Abstract
Aim
Emerging research indicates that gene mutations within the Ras-mitogen activated protein kinase (RAS-MAPK) signaling cascade, which cause Noonan syndrome and related disorders, affect neurophysiologic activity in brain regions underlying attention and executive functions. The present study examined whether children with Noonan syndrome are at heightened risk for symptoms of attention-deficit–hyperactivity disorder (ADHD) and executive dysfunction relative to an unaffected sibling comparison group, and investigated three key aspects of behavioral attention: auditory attention, sustained attention, and response inhibition.
Method
Children and adolescents with Noonan syndrome (n=32, 17 males, 15 females, mean age 11y 3mo, SD 3y) and their unaffected siblings (n=16, eight males, eight females, mean age 11y, SD 3y 6mo) were administered standardized tests of intellectual functioning and clinic-based measures of behavioral attention. Parent ratings of ADHD symptoms, executive functioning, and behavior were also obtained.
Results
Children with Noonan syndrome demonstrated higher rates of past ADHD diagnosis, as well as reduced performance, compared with unaffected siblings on behavioral attention measures. Parent-rated functional impairments in attention, social skills, working memory, and self-monitoring were more prevalent in the Noonan syndrome group. The relationship between attention regulation skills (sustained attention and inhibitory control) and intellectual test performance was significantly stronger in the Noonan syndrome group than the comparison group.
Interpretation
Clinical screening/evaluation for ADHD and executive dysfunction in Noonan syndrome is recommended to facilitate appropriate intervention and to address functional impact on daily life activities.
Gene mutations in the Ras-mitogen activated protein kinase (RAS-MAPK) signaling pathway cause Noonan syndrome and related disorders including neurofibromatosis type 1 (NF1), cardiofaciocutaneous syndrome, and Costello syndrome. These syndromes (‘RASopathies’) are associated with varying degrees of cognitive impairment, ranging from severe intellectual disability to normal or above average cognitive functioning.1,2 In recent years, the RAS-MAPK signaling pathway has emerged as a fascinating model system for understanding how particular molecular alterations can give rise to cognitive and behavioral deficits. It has become increasingly clear that the RAS-MAPK pathway is essential for normal neurophysiological functioning within multiple brain circuits,3 and that proteins in this pathway are involved in modulating release of inhibitory neurotransmitters in regions of the brain involved in attention regulation and executive functions, including the prefrontal cortex and striatum.4
Although deficits in attention skills are a well-documented phenomenon among individuals with NF1,2 no studies have systematically examined aspects of behavioral attention in other disorders affecting the RAS-MAPK pathway. Noonan syndrome, one of the most common RASopathies, is estimated to occur in 1/1000 to 1/2500 live births.5 Noonan syndrome is characterized by distinctive facial features, short stature, congenital heart disease, skeletal anomalies, and other comorbidities. Mutations in one of several genes expressed within the RAS-MAPK pathway (including PTPN11, SOS1, RAF1, KRAS, NRAS, SHOC2, RIT1, and CBL) cause about 75% of cases of Noonan syndrome.6 The underlying genetic etiology of the remaining cases has not yet been identified. The behavioral phenotype of Noonan syndrome is characterized by wide variability in most domains, with increased risk for motor delay, cognitive and language impairments, and learning difficulties.7,8
Case studies and parent surveys have documented attention challenges among some individuals with Noonan syndrome.9,10 However, it is unknown how prevalent these difficulties are in Noonan syndrome, and whether attention problems are similar to those of children with idiopathic ADHD. A first goal of the present study was to determine whether children with Noonan syndrome are at greater risk than their unaffected siblings for ADHD symptoms and associated deficits in executive functions (e.g. working memory, planning, organization, emotional control). Second, the study investigated performance of individuals with Noonan syndrome on clinic-based behavioral measures known to be challenging for children with ADHD. These included tests of auditory attention, sustained attention, and inhibitory control. Finally, the study examined whether attention skills were predictive of performance on intellectual testing, and whether the relationship between attention regulation and IQ differed between the Noonan syndrome and sibling comparison groups.
METHOD
Participants
Study participants were recruited from a cohort of families who were previously enrolled in a genotype–phenotype study directed by the third author (AER) and had provided permission to be contacted for further research, as well as at national meetings of Noonan syndrome family advocacy groups. Probands with Noonan syndrome and their unaffected siblings between the ages of 6 years and 16 years were invited to participate. A total of 50 children and adolescents enrolled at Boston Children’s Hospital (n=29), the 2011 meeting of The Noonan Syndrome Support Group (n=6), the 2013 meeting of the Noonan Syndrome Foundation (n=5), or by arrangement to visit testing sites in Wisconsin or Minnesota (n=10). All families provided written informed consent on enrollment. The study was approved by the Boston Children’s Hospital institutional review board.
The proband group included 32 children and adolescents with Noonan syndrome (17 males, 15 females). Clinical diagnostic criteria for Noonan syndrome were confirmed by a medical examination and/or review of medical records by a clinical geneticist (AER). The sample contained 22 individuals with confirmed gene mutations, including 16 individuals with PTPN11 mutations and six individuals with SOS1 mutations. The remaining 10 individuals (31%) had unknown mutations, a rate consistent with the percentage of Noonan syndrome cases with unknown mutations in recent genetics studies using clinically referred cohorts.6 One additional patient with a clinical diagnosis of Noonan syndrome received testing, but was excluded from the group analyses because of an identified mutation in the BRAF gene. There is some debate among RASopathy experts regarding whether individuals with BRAF mutations have Noonan syndrome, or should instead be diagnosed with mild cardiofaciocutaneous syndrome.11
The sibling comparison group consisted of 16 unaffected, typically developing individuals (eight males, eight females). To enable optimal group matching,12 one participant (the youngest female) who received testing was excluded from the above group. This yielded groups of participants with Noonan syndrome (mean age 11y 3mo, SD 3y) and unaffected siblings (mean age 11y, SD 3y 6mo) that did not differ significantly in chronological age, t46=0.29, d=0.09, variance ratio=0.77.
Measures
Intellectual functioning
The Wechsler Abbreviated Scale of Intelligence was administered to evaluate intellectual ability.13 This measure includes two tests of verbal reasoning (Vocabulary and Similarities) and two tests of nonverbal ability (Block Design and Matrix Reasoning). The Wechsler Abbreviated Scale of Intelligence provides estimates of Verbal IQ, Performance IQ, and Full-scale IQ (FSIQ).
Attention skills
Tasks from the Test of Everyday Attention for Children (TEA-Ch)14 were used to assess behavioral attention in three areas: auditory attention, sustained attention, and response inhibition. Each of these three tasks (Table I) has demonstrated effectiveness as an indicator of attentional impairments in children diagnosed with ADHD.15,16 Scaled scores on these measures were available for all but five participants. Two participants with Noonan syndrome (aged 6 and 8) were unable to complete these tests because of significant difficulties comprehending test instructions and attending to the tasks. Three additional participants (one proband, two comparisons) were over the age of 15 at the time of administration, and therefore outside the normative age range.
Table I:
Description of subtests from the Test of Everyday Attention for Children (TEA-Ch)14
| Subtest | Description |
|---|---|
| Score! | This tone-counting measure assesses auditory attention. Sets of computer-generated tones ranging from 9 to 15 items are presented. Children must silently count and then report the number of tones heard. Scores are based on the number of correct responses out of 10 trials. |
| Code Transmission | This vigilance task measures sustained attention over a 12min test period. Children listen to a lengthy, monotone series of numbers and are required to monitor for a target sequence (i.e. two 5s in a row). Whenever the target sequence is heard, participants must state the number immediately before the two 5s. |
| Walk, Don’t Walk | This measure is a type of ‘Go/No-Go’ task used to assess response inhibition. A response sheet comprising ‘paths’ made of 14 squares is provided. Children listen to computer-generated sounds consisting of ‘Go’ tones, indicating that they should mark a square along the path, and ‘No-Go’ tones, indicating that no mark should be made. Children hold a marker pen approximately 2cm above the sheet and are asked to mark each step as long as they hear the ‘Go’ tone, but withhold from marking if they hear the ‘No-Go’ tone. Scores are obtained based on the number of correctly marked paths out of 20 trials. |
Parent rating scales
Parents of all study participants completed the 18-item ADHD Rating Scale IV-Home Version (ADHD-RS).17 Items in the ADHD-RS, an instrument widely used clinically and in research, correspond directly to each of the nine inattentive and nine hyperactive/impulsive diagnostic criteria for ADHD as outlined in the recently updated Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5).18 Each symptom is scored on a scale from 0 points (not present) to 3 points (severe). Parents also completed the Child Behavior Checklist, a widely used parent-report assessment of childhood behavioral and emotional problems.19 Finally, the Behavior Rating Inventory of Executive Function (BRIEF) Parent Form was administered.20 This caregiver report questionnaire is composed of eight clinical scales measuring aspects of executive functioning in everyday life (Inhibition, Shift, Emotional Control, Initiate, Working Memory, Plan/Organize, Organization of Materials, and Monitor). The BRIEF manual provides evidence for the reliability, validity and diagnostic utility of this measure20, including sensitivity to differences between non-clinical and clinical groups of children with ADHD.
Procedures
Each participant was assessed individually by a trained doctoral-level examiner (EIP) within a single testing session. Participants were given the behavioral tests in a quiet, distraction-free room. Because of the observable physical characteristics associated with Noonan syndrome, it was not possible for the examiner to be blinded regarding participants’ diagnosis. Parents of study participants completed the ADHD-RS, Child Behavior Checklist, and BRIEF questionnaires in a separate room while their child engaged in testing. All forms were later scored by the examiner (using computerized scoring when available) and double-checked by an additional, blinded research assistant.
Parents also completed a demographic form for each child to obtain information about the participant’s age, medical history, and family information. Parents were asked to indicate whether their child had ever received a diagnosis of ADHD, and whether they were receiving any medications. Participants who were taking stimulant medication at the time of their testing appointment were not required to refrain from taking the medication, in order to facilitate unencumbered participation in the research, avoid interfering with physician-prescribed medication regimens, and reduce the potential for selection bias (i.e. loss of families unwilling to withhold their child’s medication). One participant in the sibling comparison group was prescribed stimulant medication, which was taken on the day of testing. Six (18.8%) participants with Noonan syndrome were currently prescribed stimulant medication, but three were not taking the medication on the date of testing (because of ‘drug holidays’).
Statistical analysis
Statistical analyses were performed using the IBM SPSS Statistics 21 package. Comparisons of the rate of ADHD diagnosis within our samples relative to community samples was conducted using one-tailed exact binomial tests. Group comparisons of children with Noonan syndrome and their siblings employed z-tests for proportions and independent sample t-tests for behavioral and parent rating measures. A Holm–Bonferroni correction21 was applied to adjust for multiple comparisons on tests that contained multiple sub-scales (i.e. TEA-Ch, BRIEF, Child Behavior Checklist). Cohen’s d was calculated as an estimate of effect size of the difference of scores between Noonan syndrome and comparison groups on behavioral measures.22
Regression analyses were conducted to examine the relationships between attention skills and IQ test scores across groups. Scores on each of the three behavioral attention measures were entered as predictors in separate analyses, with FSIQ as the outcome variable. Continuous predictors (attention tests) were centered on the median score for that measure. A binary indicator of group status (coded: Noonan syndrome=1, sibling=0) was included, as well as an interaction term (group × attention measure).
RESULTS
Intellectual functioning
Most of the Noonan syndrome cohort (66%) scored within the average range or higher on the Wechsler Abbreviated Scale of Intelligence. Two participants (6%) with Noonan syndrome scored in the IQ range consistent with intellectual disability (FSIQ <70), two participants (6%) scored in the borderline range (FSIQ 70–79), and the remaining seven participants (22%) performed mildly below average (FSIQ 80–84). Mean Verbal IQ was higher than Performance IQ in the Noonan syndrome group (mean difference=3.00, 95% confidence interval (CI) −1.25 to 7.25), but this difference did not reach statistical significance in this sample, t31=1.44, p=0.160. All of the sibling participants (100%) scored at or above the average range on the Wechsler Abbreviated Scale of Intelligence. Although the mean FSIQ for the Noonan syndrome group was within the average range, children with Noonan syndrome scored significantly lower than unaffected siblings with respect to FSIQ, t46=4.29, p<0.001, d=–1.31 (95% CI −1.95≤ d ≤–0.64) (Table II).
Table II:
Performance of children with Noonan syndrome and unaffected siblings on behavioral measures
| Measure | Noonan syndrome | Unaffected siblings | Mean difference (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Clinical impairment (≤2.0SD below mean) (%) | n | Mean (SD) | Clinical impairment (≤2.0SD below mean) (%) | ||
| Intellectual Functioning (WASI) | |||||||
| Full-scale IQ | 32 | 92.9 (14.6) | 6 | 16 | 109.8 (8.2) | 0 | −16.9 (–24.8 to −9.0) |
| Verbal IQ | 32 | 94.9 (14.7) | 6 | 16 | 109.6 (8.7) | 0 | −14.7 (–22.7 to −6.7) |
| Performance IQ | 32 | 91.9 (14.2) | 6 | 16 | 107.4 (11.5) | 0 | −15.5 (–23.7 to −7.2) |
| Attention Tests (TEA-Ch) | |||||||
| Score! | 29 | 6.8 (3.4) | 28 | 14 | 9.2 (3.7) | 14 | −2.4 (–4.7 to −0.1) |
| Code Transmission | 29 | 7.1 (3.2) | 24 | 14 | 9.8 (2.5) | 7 | −2.7 (–4.7 to −0.8) |
| Walk, Don’t Walk | 29 | 5.0 (2.7) | 45 | 14 | 8.2 (3.2) | 7 | −3.2 (–5.1 to −1.4) |
ADHD symptomatology
In the Noonan syndrome group, 10 participants (31%) had reportedly received a previous diagnosis of ADHD by a medical professional. This rate is significantly higher than the 11% diagnosis rate reported by parents in community samples23 (exact binomial test, p=0.002). Based on parent responses to the ADHD-RS administered for the present study, 11 of the 32 participants (34%) with Noonan syndrome currently met diagnostic criteria for ADHD (five predominantly inattentive type; two predominantly hyperactive–impulsive type; four combined type). Six of those 11 children were individuals who had not been previously diagnosed with ADHD. Of the 10 children with Noonan syndrome reported to have a previous diagnosis of ADHD, five did not receive current parent ratings meeting diagnostic criteria on the ADHD-RS. Three of those five were at the time taking stimulant medications, which may have impacted parent ratings.
Parents of two participants (13%) in the unaffected sibling group reported that their child had previously been diagnosed with ADHD. This rate does not differ significantly from the percentage of children reported to have received an ADHD diagnosis in the general population.23 Of the comparison participants, only one (6%) met diagnostic criteria for ADHD (combined type) based on parent ADHD-RS ratings. The child who did not meet ADHD-RS symptom criteria, but was previously diagnosed with ADHD, was taking prescribed stimulant medications.
A comparison of the proportion of children with Noonan syndrome and the proportion of unaffected siblings who met ADHD symptom criteria based on parent ratings revealed a significant group difference, with more children in the Noonan syndrome group meeting criteria for ADHD, mean difference=0.28 (95% CI 0.02 to 0.54), z=2.12, p=0.034. Attention difficulties in the Noonan syndrome group were not always accompanied by intellectual deficits, as 70% of those children with previous ADHD diagnoses and 45% of those presently meeting criteria based on ADHD-RS ratings demonstrated cognitive functioning within the average range (FSIQ ≥85).
Behavioral tests of attention
Performance was examined for children with Noonan syndrome and their siblings on the three behavioral tests of attention (Table II). The Noonan syndrome group had significantly poorer performance than the sibling group on all three measures, including auditory attention (t41=2.12, p=0.040, d=–0.69; 95% CI −1.33≤ d ≤–0.02), sustained attention (t41=2.81, p=0.008, d=–0.91; 95% CI −1.5 ≤ d ≤ −0.23), and inhibitory control (t41=3.47, p=0.001, d=–1.13; 95% CI −1.78≤ d ≤–0.43).
Parent-report measures
Parent ratings of executive functioning skills and social–emotional skills were compared for the Noonan syndrome and sibling comparison groups (Table III). Results from the BRIEF indicated that children with Noonan syndrome were rated as having significantly more problems than their unaffected siblings in two domains: working memory and self-monitoring. Scores on the eight syndrome scales of the Child Behavior Checklist were compared. Children with Noonan syndrome were rated as having significantly more problems than their unaffected siblings on both the Attention Problems and Social Problems subscales. No other significant differences were found after the Holm–Bonferroni correction was applied.
Table III:
Parent rating measures of executive functions and social-emotional functioning in individuals with Noonan syndrome and their unaffected siblings
| Measure | Noonan syndrome (n=32) | Unaffected siblings (n=16) | Mean difference (95% CI) | ||
|---|---|---|---|---|---|
| Mean (SD) | Clinically elevated (T-score ≥65) (%) | Mean (SD) | Clinically elevated (T-score ≥65) (%) | ||
| Executive Functioning (BRIEF) | |||||
| Inhibition | 57.0 (14.4) | 28 | 46.4 (12.3) | 6 | 10.6 (2.1 to 19.1) |
| Attention Shift | 54.0 (14.4) | 19 | 45.5 (9.4) | 6 | 8.5 (0.5 to 16.5) |
| Emotional Control | 54.3 (14.3) | 25 | 48.8 (14.6) | 13 | 5.4 (–3.4 to 14.3) |
| Initiation | 56.3 (13.7) | 34 | 48.8 (11.4) | 6 | 7.4 (–0.6 to 15.4) |
| Working Memory | 59.7 (13.6) | 34 | 46.8 (12.7) | 13 | 12.8 (4.6 to 21.0) |
| Plan/Organize | 57.2 (12.6) | 38 | 46.8 (12.2) | 13 | 10.4 (2.7 to 18.1) |
| Organization of Materials | 53.4 (12.2) | 25 | 49.1 (9.6) | 6 | 4.3 (–2.8 to 11.3) |
| Self-Monitor | 58.0 (13.6) | 38 | 46.2 (11.5) | 13 | 11.8 (3.8 to 19.8) |
| Social-Emotional Functioning (CBCL) | |||||
| Anxious/Depressed | 57.1 (10.1) | 16 | 58.9 (10.5) | 25 | −1.9 (–8.2 to 4.5) |
| Withdrawn/Depressed | 55.5 (8.8) | 13 | 56.6 (8.7) | 13% | −1.1 (–6.5 to 4.3) |
| Somatic Complaints | 60.6 (9.8) | 31 | 54.8 (7.9) | 13% | 5.8 (0.1 to 11.5) |
| Social Problems | 61.8 (8.2) | 38 | 54.9 (7.7) | 13% | 6.9 (2.0 to 11.9) |
| Thought Problems | 58.0 (8.7) | 22 | 54.2 (6.3) | 13% | 3.8 (–1.1 to 8.7) |
| Attention Problems | 63.3 (11.5) | 38 | 53.1 (7.4) | 6% | 10.2 (3.8 to 16.6) |
| Rule-Breaking Behavior | 54.3 (5.7) | 3 | 52.4 (5.2) | 6% | 1.9 (–1.6 to 5.3) |
| Aggressive Behavior | 56.6 (8.4) | 16 | 55.0 (9.5) | 13% | 1.6 (–3.9 to 7.0) |
Relationship between attention measures and intellectual functioning
To test the hypothesis that children with fewer attention problems would achieve higher IQ scores, we conducted a series of multiple regression analyses using both TEA-Ch subtest scores and Noonan syndrome diagnosis as predictors of FSIQ (Table IV). For all three analyses, group status was a significant predictor of IQ, with unaffected siblings performing better on intellectual testing than children with Noonan syndrome. For unaffected siblings, there was no significant association between IQ and any of the attention measures. In contrast, for the Noonan syndrome group, a 1-point increase in sustained attention predicted a 2.66-point increase in IQ. Similarly, a 1-unit increase in response inhibition predicted a 3.95-point increase in IQ. Thus, for the Noonan syndrome group, a significant positive relationship was observed between performance on attention measures and intellectual functioning (Fig. 1).
Table IV:
Results of regression models for prediction of intellectual functioning (WASI FSIQ) based on attention measures and group status
| Model | Regression coefficient (B) | 95% CI for B | t | p | Adjusted R2 |
|---|---|---|---|---|---|
| Model 1: IQ scores predicted by auditory attention (TEA-Ch: Score!) | 0.35 | ||||
| Intercept | 109.27 | 102.30 to 116.24 | |||
| Group | −13.83 | −21.93 to −5.74 | −3.46 | 0.001 | |
| Auditory Attention | 0.14 | −1.52 to 1.80 | 0.17 | 0.869 | |
| Group × Auditory Attention interaction | 1.64 | −0.42 to 3.71 | 1.61 | 0.115 | |
| Model 2: IQ scores predicted by sustained attention (TEA-Ch: Code Transmission) | 0.49 | ||||
| Intercept | 109.45 | 102.98 to 115.92 | |||
| Group | −11.85 | −19.35 to −4.34 | −3.19 | 0.003 | |
| Sustained Attention | 0.07 | −2.06 to 2.20 | 0.06 | 0.949 | |
| Group × Sustained Attention interaction | 2.66 | 0.22 to 5.09 | 2.21 | 0.033 | |
| Model 3: IQ scores predicted by response inhibition (TEA-Ch: Walk, Don’t Walk) | 0.57 | ||||
| Intercept | 110.47 | 104.53 to 116.41 | |||
| Group | −11.74 | −18.67 to −4.80 | −3.42 | 0.001 | |
| Response Inhibition | −0.41 | −1.97 to 1.16 | −0.52 | 0.603 | |
| Group × Response Inhibition interaction | 3.95 | 1.94 to 5.95 | 3.98 | <0.001 | |
Figure 1:
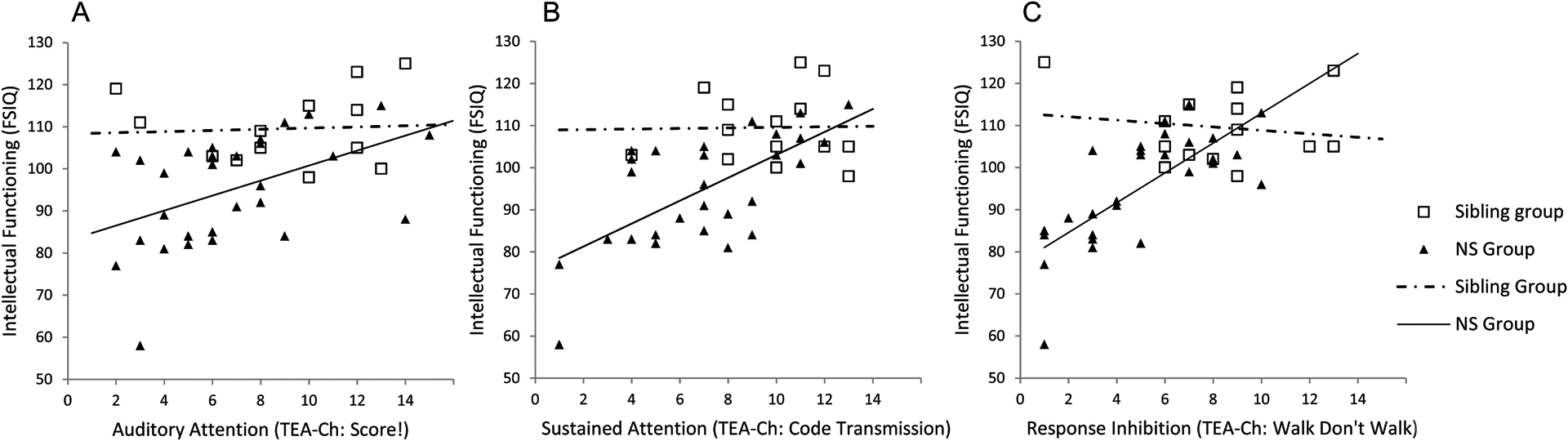
Relationship between behavioral attention measures and intellectual functioning in children with NS and unaffected siblings: (A) auditory attention; (B) sustained attention; (C) response inhibition
DISCUSSION
Symptoms of ADHD can adversely impact social functioning and quality of life throughout the lifespan, placing an individual at higher risk for problems with self-esteem, difficulties in peer relations, and poorer educational and occupational outcomes.24 Our results indicate that nearly one-third of children with Noonan syndrome in the present cohort had received a past diagnosis of ADHD from a medical professional. Further, utilizing multiple scale types (both DSM-criteria-based and broadband behavior scales), we found that children with Noonan syndrome experience significantly more problems with inattention and hyperactivity/impulsivity than their unaffected siblings. Interestingly, we observed only partial overlap (46% of cases) between participants with Noonan syndrome who had a past ADHD diagnosis and those currently exhibiting symptom criteria based on the ADHD-RS. Several factors could contribute to this finding, including developmental changes, the beneficial use of stimulant medication among some children, and/or differences in assessment practices. Clinically, diagnostic interviewing and acquisition of collateral information from school professionals are routinely used in ADHD assessment. Indeed, the lack of teacher forms to cross-reference with parent behavior ratings is a significant limitation of the current study. Nevertheless, parent rating scales are known to be highly reliable and valid measures of ADHD symptomatology, and have demonstrated sensitivity to behavioral and pharmacological treatment effects.25 In our cohort, parent ratings were generally consistent with behavioral testing; only two of the 12 individuals meeting symptom criteria on ADHD-RS (one child with Noonan syndrome, one sibling) demonstrated performance within the normal limits on all of the TEA-Ch tasks without the benefit of stimulant medication.
A particular strength of the current study was the inclusion of empirically validated behavioral tests of attention. Our results indicate that children with Noonan syndrome show greater deficits on tasks assessing attentional regulation relative to unaffected children. The largest effect size was seen on the ‘Walk, Don’t Walk’ subtest, a task that depends not only on attentional vigilance, but also on executive functions (e.g. withholding of automatic responses). For all three measures employed in the present study, children with ADHD have been shown to demonstrate reliable and disproportionate deficits compared with children without ADHD.15,16 Thus, our findings indicate some degree of similarity between the behavioral deficits observed in a portion of children with Noonan syndrome and those with idiopathic ADHD.
Our results also suggest the possibility that ADHD may be underdiagnosed in Noonan syndrome, and/or that mild-to-moderate attention problems are present among some individuals who do not meet full clinical criteria. Six individuals with Noonan syndrome (19% of the full cohort) received parent ratings consistent with DSM-5 diagnostic criteria for ADHD, but had not previously received this diagnosis. Moreover, clinically significant impairments on one or more behavioral tests of attention were present among 10 of the 22 (45%) previously undiagnosed Noonan syndrome participants.
It should also be noted that missing data from two children with Noonan syndrome who were unable to complete the behavioral tasks (because of severe attention problems) implies that the observed mean difference in attention skills in this study may represent an underestimate of the true magnitude of the difference between children with Noonan syndrome and their unaffected peers. Further, our analyses excluded a participant with a mutation in BRAF (a gene more commonly associated with cardiofaciocutaneous syndrome than Noonan syndrome), who had a previous diagnosis of ADHD and was also unable to complete the behavioral attention measures because of difficulty focusing on the tasks. This suggests that gene mutations throughout the RASopathy spectrum are associated with increased risk for significant attention problems, and that more liberal inclusion criteria could result in yet higher rates of observed attention difficulties.
With regard to impact on daily life, ADHD symptoms may interfere with a child’s ability to learn and retain information and/or to demonstrate their true cognitive capabilities.26 In our sample, performance on the attention measures explained significant variation in IQ scores obtained from study participants (Table IV). Importantly, the relationship between attention skills and IQ was of greater magnitude in children with Noonan syndrome than in the comparison group. Although these findings are limited by the relatively small sample size of the comparison group, they suggest the possibility that attention problems could contribute to poorer performance on measures of cognitive ability in Noonan syndrome. Alternatively, the close relationship between intellectual and attentional functioning in Noonan syndrome could indicate that both capacities may be impacted by neurodevelopmental differences resulting from aberrant RAS-MAPK signaling.
Certainly, the finding that children with Noonan syndrome are at heightened risk for impairments in attention and aspects of executive functioning has implications with respect to underlying neural circuitry that may be affected by pathological cellular signaling in Noonan syndrome. Recent research using mouse models has demonstrated that proteins in the RAS-MAPK signaling pathway are involved in regulating release of inhibitory neurotransmitters in prefrontal and striatal networks.4 This discovery has been further extended by research demonstrating a relationship between reduced activity in cortical–striatal circuits (especially within dorsolateral prefrontal cortex) and impaired working memory performance in humans with NF1.4 Although less extensively studied than NF1, previous research on Noonan syndrome has documented a higher rate of impairments on cognitive tasks measuring working memory capacity.8 Results of the current study expand on this finding to show that working memory impairments in Noonan syndrome may result in functional deficits in everyday life (e.g. forgetting steps within a task, becoming easily distracted during activities). Parent ratings of executive functioning in our study also identified self-monitoring (e.g. being aware of how a behavior affects others, understanding one’s own strengths/weaknesses) as a significant problem area for many individuals with Noonan syndrome.
Lastly, with respect to behavior, the two areas that most distinguished children with Noonan syndrome from unaffected siblings were attention problems and social difficulties. A key objective for future research will be to identify whether stimulant medications, which have known effectiveness in improving behavioral outcomes in NF1,27 are also beneficial for children with Noonan syndrome. Research is also needed to investigate whether social difficulties in Noonan syndrome are secondary to problems in other domains (e.g. cognitive, motor, or attention deficits), or whether these difficulties reflect a more pervasive impairment in reciprocal social interaction. Finally, preliminary unreported analyses were suggestive of a genotype-phenotype correlation with regard to risk for attention difficulties, but a small sample size and lack of identified mutations from several participants limited our ability to provide conclusive evidence. We hope to have a larger cohort to fully address this question in the future. Ultimately, identification of attention problems and other psychiatric comorbidities will be an important element in developing appropriate educational and treatment goals to benefit individuals with Noonan syndrome and other RASopathies.
What this paper adds.
Children with Noonan syndrome are at heightened risk for ADHD symptoms and associated functional deficits.
Better attention regulation is associated with higher IQ scores in children with Noonan syndrome
ACKNOWLEDGEMENTS
The authors would like to express gratitude to the children and families that participated in this research. The preparation of this manuscript was made possible by a Boston Children’s Hospital Clinical Research Program Grant awarded to the authors, with support from The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award #UL1 RR 05758 and financial contributions from Harvard University and its affiliated academic health centers). The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, or the National Institutes of Health.
ABBREVIATIONS
- ADHD-RS
ADHD Rating Scale
- BRIEF
Behavior Rating Inventory of Executive Function
- FSIQ
Full-scale IQ
Footnotes
The authors have stated that they had no interests that might be perceived as posing a conflict or bias.
REFERENCES
- 1.Cesarini L, Alfieri P, Pantaleoni F, Vasta I, Cerutti M, Petrangeli V, et al. Cognitive profile of disorders associated with dysregulation of the RAS/MAPK signaling cascade. Am J Med Genet A 2009; 149A: 140–6. [DOI] [PubMed] [Google Scholar]
- 2.Hyman SL, Shores A, North KN. The nature and frequency of cognitive deficits in children with neurofibromatosis type 1. Neurology 2005; 65: 1037–44. [DOI] [PubMed] [Google Scholar]
- 3.Samuels IS, Saitta SC, Landreth GE. MAP’ing CNS development and cognition: an ERKsome process. Neuron 2009; 61: 160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shilyansky C, Karlsgodt KH, Cummings DM, Sidiropoulou K, Hardt M, James AS, et al. Neurofibromin regulates corticostriatal inhibitory networks during working memory performance. Proc Natl Acad Sci U A 2010; 107: 13141–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mendez HM, Opitz JM, Allanson JE. Noonan syndrome: a review. Am J Med Genet 1985; 21: 493–506. [DOI] [PubMed] [Google Scholar]
- 6.Tartaglia M, Gelb BD, Zenker M. Noonan syndrome and clinically related disorders. Best Pract Res Clin Endocrinol Metab 2011; 25: 161–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pierpont EI, Ellis Weismer S, Roberts AE, Tworog-Dube E, Pierpont ME, Mendelsohn NJ, et al. The language phenotype of children and adolescents with Noonan syndrome. J Speech Lang Hear Res 2010; 53: 917–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pierpont EI, Tworog-Dube E, Roberts AE. Learning and memory in children with Noonan syndrome. Am J Med Genet A 2013; 161: 2250–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horiguchi T, Takeshita K. Neuropsychological developmental change in a case with Noonan syndrome: longitudinal assessment. Brain Dev 2003; 25: 291–3. [DOI] [PubMed] [Google Scholar]
- 10.Wood A, Massarano A, Super M, Harrington R. Behavioral aspects and psychiatric findings in Noonan’s syndrome. Arch Dis Child 1995; 72: 153–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neri G, Allanson J, Kavamura MI. No reason yet to change diagnostic criteria for Noonan, Costello and cardio-facio-cutaneous syndromes. J Med Genet 2008; 45: 832. [DOI] [PubMed] [Google Scholar]
- 12.Kover ST, Atwood AK. Establishing equivalence: methodological progress in group-matching design and analysis. Am J Intellect Dev Disabi 2013; 118: 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wechsler D Wechsler Abbreviated Scale of Intelligence. San Antonio: Pearson; 1999. [Google Scholar]
- 14.Manly T, Robertson I, Anderson V, Nimmo-Smith I. Test of Everyday Attention for Children (TEA-Ch). Bury St. Edmunds: Thames Valley Test Company; 1999. [Google Scholar]
- 15.Heaton SC, Reader SK, Preston AS, Fennell EB, Puyana OE, Gill N, et al. The Test of Everyday Attention for Children (TEA-Ch): patterns of performance in children with ADHD and clinical controls. Child Neuropsychol 2001; 7: 251–64. [DOI] [PubMed] [Google Scholar]
- 16.Manly T, Anderson V, Nimmo-Smith I, Turner A, Watson P, Robertson IH. The differential assessment of children’s attention: the Test of Everyday Attention for Children (TEA-Ch), normative sample and ADHD performance. J Child Psychol Psychiatry 2001; 42: 1065–81. [DOI] [PubMed] [Google Scholar]
- 17.DuPaul G, Power T, Anastopoulos A, Reid R. ADHD Rating Scale IV. New York: The Guilford Press; 1998. [Google Scholar]
- 18.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 19.Achenbach T, Rescorla L. Manual for the ASEBA School-Age Form & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families; 2001. [Google Scholar]
- 20.Gioia GA, Isquith PK, Guy SC, Kenworthy L. Behavior Rating Inventory of Executive Function. Lutz, FL: Psychological Assessment Resources; 2000. [Google Scholar]
- 21.Holm S A simple sequentially rejective multiple test procedure. Scand J Stat 1979; 6: 65–70. [Google Scholar]
- 22.Cohen J Statistical power analysis for the behavioral sciences, Second Edition. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- 23.Visser S, Danielson M, Bitsko R, Holbrook J, Kogan M, Ghandour R, et al. Trends in the parent-report of health care provider-diagnosed and medicated ADHD: United States, 2003—2011. J Am Acad Child Adolesc Psychiatry 2014; 53: 34–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wehmeier PM, Schacht A, Barkley RA. Social and emotional impairment in children and adolescents with ADHD and the impact on quality of life. J Adolesc Health 2010; 46: 209–17. [DOI] [PubMed] [Google Scholar]
- 25.Pelham WE Jr., Fabiano GA, Massetti GM. Evidence-based assessment of attention deficit hyperactivity disorder in children and adolescents. J Clin Child Adolesc Psychol 2005; 34: 449–76. [DOI] [PubMed] [Google Scholar]
- 26.Frazier TW, Demaree HA, Youngstrom EA. Meta-analysis of intellectual and neuropsychological test performance in attention-deficit/hyperactivity disorder. Neuropsychology 2004; 18: 543–55. [DOI] [PubMed] [Google Scholar]
- 27.Mautner VF, Kluwe L, Thakker SD, Leark RA. Treatment of ADHD in neurofibromatosis type 1. Dev Med Child Neurol 2002; 44: 164–70. [DOI] [PubMed] [Google Scholar]


