Abstract
Paeonia emodi Wall. ex Royle is commonly known as Himalayan paeony has great importance as a food and medicine. The practice of Paeonia emodi Wall. ex Royle is very ancient and it is conventionally used for a wide range of illnesses in the folk system of medicine because of its wide beneficial phytochemical profile. The main purpose of the current review was the synthesis of recent data on botany, ethnopharmacology, phytochemistry and potential pharmacological mechanisms of action of Paeonia emodi Wall. ex Royle, thus offering new prospects for the development of new adjuvant natural therapies. Using scientific databases such as PubMed/MedLine, Scopus, Web of Science, ScienceDirect, Google Scholar, Springer, and Wiley, a comprehensive literature search was performed for Paeonia emodi Wall. ex Royle. For searching, we used the next MeSH terms: “Biological Product/isolation and purification”, “Biological Products/pharmacology”, “Drug Discovery/methods”, “Ethnopharmacology, Medicine”, “Traditional/methods”, “Paeonia/chemistry”, “Plant Extracts/pharmacology”, “Phytochemicals/chemistry”, “Phytochemicals/pharmacology”, “Plants, Medicinal”. The results of the most recent studies were analyzed and the most important data were summarized in tables and figures. Phytochemical research of Paeonia emodi Wall. ex Royle has led to the isolation of triterpenes, monoterpenes, phenolic acids, fatty acids, organic compounds, steroids, free radicals and some other classes of primary metabolites. In addition, diverse pharmacological activities like antibacterial, antifungal, anticoagulant, airway relaxant lipoxygenase and beta-glucuronidase inhibiting activity, radical scavenging activity, phytotoxic and insecticidal activities have been reported for Paeonia emodi Wall. ex Royle. Different bioactive compounds of Paeonia emodi Wall. ex Royle has proven their therapeutic potential in modern pharmacological and biomedical research to cure numerous gastrointestinal and nervous disorders. In future, further in vitro and in vivo therapeutic studies are required to identify new mechanisms of action, pharmacokinetics studies, and new pharmaceutical formulations for target transport and possible interaction with allopathic drugs. Also, new research regarding quality evaluation, toxicity and safety data in humans is needed.
Keywords: Paeoniaemodi, Traditional uses, Phytochemistry, Pharmacological activities
Introduction
Paeonia emodi commonly called “Himalayan Peony” is found in the northern territories of Pakistan [1, 2]. Paeonia emodi is a medicinal plant that belongs to the Paeoniaceae family [3]. It is renowned as "the queen of herbs" [4]. Paeonia emodi Wall. ex Royle is a versatile medicinal plant of major relevance in the Himalayan region [5]. The plant is widely distributed in Pakistan, India, and Afghanistan. Paeonia was named after a Greek legend about a medical student named Paeon who healed Pluto's wound. Pluto later spared Paeon from death by transforming him into the peony, a medicinal plant that is still used today [6]. In different regions, different vernacular names are used for Paeonia emodi Wall. ex Royle for example in Urdu it is undsalib, in English it is called Paeoney rose, Himalayan paeony and in hindi it is called Pawin, Chandayra, Ud-salap [4, 6]. Because of its high therapeutic worth, the plant is intensely gathered in the region. Monoterpene glycosides, lactiflorin, paeoniflorin, peoninol, oxypaeflorine are some compounds that are reported from this plant. Previously monoterpene glycosides have been reported from the roots of Paeonia emodi Wall. ex Royle [7, 8]. Oleanolic acid, betulinic, ethyl gallate, methyl grevillate, and 1,5-dihydroxy-3-methylanthraquinone are some of the components extracted from this plant. [9]. Different constituents are monoterpene glycosides, wurdin and 15 benzoyl wurdin alongside paeoniflorin, lactiflorin and oxypaeoniflorin [7]. The different other constituents extracted from this plant incorporate a β-glucuronidase-repressing triterpene, Pa 11,beta,5alpha,23,24-pentahydroxy-30-norolean-12,20(29)-d ien-28-oic acid, oleanolic acid, betulinic acid, ethyl gallate, methyl grevillate and 1,5-dihydroxy-3-methylanthraquinone [9]. Different constituents are monoterpene glycosides, wording and benzoylwurdin alongside paeoniflorin, lactiflorin and oxypaeoniflorin [7].
The current review is an updated and novel report of traditional uses, pharmacology, the potential mechanism of actions and phytochemical constituents of Paeonia emodi Wall. ex Royle. This review paper provides a recent comprehensive literature review on the importance of its conservation and future economical sustainability. It additionally features the logical reason for future research on Paeonia emodi Wall. ex Royle and its genuine potential for the improvement of the market for homegrown therapeutic items.
Methodology
The relevant literature was collected through a bibliographic investigation conducted in the next scientific databases PubMed/MedLine, Scopus, Web of Science, ScienceDirect, Google Scholar, Springer, and Wiley using the next MeSH terms: “Biological Product/isolation & purification”, “Biological Products/pharmacology”, “Drug Discovery/methods”, “Ethnopharmacology, Medicine”, “Traditional/methods”, “Paeonia/chemistry”, “Plant Extracts/pharmacology”, “Phytochemicals/chemistry”, “Phytochemicals/pharmacology”, “Plants, Medicinal”.
Inclusion criteria: (i) relevant papers which included traditional uses, phytochemistry and modern pharmacological studies (ii) studies who included in vitro and in vivo experiments along with potential mechanisms of action (iii) papers written in the English language.
Exclusion criteria: (i) duplicates and incomplete information, ii) abstracts, letter to the editor, short communications, (iii) experiments made using homeopathic preparations associated, (iv) studies written in another language than English. Chemical constituents of the plant were identified, IUPAC names and structural and chemical formulas were confirmed from ChemSpider and PubChem. The taxonomy of the plant has been validated according to WFO [10].
Botany
Paeonia emodi Wall. ex Royle. has ternary or bi-ternary, glabrous leaves; blossom singular, white, or pale pink flowers [11]. It's a glabrous perennial herb with thick tuberous roots that grow in clumps and leaves are arranged in a ternate pattern, with decurrent whole or incised leaflets. Flowers are white-coloured (25–10 cm across) and have black, silky, and gleaming seeds [1]. As defined its botany differently is enduring herbs, up to 70 cm tall [12]. The stem is smooth. Proximate leaves 2-ternary; a few handouts fragmented; pamphlets and sections up to 15, oval elliptic or ovoid lanceolate, 9–13 × 2–3.5 cm, the two surfaces smooth, base cuneate, decurrent, highest point hone. Blooms 2–4 for each shoot, both terminal and axillary, single, 8–12 cm wide, all or simply terminal one made. Bracts 3–6, leaf-like, lanceolate. Sepal's ca. 3, suborbicular, ca. 1.5 × 1.5 cm, zenith caudate. Petals white, obovate, ca. 4.5 × 2.4 cm. Filaments 1.5–2 cm. Plate a nular. Carpel 1(or 2), light yellow tomentose, now and again glabrous. Follicles ovoid, 2–3.5 × 1–2 cm. Seeds dull, globose. The blooming period is from May to March. Leaves are ternary with lamina pale. Blooms are particular, axillary and of red concealing. Bracts are verdant, petals are 8 and seeds move from 3–5 [13]. It bears oblong-lanceolate leaves that are glabrous on both sides [14]. Under a microscope, the foliar epidermis is made up of irregularly shaped epidermal cells with undulating walls. Adaxial epidermal cells measure 71.5 m in length and 73.5 m in breadth, whereas abaxial epidermal cells measure 88.5 m in length and 76 m in width. Stomata are often anomocytic, with varying lengths and widths [15]. Flowers are white and are arranged in a terminal or axillary arrangement on branches. The blooms are mostly bracteates, with suborbicular sepals and obovate petals. The fruit is a follicle that contains ovoid seeds that are lobose black. Pollens are tricolporate, monad, and spherical in polar viewpoint, but perprolate in equatorial perspective (Fig. 1). The pole diameter (polar view) is 38.14 m, the equatorial diameter is 30.87 m, the P/E ratio is 1.23 m, the colpi length is 12.3 m, the width is 15.83 m, and the exine thickness is 2 [4].
Fig. 1.

The medicinal plant Paeonia emodi
It propagates healthy in high altitude cool, climate zones; displays well in deep, loamy, and humid soil. The plant can be found growing in narrow valleys or glens with streams. To thrive, the plant requires a lot of moisture and nutrients. The plant flourishes in the shade of Juglans regia (walnut) and Populus deloides (cottonwood poplar). Paeonia is a food source for the locals. After being collected from the forest, the leaves are cooked and kept in the shape of leaf cakes for a long time. Paeonia is eaten raw, as well as fermented and sun-dried. It is an ancient, indigenous treatment as well as a traditional technique utilized by the Bhotiya tribal community to treat stomach issues.
Ethnopharmacology
In the current period of science and innovation, individuals in the creating nations depend on conventional arrangements of medicinal services because of two main reasons, i-e low price and fewer symptoms contrasted with cutting-edge allopathic medications [16–18]. For the treatment of different sicknesses, plants have been used from the beginning of time. In the developing world, traditional practices are an important part of the primary healthcare system [19, 20].
Paeonia emodi Wall. ex Royle has been used for therapeutic purposes since ancient times. Today, most of the ethnopharmacological data of the Paeonia emodi Wall. ex Royle species is found in traditional Chinese medicine [6]. The Chinese used Paeonia emodi Wall. ex Royle to treat inflammation and hypertension. The Chinese boiled the root of the white peony and used the obtained decoction in various ailments such as joint pain, hepatitis, muscle cramps, nervous disorders, cardiovascular diseases, gynecological ailments. Its uses are recorded in the ancient Chinese medicine book "Eastern Han Dynasty" from AD 25-220 [21]. Information about this plant also appears in the "Pharmacopea of the People's Republic of China" [22]. Anti-inflammatory, analgesic, immunomodulatory, antioxidant, sedative and antimicrobial properties were attributed to the Paeonia emodi Wall. ex Royle [23].
In the local and old-fashioned systems of medicine, Paeonia emodi Wall. ex Royle has extensively been used because of its wide beneficial profile. Paeonia emodi Wall. ex Royle has been broadly used in traditional treatment for a broad range of illnesses like Stomach problems, muscle problems, intestine problems, fever, pain killers and headaches. The plant is also traditionally used for uterine illnesses, colic, epilepsy, convulsions, hysteria, obstructions, and dropsy. The whole plant is highly medicinal as a mixture of dried flowers is extremely helpful in diarrhea while seeds are cathartic and emetic [24]. Its roots, stem, leaves, seeds and flowers are used medicinally in different forms and sometimes in combination with other herbs. The tubers of Paeonia emodi Wall. ex Royle is valuable for uterine ailments, colic, nauseous impediments, dropsy, epilepsy, seizures and madness and is likewise given to kids as a blood purifier [25]. The underground plant parts are utilized to fix spinal pain, edema and brain abnormality and are likewise utilized as an energizer, vomit inducer, cleansing, blood purifier and bellyache while seeds are laxative [26]. It is likewise utilized in spine hurt, tonic, emetic, cleansing, and blood purifier. It is additionally utilized in dropsy, epilepsy and colic and body tonic.
Detailed information about plant parts used, ailments treated and mode of utilization are provided in Table 1.
Table 1.
Different ailments treated by Paeonia emodi Wall. ex Royle and their mode of utilization
| Country | Local name | Part used | Ailments | Mode of utilization | Refs. |
|---|---|---|---|---|---|
| Pakistan | – | Rhizome | Backache and tonic | – | [27] |
| Mamekh Ud-e-Saleeb | Roots, tuber, flowers, Seeds, fruit | Stomach problems, muscles problems, intestine problems, fever, pain killer, headache | – | [28] | |
| Mamakhi | Leaves | Epilepsy, blood purifier, indigestion, headache, dizziness, vomiting | Boiled extract | [29] | |
| Flowers | General body tonic and diarrhea | Decoction | [12] | ||
| Rhizome | Backbone ache, tonic, cathartic | [11] | |||
| Blood purifier, epilepsy, colic, blood tonic | |||||
| Back pain and common weakness | [30] | ||||
| Himalayan peony | Flower | Antidiarrheal, hemorrhoids, expectorant, antispasmodic | [31] | ||
| Mamekh | Seeds, tuber | Rheumatism and backache | Paste and powder | [29] | |
| Rhizome, seeds | Backache, general weakness, blood purifier, tonic | Decoction | [32] | ||
| Epilepsy, cathartic, colic, purgative | |||||
| Roots | Internal injuries | Paste | [33] | ||
| Diarrhea, rheumatic pain, gynecological disorders, vomiting | Powdered | [34, 35] | |||
| Roots, rhizome | Backbone ache, tonic, cathartic, epilepsy, purify blood |
Paste, extract decoction |
[36] | ||
| Rhizome | Stomach problems | Extract | [37] | ||
| Mamekh | Tuber, flowers, seeds, petals | Hysteria, uterine diseases, colic, convulsions, bile duct problems hemorrhoids, varicose veins problem, hypertension, obstruction, blood purifier, cathartic, diarrhea, cough | Infusion and decoction |
[35] [38] |
|
|
Whole plant – |
Dysentery | Decoction | [39] | ||
| Tonic/analgesic | [26] | ||||
| Tuber, seeds, flower, whole plant | Nervous diseases, uterine diseases, colic, bilious obstruction, dropsy, epilepsy, convulsions, hysteria, diarrhea vomiting, cholera, eye diseases, tuberculosis |
[35] [38] |
|||
| Stem, tuber | Joint pain, bone fractures, epilepsy, convulsions, hysteria, colic, uterine diseases, bilious obstructions, dropsy, blood purifier | Powder and paste |
[40] [38] |
||
| Tuber, flowers | Epileptic attacks, cholera, whooping, cough, uterus diseases, colic, bilious, obstruction, dropsy, convulsions, hysteria, diarrhea, cathartic | Infusion | [35] | ||
| Rhizome | Backache, general weakness, skeletomuscular problems | Powder | [41] | ||
| Wounds, cuts, narcotic, tonic, tumor, anticancer, stimulant | |||||
| Chandra | Roots, shoots, leaves | Whooping, cough, intestinal spasms, cuts, post-natal care | Infusion and paste | [42] | |
| Mameikh | Tuber, flowers, seeds, roots, twigs, leaves | Nervous disorders, stomach complaints, purgative | Juice | [43] | |
| Body pain, uterus disorder, blood purifier, skin diseases, backache weakness |
[44] [38] |
||||
| Mamaikh | Rhizome, seeds | Backbone ache, dropsy, epilepsy, cathartic, blood purifier, colic, purgative, tonic | Extract |
[45] [35] |
|
| Rhizome | Backbone ache, tonic, cathartic, blood purifier, dropsy, epilepsy, colic |
[46] [35] |
|||
| Pamekh, Mamekh | Rhizome | Anti-rheumatic, stomach ailments | Extract | [47] | |
| Rhizomes, roots and seeds | Backbone ache, dropsy, epilepsy, tonic, cathartic, blood purifier | [48] | |||
| Rhizome and roots | Purgative, headache, dizziness, vomiting, pregnancy, cathartic backache, headache, dizziness, vomiting, edema, epilepsy, therapeutic, blood cleanser, helps in pregnancy laxative, bellyache | [49] | |||
| Mamekh | Rhizome | Backache and stimulant | – | [27] | |
| Shoot | Body pains, heals fractured bones | – | [50] | ||
| China | – | Stem, tuber | Joint pain, bone fractures, epilepsy, convulsions, hysteria, colic | Powder and paste |
[40] [35] |
| Ethiopia | Chandra | Leaves | Uterine diseases, bilious obstructions, dropsy, blood purifier | – | [51] |
| India | Chandra | Tuber, flowers | Epileptic attacks, cholera, whooping, cough, uterus diseases | Infusion |
[24] [38] |
| Indigestion, seizures, dropsy, epilepsy, mania | |||||
| Mental disorder, rheumatism, urine complaints | |||||
| Leaves | Dysentery, blood purifier | [1] | |||
| Dhandaru | Roots | Skin diseases | Paste | [52] | |
| Chandra | Roots | Intestinal pain, dysentery, piles | Decoction | [53] | |
| Epilepsy, cathartic, colic, purgative | |||||
| Tuber and roots | Bilious, obstructions, biliousness | - | [54] | ||
| Leaves, roots | Blood dysentery, diabetes, improve lactation, hysteria, epilepsy | Powder |
[55] [38] |
||
| Roots, rhizome | Uterine diseases, biliousness, dropsy, nervous system | Infusion | [56] | ||
| Blood purifier, cathartic, diarrhea, cardiovascular | |||||
| Headache, hysteria, abdominal spasms, nervine tonic | |||||
| Respiratory illnesses, high blood pressure, atherosclerosis | |||||
| Bhoi, Pawin | Roots | Stomach problems | Decoction | [57] | |
| Udsaleeb | Roots, stem, leaves | Dyspepsia, dysentery, diarrhea, fever, blood, purifier | – |
[58] [38] |
|
| Rheumatism, urinary troubles, colic, convulsions | |||||
| Dropsy, cuts, ulcers, wounds, mental diseases | |||||
| Chandra | Leaves | Blood purifier, dysentery, digestive disorders, foul ulcer | Boiled fried | [59] | |
| Leucorrhoea | Decoction | [55] | |||
| Leaves and root | Epilepsy | Powder | [60] | ||
| Leaves | Dysentery, haemoglobin deficiency | – |
[59] [38] |
Frequently treated ailments
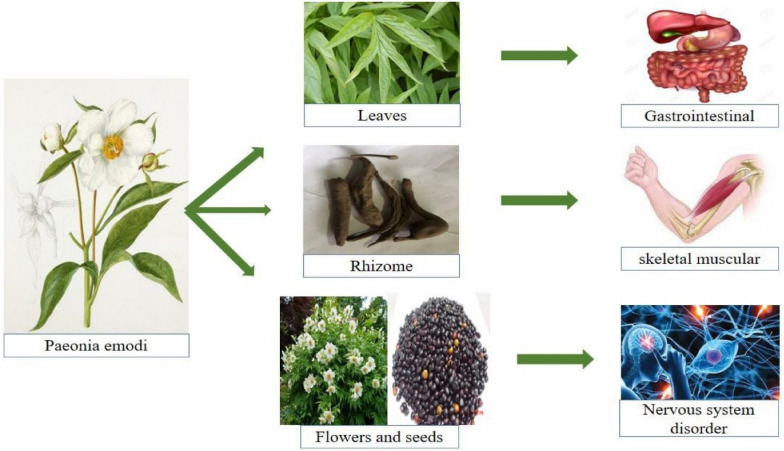
Among frequently treated ailments, the top-listed category was treated by Paeonia emodi Wall. ex Royle extracts, were gastrointestinal diseases, followed by skeletomuscular and nervous disorders; other common diseases include fever, headache, wounds, renal/urinary, gynecological, bone disorders, psychiatric disorders, respiratory and liver complaints, cardiovascular and cancer. Numerous mediators have provided detail about the pharmacokinetic importance of plants used in gastrointestinal messes [61–72]. The leaves and roots of Paeonia emodi Wall. ex Royle is used to treating many GIT problems in the form of decoction [1, 51, 57]. Paeonia emodi Wall. ex Royle rhizome powder is also used to treat pain [73]. Because of the accessibility of plants with dynamic fixings, basic oil and mixes that are profoundly compelling against colitis, gastritis, intestinal worms and disease, and so on, as industrious by ethnopharmacological considers, plant species for the cure of gastrointestinal problems play an important role in traditional medicines in the Madonie Mountains. [74–77]. Figure 2 shows major ailments treated by Paeonia emodi Wall. ex Royle.
Fig. 2.
Major aliments treated by Paeonia emodi Wall. ex Royle
Plant parts used
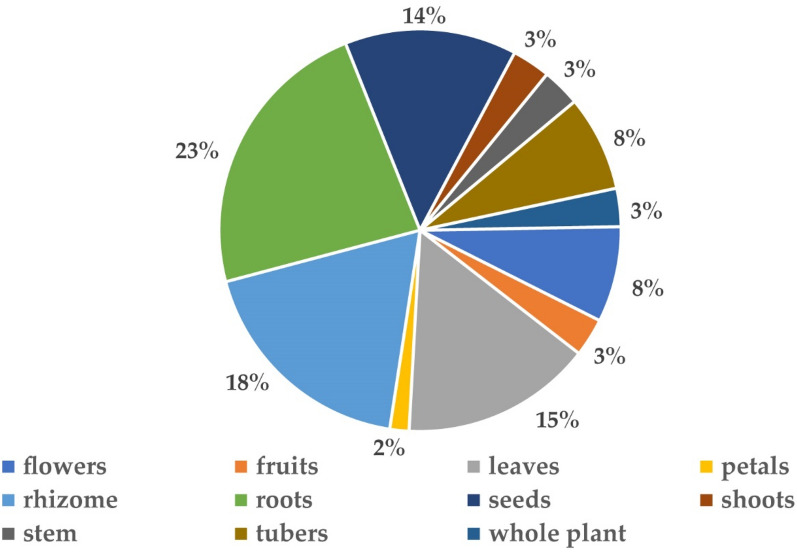
Most of the time entire plant and in many circumstances various plant parts, including root, stem, leaves, rhizome, bark, tuber, and seeds are used for the cure of different ailments. In many studies, various parts of the plant are blended for making readiness which is then used as a drug. Roots are the most often used plant parts (23%), followed by flowers (18%), leaves (15%), rhizomes (14%), tubers (8%), fruits, stem, shoots (3%) (Fig. 3). Rhizome was the main medicinal part that is used for the treatment of different diseases. For the preparation of medicine, several parts of the individual plant were used, among all 38% of species were used for their rhizome/roots [53]. Rhizome was generally used for the treatment of backache [27, 30]. Roots of Paeonia emodi Wall. ex Royle was used for treating many skin diseases [52]. The same results were obtained in the present review that rhizomes and roots were the most frequently used part for the preparation of different medicines to cure many ailments.
Fig. 3.
Different plant parts used in Paeonia emodi Wall. ex Royle
Preparation and administration
For the administration of herbal medicinal plants, distinctive readiness strategies are utilized which include decoction, combination, powder, squeeze, and glue. In our study main method of preparation is decoction, powder (19%), paste, extract (17%), followed by infusion (11%), fried (8%), cooked (3%), and juice (3%) (Fig. 4). The most widely recognized strategy for arrangement is decoction and mixture which reveals comparative discoveries [78–80]. A decoction is the major primary prescription form in Ayurveda [81]. It is created by boiling or heating the necessary plant material in water, whereas distillation is created by hanging the necessary plant material in either warm or cold water. After the plant has completely dried, the powder is produced by crushing the entire plant or a part of it. Huge numbers of arrangements are made by utilizing water as a dissolvable medium. Many people utilize castor oil, coconut oil, ginger, neem, and mustard oil in the creation of adhesive and ointment. The most common way of taking medicine is oral ingestion for internal use but in some ailments, topical application is frequently employed in the form of paste. Decoction of the leaves is administered for dyspepsia, jaundice, and cardiac and respiratory problems and is used as a tonic [82]. The major mode of utilization decoction and infusion is frequently taken as tea and broths [79, 83]. The decoction of the flowers is given for diarrhea and used as a general body tonic while root decoction is administered for various stomach problems [12, 57]. The nutritional value of Paeonia species flowers is well known. Peony blooms are high in protein, sugar, superoxide dismutase, and other nutrients, according to studies. [6]. Our results of the current review matched with the results of previous reviews in the way that the most frequently used part of Paeonia emodi Wall. ex Royle is rhizome and roots and the major mode of utilization is decoction. It might be because the rhizome is hard and its decoction is a preferred use. In previous literature of ethnomedicinal studies, the decoction was frequently used due to the activation of some chemical compounds upon heating.
Fig. 4.
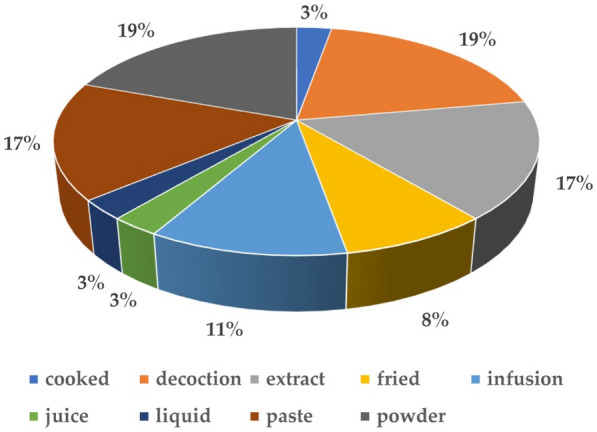
Preparation and mode of administration
For treatments of diverse diseases, the use of plant extract and its associated phytochemicals are of great importance [20, 84, 85]. Worldwide different studies have been conducted to confirm the pharmacological and medicinal effects of these phytochemicals [86–88] As per the World Health Organization, the best source to get an assortment of medications was therapeutic plants. Therefore, investigations were conducted to understand the properties, efficacy, and safety [89, 90]. The investigation of organically dynamic mixes from plants had consistently been of incredible interest to researchers [91–93]. Several constituents were isolated from Paeonia emodi Wall. ex Royle which shows significant sedative and anti-inflammatory activities [94]. The ethanolic concentrate of Paeonia emodi Wall. ex Royle was fractioned into n-hexane, ethyl acetic acid derivation, and dichloromethane while its aqueous fraction was left for phytochemical screening [2]. From the tuber of Paeonia emodi Wall. ex Royle large numbers of secondary metabolites were isolated which were used for the treatment of numerous diseases like rheumatism, uterine diseases, nervous disorders etc. [2, 94]. Qualitative phytochemical screening of Paeonia emodi Wall. ex Royle rhizome contains a large number of compounds such as carbohydrates, phenolics, tannins, reducing sugars, cardiac glycosides, anthraquinone glycosides, terpenes, steroids, resins and oxalic, tartaric, citric and ascorbic acids [95]. In Paeonia emodi Wall. ex Royle the absolute phenolic substance of hydroalcoholic extricate was seen as 375.83 ± 3.82 (mg GAE/g) while that of aqueous extract was 187.83 ± 2.52 (mg GAE/g) [95]. Previously from the roots of Paeonia emodi Wall. ex Royle monoterpene glycosides and triterpene were reported [94].
Triterpenes
In the previously conducted studies, different triterpenes were identified. In the present review, it comprises 22.7% out of all investigated phytochemicals. Primary triterpenes include emodinol, β-amyrin, lupeol, 24-methylenecycloartanol, cycloartenol, betulinic acid and oleanolic acid. Monoterpenes glycosides [7] and triterpenes [9] from Paeonia emodi Wall. ex Royle was reported in other studies. For the triterpenes, Emodinol was the first time confined from the chloroform dissolvable part of Paeonia emodi Wall. ex Royle and it showed significant β-glucuronidase inhibitory action [96]. Betulinic acid, oleanolic acid are some of the components isolated from this plant [9]. Triterpenes were the principal class of compound occurring in Paeonia emodi Wall. ex Royle. Some triterpenes were reported from time to time from the extract of this plant e.g. Emodinol [56]. Oleanolic acid, a triterpene, has been demonstrated to have anticoagulant, cardioprotective, calming effect, lipoxygenase and glucuronidase inhibitory, free-radical scavenging activities as well as significant herbicidal and antibacterial activity. 24-methylenecycloartanol was triterpene determined anti-inflammatory activity while the same chemical was reported by [56] similar pharmacological activities. β-amyrin and lupeol are two important triterpenes that showed anti-bacterial activity while the former also showed antiulcer properties [4].
Monoterpenes
In the previously conducted research studies, different monoterpenes were identified. In the present review, 12.1% of monoterpenes have been identified from all the phytochemicals. The primary monoterpenes isolated are lactiflorin, paeonin (A, B, C), wurdin, benzoyl wurdin, oxypaeoniflorin, paeoniflorin [94]. Paeonin A and paeonin B were segregated as a dismal sticky solid [96]. From the chloroform-dissolvable portion of Paeonia emodi Wall. ex Royle roots, Paeonin A and B and some new monoterpenes galactosides were isolated which showed potent lipoxygenase inhibitory activity [96]. Recent research studies on Paeonia emodi Wall. ex Royle investigated main constituents such as monoterpenes, triterpenes, and polyphenols and showed potential biological activities such as chemopreventive, cytotoxic, and cardioprotective activities [94]. According to [7] the main constituents in the phytochemical of Paeonia emodi Wall. ex Royle were monoterpenes paeoniflorin, lactiflorin, and oxypaeoniflorin are among the glycosides, wurdin, and benzoylwurdin. Numerous biological effects were ascribed to the specific chemotaxonomic indicators, paeoniflorin, and their derivatives, which were monoterpenes with a pinane skeleton [97]. Wurdin and benzoylwurdin were the two important monoterpenes showing anticoagulant activity, lipoxygenase and glucuronidase inhibitory, and free radical scavenging activities, cardioprotective and relaxing properties [56] while the same monoterpene was reported by [98] showing significant herbicidal and antibacterial activities. The crude extract of Paeonia emodi Wall. ex Royle showed maximum inhibitory activity against an obligate parasite S. typhi and a gram-negative bacteria S. flexeneri while Paeonia emodi Wall. ex Royle aerial portions had strong herbicidal action but no antifungal or antibacterial activity [48]. The two important monoterpenes (oxypaeoniflorin, Paeoniflorin) showed phytotoxic activity against Lemna aeguinoctailis and anticoagulant, cardiovascular, lipoxygenase, β-glucuronidase inhibitory, free radical scavenging and antibacterial activities [24, 56, 98]
Phenolic acids
Phenolic acid was important phytochemical derived from the aerials parts of Paeonia emodi Wall. ex Royle. In all previously conducted studies, different types of phenolic acid were identified. The present review divulges 16.6% of total phytochemicals. Paeonol, hydroxybenzoic acid, gallic acid, methyl gallate, ethyl gallate, methylgrevillate, benzoic acid, 3-hydroxy benzoic acid, 4-hydroxy benzoic acid, paeoninol, oligostilbene, and chrysophanic acid are the most common phenolic acids. [8, 56, 98]. From the extract of Paeonia emodi Wall. ex Royle specific amounts of phenol were determined using the Folin-ciocalteu reagent [99]. Paeonol and hydroxybenzoic acid were two important phenolic acids that showed inhibitory potential against the enzyme lipoxygenase and anti-oxidant activity [8]. According to [56], Gallic acid has anticoagulant, cardiovascular, and relaxing actions, as well as inhibitory and free radical quenching properties for lipoxygenase and β-glucuronidase. While the same chemical exhibit inhibitory potential against the enzyme lipoxygenase and anti-oxidant activity [8]. The phenolic acid and ethyl gallate showed significant herbicidal, antibacterial and anticoagulant activity [98]. Benzoic acid, 3-hydroxy benzoic acid, and 4-hydroxy benzoic acid all have similar anticoagulant, cardioprotective as well as inhibitory and scavenging properties for lipoxygenase, β-glucuronidase, and free radical scavenging properties. [56].
Fatty acids
Fatty acid constitutes 13.6% of the present study. Out of all the phytochemicals studied, the primary fatty acids include octanoic acid, decanoic acid, lauric acid, myristic acid, palmitic acid, palmitoleic acid, stearic acid, oleic acid, and linoleic acid [100]. The root oil of Paeonia emodi Wall. ex Royle was analyzed by [100] in which octanoic, decanoic, lauric, palmitic, stearic, oleic, linoleic, palmitoleic, myristoleic, and myristic acids were found in the saponifiable lipid. The octanoic acid showed significant antifungal and antioxidant activities. Both decanoic and lauric acid exhibit antibacterial activity while the former also exhibits antioxidant activity [100]. Probably all the fatty acids given in the table showed maximum antibacterial and antioxidant activity.
Other compounds
Previously, steroids were reported as 3% in plant. Campesterol and sitosterol were reported in the root oil of Paeonia emodi Wall. ex Royle and determined the antibacterial, antifungal and antioxidant activities [100]. Other organic compound constitutes 7.5% of all the compounds present in Paeonia emodi Wall. ex Royle. The major organic compounds are divergioic acid, chrysophanic acid, benzoylwurdin, and dichloromethane [2, 98, 101]. Divergonic acid was an important organic compound that demonstrated cytotoxicity against disease cell lines [101]. Chrysophanic acid and benzoylwurdin were reported by [98] and both organic compounds exhibited significant herbicidal and antibacterial potentials in Paeonia emodi Wall. ex Royle. Further, [2] reported free radicals scavenging, antioxidant, antibacterial, and antifungal potentials using the organic compound Dichloromethane. The other phytochemicals reported in Paeonia emodi Wall. ex Royle was d-galactose, baicalein, norhederagenin, DPPH, methyl grevillate and hydrogen peroxide [2, 8, 40, 96, 98, 101] which constitute 7.5% of all phytochemicals investigated. D-galactose was a monosaccharide sugar that showed inhibitory potentials against lipoxygenase and anti-oxidant activity [8]. The flavonoid baicalein exhibited potent lipoxygenase inhibitory activity in Paeonia emodi Wall. ex Royle [96]. Norhederagenin, a metabolite showed significant cytotoxicity against malignancy [101]. An antioxidant compound 2,2-diphenylpicrylhydrazyl displayed scavenging antioxidant activity in Paeonia emodi Wall. ex Royle [40]. Methyl grevillate was an important alkaloid that showed significant herbicidal and antibacterial potencies [98]. Hydrogen peroxide determined substantial antioxidant, antibacterial and antifungal activity [2]. These Paeonia emodi Wall. ex Royle chemicals are antioxidative, antitumor, and antipathogenic, and they help to regulate the immune system, protect the cardiovascular system, and protect the central nervous system. [102]. The main phytoconstituents of Paeonia emodi Wall. ex Royle and their pharmacological properties are summarized in Table 2.
Table 2.
Chemical constituents and their biological properties reported from Paeonia emodi Royle
| Class of compounds | Chemical compound | Sources/part of plant | Biological effect | Refs. |
|---|---|---|---|---|
| Triterpenes |
Emodinol Oleanolic acid β-amyrin Lupeol 24-methylenecycloartanol Cycloartenol Betulinic acid |
Roots/seeds/flowers/tuber |
Anticoagulant Cardioprotective ↓Lipoxygenase ↓β-glucuronidase Free-radicals Antibacterial Antiulcer |
[96] [101] [56] [4] [35] |
| Monoterpenes |
Lactiflorin, Paeonin A, B, C Wurdin Benzoyl wurdin Paeoniflorin Oxypaeoniflorin Paeoninol |
Roots/tuber/seeds |
↓Lipoxygenase Antioxidant ↓Free radicals Anticoagulant Cardioprotective Antibacterial |
[35] [94] [96] [103] [98] [48] [104] |
|
Phenolic acids |
Paeonol Hydroxybenzoic acid Gallic acid Methyl gallate Ethyl gallate Methylgrevillate Benzoic acid 3-hydroxy benzoic acid 4-hydroxy benzoic acid Oligo stilbene Chrysophanic acid Syringic acid Ethyl gallate |
Seeds/tuber/roots |
Antioxidant ↓Free radicals ↓Lipoxygenase, ↓β-glucuronidase Anticoagulant Cardioprotective |
[8] [98] [103] |
|
Fatty acids |
Octanoic acid Decanoic acid Lauric acid Myristic acid Palmitic acid Palmitoleic acid Stearic acid Oleic acid Linoleic acid Palmitoleic acid Myristoleic acid Myristic acids Butyrospermol |
Roots |
Antibacterial Antifungal Antioxidant |
[100] [35] [104] |
| Steroids |
Campesterol Sitosterol |
Roots/tuber/seeds/petals |
Antibacterial Antifungal Antioxidant |
[35] [104] |
Pharmacological properties of Paeonia emodi
Antioxidant
Natural antioxidants are a group of bioactive compounds that can help support cell integrity by neutralising free radicals, unstable molecules that the human body produces [105]. These bioactive compounds are therefore essential and indispensable for the proper functioning of the body [106–109]. Consuming antioxidants can help the body get rid of extra reactive oxygen species and free radicals [110–114]. Many unprocessed extracts and pure chemicals derived from Paeonia species have been said to have free radical-scavenging action [6]. In comparison to the diabetic nephropathy control group, the P. emodi extract treatment enhanced the level of the antioxidant enzyme. Super oxide dismutase (SOD) levels significantly rose in the pancreas, liver, and kidney [4].
Anti-inflammatory
Pain, redness and swelling are symptoms that betray inflammation and for treating these ailments conventional anti-inflammatory drugs are effective but have several side effects [115, 116]. To avoid the unwanted side effects of drugstore anti-inflammatories conventional anti-inflammatory drugs can be replaced with natural bioactive compounds [17, 19, 117, 118]. The literature is well aware of the significant anti-inflammatory properties of plant-based natural substances [91, 119–121]. It has been reported in earlier studies that the root extract of P. emodi containing poly- saccharides significantly reduced inflammation when tested in vivo on male albino rats [4]. During in vitro experiments, it is advised that P. emodi be examined to assess its anti-inflammatory potential. A focus of recent medical research has been the identification of innovative anti-inflammatory medicines derived from natural ingredients. The anti-inflammatory action of the genus Paeonia has received the most research attention [4].
Anticancer and cytotoxic properties
Cancer is a large group of diseases that vary in mode of onset, growth rate, diagnosis, detectability, invasive potential, metastasis, response to treatment and prognosis [122–126]. Numerous studies have also revealed that additional Paeonia constituents may be used to treat breast, lung, and liver cancers and leukaemia. The monoterpene 6′-O-galloylpaeoniflorin suppresses metastasis via the AMPK signalling system and has lethal effects on non-small-cell lung cancer cells [127]. There are records of the Paeonia genus being used to cure tumours, and contemporary pharmacological studies of plant extracts have partially verified its antitumor properties. Paeonia contains a variety of substances, primarily monoterpene glycosides and stilbenes, which have strong antitumor activity in vitro, further highlighting the potential benefits of Paeonia plants [6].
Anti-mutagenic
Cancer occurs as a result of somatic mutations in the cells that make up tissues [128–130]. Random mutations that constantly accumulate without having a negative impact on cell survival are passenger mutations and they do not cause clonal expansion of a malignant transformed cell, they do not promote tumour growth instead driver mutations cause oncogenesis [131, 132]. P. emodi plays a very important role in anti-mutagenic activity. The dried leaves extract of P. emodi is used in vitro to study the mutagenic effect. The extract demonstrated improved DNA protection and was able to reduce the oxidative stress brought on by the Fenton reaction [4]. However, it has been noted that more effort is required to complete the aforementioned task to achieve the proper mechanism [4].
Cardioprotective and antihyperlipidemic properties
The traditional uses of Paeonia species, which frequently entail promoting blood stasis and treating hematemesis, are connected to their cardiovascular preventive advantages. It is believed that the substances PF and paeonol may be useful in the treatment of cardiovascular diseases. The treatment of myocardial ischemia, myocardial infarction (MI), atherosclerosis, hypertension, inhibition of thrombosis, and improvement of myocardial remodelling are among the cardioprotective benefits of the genus Paeonia [4]. Hyperlipidemia is one of the primary causes of oxidative stress, a feeble antioxidant defence, diabetes, and nephropathy [133]. When fruit extracts were previously studied for the treatment of nephropathy, researchers found that this plant dramatically brought glucose levels back into the normal range [134].
Hepatoprotective
The liver is a crucial organ that manages several aspects of the digestive system and detoxifies xenobiotics produced by the body [135–137]. P. emodi extracts' hepatoprotective potential in methanol and ethanol has been studied [4].
Nephroprotective
Nephropathy is a frequent complication of life-threatening conditions like diabetes that can increase morbidity and mortality [138]. Researchers who previously investigated fruit extracts for the treatment of nephropathy discovered that this plant significantly reduced glucose levels to the normal range [139]. The root extract of P. emodi showed protective properties against diabetic nephropathy by improving blood glucose levels, associated diabetic neuropathy biomarkers, and advanced glycation end products in the kidney [4].
Antibacterial and antifungal properties
The bioactive compounds present in plants have been used for their beneficial therapeutic effects and in many preclinical pharmacological studies, their therapeutic potential for human health has been investigated. Up till now, the majority of microbes are investigated having resistance to antibiotics [140–145]. Therefore, to overcome this problem and obtain effective antimicrobial agents different plants are used by pharmaceutical companies to beat the issue of obstruction-breaking strains of microorganisms [19, 91, 146, 147]. Many scientists have paid attention to plant extracts used in herbal medicine because of the side effects and pathogenic microorganisms which have developed resistance to antibiotics [146, 148, 149]. From different parts of the world, scientists have investigated different medicinal plants with anti-microbial potential, which helped in processed food preservation, medications, natural remedies, and alternative drugs [91, 106, 117, 141, 143, 146]. Regarding the antibacterial activities of Paeonia emodi Wall. ex Royle a recent study has been carried out [39, 49]. 38 cases representing infection with nine different bacterial strains and treated with different doses of Paeonia emodi Wall. ex Royle extracts (n-hexane, chloroform, ethyl acetate, crude, ethanolic extract) have been analyzed. The different strains reported in the literature treated by E. coli, P. aeruginosa, S. aureus, S. epidermidis, S. typhi, Proteus vulgaris, and K. pneumoniae are all susceptible to Paeonia emodi Wall. ex Royle extracts in results, [49] reported maximum inhibition of P. aeruginosa and S. aureus using different extracts. In another extract maximum inhibition was observed in P. aeruginosa against (ethanolic extract), S. aureus against (ethanolic extract) and P. vulgaris against (ethanolic extract). The reason for maximum inhibition by ethanolic extract may be, that the solvent is more economical and its properties lie somewhat in between water and oil. Different fractions of Paeonia emodi Wall. ex Royle was utilized to decide the zones of inhibition of bacterial development on the agar plate [49]. It was reported by [49] Paeonia emodi Wall. ex Royle inhibited the growth of K. pneumoniae, Salmonella typhi and P. aeruginosa. E. coli showed different zone of inhibition in various extracts such as chloroform, ethyl, n-hexane and crude [49]. The different extracts were applied on Methicillin-resistant and Staphylococcus aureus and several zones of inhibition were obtained [49]. Usually, in the media MRSA was referred to as superbug and as a whole was multi-drug resistant [150]. Paeonia emodi Wall. ex Royle showed important antibacterial activity and was capable to be utilized in the treatment of irresistible ailments brought about by E. coli, P. aeruginosa, S. aureus, K. pneumonia, S. epidermidis, S. typhi, and MRSA microbes. For antifungal properties of Paeonia emodi Wall. ex Royle only a few fungal strains were tested because most of the researchers mainly focused on antibacterial activity. Paeonia emodi Wall. ex Royle demonstrated moderate antifungal movement. By negative control experiment invitro technique was applied and 400 µg of the extraction/ml of sabouraud dextrose agar was used. The ethanolic extract of Paeonia emodi Wall. ex Royle had the most substantial antifungal activity observed by the human pathogen Pseudalleschena boydii i-e 55.5% Animal pathogen Microsporum canis showed 55.1% antifungal activity by using ethanolic extract [24] while plant pathogen Fsuarium solani showed 50% antifungal activity. Further different models were used such as Trichophyton schoenleinii, Candida albicans, Aspergilus niger, Trichophyton simii, and Macrophomina phaseolina which showed significant antifungal activity. Thus, in nutshell, Paeonia emodi Wall. ex Royle displayed potential antibacterial activities against different bacterial strains (Table 3).
Table 3.
Different Paeonia emodi Wall. ex Royle mediated extracts and their antibacterial potentials
| Extract | Dose (mg/mL) | Zone of inhibition (mm) | Tested bacteria | Refs. |
|---|---|---|---|---|
| n-Hexane | 10 | 14 | Escherichia coli | [49] |
| Chloroform | 10 | 14 | E. coli | [49] |
| Ethyl acetate | 10 | 12 | E. coli | [49] |
| Crude | 10 | 16 | E. coli | [49] |
| n-Hexane | 10 | 20 | Pseudomonas aeruginosa | [49] |
| Chloroform | 10 | 18 | P. aeruginosa | [49] |
| Ethyl acetate | 10 | 20 | P. aeruginosa | [49] |
| Crude | 10 | 16 | P. aeruginosa | [49] |
| Ethanolic extracts | 30 | 18 ± 0.7 | P. aeruginosa | [39] |
| Ethanolic extracts | 20 | 12 ± 0.9 | P. aeruginosa | [39] |
| Ethanolic extracts | 10 | - | P. aeruginosa | [39] |
| n-Hexane | 10 | 10 | Klebsiella pneumoniae | [49] |
| Chloroform | 10 | 16 | K. pneumoniae | [49] |
| Ethyl acetate | 10 | 20 | K. pneumoniae | [49] |
| Crude | 10 | 14 | K. pneumoniae | [49] |
| n-Hexane | 10 | 12 | Methicillin-resistant Staphylococcus aureus (MRSA) | [49] |
| Chloroform | 10 | 14 | MRSA | [49] |
| Ethyl acetate | 10 | 18 | MRSA | [49] |
| Crude | 10 | 14 | MRSA | [49] |
| n-Hexane | 10 | 12 | Staphylococcus aureus | [49] |
| Chloroform | 10 | 12 | S. aureus | [49] |
| Ethyl acetate | 10 | 18 | S. aureus | [49] |
| Crude | 10 | 14 | S. aureus | [49] |
| Ethanolic extracts | 30 | 16 ± 0.3 | S. aureus | [39] |
| Ethanolic extracts | 20 | 12 ± 0.1 | S. aureus | [39] |
| Ethanolic extracts | 10 | – | S. aureus | [39] |
| n-Hexane | 10 | 10 | Staphylococcus epidermidis | [49] |
| Chloroform | 10 | 18 | S. Epidermidis | [49] |
| Ethyl acetate | 10 | 16 | S. Epidermidis | [49] |
| Crude | 10 | 16 | S. Epidermidis | [49] |
| n-Hexane | 10 | 8 | Salmonella typhi | [49] |
| Chloroform | 10 | 18 | S. Typhi | [49] |
| Ethyl acetate | 10 | 20 | S. Typhi | [49] |
| Crude | 10 | 16 | S. Typhi | [49] |
| Ethanolic extracts | 30 | – | Proteus vulgaris | [39] |
| Ethanolic extracts | 20 | – | P. vulgaris | [39] |
| Ethanolic extracts | 10 | 19 ± 0.3 | P. vulgaris | [39] |
| Crude | 10 | 14 | K. pneumoniae | [49] |
| Silver oxide | 30 | 1.281 | B. subtilis | [35] |
| Silver oxide | 30 | 1.519 | S. aures | |
| Silver oxide | 30 | 1.370 | Escherichia coli | |
| Silver oxide | 30 | 1.661 | P. aeruginosa | |
| Ethanolic extract | Not mention | 19.27 ± 0.23 | S. marcescens | [104] |
| Methanolic extract | Not mention | 13.28 ± 0.12 | Actinobacteria | [104] |
Neuroprotective
The brain is one of the most important organs of the body, which allows the evolution of the human being and species [17, 111, 151–153]. Brain functions are influenced by poor nutrition, stress and lack of movement, but also by natural cellular oxidation processes [19, 106, 154–157]. In addition to improving lifestyle, the brain and memory can benefit from the stimulating input of nature due to plants with a beneficial effect on brain functions [158–160]. Recent research studied the effects of a Paeonia emodi Wall. ex Royle ethanol concentrate at doses ranging from 300 to 600 mg/kg BW on pentylenetetrazole-igniting, memory impairment, oxidative damage, and anxiety without engine debilitation. Paeonia emodi Wall. ex Royle has been demonstrated to be effective in the treatment of dropsy and worried concerns because of its cell reinforcement and radical rummaging features and activities [4].
Anticonvulsant, antiepileptic, antianxiety
One of the brain disorders, epilepsy is primarily brought on by psychological, physical, and social actions [112]. In a recent study, the researchers showed that the plant extract of P. emodi had anticonvulsant and antianxiety effects that were statistically significant [4].
Enzyme inhibition and radical scavenging activities
Lipoxygenase inhibiting activity
Fruit extract of Paeonia emodi Wall. ex Royle was found to contain Paeoninol and Paeonin C, oligostilbene and monoterpene galactoside along with 4-hydroxybenzoic acid, gallic acid and methyl gallate. In the concentration-dependent method, these compounds exhibited powerful inhibitory potential against lipoxygenase enzyme showing IC50 values of 0.77 and 99.5 mM and with ABTS ± radical quenching activity with IC50 values of 147.5 and 498.2 µM [8].
β-glucuronidase inhibiting activity
Paeonia emodi Wall. ex Royle has been used to isolate emodinol from its chloroform soluble fractions. Spectral studies of arrangement 1beta, 3beta, 23-trihydroxyolean-12-en-28-oic acid have been allocated along with 2D NMR which has shown the significance of beta-glucuronidase inhibitory activities. The typical inhibitor, glucosaccharo-lactone, has an IC50 value of 1.88 mM, but emodinol derived from the roots of Paeonia emodi Wall. ex Royle has an IC50 value of 63 mM [43an]. Benzoic acid and 3-hydroxybenzoic acid have also been isolated from Paeonia emodi Wall. ex Royle [96]. From the above-ground parts of Paeonia emodi Wall. ex Royle extracts of ethanol were selected against Urease and alpha-chymotrypsin for enzyme inhibition and radical scavenging activity using the DPPH assay. Concentrates of unrefined against jack bean (74%) and Bacillus pasteurii (80%) urease indicated noteworthy catalyst restraint action and moderate movement (54%) against alpha-chymotrypsin while (83%) radical searching action was additionally gotten from the concentrate [48].
Urease activity
Mansoor and Taos (2005) evaluated the urease activity of Paeonia emodi in a 15-min experiment at 30 degrees Celsius. 5 µl of test compounds were incubated with 25ul of enzyme solution and 55ul of 100 mM urea buffers. Weather burn’s indophenol approach was used to test urease activity by generating ammonia. With the assistance of the SoftMax Pro software. The data was examined. Thiourea was an established urea inhibitor.
Antispasmodic
Paeonia emodi Wall. ex Royle was found to contain divisions of harsh concentrates from its aerial parts which indicated potential spasmolytic action. Effects of 5 mg/mL crude extract concentration were studied on jejunum rabbits inhibiting the spontaneous motility by 76%. Fractions containing ethyl acetate and chloroform showed excellent spasmolytic activities while the n-butanol fraction showed low inhibitory activities. A general spasmogenic action appeared from the portions of solvent water in the separated jejunum rabbit [48]. Tubers of P. emodi are used to study the uterotonic effect but no response was shown by the model organism [4].
Other biological properties
The rough concentration of Paeonia emodi Wall. ex Royle was evaluated for phytotoxicity against Lemna minor L. Three flasks for 500, 50, and 5 g/ml were utilized with a stock arrangement of the concentrate (20 mg/ml). Each jar held 20 mL of medium. Paraquat was employed to suppress growth. Jars were hatched in the development area for seven days before the development guideline in rate was examined using a negative control. The IC50 value was determined. The PC program was operational 95 percent of the time, according to preliminary statistics [19]. The phytotoxicity of Paeonia emodi Wall. ex Royle was also tested against Lemna aequinoctialis. KOH pellets were mixed into the medium, which was composed of purified water with a pH of 5.5–6.5. The medium was autoclaved for 15 min at 121 °C. As a starting solution, ethanol extracts were utilized. In the experiment, nine fertilized flasks were used. Each flask contained a rosette of Lemna aequinoctialis fronds. In sterile conditions, the solvent was evaporated. All flasks were kept in a growth cabinet and plugged in for seven days. The number of fronds was counted on day seven [13].
The insecticidal efficacy of Paeonia emodi Wall. ex Royle separate was modulated by direct contact application using channel paper. 3 ml of the concentrate was put on channel papers. After drying, each channel paper was put in a separate petri dish with Tribolium castaneum, Bruchus pisorum, and Rhyzopartha dominica. Permethrin, a pesticide, was employed as a control. All were kept without food for 24 h before undergoing a mortality check [19]. 70% Ethanolic extracts prepared from the roots of Paeonia emodi Wall. ex Royle was used to examining its effects on the atria, trachea, and aorta of pigs and rats. All the techniques applied were in vivo. Similarly, airway relaxant effects were examined in the lungs of the mouse. Different results were obtained demonstrating, vasodilatory, antiplatelet, and tracheal and aviation route relaxant activities. Ethanolic extracts of Paeonia emodi Wall. ex Royle justified its importance as a medicinal herbal drug in treating various cardiovascular and respiratory ailments [161].
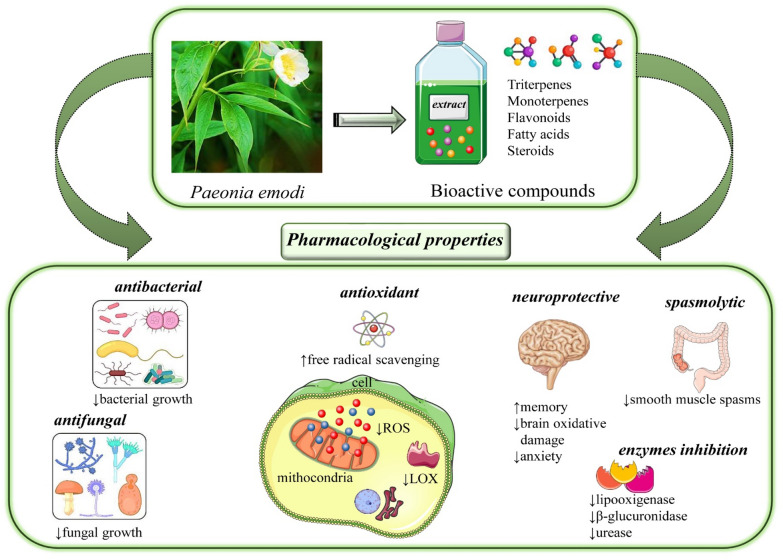
The most relevant data regarding pharmacological properties of Paeonia emodi Wall. ex Royle are summarized in Fig. 5 and Table 4.
Fig. 5.
Summarized scheme with the main phytoconstituents of Paeonia emodi Wall. ex Royle and their most relevant pharmacological properties. Abbreviations and symbols: ↑ increase, ↓decrease, lipoxygenase (LOX), reactive oxygen species (ROS)
Table 4.
The main pharmacological properties of P.emodi
| Pharmacological activity | Experimental study | Tested extract of Paeonia emodi Wall. ex Royle | IC50/dose | Main results | Refs. |
|---|---|---|---|---|---|
| Antibacterial |
In vitro Escherichia coli Proteus aeruginosa Staphylococcus aureus Staphylococcus epidermidis Salmonella typhi Proteus vulgaris Klebsiella pneumoniae |
n-hexane chloroform Ethyl acetate Ethanolic silveroxide |
NA | ↓ Bacterial growth |
[49] [39] [35] |
| Antifungal |
In vitro Pseudalleschena boydii Trichophyton schoenleinii Candida albicans Aspergilus niger Trichophyton simii Macrophomina phaseolina |
Ethanolic Methanolic |
IC50 = 400 µg/mL IC50 = 100–900 µg/mL |
Moderate antifungal activity | [5, 24] |
| Neuroprotective |
In vivo Mice PTZ epilepsy model |
Roots ethanolic extract | Dose = 300–600 mg/kg/bw |
↑Memory ↓Brain oxidative damage ↓Anxiety ↓Seizures |
[4] |
|
Enzymes inhibition |
In vitro | Fruit ethanolic extract | IC50 = 147.5–498.2 µM | ↓Lipoxygenase | [8] |
| Roots ethanolic extract | IC50 = 1.88 mM | ↓β-glucuronidase | [96] | ||
| Antispasmodic |
In vivo Rabbits |
Crude extract | Dose = 5 mg/mL/kg/bw | ↓Smooth muscle spasms | [48] |
| Anti-inflammatory |
In vivo Albino rats |
Roots aqueous extract | Dose = 20 g/5 h | ↓Pro-inflammatory factors | [4] |
| Hepatoprotective |
In vivo Albino rats |
NA/ethanol | Dose = NA/1 day | Hepatoprotective against ethanolic and methanolic toxicity | |
| Nephroprotective |
In vivo Wistar rats |
Roots/alcoholic/ Hydroalcholic extract |
Dose = 100,200, 400 mg/kg/45 days | Protective effects against kidney damages |
Symbols and abbreviations: ↑ increase, ↓decrease, the half maximal inhibitory concentration (IC50), Body Weight. (bw), Pentylenetetrazole (PTZ), nonavailable (NA)
Toxicology, side effects and safety data
For a decade, there has been a resurgence in interest in herbal remedies although the safety of herbal treatments has been repeatedly questioned [142]. There are currently misconceptions and prejudices about the safety of herbal medication [4]. According to the literature study, only a few research has been done on the toxicology of P. emodi. According to Zargar et al. [95], hydroalcoholic and aqueous plant extracts were deemed safe because they did not result in any mortality up to 2000 mg/Kg body weight. Herbal medicine side effects can have a variety of direct and indirect causes, which can be categorized. The inherent toxicity of several herbs, whether taken in overdose or at a regular therapeutic dosage, is a direct cause. Consumers may feel more secure if there is a regularity framework in place for herbal medications. The specification and regulation of herbal medications, however, differ significantly across nations. The World Health Organization (WHO) should suggest global unified planning, which includes global management standards and quality standards, radical sources of herbs, seeds and seedling breeding, planting, harvesting, and storage, rational procedures, manufacture, and quality standards to ensure the quality and safety of herbal medicines. Additionally, a system for ensuring the safety of herbal medicines should be built. This system should include prudent clinical practice and risk monitoring and play a bigger part in preserving human health [4].
Limitations and clinical gaps
Although the bioactive chemical compounds found in the Paeonia emodi Wall. ex Royle species have demonstrated a variety of biological effects in preclinical pharmacological studies, this species cannot be used as the first-line treatment for many chronic conditions for the following reasons:
There aren't enough clinical trials to back up these bioactive chemicals' toxicity, side effects, and therapeutic effects.
The absence of translational pharmacological investigations to identify the optimal therapeutic dose and administration method for achieving the best possible therapeutic impact in humans.
Insufficient experimental research on extracts to precisely describe the bioactive components after they have been purified. To ascertain the precise concentrations of bioactive chemicals that could be utilized in possible clinical trials, these extracts should be tested in several preclinical pharmacological experiments and chemically described.
A lack of research on nanomedicines would boost the bioavailability and effectiveness of these bioactive substances by including them in nanocarriers in certain target tissues. The intention is to maximize bioavailability by including phytochemicals in carrier nanoparticles, both in terms of the target tissue/organ and in terms of the moment/time in which the included bioactive compound is released.
Overall conclusions
Paeonia emodi Wall. ex Royle is an important restorative herb with a broad pharmacological spectrum. It is concluded from the present literature that Paeonia emodi Wall. ex Royle roots and rhizome are the most used part of the plant. There is an excessive possibility for the further screening of the plant against several disorders using both in vitro and in vivo animal models. A lot of work is needed to be done on the antimicrobial activity of this plant and more concern should be on data for the possible toxicity of the herb (toxicological studies, lethal dose, etc.). More phytochemical screening of this plant should be done to discover new bioactive phytochemical entities existing in the plant as this plant is the least misused species in the genus. In the future, we recommend different in vitro and in vivo biological studies using different animal models to further investigate its biopharmacological efficacies.
Acknowledgements
Not applicable.
Author contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas. That is, revising or critically reviewing the article; giving final approval of the version to be published; agreeing on the journal to which the article has been submitted; and, confirming to be accountable for all aspects of the work. All authors read and approved the final manuscript.
Funding
No funding received.
Availability of data and materials
The data supporting this review are from previously reported studies and datasets, which have been cited. The processed data are available from the corresponding author upon request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
No competing interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Nida Zahra and Javed Iqbal are equally contributed
Contributor Information
Nida Zahra, Email: nidazahra22@gmail.com.
Javed Iqbal, Email: javed89qau@gmail.com.
Muhammad Arif, Email: arifbiotech144@gmail.com.
Banzeer Ahsan Abbasi, Email: benazirahsanabbasi786@gmail.com.
Hassan Sher, Email: hassan.botany@gmail.com.
Ayesha Fazal Nawaz, Email: ayeshafazal387@gmail.com.
Tabassum Yaseen, Email: tabassumyaseen@bkuc.edu.pk.
Javad Sharifi-Rad, Email: javad.sharifirad@gmail.com.
Daniela Calina, Email: calinadaniela@gmail.com.
References
- 1.Misra S, Dhyani D, Maikhuri R (2010) Wild edible leaves: a study of their subsistence dietetic support to the inhabitants in nanda devi biosphere reserve, Uttaranchal [DOI] [PMC free article] [PubMed]
- 2.Uddin G, Sadat A, Siddiqui BS. Phytochemical screening, in vitro antioxidant and antimicrobial activities of the crude fractions ofPaeoniaemodiwall. Ex Royle. Middle-East J Sci Res. 2013;17(3):367–373. [Google Scholar]
- 3.Jalgaonkar SV, Kamble AP, Parmar UI, Kurle DG, Bedrekar MS, Gursahani M. Evaluation of the protective effect of Paeonia emodi Wall on rat model of Parkinson’s disease induced by 6 hydroxy dopamine. Int J Basic Clin Pharmacol. 2018;7(11):2137. doi: 10.18203/2319-2003.ijbcp20184317. [DOI] [Google Scholar]
- 4.Ahmad M, Malik K, Tariq A, Zhang G, Yaseen G, Rashid N, Sultana S, Zafar M, Ullah K, Khan MPZ. Botany, ethnomedicines, phytochemistry and pharmacology of Himalayan paeony (Paeonia emodi Royle.) J Ethnopharmacol. 2018;220:197–219. doi: 10.1016/j.jep.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Joshi P, Prakash P, Purohit V, Bahuguna V. Paeonia emodi: a review of multipurpose wild edible medicinal plant of western Himalaya. Int J Adv Res. 2017;5(12):480–486. doi: 10.21474/IJAR01/5982. [DOI] [Google Scholar]
- 6.Li P, Shen J, Wang Z, Liu S, Liu Q, Li Y, He C, Xiao P. Genus Paeonia: A comprehensive review on traditional uses, phytochemistry, pharmacological activities, clinical application, and toxicology. J Ethnopharmacol. 2021;269:113708. doi: 10.1016/j.jep.2020.113708. [DOI] [PubMed] [Google Scholar]
- 7.Muhammad P, Ahmad S, Rubnawaz H, Ullah N, Malik A. New monoterpene glycosides from Paeonia emodi. Zeitschrift für Naturforschung B. 1999;54(4):544–548. doi: 10.1515/znb-1999-0419. [DOI] [Google Scholar]
- 8.Riaz N, Malik A, Rehman A-u, Ahmed Z, Muhammad P, Nawaz SA, Siddiqui J, Choudhary MI. Lipoxygenase inhibiting and antioxidant oligostilbene and monoterpene galactoside from Paeonia emodi. Phytochemistry. 2004;65(8):1129–1135. doi: 10.1016/j.phytochem.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 9.Nawaz HR, Malik A, Khan PM, Shujaat S, A-u RAHMAN. A novel β-glucuronidase inhibiting triterpenoid from Paeonia emodi. Chemical Pharmaceutical Bulletin. 2000;48(11):1771–1773. doi: 10.1248/cpb.48.1771. [DOI] [PubMed] [Google Scholar]
- 10.WFO (2021) WFO The World Flora Online. http://www.worldfloraonline.org/.
- 11.Ali H, Sannai J, Sher H, Rashid A. Ethnobotanical profile of some plant resources in Malam Jabba valley of Swat, Pakistan. J Med Plants Res. 2011;5(18):4676–4687. [Google Scholar]
- 12.Haq F, Ahmad H, Alam M. Traditional uses of medicinal plants of Nandiar Khuwarr catchment (District Battagram), Pakistan. J Med Plants Res. 2011;5(1):39–48. [Google Scholar]
- 13.Wall. Ex R (1978) Flora of West Pakistan, vol 121. Pakistan Agricultural Research Council, pakistan
- 14.Misra S, Maikhuri R, Kala C, Rao K, Saxena K. Wild leafy vegetables: a study of their subsistence dietetic support to the inhabitants of Nanda Devi Biosphere Reserve, India. J Ethnobiol Ethnomed. 2008;4(1):1–9. doi: 10.1186/1746-4269-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sultana S. Taxonomic and pharmacognostic authentication of problematic medicinal plants used as herbal medicine. Quaid-i-Azam University Islamabad Pakistan, 2012.
- 16.Kitic D, Miladinovic B, Randjelovic M, Szopa A, Sharifi-Rad J, Calina D, Seidel V. Anticancer potential and other pharmacological properties of Prunus armeniaca L.: an updated overview. Plants. 2022;11(14):1885. doi: 10.3390/plants11141885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quetglas-Llabrés MM, Quispe C, Herrera-Bravo J, Catarino MD, Pereira OR, Cardoso SM, Dua K, Chellappan DK, Pabreja K, Satija S, Mehta M, Sureda A, Martorell M, Satmbekova D, Yeskaliyeva B, Sharifi-Rad J, Rasool N, Butnariu M, Bagiu IC, Bagiu RV, Calina D, Cho WC. Pharmacological properties of bergapten: mechanistic and therapeutic aspects. Oxid Med Cell Longev. 2022;2022:8615242. doi: 10.1155/2022/8615242. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Sharifi-Rad J, Quispe C, Patra JK, Singh YD, Panda MK, Das G, Adetunji CO, Michael OS, Sytar O, Polito L, Živković J, Cruz-Martins N, Klimek-Szczykutowicz M, Ekiert H, Choudhary MI, Ayatollahi SA, Tynybekov B, Kobarfard F, Muntean AC, Grozea I, Daştan SD, Butnariu M, Szopa A, Calina D. Paclitaxel: application in modern oncology and nanomedicine-based cancer therapy. Oxid Med Cell Longev. 2021;2021:3687700. doi: 10.1155/2021/3687700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Semwal P, Painuli S, Abu-Izneid T, Rauf A, Sharma A, Daştan SD, Kumar M, Alshehri MM, Taheri Y, Das R, Mitra S, Emran TB, Sharifi-Rad J, Calina D, Cho WC. Diosgenin: an updated pharmacological review and therapeutic perspectives. Oxid Med Cell Longev. 2022;2022:1035441. doi: 10.1155/2022/1035441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Popović-Djordjević J, Quispe C, Giordo R, Kostić A, Katanić Stanković JS, Tsouh Fokou PV, Carbone K, Martorell M, Kumar M, Pintus G, Sharifi-Rad J, Docea AO, Calina D. Natural products and synthetic analogues against HIV: a perspective to develop new potential anti-HIV drugs. Eur J Med Chem. 2022;233:114217. doi: 10.1016/j.ejmech.2022.114217. [DOI] [PubMed] [Google Scholar]
- 21.Luo Q, Liu CH, Gu M. Recording of classical prescriptions and materia medica in the Han Dynasty. Zhonghua Yi Shi Za Zhi. 2010;40(6):376–378. [PubMed] [Google Scholar]
- 22.Xu X, Xu H, Shang Y, Zhu R, Hong X, Song Z, Yang Z. Development of the general chapters of the Chinese Pharmacopoeia 2020 edition: a review. J Pharm Anal. 2021;11(4):398–404. doi: 10.1016/j.jpha.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang H, Li J, Wang L, Wang S, Nie X, Chen Y, Fu Q, Jiang M, Fu C, He Y. Total glucosides of paeony: a review of its phytochemistry, role in autoimmune diseases, and mechanisms of action. J Ethnopharmacol. 2020;258:112913. doi: 10.1016/j.jep.2020.112913. [DOI] [PubMed] [Google Scholar]
- 24.Ismail M, Iqbal Z, Ahmad B, Zakir S, Niaz U. Biological and pharmacological properties of two indigenous medicinal plants, Rheum emodi and Paeonia emodi. Pak J Biol Sci. 2003;6(9):984–986. doi: 10.3923/pjbs.2003.984.986. [DOI] [Google Scholar]
- 25.Chopra RN, Nayar SL, IC C. Glossary of Indian Medicinal Plants. Council of Scientific and Industrial Research, New Delhi, India; 1956.
- 26.Shinwari MI, Shinwari MI. Botanical diversity in Pakistan; past present and future. World Environ. 2010;1:85–104. [Google Scholar]
- 27.Adnan M, Hölscher D. Medicinal plants in old-growth, degraded and re-growth forests of NW Pakistan. J Econ Bot. 2011;261(11):2105–2114. [Google Scholar]
- 28.Adnan M, Hölscher D. Diversity of medicinal plants among different forest-use types of the Pakistani Himalaya. J Econ Bot. 2012;66(4):344–356. doi: 10.1007/s12231-012-9213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khan A, Akhtar T, Ambreen O, Shereni S, Ahmad I, Hussain I. Medicinal value and bio-efficacy of important traditional plants of Garam Chashma Valley Chitral. Phamaceut Res Bio Sci. 2013;2(4):207–226. [Google Scholar]
- 30.Ahmed A, Latif A. Non-timber forest products: a substitute for livelihood of the marginal community in Kalash Valley, Northern Pakistan. J Ethnobotanical Leafl. 2007;11:97–105. [Google Scholar]
- 31.Ummara U, Bokhari TZ, Altaf A, Younis U, Dasti AAJIJSER. Pharmacological study of Shogran valley flora, Pakistan. Int J Sci Eng Res. 2013;4(9):1–9. [Google Scholar]
- 32.Latif A, Shinwari Z, Hussain J, Murtaza S. NTFPS: an alternative to forest logging in Minadam and Sultanar Valley Swat. Lyonia. 2006;11(2):15–21. [Google Scholar]
- 33.ul Hassan H, Murad W, Tariq A, Ahmad A. Ethnoveterinary study of medicinal plants in Malakand Valley, district Dir (lower), Khyber Pakhtunkhwa, Pakistan. Irish Vet J. 2014;67(1):1–6. doi: 10.1186/2046-0481-67-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahmad M, Sultana S, Fazl-i-Hadi S, Ben Hadda T, Rashid S, Zafar M, Khan MA, Khan MPZ, Yaseen G. An ethnobotanical study of medicinal plants in high mountainous region of Chail valley (District Swat-Pakistan) J Ethnobiol Ethnomed. 2014;10(1):1–18. doi: 10.1186/1746-4269-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shah A, Tauseef I, Yameen MA, Haleem SK, Haq S, Shoukat S. In-vivo toxicity and therapeutic efficacy ofPaeoniaemodi-mediated zinc oxide nanoparticles: in-vitro study. Microsc Res Tech. 2022;85(1):181–192. doi: 10.1002/jemt.23894. [DOI] [PubMed] [Google Scholar]
- 36.Ishtiaq M, Mumtaz AS, Hussain T, Ghani A. Medicinal plant diversity in the flora of Leepa Valley, Muzaffarabad (AJK), Pakistan. Afr J Biotechnol. 2012;11(13):3087–3098. [Google Scholar]
- 37.Waseem M, Shah MAU, Qureshi RA, Muhammad I, Afza R, Yousaf S. Ethnopharmacological survey of plants used for the treatment of stomach, diabetes, and ophthalmic diseases in Sudhan Gali, Kashmir, Pakistan. Acta Bot Yunnanica. 2006;28(5):535. [Google Scholar]
- 38.Tehseena Jamil YB, Zahara K. An Insight into Endangered Himalayan Paeony (Paeonia emodi Royle): ethnobotany, phytochemistry and pharmacology. J Plant Environ. 2020;02(02):25–31. doi: 10.33687/jpe.002.01.3477. [DOI] [Google Scholar]
- 39.Jan S, Hamayun M, Ahmad N, Nawaz Y, Khan AL, Iqbal A, Lee I-J. Antibacterial potential of plants traditionally used for curing diarrhea in Khyber Pakhtunkhwa, Pakistan. J Med Plants Res. 2012;6(23):4039–4047. [Google Scholar]
- 40.Fazal H, Ahmad N, Khan MA. Physicochemical, phytochemical evaluation and DPPH-scavenging antioxidant potential in medicinal plants used for herbal formulation in Pakistan. Pak J Bot. 2011;43:63–67. [Google Scholar]
- 41.Akhtar N, Rashid A, Murad W, Bergmeier EJ. Diversity and use of ethno-medicinal plants in the region of Swat, North Pakistan. J Ethnobiol Ethnomed. 2013;9(1):1–14. doi: 10.1186/1746-4269-9-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Negi VS, Maikhuri R, Vashishtha D. Traditional healthcare practices among the villages of Rawain valley, Uttarkashi, Uttarakhand, India; 2011.
- 43.Ahmad S. Traditional uses of economically important plants of Chitral District, Malakand Division, NWFP, Pakistan. Pak J Bot. 2001;587–598.
- 44.Adnan SM, Khan A, Latif A, Shinwari Z. Threats to the sustainability of Ethno-Medicinal uses in Northern Pakistan (a case study of Miandam Valley, district Swat, NWFP Province, Pakistan) Lyonia. 2006;11:91–100. [Google Scholar]
- 45.Hamayun M, Khan SA, Sohn EY, Lee I-J. Folk medicinal knowledge and conservation status of some economically valued medicinal plants of District Swat, Pakistan. Lyonia. 2006;11(2):101–113. [Google Scholar]
- 46.Iqbal I, Hamayun M. Studies on the traditional uses of plants of Malam Jabba valley, District Swat, Pakistan. Ethnobot Leafl. 2004;2004(1):15. [Google Scholar]
- 47.Zakir S. Environmental awareness as a tool to promote the wise practices of wild plant resources in the Himalayas (Sudhangali-Kashmir, Pakistan). Putaj Sci. 2012;19.
- 48.Khan T, Ahmad M, Khan H, Khan MA. Biological activities of aerial parts of Paeonia emodi Wall. Afr J Biotechnol. 2005; 4 (11).
- 49.Mufti FUD, Ullah H, Bangash A, Khan N, Hussain S, Ullah F, Jamil M, Jabeen M. Antimicrobial activities of Aerva javanica and Paeonia emodi plants. Pak J Pharm Sci. 2012;25(3):565–569. [PubMed] [Google Scholar]
- 50.Ibrar M, Hussain F. Ethnobotanical studies of plants of Charkotli hills, Batkhela district, Malakand, Pakistan. Front Biol China. 2009;4(4):539–548. doi: 10.1007/s11515-009-0045-2. [DOI] [Google Scholar]
- 51.Kandari L, Phondani P, Payal K, Rao K, Maikhuri R. Ethnobotanical study towards conservation of medicinal and aromatic plants in upper catchments of Dhauli Ganga in the central Himalaya. J Mt Sci. 2012;9(2):286–296. doi: 10.1007/s11629-012-2049-7. [DOI] [Google Scholar]
- 52.Tiwari JK, Ballabha R, Tiwari P. Ethnopaediatrics in Garhwal Himalaya, Uttarakhand, India (Psychomedicine and Medicine) New York Sci J. 2010;3(4):123–126. [Google Scholar]
- 53.Bisht V, Negi J, Bh A, Sundriyal R. Traditional use of medicinal plants in district Chamoli, Uttarakhand, India. J Med Plants Res. 2013;7(15):918–929. [Google Scholar]
- 54.Sharma P, Rani S, Ojha S, Sood S, Rana J. Indian herbal medicine as hepatoprotective and hepatocurative: a review of scientific evidence. Life Sci Leafl. 2014; 50:61 to 115–161 to 115.
- 55.Rana C, Tiwari J, Dangwal L, Gairola S (2013) Faith herbal healer knowledge document of Nanda Devi Biosphere reserve, Uttarakhand, India.
- 56.Zargar BA, Masoodi MH, Khan BA, Akbar S. Paeonia emodi Royle: ethnomedicinal uses, phytochemistry and pharmacology. Phytochem Lett. 2013;6(2):261–266. doi: 10.1016/j.phytol.2013.03.003. [DOI] [Google Scholar]
- 57.Kumari P, Singh BK, Joshi GC, Tewari LM. Veterinary ethnomedicinal plants in Uttarakhand Himalayan region, India. Ethnobot Leafl. 2009;2009(10):11. [Google Scholar]
- 58.Agarwal D. Himalayan medicine system and its material medica. 2001;1–30.
- 59.Nautiyal S, Maikhuri R, Rao K, Saxena K. Medicinal plant resources in Nanda Devi Biosphere Reserve in the central Himalayas. J Herbs Spices Med Plants. 2001;8(4):47–64. doi: 10.1300/J044v08n04_06. [DOI] [Google Scholar]
- 60.Sharma J, Gairola S, Gaur R, Painuli R, Siddiqi T. Ethnomedicinal plants used for treating epilepsy by indigenous communities of sub-Himalayan region of Uttarakhand, India. J Ethnopharmacol. 2013;150(1):353–370. doi: 10.1016/j.jep.2013.08.052. [DOI] [PubMed] [Google Scholar]
- 61.Caceres A, Cano O, Samayoa B, Aguilar LJ. Plants used in Guatemala for the treatment of gastrointestinal disorders. 1. Screening of 84 plants against enterobacteria. J Ethnopharmacol. 1990;30(1):55–73. doi: 10.1016/0378-8741(90)90017-N. [DOI] [PubMed] [Google Scholar]
- 62.Rojas A, Cruz S, Rauch V, Bye R, Linares E, Mata R. Spasmolytic potential of some plants used in Mexican traditional medicine for the treatment of gastrointestinal disorders. Phytomedicine. 1995;2(1):51–55. doi: 10.1016/S0944-7113(11)80049-8. [DOI] [PubMed] [Google Scholar]
- 63.Johns T, Faubert GM, Kokwaro JO, Mahunnah R, Kimanani EK. Anti-giardial activity of gastrointestinal remedies of the Luo of East Africa. J Ethnopharmacol. 1995;46(1):17–23. doi: 10.1016/0378-8741(95)01224-2. [DOI] [PubMed] [Google Scholar]
- 64.Otshudi AL, Vercruysse A, Foriers A. Contribution to the ethnobotanical, phytochemical and pharmacological studies of traditionally used medicinal plants in the treatment of dysentery and diarrhoea in Lomela area, Democratic Republic of Congo (DRC) J Ethnopharmacol. 2000;71(3):411–423. doi: 10.1016/S0378-8741(00)00167-7. [DOI] [PubMed] [Google Scholar]
- 65.Diehl M, Atindehou KK, Téré H, Betschart B. Prospect for anthelminthic plants in the Ivory Coast using ethnobotanical criteria. J Ethnopharmacol. 2004;95(2–3):277–284. doi: 10.1016/j.jep.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 66.Maniyar Y, Bhixavatimath P, Agashikar N. Antidiarrheal activity of flowers of Ixora Coccinea Linn. in rats. J Ayurveda Integr Med. 2010;1(4):287. doi: 10.4103/0975-9476.74422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Imran I, Hussain L, Zia-Ul-Haq M, Janbaz KH, Gilani AH, De Feo V. Gastrointestial and respiratory activities of Acacia leucophloea. J Ethnopharmacol. 2011;138(3):676–682. doi: 10.1016/j.jep.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 68.Kozan E, Çankaya IT, Kahraman C, Akkol EK, Akdemir Z. The in vivo anthelmintic efficacy of some Verbascum species growing in Turkey. Exp Parasitol. 2011;129(2):211–214. doi: 10.1016/j.exppara.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 69.Rani P, Khullar N. Antimicrobial evaluation of some medicinal plants for their anti-enteric potential against multi-drug resistant Salmonella typhi. Phytothera Res. 2004;18(8):670–673. doi: 10.1002/ptr.1522. [DOI] [PubMed] [Google Scholar]
- 70.Ahmad I, Aqil F. In vitro efficacy of bioactive extracts of 15 medicinal plants against ESβL-producing multidrug-resistant enteric bacteria. J Microbiol Res. 2007;162(3):264–275. doi: 10.1016/j.micres.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 71.Dey A, Das T, Mukherjee S. In vitro antibacterial activity of n-Hexane fraction of methanolic extract of Plumeria rubra L. (Apocynaceae) stem bark. J Plant Sci. 2011;6(3):135. doi: 10.3923/jps.2011.135.142. [DOI] [Google Scholar]
- 72.Mukherjee S, Dey A, Das T. In vitro antibacterial activity of n-hexane fraction of methanolic extract of Alstonia scholaris LR Br. stem bark against some multidrug resistant human pathogenic bacteria. Eur J Med Plants. 2012;1–10.
- 73.Muhammad N, Ali N, Uddin N. Ethno-veterinary practices used for treatment of various ailments in hilly areas of Melagah valley district swat KPK, Pakistan. Int J Bot Stud. 2019;4(3):171–179. [Google Scholar]
- 74.Hawrelak JA, Cattley T, Myers SP. Essential oils in the treatment of intestinal dysbiosis: a preliminary in vitro study. Altern Med Rev 2009;14 (4). [PubMed]
- 75.Mimica-Dukic N, Bozin B. Mentha L. species (Lamiaceae) as promising sources of bioactive secondary metabolites. Curr Pharmaceut Design. 2008;14(29):3141–3150. doi: 10.2174/138161208786404245. [DOI] [PubMed] [Google Scholar]
- 76.Alexopoulos A, Kimbaris A, Plessas S, Mantzourani I, Theodoridou I, Stavropoulou E, Polissiou M, Bezirtzoglou EJA. Antibacterial activities of essential oils from eight Greek aromatic plants against clinical isolates of Staphylococcus aureus. J Anaerobe. 2011;17(6):399–402. doi: 10.1016/j.anaerobe.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 77.Gasparetto JC, Martins CAF, Hayashi SS, Otuky MF, Pontarolo R. Ethnobotanical and scientific aspects of Malva sylvestris L.: a millennial herbal medicine. J Pharm Pharmacol. 2012;64(2):172–189. doi: 10.1111/j.2042-7158.2011.01383.x. [DOI] [PubMed] [Google Scholar]
- 78.Rehecho S, Uriarte-Pueyo I, Calvo J, Vivas LA, Calvo MI. Ethnopharmacological survey of medicinal plants in Nor-Yauyos, a part of the Landscape Reserve Nor-Yauyos-Cochas, Peru. J Ethnopharmacol. 2011;133(1):75–85. doi: 10.1016/j.jep.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 79.Nadembega P, Boussim JI, Nikiema JB, Poli F, Antognoni F. Medicinal plants in baskoure, kourittenga province, Burkina Faso: an ethnobotanical study. J Ethnopharmacol. 2011;133(2):378–395. doi: 10.1016/j.jep.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 80.Alzweiri M, Al Sarhan A, Mansi K, Hudaib M, Aburjai T. Ethnopharmacological survey of medicinal herbs in Jordan, the Northern Badia region. J Ethnopharmacol. 2011;137(1):27–35. doi: 10.1016/j.jep.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 81.Sruthi C, Sindhu A. A comparison of the antioxidant property of five Ayurvedic formulations commonly used in the management of vata vyadhis. J Ayurveda Integr Med. 2012;3(1):29. doi: 10.4103/0975-9476.93945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hussain M, Ghani A. Herbal remidies used for gastrointestinal disorders in Kaghan valley, NWFP, Pakistan. Pak J Weed Sci Res. 2008;14(3–4):169–200. [Google Scholar]
- 83.Sanz-Biset J, Campos-de-la-Cruz J, Epiquién-Rivera MA, Canigueral S. A first survey on the medicinal plants of the Chazuta valley (Peruvian Amazon) J Ethnopharmacol. 2009;122(2):333–362. doi: 10.1016/j.jep.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 84.Islam MT, Quispe C, El-Kersh DM, Shill MC, Bhardwaj K, Bhardwaj P, Sharifi-Rad J, Martorell M, Hossain R, Al-Harrasi A, Al-Rawahi A, Butnariu M, Rotariu LS, Suleria HAR, Taheri Y, Docea AO, Calina D, Cho WC. A literature-based update on Benincasa hispida (Thunb.) Cogn.: traditional uses, nutraceutical, and phytopharmacological profiles. Oxid Med Cell Longev. 2021;2021:6349041. doi: 10.1155/2021/6349041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Quispe C, Herrera-Bravo J, Khan K, Javed Z, Semwal P, Painuli S, Kamiloglu S, Martorell M, Calina D, Sharifi-Rad J. Therapeutic applications of curcumin nanomedicine formulations in cystic fibrosis. Prog Biomater. 2022 doi: 10.1007/s40204-022-00198-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sharifi-Rad J, Quispe C, Bouyahya A, El Menyiy N, El Omari N, Shahinozzaman M, Ara Haque Ovey M, Koirala N, Panthi M, Ertani A, Nicola S, Lapava N, Herrera-Bravo J, Salazar LA, Changan S, Kumar M, Calina D. Ethnobotany, phytochemistry, biological activities, and health-promoting effects of the genus Bulbophyllum. Evid Based Complement Altern Med. 2022;2022:6727609. doi: 10.1155/2022/6727609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hossain R, Sarkar C, Hassan SMH, Khan RA, Arman M, Ray P, Islam MT, Daştan SD, Sharifi-Rad J, Almarhoon ZM, Martorell M, Setzer WN, Calina D. In silico screening of natural products as potential inhibitors of SARS-CoV-2 using molecular docking simulation. Chin J Integr Med. 2021;28:1–8. doi: 10.1007/s11655-021-3504-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Painuli S, Quispe C, Herrera-Bravo J, Semwal P, Martorell M, Almarhoon ZM, Seilkhan A, Ydyrys A, Rad JS, Alshehri MM, Daştan SD, Taheri Y, Calina D, Cho WC. Nutraceutical profiling, bioactive composition, and biological applications of Lepidium sativum L. Oxid Med Cell Longev. 2022;2022:2910411. doi: 10.1155/2022/2910411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rajib H, Muhammad Torequl I, Pranta R, Divya J, Abu Saim Mohammad S, Lutfun N, Anupam Das T, Satyajit S, Seyed Abdulmajid A, Miquel M, Farzad K, Natália C-M, Khattab A-K, Anca Oana D, Daniela C. Amentoflavone, new hope against SARS-CoV-2: an outlook through its scientific records and an in silico study. Pharmacogn Res. 2021;13(3):149. doi: 10.5530/pres.13.3.7. [DOI] [Google Scholar]
- 90.Sharifi-Rad J, Quispe C, Durazzo A, Lucarini M, Souto EB, Santini A, Imran M, Moussa AY, Mostafa NM, El-Shazly M, Sener B, Schoebitz M, Martorell M, Dey A, Calina D, Cruz-Martins N. Resveratrol’ biotechnological applications: enlightening its antimicrobial and antioxidant properties. J Herb Med. 2022;32:100550. doi: 10.1016/j.hermed.2022.100550. [DOI] [Google Scholar]
- 91.Taheri Y, Quispe C, Herrera-Bravo J, Sharifi-Rad J, Ezzat SM, Merghany RM, Shaheen S, Azmi L, Prakash Mishra A, Sener B, Kılıç M, Sen S, Acharya K, Nasiri A, Cruz-Martins N, Tsouh Fokou PV, Ydyrys A, Bassygarayev Z, Daştan SD, Alshehri MM, Calina D, Cho WC. Urtica dioica-derived phytochemicals for pharmacological and therapeutic applications. Evid Based Complement Alternat Med. 2022;2022:4024331. doi: 10.1155/2022/4024331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Butnariu M, Quispe C, Herrera-Bravo J, Sharifi-Rad J, Singh L, Aborehab NM, Bouyahya A, Venditti A, Sen S, Acharya K, Bashiry M, Ezzat SM, Setzer WN, Martorell M, Mileski KS, Bagiu IC, Docea AO, Calina D, Cho WC. The pharmacological activities of Crocus sativus L.: a review based on the mechanisms and therapeutic opportunities of its phytoconstituents. Oxid Med Cell Longev. 2022;2022:8214821. doi: 10.1155/2022/8214821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Alshehri MM, Quispe C, Herrera-Bravo J, Sharifi-Rad J, Tutuncu S, Aydar EF, Topkaya C, Mertdinc Z, Ozcelik B, Aital M, Kumar NVA, Lapava N, Rajkovic J, Ertani A, Nicola S, Semwal P, Painuli S, González-Contreras C, Martorell M, Butnariu M, Bagiu IC, Bagiu RV, Barbhai MD, Kumar M, Daştan SD, Calina D, Cho WC. A review of recent studies on the antioxidant and anti-infectious properties of senna plants. Oxid Med Cell Longev. 2022;2022:6025900. doi: 10.1155/2022/6025900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhao DD, Jiang LL, Li HY, Yan PF, Zhang YL. Chemical components and pharmacological activities of terpene natural products from the Genus Paeonia. Molecules. 2016;21 (10). 10.3390/molecules21101362 [DOI] [PMC free article] [PubMed]
- 95.Zargar BA, Masoodi MH, Ahmed B, Ganie SA. Antihyperlipidemic and antioxidant potential of Paeoniaemodi Royle against high-fat diet induced oxidative stress. Int Scholar Res Notices. 2014 [DOI] [PMC free article] [PubMed]
- 96.Riaz N, Anis I, Malik A, Ahmed Z, Muhammad P, Nawaz SA, Choudhary MI. Paeonins A and B, lipoxygenase inhibiting monoterpene galactosides from Paeonia emodi. Chem Pharmaceut Bull. 2003;51(3):252–254. doi: 10.1248/cpb.51.252. [DOI] [PubMed] [Google Scholar]
- 97.Duan W-J, Yang J-Y, Chen L-X, Zhang L-J, Jiang Z-H, Cai X-D, Zhang X, Qiu F. Monoterpenes from Paeonia albiflora and their inhibitory activity on nitric oxide production by lipopolysaccharide-activated microglia. J Nat Prod. 2009;72(9):1579–1584. doi: 10.1021/np9001898. [DOI] [PubMed] [Google Scholar]
- 98.Ullah S, Abbasi M, Raza M, Khan S, Muhammad B, Rehman A, Mughal M. Antibacterial activity of some selected plants of Swat valley. Biosci Res. 2011;8(1):15–18. [Google Scholar]
- 99.Spanos GA, Wrolstad RE. Influence of processing and storage on the phenolic composition of Thompson seedless grape juice. J Agric Food Chem. 1990;38(7):1565–1571. doi: 10.1021/jf00097a030. [DOI] [Google Scholar]
- 100.Asif M, Ahmad MS, Mannan A, Itohc T, Matsumoto T. Analysis ofPaeonia emodi root oil. J Am Oil Chemists Soc. 1983;60(3):581–583. doi: 10.1007/BF02679791. [DOI] [Google Scholar]
- 101.Tantry MA, Dar JA, Khuroo MA, Shawl AS. Nortriterpenoids from the roots of Paeonia emodi. Phytochem Lett. 2012;5(2):253–257. doi: 10.1016/j.phytol.2012.01.006. [DOI] [Google Scholar]
- 102.Wu L, Nie L, Wang Q, Xu Z, Wang Y, He C, Song J, Yao H. Comparative and phylogenetic analyses of the chloroplast genomes of species of Paeoniaceae. Sci Rep. 2021;11(1):1–16. doi: 10.1038/s41598-021-94137-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ibrar M, Khan MA, Abdullah NM, Khan M. Evaluation of Paeonia emodi for its cardioprotective potentials: an investigative study towards possible mechanism. J Ethnopharmacol. 2019;231:57–65. doi: 10.1016/j.jep.2018.10.041. [DOI] [PubMed] [Google Scholar]
- 104.Joshi K, Adhikari P, Bhatt ID, Pande V. Source dependent variation in phenolics, antioxidant and antimicrobial activity of Paeonia emodi in west Himalaya, India. Physiol Mol Biol Plants. 2022;28(9):1785–1798. doi: 10.1007/s12298-022-01242-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sharifi-Rad J, Dey A, Koirala N, Shaheen S, El Omari N, Salehi B, Goloshvili T, Cirone Silva NC, Bouyahya A, Vitalini S, Varoni EM, Martorell M, Abdolshahi A, Docea AO, Iriti M, Calina D, Les F, López V, Caruntu C. Cinnamomum species: bridging phytochemistry knowledge, pharmacological properties and toxicological safety for health benefits. Front Pharmacol. 2021;12:600139–600139. doi: 10.3389/fphar.2021.600139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tsoukalas D, Fragkiadaki P, Docea AO, Alegakis AK, Sarandi E, Vakonaki E, Salataj E, Kouvidi E, Nikitovic D, Kovatsi L, Spandidos DA, Tsatsakis A, Calina D. Association of nutraceutical supplements with longer telomere length. Int J Mol Med. 2019;44(1):218–226. doi: 10.3892/ijmm.2019.4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Salehi B, Prakash Mishra A, Nigam M, Karazhan N, Shukla I, Kiełtyka-Dadasiewicz A, Sawicka B, Głowacka A, Abu-Darwish MS, Hussein Tarawneh A, Gadetskaya AV, Cabral C, Salgueiro L, Victoriano M, Martorell M, Docea AO, Abdolshahi A, Calina D, Sharifi-Rad J. Ficus plants: state of the art from a phytochemical, pharmacological, and toxicological perspective. Phytother Res. 2021;35(3):1187–1217. doi: 10.1002/ptr.6884. [DOI] [PubMed] [Google Scholar]
- 108.Mititelu RR, Padureanu R, Bacanoiu M, Padureanu V, Docea AO, Calina D, Barbulescu AL, Buga AM. Inflammatory and oxidative stress markers-mirror tools in rheumatoid arthritis. Biomedicines. 2020;8 (5). 10.3390/biomedicines8050125. [DOI] [PMC free article] [PubMed]
- 109.Sharifi-Rad J, Kamiloglu S, Yeskaliyeva B, Beyatli A, Alfred MA, Salehi B, Calina D, Docea AO, Imran M, Kumar NVA, Romero-Roman ME, Maroyi A, Martorell M. Pharmacological activities of psoralidin: a comprehensive review of the molecular mechanisms of action. Front Pharmacol. 2020;11:11. doi: 10.3389/fphar.2020.571459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sharifi-Rad J, Quispe C, Imran M, Rauf A, Nadeem M, Gondal TA, Ahmad B, Atif M, Mubarak MS, Sytar O, Zhilina OM, Garsiya ER, Smeriglio A, Trombetta D, Pons DG, Martorell M, Cardoso SM, Razis AFA, Sunusi U, Kamal RM, Rotariu LS, Butnariu M, Docea AO, Calina D. Genistein: an integrative overview of its mode of action, pharmacological properties, and health benefits. Oxid Med Cell Longev. 2021;2021:3268136. doi: 10.1155/2021/3268136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Salehi B, Sestito S, Rapposelli S, Peron G, Calina D, Sharifi-Rad M, Sharopov F, Martins N, Sharifi-Rad J. Epibatidine: a promising natural alkaloid in health. Biomolecules. 2019;9(1):6. doi: 10.3390/biom9010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sharifi-Rad J, Quispe C, Herrera-Bravo J, Martorell M, Sharopov F, Tumer TB, Kurt B, Lankatillake C, Docea AO, Moreira AC, Dias DA, Mahomoodally MF, Lobine D, Cruz-Martins N, Kumar M, Calina D. A pharmacological perspective on plant-derived bioactive molecules for epilepsy. Neurochem Res. 2021;46(9):2205–2225. doi: 10.1007/s11064-021-03376-0. [DOI] [PubMed] [Google Scholar]
- 113.Georgiadis G, Zisis IE, Docea AO, Tsarouhas K, Fragkiadoulaki I, Mavridis C, Karavitakis M, Stratakis S, Stylianou K, Tsitsimpikou C, Calina D, Sofikitis N, Tsatsakis A, Mamoulakis C. Current concepts on the reno-protective effects of phosphodiesterase 5 inhibitors in acute kidney injury: systematic search and review. J Clin Med. 2020; 9 (5). 10.3390/jcm9051284. [DOI] [PMC free article] [PubMed]
- 114.Rahaman MM, Hossain R, Herrera-Bravo J, Islam MT, Atolani O, Adeyemi OS, Owolodun OA, Kambizi L, Daştan SD, Calina D, Sharifi-Rad J. Natural antioxidants from some fruits, seeds, foods, natural products, and associated health benefits: an update. Food Sci Nutr. 10.1002/fsn3.3217. [DOI] [PMC free article] [PubMed]
- 115.Jiang L, Chi C, Yuan F, Lu M, Hu D, Wang L, Liu X. Anti-inflammatory effects of anemonin on acute ulcerative colitis via targeted regulation of protein kinase C-θ. Chinese Med. 2022;17(1):39. doi: 10.1186/s13020-022-00599-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu F-J, Yang J, Chen X-Y, Yu T, Ni H, Feng L, Li P, Li H-J. Chemometrics integrated with in silico pharmacology to reveal antioxidative and anti-inflammatory markers of dandelion for its quality control. Chinese Med. 2022;17(1):125. doi: 10.1186/s13020-022-00679-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Scheau C, Caruntu C, Badarau IA, Scheau AE, Docea AO, Calina D, Caruntu A. Cannabinoids and inflammations of the gut-lung-skin barrier. J Pers Med. 2021;11(6):494. doi: 10.3390/jpm11060494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tsoukalas D, Zlatian O, Mitroi M, Renieri E, Tsatsakis A, Izotov BN, Burada F, Sosoi S, Burada E, Buga AM, Rogoveanu I, Docea AO, Calina D. A novel nutraceutical formulation can improve motor activity and decrease the stress level in a murine model of middle-age animals. J Clin Med. 2021;10(4):624. doi: 10.3390/jcm10040624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sharifi-Rad J, Herrera-Bravo J, Kamiloglu S, Petroni K, Mishra AP, Monserrat-Mesquida M, Sureda A, Martorell M, Aidarbekovna DS, Yessimsiitova Z, Ydyrys A, Hano C, Calina D, Cho WC. Recent advances in the therapeutic potential of emodin for human health. Biomed Pharmacother. 2022;154:113555. doi: 10.1016/j.biopha.2022.113555. [DOI] [PubMed] [Google Scholar]
- 120.Custódio L, Vizetto-Duarte C, Cebeci F, Özçelik B, Sharopov F, Gürer ES, Kumar M, Iriti M, Sharifi-Rad J, Calina D. Natural products of relevance in the management of attention deficit hyperactivity disorder. eFood. 2023;4(1):e57. doi: 10.1002/efd2.57. [DOI] [Google Scholar]
- 121.Coêlho ML, Islam MT, da Silva L, Oliveira G, Barros O, de Alencar MV, de Oliveira V, Santos J, Campinho dos Reis A, Ferreira O, da Mata AM, Correia Jardim Paz MF, Docea AO, Calina D, Sharifi-Rad J, de Carvalho A, Melo-Cavalcante A. Cytotoxic and antioxidant properties of natural bioactive monoterpenes nerol, estragole, and 3,7-dimethyl-1-octanol. Adv Pharmacolog Pharmaceut Sci. 2022;2022:8002766. doi: 10.1155/2022/8002766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jain D, Chaudhary P, Varshney N, Bin Razzak KS, Verma D, Zahra TRK, Janmeda P, Sharifi-Rad J, Dastan SD, Mahmud S, Docea AO, Calina D. Tobacco smoking and liver cancer risk: potential avenues for carcinogenesis. J Oncol. 2021 doi: 10.1155/2021/5905357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Javed Z, Khan K, Herrera-Bravo J, Naeem S, Iqbal MJ, Raza Q, Sadia H, Raza S, Bhinder M, Calina D, Sharifi-Rad J, Cho WC. Myricetin: targeting signaling networks in cancer and its implication in chemotherapy. Cancer Cell Int. 2022;22(1):239. doi: 10.1186/s12935-022-02663-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Varela C, Melim C, Neves BG, Sharifi-Rad J, Calina D, Mamurova A, Cabral C. Cucurbitacins as potential anticancer agents: new insights on molecular mechanisms. J Transl Med. 2022;20(1):630. doi: 10.1186/s12967-022-03828-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Garzoli S, Alarcón-Zapata P, Seitimova G, Alarcón-Zapata B, Martorell M, Sharopov F, Fokou PVT, Dize D, Yamthe LRT, Les F, Cásedas G, López V, Iriti M, Rad JS, Gürer ES, Calina D, Pezzani R, Vitalini S. Natural essential oils as a new therapeutic tool in colorectal cancer. Cancer Cell Int. 2022;22(1):407. doi: 10.1186/s12935-022-02806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Iqbal MJ, Javed Z, Herrera-Bravo J, Sadia H, Anum F, Raza S, Tahir A, Shahwani MN, Sharifi-Rad J, Calina D, Cho WC. Biosensing chips for cancer diagnosis and treatment: a new wave towards clinical innovation. Cancer Cell Int. 2022;22(1):354. doi: 10.1186/s12935-022-02777-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gao J, Song L, Xia H, Peng L, Wen Z. 6'-O-galloylpaeoniflorin regulates proliferation and metastasis of non-small cell lung cancer through AMPK/miR-299-5p/ATF2 axis. Respir Res. 2020;21(1):39. doi: 10.1186/s12931-020-1277-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Prasher P, Sharma M, Sharma AK, Sharifi-Rad J, Calina D, Hano C, Cho WC. Key oncologic pathways inhibited by Erinacine A: a perspective for its development as an anticancer molecule. Biomed Pharmacother. 2023;160:114332. doi: 10.1016/j.biopha.2023.114332. [DOI] [PubMed] [Google Scholar]
- 129.Dhyani P, Sati P, Sharma E, Attri DC, Bahukhandi A, Tynybekov B, Szopa A, Sharifi-Rad J, Calina D, Suleria HAR, Cho WC. Sesquiterpenoid lactones as potential anti-cancer agents: an update on molecular mechanisms and recent studies. Cancer Cell Int. 2022;22(1):305. doi: 10.1186/s12935-022-02721-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sharma E, Attri DC, Sati P, Dhyani P, Szopa A, Sharifi-Rad J, Hano C, Calina D, Cho WC. Recent updates on anticancer mechanisms of polyphenols. Front Cell Develop Biol. 2022 doi: 10.3389/fcell.2022.1005910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Motyka S, Jafernik K, Ekiert H, Sharifi-Rad J, Calina D, Al-Omari B, Szopa A, Cho WC. Podophyllotoxin and its derivatives: potential anticancer agents of natural origin in cancer chemotherapy. Biomed Pharmacother. 2023;158:114145. doi: 10.1016/j.biopha.2022.114145. [DOI] [PubMed] [Google Scholar]
- 132.Pezzani R, Jiménez-Garcia M, Capó X, Sönmez Gürer E, Sharopov F, Rachel TYL, Ntieche Woutouoba D, Rescigno A, Peddio S, Zucca P, Tsouh Fokou PV, Martorell M, Gulsunoglu-Konuskan Z, Ydyrys A, Bekzat T, Gulmira T, Hano C, Sharifi-Rad J, Calina D. Anticancer properties of bromelain: State-of-the-art and recent trends. Front Oncol. 2023 doi: 10.3389/fonc.2022.1068778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vijayaraj P, Muthukumar K, Sabarirajan J, Nachiappan V. Antihyperlipidemic activity of Cassia auriculata flowers in triton WR 1339 induced hyperlipidemic rats. Exp Toxicol Pathol. 2013;65(1–2):135–141. doi: 10.1016/j.etp.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 134.Kishore L, Kaur N, Singh R. Nephroprotective effect of Paeonia emodi via inhibition of advanced glycation end products and oxidative stress in streptozotocin–nicotinamide induced diabetic nephropathy. J Food Drug Anal. 2017;25(3):576–588. doi: 10.1016/j.jfda.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cioboată R, Găman A, Traşcă D, Ungureanu A, Docea AO, Tomescu P, Gherghina F, Arsene AL, Badiu C, Tsatsakis AM, Spandidos DA, Drakoulis N, Călina D. Pharmacological management of non-alcoholic fatty liver disease: atorvastatin versus pentoxifylline. Exp Ther Med. 2017;13(5):2375–2381. doi: 10.3892/etm.2017.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kamal AM, Mitrut P, Docea AO, Sosoi SS, Kamal CK, Mitrut R, Margaritescu D, Calina D, Banciu C, Tica OS, Tica AA, Alexandru DO. Double therapy with pegylated interferon and ribavirin for chronic hepatitis c. A pharmacogenetic guide for predicting adverse events. Farmacia. 2017;65(6):877–884. [Google Scholar]
- 137.Popescu MS, Firu DM, Padureanu V, Marginean CM, Pirvu DC, Osman A, Mitrut R, Parvu S, Margaritescu DN, Calina D, Docea AO, Cobelschi PC, Mitrut P. Changes in biochemical parameters in diabetes after successful direct-acting antiviral treatment of hepatitis c virus. Farmacia. 2022;70(4):643–651. doi: 10.31925/farmacia.2022.4.9. [DOI] [Google Scholar]
- 138.Iordache AM, Docea AO, Buga AM, Zlatian O, Ciurea ME, Rogoveanu OC, Burada F, Sosoi S, Mitrut R, Mamoulakis C, Albulescu D, Vasile RC, Tsatsakis A, Calina D. Sildenafil and tadalafil reduce the risk of contrast-induced nephropathy by modulating the oxidant/antioxidant balance in a murine model. Food Chem Toxicol. 2020;135:9. doi: 10.1016/j.fct.2019.111038. [DOI] [PubMed] [Google Scholar]
- 139.Amin R, Thalluri C, Docea AO, Sharifi-Rad J, Calina D. Therapeutic potential of cranberry for kidney health and diseases. eFood. 2022;3(5):e33. doi: 10.1002/efd2.33. [DOI] [Google Scholar]
- 140.Taheri Y, Jokovic N, Vitorovic J, Grundmann O, Maroyi A, Calina D. The burden of the serious and difficult-to-treat infections and a new antibiotic available: cefiderocol. Front Pharmacol. 2021;11:18. doi: 10.3389/fphar.2020.578823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zlatian O, Balasoiu AT, Balasoiu M, Cristea O, Docea AO, Mitrut R, Spandidos DA, Tsatsakis AM, Bancescu G, Calina D. Antimicrobial resistance in bacterial pathogens among hospitalised patients with severe invasive infections. Exp Ther Med. 2018;16(6):4499–4510. doi: 10.3892/etm.2018.6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sharifi-Rad J, Quispe C, Turgumbayeva A, Mertdinç Z, Tütüncü S, Aydar EF, Özçelik B, Anna S-W, Mariola S, Koziróg A, Otlewska A, Antolak H, Sen S, Acharya K, Lapava N, Emamzadeh-Yazdi S, Martorell M, Kumar M, Varoni EM, Iriti M, Calina D. Santalum Genus: phytochemical constituents, biological activities and health promoting-effects. Z Naturforschung C. 2022 doi: 10.1515/znc-2022-0076. [DOI] [PubMed] [Google Scholar]
- 143.Ungureanu A, Zlatian O, Mitroi G, Drocas A, Tirca T, Calina D, Dehelean C, Docea AO, Izotov BN, Rakitskii VN, Cioboata R, Spandidos DA, Tsatsakis AM, Gaman A. Staphylococcus aureus colonisation in patients from a primary regional hospital. Mol Med Rep. 2017;16(6):8771–8780. doi: 10.3892/mmr.2017.7746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lu L, Zhao Y, Yi G, Li M, Liao L, Yang C, Cho C, Zhang B, Zhu J, Zou K, Cheng Q. Quinic acid: a potential antibiofilm agent against clinical resistant Pseudomonas aeruginosa. Chinese Med. 2021;16(1):72. doi: 10.1186/s13020-021-00481-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lv S, Qiu Q, Wang Q, Kuang H. A comprehensive review of the botany, ethnopharmacology, biochemistry, pharmacology, pharmacokinetics and toxicity of Filifolium sibiricum (L.) Kitam. Chinese Med. 2021;16(1):83. doi: 10.1186/s13020-021-00471-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tanase A, Colita A, Ianosi G, Neagoe D, Branisteanu DE, Calina D, Docea AO, Tsatsakis A, Ianosi SL. Rare case of disseminated fusariosis in a young patient with graft vs. host disease following an allogeneic transplant. Exp Ther Med. 2016;12(4):2078–2082. doi: 10.3892/etm.2016.3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Shen X, Zhang W, Peng C, Yan J, Chen P, Jiang C, Yuan Y, Chen D, Zhu W, Yao M. In vitro anti-bacterial activity and network pharmacology analysis of Sanguisorba officinalis L. against Helicobacter pylori infection. Chinese Med. 2021;16(1):33. doi: 10.1186/s13020-021-00442-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hossain R, Quispe C, Herrera-Bravo J, Islam MS, Sarkar C, Islam MT, Martorell M, Cruz-Martins N, Al-Harrasi A, Al-Rawahi A, Sharifi-Rad J, Ibrayeva M, Daştan SD, Alshehri MM, Calina D, Cho WC. Lasia spinosa chemical composition and therapeutic potential: a literature-based review. Oxid Med Cell Longev. 2021;2021:1602437. doi: 10.1155/2021/1602437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sharifi-Rad J, Quispe C, Bouyahya A, El Menyiy N, El Omari N, Shahinozzaman M, Ara Haque Ovey M, Koirala N, Panthi M, Ertani A, Nicola S, Lapava N, Herrera-Bravo J, Salazar LA, Changan S, Kumar M, Calina D. Ethnobotany, phytochemistry, biological activities, and health-promoting effects of the genus Bulbophyllum. Evid Based Complement Alternat Med. 2022;2022:6727609. doi: 10.1155/2022/6727609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dettenkofer M, Widmer A, Kern W. MRSA and other multi-resistant pathogens: an ever increasing problem even in ambulant medicine. J Deutsche Med Wochenschrift. 2008;133(8):370–371. doi: 10.1055/s-2008-1046722. [DOI] [PubMed] [Google Scholar]
- 151.Yeni Y, Cakir Z, Hacimuftuoglu A, Taghizadehghalehjoughi A, Okkay U, Genc S, Yildirim S, Saglam YS, Calina D, Tsatsakis A, Docea AO. A selective histamine H4 receptor antagonist, JNJ7777120, role on glutamate transporter activity in chronic depression. J Personalized Med. 2022;12(2):246. doi: 10.3390/jpm12020246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Amin R, Quispe C, Docea AO, Ydyrys A, Kulbayeva M, Durna Daştan S, Calina D, Sharifi-Rad J. The role of tumour necrosis factor in neuroinflammation associated with Parkinson's disease and targeted therapies. Neurochem Int. 2022;158:105376. doi: 10.1016/j.neuint.2022.105376. [DOI] [PubMed] [Google Scholar]
- 153.Tsoukalas D, Buga AM, Docea AO, Sarandi E, Mitrut R, Renieri E, Spandidos DA, Rogoveanu I, Cercelaru L, Niculescu M, Tsatsakis A, Calina D. Reversal of brain aging by targeting telomerase: a nutraceutical approach. Int J Mol Med. 2021; 48 (5). 10.3892/ijmm.2021.5032. [DOI] [PMC free article] [PubMed]
- 154.Salehi B, Sharifi-Rad J, Cappellini F, Reiner A, Zorzan D, Imran M, Sener B, Kilic M, El-Shazly M, Fahmy NM, Al-Sayed E, Martorell M, Tonelli C, Petroni K, Docea AO, Calina D, Maroyi A. The therapeutic potential of anthocyanins: current approaches based on their molecular mechanism of action. Front Pharmacol. 2020;11:20. doi: 10.3389/fphar.2020.01300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Calina D, Buga AM, Mitroi M, Buha A, Caruntu C, Scheau C, Bouyahya A, El Omari N, El Menyiy N, Docea AO. The treatment of cognitive, behavioural and motor impairments from brain injury and neurodegenerative diseases through cannabinoid system modulation-evidence from in vivo studies. J Clin Med. 2020;9(8):28. doi: 10.3390/jcm9082395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Calina D, Carvalho F, Oana Docea A. Chapter 43—Toxicity of psychedelic drugs. In: Tsatsakis AM, editor. Toxicological risk assessment and multi-system health impacts from exposure. Academic Press; 2021. pp. 545–556. [Google Scholar]
- 157.Tsoukalas D, Fragkiadaki P, Docea AO, Alegakis AK, Sarandi E, Thanasoula M, Spandidos DA, Tsatsakis A, Razgonova MP, Calina D. Discovery of potent telomerase activators: unfolding new therapeutic and anti-aging perspectives. Mol Med Rep. 2019;20(4):3701–3708. doi: 10.3892/mmr.2019.10614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Konovalov DA, Cáceres EA, Shcherbakova EA, Herrera-Bravo J, Chandran D, Martorell M, Hasan M, Kumar M, Bakrim S, Bouyahya A, Cho WC, Sharifi-Rad J, Suleria HAR, Calina D. Eryngium caeruleum: an update on ethnobotany, phytochemistry and biomedical applications. Chinese Med. 2022;17(1):114. doi: 10.1186/s13020-022-00672-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Asgharian P, Quispe C, Herrera-Bravo J, Sabernavaei M, Hosseini K, Forouhandeh H, Ebrahimi T, Sharafi-Badr P, Tarhriz V, Soofiyani SR, Helon P, Rajkovic J, Durna Daştan S, Docea AO, Sharifi-Rad J, Calina D, Koch W, Cho WC. Pharmacological effects and therapeutic potential of natural compounds in neuropsychiatric disorders: an update. Front Pharmacol. 2022 doi: 10.3389/fphar.2022.926607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sharifi-Rad J, Rapposelli S, Sestito S, Herrera-Bravo J, Arancibia-Diaz A, Salazar LA, Yeskaliyeva B, Beyatli A, Leyva-Gómez G, González-Contreras C, Gürer ES, Martorell M, Calina D. Multi-target mechanisms of phytochemicals in Alzheimer's disease: effects on oxidative stress, neuroinflammation and protein aggregation. J Pers Med. 2022;12(9):793. doi: 10.3390/jpm12091515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ghayur MN, Gilani AH, Rasheed H, Khan A, Iqbal Z, Ismail M, Saeed SA, Janssen L. Cardiovascular and airway relaxant activities of peony root extract. Can J Physiol Pharmacol. 2008;86(11):793–803. doi: 10.1139/Y08-084. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting this review are from previously reported studies and datasets, which have been cited. The processed data are available from the corresponding author upon request.