Abstract
The gut microbiota is an important contributor to both health and disease. While previous studies have reported on the beneficial influences of the gut microbiota and probiotic supplementation on bone health, their role in recovery from skeletal injury and resultant systemic sequelae remains unexplored. This study aimed to determine the extent to which probiotics could modulate bone repair by dampening fracture-induced systemic inflammation. Our findings demonstrate that femur fracture induced an increase in gut permeability lasting up to 7 days after trauma before returning to basal levels. Strikingly, dietary supplementation with Bifidobacterium adolescentis augmented the tightening of the intestinal barrier, dampened the systemic inflammatory response to fracture, accelerated fracture callus cartilage remodeling, and elicited enhanced protection of the intact skeleton following fracture. Together, these data outline a mechanism whereby dietary supplementation with beneficial bacteria can be therapeutically targeted to prevent the systemic pathologies induced by femur fracture.
Keywords: Probiotic, Intestinal permeability, Trauma, Inflammation, Microbiome, Dietary supplement
1. Introduction
Long bone fractures are common traumatic injuries that have a remarkable capacity to heal in most individuals. These fractures primarily heal through secondary bone healing, which largely progresses through endochondral ossification. Secondary bone healing is a well-orchestrated series of overlapping events that is initiated by an initial reactive inflammatory stage [1–4]. The local inflammatory environment is critically important for the synthesis of matrix, recruitment of mesenchymal stem cells, and vascularization of the callus [1,5]. However, fractures have far-reaching effects beyond the local injured tissue environment, including induction of systemic inflammation, suppression of anti-inflammatory mediators (e.g., IL-10), and bone loss within the intact skeleton [6–8]. Traumatic injuries, such as femur fracture, can also disrupt gut physiology by disturbing the intestinal barrier and increasing various T-cell populations within the intestinal Peyer’s patches [7,9]. These alterations can facilitate paracellular antigen translocation and endotoxemia, culminating in increased systemic inflammation.
Traumatic injuries can also rapidly induce dysbiosis by decreasing bacterial diversity [10]. In healthy states, the microbiota that reside within the gastrointestinal tract can positively influence health, largely by preventing pathogen colonization and by the generation of immunomodulatory factors and nutrients. These health effects stretch beyond digestive tissues extending to various organs in the body, including bone [11–16]. Transiently altering the indigenous gut microbiome using probiotic supplements has also been shown to benefit bone health in a variety of models. Many of the promoted probiotic bacteria are lactic acid bacteria, including species from the Lactobacillus and Bifidobacterium genera. Recently, administration of the probiotic strain Lactobacillus casei Shirota was shown to significantly improve pain and functional outcomes in elderly patients with distal radius and humerus fractures [17,18]. Bifidobacterium species are also one of the most well studied and widely consumed probiotic bacteria, benefitting the host by altering the diversity of the gut microbiota, modulating immune responses, and improving epithelial barrier function [19,20]. Two Bifidobacterium species predominate in the gut of healthy adults, Bifidobacterium adolescentis (B. adolescentis) and Bifidobacterium longum (B. longum) [21]. B. adolescentis is considered a genuine symbiont that does not promote systemic or intestinal inflammation in a healthy state. However, it does have potent immunomodulatory properties in diseased states that can attenuate the inflammatory host response to intestinal injury and pathogen invasion [22,23].
It is now recognized that the gut microbiome and individual probiotic species have potent immunomodulatory functions that can indirectly influence bone metabolism; however, the role of the gut microbiota during secondary bone healing remains unknown. Herein, we first describe an increase in gut permeability in response to femur fracture during the early stages of healing that is corrected by altered expression of tight junction-associated genes. We then demonstrate that supplementation with a single probiotic species, namely B. adolescentis ATCC 15703 tightened the intestinal barrier, dampened systemic inflammation, and altered the intestinal microbiota community structure during fracture healing. These responses resulted in protection against post-traumatic bone loss and accelerated callus cartilage remodeling. Our data outline a potential therapeutic intervention where dietary supplementation with beneficial bacteria may prevent the systemic pathologies induced by femur fracture.
2. Methods
2.1. Animal husbandry
Eight-week-old male C57BL/6 J mice were obtained from Jackson Laboratories and allowed to acclimate to the vivarium for two weeks prior to the start of all experiments. Male mice were used in this study because of the higher incidence of fractures in young males compared to young females [24]. Mice had ab libitum access to autoclaved food (Envigo #2018S) and water (0.1 μm filtered). All mice were housed at the Atlanta Veterans Affairs Medical Center (VAMC) animal facility in specific pathogen free cages and controlled conditions (temperature, 21–24 °C; humidity, 40–70 %; light/dark cycle, 12/12 h). All experiments were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Atlanta Veterans Affairs Medical Center Institutional Animal Care and Use Committee (Approval #V006–17 and V017–19).
2.2. Assessment of gut permeability after fracture
Naïve twelve-week old male C57BL/6 J mice were fasted for 4 h with access to water then orally gavaged with 150 μl/mouse of 80 mg/ml fluorescein isothiocyanate–dextran 4 (FITC-Dextran MW: 3–5 kDA; Sigma-Aldrich #FD4) in sterile PBS (Invitrogen). After an additional four hour fast without food or water the tail was cleaned with alcohol then nicked using a sterile scalpel blade. Blood was collected using heparinized capillary tubes and plasma isolated by centrifugation for 10 min at 5000 rpm at 4 °C. Plasma concentration of FITC-dextran was then determined by measuring the fluorescence at 528 nm with excitation at 485 nm using a Spectramax M2 plate reader (Molecular Devices). Assessment of plasma FITC-dextran concentrations were assessed in the same mice at two days prior to femur fracture and at day 3, 7, 10, and 18 post-fracture.
2.3. Probiotic supplementation
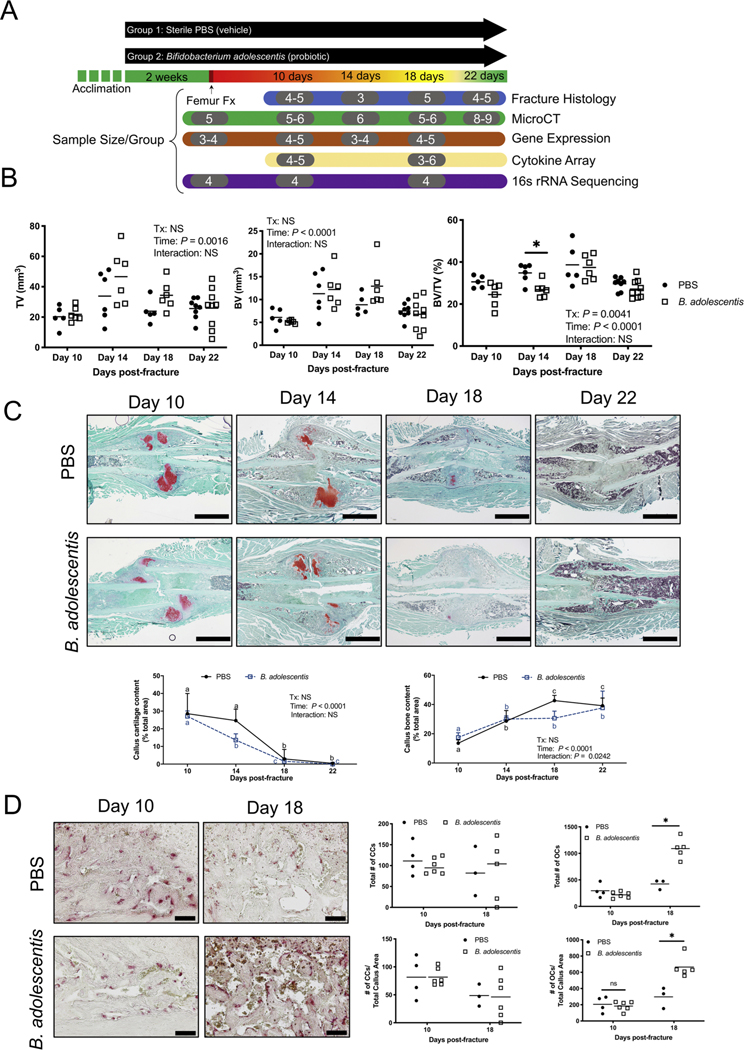
Ten-week old male mice were randomly assigned to receive either sterile PBS (Life Technologies) or 1 × 108 CFU of Bifidobacterium adolescentis (B. adolescentis, ATCC 15703) through oral gavage 5 times per week. Probiotic and control animals were housed separately with ad libitum access to autoclaved food (Envigo #2018S) and water (0.1 μm filtered) and unrestricted cage activity. After two weeks of supplementation, one cohort of mice (n = 5/treatment) was randomly selected to be sacrificed prior to fracture and referred to as Day 0, while the remaining mice were subjected to a closed mid-diaphyseal fracture as described below. All mice continued to receive either B. adolescentis or PBS vehicle control for 10, 14, 18, and 22 days post-fracture. The experimental design is depicted in Fig. 2A. Mice were weighed weekly throughout the duration of the study.
Fig. 2. Supplementation with B. adolescentis increases cartilage remodeling.
(A) Study design and sample sizes for each timepoint. 10-week old male mice were randomly assigned to receive PBS (vehicle control) or B. adolescentis (probiotic) supplements five times per week by oral gavage. After two weeks of supplementation, a mid-diaphyseal fracture (Fx) was created and healing and systemic effects were assessed at day 10, 14, 18, and 22. (B) MicroCT analyses of fracture calluses did not reveal any significant differences in callus size (TV) or bone volume (BV) between groups but did show significantly less bone volume fraction (BV/TV) at day 14 in probiotic treated mice. Two-way ANOVA followed by Bonferroni’s multiple comparison testing to determine effect of treatment (Tx), *P < 0.05 (n = 5–9 per group). (C) B. adolescentis supplementation resulted in faster remodeling of the cartilaginous callus between day 10 and 14. Scale bar, 100 μm. Data analyzed by two-way ANOVA followed by Bonferroni’s multiple comparison testing to determine effect of time. Values represent mean ± SD, values of the same color not sharing a common letter are significantly different (n = 4–6 per group per timepoint). (D) Significant increase in the number of TRAP + osteoclasts (OC) but not chondroclasts (CC) within the fracture calluses of B. adolescentis supplemented mice. Scale bar, 40 μm. Significance determined by unpaired two-tailed Student’s t-test, *P < 0.05 (n = 3–6).
2.4. Fracture model
Femoral fractures were generated as we have previously described [25–28]. Twelve-week old mice were anesthetized with isoflurane inhalation, given analgesics (Buprenorphine SR, 0.05 ml/mouse), and the left hind limb shaved and sterilized with chlorohexidine and alcohol. The articular surface of the femoral intercondylar notch was then perforated with a 25-gauge needle through the skin, followed by insertion of a precut stainless steel 316LVM wire (Small Parts, diameter 0.15 inch) into the medullary canal with the use of a retrograde approach. A transverse mid-diaphyseal fracture was then created using three-point bending via a blunt guillotine device. The fractured limbs were radiographically examined by digital X-ray (Bruker) immediately post-fracture to confirm fracture location and pin placement. Animals with comminuted, distal, or proximal fractures were excluded from the histological and μCT studies, but were used for other outcomes used to assess systemic effects of fracture. Mice were allowed to fully weight-bear without any restrictions on activity after recovery from anesthesia. Mice were then randomly assigned to be euthanized by CO2 asphyxiation followed by cervical dislocation at either 10, 14, 18, or 22 days post-fracture.
2.5. Bacterial culture
B. adolescentis was cultured under anaerobic conditions in reinforced clostridial media containing 9 % Oxyrase (Sigma-Aldrich #SAE0013) at 37 °C for approximately 24 h. Each day prior to gavage the bacteria were centrifuged at 3000×g, media aspirated, and washed in sterile PBS for a total of two times. The washed pellet was resuspended in 3 ml of sterile PBS and used for gavage.
2.6. Micro-computed tomography
Micro-Computed tomography (μCT) was performed on the fractured femur to determine callus bone. μCT was also performed on the un- fractured contralateral femur and 3rd lumbar (L3) vertebrae ex vivo to assess trabecular and cortical bone microarchitecture using a μCT40 scanner (Scanco Medical AG, Brüttisellen, Switzerland) that was calibrated weekly using a factory-supplied phantom. Bones were first fixed for 1 week in 10 % neutral buffered formalin at 4 °C followed by scanning in PBS medium. For fracture callus analyses, the entire callus was manually segmented to exclude existing cortical bone and any bone fragments. The following measures of callus structure and composition were quantified for each fracture callus: total callus volume (TV); mineralized callus volume (BV); and bone volume fraction (BV/TV).
Microarchitecture of the unfractured contralateral femurs was analyzed from 99 tomographic slices taken from the distal femoral metaphysis starting 0.5 mm proximal from distal growth plate. Trabecular bone was manually segmented from the cortical shell at a voxel size of 6 μm (70 kVp and 114 mA, and with 200 ms integration time). Cortical bone was quantified at the femoral mid-diaphysis from 104 tomographic slices. Projection images were reconstructed using the auto-contour function for vertebral body trabecular bone between the cranial and caudal growth plates from approximately 350 tomographic slices. Representative 3D images of vertebral body trabecular bone based on mean BV/TV. The following indices were analyzed: bone volume fraction (BV/TV, %), trabecular number (Tb.N, mm1), trabecular thickness (Tb.Th, μm), trabecular separation (Tb.Sp, μm), volumetric bone mineral density (vBMD, mg HA/cm3), cortical thickness (Ct.Th, mm), cortical porosity (Ct.Po, %), and cortical area (Ct.Ar, mm2). All indices and units were standardized according to published guidelines [29].
2.7. Histology and static histomorphometry
After μCT scanning fractured femurs were decalcified in 14 % EDTA (pH 7.2) for ≥2 weeks before embedding in paraffin. Five micrometer thick sections were obtained and stained with hematoxylin and eosin and Safranin O/Fast green. The percent of cartilage and bone within the fracture callus was quantified using Osteomeasure (Osteometrics) by normalizing the amount of each tissue type to the size of the callus. Histological sections were also stained for TRAP (Sigma) and the number of osteoclasts (TRAP + cells adjacent to bone) and chondroclasts (TRAP + cells adjacent to cartilage) within the fracture callus were quantified. For intestinal histology, the small intestine was equally divided into three parts. The ileum was flushed and fixed with methanol Carnoy solution (60 % methanol, 30 % chloroform, 10 % glacial acetic acid), cut open longitudinally and coiled into a Swiss-roll, which were fixed in 10 % neutral buffered formalin and processed for analysis of goblet cell numbers. Eight-micrometer thick sections of the ilea were used for histological staining [30]. Slides were stained using an Alcian Blue (pH 2.5) stain kit (Poly Scientific R&D Corporation #S111A) and counter-stained with nuclear fast red. The number of Goblet cells per villi were manually counted from 2 to 5 representative fields per slide and averaged per mouse.
2.8. Gene expression
A 1 cm section of the small intestines corresponding to the duodenum and proximal colon was collected, flash frozen, and stored at −80 °C until analysis. The frozen small intestine and colon was pulverized using a SPEX Freezer/Mill and total RNA was isolated using TRIzol (Invitrogen). Whole bone marrow was obtained from the tibia (contralateral unfractured hindlimb) and flash frozen prior to RNA isolation using TRIzol (Invitrogen). First-strand cDNA was synthesized with oligo(dT) and random primers using qScript cDNA SuperMix (Quantabio). All qRT-PCR were performed on an Analytik Jena qTower3 G Real-Time PCR Detection System using PerfeCTa SYBR Green FastMix (Quantabio). Amplicon authenticity was confirmed by melt curve analysis. Primer sequences are provided in Supplementary Table 1 and β-actin was used as the normalization control. The data were analyzed for fold change using the ΔΔCT method.
2.9. Measurement of serum inflammatory and bone turnover markers
Whole blood was obtained by cardiac puncture at time of harvest, allowed to clot at room temperature for ≥30 min, then centrifuged at 10,000g for 10 min. Systemic cytokines were assayed using a Meso Scale Discovery U-Plex electrochemiluminescence assay for a total of 20 cytokines according to manufacturer’s instructions at the Emory Multiplex Immunoassay core. The investigated markers were EPO, GM-CSF, IFN-γ, IL-1β, IL-4, IL-6, IL-10, IL-13, IL-16, IL-17a, IL-17c, IL-17e/IL-25, IL-17f, IL-21, IL-22, IL-23, IL-33, MCP-1, TNF-α, and VEGF-a. Undetectable concentrations of any cytokine were recorded as one-half of the lower limit of quantification, with the exception of GM-CSF, IL-13, IL-4, IL- 17c, IL-17e/IL-25, and IL-17f which were not detected in any sample and were excluded from analyses. N-terminal propeptide of type I procollagen (PINP) (ImmunoDiagnostic Systems) were assayed using an Enzyme-Linked Immunosorbent Assay kit according to the manufacturer’s recommendations.
2.10. Measurement of serum endotoxin/lipopolysaccharide
To assess serum endotoxin levels, serum samples were diluted 100-fold in sterile, pyrogen-free water and heated at 70 °C for 15 min. Endotoxin levels were quantified using the Pierce Chromogenic Endotoxin Quant Kit (#A39552; Thermo Scientific) according to the manufacturer’s directions. The absorbance of each sample was measured spectrophotometrically at 405 nm, and the concentration of endotoxin was calculated from a standard curve.
2.11. 16 s rRNA sequencing
Bacterial 16S rRNA gene V4 amplicons from fecal samples were generated on an Illumina MiSeq using the 300-bp paired-end kit (v.3) by Microbiome Insights Inc. (Vancouver, Canada). Sequences were denoised, taxonomically classified using Greengenes (v.13_8) as the reference database, and clustered into 97 %-similarity operational taxonomic units (OTUs) with the mothur software package (v. 1.39.5) [31]. The resulting dataset had 5535 OTUs (including those occurring once with a count of 1, or singletons). An average of 35389 quality-filtered reads were generated per sample. Co-sequencing DNA amplified from fecal samples, template-free controls, and extraction kit reagents processed the same way as the samples were used to address contamination. Any OTU with a mean abundance that reached or exceeded 25 % of their mean abundance in specimens were considered putative contaminants and were removed.
2.12. Statistical & bioinformatic analysis
Results are shown as mean ± SD. Statistical significance was determined by either paired two-tailed Student’s t-test, unpaired two-tailed Student’s t-test, Mann-Whitney U Test, one-way or two-way ANOVA as indicated in the figure legend using GraphPad Prism software (version 8.3.0). All statistical tests were performed at the 5 % significance level. The presence of outliers was determined using the ROUT method (Q = 1%). Prior to principal component analysis (PCA), serum cytokine concentrations were log-transformed then converted to Z-scores. PCA of serum cytokine data was carried out as a tool of exploratory data analysis using the prcomp function in R (version 6.3.2) and scatter plots of PC1 and PC2 where generated using ggbiplot R package. Each dot represents a sample projected onto the two main principal components and the color of the dot represents the treatment. Microbial alpha diversity was estimated with the Shannon index on raw OTU abundance tables after filtering out contaminants. To estimate beta diversity across samples, OTUs occurring with a count of less than 3 in at least 10 % of the samples were excluded and then used to computed Bray-Curtis indices. Beta diversity was visualized using Principal Coordinates Analysis (PCoA) ordination and variation in community structure was assessed with permutational multivariate analyses of variance (PERMANOVA) with treatment group as the main fixed factor and using 9999 permutations for significance testing. All analyses were conducted in the R environment by Microbiome Insights (Vancouver, Canada).
3. Results
3.1. Fracture induces increased gut permeability in mice up to day 7 following trauma
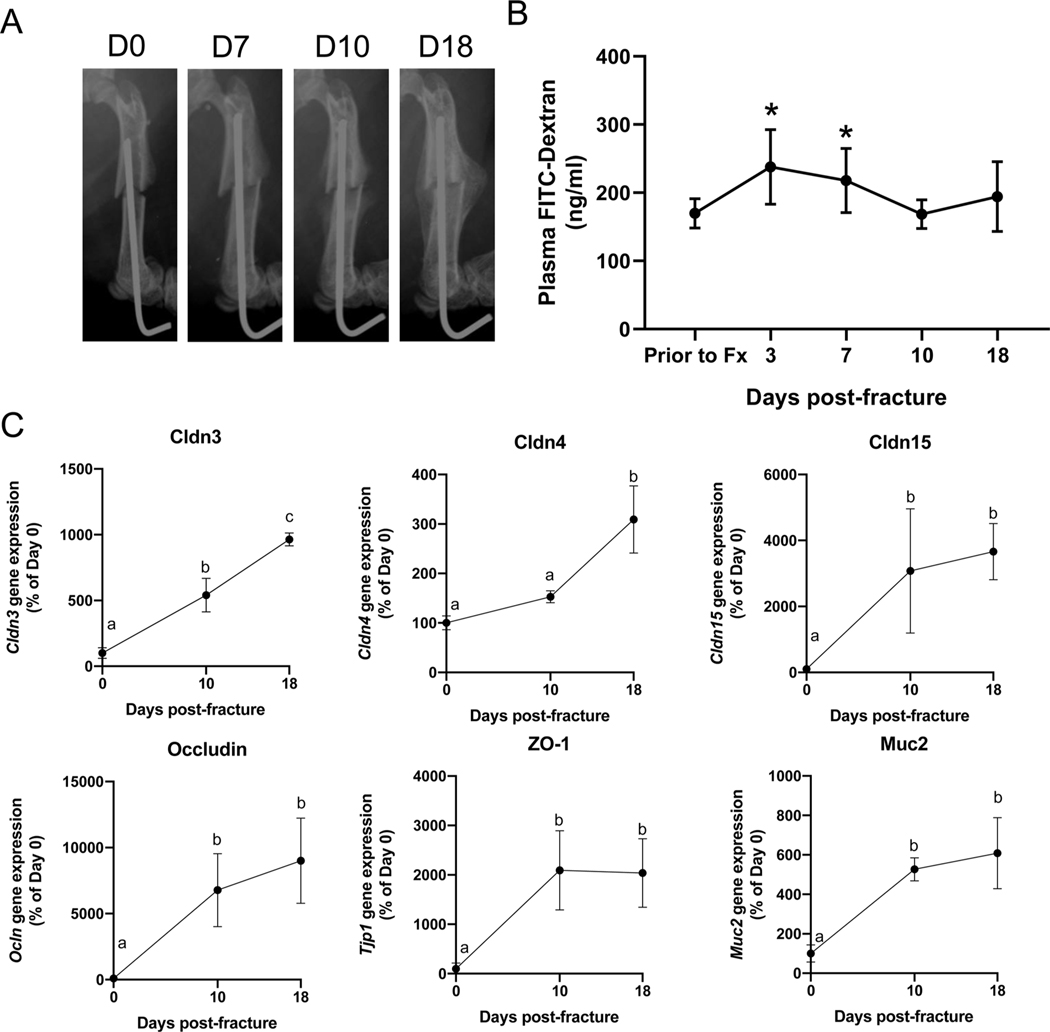
Severe traumatic injuries can disrupt the intestinal barrier and permit paracellular translocation of bacteria and bacterial by-products, such as endotoxin/lipopolysaccharide [32–34]. However, whether simple bone fractures also impair gut barrier function throughout the healing process has not been fully described. Thus, we sought to determine if fracture would also impact gut barrier function throughout secondary bone healing by measuring gut permeability in the context of an established model of closed femur fracture (Fig. 1A). Compared to pre-fracture levels, induction of a closed femur fracture significantly increased gut permeability, as measured by plasma FITC-Dextran levels at day 3 and day 7 post-fracture (Fig. 1B). At day 10 and 18 post-fracture, the gut permeability had returned to baseline pre-fracture levels (Fig. 1B). Consistent with the observations of a return to baseline permeability levels by day 10, we detected a significant increase in the gene expression of small intestine tight junction proteins, including Claudin-3, −4, and −15, Occludin, and ZO-1 at both day 10 and 18 post-fracture compared to pre-fracture expression levels (Fig. 1C). In addition, gene expression of the mucus subunit protein Mucin-2 (Muc2) was significantly elevated at 10- and 18-days post-fracture compared to pre-fracture expression levels (Fig. 1C). These data indicate that fracture induces a leaky gut phenotype in mice up to about 10 days post-fracture, which then resolves and returns to baseline levels. Elevated levels of gut epithelial tight junction proteins correlated with a return to baseline levels of gut permeability.
Fig. 1. Fracture induces an early increase in intestinal permeability.
(A) Radiographs of a representative fractured femur immediately after fracture (Day 0) and throughout healing. (B) Plasma FITC-Dextran concentrations were significantly higher at day 3 and 7 post-fracture compared pre-fracture levels, with return to pre- fracture levels by day 10 (n = 6). Two-tailed paired Student’s t-test, *P < 0.05 vs prior to fracture (Fx) control. (C) Fracture upregulates gene expression of tight junction-associated proteins and Muc2 within the small intestines (n = 4). Significance determine with one-way ANOVA followed by Tukey’s multiple comparisons test. Data not sharing a common letter are significantly different at P < 0.05. Data represent mean ± SD.
3.2. Probiotic supplementation accelerates callus cartilage remodeling
Probiotics can tighten the intestinal barrier and decrease gut permeability [15,35,36]. Thus, we next sought to determine if prophylactic dietary supplementation with a candidate beneficial bacterium, which is analogous to consuming a probiotic supplement as part of normal daily health routine, would prime the body to respond to an unanticipated traumatic fracture that typically occur in young adults. Mice were randomly assigned to receive an oral gavage of sterile PBS (vehicle control) or the candidate probiotic Bifidobacterium adolescentis (B. adolescentis) daily for two weeks before fracture (Fig. 2A). Thereafter, a mid-diaphyseal femur fracture was inflicted, and healing assessed at 10, 14, 18, and 22 days post-fracture (Fig. 2A). The mice continued to receive their respective dietary supplement throughout the 22 day post-fracture period. Supplementation of B. adolescentis did not influence total body weight, spleen weight, or macroscopic appearance of the intestines throughout the study, suggesting there are no detectable adverse effects of daily consumption of B. adolescentis (Supplementary Fig. 1A–C). Micro-CT analyses of fracture calluses did not reveal any significant differences in callus size (TV) or bone volume (BV) between groups; however, temporal differences were detected (Fig. 2B). In particular, there was a significant difference of dietary supplementation with B. adolescentis on callus bone volume fraction (BV/TV), with the probiotic-supplemented mice exhibiting significantly less bone at day 14 post-fracture (Fig. 2B). B. adolescentis supplementation resulted in accelerated remodeling of the cartilaginous callus between day 10 and 14 post-fracture, which was not observed in the PBS control group (Fig. 2C). Static histomorphometry also revealed an increase in callus bone throughout fracture healing in both groups that was move evident in the control group (Fig. 2C). TRAP staining of fracture calluses at day 10 and 18 post-fracture and quantification of TRAP + cells did not reveal any significant differences in chondroclasts or osteoclasts between groups at day 10 post-fracture (Fig. 2D). However, there was a significant increase in the number of osteoclasts within the calluses of B. adolescentis supplemented mice at day 18 post-fracture (Fig. 2D). These data indicate that dietary supplementation with B. adolescentis before and following a mid-diaphyseal femur fracture accelerated callus cartilage remodeling and influences callus osteoclasts.
3.3. B. adolescentis dampens the systemic inflammatory response to fracture
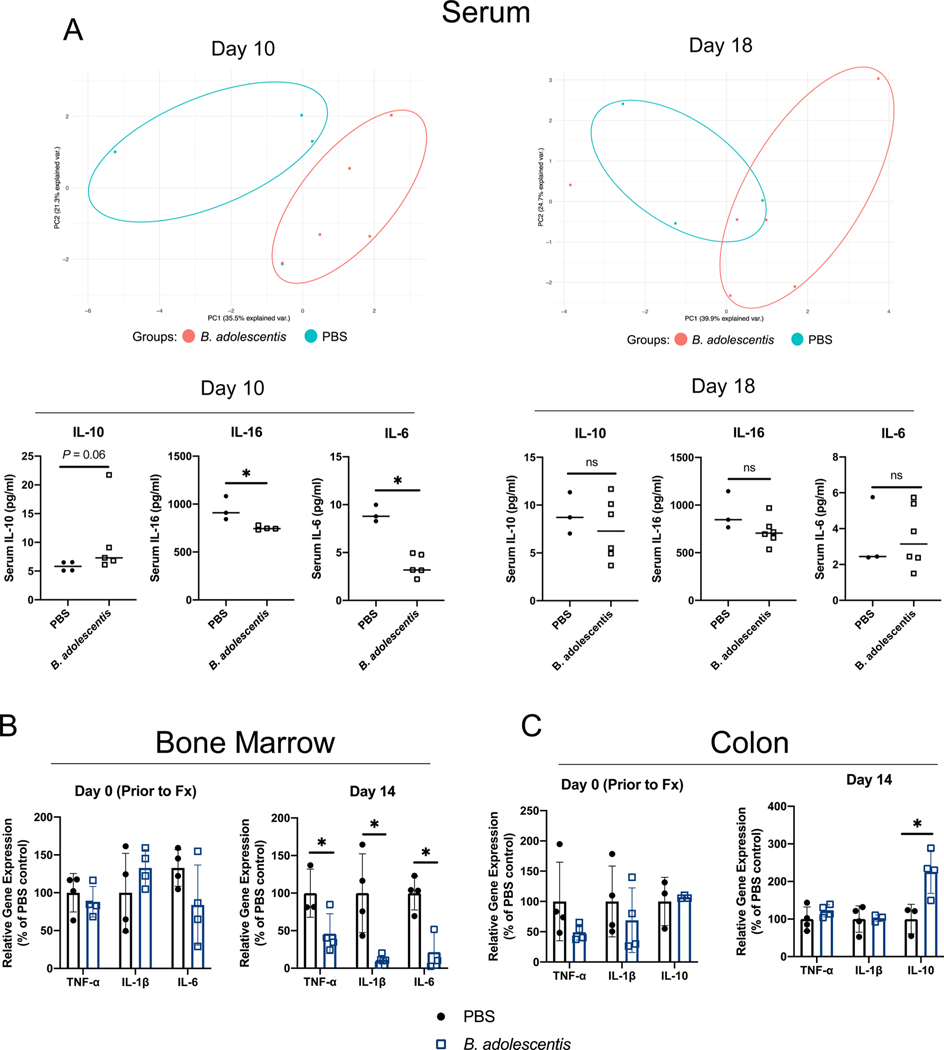
Beyond the local inflammatory response to injury, fracture can also initiate systemic inflammation and lead to inflammatory response syndrome which can be detrimental for bone healing [6,37]. Serum from PBS and B. adolescentis supplemented mice was subjected to a multiplex immunoassay to determine systemic levels of 20 cytokines. Principal component analyses (PCA) revealed that B. adolescentis supplementation induced a distinct inflammatory profile particularly evident at day 10 post-fracture (Fig. 3A). This included significantly lower levels of pro-inflammatory cytokines IL-16 and IL-6 at day 10 post-fracture (Fig. 3A). The serum levels of the anti-inflammatory cytokine IL-10 were also higher, although the differences did not reach the threshold for statistical significance (Fig. 3A). No significant differences were observed in the assessed serum cytokine levels at day 18 post-fracture (Fig. 3A). Furthermore, no significant differences were detected in the other measured cytokines at day 10 or 18 post-fracture (Supplemental Fig. 2). Assessment of pro-inflammatory cytokine gene expression within the bone marrow compartment of the contralateral tibia did not reveal any significant differences after two weeks of B. adolescentis supplementation (Fig. 3B). However, at day 14 post-fracture gene expression of IL-1β, TNFα, and IL-6 was significantly lower within the bone marrow of mice given B. adolescentis (Fig. 3B). Similarly, there were no differences in cytokine gene expression within the colon between groups prior to fracture (Fig. 3C). At day 14 post-fracture, there were no significant differences in expression of IL-1β and TNFα; however, there was a significant increase in gene expression of the anti-inflammatory cytokine IL-10 in the colon of B. adolescentis supplemented mice (Fig. 3C). Gene expression of IL-6 was not detected in the colon samples at either timepoint (data not shown). Together, these data show that B. adolescentis supplementation significantly dampens the systemic pro-inflammatory response that occurs following fracture.
Fig. 3. Probiotic supplementation dampens the systemic inflammatory response to fracture.
(A) Principal component analyses of serum cytokines revealed that B. adolescentis supplementation created an inflammatory profile that was distinct from control mice at day 10 and 18 post-fracture. B. adolescentis supplementation decreased serum levels of IL-16 and IL-6 at day 10 post-fracture. Data analyzed by Mann-Whitney U-test, *P < 0.05. n = 3–6 in each group and timepoint. (B) At day 14 post-fracture B. adolescentis supplementation repressed the expression of IL-1β, TNFα, and IL-6 within the bone marrow compartment of the unfractured contralateral tibia. (C) At day 14 post-fracture there was a significant increase in colonic IL-10 gene expression. Significance of gene expression was determined by unpaired two-tailed Student’s t-test, *P < 0.05 (n = 3–4 in each group). Abbreviations: Fx, fracture; B. adolescentis, Bifidobacterium adolescentis.
3.4. Probiotic supplementation increases expression of intestinal tight junction proteins
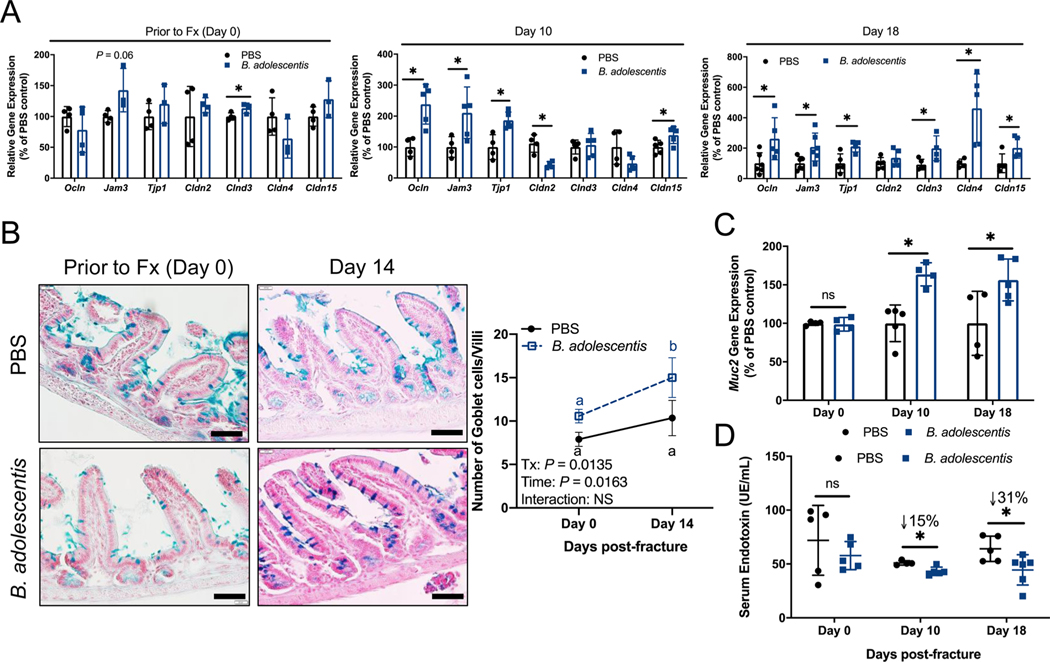
One mechanism whereby probiotics influence human health and systemic inflammation is by buttressing the intestinal epithelial barrier [38]. Considering that femoral fracture induces gut permeability (Fig. 1), we sought to determine whether B. adolescentis supplementation would augment the expression of intestinal tight junction proteins to tighten the gut epithelial barrier and prevent translocation of pro-inflammatory bacterial products such as endotoxin to sub-epithelial compartments. Before fracture, two weeks of B. adolescentis supplementation induced a significant increase in the expression of Claudin 3 (Cldn3) within the small intestine but did not alter the expression of other tight junction proteins within the small intestine or colon (Fig. 4A and Supplemental Fig. 3). At day 10 post-fracture, B. adolescentis supplementation significantly increased small intestine expression of Ocln (Occludin), Jam3, Tjp1 (ZO-1), and Cldn15 (Claudin 15) and decreased the expression of Cldn2 compared to PBS supplemented mice (Fig. 4A). All tight junction-associated genes examined were significantly up-regulated, with the exception of Cldn2, in the small intestine in response to B. adolescentis supplementation at day 18 post-fracture (Fig. 4A). Histological assessment of the small intestine revealed a significant increase in the number of mucin-producing Goblet cells per villus at 14 days post-fracture in B. adolescentis supplemented mice relative to baseline (Fig. 4B). Similar to tight junction protein gene expression, no differences were observed in the expression of the predominate intestinal mucus glycoprotein Mucin 2 (Muc2) prior to fracture; however, at day 10 and 18 post-fracture there was a significant increase in small intestine Muc2 expression in B. adolescentis supplemented mice (Fig. 4C). Within the colon, expression of Muc2 was also significantly increased in the probiotic group at day 14 post-fracture (Supplemental Fig. 3). We measured serum endotoxin levels to determine whether these changes in gut barrier defense-related genes translated to reduced gut permeability. Prior to fracture no differences in serum endotoxin were observed between groups; however, B. adolescentis supplementation resulted in lower serum endotoxin at both day 10 and 18 post-fracture (Fig. 4D). These data show that B. adolescentis supplementation increases expression of intestinal tight junction proteins which is particularly strong during the period following fracture (between day 7 and day 10) when gut barrier integrity is restored to baseline levels following the fracture induced leaky gut.
Fig. 4. B. adolescentis tightens the intestinal barrier.
(A) Prior to fracture (Fx; Day 0) B. adolescentis supplementation increased the expression of Cldn3. At day 10 post-fracture, B. adolescentis supplementation significantly increased the gene expression of Ocln, Jam3, Tjp1, and Cldn15, and decreased the expression of Cldn2 within the small intestine. B. adolescentis supplementation significantly increased the expression of Ocln, Jam3, Tjp1, Cldn3, Cldn4, and Cldn15 at day 18 post-fracture compared to control mice. n = 4–6 per group. (B) Representative histological sections of small intestine isolated from control and B. adolescentis supplemented mice prior to fracture and at day 14 post-fracture. Scale bar, 40 μm. There was a significant increase in the number of goblet cells per villi in the probiotic supplemented mice at day 14 post-fracture. Two-way ANOVA followed by Bonferroni’s multiple comparisons testing to determine effect of time on goblet cell number. Values represent mean ± SD, values of the same color not sharing a common letter are significantly different, P < 0.05 (n = 3–4 per group). (C) Significant increase in Muc2 expression at day 10 and 18 post-fracture in mice supplemented with B. adolescentis. n = 4–5 per group per timepoint. (D) At post-fracture day 10 and 18 there was significantly less endotoxin in B. adolescentis mice. n = 5–6 per group per timepoint. Gene expression and serum endotoxin data analyzed using an unpaired two-tailed Student’s t-test, *P < 0.05. Abbreviations: Fx, fracture; B. adolescentis, Bifidobacterium adolescentis; Tx, treatment.
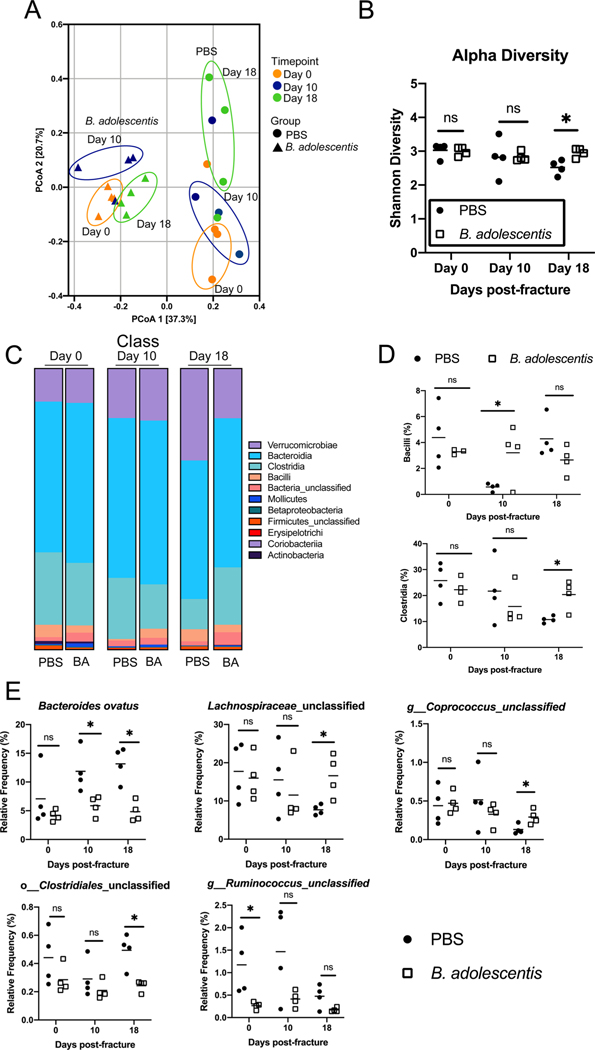
3.5. B. adolescentis supplementation alters the community structure of the gut microbiome
Previous reports have shown that probiotic supplementation can alter the gut microbiome community structure [16]. In order to assess the extent to which B. adolescentis supplementation may elicit a similar effect, the microbiome of groups of mice were normalized by repeated rounds of bedding exchanges. Mice were then supplemented with B. adolescentis or PBS for 2 weeks before sequence analysis of bacterial 16S rRNA V4 region was conducted on fecal samples collected prior to fracture (Day 0), and at days 10 and 18 post-fracture. Principal coordinates analysis (PCoA) ordination visualization of beta diversity demonstrate a clear separation between each treatment groups (Fig. 5A) (PERMANOVA, p = 0.0001, R2 = 0.3520); indicating that there is dissimilarity between the gut microbiota of PBS and B. adolescentis supplemented mice. PERMANOVA did not reveal a significant effect of time on microbiota composition (p = 0.45, R2 = 0.075). However, visual inspection of the PCoA plots indicates, that in B. adolescentis-supplemented mice, there was an initial shift in beta diversity at day 10 post-fracture that becomes more similar to the non-fractured mice at day 18 post-fracture. In the control PBS supplemented mice, there was a progressive shift in the beta-diversity throughout healing, characterized by a large shift in the microbiota diversity at day 18 post-fracture (Fig. 5A). Microbial alpha diversity was also significantly increased in B. adolescentis-supplemented mice at day 18 post-fracture (Fig. 5B). B. adolescentis supplementation did not significantly alter the taxonomic composition at the class level prior to fracture (day 0) (Fig. 5C and D). However, at day 10 post-fracture the frequency of Bacilli in the B. adolescentis-treated mice (3 %) was significantly higher than the control mice (0.56 %) (Fig. 5C and D). At day 18 post-fracture, B. adolescentis significantly increased the relative frequency of Clostridia within the intestine (20 % versus 10.8 %) (Fig. 5C and D). Similar results were observed at the species level, in that B. adolescentis supplementation did not significantly alter the species prior to fracture with the exception of unclassified Ruminococcus (Fig. 5E). However, at 10 days post-fracture probiotic supplementation lead to a decrease in the proportion of Bacteroides ovatus, which persisted at day 18 post-fracture compared to control mice (Fig. 5E). At day 18 post-fracture, there was a significant increase in the relative proportions of unclassified Lachnospiraceae, unclassified Coprococcus, but a decrease in unclassified Clostridiales in B. adolescentis supplemented mice compared to control mice (Fig. 5E). These data show that B. adolescentis supplementation alters the community structure of the gut microbiome which may be a contributory factor in the observed differences in fracture healing between B. adolescentis and PBS supplemented groups.
Fig. 5. B. adolescentis supplementation influences the composition of the intestinal microbiome.
(A) Principal coordinates ordination analyses of fecal beta diversity (n = 4). (B) B. adolescentis increased bacterial alpha-diversity at day 18 post-fracture. (C) Detailed relative abundance of bacterial taxa at the class level within fecal pellets at day 0 (prior to fracture) and day 10 and 18 post-fracture. (D) B. adolescentis increases the relative frequency of Bacilli at day 10 post-fracture and Clostridia at day 18 post-fracture in the gut. (E) Bacteroides ovatus was significantly lower in B. adolescentis supplemented mice at day 10 and 18 post-fracture. B. adolescentis increased the relative frequency of unclassified Lachnospiraceae, unclassified Coprococcus, and unclassified Clostridiales at day 18 post-fracture. Each dot represents an individual animal and the line represents the mean. Unpaired two-tailed Student’s t-test, *P < 0.05.
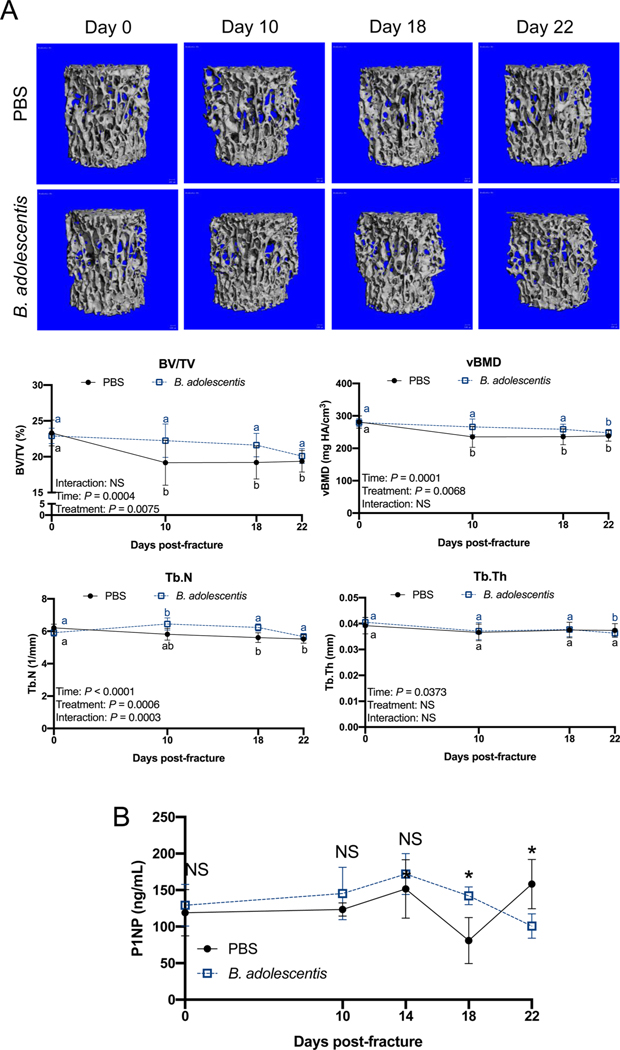
3.6. Probiotic supplementation protects against post-traumatic bone loss
Fractures can lead to bone loss within the intact skeleton, such as the vertebrae, through a mechanism largely attributed to heightened inflammation [6]. We sought to determine if the immunosuppressive activities of B. adolescentis (Fig. 3) would prevent post-traumatic bone loss within the intact skeleton after fracture. In the control mice supplemented with PBS, there was an initial loss of trabecular bone volume fraction (BV/TV), volumetric bone mineral density (vBMD), and trabecular number (Tb.N) in the L3 vertebral body that was sustained until day 22 post-fracture (Fig. 6A). This early post-traumatic decrease in L3 trabecular bone microarchitecture was blunted in the B. adolescentis supplemented mice that may have stemmed from a significant increase in Tb.N (Fig. 6A). No differences were observed between groups in the trabecular or cortical bone of the intact contralateral femur (Supplemental Fig. 4). Assessment of serum procollagen type I N-terminal propeptide (P1NP), a biomarker of bone formation, did not reveal significant differences between groups prior to fracture (day 0) or at days 10 and 14 post-fracture (Fig. 6B). At day 18 post-fracture, serum levels of P1NP were significantly higher in mice given B. adolescentis supplements; however, at day 22 post-fracture P1NP was significantly lower in the B. adolescentis supplemented group (Fig. 6B). These data show that B. adolescentis supplementation protects against post-traumatic bone loss, likely through dampening the systemic inflammatory response elicited by bone fracture.
Fig. 6. B. adolescentis supplementation protects against systemic bone loss.
(A) Representative microCT 3D reconstructions of the L3 vertebrae trabecular bone and volumetric analyses show decrease in trabecular bone volume fraction (BV/TV), volumetric bone mineral density (vBMD) and trabecular number (Tb.N) in control mice that was attenuated in the B. adolescentis supplemented mice after fracture. Data analyzed by two-way ANOVA followed by Bonferroni’s multiple comparison testing to assess effect of time. Values of the same color not sharing a common letter are significantly different, P < 0.05 (n = 5–9 per group). (B) Serum P1NP was significantly higher in B. adolescentis supplemented mice at day 18 post-fracture, but significantly lower at day 22 post-fracture. Assessed by unpaired two- tailed Student’s t-test, *P < 0.05 (n = 4 per group).
4. Discussion
In this study, we investigated whether prophylactic dietary supplementation with B. adolescentis would prime the body for improved outcomes in the event of an unanticipated fracture and its effects throughout secondary fracture healing. It is established that fracture results in the systemic elevation of pro-inflammatory cytokines. Here, we demonstrate that fracture also induces a leaky gut phenotype up to 7 days following infliction of trauma, and the leaky gut phenotype is resolved and restored to pre-fracture basal levels by day 10 following fracture. During the period of tightening of the leaky gut, probably between days 7–10 following fracture, we detected an increase in the expression of tight junction proteins within the gut epithelium. Critically, we also showed that dietary supplementation with B. adolescentis elicited reduced intestinal permeability, and increased expression of tight junction proteins within the gut epithelium. Consistently, B. adolescentis supplemented mice had lower systemic inflammation following fracture, and exhibited accelerated remodeling of the cartilaginous callus likely by reducing systemic inflammation. Finally, we show that B. adolescentis supplemented mice had altered microbiome community structure compared to PBS supplemented mice.
The inflammatory changes stemming from traumatic injuries are thought to be secondary to increased gut permeability. Injury leads to increased intestinal permeability that drives a systemic inflammatory response driven by paracellular transport of bacterial proteins and products that permeate to sub-epithelial compartment of the gut [39–41]. Furthermore, the severity of trauma is strongly associated with the degree of intestinal permeability [42]. Simple bone fractures can also impact gut function by increasing intestinal permeability as soon as 24 h after femur fracture [9]. We also observed an increase in gut permeability during the early stages of fracture healing in our mouse model of fracture infliction; however, in our femur fracture model permeability persisted until one-week post-fracture. This increase in gut permeability corresponds to the inflammatory reactive stage when systemic inflammation is highest, which may be synergistically driven by gut-derived pro-inflammatory bacterial proteins and products and intrinsic host derived factors. We also observed that expression of many intestinal tight junction-associated genes were upregulated 10 days after fracture, which corresponded to the return to basal levels of gut permeability. This increased expression likely reflects the intestine’s response to correct the fracture-induced disruption of the intestinal barrier function that may be an evolutionary adapted mechanism to evoke a heightened immune response immediately following fracture.
Supplementing the existing microbiota with probiotics for improved bone repair is therapeutically promising. Our data hint at accelerated fracture healing in mice that received B. adolescentis supplements. This was indicated by faster cartilaginous callus remodeling, which likely stemmed from increased osteoclast numbers within the fracture calluses. To our knowledge, this is the first study to report an acceleration of fracture healing by consuming probiotic supplements. Two prior studies have suggested that fracture healing was accelerated in elderly individuals who consumed a supplement containing Lactobacillus casei Shirota; however, these studies only examined pain and functional outcomes rather than radiographic indicators of healing [17,18]. Nonetheless, our study adds to the growing body of evidence that consumption of probiotics can positively influence fracture healing.
Mechanistically, probiotics, including B. adolescentis, partly benefit the host by influencing gut physiology [43]. This involves preservation of tight junction integrity through regulating the expression and assembly of tight junction proteins that function to firmly anchor adjacent epithelial cells to each other [35]. Our data suggests that B. adolescentis supplementation functions in a similar capacity in the post-fracture period by protecting against fractured-mediated dysfunction of intercellular tight junctions in the small intestine. At day 10 post-fracture, there was a significant increase in the expression of several tight junction genes in the probiotic group that remained significantly higher than the control group at day 18 post-fracture. ZO-1 (Tjp1) is a scaffolding protein that forms the link between the transmembrane tight junction proteins (e.g., Occludin, Claudins, Jams) and the cytoskeleton to stabilize the tight junction [44]. Upregulated expression of ZO-1 may facilitate the assembly of bicellular tight junctions consisting of Occludin and Jam3. Interestingly, Claudin 2 (Cldn2) levels were significantly downregulated by probiotic supplementation. Claudin 2 is a pore-forming claudin that is consistently upregulated in inflammatory conditions [45,46] and in response to high levels of certain cytokines, such as IL-6 [47,48], TNF-α [49], and IL-13 [50]. Elevated levels of Claudin 2 can decrease the transepithelial resistance when inserted into the tight junction; thereby, increasing permeability [51]. At day 18 post-fracture Cldn2 levels did not differ from the control mice, but expression of other barrier forming claudins (i.e., Cldn3, Cldn4) and Cldn15 were significantly up-regulated in the probiotic-supplemented mice suggesting that tight junction activity may have been augmented. We also observed a significant increase in the gene expression of the predominate intestinal mucus protein Muc2 in the small intestine and colon as well as increased number of mucin-producing goblet cells within the small intestine in B. adolescentis-supplemented mice. The mucus layer acts as a physical shield to prevent direct contact of luminal content with the epithelial cells [52]. It also functions to prevent dilution of antimicrobial compounds secreted by Paneth cells and enterocytes, thereby generating an anti-microbial gradient from the epithelial cells towards the lumen [53]. The increase in IL-10 expression in B. adolescentis supplemented mice may have driven the increase in mucin production as IL-10 facilitates protein folding and stimulates mucus production by goblet cells [54]. Together these data suggest that the probiotic B. adolescentis is able to fortify intestinal barrier defenses after fracture through upregulation of tight junction proteins and mucin production.
Improved gut function in B. adolescentis supplemented mice likely resulted in the significantly lower serum endotoxin levels observed after fracture. Endotoxin or lipopolysaccharide (LPS) is a product of the outer membrane of gram-negative bacteria that is a potent trigger of inflammation [55,56]. LPS-induced systemic inflammation has previously been shown to influence bone regeneration by impairing vascularization and decreasing bone turnover by osteoblasts and osteoclasts and lead to hypertrophic and immature calluses [57,58]. The lower levels of endotoxin may also be responsible for the dampened inflammatory response observed in the serum and bone marrow of probiotic-supplemented mice. This dampened inflammation could have contributed to the accelerated fracture healing phenotype and protected the vertebrae trabecular bone against post-traumatic bone loss.
Fracture induces systemic pathologies that extend beyond the fractured bone to impact the intact skeleton. Incident fractures accelerate loss of cancellous bone of the vertebral body and hip bone mineral density by increasing osteoclast numbers [6,59,60]. Similar to these studies, we observed a significant decrease in trabecular microarchitectural indices within the L3 vertebral body in the control mice; however, this bone loss was attenuated in the B. adolescentis supplemented mice. Importantly, our study is consistent with previous reports and shows that the detrimental effects of fracture on the intact skeleton primarily affects the cancellous bone within the vertebral body rather than that of the femur, which likely stems from compensatory loading on the unfractured limb [6]. The bone formation biomarker P1NP was significantly higher at day 18 post-fracture in mice given the probiotic. Interestingly, at day 22 the control mice had higher levels of P1NP. Levels of P1NP naturally increase throughout secondary fracture healing as new bone is being formed, returning to baseline levels during the later remodeling stages (21–23 days post-fracture) of fracture healing [61]. This increase in P1NP in control mice may reflect recovery from this initial bone loss, which occurs by 4–6 weeks in young mice [6].
Intriguingly, the majority of the observed beneficial effects of B. adolescentis supplementation were only observed after fracture. Our findings are supported by previous studies that classify B. adolescentis as a bona fide symbiont that does not induce intestinal or systemic inflammation in a healthy host [62]. However, in the context of an inflammatory host response B. adolescentis can impart anti-inflammatory potential. For example, B. adolescentis was able to decelerate induction of intestinal inflammation in a dextran sodium sulfate-induced colitis model and led to a reduction in the Yersinia invasion-triggered IL-8 production by epithelial cells [22]. These data along with our study suggest that B. adolescentis requires an injury or assault to exert its anti-inflammatory effect; however, the mechanism by which B. adolescentis imparts these beneficial effects remains enigmatic. B. adolescentis has been shown to produce and secrete metabolites that can suppress NSAID-induced intestinal ulcer formation [63]. The production of post-biotics (e.g., lactate, formate and acetate) may be partly responsible for the observed effects. Both acetate and formate can improve tight junction barrier function by upregulating the expression of tight junction proteins [64]. Beyond these metabolites, B. adolescentis has been shown to metabolize various dietary phenolic compounds such as quercetin and fisetin into bioactive anti-inflammatory molecules [65, 66].
Another explanation for the observed effects during the post-fracture period may be attributable to changes in the microbial community structure or activity of resident microbes within the gut. In fact, it is theorized that the health benefits of probiotics are not directly a result of the strain consumed; rather, a result of interactions with the indigenous gut microbiota that can significantly alter the activity, without influencing the composition [67–69]. B. adolescentis is a lactic acid bacteria that can not only ferment dietary prebiotics into lactate but also partially breakdown dietary fibers which can support the growth of other populations of bacterial species through cross-feeding [70]. Furthermore, the metabolic interactions can lead to changes in the bacterial community landscape within the intestine and colon through changes in pH driven by fermentation products. In our study, we observed a general stability in the gut microbiome diversity during fracture healing in mice supplemented with B. adolescentis. We also observed an increase in the relative frequency of Clostridia, a major SCFA-producing class of bacteria, that may have contributed to accelerated fracture healing and bone-protective effects observed [71,72]. SCFAs, primarily butyrate, produced by these bacteria have important roles in maintaining and restoring intestinal barrier integrity by controlling the expression of tight junction proteins [73–77]. Butyrate also induces mucin production, which we observed in our study [78]. Although we did not assess SCFA levels in the present study it remains conceivable that the levels would be elevated in B. adolescentis supplemented mice. We also observed differences in several bacterial species in B. adolescentis supplemented mice, mainly the Gram-negative species Bacteroides ovatus. In inflammatory bowel disease (IBD) patients, Bacteroides ovatus was shown to be one the predominant commensal species that induces a systemic antibody response [79]. It also increases IgG and IgA production in mono-colonized germ-free mice [80,81]. B. ovatus may have exaggerated the inflammatory response to fracture in control mice, which was repressed with B. adolescentis supplementation. Ultimately, we cannot ascribe causality to these microbial changes and future work will need to experimentally determine the significance of these changes in the context of fracture healing. We also did not examine the changes at a later time-point to determine if the diversity would revert back to pre-fracture levels after successful fracture healing. I.e., if the effects were a transient response to femur fracture or long-lasting.
Whether the observed effects of B. adolescentis on fracture healing and the intact skeleton are specific to this species and strain remains to be determined. Currently, there are seven core genera of microbial organisms with identified probiotic properties, including Bifidobacterium, Lactobacillus, Saccharomyces, Enterococcus, Streptococcus, Bacillus, and Escherichia. Many of these species have demonstrated protective effects on bone mass in ovariectomy and glucocorticoid-induced osteoporosis models [15,82]. Thus, it is conceivable that one or more probiotic species may also have similar effects, especially considering that many probiotics influence health in a similar mechanism as B. adolescentis. In human studies, Lactobacillus casei Shirota was found to benefit pain and functional outcomes in elderly patients with radius and humerus fractures [17,18]. However, whether or not this probiotic supplement influences fracture healing remains unknown. Future studies are certainly warranted to determine if other probiotic species can elicit similar effects.
In summary, this study demonstrates that transiently altering the intestinal microbiota using probiotic supplements, such as B. adolescentis, can influence bone healing and limit the systemic pathologies induced by fracture. Collectively, our study argues that probiotic supplements may be a convenient, yet clinically relevant strategy to prevent the negative consequences of fracture-induced gut leakiness.
Supplementary Material
Funding
This work was supported by the National Institutes of Health-R21AG065977 (to H.D.), the Burroughs Wellcome Fund, and Emory University.
Footnotes
Declaration of Competing Interest
The authors declare that there are no conflicts of interests.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.biopha.2020.110831.
References
- [1].Marsell R, Einhorn TA, The biology of fracture healing, Injury 42 (2011) 551–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].McKibbin B, The biology of fracture healing in long bones, J. Bone Joint Surg. Br 60-B (1978) 150–162. [DOI] [PubMed] [Google Scholar]
- [3].Einhorn TA, Gerstenfeld LC, Fracture healing: mechanisms and interventions, Nat. Rev. Rheumatol 11 (2015) 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Roberts JL, Drissi H, Advances and promises of nutritional influences on natural bone repair, J. Orthop. Res 38 (2020) 695–707. [DOI] [PubMed] [Google Scholar]
- [5].Baht GS, Vi L, Alman BA, The role of the immune cells in fracture healing, Curr. Osteoporos. Rep 16 (2018) 138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Emami AJ, Toupadakis CA, Telek SM, Fyhrie DP, Yellowley CE, Christiansen BA, Age dependence of systemic bone loss and recovery following femur fracture in mice, J. Bone Miner. Res 34 (2019) 157–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Buzdon MM, Napolitano LM, Shi HJ, Ceresoli DM, Rauniya R, Bass BL, Femur fracture induces site-specific changes in T-cell immunity, J. Surg. Res 82 (1999) 201–208. [DOI] [PubMed] [Google Scholar]
- [8].Pfeifer R, Darwiche S, Kohut L, Billiar TR, Pape HC, Cumulative effects of bone and soft tissue injury on systemic inflammation: a pilot study, Clin. Orthop. Relat. Res 471 (2013) 2815–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Napolitano LM, Koruda MJ, Meyer AA, Baker CC, The impact of femur fracture with associated soft tissue injury on immune function and intestinal permeability, Shock 5 (1996) 202–207. [DOI] [PubMed] [Google Scholar]
- [10].Howard BM, Kornblith LZ, Christie SA, Conroy AS, Nelson MF, Campion EM, et al. , Characterizing the gut microbiome in trauma: significant changes in microbial diversity occur early after severe injury, Trauma Surg. Acute Care Open. 2 (2017), e000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Schepper JD, Collins FL, Rios-Arce ND, Raehtz S, Schaefer L, Gardinier JD, et al. , Probiotic Lactobacillus reuteri prevents postantibiotic bone loss by reducing intestinal dysbiosis and preventing barrier disruption, J. Bone Miner. Res 34 (2019) 681–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Collins FL, Rios-Arce ND, Schepper JD, Parameswaran N, McCabe LR, The potential of probiotics as a therapy for osteoporosis, Microbiol. Spectr (2017) 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Collins FL, Irwin R, Bierhalter H, Schepper J, Britton RA, Parameswaran N, et al. , Lactobacillus reuteri 6475 increases bone density in intact females only under an inflammatory setting, PLoS One 11 (2016), e0153180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Britton RA, Irwin R, Quach D, Schaefer L, Zhang J, Lee T, et al. , Probiotic L. reuteri treatment prevents bone loss in a menopausal ovariectomized mouse model, J. Cell. Physiol 229 (2014) 1822–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Li J-Y, Chassaing B, Tyagi AM, Vaccaro C, Luo T, Adams J, et al. , Sex steroid deficiency–associated bone loss is microbiota dependent and prevented by probiotics, J. Clin. Invest 126 (2016) 2049–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tyagi AM, Yu M, Darby TM, Vaccaro C, Li J-Y, Owens JA, et al. , The microbial metabolite butyrate stimulates bone formation via t regulatory cell- mediated regulation of WNT10B expression, Immunity 49 (2018) 1116–1131, e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhang C, Xue S, Wang Y, Yu D, Hua L, Guo C, et al. , Oral administration of Lactobacillus casei Shirota improves recovery of hand functions after distal radius fracture among elder patients: a placebo-controlled, double-blind, and randomized trial, J. Orthop. Surg. Res 14 (2019) 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lei M, Hua LM, Wang DW, The effect of probiotic treatment on elderly patients with distal radius fracture: a prospective double-blind, placebo-controlled randomised clinical trial, Benef. Microbes 7 (2016) 631–637. [DOI] [PubMed] [Google Scholar]
- [19].Picard C, Fioramonti J, Francois A, Robinson T, Neant F, Matuchansky C, Review article: bifidobacteria as probiotic agents – physiological effects and clinical benefits, Aliment. Pharmacol. Ther 22 (2005) 495–512. [DOI] [PubMed] [Google Scholar]
- [20].Arboleya S, Watkins C, Stanton C, Ross RP, Gut bifidobacteria populations in human health and aging, Front. Microbiol 7 (2016) 1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Turroni F, Peano C, Pass DA, Foroni E, Severgnini M, Claesson MJ, et al. , Diversity of bifidobacteria within the infant gut microbiota, PLoS One 7 (2012), e36957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Frick JS, Fink K, Kahl F, Niemiec MJ, Quitadamo M, Schenk K, et al. , Identification of commensal bacterial strains that modulate Yersinia enterocolitica and dextran sodium sulfate-induced inflammatory responses: implications for the development of probiotics, Infect. Immun 75 (2007) 3490–3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Guo Y, Xie JP, Deng K, Li X, Yuan Y, Xuan Q, et al. , Prophylactic effects of Bifidobacterium adolescentis on anxiety and depression-like phenotypes after chronic stress: a role of the gut microbiota-inflammation Axis, Front. Behav. Neurosci 13 (2019) 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Farr JN, Melton LJ 3rd., Achenbach SJ, Atkinson EJ, Khosla S, Amin S, Fracture Incidence and characteristics in young adults aged 18 to 49 years: a population-based study, J. Bone Miner. Res 32 (2017) 2347–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Soung DY, Talebian L, Matheny CJ, Guzzo R, Speck ME, Lieberman JR, et al. , Runx1 dose-dependently regulates endochondral ossification during skeletal development and fracture healing, J. Bone Miner. Res 27 (2012) 1585–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kaback LA, Soung DY, Naik A, Geneau G, Schwarz EM, Rosier RN, et al. , Teriparatide (1–34 human PTH) regulation of Osterix during fracture repair, J. Cell. Biochem 105 (2008) 219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Naik AA, Xie C, Zuscik MJ, Kingsley P, Schwarz EM, Awad H, et al. , Reduced COX-2 expression in aged mice is associated with impaired fracture healing, J. Bone Miner. Res 24 (2009) 251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Paglia DN, Diaz-Hernandez ME, Roberts JL, Kalinowski J, Lorenzo J, Drissi H, Deletion of Runx1 in osteoclasts impairs murine fracture healing through progressive woven bone loss and delayed cartilage remodeling, J. Orthop. Res (2019). [DOI] [PubMed] [Google Scholar]
- [29].Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Müller R, Guidelines for assessment of bone microstructure in rodents using micro–computed tomography, J. Bone Miner. Res 25 (2010) 1468–1486. [DOI] [PubMed] [Google Scholar]
- [30].Fernandes LM, Al-Dwairi A, Simmen RCM, Marji M, Brown DM, Jewell SW, et al. , Malic Enzyme 1 (ME1) is pro-oncogenic in Apc(Min/+) mice, SSci. Rep 8 (2018) 14268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, et al. , Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities, Appl. Environ. Microbiol 75 (2009) 7537–7541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].He W, Wang Y, Wang P, Wang F, Intestinal barrier dysfunction in severe burn injury, Burns Trauma 7 (2019) 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wrba L, Ohmann JJ, Eisele P, Chakraborty S, Braumuller S, Braun CK, et al. , Remote intestinal injury early after experimental polytrauma and hemorrhagic shock, Shock 52 (2019) e45–e51. [DOI] [PubMed] [Google Scholar]
- [34].Walsh DS, Thavichaigarn P, Dheeradhada C, Jiarakul N, Pearce FC, Wiesmann WP, et al. , Prolonged alteration in gut permeability following nonthermal injury, Injury 27 (1996) 491–494. [DOI] [PubMed] [Google Scholar]
- [35].Rao RK, Samak G, Protection and restitution of gut barrier by probiotics: nutritional and clinical implications, Curr. Nutr. Food Sci 9 (2013) 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ohland CL, Macnaughton WK, Probiotic bacteria and intestinal epithelial barrier function, Am. J. Physiol. Gastrointest. Liver Physiol 298 (2010) G807–19. [DOI] [PubMed] [Google Scholar]
- [37].Vester H, Huber-Lang MS, Kida Q, Scola A, van Griensven M, Gebhard F, et al. , The immune response after fracture trauma is different in old compared to young patients, Immun. Ageing 11 (2014) 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Plaza-Diaz J, Ruiz-Ojeda FJ, Gil-Campos M, Gil A, Mechanisms of action of probiotics, Adv. Nutr 10 (2019) S49–S66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Moore EE, Synergy of bone fractures, soft tissue disruption, and hemorrhagic shock in the genesis of postinjury immunochaos: the pathway to multiple organ failure, Crit. Care Med 26 (1998) 1305–1306. [DOI] [PubMed] [Google Scholar]
- [40].Guo W, Ding J, Huang Q, Jerrells T, Deitch EA, Alterations in intestinal bacterial flora modulate the systemic cytokine response to hemorrhagic shock, Am. J. Physiol 269 (1995) G827–32. [DOI] [PubMed] [Google Scholar]
- [41].Levy RM, Prince JM, Yang R, Mollen KP, Liao H, Watson GA, et al. , Systemic inflammation and remote organ damage following bilateral femur fracture requires Toll-like receptor 4, Am. J. Physiol. Regul. Integr. Comp. Physiol 291 (2006) R970–6. [DOI] [PubMed] [Google Scholar]
- [42].Faries PL, Simon RJ, Martella AT, Lee MJ, Machiedo GW, Intestinal permeability correlates with severity of injury in trauma patients, J. Trauma 44 (1998) 1031–1035, discussion 5–6. [DOI] [PubMed] [Google Scholar]
- [43].Reichold A, Brenner SA, Spruss A, Forster-Fromme K, Bergheim I, Bischoff SC, Bifidobacterium adolescentis protects from the development of nonalcoholic steatohepatitis in a mouse model, J. Nutr. Biochem 25 (2014) 118–125. [DOI] [PubMed] [Google Scholar]
- [44].Van Itallie CM, Fanning AS, Bridges A, Anderson JM, ZO-1 stabilizes the tight junction solute barrier through coupling to the perijunctional cytoskeleton, Mol. Biol. Cell 20 (2009) 3930–3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Weber CR, Nalle SC, Tretiakova M, Rubin DT, Turner JR, Claudin-1 and claudin-2 expression is elevated in inflammatory bowel disease and may contribute to early neoplastic transformation, Lab. Invest 88 (2008) 1110–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Zeissig S, Burgel N, Gunzel D, Richter J, Mankertz J, Wahnschaffe U, et al. , Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease, Gut 56 (2007) 61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Suzuki T, Yoshinaga N, Tanabe S, Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium, J. Biol. Chem 286 (2011) 31263–31271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Al-Sadi R, Ye D, Boivin M, Guo S, Hashimi M, Ereifej L, et al. , Interleukin-6 modulation of intestinal epithelial tight junction permeability is mediated by JNK pathway activation of claudin-2 gene, PLoS One 9 (2014), e85345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Mankertz J, Amasheh M, Krug SM, Fromm A, Amasheh S, Hillenbrand B, et al. , TNFalpha up-regulates claudin-2 expression in epithelial HT-29/B6 cells via phosphatidylinositol-3-kinase signaling, Cell Tissue Res. 336 (2009) 67–77. [DOI] [PubMed] [Google Scholar]
- [50].Weber CR, Raleigh DR, Su L, Shen L, Sullivan EA, Wang Y, et al. , Epithelial myosin light chain kinase activation induces mucosal interleukin-13 expression to alter tight junction ion selectivity, J. Biol. Chem 285 (2010) 12037–12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Van Itallie CM, Holmes J, Bridges A, Gookin JL, Coccaro MR, Proctor W, et al. , The density of small tight junction pores varies among cell types and is increased by expression of claudin-2, J. Cell. Sci 121 (2008) 298–305. [DOI] [PubMed] [Google Scholar]
- [52].Birchenough GM, Johansson ME, Gustafsson JK, Bergstrom JH, Hansson GC, New developments in goblet cell mucus secretion and function, Mucosal Immunol. 8 (2015) 712–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Dupont A, Heinbockel L, Brandenburg K, Hornef MW, Antimicrobial peptides and the enteric mucus layer act in concert to protect the intestinal mucosa, Gut Microbes 5 (2014) 761–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Hasnain SZ, Tauro S, Das I, Tong H, Chen AC, Jeffery PL, et al. , IL-10 promotes production of intestinal mucus by suppressing protein misfolding and endoplasmic reticulum stress in goblet cells, Gastroenterology 144 (2013) 357–368, e9. [DOI] [PubMed] [Google Scholar]
- [55].Ngkelo A, Meja K, Yeadon M, Adcock I, Kirkham PA, LPS induced inflammatory responses in human peripheral blood mononuclear cells is mediated through NOX4 and Gialpha dependent PI-3kinase signalling, J Inflamm (Lond) 9 (2012) 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Yucel G, Zhao Z, El-Battrawy I, Lan H, Lang S, Li X, et al. , Lipopolysaccharides induced inflammatory responses and electrophysiological dysfunctions in human-induced pluripotent stem cell derived cardiomyocytes, Sci. Rep 7 (2017) 2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Behrends DA, Hui D, Gao C, Awlia A, Al-Saran Y, Li A, et al. , Defective bone repair in C57Bl6 mice with acute systemic inflammation, Clin. Orthop. Relat. Res 475 (2017) 906–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Reikeras O, Shegarfi H, Wang JE, Utvag SE, Lipopolysaccharide impairs fracture healing: an experimental study in rats, Acta Orthop. 76 (2005) 749–753. [DOI] [PubMed] [Google Scholar]
- [59].Christiansen BA, Harrison SL, Fink HA, Lane NE, Study of Osteoporotic Fractures Research G. Incident fracture is associated with a period of accelerated loss of hip BMD: the Study of Osteoporotic Fractures, Osteoporos. Int 29 (2018) 2201–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Fischer V, Haffner-Luntzer M, Prystaz K, Vom Scheidt A, Busse B, Schinke T, et al. , Calcium and vitamin-D deficiency marginally impairs fracture healing but aggravates posttraumatic bone loss in osteoporotic mice, Sci. Rep 7 (2017) 7223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Coulibaly MO, Sietsema DL, Burgers TA, Mason J, Williams BO, Jones CB, Recent advances in the use of serological bone formation markers to monitor callus development and fracture healing, Crit. Rev. Eukaryot. Gene Expr 20 (2010) 105–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Tan TG, Sefik E, Geva-Zatorsky N, Kua L, Naskar D, Teng F, et al. , Identifying species of symbiont bacteria from the human gut that, alone, can induce intestinal Th17 cells in mice, Proc. Natl. Acad. Sci 113 (2016) E8141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Kinouchi T, Kataoka K, Bing SR, Nakayama H, Uejima M, Shimono K, et al. , Culture supernatants of Lactobacillus acidophilus and Bifidobacterium adolescentis repress ileal ulcer formation in rats treated with a nonsteroidal antiinflammatory drug by suppressing unbalanced growth of aerobic bacteria and lipid peroxidation, Microbiol. Immunol 42 (1998) 347–355. [DOI] [PubMed] [Google Scholar]
- [64].Hsieh CY, Osaka T, Moriyama E, Date Y, Kikuchi J, Tsuneda S, Strengthening of the intestinal epithelial tight junction by Bifidobacterium bifidum, Physiol. Rep (2015) 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Kawabata K, Sugiyama Y, Sakano T, Ohigashi H, Flavonols enhanced production of anti-inflammatory substance(s) by Bifidobacterium adolescentis: prebiotic actions of galangin, quercetin, and fisetin, Biofactors. 39 (2013) 422–429. [DOI] [PubMed] [Google Scholar]
- [66].Kawabata K, Baba N, Sakano T, Hamano Y, Taira S, Tamura A, et al. , Functional properties of anti-inflammatory substances from quercetin-treated Bifidobacterium adolescentis, Biosci. Biotechnol. Biochem 82 (2018) 689–697. [DOI] [PubMed] [Google Scholar]
- [67].Cani PD, Van Hul M, Novel opportunities for next-generation probiotics targeting metabolic syndrome, Curr. Opin. Biotechnol 32 (2015) 21–27. [DOI] [PubMed] [Google Scholar]
- [68].Scott KP, Antoine JM, Midtvedt T, van Hemert S, Manipulating the gut microbiota to maintain health and treat disease, Microb. Ecol. Health Dis 26 (2015) 25877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Eloe-Fadrosh EA, Brady A, Crabtree J, Drabek EF, Ma B, Mahurkar A, et al. , Functional dynamics of the gut microbiome in elderly people during probiotic consumption, mBio. (2015) 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Belenguer A, Duncan SH, Calder AG, Holtrop G, Louis P, Lobley GE, et al. , Two routes of metabolic cross-feeding between Bifidobacterium adolescentis and butyrate-producing anaerobes from the human gut, Appl. Environ. Microbiol 72 (2006) 3593–3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Lopetuso LR, Scaldaferri F, Petito V, Gasbarrini A, Commensal Clostridia: leading players in the maintenance of gut homeostasis, Gut Pathog. 5 (2013) 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Ilinskaya ON, Ulyanova VV, Yarullina DR, Gataullin IG, Secretome of intestinal Bacilli: a natural guard against pathologies, Front. Microbiol 8 (2017) 1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Gonzalez A, Krieg R, Massey HD, Carl D, Ghosh S, Gehr TWB, et al. , Sodium butyrate ameliorates insulin resistance and renal failure in CKD rats by modulating intestinal permeability and mucin expression, Nephrol. Dial. Transplant 34 (2019) 783–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Feng W, Wu Y, Chen G, Fu S, Li B, Huang B, et al. , Sodium butyrate attenuates diarrhea in weaned piglets and promotes tight junction protein expression in Colon in a GPR109A-Dependent manner, Cell. Physiol. Biochem 47 (2018) 1617–1629. [DOI] [PubMed] [Google Scholar]
- [75].Hu ED, Chen DZ, Wu JL, Lu FB, Chen L, Zheng MH, et al. , High fiber dietary and sodium butyrate attenuate experimental autoimmune hepatitis through regulation of immune regulatory cells and intestinal barrier, Cell. Immunol 328 (2018) 24–32. [DOI] [PubMed] [Google Scholar]
- [76].Matheus VA, Monteiro L, Oliveira RB, Maschio DA, Collares-Buzato CB, Butyrate reduces high-fat diet-induced metabolic alterations, hepatic steatosis and pancreatic beta cell and intestinal barrier dysfunctions in prediabetic mice, Exp. Biol. Med. (Maywood) 242 (2017) 1214–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Han X, Song H, Wang Y, Sheng Y, Chen J, Sodium butyrate protects the intestinal barrier function in peritonitic mice, Int. J. Clin. Exp. Med 8 (2015) 4000–4007. [PMC free article] [PubMed] [Google Scholar]
- [78].Cornick S, Tawiah A, Chadee K, Roles and regulation of the mucus barrier in the gut, Tissue Barriers 3 (2015), e982426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Saitoh S, Noda S, Aiba Y, Takagi A, Sakamoto M, Benno Y, et al. , Bacteroides ovatus as the predominant commensal intestinal microbe causing a systemic antibody response in inflammatory bowel disease, Clin. Diagn. Lab. Immunol 9 (2002) 54–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Shiba T, Aiba Y, Ishikawa H, Ushiyama A, Takagi A, Mine T, et al. , The suppressive effect of bifidobacteria on Bacteroides vulgatus, a putative pathogenic microbe in inflammatory bowel disease, Microbiol. Immunol 47 (2003) 371–378. [DOI] [PubMed] [Google Scholar]
- [81].Yang C, Mogno I, Contijoch EJ, Borgerding JN, Aggarwala V, Li Z, et al. , Fecal IgA levels are determined by strain-level differences in Bacteroides ovatus and are modifiable by gut microbiota manipulation, Cell Host Microbe. (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Schepper JD, Collins F, Rios-Arce ND, Kang HJ, Schaefer L, Gardinier JD, et al. , Involvement of the gut microbiota and barrier function in glucocorticoid-induced osteoporosis, J. Bone Miner. Res (2019). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.