Abstract
Background
The prevalence of prediabetes is increasing in young adults and patients undergoing coronary angiography. However, whether prediabetes is a considerable risk factor for all-cause mortality remains undetermined in young patients undergoing coronary angiography.
Methods
In this study, we retrospectively included 8868 young patients (men aged < 45 years, women aged < 55 years) who underwent coronary angiography (CAG). Patients were categorized as normoglycemic, prediabetes and diabetes according to the HbA1c level or documented history of diabetes. The association of all-cause mortality with diabetes and prediabetes was detected by Cox proportional hazards regression analysis.
Results
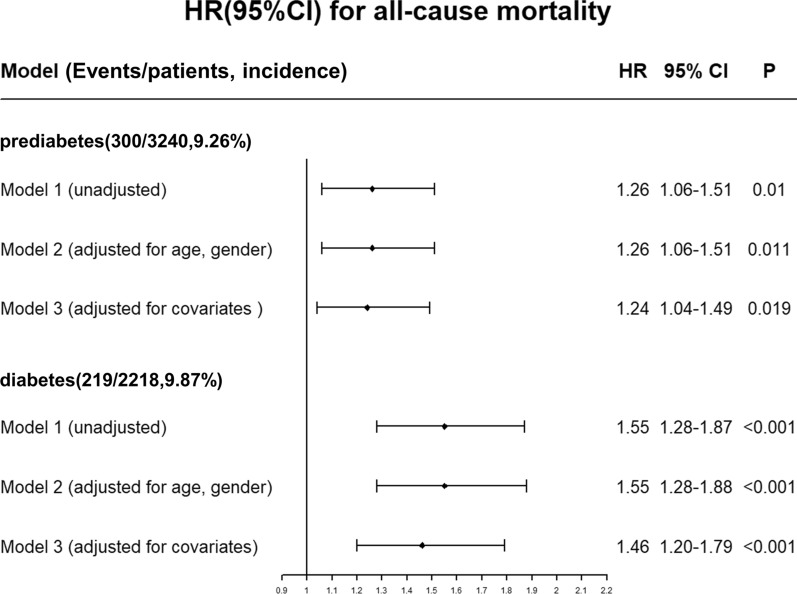
A total of 3240 (36.5%) among 8868 young patients receiving CAG were prediabetes and 2218 (25.0%) were diabetes. 728 patients died during a median follow-up of 4.92 years. Compared to the normoglycemic group, prediabetes increased the risk of all-cause mortality in young CAG patients by 24%(adjusted HR: 1.24, 95% CI: 1.04–1.49, p = 0.019) and diabetes increased the risk of all-cause mortality by 46%(adjusted HR:1.46, 95% CI:1.2–1.79, p < 0.001). Subgroup analysis showed that diabetes and prediabetes increased the risk of death mainly in patients without comorbidities.
Conclusion
Prediabetes accounts for more than one-third of the young adults undergoing CAG and was associated with an increased risk of all-cause mortality, active prevention strategy should be considered for these patients.
Keywords: Coronary artery angiography, Mortality, Prediabetes, Young adults
Introduction
Prediabetes is defined as the intermediate metabolic state between normoglycemia and diabetes mellitus. According to the American Diabetes Association (ADA) guidelines, prediabetes is defined as the level of HbA1c ranged from 38.8 mmol/mol (5.7%) to 47.5 mmol/mol (6.4%) for patients without known diabetes [1]. Previous studies have demonstrated the current prevalence of prediabetes is elevated, especially in the young population [2, 3]. In a survey of the Chinese general population, the prevalence of prediabetes in adults aged 30–39 years has reached 29.9% [4]. On the other hand, prediabetes was reported more prevalent in patients undergoing coronary angiography (CAG) than in the general population [5, 6]. Marín also found that prediabetes is common in young patients with ST-elevation myocardial infarction (STEMI). As a conventional method for diagnosis of coronary artery disease (CAD), the number of people undergoing coronary artery angiography is increasing [7], with a notable increase in young adults [8]. However, there is still a lack of research on the prevalence and impact of prediabetes on young patients undergoing CAG in China.
Studies have shown that prediabetes increases the risk of cardiovascular disease (CVD) and kidney disease with increased mortality [9, 10], while reversing to normoglycemia from prediabetes prompted reducing the corresponding risk [11]. Compared with old adults, young adults with abnormal blood glucose are reported with a higher risk of mortality [12]. However, it has also been found that in patients with CAD, prediabetes is not associated with the risk of cardiovascular mortality and all-cause mortality [13]. The relationship between prediabetes and cardiovascular disease and all-cause mortality remains equivocal in young patients.
In this study, we aim to investigate the prevalence and effect of prediabetes on all-cause mortality in a large, multi-center cohort of young patients undergoing coronary angiography in China.
Methods
Study population
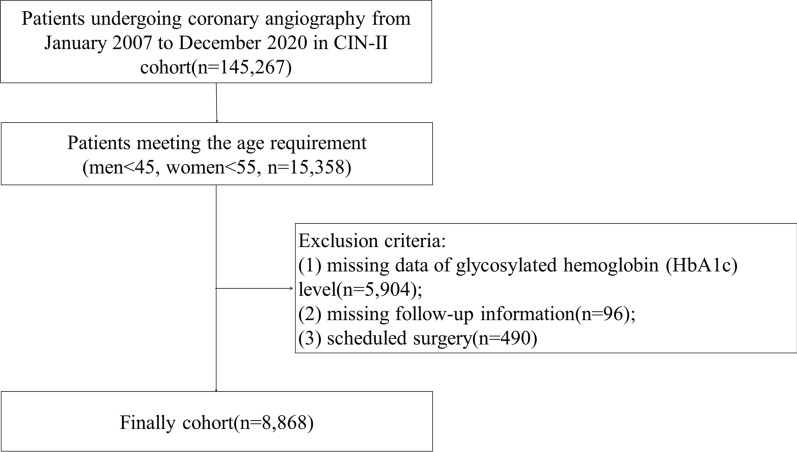
This cohort study analyzed data from the Cardiorenal Improvement II (CIN-II) study, which is a multi-center cohort study with patients enrolled at five large tertiary hospitals (Cardiorenal Improvement II, ClinicalTrials.gov NCT05050877) in China. A total of 145,267 patients undergoing CAG from January 2007 to December 2020 were enrolled. We included patients of young age (men < 45, women < 55; n = 15,358). Patients with missing data on glycosylated hemoglobin (HbA1c) level (n = 5,904), or follow-up information (n = 96), with scheduled cardiac surgery (n = 490) were excluded. Eventually, 8868 patients undergoing CAG were enrolled (Fig. 1). The study was approved by the ethics committee of the participant hospital and complied with the Declaration of Helsinki.
Fig. 1.
Flowchart of the study
Data collection
Data was extracted from the electronic clinical management system (ECMS) of each participant hospital. The baseline data comprised the demographic details, medical history, laboratory examination and other clinical information. Patients were subjected to follow-up by trained nurses or assistants after discharge and the follow-up data was obtained by telephone or clinical visits to the patients, otherwise the National Death Registry Database was searched for mortality outcome if necessary.
Definition and outcome
The ADA’s standards in HbA1c were adopted for the definition of prediabetes and diabetes. Patients with HbA1c lower than 38.8 mmol/mol (5.7%) and no prior diagnosis of all kinds of diabetes were categorized as normoglycemic, with HbA1c ranging from 38.8 mmol/mol (5.7%) to 47.5 mmol/mol (6.4%) and no prior diagnosis of all kinds all diabetes were defined as prediabetes while HbA1c higher than 47.5 mmol/mol (6.5%) or with documented hypoglycemic therapy were defined as diabetes (Type II). The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [16], and chronic kidney disease (CKD) was defined as eGFR < 60 mL/min/1.73m2 [17]. Congestive heart failure (CHF) was defined as New York Heart Association class > 2 or Killip class > 1. Acute myocardial infarction (AMI), type 2 diabetes mellitus, and hypertension (HT) were defined according to the 10th Revision Codes of the International Classification of Diseases (ICD-10).
The primary outcome was the all-cause mortality which was acquired from the follow-up information recorded by trained staff, the research in the National Death Registry Database was also available for mortality outcome if necessary.
Statistical analysis
Continuous variables were presented as means (standard deviations [SD]) or median quartiles (IQRs), categorical variables were presented as frequency counts and percentages. Patients' demographic characteristics, medical histories, admission information, and clinical features were listed. One-way analysis of variance (ANOVA) was used to investigate differences between each group. Kaplan–Meier curves were constructed to visually represent time-to-event data, and log-rank tests were employed to evaluate survival across the groups. Covariates enrolled in the multivariate model were screened by stepwise cox regression analysis and based on the clinical significance, including age, gender, triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), CKD, CHF, CAD, AMI, percutaneous coronary intervention (PCI), angiotensin-converting enzyme inhibitor/ angiotensin receptor blocker (ACEI/ARB), beta-blocker, calcium channel blocker, uremic acid, estimated glomerular filtration rate, systolic blood pressure and history of smoking. Multiple imputation was performed for the missing data. To avoid the potential collinearity between variables, variance inflation factors (VIF) were calculated. Subgroup analyses were also performed according to different comorbidities including age, CAD, CHF, CKD, AMI, and PCI. P values derived from two-tailed tests, and values < 0.05 were deemed statistically significant. All statistical analyses were performed using R, version 4.0.3 software (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline characteristics
A total of 8868 young patients (mean age 43.99 ± 6.90 years, 53.6% were men) undergoing CAG were enrolled in the study. 2218 (25.0%) patients were categorized as diabetes while 3240 (36.5%) were prediabetes. There were 4777 (54.4%) patients were diagnosed with CAD, 3608 (40.7%) patients underwent PCI, 3195 (36.4%) with hypertension, 1754 (20.0%) with acute myocardial infarction (AMI), 984 (11.2%) with CHF, and 511 (5.8%) with CKD. Compared to the normoglycemic group, patients with prediabetes and diabetes were older, more likely to be female and have comorbidities such as AMI, CHF, CKD, CAD, and hypertension. Their TG, TC and LDL-C were higher than those with normoglycemic, while the levels of high-density lipoprotein cholesterol (HDL-C) were lower. The details of the clinical baseline characteristics are shown in Table 1.
Table 1.
Baseline characteristics according to the glycemic status
| Characteristic | Metabolic status | ||||
|---|---|---|---|---|---|
| Overall | Normoglycemia | Prediabetes | Diabetes | P-value | |
| n = 8868 | n = 3410 | n = 3240 | n = 2218 | ||
| Demographic characteristics | |||||
| Age(years) | 44.0 ± 6.9 | 42.5 ± 7.2 | 44.8 ± 6.5 | 45.0 ± 6.6 | < 0.001 |
| Female | 4112(46.4%) | 1408(41.3%) | 1585(48.9%) | 1119(50.5%) | < 0.001 |
| Medical history and clinical condition | |||||
| Smoke | 0.197 | ||||
| None | 4533(74.5) | 1688(73.4) | 1742(74.3) | 1103(76.5) | |
| Current | 1316(21.6) | 517(22.5) | 518(22.1) | 281(19.5) | |
| Past | 235(3.9) | 94(4.1) | 84(3.6) | 57(4.0) | |
| AMI | 1754(20.0) | 665(19.6) | 526(16.5) | 563(25.6) | < 0.001 |
| CHF | 984(11.2) | 311(9.2) | 327(10.2) | 346(15.7) | < 0.001 |
| CKD | 511(5.8) | 135(4.0) | 159(4.9) | 217(9.8) | < 0.001 |
| CAD | 4777(54.4) | 1685(49.7) | 1579(49.4) | 1513(68.7) | < 0.001 |
| PCI | 3608(40.7) | 1230(36.1) | 1189(36.7) | 1189(53.6) | < 0.001 |
| COPD | 11(0.1) | 5(0.1) | 2(0.1) | 4(0.2) | 0.428 |
| HT | 3195(36.4) | 999(29.5) | 1109(34.7) | 1087(49.4) | < 0.001 |
| AF | 338(3.8) | 98(2.9) | 157(4.9) | 83(3.8) | < 0.001 |
| SBP (mmHg) | 126.8 ± 18.7 | 125.6 ± 18.0 | 126.3 ± 18.5 | 129.5 ± 19.8 | < 0.001 |
| Laboratory examination | |||||
| HbA1c (%) | 6.2 ± 1.4 | 5.3 ± 0.3 | 6.0 ± 0.2 | 7.8 ± 1.8 | < 0.001 |
| Neutrophil(× 1012/L) | 5.3 ± 2.9 | 5.1 ± 2.8 | 5.0 ± 2.7 | 5.8 ± 3.1 | < 0.001 |
| Lymphocyte(× 1012/L) | 2.1 ± 0.7 | 2.0 ± 0.7 | 2.2 ± 0.7 | 2.2 ± 0.8 | < 0.001 |
| Albumin(g/L) | 39.2 ± 4.3 | 39.6 ± 4.2 | 39.2 ± 4.2 | 38.7 ± 4.7 | < 0.001 |
| TG (mmol/L) | 1.9 ± 1. 6 | 1.6 ± 1.3 | 1.8 ± 1.3 | 2.4 ± 2.1 | < 0.001 |
| TC (mmol/L) | 4.8 ± 1.3 | 4.7 ± 1.3 | 4.9 ± 1.3 | 4.9 ± 1.4 | < 0.001 |
| HDL-C(mmol/L) | 1.1 ± 0.3 | 1.1 ± 0.3 | 1.1 ± 0.3 | 1.0 ± 0.3 | < 0.001 |
| LDL-C(mmol/L) | 3. 0 ± 1.1 | 2.9 ± 1.0 | 3.1 ± 1.1) | 3.0 ± 1.1 | < 0.001 |
| eGFR(ml/min/1.73m2) | 100.4(85.2, 108.8) | 101.8(87.3, 109.7) | 99.5(85.0, 107.4) | 100.4(80.9, 109.4) | < 0.001 |
| Uric acid(μmol/L) | 382.6 ± 114.8 | 374.3 ± 110.3 | 385.4 ± 111.6 | 391.6 ± 124.8 | < 0.001 |
| Medication during hospitalization | |||||
| ACEI | 2985(36.0) | 1056(34.0) | 1062(34.7) | 867(40.7) | < 0.001 |
| ARB | 1535(18.5) | 468(15.1) | 544(17.8) | 523(24.6) | < 0.001 |
| β-blockers | 5617(67.7) | 1996(64.3) | 1986(64.9) | 1635(76.8) | < 0.001 |
| CCB | 1986(23.9) | 737(23.7) | 681(22.3) | 568(26.7) | 0.001 |
| Statins | 6082(73.3) | 2164(69.7) | 2167(70.8) | 1751(82.2) | < 0.001 |
| Aspirin | 5534(66.7) | 1975(63.6) | 1907(62.3) | 1652(77.6) | < 0.001 |
| Diuretic | 1198(14.4) | 355(11.4) | 480(15.7) | 363(17.1) | < 0.001 |
AMI acute myocardial infarction, CHF congestive heart failure, CKD chronic kidney disease, CAD coronary artery disease, PCI percutaneous interventions, COPD chronic obstructive pulmonary disease, HT hypertension, AF atrial fibrillation, SBP Systolic blood pressure, HbA1c glycosylated hemoglobin, TG triglycerides, TC total cholesterol, LDL-C low-density lipoprotein cholesterol, HDL-C High-density lipoprotein cholesterol, eGFR estimated glomerular filtration rate, ACEI angiotensin-converting enzyme inhibitor, ARB angiotensin receptor blocker, CCB calcium channel blocker
Prediabetes and clinical outcomes
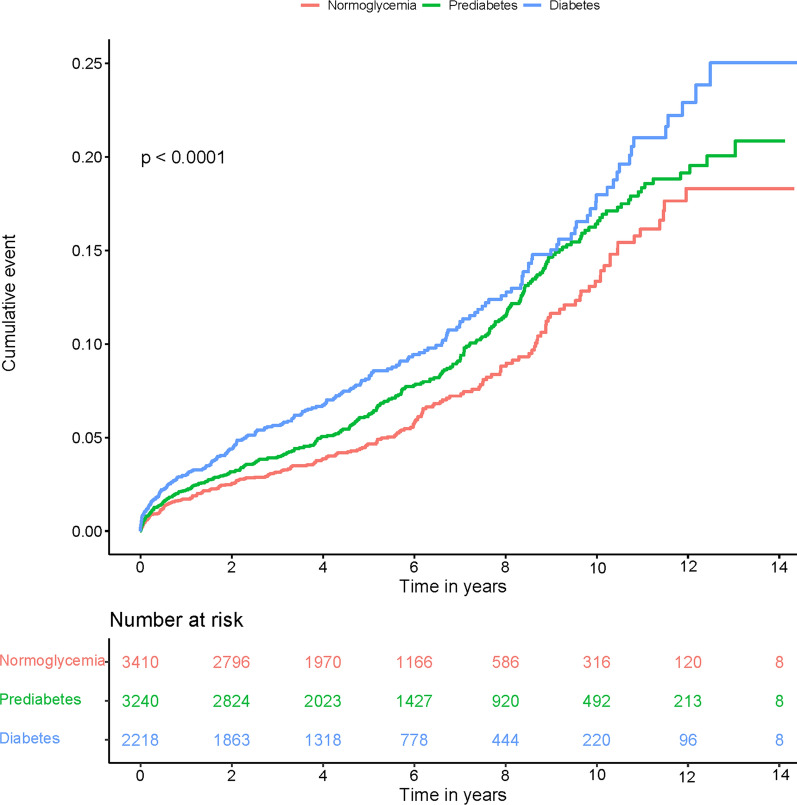
During a mean follow-up of 4.92 years, a total of 728 patients died including 209 in the normoglycemia group, 300 in the prediabetes group and 219 in the diabetes group. The time-to-event curves showed that patients with prediabetes had a increased risk in all-cause mortality compared with normoglycemic patients, and diabetes was associated with a higher risk of mortality (Fig. 2). Cox regression analysis showed that prediabetes and diabetes significantly increased all-cause mortality in young CAG patients by comparison to the normoglycemic group (adjusted HR: 1.24, 95% CI: 1.04–1.49, p = 0.019; adjusted HR:1.46, 95% CI:1.2–1.79, p < 0.001) (Fig. 3). The variance inflation factors showed no significant covariance among each of the incorporated covariates (VIF < 5).
Fig. 2.
Kaplan–Meier curves of all-cause mortality
Fig. 3.
Unadjusted and adjusted HRs and 95% CIs for the primary end point (all-cause mortality) of diabetes and prediabetes Model 1: unadjusted; Model 2: adjusted for age and gender; Model 3: adjusted for age, gender, triglycerides, low-density lipoprotein cholesterol, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker, beta-blocker, calcium channel blocker, uremic acid, estimated glomerular filtration rate, systolic blood pressure, history of smoking, and comorbidities including chronic kidney disease, congestive heart failure, coronary artery disease, acute myocardial infarction, percutaneous coronary intervention
Subgroup analysis
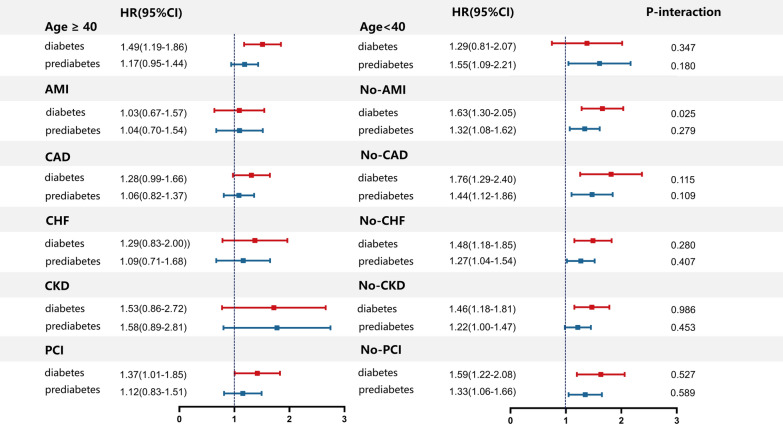
In the subgroup analysis, Cox regression analysis showed that diabetes increased the risk of mortality in patients without AMI, CAD, CHF, CKD, age ≥ 40 years old and those with or without receiving PCI at baseline (Fig. 4). On the other hand, prediabetes was associated with the elevated risk of mortality in patients without AMI, CAD, CHF, CKD, PCI and age < 40. However, the interactions between the subgroups regarding the effect of prediabetes and diabetes on mortality were insignificant generally.
Fig. 4.
Forest plots of hazard ratio for all-cause mortality in subgroup
Discussion
In summary, our data showed that prediabetes increased the risk of all-cause mortality in young patients undergoing CAG by 24% and diabetes increased the risk of all-cause mortality by 45% compared to the normoglycemic group. It emphasizes the additional risk of prediabetes for young patients beyond diabetes, which should be considered earlier in prevention.
There are few studies demonstrating the prevalence and importance of prediabetes in young adults who underwent CAG. Among young STEMI patients, the prevalence of prediabetes was 24% [7]. In addition, the prevalence of prediabetes was significantly higher in young patients receiving CAG compared to the corresponding age group in the general population [3]. Our results indicated that over 1/3 of young patients were characterized as prediabetes, which emphasized the importance of routine testing for prediabetes in young patients undergoing CAG.
It was controversial whether prediabetes increases the risk of cardiovascular disease and all-cause mortality [14–16]. Previous studies have demonstrated the increased risk of CVD and all-cause mortality were associated with the presence of prediabetes in young adults without diabetes and prior CVD [17]. In Japanese workers, the relationship between prediabetes and all-cause mortality has also been investigated [18]. However, an innovative cross-sectional study to reduce cardiovascular complications in diabetes (ARTEMIS) showed that prediabetes is not associated with the risk of cardiovascular mortality and all-cause mortality in patients with CAD [13]. In older adults, prediabetes and newly diagnosed diabetes are not significantly associated with a higher risk of all-cause mortality [19–21]. Huang et al. found no significant association between prediabetes alone and long-term mortality in the general population, but the predictive power of prediabetes for the mortality risk appears to be stronger among low-risk populations (younger and White participants) [22].
Prediabetes was also defined according to various criteria in different guidelines and studies, including impaired glucose tolerance (IGT) and impaired fasting glucose (IFG). IGT is defined as an oral glucose tolerance test 2-h plasma glucose of 7.8–11.0 mmol/L, while IFG is defined by the World Health Organization (WHO) and the ADA as fasting blood glucose of 6.1–6.9 mmol/L (IFG-WHO) and 5.6–6.9 mmol/L (IFG-ADA), respectively [23, 24]. According to Echouffo-Tcheugui’s study, prediabetes in different definitions was related to adverse prognostic risks, including cardiovascular disease, renal disease, and all-cause mortality, with varying effect sizes, depending on the definitions used [23]. Warren’s study reported that HbA1c was more specific than postprandial glucose in screening for prediabetes, improving risk discrimination for clinical complications [25]. On the other hand. a large cross-sectional study has shown that prediabetes is associated with worse outcomes, regardless of the definition adopted [26]. Huang et al. have revealed through meta-analysis that prediabetes was associated with an increased risk of adverse events regardless of different definitions both in the general population and patients with atherosclerotic cardiovascular disease [9, 27]. Nonetheless, further studies are warranted to verify whether the definition of prediabetes would affect the prognosis in the large-scale cohort study of young patients.
Our study showed that prediabetes and diabetes detected by HbA1c can predict a higher risk of all-cause mortality in young adults undergoing CAG. However, in the subgroup analysis, prediabetes and diabetes increased the risk of long-term mortality mainly in young patients received CAG without comorbidities at baseline and the association was insignificant in those patients with AMI, CAD, CHF, CKD or undergoing PCI at baseline. This may partially explain the controversy over prediabetes as a risk factor for all-cause mortality. In patients with existing comorbidities or older age, who have higher mortality and complex risk factors, the role of type 2 diabetes and prediabetes may be masked. However, in younger adults with fewer risk factors, prediabetes and type 2 diabetes may turn out to be one of the primary risk factors. In this study, we confirmed that in young patients undergoing CAG, prediabetes still appeared to be significantly associated with all-cause mortality. Therefore, prediabetes may remain a concern for young patients undergoing CAG.
Limitation
Several limitations exist in the study. First, as an observational study, our results were influenced by its nature and do not reflect direct cause-and-effect relationships. However, we included more patients compared with previous studies [28–30] and our results still provided a reference for the debates on the prognostic impact of prediabetes. Second, although we have made adjustments for variables as much as we can, there could be potential confounding factors that we may have overlooked. We were unable to evaluate data from additional aspects since our data did not contain concrete causes of mortality and other adverse events. Third, we failed to investigate the evolution in glucose metabolism over time and we did not have sufficient data on fasting glycemia to evaluate the effect of prediabetes on mortality in various definitions. The possibility that the increase in mortality resulted from the conversion of prediabetes to diabetes cannot be excluded. Further study on tracking the changes of prediabetes in young patients receiving CAG is recommended.
Conclusion
In summary, our study demonstrated that prediabetes was common among young patients undergoing CAG and prediabetes was an independent risk factor for all-cause mortality among them, especially in patients without previous complications.
Acknowledgements
We appreciate the contribution of all study participants. The authors thank the follow-up staff of the Department of Cardiology, Guangdong Cardiovascular Institute, Guangdong Provincial People’s Hospital, for their excellent work.
Abbreviations
- ACEI
Angiotensin-converting enzyme inhibitor
- ARB
Angiotensin receptor blocker
- ADA
American Diabetes Association
- AMI
Acute myocardial infarction
- CAG
Coronary artery angiography
- CAD
Coronary artery disease
- CKD
Chronic kidney disease
- CHF
Congestive heart failure
- ECMS
Electronic clinical management system
- eGFR
Estimated glomerular filtration rate
- HT
Hypertension
- LDL-C
Low-density lipoprotein cholesterol
- PCI
Percutaneous coronary intervention
- STEMI
ST-elevation myocardial infarction
- TC
Total cholesterol
- TG
Triglycerides
Author contributions
YBH, HYL, YHL, JL, SJY, ZYZ and TC substantially contributed to the conception or design of the work. YBH, HYL, YHL, JYC, SQC and YL contributed to the acquisition of the data. JL, SJY, ZYZ, and TC analyzed and interpreted the work. YBH and HYL drafted the article. All authors revised, reviewed and approved the final version of the manuscript. YL is the guarantor of this work.
Funding
This work was supported by grants from Guangdong Provincial science and technology project (2020B1111170011); Guangdong Provincial science and technology project (KJ022021049); Guangdong Provincial Key Laboratory of Coronary Heart Disease Prevention (No.2017B030314041; Y0120220151), National Science Foundation for Young Scientist of China (Grant No.82070360) and High-level Hospital Construction Project (DFJH2020026). The work was not funded by any industry sponsors. The study sponsor/funder was not involved in the design of the study; the collection, analysis, and interpretation of data; writing the report; and did not impose any restrictions regarding the publication of the report.
Availability of data and materials
The datasets generated and analyzed during the current study are not publicly available due to the institution policy but are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The Ethics Committee of the Guangdong Provincial People's Hospital approved the study (No. GDREC2019-555H-2). All participating sites received institutional review board approval from their own ethics committees. It was conducted in accordance with the principles of the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
The original online version of this article was revised: The error in the funding note has been corrected.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yibo He, Hongyu Lu, and Yihang Ling contributed equally to this work and share first authorship
Change history
6/29/2023
A Correction to this paper has been published: 10.1186/s12933-023-01902-8
Contributor Information
Yong Liu, Email: liuyong@gdph.org.cn.
Shiqun Chen, Email: shiqunchen@126.com.
Jiyan Chen, Email: chenjiyandr@126.com.
References
- 1.Marx N, Davies MJ, Grant PJ, Mathieu C, Petrie JR, Cosentino F, et al. Guideline recommendations and the positioning of newer drugs in type 2 diabetes care. Lancet Diabetes Endocrinol. 2021;9(1):46–52. doi: 10.1016/S2213-8587(20)30343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andes LJ, Cheng YJ, Rolka DB, Gregg EW, Imperatore G. Prevalence of prediabetes among adolescents and young adults in the United States, 2005–2016. JAMA Pediatr. 2020;174(2):e194498. doi: 10.1001/jamapediatrics.2019.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saeedi P, Salpea P, Karuranga S, Petersohn I, Malanda B, Gregg EW, et al. Mortality attributable to diabetes in 20–79 years old adults, 2019 estimates: results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract. 2020;162:108086. doi: 10.1016/j.diabres.2020.108086. [DOI] [PubMed] [Google Scholar]
- 4.Li Y, Teng D, Shi X, Qin G, Qin Y, Quan H, et al. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American Diabetes Association: national cross sectional study. BMJ. 2020;369:m997. doi: 10.1136/bmj.m997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bullard KM, Saydah SH, Imperatore G, Cowie CC, Gregg EW, Geiss LS, et al. Secular changes in U.S. Prediabetes prevalence defined by hemoglobin A1c and fasting plasma glucose: National Health and Nutrition Examination Surveys, 1999–2010. Diabetes Care. 2013;36(8):2286–2293. doi: 10.2337/dc12-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang JS, Lee IT, Lee WJ, Lin SY, Fu CP, Lee WL, et al. Comparing HbA1c, fasting and 2-h plasma glucose for screening for abnormal glucose regulation in patients undergoing coronary angiography. Clin Chem Lab Med. 2015;53(9):1441–1449. doi: 10.1515/cclm-2014-0860. [DOI] [PubMed] [Google Scholar]
- 7.Mata Marín LA, Schmucker J, Fach A, Osteresch R, Rühle S, Garstka D, et al. Prevalence and clinical characteristics of prediabetes and diabetes mellitus in young patients with ST-segment elevation myocardial infarction. Clin Res Cardiol. 2021;110(10):1647–1658. doi: 10.1007/s00392-021-01868-1. [DOI] [PubMed] [Google Scholar]
- 8.Park JS, Lee HJ, Kim YJ, Seong IW, Lee JW, Kim JJ, et al. The epidemiological and clinical characteristics of patients admitted for coronary angiography to evaluate ischemic heart disease. Korean J Intern Med. 2007;22(2):87–92. doi: 10.3904/kjim.2007.22.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang Y, Cai X, Mai W, Li M, Hu Y. Association between prediabetes and risk of cardiovascular disease and all cause mortality: systematic review and meta-analysis. BMJ. 2016;355:i5953. doi: 10.1136/bmj.i5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park KS, Hwang SY. Lifestyle-related predictors affecting prediabetes and diabetes in 20–30-year-old young Korean adults. Epidemiol Health. 2020;42:e2020014. doi: 10.4178/epih.e2020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu X, Wu S, Song Q, Wang X. Reversion from pre-diabetes mellitus to normoglycemia and risk of cardiovascular disease and all-cause mortality in a Chinese population: a prospective cohort study. J Am Heart Assoc. 2021;10(3):e019045. doi: 10.1161/JAHA.120.019045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rhee EJ, Jung I, Kwon H, Park SE, Kim YH, Han KD, et al. Increased mortality burden in young asian subjects with dysglycemia and comorbidities. J Clin Med. 2020;9(4):1042. doi: 10.3390/jcm9041042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiviniemi AM, Lepojärvi ES, Tulppo MP, Piira OP, Kenttä TV, Perkiömäki JS, et al. Prediabetes and risk for cardiac death among patients with coronary artery disease: the ARTEMIS study. Diabetes Care. 2019;42(7):1319–1325. doi: 10.2337/dc18-2549. [DOI] [PubMed] [Google Scholar]
- 14.Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41(2):255–323. doi: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]
- 15.Welsh C, Welsh P, Celis-Morales CA, Mark PB, Mackay D, Ghouri N, et al. Glycated hemoglobin, prediabetes, and the links to cardiovascular disease: data from UK biobank. Diabetes Care. 2020;43(2):440–445. doi: 10.2337/dc19-1683. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X, Wu H, Fan B, Shi M, Lau ESH, Yang A, et al. The role of age on the risk relationship between prediabetes and major morbidities and mortality: analysis of the Hong Kong diabetes surveillance database of 2 million Chinese adults. Lancet Reg Health West Pac. 2023;30:100599. doi: 10.1016/j.lanwpc.2022.100599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim SM, Lee G, Choi S, Kim K, Jeong SM, Son JS, et al. Association of early-onset diabetes, prediabetes and early glycaemic recovery with the risk of all-cause and cardiovascular mortality. Diabetologia. 2020;63(11):2305–2314. doi: 10.1007/s00125-020-05252-y. [DOI] [PubMed] [Google Scholar]
- 18.Islam Z, Akter S, Inoue Y, Hu H, Kuwahara K, Nakagawa T, et al. Prediabetes, diabetes, and the risk of all-cause and cause-specific mortality in a Japanese working population: Japan epidemiology collaboration on occupational health study. Diabetes Care. 2021;44(3):757–764. doi: 10.2337/dc20-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang O, Matsushita K, Coresh J, Sharrett AR, McEvoy JW, Windham BG, et al. Mortality implications of prediabetes and diabetes in older adults. Diabetes Care. 2020;43(2):382–388. doi: 10.2337/dc19-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao Q, Xin Z, He R, Wang T, Xu M, Lu J, et al. Age-specific difference in the association between prediabetes and subclinical atherosclerosis: an analysis of a chinese prospective cohort study. Cardiovasc Diabetol. 2022;21(1):153. doi: 10.1186/s12933-022-01592-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deedwania P, Patel K, Fonarow GC, Desai RV, Zhang Y, Feller MA, et al. Prediabetes is not an independent risk factor for incident heart failure, other cardiovascular events or mortality in older adults: findings from a population-based cohort study. Int J Cardiol. 2013;168(4):3616–3622. doi: 10.1016/j.ijcard.2013.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang YQ, Liu L, Huang JY, Chen CL, Yu YL, Lo K, et al. Prediabetes and risk for all-cause and cardiovascular mortality based on hypertension status. Ann Transl Med. 2020;8(23):1580. doi: 10.21037/atm-20-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Echouffo-Tcheugui JB, Selvin E. Prediabetes and what it means: the epidemiological evidence. Annu Rev Public Health. 2021;42:59–77. doi: 10.1146/annurev-publhealth-090419-102644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.International Expert Committee International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32(7):1327–1334. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Warren B, Pankow JS, Matsushita K, Punjabi NM, Daya NR, Grams M, et al. Comparative prognostic performance of definitions of prediabetes: a prospective cohort analysis of the Atherosclerosis Risk in Communities (ARIC) study. Lancet Diabetes Endocrinol. 2017;5(1):34–42. doi: 10.1016/S2213-8587(16)30321-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ali MK, Bullard KM, Saydah S, Imperatore G, Gregg EW. Cardiovascular and renal burdens of prediabetes in the USA: analysis of data from serial cross-sectional surveys, 1988–2014. Lancet Diabetes Endocrinol. 2018;6(5):392–403. doi: 10.1016/S2213-8587(18)30027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cai X, Zhang Y, Li M, Wu JH, Mai L, Li J, et al. Association between prediabetes and risk of all cause mortality and cardiovascular disease: updated meta-analysis. BMJ. 2020;370:m2297. doi: 10.1136/bmj.m2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maroszyńska-Dmoch EM, Wożakowska-Kapłon B. Clinical and angiographic characteristics of coronary artery disease in young adults: a single centre study. Kardiol Pol. 2016;74(4):314–321. doi: 10.5603/KP.a2015.0178. [DOI] [PubMed] [Google Scholar]
- 29.Juan-Salvadores P, Jiménez Díaz VA, Iglesia Carreño C, Guitián González A, Veiga C, Martínez Reglero C, et al. Coronary artery disease in very young patients: analysis of risk factors and long-term follow-up. J Cardiovasc Dev Dis. 2022;9(3):82. doi: 10.3390/jcdd9030082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faisal AWK, Habib G, Yasmin S, Latif W, Ahmed S. Angiographic patterns of coronary artery disease in young patients presenting at a tertiary cardiac center. Pak J Med Sci. 2022;38(8):2107–2111. doi: 10.12669/pjms.38.8.6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study are not publicly available due to the institution policy but are available from the corresponding author on reasonable request.