Thorac Surg. 2011;92(3):1083–1089. doi: 10.1016/j.athoracsur.2011.02.088. [DOI] [PubMed] [Google Scholar]
- 41.Furnée EJ, et al. Surgical reintervention after failed antireflux surgery: a systematic review of the literature. J Gastrointest Surg. 2009;13(8):1539–1549. doi: 10.1007/s11605-009-0873-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mertens AC, et al. Morbidity and mortality in complex robot-assisted hiatal hernia surgery: 7-year experience in a high-volume center. Surg Endosc. 2019;33:2152–2161. doi: 10.1007/s00464-018-6494-4. [DOI] [PubMed] [Google Scholar]
- 43.Neuhauser B, Hinder RA. Laparoscopic reoperation after failed antireflux surgery. Semin Laparosc Surg. 2001;8(4):281–286. doi: 10.1053/slas.2001.30172. [DOI] [PubMed] [Google Scholar]
- 44.Khajanchee YS, Dunst CM, Swanstrom LL. Outcomes of Nissen fundoplication in patients with gastroesophageal reflux disease and delayed gastric emptying. Arch Surg. 2009;144(9):823–828. doi: 10.1001/archsurg.2009.160. [DOI] [PubMed] [Google Scholar]
- 45.Hamrick MC, et al. Incidence of delayed gastric emptying associated with revisional laparoscopic paraesophageal hernia repair. J Gastrointest Surg. 2013;17(2):213–217. doi: 10.1007/s11605-012-1989-0. [DOI] [PubMed] [Google Scholar]
- 46.Lu D, et al. Investigating rates of reoperation or postsurgical gastroparesis following fundoplication or paraesophageal hernia repair in New York State. Surg Endosc. 2019;33(9):2886–2894. doi: 10.1007/s00464-018-6588-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.