Abstract
Background
Trichomonas vaginalis (T. vaginalis) is a microaerophilic protozoan parasite which is responsible for trichomoniasis, the most common non-viral sexually transmitted infection in the world. The infection greatly damages the reproductive system. However, whether T. vaginalis infection can cause reproductive system cancer remains controversial.
Methods
This study systematically searched PubMed, EMBASE, Ovid and Google scholar, and 144 relevant articles were retrieved and classified into three categories: epidemiological investigations (68), reviews (30) and research articles (46). These three types of articles were verified according to their respective inclusion and exclusion criteria. Stata 16 was used to conduct a meta-analysis on the articles of epidemiological investigations for analysing the correlation between T. vaginalis infection and reproductive system cancer.
Results
The result of meta-analysis indicated that the rate of T. vaginalis infection in the cancer group was significantly higher than that in the non-cancer group (OR = 1.87, 95% CI 1.29–2.71, I2 = 52%). Moreover, the cancer rate of the population infected with T. vaginalis was significantly higher than that of the population without T. vaginalis infection (OR = 2.77, 95% CI 2.37–3.25, I2 = 31%). The review articles and most research articles stated that the infection of T. vaginalis could lead to cancer and the pathogenic mechanisms were as follows: T. vaginalis promoting inflammatory response, T. vaginalis infection changing the internal environment around parasitic sites and signal transduction pathway, the metabolites secreted by T. vaginalis inducing carcinogenesis and T. vaginalis increasing other pathogenic microbial infection to promote the occurrence of cancer.
Conclusions
Our study confirmed that there was a correlation between the infection of T. vaginalis and reproductive system cancer, and provided some possible research directions for clarifying the carcinogenic mechanisms caused by T. vaginalis infection.
Keywords: Trichomonas vaginalis, Infection, Cancer, Correlation, Systematic review
Background
Trichomoniasis is the most common non-viral sexually transmitted infection (STI) in human beings which is responsible for a range of symptoms such as increasing vaginal secretion, pruritus and irritation of perineum in female [1]. According to the survey of the World Health Organization (WHO), at least 370 million people worldwide suffered from trichomoniasis. The global prevalence of trichomoniasis was 5.3% in females and 0.6% in males with a growing trend [2]. Trichomonas vaginalis (T. vaginalis) infection prevalence was 1.8% and 0.5% among females and males in the U.S [3]. In France, the total infection rates of T. vaginalis was 1.7% [4]. In Africa, the prevalence was as higher as 29% in Natal and 7.1% in Tanzania [5, 6]. In China, the prevalence was varied in different regions, for instance, 13.9% in Zhengzhou, 1.6% in Xinxiang [7] and 0.7% in Sichuan [8].
The infection of T. vaginalis can cause female vaginitis, cervicitis and adverse birth outcomes [9, 10]. Most of male infected with T. vaginalis are asymptomatic, but the infection can also cause urethritis or prostatitis and even lead to infertility [11]. Moreover, T. vaginalis usually increases the risk of other pathogens infection [12], for example HIV, which greatly threaten public health [13]. Some studies indicated that T. vaginalis infection might be a risk factor for cervical cancer and prostate cancer [14, 15].
As is known to all, the main pathogenic pathway of T. vaginalis is to cause the inflammatory reaction of parasitic sites, and the repeated inflammation may induce carcinogenesis. It was reported that T. vaginalis infection could lead to cervical precancerous lesions and neoplastic lesions [16]. The risk of cervical cancer increased about twice in the presence of T. vaginalis [17], and T. vaginalis infection could promote prostate cancer by damaging prostate epithelial cells [18]. Moreover, T. vaginalis infection was associated with hrHPV, the causative agent in most cervical cancers [13, 16].
Although many published articles have shown that T. vaginalis infection can increase the risk of cervical cancer or prostate cancer, the correlation between T. vaginalis infection and reproductive system cancer and whether T. vaginalis can cause reproductive system cancer remain unclear. Therefore, in this study, we analyzed the correlation between T. vaginalis infection and reproductive system cancer through meta-analysis of relevant epidemiological data. In addition, we summarized the potential pathogenic mechanism of cancer caused by T. vaginalis infection through consulting the relevant review and research articles. The results of the study provided a direction for exploring the pathogenic mechanism of T. vaginalis leading to cancer.
Methods
Search strategy and classification
The literature retrieval databases mainly included PubMed database, EMBASE, Ovid MEDLINE medical literature library, Web of Science, Science Direct and Google Scholar, and the literature search time was up to December 2021. The keywords searched by MeSH and commonly used for literature retrieval were used alone or in combination: “Trichomonas vaginalis”, “Trichomonas infections”, “Trichomoniasis”, “Trichomonas vaginitis”, “Neoplasms”, “cancer”, “neoplasia”, “carcinoma in situ”, “canceration” and “tumour” with “OR” and / or “AND” operators. The systematic search of literature was performed by two independent researchers. The retrieved articles were manually checked the title, abstract and full-text by two independent researchers, and the irrelevant articles and duplicate were removed. The retained articles were categorized as epidemiological investigations, reviews, and research articles. This systematic review with meta-analysis was registered in the International Prospective Register of Systematic Reviews (PROSPERO, https://www.crd.york.ac.uk/prospero) and a registration ID was assigned (CRD42022340263).
Inclusion criteria and exclusion criteria
Inclusion criteria for epidemiological investigations
1. The article contains statistical data on cancer in people infected with T. vaginalis and without T. vaginalis. 2. The article contains statistical data on T. vaginalis infection in cancer patients and noncancer patients. 3. In the articles, the data of T. vaginalis infection in cancer patients and noncancer patients are compared. 4. All articles have clear data sources. 5. For the literature with repeated relevant data, the latest and most comprehensive article is selected.
Exclusion criteria for epidemiological investigations
1. The effect of T. vaginalis on cancer is not mentioned in the article, and the relevant data is incomplete. 2. The article only contains data on cancer in people infected with T. vaginalis with no corresponding data from people without T. vaginalis infection. 3. The article only contains data on T. vaginalis infection in cancer patients without corresponding data from noncancer patients. 4. In the articles, the sample size is too small.
Inclusion criterion for review articles
1. The article concerns the correlation between T. vaginalis infection and cancer. 2. The article has a clear conclusion.
Exclusion criterion for review articles
1. The article does not concern the correlation between T. vaginalis infection and cancer. 2. The conclusion about the correlation between T. vaginalis infection and cancer is ambiguous.
Inclusion criterion for research articles
There is a clear carcinogenic mechanism caused by T. vaginalis infection in the article.
Exclusion criterion for research articles
The relevant mechanism of cancer caused by T. vaginalis infection was not written in the article.
Quality assessment and data analysis for epidemiological investigations
Newcastle–Ottawa scale (NOS) was used to evaluate the quality of the epidemiological investigations with a total score of 9 points, and the articles with a score ≥ 6 points were included in statistics. NOS considers the following items comprising selection criteria (0–4 points), subject comparability (0–2 points) and exposure (0–3 points). The selected epidemiological investigations were analyzed by Stata 16 software, and the forest plot was drawn. I2 test was used for heterogeneity analysis. If P < 0.05 or I2 ≥ 50%, it was considered that there was heterogeneity among the research, and the origin of heterogeneity should be further analyzed by the random-effect model and Galbraith plot. The funnel chart was used to test the publication bias of the included articles, and the Begg and egger tests were further used to evaluate the publication bias. If there was publication bias, we excluded articles one by one to make a sensitivity test, and the total effect quantity was observed to judge the stability of the analysis.
Results
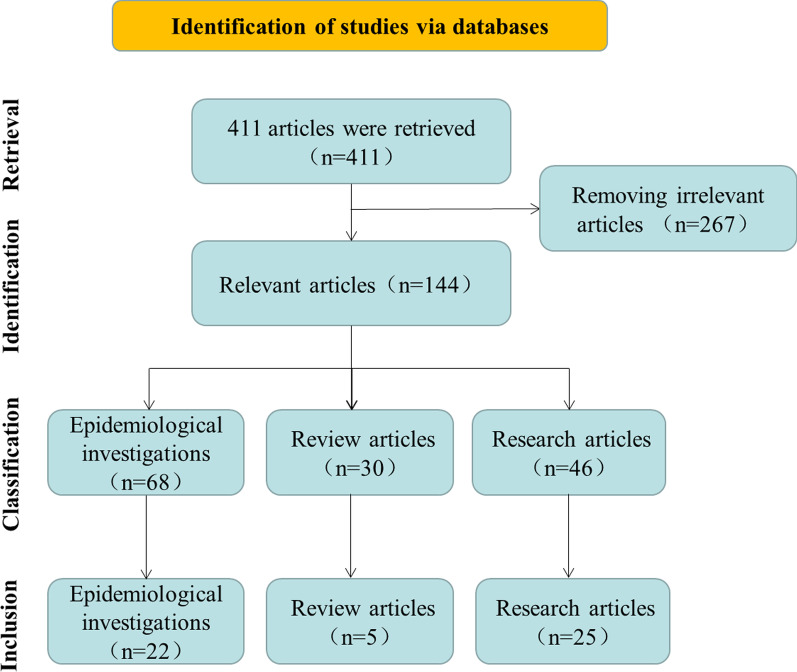
In this study, a total of 411 articles were retrieved (Fig. 1), and the title, abstract and full-text of these articles were checked. After removing 267 irrelevant articles which did not mention both “T. vaginalis” and “Neoplasms”, the remaining 144 articles were classified into three categories, 68 epidemiological investigations, 30 review articles, and 46 research articles. In the 144 articles, 143 articles were about T. vaginalis and reproductive system cancer (cervical cancer, prostate cancer and vaginal cancer), and the other one was about T. vaginalis and anal canal carcinoma.
Fig. 1.
Flow diagram of articles retrieval, identification, classification and inclusion
Epidemiological investigations
Literature inclusion
The 68 epidemiological selected articles were identified according to the NOS (NOS score ≥ 6), inclusion and exclusion criteria. Finally, 22 relevant articles were obtained. Fourteen articles (Table 1) were concerned the infection of T. vaginalis in reproductive system cancer patients. In these articles, we set the cancer patients as the experimental group and healthy people or noncancer patients as the control group. The other eight articles were concerned the reproductive system cancer incidence in T. vaginalis infected people. The people infected with T. vaginalis were set as the experimental group, and the people without T. vaginalis infection were set as the control group. The samples of these articles were sufficient for meta-analysis, which were deemed as high-quality articles based on the NOS quality assessment score (NOS score ≥ 6).
Table 1.
The information of relevant epidemiological articles
| Number | Title | First author | Year | Location | Total sample | Experimental group | Control group | Nos |
|---|---|---|---|---|---|---|---|---|
| 1 | An epidemiologic study of carcinoma in situ and squamous dysplasia of the uterine cervix | Thomas DB | 1973 | USA | 626 | 49/324 (15.1%) | 18/302 (6.0%) | 8 |
| 2 | The association of sexually transmitted diseases with cervical intraepithelial neoplasia: a case–control study | Guijon FB | 1985 | Canada | 84 | 1/32 (3%) | 1/52 (2%) | 6 |
| 3 | Vaginal microbial flora as a cofactor in the pathogenesis of uterine cervical intraepithelial neoplasia | Guijon F | 1992 | Canada | 185 | 4/106 (3.8%) | 4/79 (5.1%) | 8 |
| 4 | The association between sexually transmitted pathogens and cervical intra-epithelial neoplasia in a developing community | Kharsany AB | 1993 | Durban | 48 | 11/28 (39%) | 3/20 (15%) | 6 |
| 5 | Trichomonas vaginalis and cervical cancer. A prospective study in China | Zhang ZF | 1995 | China | 16,797 | 7/421 (1.7%) | 92/16376 (0.6%) | 7 |
| 6 | Atypical squamous cells of undetermined significance: Bethesda classification and association with Human Papillomavirus | Barcelos AC | 2011 | Brazil | 170 | 4/140 (2.85%) | 0/30 (0) | 6 |
| 7 | Prospective study of effect modification by Toll-like receptor 4 variation on the association between Trichomonas vaginalis serostatus and prostate cancer | Chen YC | 2013 | USA | 1382 | 71/690 (10%) | 61/692 (9%) | 6 |
| 8 | Bacterial vaginosis, aerobic vaginitis, vaginal inflammation and major Pap smear abnormalities Prevalence of human papillomavirus and co-existent sexually transmitted | Vieira P | 2016 | Portugal | 1244 | 31/905 (3.4%) | 11/339 (3.2%) | 6 |
| 9 | Prevalence of human papillomavirus, Chlamydia trachomatis, and Trichomonas vaginalis infections in Amazonian women with normal and abnormal cytology | Costa-Lira | 2017 | Brazil | 180 | 0/47 (0) | 24/133 (18%) | 6 |
| 10 | Correlation between Common Lower Genital Tract Microbes and High-Risk Human Papillomavirus Infection | Panpan Lv | 2019 | China | 826 | 8/254 (3.1%) | 1/572 (0.2%) | 6 |
| 11 | Prevalence of human papillomavirus, human immunodeficiency virus and other sexually transmitted infections among female sex workers in Togo: a national cross-sectional survey | Ferré VM | 2019 | Togo | 310 | 9/102 (8.8%) | 11/208 (5.3%) | 6 |
| 12 | Association between Trichomonas vaginalis infection and cervical lesions: a population-based, nested case–control study in Taiwan | Su RY | 2020 | Taiwan | 270,015 | 12/54003 (0.02%) | 18/216012 (0.008%) | 6 |
| 13 | Trichomonas vaginalis serostatus and prostate cancer risk in Egypt: a case–control study | Saleh NE | 2021 | Egypt | 445 | 57/325 (17.5%) | 10/120 (8.3%) | 6 |
| 14 | Prevalence of cervical HPV infection, sexually transmitted infections and associated antimicrobial resistance in women attending cervical cancer screening in Mali | Jary A | 2021 | Mali | 144 | 7/90 (7.8%) | 3/54 (5.6%) | 7 |
| 15 | Infection with Trichomonas vaginalis in a black population | Miller JM | 1989 | USA | 3005 | 140/745 (18.8%) | 169/2260 (7.5%) | 6 |
| 16 | Gynaecological infections as risk determinants of subsequent cervical neoplasia | Viikki M | 2000 | Finland | 19,114 | 16/1544 (1.0%) | 82/17600 (0.5%) | 6 |
| 17 | Association of Chlamydia trachomatis with persistence of high-risk types of human papillomavirus in a cohort of female adolescents | Samoff E | 2005 | USA | 261 | 7/18 (39%) | 83/234 (35.8%) | 6 |
| 18 | Results of longterm hospital based cytological screening in asymptomatic women | Misra JS | 2006 | India | 20,417 | 54/671 (8.1%) | 485/19746 (2.5%) | 6 |
| 19 | Human Papillomaviruses and genital co-infections in gynaecological Outpatients | Verteramo R | 2008 | Italy | 857 | 3/10 (0.3%) | 263/847 (1.2%) | 7 |
| 20 | Prevalence and risk factors for cervical neoplasia: a cervical cancer screening program in Beijing | Tao L | 2014 | China | 748,105 | 9/5104 (1.8%) | 504/697064 (0.07%) | 6 |
| 21 | Association between high risk human papillomavirus infection and co-infection with Candida spp. and Trichomonas vaginalis in women with cervical premalignant and malignant lesions | Ghosh I | 2017 | India | 225 | 130/177 (73.4%) | 27/48 (56.2%) | 6 |
| 22 | Trichomonas vaginalis as a risk factor for human papillomavirus: a study with women undergoing cervical cancer screening in a northeast region of Brazil | Belfort IKP | 2021 | Brazil | 562 | 21/107 (20%) | 27/455 (5.9%) | 7 |
1–14 Experimental group: the number (incidence) of T. vaginalis infection among patients with cancer; Control group: the number (incidence) of T. vaginalis infection among people without cancer. 15–22 Experimental group: the number (incidence) of cancer among people with T. vaginalis infection; Control group: the number (incidence) of cancer among people without T. vaginalis infection
Meta analysis of the epidemiological investigations
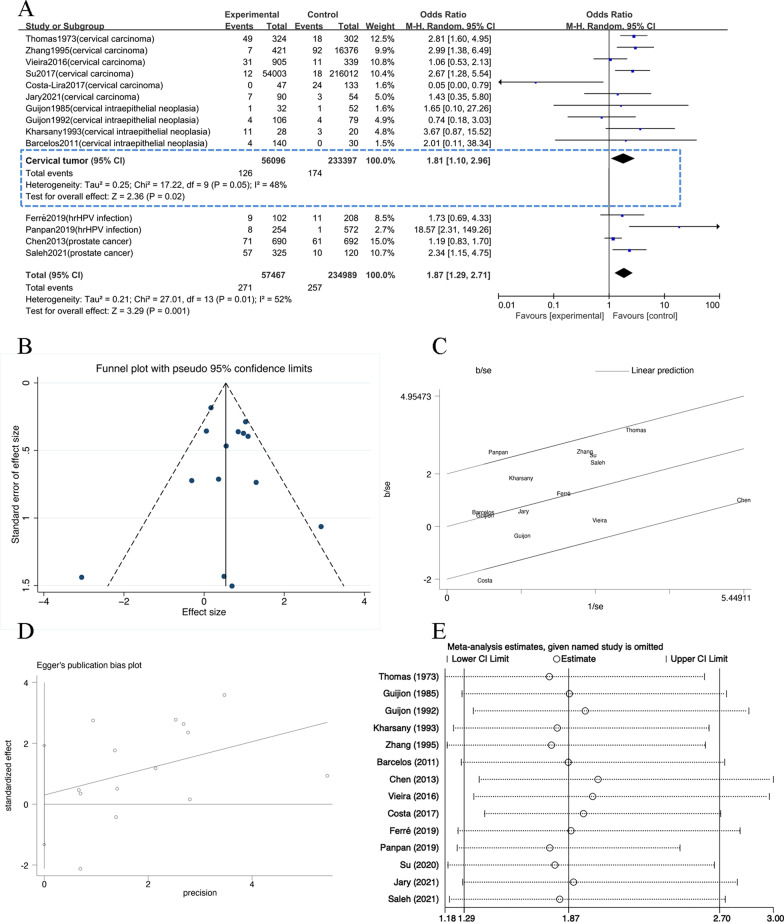
The data of the 14 articles on T. vaginalis infection in cancer patients were analyzed by Stata 16 software. The results showed that 292,456 research subjects were included in the articles, including 57,467 in the experimental group (cancer patients) and 234,989 in the control group (healthy or non-cancer patients) [15, 19–31]. As shown in the forest plot (Fig. 2a), P = 0.01, I2 = 52% which indicated that there was heterogeneity among the research results (P < 0.1, I2 ≥ 50%). So random-effect model was used for further analysis, and Galbraith plot analysis was performed for the included articles (Fig. 2c). In the plot, the two articles, Panpan (2019) and Costa (2017), had influence on heterogeneity. The reason for the heterogeneity of Panpan (2019) was that the sample size was relatively small, while the reason for the heterogeneity of Costa (2017) was that the sample size of the experimental group and the control group was quite different.
Fig. 2.
The articles concerned the incidence of T. vaginalis infection among patients with cancer. A Forest plot of the meta-analysis. The heterogeneity test yielded Chi2 = 27.01, p = 0.01, and I2 = 52%. The OR and 95% confidence interval were calculated as 1.87 and 1.29–2.71, respectively, and z and p values of the combined effect size were as 3.29 and less than 0.001. T. vaginalis infection among patients with cervical tumor: The heterogeneity test yielded Chi2 = 17.22, p = 0.05, and I2 = 48%. The OR and 95% confidence interval were calculated as 1.81 and 1.10–2.96, respectively, and z and p values of the combined effect size were as 2.36 and 0.02. B Funnel plot of the meta-analysis. C Galbraith plot. D Begg and Egger plot of the meta-analysis. P = 0.696 (P > 0.1) E Sensitivity analysis of the articles
Combining the data of these 14 articles, the pooled OR was 1.87 (95% CI 1.29–2.71). The results of the combined effect were Z = 3.29 and P = 0.001 which meant that there was a significant difference in T. vaginalis infection between the experimental group and the control group (P < 0.05). As shown in the funnel plot (Fig. 2b), the excellent distribution symmetry of the individual sample points indicated that there was no publication bias. Further, the Begg and Egger plot was used to test (Fig. 2d) the bias, and the result was found that P = 0.696 (P > 0.1) and showed that there was no publication bias in these data. The sensitivity analysis (Fig. 2e) found that the impact on the analysis was not significant after excluding each article, and the 95% confidence interval was still within 1.29–2.70, indicating that the analysis was relatively stable.
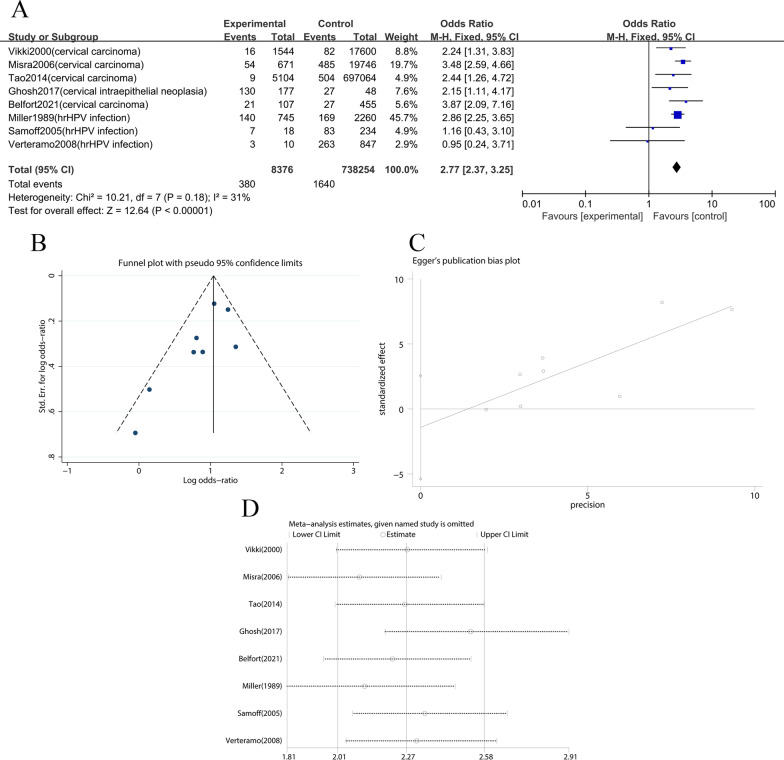
In addition, 8 articles on cancer among patients infected with T. vaginalis were screened for meta-analysis (Fig. 3a). The results showed that 746,630 subjects were included in the articles, including 8376 cases in the experimental group (with T. vaginalis infection) and 738,254 cases in the control group (without T. vaginalis infection) [32–38]. The pooled OR was 2.77 (95% Cl: 2.37–3.25). The results of the combined effect were Z = 12.64 and P = 0 (P < 0.05) which meant that there was a significant difference in cancer patients between the experimental group and the control group (P < 0.05). The results of I2 = 31%, P = 0.18 proved that the heterogeneity was small (P > 0.1). The funnel plot (Fig. 3b) were asymmetric, indicating that there might be publication bias. The Begg and Egger plot was used to test (Fig. 3c), P = 0.051 (P < 0.1) proved that there was publication bias. Because few retrieved articles were included, so it was necessary to add more articles for further study. The sensitivity analysis (Fig. 3d) indicated that the analysis was relatively stable (95%Cl: 2.01–2.58).
Fig. 3.
The articles concerned the incidence of cancer among people with T. vaginalis infection. A Forest plot of the meta-analysis. The heterogeneity test yielded Chi2 = 10.21, p = 0.18, and I2 = 31%. The OR and 95% confidence interval were calculated as 2.77 and 2.37–3.25, respectively, and z and p values of the combined effect size were as 12.64 and 0 (P < 0.05). B Funnel plot of the meta-analysis. C Begg and Egger plot of the meta-analysis. P = 0.051 (P < 0.1). D Sensitivity analysis of the articles
Review articles
In this study, 30 reviews were selected and five articles were finally obtained after screening by inclusion and exclusion criteria. As shown in Table 2, we analyzed the conclusions of each article. These five articles suggested that T. vaginalis could lead to cervical cancer and prostate cancer [16, 17, 39–41]. The possible reasons were cell carcinogenesis caused by long-term inflammatory response [16, 17, 39, 40] and cancer caused by T. vaginalis increasing the infection of other pathogens (hrHPV, Chlamydia) [41].
Table 2.
The information of review articles
| Title | First author | Year | Conclusion | T. vaginalis causes cancer |
|---|---|---|---|---|
| Infection and cervical intraepithelial neoplasia | Boyle DC | 1999 | The risk of cervical neoplasia in the presence of T. vaginalis is about twice that in normal, which may be related to the production of nitrosamine | Yes |
| Trichomonas vaginalis: paradigm of a successful sexually transmitted organism | Rughooputh S | 2005 | T. vaginalis can be classified as one of the most important auxiliary factors in the pathogenesis of cervical cancer | Yes |
| Sexually transmitted infections and risk of prostate cancer: review of historical and emerging hypotheses | Sutcliffe S | 2014 | T. vaginalis promotes prostate cancer through an IgE mediated anti flagellar hormone inflammatory immune mechanism, while T. vaginalis may promote cancer by directly damaging or dissolving prostate epithelial cells | Yes |
| Association of Genital Infections Other Than Human Papillomavirus with Pre-Invasive and Invasive Cervical Neoplasia | Ghosh I | 2016 | T. vaginalis infection has a higher risk of cervical precancerous lesions and neoplastic lesions | Yes |
| The dawn of novel STI prevention methods: modelling potential unintended effects of changes in cervical cancer screening guidelines on trichomoniasis | Rönn MM | 2018 | Patients infected with hrHPV are more likely to be infected with T. vaginalis than those not infected with hrHPV | Yes |
Research articles
In this study, 48 research articles were selected and 25 articles were obtained according to the inclusion and exclusion criteria of research articles, of which 24 articles were related to T. vaginalis infection and reproductive system cancer, and the other articles involved T. vaginalis infection and anal canal carcinoma [18, 42–67]. As shown in Table 3, we listed the information of the articles and concluded the mechanism of cancer caused by T. vaginalis infection. Most of the articles showed that T. vaginalis infection could promote cancer procession but 3 articles hold the opposite view. As described in these articles, the main pathogenic mechanisms were as follows: (1) T. vaginalis promoted inflammatory reaction through various ways and leaded to cell carcinogenesis; (2) The metabolites secreted by T. vaginalis promoted the occurrence of cancer; (3) The infection of T. vaginalis affected the vivo environment and signal transduction pathway which was associated with cancer; (4) T. vaginalis increasing other pathogenic microbial infection (HPV) promoted the occurrence of cancer.When phagocytosis of Candida spp. by T. vaginalis occurs, Candida spp. are protected by T. vaginalis from the defences of the host and the inhibitory effects of antimycotic drugs used for treatment finally lead to anal canal carcinoma
Table 3.
The information of research articles
| Title | First author | Year | Conclusion | T. vaginalis causes cancer |
|---|---|---|---|---|
| Significance of variations in the size of Trichomonas vaginalis in patients with dysplasia, intrapithelial and invasive planocellular carcinoma of the uterine cervix | Mekki F | 1979 | Small forms of T. vaginalis are more pathogenic than large ones and might be one of the causative agents of the atypical transformation of the squamous epithelium of the uterine cervix | Yes |
| Enhancement versus tumor resistance induced by different levels of immunodepression in BALB/c mice with protozoan infections | Landolfo S | 1979 | In T. vaginalis infected mice, a slight and transient depression of both humoral and cellular immune reactivity induces an enhanced tumor growth | Yes |
| Gas chromatographic studies on propionic acid, butyric acid and valeric acid in culture fluid of Trichomonas vaginalis | Ishiguro T | 1984 | Propionic acid or iso-valeric acid produced by T. vaginalis had a promoter-like activity and/or promoter-enhancing effect, which is, at least in part, responsible for the promotion of cervical cancer or vaginal cancer | Yes |
| Pseudocyst forms of Trichomonas vaginalis from cervical neoplasia | Afzan MY | 2012 | T. vaginalis phenotypic variant forms of pseudocysts does exist and this phenotype with higher nuclear content and more rough and creased surface with higher numbers of deep micropores with larger numbers of chromatin masses, vacuoles, and hydrogenosomes play a role in exacerbating cervical cancer | Yes |
| Phenotypic 'variant' forms of Trichomonas vaginalis trophozoites from cervical neoplasia patients | Yusof AM | 2012 | T. vaginalis trophozoites in cervical neoplasia isolates showed more rough and creased surface with numerous deep micropores, and there was higher numbers of vacuoles and hydrogenosomes in these forms. These were virulent forms which could aggravate or exacerbate cervical neoplasia conditions | Yes |
| Light microscopic observation on phagocytosis of Candida spp. blastospores by Trichomonas vaginalis in a patient with anal canal carcinoma | Oz ZS | 2012 | Yes | |
| Epitopes of the highly immunogenic Trichomonas vaginalis α-actin in are serodiagnostic targets for both women and men | Neace CJ | 2013 | There is a relationship between seropositivity for α-actinin truncated protein of T. vaginalis and prostate cancer | Yes |
| Association of Trichomonas vaginalis with its symbiont Mycoplasma hominis synergistically upregulates the in vitro proinflammatory response of human monocytes | Fiori PL | 2013 | The synergistic upregulation of the macrophage proinflammatory response might also affect some important clinical conditions associated with T. vaginalis infection, such as the increased risk of acquiring cervical cancer or HIV, which are thought to be affected by the inflammatory milieu during trichomoniasis | Yes |
| Trichomonas vaginalis homolog of macrophage migration inhibitory factor induces prostate cell growth, invasiveness, and inflammatory responses | Twu O | 2014 | Chronic T. vaginalis infections may result in TvMIF-driven inflammation and cell proliferation, thus triggering pathways that contribute to the promotion and progression of prostate cancer | Yes |
| Trichomonas vaginalis: a possible foe to prostate cancer | Zhu Z | 2016 | T. vaginalis inhibits the growth and development of prostate cancer | No |
| Signalling pathways associated with IL-6 production and epithelial-mesenchymal transition induction in prostate epithelial cells stimulated with Trichomonas vaginalis | Han IH | 2016 | Inflammatory conditions induced by T. vaginalis infections have been shown to promote epithelial-mesenchymal transition (EMT) | Yes |
| Proliferation of Prostate Stromal Cell Induced by Benign Prostatic Hyperplasia Epithelial Cell Stimulated With Trichomonas vaginalis via Crosstalk With Mast Cell | Kim JH | 2016 | The inflammatory response by benign prostatic hyperplasia epithelial cells stimulated with T. vaginalis induce the proliferation of prostate stromal cells via crosstalk with mast cells | Yes |
| Inflammatory Responses in a Benign Prostatic Hyperplasia Epithelial Cell Line (BPH-1) Infected with Trichomonas vaginalis | Kim SS | 2016 | The level of IL-6 in BPH-1 cells infected with T. vaginalis increased, and IL-6 is considered to promote the development of benign prostatic hyperplasia and prostate cancer | Yes |
| Trichomonas Vaginalis Inhibits HeLa Cell Growth Through Modulation of Critical Molecules for Cell Proliferation and Apoptosis | Zhu Z | 2018 | T. vaginalis culture supernatant inhibited the growth of HeLa cervical cancer cells by inhibiting cell proliferation and promoting apoptosis | No |
| Druggability of the guanosine/adenosine/cytidine nucleoside hydrolase from Trichomonas vaginalis | Alam R | 2018 | Individuals infected with T. vaginalis have a higher susceptibility to more serious conditions such as cervical and prostate cancer | Yes |
| Inflammatory mediators of prostate epithelial cells stimulated with Trichomonas vaginalis promote proliferative and invasive properties of prostate cancer cells | Han IH | 2019 | T. vaginalis infection may be one of the factors creating the supportive microenvironment to promote proliferation and invasiveness of PCa cells | Yes |
| Experimental rat prostatitis caused by Trichomonas vaginalis infection | Jang KS | 2019 | T. vaginalis has been detected in prostatic tissue of patients with prostatitis and reported to be associated with chronic prostatitis and benign prostatic hyperplasia as well as prostate cancer | Yes |
| IL-6 produced by prostate epithelial cells stimulated with Trichomonas vaginalis promotes proliferation of prostate cancer cells by inducing M2 polarization of THP-1-derived macrophages | Han IH | 2020 | When T. vaginalis infection causes inflammation, prostate epithelial cells produce IL-6, macrophages polarize into M2 type, and M2 macrophages promote the proliferation and migration of cancer cells | Yes |
| Gardnerella vaginalis and Trichomonas vaginalis infections and the risk of persistence or progression of low-grade cervical intraepithelial neoplasia | Raffone A | 2020 | T. vaginalis infection alone does not significantly affect the risk of persistence or progression of such lesions, while it may greatly increase the risk of progression when associated with G. vaginalis infection | Yes |
| Polarization of M2 Macrophages by Interaction between Prostate Cancer Cells Treated with Trichomonas vaginalis and Adipocytes | Chung HY | 2020 | Interaction between inflamed PCa treated with T. vaginalis and adipocytes causes M2 macrophage polarization, so contributing to the progression of PCa | Yes |
| The Role of Purinergic Signaling in Trichomonas vaginalis Infection | Ferla M | 2020 | T. vaginalis infect the prostate and make prostate epithelial cells express P2X1, P2X2 and P2X7 receptors, affecting the purinergic signaling of host, which may be related to prostate cancer | Yes |
| Inflammation driven tumor-like signaling in prostatic epithelial cells by sexually transmitted Trichomonas vaginalis | Kushwaha B | 2020 | The initiation of inflammation driven tumor-like cell signaling in parasite-infected human prostatic epithelial cells is apparent, with the prostate tumor (DU145) cells being more sensitive to T. vaginalis than normal (RWPE-1) prostatic cells | Yes |
| Investigation of viral etiology in potentially malignant disorders and oral squamous cell carcinomas in non-smoking, non-drinking patients | Pérot P | 2020 | T. vaginalis can induce the production of a large number of different proinflammatory cytokines, which is associated with a high risk of high-grade or metastatic prostate cancer | Yes |
| Molecular Examination of Trichomonas vaginalis Infection and Risk of Prostate Cancer in the Biopsy of Patients with Different Prostate Lesions | Kamarkhani Z | 2021 | T. vaginalis may have no pathogenic effect on different prostate lesions | No |
| Signaling Role of Adipocyte Leptin in Prostate Cell Proliferation Induced by Trichomonas vaginalis | Kim JH | 2021 | T. vaginalis contributes to prostate enlargement in BPH via adipocyte leptin released as a result of inflammation of the prostate | Yes |
Discussion
In recent years, there are increasing number of studies on T. vaginalis, and many studies indicated that T. vaginalis was closely related to reproductive system cancer. In this study, we searched for 14 articles on incidence of T. vaginalis infection in cancer patients. By meta-analysis, the combined effect size of the forest plot was determined to Z = 3.29, P = 0.001, which was statistically significant. However, the analysis result of forest plot (P = 0.01, I2 = 52%) indicated a moderate degree of heterogeneity in these articles. Galbraith plot indicated that there was heterogeneity in Panpan (2019) and Costa (2017), but sensitivity analysis showed that the removal of the 2 articles has little impact on the results, and the data was stable. These results signified that the infection rate of T. vaginalis in cancer patients was significantly higher than that in healthy group or non cancer group, which indicated there was a correlation between T. vaginalis infection and cancer. By analyzing funnel plot, Begg and Egger plot, we found that there was no publication bias in our analysis. However, the heterogeneity still affected the quality of the analysis. Although the result indicated that T. vaginalis infection was related to cancer, more epidemiological data should be further excavated.
In addition, there were eight epidemiological investigations on incidence of cancer in people with T. vaginalis infection. In the study, the people infected with T. vaginalis were set as the experimental group, and the people without T. vaginalis infection were set as the control group. By comparing the prevalence of cancer between the two groups, the results indicated that the group infected with T. vaginalis had a high proportion of cancer. There was no heterogeneity in the data (I2 = 31%, P = 0.18). However, the Begg and egger analysis of the data (P = 0.051, P < 0.1) showed publication bias in the result. The reason for this might be related to the small number of such articles in this study. Therefore, the epidemiological investigations on the prevalence of cancer in the people infected with T. vaginalis need further research and more relevant articles need to be included to consolidate the results.
Several review articles on T. vaginalis and cancer were selected in this study. By consulting these articles, we found that T. vaginalis plays a positive role in the occurrence and evolution of cancer. T. vaginalis infection could directly or indirectly lead to cancer. It was found that T. vaginalis infection in women mainly caused inflammation of reproductive tract and the release of proinflammatory factors, changed in vaginal environment and promoted pathogenic microbial infections (HPV) [16, 41] which led to cervical cancer. The main factors leading to prostate cancer in men infected with T. vaginalis were the inflammatory response induced by T. vaginalis or its cytotoxic effect. The infection of T. vaginalis could induce the production of a large number of different proinflammatory cytokines [40].
From the research articles, we found several potential mechanisms of cancer induced by T. vaginalis infection.
The infection of T. vaginalis can promote the inflammatory response and lead to cell carcinogenesis in a variety of ways. Chronic T. vaginalis infection leads to the production of macrophage migration factor and macrophage polarization to M2 type, driving inflammation and abnormal cell proliferation to promote the progress of cervical and prostate cancer [60, 62]. The inflammatory response by BPH (benign prostatic hyperplasia) epithelial cells stimulated with T. vaginalis induce the proliferation of prostate stromal cells via crosstalk with mast cells [55, 67]. Chronic T. vaginalis infections result in TvMIF (T. vaginalis macrophage migration inhibitory factor)-driven inflammation and cell proliferation, thus triggering pathways that contribute to progression of prostate cancer [51]. The initiation of inflammation driven tumor-like cell signaling in parasite-infected human prostatic epithelial cells and the prostate tumor cells are more sensitive to T. vaginalis than normal prostatic cells [63]. The level of IL-6 in BPH-1 cells infected with T. vaginalis is increased, and IL-6 is considered to be a factor promoting the development of benign prostatic hyperplasia and prostate cancer [54, 66].
During the metabolism of T. vaginalis, the contents of propionic acid and iso-valeric acid increase [44]. Both of them had a promoter-like activity and/or promoter-enhancing effect in vitro studies on viral oncology, which could promote the occurrence of cervical cancer or vaginal cancer. Moreover, T. vaginalis infect the prostate cells and lead prostate epithelial cells to express P2X1, P2X2 and P2X7 receptors, affecting the purinergic signaling of host, which is related to prostate cancer [18, 47]. T. vaginalis count and growth rates were significantly higher in trophozoites from CN (cervical neoplasia) and cervical neoplasia proliferates, and the trophozoite surface of CN isolate was creased and rough implying that these were virulent forms which could aggravate cervical neoplasia conditions [47]. There is a relationship between seropositivity for ACT-P2 (truncated protein of trichomonad α-actinin) of T. vaginalis and prostate cancer [49]. T. vaginalis may promote cancer by directly damaging or dissolving prostate epithelial cells [52].
T. vaginalis infection associated with other pathogen infection increase the risk of cancer. The presence of Mycoplasma hominis (M. hominis) in T. vaginalis play a key role in inflammation. The synergistic upregulation of the macrophage proinflammatory response also increase the risk of acquiring cervical cancer [50]. When phagocytosis of Candida spp by T. vaginalis occurs, Candida spp are protected by T. vaginalis from the defences of the host and the inhibitory effects of antimycotic drugs used for treatment finally lead to anal canal carcinoma [48]. T. vaginalis infection associated with Gardnerella vaginalis infection might increase the progression of low-grade cervical intraepithelial neoplasia [61]. Morever, some published articles indicated that T. vaginalis significantly increased the infection of hrHPV [41]. However, which mechanism plays a major role in cancer induced by T. vaginalis infection needs to be further studied.
Conclusions
Through meta-analysis of relevant epidemiological data, we found that there was a correlation between T. vaginalis infection and reproductive system cancer. By consulting the relevant review and research articles, we discovered that T. vaginalis infection could lead to cervical cancer or prostate cancer, and the articles showed that the main potential carcinogenic mechanisms involved inflammatory reaction change the environment around the parasitic site and signal transduction pathway, and aggravate the infection of other carcinogenic pathogenic microorganisms. Our study provides a foundation for further investigation to the mechanism of cancer caused by T. vaginalis in the future.
Acknowledgements
Not applicable.
Abbreviations
- T. vaginalis
Trichomonas vaginalis
- STI
Sexually transmitted infection
- BPH
Benign prostatic hyperplasia
- CN
Cervical neoplasia
- ACT-P2
Truncated protein of trichomonad α-actinin
- TvMIF
T. vaginalis Macrophage migration inhibitory factor
- GV
Gardnerella vaginalis
- hrHPV
High risk Human papilloma virus
- M. hominis
Mycoplasma hominis
- OR
Odds ratio
- NOS
Newcastle–Ottawa scale
Author contributions
ZCZ and XM conceived and designed the study. DL, YL and XY collected the relevant literatures. DL and RZ classified the articles. XX and YY arranged epidemiological investigations. XY and YL performed the meta-analysis. XT, ZY and SW arranged review articles and research articles. LZ prepared the figures and tables. ZCZ and XM analysed the data and wrote the paper. All authors read and approved the final manuscript.
Funding
This study obtained the funding from the National Natural Science Foundation of China (No. 81802028), the Scientific and Technological Projects of Henan Province (No. 212102310820), the Science and Technology Planning Project of Xinxiang City (No. GG2021011), and the Doctoral Scientific Research Activation Foundation of Xinxiang Medical University (Nos. XYBSKYZZ202140 and XYBSKYZZ201631). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
All data generated or analyzed during this study are included in this article and additional information files.
Declarations
Ethics approval and consent to participate
The study was reviewed and approved by the Ethics Review Committee of Xinxiang Medical University (Reference No. 2020206).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ryan CM, de Miguel N, Johnson PJ. Trichomonas vaginalis: current understanding of host-parasite interactions. Essays Biochem. 2011;51:161–175. doi: 10.1042/bse0510161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rowley J, Vander Hoorn S, Korenromp E, Low N, Unemo M, et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull World Health Organ. 2019;97:548–562P. doi: 10.2471/BLT.18.228486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel EU, Gaydos CA, Packman ZR, Quinn TC, Tobian AAR. Prevalence and correlates of Trichomonas vaginalis infection among men and women in the United States. Clin Infect Dis. 2018;67:211–217. doi: 10.1093/cid/ciy079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pereyre S, Laurier Nadalie C, Bebear C, Investigator G. Mycoplasma genitalium and Trichomonas vaginalis in France: a point prevalence study in people screened for sexually transmitted diseases. Clin Microbiol Infect. 2017;23(122):e121–122e127. doi: 10.1016/j.cmi.2016.10.028. [DOI] [PubMed] [Google Scholar]
- 5.Moodley P, Wilkinson D, Connolly C, Moodley J, Sturm AW. Trichomonas vaginalis is associated with pelvic inflammatory disease in women infected with human immunodeficiency virus. Clin Infect Dis. 2002;34:519–522. doi: 10.1086/338399. [DOI] [PubMed] [Google Scholar]
- 6.Juliana NCA, Deb S, Ouburg S, Chauhan A, Pleijster J, et al. The prevalence of chlamydia trachomatis and three other non-viral sexually transmitted infections among pregnant women in Pemba Island Tanzania. Pathogens. 2020;9:128. doi: 10.3390/pathogens9080625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Z, Kang L, Wang W, Zhao X, Li Y, et al. Prevalence and genetic diversity of Trichomonas vaginalis clinical isolates in a targeted population in Xinxiang City, Henan Province, China. Parasit Vectors. 2018;11:124. doi: 10.1186/s13071-018-2753-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yongjun T, Samuelson J, Qingsheng D, Ali MM, Li X, et al. The prevalence of sexually transmitted and other lower reproductive tract infections among rural women in Sichuan Province, China. Southeast Asian J Trop Med Public Health. 2009;40:1038–1047. [PubMed] [Google Scholar]
- 9.Bhakta SB, Moran JA, Mercer F. Neutrophil interactions with the sexually transmitted parasite Trichomonas vaginalis: implications for immunity and pathogenesis. Open Biol. 2020;10:200192. doi: 10.1098/rsob.200192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Gerwen OT, Craig-Kuhn MC, Jones AT, Schroeder JA, Deaver J, et al. Trichomoniasis and adverse birth outcomes: a systematic review and meta-analysis. BJOG. 2021;128:1907–1915. doi: 10.1111/1471-0528.16774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edwards T, Burke P, Smalley H, Hobbs G. Trichomonas vaginalis: Clinical relevance, pathogenicity and diagnosis. Crit Rev Microbiol. 2016;42:406–417. doi: 10.3109/1040841X.2014.958050. [DOI] [PubMed] [Google Scholar]
- 12.Kanyina EW, Kamau L, Muturi M. Cervical precancerous changes and selected cervical microbial infections, Kiambu County, Kenya, 2014: a cross sectional study. BMC Infect Dis. 2017;17:647. doi: 10.1186/s12879-017-2747-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McClelland RS, Sangare L, Hassan WM, Lavreys L, Mandaliya K, et al. Infection with Trichomonas vaginalis increases the risk of HIV-1 acquisition. J Infect Dis. 2007;195:698–702. doi: 10.1086/511278. [DOI] [PubMed] [Google Scholar]
- 14.Sutcliffe S, Giovannucci E, Alderete JF, Chang TH, Gaydos CA, et al. Plasma antibodies against Trichomonas vaginalis and subsequent risk of prostate cancer. Cancer Epidemiol Biomark Prev. 2006;15:939–945. doi: 10.1158/1055-9965.EPI-05-0781. [DOI] [PubMed] [Google Scholar]
- 15.Zhang ZF, Graham S, Yu SZ, Marshall J, Zielezny M, et al. Trichomonas vaginalis and cervical cancer. A prospective study in China. Ann Epidemiol. 1995;5:325–332. doi: 10.1016/1047-2797(94)00101-X. [DOI] [PubMed] [Google Scholar]
- 16.Ghosh I, Mandal R, Kundu P, Biswas J. Association of genital infections other than human papillomavirus with pre-invasive and invasive cervical neoplasia. J Clin Diagn Res. 2016;10:XE01–XE06. doi: 10.7860/JCDR/2016/15305.7173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyle DC, Smith JR. Infection and cervical intraepithelial neoplasia. Int J Gynecol Cancer. 1999;9:177–186. doi: 10.1046/j.1525-1438.1999.99007.x. [DOI] [PubMed] [Google Scholar]
- 18.Ferla M, Tasca T. The role of purinergic signaling in Trichomonas vaginalis infection. Curr Top Med Chem. 2021;21:181–192. doi: 10.2174/1568026620999200904122212. [DOI] [PubMed] [Google Scholar]
- 19.Thomas DB. An epidemiologic study of carcinoma in situ and squamous dysplasia of the uterine cervix. Am J Epidemiol. 1973;98:10–28. doi: 10.1093/oxfordjournals.aje.a121528. [DOI] [PubMed] [Google Scholar]
- 20.Guijon FB, Paraskevas M, Brunham R. The association of sexually transmitted diseases with cervical intraepithelial neoplasia: a case-control study. Am J Obstet Gynecol. 1985;151:185–190. doi: 10.1016/0002-9378(85)90009-2. [DOI] [PubMed] [Google Scholar]
- 21.Guijon F, Paraskevas M, Rand F, Heywood E, Brunham R, et al. Vaginal microbial flora as a cofactor in the pathogenesis of uterine cervical intraepithelial neoplasia. Int J Gynaecol Obstet. 1992;37:185–191. doi: 10.1016/0020-7292(92)90379-W. [DOI] [PubMed] [Google Scholar]
- 22.Kharsany AB, Hoosen AA, Moodley J, Bagaratee J, Gouws E. The association between sexually transmitted pathogens and cervical intra-epithelial neoplasia in a developing community. Genitourin Med. 1993;69:357–360. doi: 10.1136/sti.69.5.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barcelos AC, Michelin MA, Adad SJ, Murta EF. Atypical squamous cells of undetermined significance: Bethesda classification and association with Human Papillomavirus. Infect Dis Obstet Gynecol. 2011;2011:904674. doi: 10.1155/2011/904674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen YC, Huang YL, Platz EA, Alderete JF, Zheng L, et al. Prospective study of effect modification by Toll-like receptor 4 variation on the association between Trichomonas vaginalis serostatus and prostate cancer. Cancer Causes Control. 2013;24:175–180. doi: 10.1007/s10552-012-0103-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vieira-Baptista P, Lima-Silva J, Pinto C, Saldanha C, Beires J, et al. Bacterial vaginosis, aerobic vaginitis, vaginal inflammation and major Pap smear abnormalities. Eur J Clin Microbiol Infect Dis. 2016;35:657–664. doi: 10.1007/s10096-016-2584-1. [DOI] [PubMed] [Google Scholar]
- 26.Costa-Lira E, Jacinto A, Silva LM, Napoleao PFR, Barbosa-Filho RAA, et al. Prevalence of human papillomavirus, Chlamydia trachomatis, and Trichomonas vaginalis infections in Amazonian women with normal and abnormal cytology. Genet Mol Res. 2017;16:89–180. doi: 10.4238/gmr16029626. [DOI] [PubMed] [Google Scholar]
- 27.Lv P, Zhao F, Xu X, Xu J, Wang Q, et al. Correlation between Common lower genital tract microbes and high-risk human papillomavirus infection. Can J Infect Dis Med Microbiol. 2019;2019:9678104. doi: 10.1155/2019/9678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferre VM, Ekouevi DK, Gbeasor-Komlanvi FA, Collin G, Le Hingrat Q, et al. Prevalence of human papillomavirus, human immunodeficiency virus and other sexually transmitted infections among female sex workers in Togo: a national cross-sectional survey. Clin Microbiol Infect. 2019;25(1560):e1561–1560 e1567. doi: 10.1016/j.cmi.2019.04.015. [DOI] [PubMed] [Google Scholar]
- 29.Su RY, Ho LJ, Yang HY, Chung CH, Yang SS, et al. Association between Trichomonas vaginalis infection and cervical lesions: a population-based, nested case-control study in Taiwan. Parasitol Res. 2020;119:2649–2657. doi: 10.1007/s00436-020-06759-4. [DOI] [PubMed] [Google Scholar]
- 30.Saleh NE, Alhusseiny SM, El-Zayady WM, Aboelnaga EM, El-Beshbishi WN, et al. Trichomonas vaginalis serostatus and prostate cancer risk in Egypt: a case-control study. Parasitol Res. 2021;120:1379–1388. doi: 10.1007/s00436-020-06942-7. [DOI] [PubMed] [Google Scholar]
- 31.Jary A, Teguete I, Sidibe Y, Kodio A, Dolo O, et al. Prevalence of cervical HPV infection, sexually transmitted infections and associated antimicrobial resistance in women attending cervical cancer screening in Mali. Int J Infect Dis. 2021;108:610–616. doi: 10.1016/j.ijid.2021.06.024. [DOI] [PubMed] [Google Scholar]
- 32.Miller JM, Chambers DC, Miller JM. Infection with Trichomonas vaginalis in a black population. J Natl Med Assoc. 1989;81:701–702. [PMC free article] [PubMed] [Google Scholar]
- 33.Samoff E, Koumans EH, Markowitz LE, Sternberg M, Sawyer MK, et al. Association of Chlamydia trachomatis with persistence of high-risk types of human papillomavirus in a cohort of female adolescents. Am J Epidemiol. 2005;162:668–675. doi: 10.1093/aje/kwi262. [DOI] [PubMed] [Google Scholar]
- 34.Misra JS, Singh U. Results of longterm hospital based cytological screening in asymptomatic women. Diagn Cytopathol. 2006;34:184–187. doi: 10.1002/dc.20377. [DOI] [PubMed] [Google Scholar]
- 35.Verteramo R, Pierangeli A, Mancini E, Calzolari E, Bucci M, et al. Human Papillomaviruses and genital co-infections in gynaecological outpatients. BMC Infect Dis. 2009;9:16. doi: 10.1186/1471-2334-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tao L, Han L, Li X, Gao Q, Pan L, et al. Prevalence and risk factors for cervical neoplasia: a cervical cancer screening program in Beijing. BMC Public Health. 2014;14:1185. doi: 10.1186/1471-2458-14-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ghosh I, Muwonge R, Mittal S, Banerjee D, Kundu P, et al. Association between high risk human papillomavirus infection and co-infection with Candida spp. and Trichomonas vaginalis in women with cervical premalignant and malignant lesions. J Clin Virol. 2017;87:43–48. doi: 10.1016/j.jcv.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 38.Belfort IKP, Cunha APA, Mendes FPB, Galvao-Moreira LV, Lemos RG, et al. Trichomonas vaginalis as a risk factor for human papillomavirus: a study with women undergoing cervical cancer screening in a northeast region of Brazil. BMC Womens Health. 2021;21:174. doi: 10.1186/s12905-021-01320-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rughooputh S, Greenwell P. Trichomonas vaginalis: paradigm of a successful sexually transmitted organism. Br J Biomed Sci. 2005;62:193–200. doi: 10.1080/09674845.2005.11732710. [DOI] [PubMed] [Google Scholar]
- 40.Mercer F, Diala FG, Chen YP, Molgora BM, Ng SH, et al. Leukocyte lysis and cytokine induction by the human sexually transmitted parasite Trichomonas vaginalis. PLoS Negl Trop Dis. 2016;10:e0004913. doi: 10.1371/journal.pntd.0004913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ronn MM, Turner KM. The dawn of novel STI prevention methods: modelling potential unintended effects of changes in cervical cancer screening guidelines on trichomoniasis. Sex Transm Infect. 2018;94:161–162. doi: 10.1136/sextrans-2018-053534. [DOI] [PubMed] [Google Scholar]
- 42.Mekki F, Ivic J. Significance of variations in the size of Trichomonas vaginalis in patients with dysplasia, intrapithelial and invasive planocellular carcinoma of the uterine cervix. Jugosl Ginekol Opstet. 1979;18:15–19. [PubMed] [Google Scholar]
- 43.Landolfo S, Giovarelli M, Martinotti MG, Varesio L, Cappuccinelli P. Enhancement versus tumor resistance induced by different levels of immunodepression in BALB/c mice with protozoan infections. Eur J Cancer. 1979;15:27–33. doi: 10.1016/0014-2964(79)90201-9. [DOI] [PubMed] [Google Scholar]
- 44.Ishiguro T. Gas chromatographic studies on propionic acid, butyric acid and valeric acid in culture fluid of Trichomonas vaginalis. Nihon Sanka Fujinka Gakkai Zasshi. 1984;36:363–368. [PubMed] [Google Scholar]
- 45.Viikki M, Pukkala E, Nieminen P, Hakama M. Gynaecological infections as risk determinants of subsequent cervical neoplasia. Acta Oncol. 2000;39:71–75. doi: 10.1080/028418600431003. [DOI] [PubMed] [Google Scholar]
- 46.Afzan MY, Suresh K. Pseudocyst forms of Trichomonas vaginalis from cervical neoplasia. Parasitol Res. 2012;111:371–381. doi: 10.1007/s00436-012-2848-3. [DOI] [PubMed] [Google Scholar]
- 47.Yusof AM, Kumar S. Phenotypic 'variant' forms of Trichomonas vaginalis trophozoites from cervical neoplasia patients. Exp Parasitol. 2012;131:267–273. doi: 10.1016/j.exppara.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 48.Oz ZS, Gun BD, Ozdamar SO. Light microscopic observation on phagocytosis of Candida spp. blastospores by Trichomonas vaginalis in a patient with anal canal carcinoma. Cytopathology. 2012;23:207–209. doi: 10.1111/j.1365-2303.2011.00858.x. [DOI] [PubMed] [Google Scholar]
- 49.Neace CJ, Alderete JF. Epitopes of the highly immunogenic Trichomonas vaginalis alpha-actinin are serodiagnostic targets for both women and men. J Clin Microbiol. 2013;51:2483–2490. doi: 10.1128/JCM.00582-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fiori PL, Diaz N, Cocco AR, Rappelli P, Dessi D. Association of Trichomonas vaginalis with its symbiont Mycoplasma hominis synergistically upregulates the in vitro proinflammatory response of human monocytes. Sex Transm Infect. 2013;89:449–454. doi: 10.1136/sextrans-2012-051006. [DOI] [PubMed] [Google Scholar]
- 51.Twu O, Dessi D, Vu A, Mercer F, Stevens GC, et al. Trichomonas vaginalis homolog of macrophage migration inhibitory factor induces prostate cell growth, invasiveness, and inflammatory responses. Proc Natl Acad Sci USA. 2014;111:8179–8184. doi: 10.1073/pnas.1321884111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sutcliffe S. Sexually transmitted infections and risk of prostate cancer: review of historical and emerging hypotheses. Future Oncol. 2010;6:1289–1311. doi: 10.2217/fon.10.95. [DOI] [PubMed] [Google Scholar]
- 53.Zhu Z, Davidson KT, Brittingham A, Wakefield MR, Bai Q, et al. Trichomonas vaginalis: a possible foe to prostate cancer. Med Oncol. 2016;33:115. doi: 10.1007/s12032-016-0832-y. [DOI] [PubMed] [Google Scholar]
- 54.Han IH, Kim JH, Kim SS, Ahn MH, Ryu JS. Signalling pathways associated with IL-6 production and epithelial-mesenchymal transition induction in prostate epithelial cells stimulated with Trichomonas vaginalis. Parasite Immunol. 2016;38:678–687. doi: 10.1111/pim.12357. [DOI] [PubMed] [Google Scholar]
- 55.Kim JH, Kim SS, Han IH, Sim S, Ahn MH, et al. Proliferation of prostate stromal cell induced by benign prostatic hyperplasia epithelial cell stimulated with Trichomonas vaginalis via crosstalk with mast cell. Prostate. 2016;76:1431–1444. doi: 10.1002/pros.23227. [DOI] [PubMed] [Google Scholar]
- 56.Zhu Z, Zhao L, Brittingham A, Bai Q, Wakefield MR, et al. Trichomonas vaginalis inhibits HeLa cell growth through modulation of critical molecules for cell proliferation and apoptosis. Anticancer Res. 2018;38:5079–5086. doi: 10.21873/anticanres.12827. [DOI] [PubMed] [Google Scholar]
- 57.Alam R, Barbarovich AT, Caravan W, Ismail M, Barskaya A, et al. Druggability of the guanosine/adenosine/cytidine nucleoside hydrolase from Trichomonas vaginalis. Chem Biol Drug Des. 2018;92:1736–1742. doi: 10.1111/cbdd.13341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Han IH, Kim JH, Jang KS, Ryu JS. Inflammatory mediators of prostate epithelial cells stimulated with Trichomonas vaginalis promote proliferative and invasive properties of prostate cancer cells. Prostate. 2019;79:1133–1146. doi: 10.1002/pros.23826. [DOI] [PubMed] [Google Scholar]
- 59.Jang KS, Han IH, Lee SJ, Yoo J, Kim YS, et al. Experimental rat prostatitis caused by Trichomonas vaginalis infection. Prostate. 2019;79:379–389. doi: 10.1002/pros.23744. [DOI] [PubMed] [Google Scholar]
- 60.Han IH, Song HO, Ryu JS. IL-6 produced by prostate epithelial cells stimulated with Trichomonas vaginalis promotes proliferation of prostate cancer cells by inducing M2 polarization of THP-1-derived macrophages. PLoS Negl Trop Dis. 2020;14:e0008126. doi: 10.1371/journal.pntd.0008126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Raffone A, Travaglino A, Angelino A, Esposito R, Pontillo M, et al. Gardnerella vaginalis and Trichomonas vaginalis infections and the risk of persistence or progression of low-grade cervical intraepithelial neoplasia. Pathol Res Pract. 2020;216:153234. doi: 10.1016/j.prp.2020.153234. [DOI] [PubMed] [Google Scholar]
- 62.Chung HY, Kim JH, Han IH, Ryu JS. Polarization of M2 macrophages by interaction between prostate cancer cells treated with Trichomonas vaginalis and adipocytes. Korean J Parasitol. 2020;58:217–227. doi: 10.3347/kjp.2020.58.3.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kushwaha B, Devi A, Maikhuri JP, Rajender S, Gupta G. Inflammation driven tumor-like signaling in prostatic epithelial cells by sexually transmitted Trichomonas vaginalis. Int J Urol. 2021;28:225–240. doi: 10.1111/iju.14431. [DOI] [PubMed] [Google Scholar]
- 64.Perot P, Falguieres M, Arowas L, Laude H, Foy JP, et al. Investigation of viral etiology in potentially malignant disorders and oral squamous cell carcinomas in non-smoking, non-drinking patients. PLoS ONE. 2020;15:e0232138. doi: 10.1371/journal.pone.0232138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kamarkhani Z, Rafiei-Sefiddashti R, Haghighi L, Badirzadeh A, Hadighi R. Molecular examination of Trichomonas vaginalis infection and risk of prostate cancer in the biopsy of patients with different prostate lesions. Ethiop J Health Sci. 2021;31:237–240. doi: 10.4314/ejhs.v31i2.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim SS, Kim JH, Han IH, Ahn MH, Ryu JS. Inflammatory responses in a benign prostatic hyperplasia epithelial cell line (BPH-1) infected with Trichomonas vaginalis. Korean J Parasitol. 2016;54:123–132. doi: 10.3347/kjp.2016.54.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim JH, Han IH, Shin SJ, Park SY, Chung HY, et al. Signaling role of adipocyte leptin in prostate cell proliferation induced by Trichomonas vaginalis. Korean J Parasitol. 2021;59:235–249. doi: 10.3347/kjp.2021.59.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article and additional information files.