Abstract
Phthalimides have diverse bioactivities and are attractive molecules for drug discovery and development. Here, we explored new synthesized phthalimide derivatives (compounds 1–3) in improving memory impairment associated with Alzheimer’s disease (AD), using in vitro and ex vivo acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE) inhibition and in vivo models, including Y-maze test and novel object recognition test (NORT). Compounds 1–3 exhibited significant AChE activity with IC50 values of 10, 140, and 18 μM and BuChE with IC50 values of 80, 50, and 11 μM, respectively. All compounds 1–3 showed excellent antioxidant potential in DPPH and ABTS assays with IC50 values in the range of 105–340 and 205–350 μM, respectively. In ex vivo studies, compounds 1–3 also significantly inhibited both enzymes in a concentration-dependent manner along with significant antioxidant activities. In in vivo studies, compounds 1–3 reversed scopolamine-induced amnesia as indicated by a significant increase in the spontaneous alternation in the Y-maze test and an increase in the discrimination index in the NORT. Molecular docking was also conducted for compounds 1–3 against AChE and BuChE, which showed that compounds 1 and 3 have excellent binding with AChE and BuChE as compared to 2. These findings suggest that compounds 1–3 possess significant antiamnesic potential and may serve as useful leads to develop novel therapeutics for the symptomatic management and treatment of AD.
1. Introduction
Alzheimer’s disease (AD) is a neurodegenerative disease of the brain and is one of the foremost public health issues all over the world. It has a major health, financial, and social burden on society.1,2 It is predicted that 36 million people are affected with AD or some other kinds of dementia, and around 65 million people are projected to be affected by AD in 2030, and in 2050, this number will almost double.3 AD has affected more than 3 million people in the USA alone and is the leading cause of death in America.4 Hallucinations and delusion have been described as the most common symptoms in patients who are affected by this disease and the presence of these symptoms can lead to prompt institutionalization.5
Age is the main risk factor in the development of AD and is a prominent reason for mental damage in old aged people.6 The risk of AD increases with age, and after 65 years, the ratio almost doubles after every 5 years.3 Family history is the second most important risk factor for AD following advanced age. Genetic factors play a role in approximately 80% of AD cases as shown by twin and family studies. It is believed to be a genetically dichotomous disease, involving an early-onset (<60 years) familial type and late-onset (≥60 years) AD or LOAD.7 The familial AD is caused by rare mutations in PSEN1, PSEN2, and APP(8) and accounts for less than 10% of all AD cases, whereas common gene polymorphisms, such as ε4 and ε2 variants of the APOE gene, can influence susceptibility for the most predominant type of AD, late-onset AD. The “sporadic” AD strongly involves genetic changes combined with life exposure factors.7
Numerous pieces of evidence propose that cerebrovascular diseases (CVDs) also play an important role in the progression and development of AD as both AD and CVD are associated with increasing age and are among the leading causes of death. Individuals who are suffering from stroke, coronary heart disease, heart failure, hypertension, and diabetes have the greatest risk of developing AD9 as they all increase the risk of atherosclerosis.
Cholinergic deficiency with loss of cholinergic neurons in AD appears to be an important factor in producing dementia. AD is categorized by progressive damage in cognitive functions and loss of memory.10,11 Studies of the brain, hippocampus, and neurocortex have shown the presence of numerous different neuropathological changes, comprising intracellular neurofibrillary tangles containing abnormally hyperphosphorylated protein, extracellular â-amyloid (Aâ) containing plaques, and also the disintegration of cholinergic neurons of the basal forebrain.12
In the past 20 years, many studies have been carried out to recognize the molecular pathogenesis of AD and to provide background to improve actual pharmacological cures. The main therapeutic strategy involves the improvement of the central cholinergic function, which increases the level of acetylcholine in the brain. These include the drugs that inhibit the acetylcholinesterase (AChE) enzyme, such as rivastigmine, tacrine, and galantamine, and recently, donepezil has been introduced in the market for the symptomatic treatment of AD.13
Phthalimide derivatives are very significant as they possess numerous biological activities, including anti-inflammatory, anticancer, and anticonvulsant.14 Recently, phthalimide derivatives have been reported to possess potential anticholinesterase activity.15,16 Compounds based on the phthalimide structure have similar pharmacophoric portions like indanone ring of the donepezil and thus can act as peripheral binding site inhibitors of AChE and BuChE. In the current investigation, we focused on the design and synthesis of novel anticholinesterase inhibitors with a phthalimide-based structure (Figure 1) to obtain more active analogues as potential therapeutics for the symptomatic management of AD. We evaluated the AChE and BuChE inhibitory potential of compounds 1–3 (Figure 1) using in vitro and ex vivo assays. The in silico binding mode of phthalimide ligands in the binding sites of enzymes in comparison with donepezil as a reference drug by docking procedure was also assessed. Furthermore, the proposed ligands were evaluated for their memory enhancement effects using scopolamine-induced mouse amnesic models by conducting Y-maze test and NORT.
Figure 1.
Chemical structures of the synthesized compounds of phthalimide derivatives: 2-(4-(trifluoromethyl)phenyl)isoindoline-1,3-dione (1), 2-(4-bromophenyl)isoindoline-1,3-dione (2), and 2-(2,4-dimethylphenyl)isoindoline-1,3-dione (3).
2. Materials and Methods
2.1. Chemicals
Acetylcholine iodide, butyrylcholine iodide, 5,5-dithio-bis-nitrobenzoic acid (DTNB), electric eel acetylcholinesterase (type-VI-S), equine butyrylcholinesterase, 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2′-azinobis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS), HPLC-grade methanol, donepezil, and scopolamine were purchased from Sigma-Aldrich. All chemicals and solvents used were of analytical grades.
2.2. Chemistry
2.2.1. General
All of the chemicals were purchased from Sigma Chemical Company. Silica gel 60H with particle size 5–40 μM was obtained from Merck and used for column chromatography (CC), whereas precoated silica (0.25 mm, 60F254) aluminum-backed Merck plates were used for thin-layer chromatography (TLC). 1H NMR and 13C NMR spectra were recorded on a Varian Gemini spectrometer (Palo Alto, California) (1H, 400 MHz; 13C, 100 MHz) in CDCl3 (Sigma-Aldrich) with tetramethylsilane as an internal standard. Mass spectra were carried out on a Thermo Finnigan (Waltham, Massachusetts) PolarisQ Ion Trap system using a direct exposure probe.
2.3. General Procedure for the Synthesis of Compound Phthalimide Derivatives 1–3
In a 100 mL round bottom flask, 1 equiv of phthalic anhydride and 1 equiv of amine were placed provided with a reflux condenser (Scheme 1). Forty milliliters of glacial acetic acid was added as a solvent. The mixture of reaction was refluxed with stirring for 1 h at(room temp) so that imide was synthesized. Glacial acetic acid was eliminated by extracting the mixture of reaction with water, ethyl acetate, and chloroform. The crude product was purified using column chromatography giving a yield of 94–96%.
Scheme 1. Synthesis of Phthalimide Derivatives 1–3.
2.3.1. 2-(4-(Trifluoromethyl)phenyl)isoindoline-1,3-dione (1)
White solid. 1H NMR (CDCl3) δ ppm: 8.07 ddd (J = 7.80, 1.32, 0.55 Hz, 2H), 7.95 ddd (7.80, 7.55, 1.33 Hz, 2H), 7.61 ddd (8.22, 1.9, 0.53 Hz, 2H), 7.39 ddd (8.22, 1.57, 0.53 Hz, 2H). 13C NMR (CDCl3) δ ppm: 167.2, 139.3, 135.5, 131.8, 127.1, 125.1, 124.9, 123.2, 113.4. HRMS (ESI) [MH]+ calcd for C15H9F3NO2, 292.2306; found, 292.2302.
2.3.2. 2-(4-Bromophenyl)isoindoline-1,3-dione (2)
White solid. 1H NMR (CDCl3) δ ppm: 8.08 ddd (J = 7.81, 1.31, 0.54 Hz, 2H), 7.97 ddd (7.80, 7.78, 1.31 Hz, 2H), 7.52 ddd (8.24, 1.70, 0.55 Hz, 2H), 7.46 ddd (.23, 1.43, 0.56 Hz, 2H). 13C NMR (CDCl3) δ ppm: 167.4, 135.3, 133.3. 132.0, 130.0, 127.6, 123.1, 119.3. HRMS (ESI) [MH]+ calcd for C14H9BrNO2, 303.1287; found, 3031282.
2.3.3. 2-(2,4-Dimethylphenyl)isoindoline-1,3-dione (3)
White solid. 1H NMR (CDCl3) δ ppm: 8.05 ddd (J = 7.82, 1.31, 0.55 Hz, 2H), 7.94 ddd (7.80, 7.58, 1.33 Hz, 2H), 7.14 dd (8.21, 1.25 Hz, 1H), 7.05 dd (8.21, 1.25 Hz, 1H), 6.97 dd (.1.25, 0.55 Hz, 1H), 2.23 (s, 3H), 2.20 (s, 3H). 13C NMR (CDCl3) δ ppm: 167.1, 134.9, 133.8, 131.5, 129.1, 128.9, 127.4, 123.1, 122.4, 20.5, 17.1. HRMS (ESI) [MH]+ calcd for C16H14NO2 252.2351, found 252.2346.
2.4. DPPH Free Radical Scavenging Assay
DPPH free radical was evaluated via the previously described procedure.17 Different dilutions of synthesized compounds were prepared, and 0.1 mL of each solution was added to the methanolic (0.004%) solution of DPPH. The solution mixture was incubated for 30 min. After incubation, absorbance was determined at 517 nm using a UV spectrophotometer. The percent % scavenging activity was estimated by the following formula
2.5. ABTS Scavenging Assay
The capability of various phthalimide derivatives to scavenge a 2,2-azino-bis-(ethylbenzthiozoline-6-sulfonic acid) radical cation was measured. The radical cation was prepared by mixing 2.45 mM potassium persulfate and 7 mM ABTS stock solution and allowed the reaction to complete for 4–16 h. The ABTS solution was diluted with ethanol for absorbance at 734 nm. Then, 0.9 mL of ABTS solution and 0.1 mL of 100 and 200 μM tested samples were mixed for 45 s. Measurement was noted at 734 nm after 15 min. The antioxidant potential of the tested samples was calculated using the following formula17
where Ac and At are respective absorbance of ABTS and tested samples.
2.6. Enzyme Inhibition of Acetylcholinesterase and Butyrylcholinesterase
The AChE inhibitory potential of the phthalimide derivatives was evaluated by Ellman’s colorimetric method. Initially, 20 μL of tested solution (0.25–1 mM), 40 μL of 0.02 U/mL AChE, and 1900 μL of 50 mM Tris–HCl buffer (pH 8.0) were blended for 30 min at 4 °C preincubation period. The response arose with the addition of 20 μL of 12 mM ATChI and 20 μL of 10 mM DTNB. Acetyl and BuChE activities were determined spectrophotometrically by calculating the alteration in UV absorbance of the test solution at 413 nm for a period of 10 min at 25 °C. Galantamine was used as a positive control (0.03–1 mM). To calculate enzyme inhibition, the percentage of enzymatic activity was compared in the absence and presence of an inhibitor. The experiment was carried out in triplicate.18
2.7. Animals
A set of 72 healthy Swiss male albino mice weighing between 22–30 g was taken from the National Institutes of Health, Islamabad, Pakistan, and kept in the animal house of the Department of Pharmacy, University of Malakand. The mice were segregated into groups of six in individual cages made from stainless steel with softwood shavings as bedding, provided with water and a normal pellet diet, and maintained under normal laboratory conditions, such as temperature, humidity, and 12 h light–dark cycle. Activities were carried out, according to the accepted guidelines of the Animal (Scientific Procedures) Act UK 1986.19 Each experimental trial was carried out in strict compliance with the approved procedures (DAEC/PHARM/2012/15) by the Department Ethics Committee based on the Animals (Scientific Procedures) Act 1986.
2.8. Acute Toxicity Studies
Acute toxicity of compounds 1–3 was measured at the doses of 100 and 200 mg/kg using mice. Animals were observed for initial 4 h for any major changes in their bodies, including tremors, diarrhea, sleep, lethargy, salivation, behavioral patterns, and effects on eyes, skin, and fur. Animals were observed for 48 h to investigate any gross changes and mortality in the animals.20,21
2.9. In Vivo Studies
2.9.1. Experimental Design
Mice weighing 24 ± 4 g were used for in vivo activities. Animals were divided into 12 separate groups from groups I–XII. Each group comprises eight mice (n = 8). Groups I–III received normal saline, scopolamine, and scopolamine plus donepezil, respectively. Groups IV–VI received compound 1 at the doses of 1, 10, and 30 mg/kg plus scopolamine, groups VII–IX received compound 2 at the doses of 1, 10, and 30 mg/kg plus scopolamine, and groups X–XII were given compound 3 at the doses of 1, 10, and 30 mg/kg plus scopolamine.
2.9.2. Y-Maze Spontaneous Alternations
To evaluate the short-term memory-enhancing effect and exploratory activity, the Y-maze model was used to evaluate the effect of the synthesized compounds on memory and locomotor activities. The Y-maze task is widely used to measure spatial working through the spontaneous alternation of behavior. Y-maze consists of three equal arms about 20 cm long, 6 cm wide, and 16 cm high and converges at an equal angle. Every mouse is released in one arm of the maze and permits it to freely move for 5 min. The maze was explored in the mice systematically due to entering every arm in turn. The capability to change the need that the mice knew what arm they have visited before. The chain of arm entrances and returns that are possible into the previous arm were visually recorded. Change is referred to as a number of consecutive entrances into all three arms on intersecting triplet sets. The percentage ratio of change is calculated as compared to the actual change ratio, which is defined as the total number of arm entrances minus two and then multiplied by a hundred.22,23
The number of alternations means the continuous entries into three different arms in overlapping triplet sets (e.g., ABCBACA = 3). Total arm entries are simply the total number of arms entered (e.g., ABCBACA = 7). The percentage of alternation (% alteration) was calculated using the following formula
2.9.3. Novel Object Recognition Test (NORT)
The potential of phthalimide derivatives to increase animal working memory and long-term memory was evaluated by NORT.17 It consists of a 40 cm wide, 40 cm long, and 66 cm high box. NORT involves a 2-day habituation, 2 days training period, and then a testing period. The habituation period involves an empty box, whereas two identical objects were placed at the same position in two corners of the box during the sample phase. In the test session, one object is replaced with one novel object at the time of testing. For STM, the test phase was performed 5 min after the sample phase. Five days of washout period before the NORT was provided to the mice for the assessment of their long-term memory. The process was like the STM process, except that the mice will be released in the test phase after 24 h of exposure to the sample phase. The time to explore every object by mice in every phase was manually recorded by a stopwatch. All of the sessions were also videotaped for double-checking of data by an independent observer. When the mouse positioned its head in the direction of the object (with a distance of 2 cm) or made contact via nose with the object, the mouse was declared as exploring the object.
2.9.4. Ex Vivo Analysis for AChE and BuChE Activities
The ex vivo analysis of brain BuChE was carried out according to the method described previously.24 All mice were euthanized by cervical dislocation at the end of NORT, and the whole-brain samples were dissected out for further studies. Brain tissues were homogenized in 10 volumes of ice-cold 20 mM phosphate buffer (pH 7.4) containing EDTA (1 mM) and phenylmethylsulfonyl fluoride (1 mM). The homogenates were centrifuged at 8000g for 15 min, and the supernatant was collected for biochemical analysis. Total AChE and BuChE activities were measured as triplicate in aliquots of brain homogenate using Ellman assays, and the enzymatic inhibition is expressed as the percentage of the control.
2.10. Molecular Docking
Molecular docking was conducted on a molecular operating environment (MOE) docking suite [www.chemcomp.com]. The three-dimensional (3D) structure of AChE in complex with the drug Aricept (donepezil) was downloaded from the RCSB protein databank (PDB code: 1EVE). We identified six water molecules (wat1158, wat1159, wat1160, wat1161, wat1249, wat111254) previously that play an important role in protein–ligand interaction. These water molecules were retained while the rest were removed from the protein file. Hydrogen atoms were added to residues, and water molecules of protein and partial charges were calculated by the MMFF94x force field. The protonation state of all atoms was set by protonating 3D command of MOE. The 2D coordinates of compounds 1–3 were prepared by Chemdraw and converted into 3D form by MOE wash module, which adds hydrogen on ligand atoms, calculates partial charges of each atom of ligand, and minimizes the structure until the root mean square (RMS) gradient reaches to 0.1 kcal/mol/A2. For BuChE, the crystal structure of human BuChE in complex with the drug rivastigmine (PDB code: 5DYT) was chosen. Water molecules (wat703 and wat812) form interactions with the ligand moiety and therefore retained in the protein file during docking, while the rest of the water molecules were removed. The protein file was prepared as mentioned above. Docking was carried out with the Triangle matcher docking algorithm and LondondG scoring function. The force field refinement method was used with the GBVI/WSA dG rescoring method.
3. Results
3.1. Acute Toxicity Studies
Pharmacological evaluation is important to study various bioactivities of synthesized compounds. Acute toxicity study is crucial because numerous compounds possess potential therapeutic effects, however, their toxic nature may lose their therapeutic purposes. In our experiment, all three synthesized compounds possess a safety profile of up to 200 mg/kg, where no mortality and behavioral changes were noted in the animals.
3.2. Free Radical Scavenging Assay
3.2.1. DPPH Scavenging Assay
DPPH free radical scavenging potential of compound 1 showed an IC50 value of 105 μM. Similarly, compound 3 showed was found to exert the DPPH free radical scavenging activity at an IC50 value of 150 μM (Table 1) However, compound 2 demonstrated the least activity with 69.87 ± 0.23 inhibition potential at 1000 μM and an IC50 value of 340 μM. Ascorbic acid was used as a positive control, which exhibited IC50 = 63 μM against DPPH.
Table 1. Percent DPPH and ABTS Free Radical Scavenging Activity of Compounds 1–3a.
| sample | concentration (μM) | % DPPH scavenging mean ± SEM | IC50 (μM) | % ABTS scavenging mean ± SEM | IC50 (μM) |
|---|---|---|---|---|---|
| compound 1 | 1000 | 85.87 ± 0.23 | 105 | 70.55 ± 0.32 | 205 |
| 500 | 76.65 ± 0.30 | 66.65 ± 0.61 | |||
| 250 | 67.85 ± 0.18 | 49.72 ± 0.72 | |||
| 125 | 52.16 ± 0.15 | 42.10 ± 1.11 | |||
| 62.5 | 46.03 ±. 032 | 28.21 ± 0.32 | |||
| 31.25 | 30.21 ± 0.17 | 11.47 ± 0.54 | |||
| compound 2 | 1000 | 69.87 ± 0.23 | 340 | 62.57 ± 0.50 | 350 |
| 500 | 56.65 ± 0.30 | 57.76 ± 0.75 | |||
| 250 | 47.85 ± 0.17 | 41.72 ± 0.81 | |||
| 125 | 40.16 ± 0.14 | 34.11 ± 1.21 | |||
| 62.5 | 30.03 ±. 031 | 22.12 ± 0.32 | |||
| 31.25 | 15.21 ± 0.19 | 10.47 ± 0.25 | |||
| compound 3 | 1000 | 80.87 ± 0.25 | 150 | 73.57 ± 0.41 | 220 |
| 500 | 77.65 ± 0.31 | 68.76 ± 0.72 | |||
| 250 | 55.85 ± 0.18 | 54.72 ± 0.80 | |||
| 125 | 40.16 ± 0.15 | 46.11 ± 1.21 | |||
| 62.5 | 35.03 ±. 032 | 35.22 ± 0.50 | |||
| 31.25 | 25.21 ± 0.15 | 18.47 ± 0.83 | |||
| ascorbic acid | 1000 | 85.08 ± 0.32 | 61 | 82.14 ± 0.19 | 125 |
| 500 | 74.44 ± 0.48 | 73.22 ± 0.21 | |||
| 250 | 65.19 ± 0.29 | 63.11 ± 1.22 | |||
| 125 | 52.56 ± 0.69 | 51.89 ± 0.59 | |||
| 62.5 | 47.65 ± 0.2a | 44.58 ± 1.11 | |||
| 31.25 | 29.20 ± 1.03 | 35.27 ± 0.67 |
Data are given as mean ± SEM (n = 3).
3.2.2. ABTS Scavenging Assay
In ABTS free radical scavenging assay, compound 1 showed ABTS inhibition with an IC50 value of 205 μM. The IC50 values of compounds 2 and 3 were found to be 350 and 220 μM, respectively. The activity of compounds 1–3 was comparable to the inhibition of ascorbic acid (positive control), which exhibited IC50 = 125 μM and displayed a response dependent on concentration. The results are tabulated in Table 1.
3.3. AChE and BuChE Inhibitory Potential of Compounds 1–3 at Different Concentrations
Table 2 shows the % inhibition of compounds 1–3 against AChE and BuChE with their IC50 values. All three compounds were used at different concentrations. At 1000 μM, compound 1 exhibited maximum inhibitory potential (98.51 ± 0.65) for AChE, while compound 3 exhibited 95.70 ± 0.12% inhibition against AChE, whereas compound 2 was found to be least active at this dose (66.70 ± 5.14% inhibition). Donepezil was used as a positive control (% inhibition = 97.31 ± 0.5 at 1000 μM), whose activity gradually decreased with the decreasing concentration. Against BuChE, the % inhibition of compound 3 (% inhibition = 87.29 ± 0.03) was higher than compound 2 (% inhibition = 78.20 ± 0.07), which was more potent than compound 1 (% inhibition = 75.38 ± 1.39). At 31.25 μM, compound 3 showed 65.25 ± 0.75% inhibition of AChE and 59.49 ± 0.11% inhibition of BChE as compared to donepezil, which shows 74.31 ± 0.04% inhibition at this concentration. Compounds 1 and 3 showed higher inhibition of AChE as compared to BuChE, whereas compound 2 exhibited higher potency for BuChE than AChE. For AChE, the order of activity of the three compounds was 1 > 3 > 2, while the order of activity against BuChE was 3 > 2 > 1.
Table 2. % Inhibition of AChE and BuChE by Compounds 1–3 at Different Concentrationsa.
| samples name | concentration (μM) | % AChE inhibition mean ± SEM | IC50 (μM) | % BuChE inhibition mean ± SEM | IC50 (μM) |
|---|---|---|---|---|---|
| compound 1 | 1000 | 98.51 ± 0.65 | 10 | 75.38 ± 1.39 | 80 |
| 500 | 81.44 ± 0.29 | 67.39 ± 0.89 | |||
| 250 | 84.39 ± 1.39 | 61.76 ± 0.49 | |||
| 125 | 77.39 ± 0.90 | 54.29 ± 0.19 | |||
| 62.5 | 61.77 ± 0.49 | 47.29 ± 0.12 | |||
| 31.25 | 55.68 ± 0.52 | 41.39 ± 0.07 | |||
| compound 2 | 1000 | 66.70 ± 5.14 | 140 | 78.20 ± 0.07 | 50 |
| 500 | 61.1 ± 1.95 | 71.25 ± 0.12 | |||
| 250 | 54.33 ± 0.22 | 64.36 ± 0.03 | |||
| 125 | 47.14 ± 0.09 | 56.28 ± 0.03 | |||
| 62.5 | 41.35 ± 0.13 | 51.34 ± 0.03 | |||
| 31.25 | 38.36 ± 0.22 | 45.45 ± 0.22 | |||
| compound 3 | 1000 | 95.24 ± 0.12 | 18 | 87.29 ± 0.03 | 11 |
| 500 | 91.47 ± 0.26 | 82.4 ± 0.06 | |||
| 250 | 84.18 ± 0.10 | 75.65 ± 0.03 | |||
| 125 | 77.32 ± 0.21 | 69.37 ± 0.02 | |||
| 62.5 | 52.46 ± 0.92 | 63.41 ± 0.14 | |||
| 31.25 | 65.25 ± 0.75 | 59.49 ± 0.11 | |||
| donepezil | 1000 | 97.31 ± 0.5 | 5.15 | 91.29 ± 0.06 | 3.89 |
| 500 | 93.52 ± 0.12 | 86.42 ± 0.11 | |||
| 250 | 89.40 ± 0.11 | 82.49 ± 0.08 | |||
| 125 | 85.40 ± 0.06 | 78.39 ± 0.06 | |||
| 62.5 | 79.36 ± 0.08 | 74.42 ± 0.06 | |||
| 31.25 | 74.31 ± 0.04 | 69.31 ± 0.06 |
All values were expressed as mean ± SEM (n = 3).
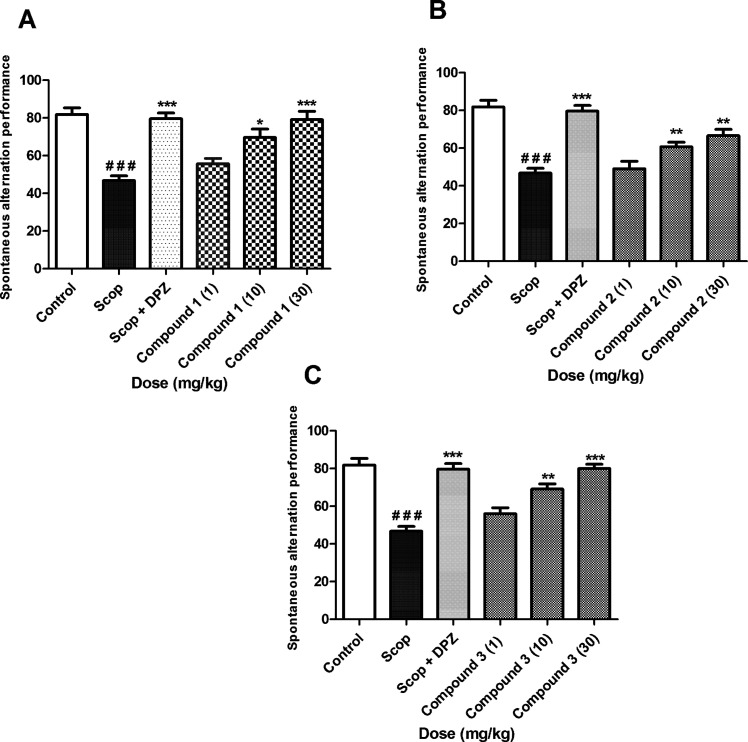
3.4. Y-Maze Spontaneous Alteration
The results of the Y-maze test are shown in Figure 2. Scopolamine significantly reduced spontaneous alteration from 86.67 ± 1.63 to 47.83 ± 1.94. The spontaneous alteration is normalized by donepezil, which increased the % alteration to 83.83 ± 2.64. Compounds 1–3 also increased the % alteration in a dose-dependent manner. Compound 1 showed a significant increase (*p < 0.05, **p < 0.01; ***p < 0.001) in spontaneous alternation at 10 and 30 mg/kg as compared to the scopolamine group. The increase in spontaneous alternation (***p < 0.001) observed with compounds 1 and 3 at 30 mg/kg was comparable to the reference drug donepezil at 2 mg/kg.
Figure 2.
Effect of compound 1 (A), compound 2 (B), and compound 3 (C) on spontaneous alteration for working memory in Y-maze test. All values were expressed as mean ± SEM (n = 8). p * <0.05, ***0.001 as compared to the scopolamine group; ###p < 0.001 compared to control, using one-way ANOVA followed by Tukey’s multiple comparison test.
3.5. Novel Object Recognition Test
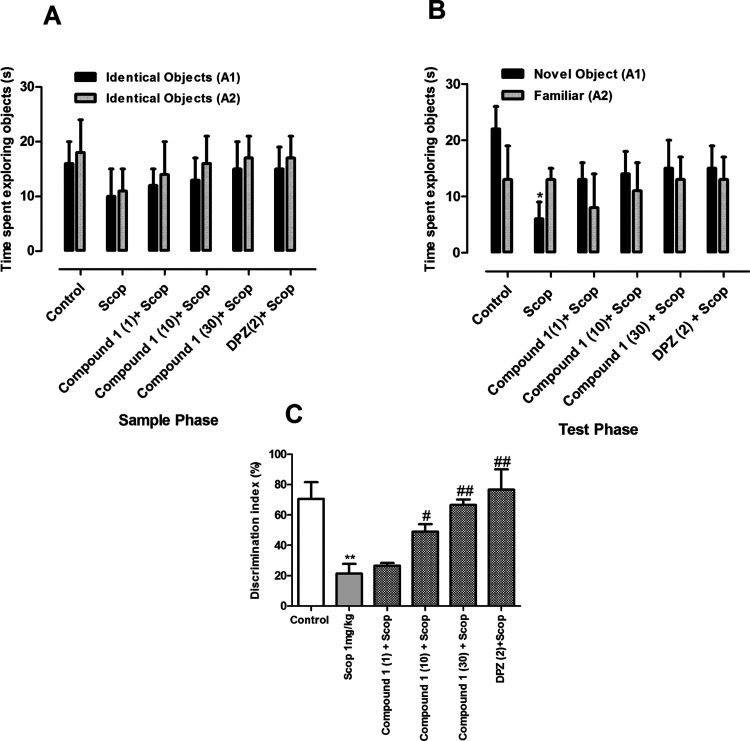
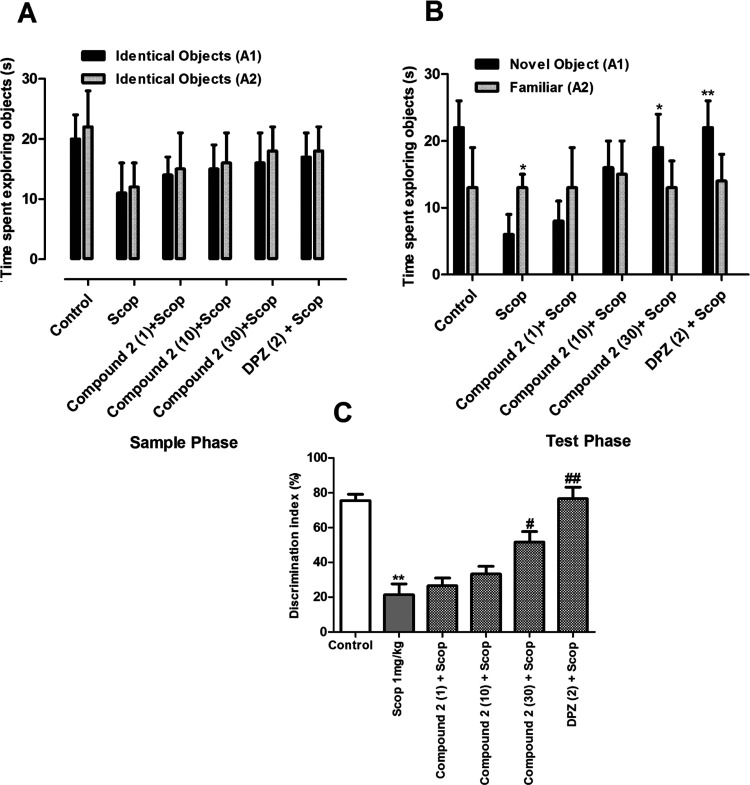
3.5.1. Memory-Enhancing Effects of Compound 1
3.5.1.1. Short-Term Task
A novel object recognition test was carried out to study short-term memory tasks. The results are shown in Figure 3. In the sample phase, no significant difference in the total time spent exploring the two objects was observed (Figure 3A). Similarly, no significant difference was observed between compound 1 treated groups and scopolamine groups in the time spent exploring each identical object. However, in the test phase, the animals pretreated with compound 1 (1, 10, and 30 mg/kg) or donepezil (2 mg/kg) and scopolamine (1 mg/kg) spent more time with the novel object than the identical one; but this difference in activity was statistically not significant. In contrast, the group treated with scopolamine alone spent a significantly longer time with the familiar object (p < 0.05) (Figure 3B). The % of discrimination index (% DI) was significantly greater for compound 1 (10 and 30 mg/kg), DPZ, and scopolamine (p < 0.05; p < 0.01) as compared to scopolamine-treated group alone. The % DI for the scopolamine-treated group was significantly lower (p < 0.01) when compared to the control group (Figure 3C).
Figure 3.
Effect of compound 1 (1, 10, and 30 mg/kg) in short-term memory NORT: (A) exploration time in the sample phase, (B) exploration time in the test phase, and (C) discrimination index. **p < 0.01 vs control and #p < 0.05, ##p < 0.01 vs SCOP 1 mg/kg using one-way ANOVA followed by Tukey’s multiple comparison test.
3.5.1.2. Long-Term Memory Task
The results of the sample phase of the long-term memory task were similar to those of the short-term memory task. In the sample phase, no significant difference was observed among the groups in exploring both objects. Similarly, there was no significant difference among various groups in exploring the two identical objects (p > 0.05) (Figure 4A). However, in the test phase, the groups treated with compound 1 (30 mg/kg) and DPZ (2 mg/kg) spent a significantly longer time with the novel object than the familiar one (p < 0.05) (Figure 4B). Similarly, the % DI was significantly higher for compound 1 at 10 and 30 mg/kg and DPZ (p < 0.05; 0.01) (Figure 4C).
Figure 4.
Effect of compound 1 (1, 10, and 30 mg/kg) in long-term memory NORT. (A) Exploration time in the sample phase, (B) exploration time in the test phase, and (C) discrimination index. *p < 0.05; *p < 0.01 vs control and #p < 0.05, ##p < 0.01 vs SCOP 1 mg/kg using one-way ANOVA followed by Tukey’s multiple comparison test.
3.5.2. Memory-Enhancing Effects of Compound 2
3.5.2.1. Short-Term Memory Task
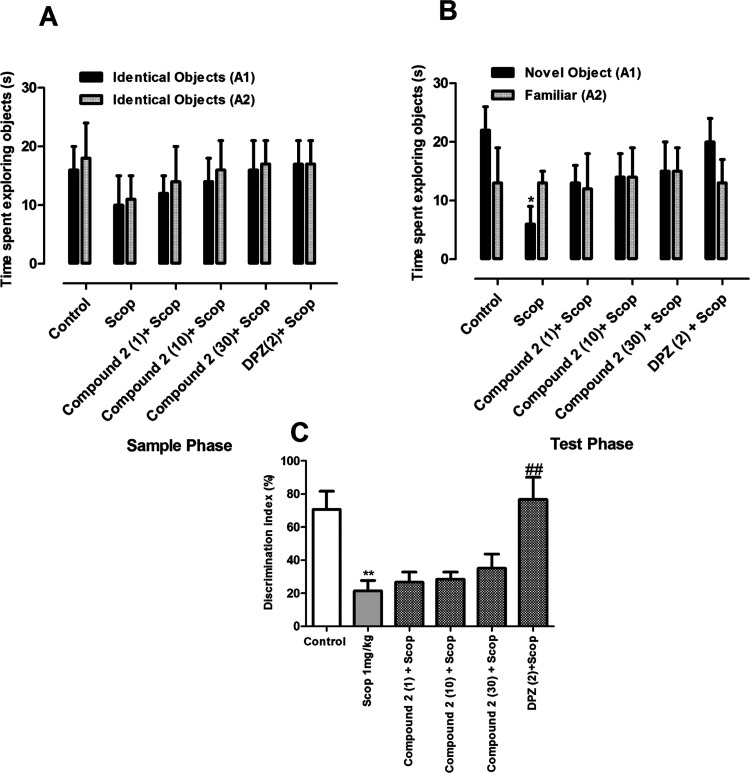
The results obtained with compound 2 in the short-term task of NORT are shown in Figure 5. Compound 2 did not exert a significant effect in the exploration of two objects in both the sample phase and test phase of the short-term memory task. However, in the test phase, scopolamine-treated animals spent a significantly longer time with the familiar object as compared to the vehicle control animals (Figure 5A).
Figure 5.
Effect of compound 1 (1, 10, and 30 mg/kg) in short-term memory NORT: (A) exploration time in the sample phase, (B) exploration time in the test phase, and (C) discrimination index. *p < 0.01 vs control and #p < 0.05, ##p < 0.01 vs SCOP 1 mg/kg using one-way ANOVA followed by Tukey’s multiple comparison test.
3.5.2.2. Long-Term Memory Task
The results of the sample phase of the long-term memory task were similar to those of the short-term memory task (Figure 6). No significant difference in exploring both objects was observed among the groups. Similarly, there was no significant difference among various groups in exploring the two identical objects (p > 0.05) (Figure 6B). However, in the test phase, the groups treated with compound 1 (30 mg/kg) and DPZ (2 mg/kg) spent a significantly longer time with the novel object than the familiar one (p < 0.05). Similarly, the % DI was significantly higher for compound 1 at 30 mg/kg and DPZ (p < 0.05; 0.01) (Figure 6C).
Figure 6.
Effect of compound 2 (1, 10, and 30 mg/kg) in long-term memory NORT. (A) Exploration time in the sample phase, (B) exploration time in the test phase, and (C) discrimination index. *p < 0.05; *p < 0.01 vs control and #p < 0.05, ##p < 0.01 vs SCOP 1 mg/kg using one-way ANOVA followed by Tukey’s multiple comparison test.
3.5.3. Memory-Enhancing Effects of Compound 3
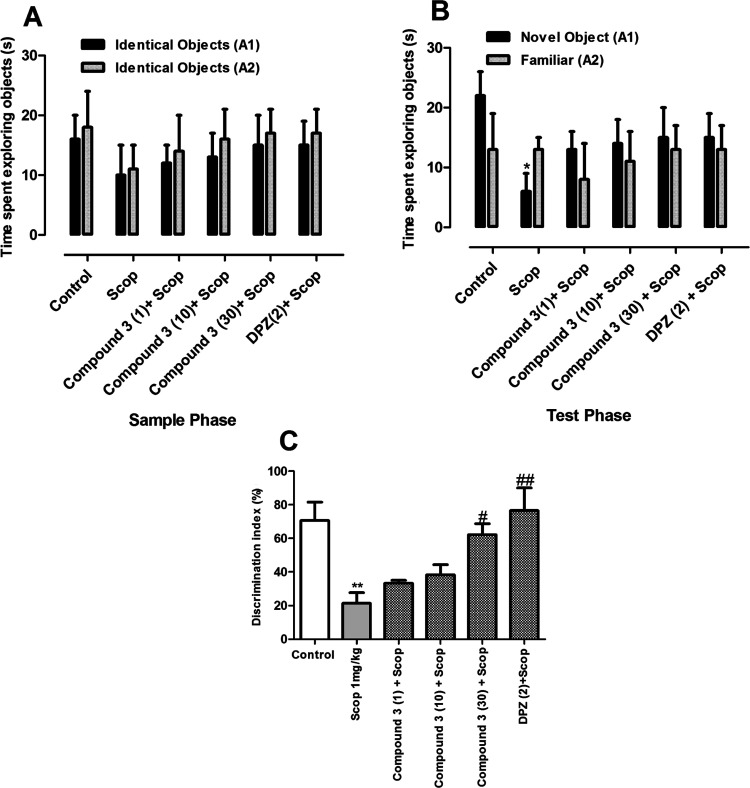
3.5.3.1. Short-Term Memory Task
The results obtained with compound 3 in the short-term task of NORT are shown in Figure 7. Similar to compound 1, compound 3 did not exert a significant effect in the exploration of two objects in the sample phase of the short-term memory task (Figure 4A). Similarly, no significant difference was observed between compound 3 or DPZ and scopolamine-treated groups in the time spent exploring each identical object. In contrast, the scopolamine-only treated animals spent a significantly longer time with the familiar object (p < 0.05). The % DI for compound 3 (30 mg/kg) and DPZ were significantly higher than the group treated with scopolamine alone.
Figure 7.
Effect of compound 3 (1, 10, and 30 mg/kg) in short-term memory NORT. (A) Exploration time in the sample phase, (B) exploration time in the test phase, and (C) discrimination index. *p < 0.01 vs control and #p < 0.05, ##p < 0.01 vs SCOP 1 mg/kg using one-way ANOVA followed by Tukey’s multiple comparison test.
3.5.3.2. Long-Term Memory Task
The results of the sample phase of the long-term memory task are shown in Figure 8. No significant difference in exploring both identical objects was observed among the groups (p > 0.05). However, in the test phase, the groups treated with compound 3 at the dose of 30 mg/kg and DPZ (2 mg/kg) spent a significantly longer time with the novel object than the familiar one (p < 0.05). Similarly, the % DI was significantly higher for compound 3 at 10 and 30 mg/kg and DPZ (p < 0.05; 0.01).
Figure 8.
Effect of compound 3 (1, 10, and 30 mg/kg) in long-term memory NORT. (A) Exploration time in the sample phase, (B) exploration time in the test phase, and (C) discrimination index. *p < 0.05; *p < 0.01 vs control and #p < 0.05, ##p < 0.01 vs SCOP 1 mg/kg using one-way ANOVA followed by Tukey’s multiple comparison test.
3.5.4. Effect of Compounds 1–3 on Ex Vivo AChE Activity
To correlate the in vitro and in vivo findings, the AChE and BuChE inhibition potential of compounds 1–3 was determined in whole-brain tissues after NORT. As shown in Table 3, the AChE activity was increased in the scopolamine-only treated animals. The groups treated with donepezil and compounds 1–3 showed a significant decrease in the activity compared to the scopolamine-treated group. Similar results were observed with inhibition of BuChE. Compounds 1–3 significantly decreased BuChE activity at the dose of 30 mg/kg. However, compounds 1 and 3 were found to be more efficacious (54 ± 6.4 and 50 ± 4.6%) than compound 2 (40 ± 5.5%) (Table 4). The enzyme inhibitory potential of compounds 1–3 were found to be in the order of compounds 1 > 3 > 2, similar to the one observed in in vivo studies. Thus, it was deduced that compounds 1–3 possessed AChE inhibition effect both in vivo and in vitro.
Table 3. Effect of Compounds 1–3 on the Activity of AChE in the Brain after NORTa.
| treatment groups | dose (mg/kg) | AChE inhibition (%) |
|---|---|---|
| control | 100 | |
| scopolamine | 1 | 20 ± 2.5b |
| donepezil | 2 | 75 ± 4.2e |
| compound 1 | 1 | 32 ± 3.6 |
| 10 | 60 ± 5.1d | |
| 30 | 74 ± 6.4e | |
| compound 2 | 1 | 25 ± 3.1 |
| 10 | 37 ± 4.3c | |
| 30 | 44 ± 5.5c | |
| compound 3 | 1 | 28 ± 3.4 |
| 10 | 51 ± 4.2d | |
| 30 | 70 ± 4.6e |
All values were expressed as mean ± SEM (n = 3).
p < 0.001 vs control.
p < 0.05.
p < 0.01.
p < 0.001 vs SCOP 1 mg/kg using one-way ANOVA followed by Tukey’s multiple comparison test.
Table 4. Effect of Compounds 1–3 on the Activity of BuChE in the Braina.
| treatment groups | dose (mg/kg) | BuChE inhibition (%) |
|---|---|---|
| control | 100 | |
| scopolamine | 1 | 20 ± 2.5b |
| donepezil | 2 | 55 ± 4.2d |
| compound 1 | 1 | 22 ± 3.6 |
| 10 | 25 ± 5.1 | |
| 30 | 54 ± 6.4d | |
| compound 2 | 1 | 25 ± 3.1 |
| 10 | 23 ± 4.3 | |
| 30 | 40 ± 5.5c | |
| compound 3 | 1 | 24 ± 3.4 |
| 10 | 26 ± 4.2 | |
| 30 | 50 ± 4.6d |
All values were expressed as mean ± SEM (n = 3).
p < 0.001 vs control.
p < 0.05.
p < 0.01 vs SCOP 1 mg/kg using one-way ANOVA followed by Tukey’s multiple comparison test.
3.6. Molecular Docking
The protein–ligand interactions at the molecular level were analyzed by in silico molecular docking. Before docking compounds 1–3, we scrutinized the performance of the docking program by redocking experiment. Redocking of the complex ligand donepezil was conducted, which suggests that donepezil binds at the same binding site at the X-ray crystal structure. The docked orientation is like the X-ray-determined confirmation, with the same interactions, and the RMSD of the docked pose is 1.78 Å. The docking score of donepezil is −9.79. The redocking results suggest that the docking program is robust enough to determine the binding mechanism of compounds 1–3.
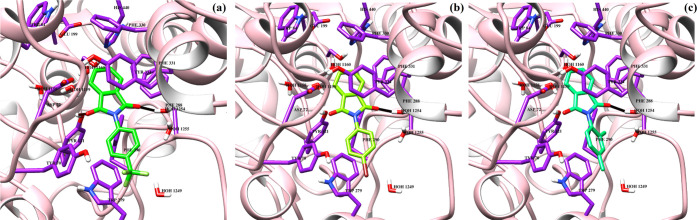
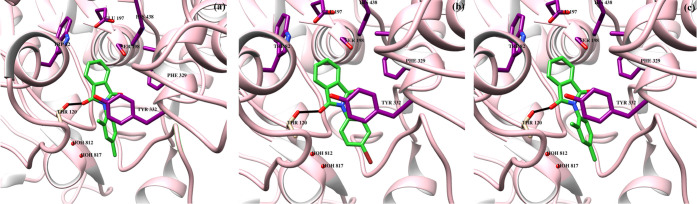
The docked orientation of all compounds (1–3) depicts that these compounds bind at the same binding site at the entrance of the gorge of AChE. The carbonyl moieties of compound 1 mediate hydrogen bonding with the side chain hydroxyl group of Tyr121 (2.40 Å) and Wat1254 (2.18 Å). Moreover, the side chain of Trp279 provides π–π interactions to the trifluoromethyl-substituted phenyl ring. Compound 2 formed a hydrogen bond with wat1254 (2.20 Å) while lost hydrogen bonding with Tyr121. Similarly, compound 3 mediated hydrogen bonding with wat1254 (2.19 Å), and side chains of Phe290 and Trp279 stabilized the compound by π–π interactions. The binding scores of compounds 1–3 are −5.77, 3.31, and −5.58, respectively. The docking score correlates with the experimental findings. The docked poses of compounds 1–3 are displayed in Figure 9. The binding interactions of compounds 1–3 in the active side gorge of BuChE are depicted in Figure 10. Initially, rivastigmine was redocked in the binding cavity. Rivastigmine molecules bind with the score −9.26, and RMSD 2.2 Å, with X-ray, deduced binding mode. The most active compound (3) binds at the interface of the BuChE binding site. The substituted carbonyl group formed an H-bond with the side chain −OH of Thr120 (3.30 Å), while the side chain of Trp82 provides hydrophobic interactions to the isoindoline group of the compound. All of the compounds (1–3) formed hydrogen bonding with Thr120 at more than 3.3 Å distances. However, these compounds lack hydrophobic interactions and water-mediated bridging with the protein residues. The docking scores of compounds 1–3 are −4.34, −5.28, and −5.95, respectively. The binding score is in complete agreement with the experimental results.
Figure 9.
Binding interactions of compounds 1–3 (a–c) are shown in the active site of acetylcholinesterase. The ligands are depicted in green sticks, interacting residues are shown in purple sticks, hydrogen bonds are displayed in black lines, and protein is shown in pink ribbon.
Figure 10.
Binding interactions of compounds 1–3 (a–c) are shown in the active site of human butyrylcholinesterase. The ligands are depicted in green sticks, interacting residues are shown in magenta sticks, hydrogen bonds are displayed in black lines, and protein is shown in pink ribbon.
4. Discussion
In this study, we have evaluated the antiamnesic effects of phthalimide derivatives including compounds 1–3 for their ability to prevent and treat learning and memory deficits in an animal model of scopolamine (SCOP)-induced amnesia. We investigated whether compounds 1–3 attenuate scopolamine (a muscarinic acetylcholine receptor antagonist)-induced learning and memory impairments by conducting Y-maze and novel object recognition tests. SCOP causes learning and memory dysfunction and subsequently interferes with both short-term and long-term memories. Furthermore, cholinergic neurons in the CNS are implicated in mediating reference (long-term) as well as working (short-term) memories of both animals and humans.25 Thus, any disruption of the cholinergic neurotransmission system plays a critical role in the development of early stage of AD.26,27
Y-maze spontaneous alteration is also used for studying the working memory of animals. SCOP-induced reduction in spontaneous alternation score was reversed by compounds 1–3 at the doses of 10 and 30 mg/kg, i.p. All three compounds significantly increased spontaneous alternation score at these doses. The order of activity of these compounds was compound 1 > compound 3 > compound 2. The results suggest that compounds 1–3 may improve memory function by rescuing the acetylcholine system from SCOP-induced deficits.
Novel object recognition test (NORT) is used for studying both short- and long-term memory tasks.28 The general principle of the novel object recognition test is based on exploring new objects. The rodents spend more time with the unfamiliar object as compared to the familiar object. During the experiment, animals are familiarized with two similar objects, and after a washout period, one object in the closed box is replaced with a new unfamiliar object.29 In this study, compounds 1–3 were evaluated for their effect on reference and working memories in NORT. In both the sample phase and test phase of the short-term memory task, no significant difference was observed in exploring the two objects for compounds 1–3. In contrast, the group treated with scopolamine spent a significantly longer time with the familiar object than the novel object (p < 0.05). The % discrimination index (% DI) was significantly higher for compound 1 at 10 and 30 mg/kg (p < 0.05; p < 0.01) and compound 3 at 30 mg/kg (p < 0.05) in the short-term memory task. Similarly, the % DI was also significantly higher for DPZ (2 mg/kg) (p < 0.01).
The results obtained in the sample phase of the long-term memory task were similar to that of the short-term memory task. However, in the test phase of the long-term memory task, compounds 1 and 3 (10 and 30 mg/kg) significantly increased the exploration time of the novel object than the familiar object compared to the scopolamine group (p < 0.05; p < 0.01). Similarly, the % DI was also significantly higher for compounds 1 and 3 (10 and 30 mg/kg) (p < 0.05; p < 0.01). The group treated with DPZ (2 mg/kg) also spent a significantly longer time with the novel object than the familiar one (p < 0.01).
The findings of in vitro and in vivo studies were corroborated by determining the ability of compounds 1–3 to inhibit the enzyme AChE in brain homogenates. The results indicated that donepezil and compounds 1–3 significantly inhibited AChE compared to the scopolamine-treated group, with a similar order of efficacy as found in the in vivo studies. Compound 1 was found to be more efficacious, followed by compounds 3 and 1.
Previous molecular docking study shows that phthalimide derivatives have acetylcholine esterase and butyrylcholinesterase binding affinity.30 Studies have revealed that the phthalimide structure interacts with the active site of AChE and BuChE, and several novel AChE inhibitors were designed based on this pharmacophore.31,32 Meanwhile, sulfonamide derivatives are another important class of pharmacophores in medicinal chemistry effective in a number of different therapeutic areas, including Alzheimer’s disease.33 They act as antibacterial, diuretics, carbonic anhydrase inhibitors, anticonvulsants, anti-inflammatory, anticancer, antihypertensive, and AChE inhibitors. In this study, we designed a series of 4-phthalimidobenzenesulfonamide derivatives as potent cholinesterase inhibitors.34 In this study, compounds 1–3 significantly inhibited the enzymes AChE and BuChE with IC50 values of 10 and 140, 18 and 80, and 50 and 11 μM, respectively. Similarly, donepezil also inhibited AChE and BuChE with IC50 values of 10.1 and 3.89 μM, respectively. Ex vivo findings in this study also showed inhibition of AChE and BuChE by compounds 1–3 with significantly higher efficacy toward AChE.
Thus, in the light of in vitro, in silico, and ex vivo results, it shows that the memory-enhancing effect of compounds 1-3 on both short-term and long-term memory tasks may be due to their ability to inhibit the activity of acetylcholinesterase, an enzyme primarily responsible for the degradation of acetylcholine. As the administration of donepezil significantly decreased the activity of this enzyme in the brains of amnesic mice, it may be suggested that compounds 1–3 had the same effect. These findings suggest that the mechanism(s) underlying the antiamnesic effects of compounds 1–3 in scopolamine-induced amnesic animals may be similar to that involved in the action of donepezil. These findings indicate that compounds 1–3 may exhibit a therapeutic effect on short- and long-term memory deficits associated with Alzheimer’s disease by improving the dysfunction of central cholinergic systems.
Oxidative damage is widespread in the brain in age-related cognitive decline and associated with AD in the elderly.17 The free radicals cause damage to DNA, proteins, lipids, and mitochondria and may interfere with the cell cycle, thus overcoming endogenous antioxidant defenses in the brain, and contribute to neuronal damage.35 Thus, antioxidant treatments could potentially affect key pathogenic mechanisms and may reduce the risk of AD. Karthik et al. have synthesized different phthalimide derivatives, such as 2-(3-fluoro-5-(trifluoromethyl)benzyl)isoindoline-1,3-dione (3b) and 2-(4-bromo-2-chloro-6-methylphenyl)isoindoline-1,3-dione (3c). These compounds have antioxidant activity and were found to possess neuroprotective activity.36 In the current study, compounds 1–3 inhibited DPPH and ABTS free radicals, demonstrating their antioxidant potential. Compounds 1–3 inhibited DPPH with IC50 values of 105, 340, and 150 μM and ABTS with IC50 values of 205, 350, and 220 μM, respectively. Ascorbic acid, the reference standard drug, also inhibited DPPH and ABTS with IC50 values of 61 and 125 μM, respectively.
In conclusion, phthalimide derivatives 1–3 possess antioxidant effects and inhibit choline esterase enzymes in vitro complemented by molecular docking studies. Furthermore, these compounds also exhibited significant antiamnesic effects, in in vivo animal models, an action that could be related to their antioxidant and anti-AchE properties. Molecular docking analysis of the AchE molecular target predicted the possible mode of action relating to the anti-Alzheimer activities of compounds 1–3. To our knowledge, this is the first study reporting the antioxidant, anticholinesterases, and memory-enhancing effects of compounds 1–3 in in vitro, in silico, ex vivo, and in vivo studies. Thus, compounds 1–3 could be useful leads for the development of novel therapeutic agents for treating memory impairment in Alzheimer’s disease.
Acknowledgments
The authors would like to thank the Deanship of Scientific Research at Umm Al-Qura University for supporting this work by Grant Code: (22UQU4331128DSR46). This work was supported by grant from The Oman Research Council (TRC) through the funded project (BFP/RGP/CBS/21/002). Dr. Nasiara Karim acknowledges funding (National research program for universities; NRPU 20-3425) from the Higher Education Commission of Pakistan for the completion of this research work.
Author Contributions
N.K., A.K., and A.A.-H. conceived and designed the study. N.K. and I.K. performed all experiments. S.A.H., N.U.R., and A.K. performed all computational studies and analyzed the data. A.K. and A.N.A. helped in analysis of data. N.K. and S.A.H. wrote the manuscript with input and comments from all co-authors. All authors have read and approved the final version of the manuscript.
This work was supported by grant from The Oman Research Council (TRC) through the funded project (BFP/RGP/CBS/21/002). The authors would like to thank the Deanship of Scientific Research at Umm Al-Qura University for supporting this work by Grant Code: (22UQU4331128DSR46).
The authors declare no competing financial interest.
Notes
Each experimental trial was carried out in strict compliance with the approved procedures (DAEC/PHARM/2012/15) by the Department Ethics Committee based on the Animals (Scientific Procedures) Act 1986.
References
- Dharmarajan T. S.; Gunturu S. G. Alzheimer’s disease: a healthcare burden of epidemic proportion. Am. Health Drug Benefits 2009, 2, 39–47. [PMC free article] [PubMed] [Google Scholar]
- Elmaidomy A. H.; Abdelmohsen U. R.; Alsenani F.; Aly H. F.; Shams S. G. E.; Younis E. A.; Ahmed K. A.; Sayed A. M.; Owis A. I.; Afifi N.; et al. The anti-Alzheimer potential of Tamarindus indica: an in vivo investigation supported by in vitro and in silico approaches. RSC Adv. 2022, 12, 11769–11785. 10.1039/D2RA01340A. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Qiu C.; Kivipelto M.; von Strauss E. Epidemiology of Alzheimer’s disease: occurrence, determinants, and strategies toward intervention. Dialogues Clin. Neurosci. 2009, 11, 111–128. 10.31887/DCNS.2009.11.2/cqiu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong W. Economic burden of Alzheimer disease and managed care considerations. Am. J. Managed Care 2020, 26, S177–S183. 10.37765/ajmc.2020.88482. [DOI] [PubMed] [Google Scholar]
- Scarmeas N.; Brandt J.; Albert M.; Hadjigeorgiou G.; Papadimitriou A.; Dubois B.; Sarazin M.; Devanand D.; Honig L.; Marder K.; Bell K.; Wegesin D.; Blacker D.; Stern Y. Delusions and hallucinations are associated with worse outcome in Alzheimer disease. Arch. Neurol. 2005, 62, 1601–8. 10.1001/archneur.62.10.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenebe Y.; Akele B.; M W. S.; Necho M. Prevalence and determinants of depression among old age: a systematic review and meta-analysis. Ann. Gen. Psychiatr. 2021, 20, 55 10.1186/s12991-021-00375-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzi R. E. The genetics of Alzheimer disease. Cold Spring Harbor Perspect. Med. 2012, 2, a006296 10.1101/cshperspect.a006296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Broeckhoven C. L. Molecular genetics of Alzheimer disease: identification of genes and gene mutations. Eur. Neurol. 1995, 35, 8–19. 10.1159/000117083. [DOI] [PubMed] [Google Scholar]
- Barnes D. E.; Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011, 10, 819–828. 10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarawneh R.; Holtzman D. M. The clinical problem of symptomatic Alzheimer disease and mild cognitive impairment. Cold Spring Harbor Perspect. Med. 2012, 2, a006148 10.1101/cshperspect.a006148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani R. J.; Rolston R. K.; Smith M. A. Alzheimer disease. Disease-a-Month 2010, 56, 484–546. 10.1016/j.disamonth.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez S. E.; Raghanti M. A.; Hof P. R.; Kramer L.; Ikonomovic M. D.; Lacor P. N.; Erwin J. M.; Sherwood C. C.; Mufson E. J. Alzheimer’s disease pathology in the neocortex and hippocampus of the western lowland gorilla (Gorilla gorilla gorilla). J. Comp. Neurol. 2013, 521, 4318–4338. 10.1002/cne.23428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaria R. N.; Maestre G. E.; Arizaga R.; Friedland R. P.; Galasko D.; Hall K.; Luchsinger J. A.; Ogunniyi A.; Perry E. K.; Potocnik F.; Prince M.; Stewart R.; Wimo A.; Zhang Z. X.; Antuono P. Alzheimer’s disease and vascular dementia in developing countries: prevalence, management, and risk factors. Lancet Neurol. 2008, 7, 812–26. 10.1016/S1474-4422(08)70169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma U.; Kumar P.; Kumar N.; Singh B. Recent advances in the chemistry of phthalimide analogues and their therapeutic potential. Mini Rev. Med. Chem. 2010, 10, 678–704. 10.2174/138955710791572442. [DOI] [PubMed] [Google Scholar]
- Aliabadi A.; Foroumadi A.; Mohammadi-Farani A.; Garmsiri Mahvar M. Synthesis and Evaluation of Anti-acetylcholinesterase Activity of 2-(2-(4-(2-Oxo-2-phenylethyl)piperazin-1-yl) ethyl)Isoindoline-1,3-dione Derivatives with Potential Anti-Alzheimer Effects. Iran. J. Basic Med. Sci. 2013, 16, 1049–1054. [PMC free article] [PubMed] [Google Scholar]
- Alonso D.; Dorronsoro I.; Rubio L.; Muñoz P.; García-Palomero E.; Del Monte M.; Bidon-Chanal A.; Orozco M.; Luque F. J.; Castro A.; Medina M.; Martínez A. Donepezil-tacrine hybrid related derivatives as new dual binding site inhibitors of AChE. Bioorg. Med. Chem. 2005, 13, 6588–6597. 10.1016/j.bmc.2005.09.029. [DOI] [PubMed] [Google Scholar]
- Karim N.; Khan I.; Abdelhalim A.; Abdel-Halim H.; Hanrahan J. R. Molecular docking and antiamnesic effects of nepitrin isolated from Rosmarinus officinalis on scopolamine-induced memory impairment in mice. Biomed. Pharmacother. 2017, 96, 700–709. 10.1016/j.biopha.2017.09.121. [DOI] [PubMed] [Google Scholar]
- Kostelnik A.; Pohanka M. Inhibition of Acetylcholinesterase and Butyrylcholinesterase by a Plant Secondary Metabolite Boldine. BioMed Res. Int. 2018, 2018, 9634349 10.1155/2018/9634349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelhalim A.; Karim N.; Chebib M.; Aburjai T.; Khan I.; Johnston G. A.; Hanrahan J. Antidepressant, Anxiolytic and Antinociceptive Activities of Constituents from Rosmarinus Officinalis. J. Pharm. Pharm. Sci. 2015, 18, 448–459. 10.18433/J3PW38. [DOI] [PubMed] [Google Scholar]
- Jothy S. L.; Zakaria Z.; Chen Y.; Lau Y. L.; Latha L. Y.; Sasidharan S. Acute oral toxicity of methanolic seed extract of Cassia fistula in mice. Molecules 2011, 16, 5268–5282. 10.3390/molecules16065268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin S. Comprehensive observational assessment: Ia. A systematic, quantitative procedure for assessing the behavioral and physiologic state of the mouse. Psychopharmacologia 1968, 13, 222–257. 10.1007/BF00401402. [DOI] [PubMed] [Google Scholar]
- Ashwini G.; Pranay P.; Thrinath G.; Karnaker Reddy T.; Giri Prasad V. Pharmacological evalution of Marsilea qudrifolia plant extracts against Alzheimer’s disease. Int. J. Drug Dev. Res. 2012, 4, 153–158. [Google Scholar]
- Hughes R. N. The value of spontaneous alternation behavior (SAB) as a test of retention in pharmacological investigations of memory. Neurosci. Biobehav. Rev. 2004, 28, 497–505. 10.1016/j.neubiorev.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Konrath E. L.; Neves B. M.; Lunardi P. S.; Passos Cdos S.; Simões-Pires A.; Ortega M. G.; Gonçalves C. A.; Cabrera J. L.; Moreira J. C.; Henriques A. T. Investigation of the in vitro and ex vivo acetylcholinesterase and antioxidant activities of traditionally used Lycopodium species from South America on alkaloid extracts. J. Ethnopharmacol. 2012, 139, 58–67. 10.1016/j.jep.2011.10.042. [DOI] [PubMed] [Google Scholar]
- Deiana S.; Platt B.; Riedel G. The cholinergic system and spatial learning. Behav. Brain Res. 2011, 221, 389–411. 10.1016/j.bbr.2010.11.036. [DOI] [PubMed] [Google Scholar]
- Daulatzai M. A. Early Stages of Pathogenesis in Memory Impairment during Normal Senescence and Alzheimer’s Disease. J. Alzheimer’s Dis. 2010, 20, 355–367. 10.3233/JAD-2010-1374. [DOI] [PubMed] [Google Scholar]
- Swerdlow R. H. Pathogenesis of Alzheimer’s disease. Clin. Interventions Aging 2007, 2, 347–59. [PMC free article] [PubMed] [Google Scholar]
- Lueptow L. M. Novel Object Recognition Test for the Investigation of Learning and Memory in Mice. J. Vis. Exp. 2017, 126, e55718 10.3791/55718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwashita H.; Sano M.; Chiba A. Diazepam induces retrograde facilitation of object recognition and object location memory in male mice. NeuroReport 2023, 34, 10-1097 10.1097/WNR.0000000000001869. [DOI] [PubMed] [Google Scholar]
- Sang Z.; Wang K.; Wang H.; Yu L.; Wang H.; Ma Q.; Ye M.; Han X.; Liu W. Design, synthesis and biological evaluation of phthalimide-alkylamine derivatives as balanced multifunctional cholinesterase and monoamine oxidase-B inhibitors for the treatment of Alzheimer’s disease. Bioorg. Med. Chem. Lett. 2017, 27, 5053–5059. 10.1016/j.bmcl.2017.09.055. [DOI] [PubMed] [Google Scholar]
- Soyer Z.; Uysal S.; Parlar S.; Tarikogullari Dogan A. H.; Alptuzun V. Synthesis and molecular docking studies of some 4-phthalimidobenzenesulfonamide derivatives as acetylcholinesterase and butyrylcholinesterase inhibitors. J. Enzyme Inhib. Med. Chem. 2017, 32, 13–19. 10.1080/14756366.2016.1226298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi-Farani A.; Ahmadi A.; Nadri H.; Aliabadi A. Synthesis, docking and acetylcholinesterase inhibitory assessment of 2-(2-(4-Benzylpiperazin-1-yl)ethyl)isoindoline-1,3-dione derivatives with potential anti-Alzheimer effects. DARU, J. Pharm. Sci. 2013, 21, 47 10.1186/2008-2231-21-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bag S.; Tulsan R.; Sood A.; Cho H.; Redjeb H.; Zhou W.; LeVine H.; Török B.; Török M. Sulfonamides as multifunctional agents for Alzheimer’s disease. Bioorg. Med. Chem. Lett. 2015, 25, 626–630. 10.1016/j.bmcl.2014.12.006. [DOI] [PubMed] [Google Scholar]
- Akin Kazancioglu E.; Senturk M. Synthesis of N-phenylsulfonamide derivatives and investigation of some esterase enzymes inhibiting properties. Bioorg. Chem. 2020, 104, 104279 10.1016/j.bioorg.2020.104279. [DOI] [PubMed] [Google Scholar]
- Galasko D. R.; Peskind E.; Clark C. M.; Quinn J. F.; Ringman J. M.; Jicha G. A.; Cotman C.; Cottrell B.; Montine T. J.; Thomas R. G.; Aisen P. Antioxidants for Alzheimer disease: a randomized clinical trial with cerebrospinal fluid biomarker measures. Arch. Neurol. 2012, 69, 836–841. 10.1001/archneurol.2012.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karthik C. S.; Mallesha L.; Mallu P. Investigation of Antioxidant Properties of Phthalimide Derivatives. Can. Chem. Trans. 2015, 3, 199–206. 10.13179/canchemtrans.2015.03.02.0194. [DOI] [Google Scholar]