Abstract
Background
Albumin-bound paclitaxel (nab-paclitaxel), as a special targeted preparation of paclitaxel, has the advantages of good curative effect and less side effects in anti-tumor therapy. The existence of the plasma-peritoneal barrier and insufficient blood supply make intravenous drugs hard to reach the peritoneum, while hyperthermic intraperitoneal chemotherapy can solve the difficulty. And compared with systemic medications, HIPEC can also give higher concentrations of chemotherapy drugs in the abdominal cavity, while ensuring lower systemic toxicity. However, at present, there is no relevant report on the clinical study of nab-paclitaxel during intraperitoneal hyperthermic chemotherapy, and its stability under special temperature conditions has not been reported either.
Methods
In this study, We examined three batches of albumin-bound paclitaxel dissolved in saline at different temperatures (25 °C, 37 °C, 41 °C, 42 °C and 43 °C) for the changes of human serum albumin content, human serum albumin polymer content, related substance content, in-vitro release rate, paclitaxel binding rate and paclitaxel content at different temperatures.
Results
Our results demonstrated that the indicators including human serum albumin content, human serum albumin polymer content, in-vitro release rate, paclitaxel binding rate and paclitaxel content were stable to the several temperatures, except that Taxane (0.1%) and other individual impurities in the determination of related substance content fluctuated comparatively widely with the change of temperature. In addition, only Taxane (0.1%) and 7-Epitaxol (1%) were detected.
Conclusions
Overall, albumin-bound paclitaxel is relatively stable to different temperatures (25 °C, 37 °C, 41 °C, 42 °C and 43 °C). This study will lay a foundation for further studies on the albumin-bound paclitaxel during hyperthermic intraperitoneal chemotherapy.
Keywords: Albumin-bound paclitaxel, Thermal stability, Temperature, Hyperthermic intraperitoneal chemotherapy
Introduction
Globally, gastric cancer is the third leading cause of cancer death [1–3]. Approximately 15–50% of patients with advanced gastric cancer have peritoneal carcinomatosis (PC) at diagnosis, and PC was the main cause of post-op recurrence in 35–50% of patients [4, 5]. Hyperthermic intraperitoneal chemotherapy (HIPEC), as a comprehensive therapy involving intraperitoneal perfusion, warming effect, and chemotherapeutic drugs, has been used in primary and secondary peritoneal tumors, as well as an adjuvant treatment for abdominal malignant tumors [6–11].
In recent years, the dosage form of paclitaxel has been increasing continuously, overcoming the shortcomings of traditional paclitaxel injection. Among them, albumin-bound paclitaxel (nab-paclitaxel) is a solvent-free, 130 nm albumin-paclitaxel complex [12, 13]. Compared with traditional paclitaxel, nab-paclitaxel, which does not contain polyethoxylated castor oil, can minimize the risk of hypersensitivity reactions [14, 15]. In addition, since this form does not require hydrated ethanol as a solvent, it can be used for patients with alcohol intolerance. Furthermore, compared to solvent-based paclitaxel, high-dose nab-paclitaxel can be administered in a shorter infusion time. At the same dose, the paclitaxel dose of nab-paclitaxel is 33% higher than that of solvent-based paclitaxel, which indicates that the intratumoral accumulation of nab-paclitaxel is more effective [16, 17]. To date, nab-paclitaxel has been approved for the treatment of metastatic breast cancer [18–20], locally advanced or metastatic non-small cell-lung cancer [21–23], and metastatic pancreatic cancer [24–26]. Not only that, more and more evidences show that nab-paclitaxel is also effective for gastric cancer and peritoneal cancer [27–33].
At present, there are few studies on nab-paclitaxel in the treatment of gastric cancer with peritoneal metastatic cancer by intraperitoneal hyperthermia. In the recent 3 years, nab-paclitaxel is still mainly administered intravenously [34–36], but Markman M and coworkers proved that paclitaxel can have acceptable toxicity and pharmacokinetic advantage for cavity exposure by the intraperitoneal administration [37]. Manzanedo and colleagues used paclitaxel as part of an HIPEC procedure for 1 hour and showed that paclitaxel didn’t negatively affect the prognosis of patients with advanced ovarian cancer [38]. Therefore, we considered whether HIPEC paclitaxel or nab-paclitaxel had the same therapeutic effect in treating patients with gastric cancer. In addition, the stability of hyperthermia drugs plays an important role in efficacy and safety during HIPEC. HIPEC mainly uses the special differences of temperature tolerance between cancer cells and normal tissues to heat the chemotherapy drugs and perfusion fluid to a certain temperature through the intraperitoneal hyperthermic perfusion therapy system, and then continuously circulates and perfuses into the abdominal cavity of patients at constant temperature, so as to remove free cancer cells and small metastatic lesions in the abdominal cavity. Normal tissue cells can continuously tolerate 47 °C for 1 hour under high temperature conditions, while malignant tumor cells can only tolerate 43 °C for 1 hour. 47 °C and 43 °C for 1 hour are regarded as the critical temperatures for irreversible damage of normal tissue cells and malignant tumor cells. However, albumin may be degraded at high temperature. If albumin-bound paclitaxel cannot be guaranteed to be stable under the condition of intraperitoneal hyperthermic perfusion, the therapeutic effect will be affected. Therefore, this topic investigated the stability of albumin-bound paclitaxel drugs at different temperatures, including the determination of the drug content of albumin-bound paclitaxel at 25 °C, 37 °C, 41 °C, 42 °C, and 43 °C, and paclitaxel binding rate, in vitro release rate, determination of human serum albumin content, determination of human serum albumin polymer content, determination of related substances content, etc. This study has laid a foundation for the later study of albumin-bound paclitaxel intraperitoneal hyperthermic perfusion.
Materials and methods
Materials
Human albumin reference substance (SLBX9574, purity > 98%) was purchased from Merck. Albumin-bound paclitaxel (B042001249, B042001250, B042001251) was purchased from CSPC Ouyi Pharmaceutical Co., Ltd. (Shijiazhuang, China). 0.9% sodium chloride solution was purchased from Shijiazhuang No.4 Pharmaceutical Co., Ltd. Dipotassium hydrogen phosphate solution (20180625) was purchased from Sinopharm Chemical Reagent Co., Ltd. Paclitaxel reference substance (100382–201,603, purity: 98%) was purchased from National Institutes for Food and Drug Control. Acetonitrile (SHBM9830), dehydrated ethanol (K52258727010)and Methanol was purchased from Merck. Purified water was purchased from Hangzhou Wahaha Co., Ltd. Potassium hydroxide (KOH) (10017018) was purchased from Sinopharm Chemical Reagent Co., Ltd. Glacial acetic acid (20210127) was purchased from Tianjin Kemiou Chemical Reagent Co., Ltd. BAKERBOND Ociadecyl (C18) Disposable Extraction was purchased from J.T.Baker. Cephalomannine reference substance (100926–201,503, HPLC system performance test) was purchased from National Institutes for Food and Drug Control.
Instruments
High performance liquid chromatography (Waters e2695) was purchased from (Waters, USA), Electronic balance (XS105) was purchased from (Mettler, Switzerland). High performance liquid chromatography (UltiMate 3000) was purchased from (Thermo Electron Corporation, USA). Vortex mixer (IKA MS3) was purchased from (Ikachina, China).
Determination of human serum albumin content
Preparation of standard curve solution and system suitability test: Placed 65.54 mg human albumin reference substance in a 10 mL measuring flask and diluted to the scale with 0.9% sodium chloride solution, as the control solution (1). Precisely measured 5 mL and 2.5 mL control solution (1) respectively, and placed them in two 10 mL measuring flasks, diluted to the scale with 0.9% sodium chloride solution, as the control solution (2) and (3) (left at room temperature, can be used within 66 h).
Preparation of the test solution: Transferred the albumin-bound paclitaxel with 0.9% sodium chloride solution into a 250 mL measuring flask by subsections and was diluted to the scale with 0.9% sodium chloride solution. Then took 3 mL diluted solution in a water bath at 25 °C, 37 °C, 41 °C, 42 °C and 43 °C for 60 minutes respectively and then determined according to the method.
Analytical condition: Tosohaas TSK G3000 SWXL column (300 × 7.8 mm) was used, column number: 010D04207D, column temperature: 30 °C; detector: UV, wavelength: 232 nm; mobile phase: 0.1 mol/L dipotassium hydrogen phosphate solution (adjusted pH to 7.0 with hydrochloric acid). The flow rate was set at 0.7 mL/min.
Determination of human serum albumin polymer content
Preparation of test solution: The test solution was taken in the determination of human albumin content section.
Analytical condition: Same condition as the determination of human albumin section, the detection wavelength was set at 280 nm.
Determination of related substances content
Chromatographic condition: Lichrospher RP C18 analytical column (250 × 4.0 mm), column temperature: 24 °C; Ultraviolet-light detector, wavelength: 232 nm; mobile phase A: methanol-water (1:1), mobile phase B: gradient elution according to the Table 1:
Table 1.
Gradient elution program
| Time(min) | A(%) | B(%) | Flow rate (mL/min) |
|---|---|---|---|
| 0 | 76 | 24 | 1.0 |
| 50 | 76 | 24 | 1.0 |
| 60 | 0 | 100 | 1.0 |
| 65 | 0 | 100 | 1.5 |
| 70 | 0 | 100 | 1.5 |
| 71 | 76 | 24 | 1.0 |
| 90 | 76 | 24 | 1.0 |
Preparation of system suitability solutions A: Placed 20.33 mg paclitaxel reference substance in a 25 mL measuring flask and added 0.01 mol/L potassium hydroxide ethanol solution (added 0.07 g potassium hydroxide in a 100 mL measuring flask and 0.4 mL water to dissolve, diluted to the scale with anhydrous ethanol and mixed well) to 1 mL, shook for 1 minute and then diluted to the scale with 0.1% (V/V) glacial acetic acid methanol solution and shook well. Stored at 4 °C for at least 30 minutes before feeding (stored at 4 °C, can be used within 30 months).
Preparation of system suitability solutions B: Placed 20.33 mg paclitaxel reference substance in a 100 mL measuring flask, dissolved with acetonitrile and diluted to the scale, shook well. Placed 3 mL precisely in a 25 mL measuring flask, diluted to the scale with acetonitrile and shook well. Stored at 4 °C for at least 30 minutes before feeding (stored at 4 °C, can be used within 5 days).
Preparation of system suitability solutions C: Added 0.6 mL system suitability solutions B into a 25 mL measuring flask, diluted to the scale with acetonitrile and shook well. Stored at 4 °C for at least 30 minutes before feeding (stored at 4 °C, can be used within 5 days).
According to elution procedure 1, 10 μL system suitability solution A was injected into the liquid chromatograph and the chromatogram was recorded.
According to elution procedure 2, 10 μL each of system suitability solutions B and C were precisely measured and injected into the liquid chromatograph respectively, and the chromatogram was recorded with 6 consecutive injections.
Formula:
Ai is the impurity peak area, A is the sum of the peak areas, and RRF is relative response factor.
The steps of solid phase extraction: (1) The solid phase extraction column (SPE C18) was activated with 3 mL acetonitrile, followed by two washes with water, 3 mL each time. SPEC18 column was not allowed to run dry. (2) Added 1 mL water into each SPE column, poured the test solution into the SPE column and filled the column with water and left to stand for 10 minutes. (3) Let the test solution slowly pass through the column without running dry, wash three times with water, 3 mL each time, and let the column run dry until the last wash, wiped the receiver down. (4) Added 1 mL acetonitrile to each SPE column and collected the eluate into an HPLC vial. The solution was allowed to store at 4 °C for at least 30 minutes before feeding into the sample.
Preparation of test solution: Took 1 bottle of the product, added 20 mL of 0.9% sodium chloride solution, shook gently to disperse evenly, heated in a water bath at 25 °C, 37 °C, 41 °C, 42 °C and 43 °C for 60 minutes respectively, then placed 305 μL in a 2 mL centrifuge tube, added 600 μL acetonitrile, vortexed for 30 seconds, then extracted by solid phase extraction, the resulting solution was the test solution.
Preparation of blank solution 1: Placed 84 μL human albumin (20%) in a 2 mL centrifuge tube, 600 μL acetonitrile was added precisely, vortexed for 30 seconds, and then the solution was extracted by solid phase extraction. The resulting solution was the blank solution 1.
Preparation of blank solution 2: 600 μL acetonitrile was used for solid phase extraction and the resulting solution was blank solution 2.
Determination method: The test solution and the blank solution 1 and 2 were each measured 10 μL, injected into the liquid chromatograph respectively, eluted according to the gradient procedure 1 in Table 1, recorded the chromatogram, deducted the solvent peak and the impurity peak with impurity content that less than 0.05% according to the corrected peak area normalization method, and calculated the impurity content according to the corrected peak area normalization method.
In-vitro release rate
Preparation of the test solution: Took 1 bottle of paclitaxel for injection (albumin-binding type), added 20 mL 0.9% sodium chloride solution, shook gently to make it evenly dispersed, took 3 mL and heated it in a water bath at 25 °C, 37 °C, 41 °C, 42 °C and 43 °C for 60 minutes respectively, then took 1 mL of the above solution, put it into a 250 mL measuring bottle. Added simulated human plasma solution (commercially available human albumin preparation mixed with 0.9% sodium chloride solution to obtain simulated plasma solution with a concentration of 5% human albumin) and diluted to the scale. Added 1 mL to a 2 mL round bottom centrifuge tube, prepared 2 portions in parallel, centrifuged at 21,000 g for 60 minutes, put 0.5 mL supernatant into a 5 mL flask, added acetonitrile, sonicate to disperse well, diluted with acetonitrile to the scale and shook well, filtered and take the filtrate as the test solution.
Preparation of the control solution: Placed 10.31 mg paclitaxel reference substance in a 50 mL measuring flask, dissolved and diluted to the scale with acetonitrile, shook well and placed 5 mL in a 50 mL measuring flask, diluted to the scale with acetonitrile, shook well, and 1 mL was measured precisely and placed in a 10 mL measuring flask, diluted to the scale with acetonitrile, shook well. The average of the results of two determinations was taken as the amount of paclitaxel released and recorded as CP2 (mg/mL). The amount of paclitaxel measured in the determination of paclitaxel content section was taken as the total amount of paclitaxel, and recorded as CP (mg/bottle), and the release rate of paclitaxel was calculated.
Formula:
C P2 (mg/mL): the amount of paclitaxel that has been released.
C P (mg/bottle): the amount measured in the determination of paclitaxel content section is the amount of total paclitaxel.
Analytical condition: same as determination of paclitaxel content section.
Paclitaxel binding rate
Preparation of the test solution: Took 1 bottle of the product, added 20 mL 0.9% sodium chloride solution, shook gently to disperse evenly and took 1 mL into a 2 mL round bottom centrifuge tube, centrifuged at 21000 g for 60 minutes, put 0.5 mL supernatant into a 5 mL measuring flask, added an appropriate amount of acetonitrile, and sonicated to disperse evenly, diluted with acetonitrile to the scale and shook well, filtered, and the continuous filtrate was used as the test solution. In addition, took the control solution in the determination of paclitaxel content section, and determined with the same method. Calculated the peak area in external standard method. Took the average of the results of the two test samples as the amount of unconjugated paclitaxel, which was recorded as CP1 (mg/mL). Took the content measured in the determination of paclitaxel content section as the total amount of paclitaxel, recorded as CP (mg/bottle), and calculated the binding rate of paclitaxel.
Formula:
C p1 (mg/mL): the amount of unconjugated paclitaxel.
C P (mg/bottle): taking the content measured in the determination of paclitaxel content section as the total amount of paclitaxel.
Analytical condition: same as the analytical condition in determination of paclitaxel content section.
Determination of paclitaxel content
Preparation of the control solution: Added 10.11 mg and 10.20 mg paclitaxel reference substance in 50 mL volumetric flasks respectively, dissolved and diluted to the scale with acetonitrile, shook well, accurately measured 5 mL, and put them in a 50 mL volumetric flask, and diluted with acetonitrile to the scale, shook well, as a reference solution, put at 4 °C for at least 30 minutes before injection (stored at 4 °C, can be used within 120 hours).
Preparation of the test solution: Took 10 bottles of the product, dispersed them with a small amount of 0.9% sodium chloride solution, and transferred them to a 1000 mL measuring flask with 0.9% sodium chloride solution, diluted to the scale with 0.9% sodium chloride solution, sonicated for 1 minute, measure 5.0 mL precisely, and placed in a 25 mL measuring flask, put in a water bath at 25 °C, 37 °C, 41 °C, 42 °C, 43 °C, respectively, after 60 minutes, diluted to the scale with acetonitrile and sonicated for 5 minutes as the test solution, left at least 30 minutes at 4 °C before sampling (stored at 4 °C, can be used within 28 hours).
Added 20.33 mg paclitaxel reference substance in a 100 mL volumetric flask, dissolved it with acetonitrile and diluted it to the scale, shook well, and used it as the reference substance solution A. Added 12.54 mg cephalomannine reference substance in a 25 mL volumetric flask, dissolved it with acetonitrile and diluted to the scale, shook well, and used it as reference substance solution B. Precisely measure 3 mL reference substance solution A and 1 mL reference substance solution, put them in a 25 mL volumetric flask, diluted to the scale with acetonitrile, and shook well. Left at 4 °C for at least 30 minutes before injection (store at 4 °C, use within 120 hours).
Analytical conditions: Agilent Zorbax SB C18 column (150 × 4.6 mm), column number: USCM053630, column temperature: 30 °C; detector: UV, detection wavelength: 228 nm; mobile phase: acetonitrile-water (1:1).
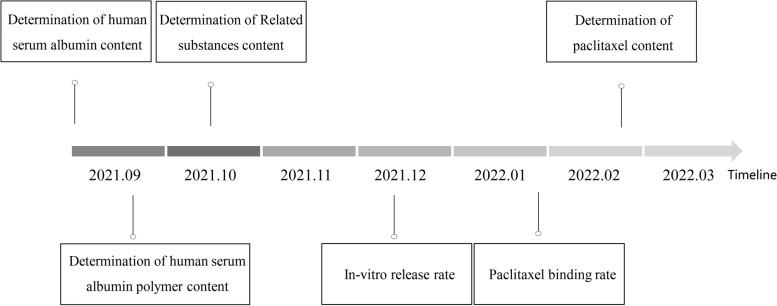
Above all, we made a schematic representation to intuitively understand the experimental design (Fig. 1).
Fig. 1.
The experimental design with timeline
Results
Determination of human serum albumin content
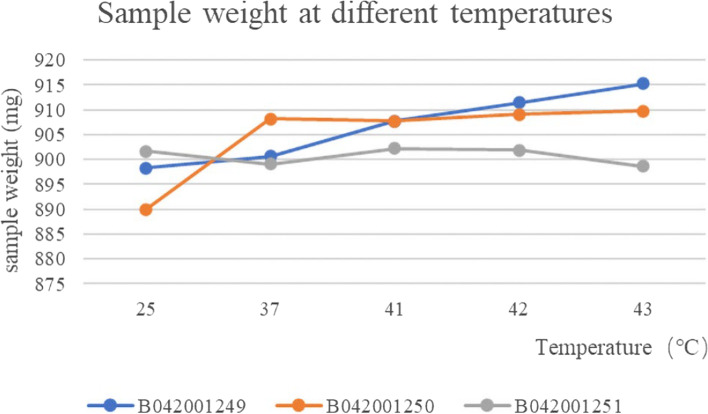
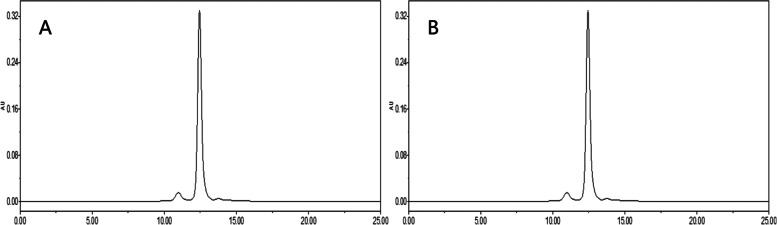
The weight of the three batches of albumin-bound paclitaxel was essentially constant at different temperatures and the RSD values for weight at different temperatures were 0.79, 0.93 and 0.19% respectively, which were stable to temperature (Fig. 2 and Table 2). Chromatograms of dimer (the left peak) and monomer (the right peak) of human albumin reference substance (A) and albumin-bound paclitaxel (B) were shown in Fig. 3.
Fig. 2.
The results of weight changes of three batches of albumin-bound paclitaxel at different temperatures
Table 2.
The results of content determination of three batches of albumin-bound paclitaxel at different temperatures
| Batch number | B042001249 | B042001250 | B042001251 |
|---|---|---|---|
| Temperature(°C) | (mg/bottle) | ||
| 25 | 898.3 | 890.0 | 901.7 |
| 37 | 900.7 | 908.2 | 899.1 |
| 41 | 907.8 | 907.7 | 902.2 |
| 42 | 911.4 | 909.1 | 901.9 |
| 43 | 915.3 | 909.8 | 898.6 |
Fig. 3.
Chromatograms of human albumin reference substance (A) and albumin-bound paclitaxel (B)
Determination of human serum albumin polymer content
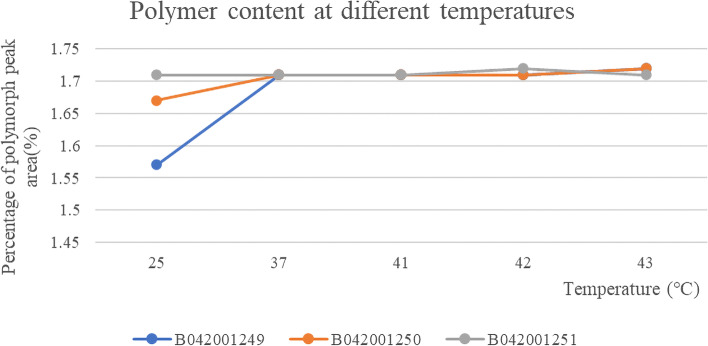
The content of polymer was basically unchanged in the three batches of samples at different temperatures, and the RSD values of the peak area of polymers at different temperatures were: 3.79, 1.14 and 0.26%, respectively. The polymer did not increase at different temperatures, indicating that paclitaxel (albumin-bound) for injection was stable in saline at different temperatures (Fig. 4 and Table 3). A typical chromatogram of human albumin polymer is shown in Fig. 5.
Fig. 4.
The results of content changes of three batches of polymer of albumin-bound paclitaxel at different temperatures
Table 3.
The results of polymer peak area of three batches of albumin-bound paclitaxel at different temperatures
| Batch number | Temperature (°C) | Polymorph peak area | Total area | Result (%) |
|---|---|---|---|---|
| B042001249 | 25 | 47,721 | 1,518,743 | 1.57 |
| 37 | 52,150 | 1,525,324 | 1.71 | |
| 41 | 52,201 | 1,526,191 | 1.71 | |
| 42 | 52,203 | 1,524,651 | 1.71 | |
| 43 | 52,153 | 1,518,980 | 1.72 | |
| B042001250 | 25 | 51,013 | 1,525,879 | 1.67 |
| 37 | 52,083 | 1,523,291 | 1.71 | |
| 41 | 52,070 | 1,525,968 | 1.71 | |
| 42 | 52,090 | 1,520,404 | 1.71 | |
| 43 | 52,196 | 1,521,527 | 1.72 | |
| B042001251 | 25 | 52,152 | 1,524,698 | 1.71 |
| 37 | 52,080 | 1,524,160 | 1.71 | |
| 41 | 52,041 | 1,523,870 | 1.71 | |
| 42 | 52,132 | 1,519,462 | 1.72 | |
| 43 | 52,003 | 1,517,695 | 1.71 |
Fig. 5.
Chromatograms of Polymer (1), Unknown impurities (2), Dimer (3) and Monomer (4)
Determination of related substances content
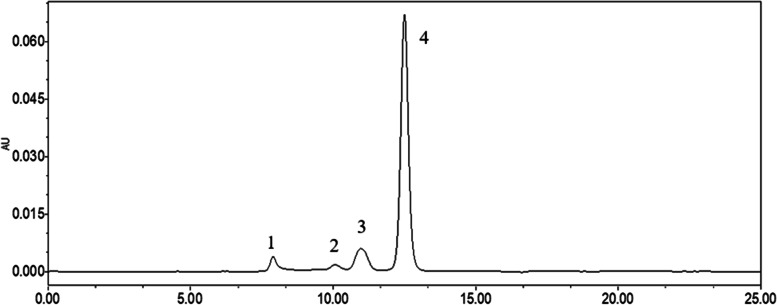
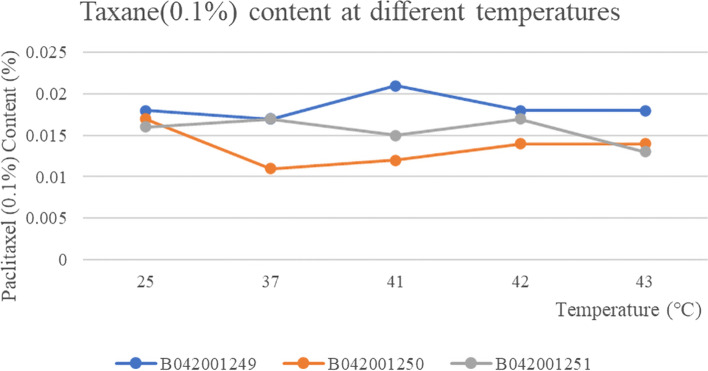
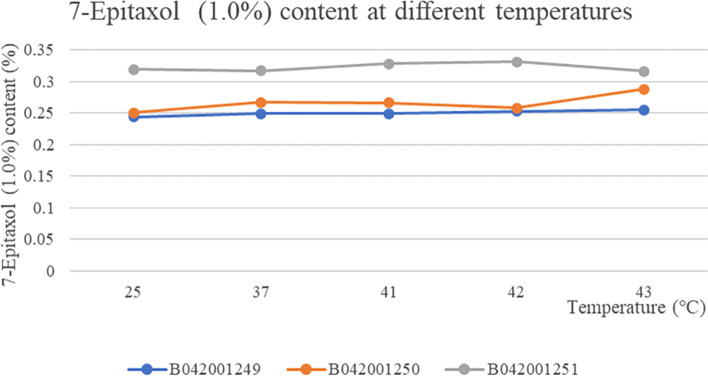
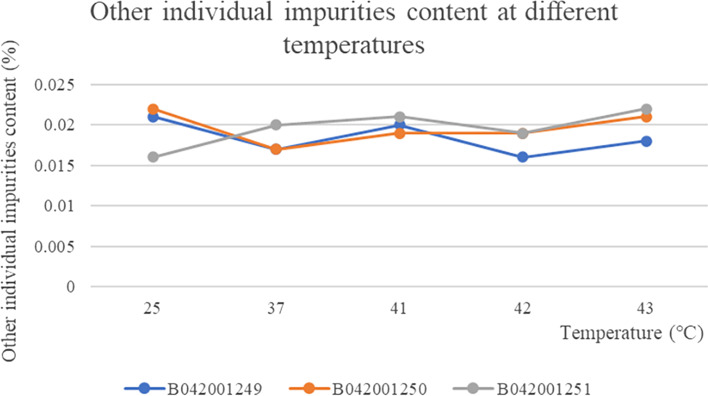
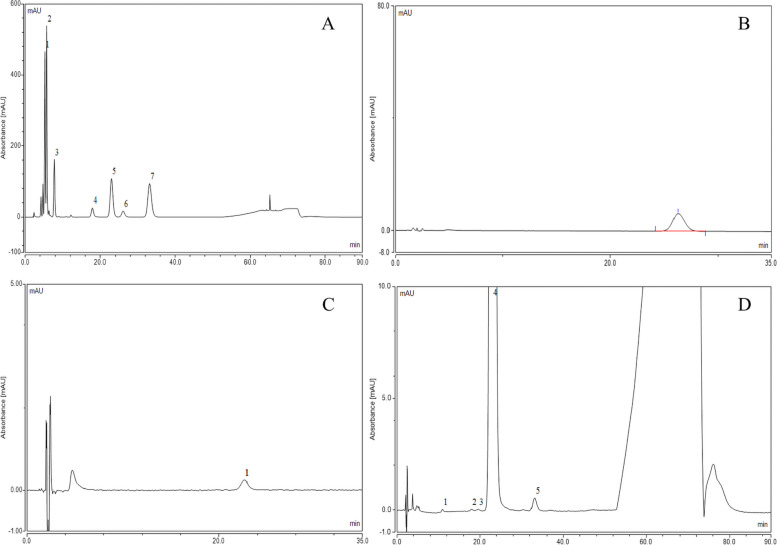
The three batches of taxane of albumin-bound paclitaxel at different temperatures showed no change with the change of temperature and were stable at all temperatures The RSD values for weight at different temperatures were 0.79, 0.93 and 0.19% respectively, which were stable to temperature (Fig. 6). The content of the substance 7-Epitaxol (1%) in the three batches of samples was basically the same at different temperatures and the RSD values for the content of 7-Epitaxol (1.0%) at different temperatures in the three batches of samples were 1.70, 5.23 and 2.12% respectively, so the substance 7-Epitaxol (1%) was stable to temperature (Fig. 7). The other individual maximum impurities at different temperatures were stable at different temperatures with no increase. No new impurities were produced with increasing temperature and the samples were stable at different temperatures (Fig. 8). In addition, Taxane (0.1%) and 7-Epitaxol (1.0%) were detected which was shown in every batch of albumin-bound paclitaxel at different temperatures (Table 4). The chromatogram of related substances can be seen in Fig. 9.
Fig. 6.
The results of content changes of three batches of taxane of albumin-bound paclitaxel at different temperatures
Fig. 7.
The results of content changes of three batches of 7-Epitaxol of albumin-bound paclitaxel at different temperatures
Fig. 8.
The results of content changes of three batches of other individual impurities of albumin-bound paclitaxel at different temperatures
Table 4.
The relative retention time (RRT) and relative response factor (RRF) of three batches of related substances of albumin-bound paclitaxel at different temperatures
| Batch number: B042001249 | |||||||
| Impurities and degradation products, limits | RRT | RRF | 25 °C | 37 °C | 41 °C | 42 °C | 43 °C |
| (2R,3S)-N-benzoyl-3-phenylisoserine methyl ester (0.1%) | 0.21 | 0.90 | Not detected | Not detected | Not detected | Not detected | Not detected |
| Baccatin III (0.1%) | 0.22–0.24 | 1.00 | Not detected | Not detected | Not detected | Not detected | Not detected |
| (2R,3S)-N-benzoyl-3-phenylisoserine methyl ester (0.1%) | 0.24–0.26 | 0.90 | Not detected | Not detected | Not detected | Not detected | Not detected |
| C3-C11 bridge paclitaxel isomers (0.1%) | 0.41 | 1.00 | Not detected | Not detected | Not detected | Not detected | Not detected |
| 10-Deacetylpaclitaxel (0.1%) | 0.79–0.80 | 1.00 | Not detected | Not detected | Not detected | Not detected | Not detected |
| Taxane (0.1%) | 0.83–0.84 | 1.00 | 0.018 | 0.017 | 0.021 | 0.018 | 0.018 |
| Cephalomannine (0.4%) | 0.89 | 1.33 | Not detected | Not detected | Not detected | Not detected | Not detected |
| 10-Deacetyl-7-epipaclitaxel (0.1%) | 1.14 | 1.00 | Not detected | Not detected | Not detected | Not detected | Not detected |
| 7-Epicephalomannine (0.1%) | 1.26–1.28 | 1.31 | Not detected | Not detected | Not detected | Not detected | Not detected |
| 7-Epitaxol (1.0%) | 1.41–1.45 | 1.00 | 0.244 | 0.249 | 0.249 | 0.253 | 0.255 |
| Paclitaxel C (0.1%) | 1.89–2.08 | 1.80 | Not detected | Not detected | Not detected | Not detected | Not detected |
| N-methyl-paclitaxel C (0.1%) | RT = 57-60 min | 1.68 | Not detected | Not detected | Not detected | Not detected | Not detected |
| Other single impurities (0.1%) | – | 1.00 | 0.021 | 0.017 | 0.020 | 0.016 | 0.018 |
| Total impurities (1.5%) | – | – | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Batch number: B0420012450 | |||||||
| Impurities and degradation products, limits | RRT | RRF | 25 °C | 37 °C | 41 °C | 42 °C | 43 °C |
| (2R,3S)-N-benzoyl-3-phenylisoserine methyl ester (0.1%) | 0.21 | 0.90 | Not detected | Not detected | Not detected | Not detected | Not detected |
| Baccatin III (0.1%) | 0.22–0.24 | 1.00 | Not detected | Not detected | Not detected | Not detected | Not detected |
| (2R,3S)-N-benzoyl-3-phenylisoserine methyl ester (0.1%) | 0.24–0.26 | 0.90 | Not detected | Not detected | Not detected | Not detected | Not detected |
| C3-C11 bridge paclitaxel isomers (0.1%) | 0.41 | 1.00 | Not detected | Not detected | Not detected | Not detected | Not detected |
| 10-Deacetylpaclitaxel (0.1%) | 0.79–0.80 | 1.00 | Not detected | Not detected | Not detected | Not detected | Not detected |
| Taxane (0.1%) | 0.83–0.84 | 1.00 | 0.017 | 0.011 | 0.012 | 0.014 | 0.014 |
| Cephalomannine (0.4%) | 0.89 | 1.33 | Not detected | Not detected | Not detected | Not detected | Not detected |
| 10-Deacetyl-7-epipaclitaxel (0.1%) | 1.14 | 1.00 | Not detected | Not detected | Not detected | Not detected | Not detected |
| 7-Epicephalomannine (0.1%) | 1.26–1.28 | 1.31 | Not detected | Not detected | Not detected | Not detected | Not detected |
| 7-Epitaxol (1.0%) | 1.41–1.45 | 1.00 | 0.251 | 0.267 | 0.266 | 0.258 | 0.288 |
| Paclitaxel C (0.1%) | 1.89–2.08 | 1.80 | Not detected | Not detected | Not detected | Not detected | Not detected |
| N-methyl-paclitaxel C (0.1%) | RT = 57-60 min | 1.68 | Not detected | Not detected | Not detected | Not detected | Not detected |
| Other single impurities (0.1%) | – | 1.00 | 0.022 | 0.017 | 0.019 | 0.019 | 0.021 |
| Total impurities (1.5%) | – | – | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Batch number: B042001251 | |||||||
| Impurities and degradation products, limits | RRT | RRF | 25 °C | 37 °C | 41 °C | 42 °C | 43 °C |
| (2R,3S)-N-benzoyl-3-phenylisoserine methyl ester (0.1%) | 0.21 | 0.90 | Not detected | Not detected | Not detected | Not detected | Not detected |
| Baccatin III (0.1%) | 0.22–0.24 | 1.00 | Not detected | Not detected | Not detected | Not detected | Not detected |
| (2R,3S)-N-benzoyl-3-phenylisoserine methyl ester (0.1%) | 0.24–0.26 | 0.90 | Not detected | Not detected | Not detected | Not detected | Not detected |
| C3-C11 bridge paclitaxel isomers (0.1%) | 0.41 | 1.00 | Not detected | Not detected | Not detected | Not detected | Not detected |
| 10-Deacetylpaclitaxel (0.1%) | 0.79–0.80 | 1.00 | Not detected | Not detected | Not detected | Not detected | Not detected |
| Taxane (0.1%) | 0.83–0.84 | 1.00 | 0.016 | 0.017 | 0.015 | 0.017 | 0.013 |
| Cephalomannine (0.4%) | 0.89 | 1.33 | Not detected | Not detected | Not detected | Not detected | Not detected |
| 10-Deacetyl-7-epipaclitaxel (0.1%) | 1.14 | 1.00 | Not detected | Not detected | Not detected | Not detected | Not detected |
| 7-Epicephalomannine (0.1%) | 1.26–1.28 | 1.31 | Not detected | Not detected | Not detected | Not detected | Not detected |
| 7-Epitaxol (1.0%) | 1.41–1.45 | 1.00 | 0.319 | 0.317 | 0.328 | 0.331 | 0.316 |
| Paclitaxel C (0.1%) | 1.89–2.08 | 1.80 | Not detected | Not detected | Not detected | Not detected | Not detected |
| N-methyl-paclitaxel C (0.1%) | RT = 57-60 min | 1.68 | Not detected | Not detected | Not detected | Not detected | Not detected |
| Other single impurities (0.1%) | – | 1.00 | 0.016 | 0.020 | 0.021 | 0.019 | 0.022 |
| Total impurities (1.5%) | – | – | 0.4 | 0.4 | 0.4 | 0.4 | |
RRT Relative retention time, RRF Relative response factor
Fig. 9.
Chromatograms of system suitability solution A (A): 2-Baccatin III (2), 1-(2R,3S)-N-benzoyl-3-phenylisoserine methyl ester (3), 5-Paclitaxel (5), 10-Deacetyl-6-7-epipaclitaxel (6), 7–7-Epipaclitaxel (7), system suitability solution B (B), system suitability solution C (C) and albumin-bound paclitaxel (D): 1–20-Taxane (3), 3-Paclitaxel (4), 4–7-Epipaclitaxel (5)
In-vitro release rate
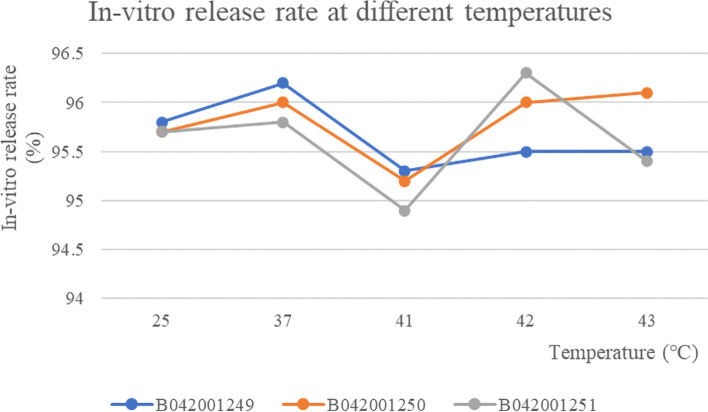
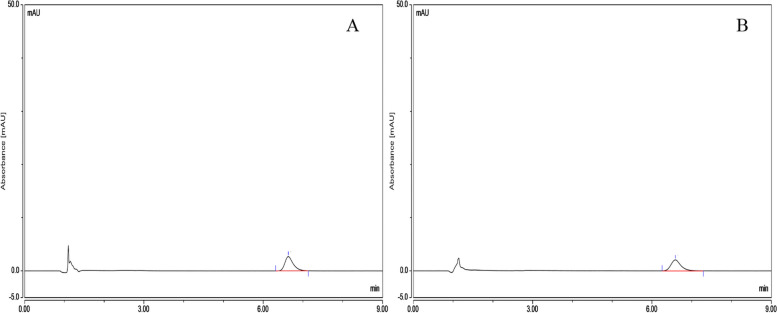
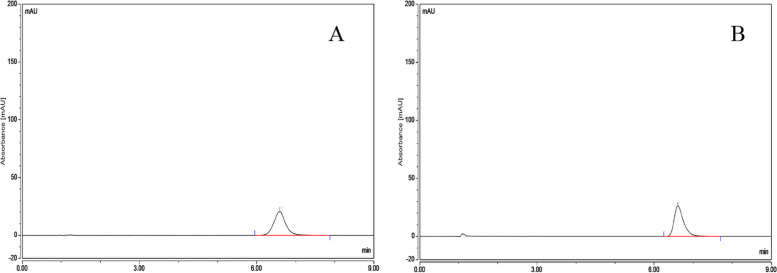
The In-vitro release rate of three batches of albumin-bound paclitaxel was shown in Table 5 and the RSD values for the in-vitro release rate of the three batches of samples at different temperatures were 0.37, 0.38 and 0.54% respectively, indicating that the temperature had a small effect on the release rate of the drug and the in-vitro release rate were basically the same at different temperatures (Fig. 10). The chromatogram of control solution (A): RT (retention time) was 6.630 minutes and test solution (B): RT (retention time) was 6.590 minutes (Fig. 11).
Table 5.
The results of peak area, CP and release rate CP2 of three batches of albumin-bound paclitaxel at different temperatures
| Batch number | Temperature (°C) | Peak area | CP (%) | Release rate CP2 (%) | Mean value (%) |
|---|---|---|---|---|---|
| B042001249 | 25 | 0.6286 | 101.55 | 96.23 | 95.8 |
| 0.6224 | 101.55 | 95.28 | |||
| 37 | 0.6282 | 101.55 | 96.17 | 96.2 | |
| 0.6289 | 101.55 | 96.28 | |||
| 41 | 0.6241 | 101.55 | 95.54 | 95.3 | |
| 0.6206 | 101.55 | 95.01 | |||
| 42 | 0.6217 | 101.55 | 95.17 | 95.5 | |
| 0.6254 | 101.55 | 95.74 | |||
| 43 | 0.6228 | 101.55 | 95.34 | 95.5 | |
| 0.6245 | 101.55 | 95.6 | |||
| B042001250 | 25 | 0.6242 | 101.36 | 95.74 | 95.7 |
| 0.6231 | 101.36 | 95.57 | |||
| 37 | 0.6268 | 101.36 | 96.13 | 96.0 | |
| 0.625 | 101.36 | 95.86 | |||
| 41 | 0.6226 | 101.36 | 95.49 | 95.2 | |
| 0.6193 | 101.36 | 94.98 | |||
| 42 | 0.6242 | 101.36 | 95.74 | 96.0 | |
| 0.627 | 101.36 | 96.16 | |||
| 43 | 0.6271 | 101.36 | 96.18 | 96.1 | |
| 0.6256 | 101.36 | 95.95 | |||
| B042001251 | 25 | 0.6231 | 101.54 | 95.4 | 95.7 |
| 0.6268 | 101.54 | 95.96 | |||
| 37 | 0.6306 | 101.54 | 96.55 | 95.8 | |
| 0.6211 | 101.54 | 95.09 | |||
| 41 | 0.6197 | 101.54 | 94.88 | 94.9 | |
| 0.6202 | 101.54 | 94.95 | |||
| 42 | 0.6279 | 101.54 | 96.13 | 96.3 | |
| 0.6301 | 101.54 | 96.47 | |||
| 43 | 0.6228 | 101.54 | 95.35 | 95.4 | |
| 0.6229 | 101.54 | 95.37 |
Fig. 10.
The results of in-vitro release rate of three batches of albumin-bound paclitaxel at different temperatures
Fig. 11.
Chromatograms of control solution (A) and test solution (B)
Paclitaxel binding rate
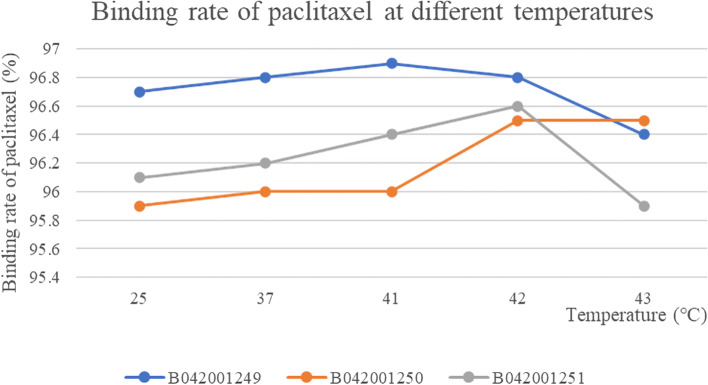
The RSD value of paclitaxel binding rate at different temperatures for the three batches of samples were: 0.20, 0.31 and 0.28%, respectively. The effect of temperature on the binding rate of paclitaxel was small and stable to temperature (Fig. 12). The peak area, CP, CP1 and paclitaxel binding rate of three batches of albumin-bound paclitaxel at different temperatures were shown in Table 6.
Fig. 12.
The results of binding rate of three batches of albumin-bound paclitaxel at different temperatures
Table 6.
The peak area, CP, CP1 and paclitaxel binding rate of three batches of albumin-bound paclitaxel at different temperatures
| Batch number | Temperature (°C) | Peak area | CP | CP1 | Paclitaxel binding rate (%) | Mean value (%) |
|---|---|---|---|---|---|---|
| B042001249 | 25 | 6.0256 | 101.55 | 0.016708544 | 96.70 | 96.7 |
| 6.0806 | 101.55 | 0.016861054 | 96.67 | |||
| 37 | 5.9337 | 101.55 | 0.016453712 | 96.75 | 96.8 | |
| 5.9248 | 101.55 | 0.016429033 | 96.76 | |||
| 41 | 5.685 | 101.55 | 0.015764085 | 96.89 | 96.9 | |
| 5.6808 | 101.55 | 0.015752439 | 96.89 | |||
| 42 | 5.7725 | 101.55 | 0.016006716 | 96.84 | 96.8 | |
| 5.7651 | 101.55 | 0.015986196 | 96.85 | |||
| 43 | 6.5324 | 101.55 | 0.018113862 | 96.43 | 96.4 | |
| 6.54 | 101.55 | 0.018134937 | 96.42 | |||
| B042001250 | 25 | 7.5575 | 101.36 | 0.020956389 | 95.86 | 95.9 |
| 7.5572 | 101.36 | 0.020955557 | 95.86 | |||
| 37 | 7.3853 | 101.36 | 0.020478891 | 95.95 | 96.0 | |
| 7.371 | 101.36 | 0.020439238 | 95.96 | |||
| 41 | 7.3002 | 101.36 | 0.020242915 | 96.00 | 96.0 | |
| 7.2888 | 101.36 | 0.020211304 | 96.01 | |||
| 42 | 6.3328 | 101.36 | 0.017560386 | 96.53 | 96.5 | |
| 6.3331 | 101.36 | 0.017561218 | 96.53 | |||
| 43 | 8.2648 | 101.36 | 0.02291768 | 95.47 | 95.5 | |
| 8.2573 | 101.36 | 0.022896883 | 95.48 | |||
| B042001251 | 25 | 7.2225 | 101.54 | 0.020027459 | 96.05 | 96.1 |
| 7.2217 | 101.54 | 0.02002524 | 96.05 | |||
| 37 | 6.9538 | 101.54 | 0.019282374 | 96.20 | 96.2 | |
| 6.9525 | 101.54 | 0.019278769 | 96.20 | |||
| 41 | 6.5789 | 101.54 | 0.018242804 | 96.40 | 96.4 | |
| 6.5866 | 101.54 | 0.018264155 | 96.40 | |||
| 42 | 6.2865 | 101.54 | 0.017432 | 96.56 | 96.6 | |
| 6.2864 | 101.54 | 0.017431723 | 96.56 | |||
| 43 | 7.5801 | 101.54 | 0.021019057 | 95.85 | 95.9 | |
| 7.5687 | 101.54 | 0.020987446 | 95.86 |
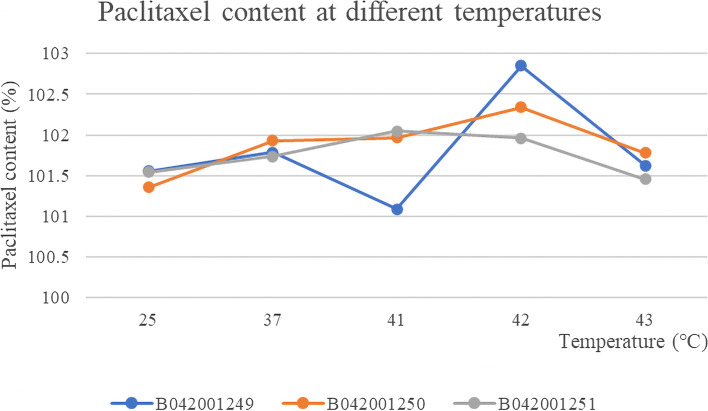
Determination of paclitaxel content
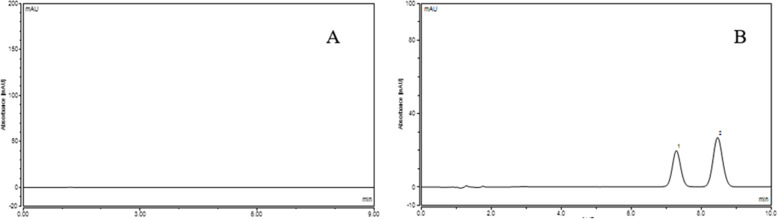
The results (peak area and content) of three batches of albumin-bound paclitaxel in water bath at 25 °C, 37 °C, 41 °C, 42 °C, 43 °C were shown in Table 7, 8, 9, 10 and 11 and the Summary of content results of three batches of albumin-bound paclitaxel in water bath at various temperature conditions was shown in Table 12.The RSDs of paclitaxel content at different temperatures of the three batches of samples were 0.64, 0.35 and 0.26%, respectively. As can be seen from the Fig. 13, the paclitaxel content remained basically the same at different temperatures, indicating that paclitaxel is stable to temperature. The chromatogram of control solution (A): RT (retention time) was 6.588 minutes and test solution (B): RT (retention time) was 6.608 minutes (Fig. 14). Solvent chromatogram (A) and the chromatogram of system suitability solution(B): 1-Cephalomannine (1), 2-Paclitaxel (2) were shown in Fig. 15.
Table 7.
The results (peak area and content) of three batches of albumin-bound paclitaxel in water bath at 25 °C
| Temperature | Batch number | Sample number | Peak area | Content (%) | Mean value (%) |
|---|---|---|---|---|---|
| 25 °C | B042001249 | 1 | 7.3661 | 102.12 | 101.554 |
| 2 | 7.3004 | 101.21 | |||
| 3 | 7.3212 | 101.5 | |||
| 4 | 7.3094 | 101.34 | |||
| 5 | 7.3016 | 101.23 | |||
| 6 | 7.3864 | 102.4 | |||
| 7 | 7.3242 | 101.54 | |||
| 8 | 7.3404 | 101.77 | |||
| 9 | 7.2974 | 101.17 | |||
| 10 | 7.304 | 101.26 | |||
| B042001250 | 1 | 7.2889 | 101.05 | 101.36 | |
| 2 | 7.1917 | 99.71 | |||
| 3 | 7.2653 | 100.73 | |||
| 4 | 7.3685 | 102.16 | |||
| 5 | 7.4095 | 102.72 | |||
| 6 | 7.2797 | 100.93 | |||
| 7 | 7.1981 | 99.79 | |||
| 8 | 7.2755 | 100.87 | |||
| 9 | 7.398 | 102.57 | |||
| 10 | 7.4347 | 103.07 | |||
| B042001251 | 1 | 7.3198 | 101.48 | 101.542 | |
| 2 | 7.3458 | 101.84 | |||
| 3 | 7.2917 | 101.09 | |||
| 4 | 7.3231 | 101.53 | |||
| 5 | 7.3709 | 102.19 | |||
| 6 | 7.3014 | 101.23 | |||
| 7 | 7.3338 | 101.68 | |||
| 8 | 7.2636 | 100.7 | |||
| 9 | 7.3193 | 101.47 | |||
| 10 | 7.3721 | 102.21 |
Table 8.
The results (peak area and content) of three batches of albumin-bound paclitaxel in water bath at 37 °C
| Temperature | Batch number | Sample number | Peak area | Content (%) | Mean value (%) |
|---|---|---|---|---|---|
| 37 °C | B042001249 | 1 | 7.3958 | 102.54 | 101.786 |
| 2 | 7.3504 | 101.91 | |||
| 3 | 7.3685 | 102.16 | |||
| 4 | 7.2965 | 101.16 | |||
| 5 | 7.2936 | 101.12 | |||
| 6 | 7.4178 | 102.84 | |||
| 7 | 7.3465 | 101.85 | |||
| 8 | 7.3573 | 102 | |||
| 9 | 7.3167 | 101.44 | |||
| 10 | 7.2736 | 100.84 | |||
| B042001250 | 1 | 7.3061 | 101.29 | 101.927 | |
| 2 | 7.3012 | 101.22 | |||
| 3 | 7.3789 | 102.3 | |||
| 4 | 7.3939 | 102.51 | |||
| 5 | 7.3975 | 102.56 | |||
| 6 | 7.2903 | 101.07 | |||
| 7 | 7.3314 | 101.64 | |||
| 8 | 7.3808 | 102.33 | |||
| 9 | 7.3581 | 102.01 | |||
| 10 | 7.3818 | 102.34 | |||
| B042001251 | 1 | 7.2706 | 100.8 | 101.735 | |
| 2 | 7.3919 | 102.48 | |||
| 3 | 7.3353 | 101.7 | |||
| 4 | 7.3306 | 101.63 | |||
| 5 | 7.3376 | 101.73 | |||
| 6 | 7.2938 | 101.12 | |||
| 7 | 7.4079 | 102.7 | |||
| 8 | 7.3152 | 101.42 | |||
| 9 | 7.3633 | 102.08 | |||
| 10 | 7.3352 | 101.69 |
Table 9.
The results (peak area and content) of three batches of albumin-bound paclitaxel in water bath at 41 °C
| Temperature | Batch number | Sample number | Peak area | Content (%) | Mean value (%) |
|---|---|---|---|---|---|
| 41 °C | B042001249 | 1 | 7.3442 | 101.82 | 101.089 |
| 2 | 7.2764 | 100.88 | |||
| 3 | 7.2547 | 100.58 | |||
| 4 | 7.2691 | 100.78 | |||
| 5 | 7.272 | 100.82 | |||
| 6 | 7.3491 | 101.89 | |||
| 7 | 7.3631 | 102.08 | |||
| 8 | 7.2501 | 100.51 | |||
| 9 | 7.2732 | 100.84 | |||
| 10 | 7.2625 | 100.69 | |||
| B042001250 | 1 | 7.3008 | 101.22 | 101.967 | |
| 2 | 7.3877 | 102.42 | |||
| 3 | 7.3871 | 102.41 | |||
| 4 | 7.34 | 101.76 | |||
| 5 | 7.3447 | 101.83 | |||
| 6 | 7.3331 | 101.67 | |||
| 7 | 7.3324 | 101.66 | |||
| 8 | 7.3788 | 102.3 | |||
| 9 | 7.3763 | 102.26 | |||
| 10 | 7.3671 | 102.14 | |||
| B042001251 | 1 | 7.3122 | 101.38 | 102.049 | |
| 2 | 7.3507 | 101.91 | |||
| 3 | 7.3686 | 102.16 | |||
| 4 | 7.3974 | 102.56 | |||
| 5 | 7.3766 | 102.27 | |||
| 6 | 7.2984 | 101.18 | |||
| 7 | 7.3435 | 101.81 | |||
| 8 | 7.358 | 102.01 | |||
| 9 | 7.4124 | 102.77 | |||
| 10 | 7.3886 | 102.44 |
Table 10.
The results (peak area and content) of three batches of albumin-bound paclitaxel in water bath at 42 °C
| Temperature | Batch number | Sample number | Peak area | Content (%) | Mean value (%) |
|---|---|---|---|---|---|
| 42 °C | B042001249 | 1 | 7.4453 | 103.22 | 102.853 |
| 2 | 7.3779 | 102.29 | |||
| 3 | 7.3931 | 102.5 | |||
| 4 | 7.4412 | 103.16 | |||
| 5 | 7.4651 | 103.5 | |||
| 6 | 7.4636 | 103.48 | |||
| 7 | 7.3539 | 101.95 | |||
| 8 | 7.3773 | 102.28 | |||
| 9 | 7.3978 | 102.56 | |||
| 10 | 7.4719 | 103.59 | |||
| B042001250 | 1 | 7.3539 | 101.95 | 102.338 | |
| 2 | 7.3376 | 101.73 | |||
| 3 | 7.385 | 102.39 | |||
| 4 | 7.4687 | 103.55 | |||
| 5 | 7.3915 | 102.48 | |||
| 6 | 7.3423 | 101.79 | |||
| 7 | 7.3271 | 101.58 | |||
| 8 | 7.3617 | 102.06 | |||
| 9 | 7.481 | 103.72 | |||
| 10 | 7.3666 | 102.13 | |||
| B042001251 | 1 | 7.2911 | 101.08 | 101.96 | |
| 2 | 7.3901 | 102.46 | |||
| 3 | 7.3333 | 101.67 | |||
| 4 | 7.3812 | 102.33 | |||
| 5 | 7.3567 | 101.99 | |||
| 6 | 7.3112 | 101.36 | |||
| 7 | 7.3781 | 102.29 | |||
| 8 | 7.3311 | 101.64 | |||
| 9 | 7.3826 | 102.35 | |||
| 10 | 7.388 | 102.43 |
Table 11.
The results (peak area and content) of three batches of albumin-bound paclitaxel in water bath at 43 °C
| Temperature | Batch number | Sample number | Peak area | Content (%) | Mean value (%) |
|---|---|---|---|---|---|
| 43 °C | B042001249 | 1 | 7.3777 | 102.28 | 101.627 |
| 2 | 7.3343 | 101.68 | |||
| 3 | 7.3121 | 101.37 | |||
| 4 | 7.3222 | 101.51 | |||
| 5 | 7.3126 | 101.38 | |||
| 6 | 7.3758 | 102.26 | |||
| 7 | 7.3535 | 101.95 | |||
| 8 | 7.3131 | 101.39 | |||
| 9 | 7.3212 | 101.5 | |||
| 10 | 7.2816 | 100.95 | |||
| B042001250 | 1 | 7.2478 | 100.48 | 101.779 | |
| 2 | 7.31 | 101.35 | |||
| 3 | 7.3119 | 101.37 | |||
| 4 | 7.4031 | 102.64 | |||
| 5 | 7.4141 | 102.79 | |||
| 6 | 7.2651 | 100.72 | |||
| 7 | 7.3212 | 101.5 | |||
| 8 | 7.3271 | 101.58 | |||
| 9 | 7.392 | 102.48 | |||
| 10 | 7.4208 | 102.88 | |||
| B042001251 | 1 | 7.3407 | 101.77 | 101.457 | |
| 2 | 7.3106 | 101.35 | |||
| 3 | 7.3262 | 101.57 | |||
| 4 | 7.3377 | 101.73 | |||
| 5 | 7.3047 | 101.27 | |||
| 6 | 7.3478 | 101.87 | |||
| 7 | 7.2745 | 100.85 | |||
| 8 | 7.3221 | 101.51 | |||
| 9 | 7.3416 | 101.78 | |||
| 10 | 7.2754 | 100.87 |
Table 12.
The Summary of content results of three batches of albumin-bound paclitaxel in water bath at 25 °C, 37 °C, 41 °C, 42 °C, 43 °C
| Batch number | B042001249 | B042001250 | B042001251 |
|---|---|---|---|
| Temperature | Content (%) | ||
| 25 °C | 101.55 | 101.36 | 101.54 |
| 37 °C | 101.79 | 101.93 | 101.74 |
| 41 °C | 101.09 | 101.97 | 102.05 |
| 42 °C | 102.85 | 101.96 | 101.63 |
| 43 °C | 101.63 | 101.78 | 101.46 |
Fig. 13.
The content results of paclitaxel of three batches of albumin-bound paclitaxel at different temperatures
Fig. 14.
Chromatograms of control solution (A) and test solution (B)
Fig. 15.
Solvent chromatogram (A) and the chromatogram of system suitability solution (B): 1-Cephalomannine (1), 2-Paclitaxel (2)
Discussion
Gastric cancer is a major disease in modern time and now it is the fifth most common cancer [39]. In the past few decades, surgical resection has played a crucial role in the treatment of GC [40]. Currently, there are relatively few studies on HIPEC in gastric cancer patients, but the number of related studies is gradually increasing.
Considering that there is a lack of basic research on albumin-bound paclitaxel in intraperitoneal hyperthermia chemotherapy and the therapy requires a temperature of 43 °C, this experiment was designed to investigate the stability of nab-paclitaxel at this temperature and at 25 °C, 37 °C, 41 °C, 42 °C and 43 °C. In the process of hyperthermic intraperitoneal chemotherapy, adequate physicochemical stability is critical to ensure safety and efficacy on clinically relevant conditions. Therefore, we mainly focus on the content of human albumin, polymer content, related substance content, in-vitro release rate, paclitaxel binding rate, paclitaxel content, changes in substance content to determine whether there were significant differences in the stability of the drugs at different temperatures. The results that we described show that albumin-bound paclitaxel is relatively stable to different temperatures.
Compared with intravenous injection, hyperthermic intraperitoneal chemotherapy increases the antitumor effect of hyperthermia and the synergistic effect of chemotherapy drugs on the basis of intraperitoneal infusion chemotherapy. At the same time, hyperthermia can also increase the penetration of the drug in the tissue, and the systemic adverse reactions are small, which can improve the quality of life of patients with abdominal metastasis. However, there are few studies on the thermal stability of antitumor drugs under the temperature conditions required for intraperitoneal thermal perfusion.
From the above discussion, although we have proved that nab-paclitaxel is stable at different temperatures when dissolved in saline, this experiment lacks the investigation of the effect of concentration on the stability of nab-paclitaxel at different temperatures and the maximum time to maintain stability. Moreover, The entire experiment was conducted in vitro, ignoring the situation of the stability of nab-paclitaxel in vivo and its metabolism, so it’s necessary to conduct animal experiments under the premise of further refinement of the experimental procedure to lay a good foundation for future clinical application.
Conclusion
Three batches of albumin-bound paclitaxel were dissolved in saline at different temperatures (25 °C, 37 °C, 41 °C, 42 °C and 43 °C) to examine that the changes of human blood albumin content, polymer content, in-vitro release rate, paclitaxel binding rate and paclitaxel content were stable to the several temperatures. With the change in temperature, Taxane (0.1%) and other impurities in the determination of related substance content fluctuated comparatively widely. Aside from Taxane (0.1%), only 7-Epitaxol (1%) was detected. Overall, the drug is relatively stable to temperature.
Acknowledgements
The project was financially supported by Natural Science Foundation of Hebei Province (H2022206533). Thanks to the Department of Pharmacy, The Fourth Hospital of Hebei Medical University for the support of instrument.
Authors’ contributions
Jingjing Zhang and Luya Li: research idea, study design, manuscript editing and performed the experimental studies. Jintuo Yin and Xidong Zhang: performed the experimental studies. Ying Zheng and Rui Feng: carried out the study concepts. The author(s) read and approved the final manuscript.
Funding
This research is funded by Natural Science Foundation of Hebei Province, China (H2022206533).
Availability of data and materials
Data analyzed and used for this manuscript are available within the manuscript.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jingjing Zhang and Luya Li are co-first author and contributed equally to this work.
Contributor Information
Ying Zheng, Email: zheng-ying-521@163.com.
Rui Feng, Email: fengrui-125@163.com.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Li L, Chen Y, Shen Z, Zhang X, Sang J, Ding Y, Yang X, Li J, Chen M, Jin C, Chen C, Yu C. Convolutional neural network for the diagnosis of early gastric cancer based on magnifying narrow band imaging. Gastric Cancer. 2020;23(1):126–132. doi: 10.1007/s10120-019-00992-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee JH, Park B, Joo J, Kook MC, Kim YI, Lee JY, Kim CG, Choi IJ, Eom BW, Yoon HM, Ryu KW, Kim YW, Cho SJ. Body mass index and mortality in patients with gastric cancer: a large cohort study. Gastric Cancer. 2018;21(6):913–924. doi: 10.1007/s10120-018-0818-x. [DOI] [PubMed] [Google Scholar]
- 4.Arai H, Iwasa S, Boku N, Kawahira M, Yasui H, Masuishi T, Muro K, Minashi K, Hironaka S, Fukuda N, Takahari D, Nakajima TE. Fluoropyrimidine with or without platinum as first-line chemotherapy in patients with advanced gastric cancer and severe peritoneal metastasis: a multicenter retrospective study. BMC Cancer. 2019;19(1):652. doi: 10.1186/s12885-019-5720-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hao Y, Liu Y, Ishibashi H, Wakama S, Nishino E, Yonemura Y. Downstaging of lymph node metastasis after neoadjuvant intraperitoneal and systemic chemotherapy in gastric carcinoma with peritoneal metastasis. Eur J Surg Oncol. 2019;45(8):1493–1497. doi: 10.1016/j.ejso.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 6.Badgwell B, Ikoma N, Murphy MB, Wang X, Estrella J, Roy-Chowdhuri S, Das P, Minsky BD, Lano E, Song S, Mansfield P, Ajani J. A phase II trial of Cytoreduction, gastrectomy, and Hyperthermic intraperitoneal perfusion with chemotherapy for patients with gastric Cancer and Carcinomatosis or positive cytology. Ann Surg Oncol. 2021;28(1):258–264. doi: 10.1245/s10434-020-08739-5. [DOI] [PubMed] [Google Scholar]
- 7.Lu Y, Jin Z, Zheng S, Bai Y, Sun Y. Hyperthermic intraperitoneal chemotherapy combined with systemic chemotherapy for gastric cancer peritoneal carcinomatosis: a protocol for systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 2020;99(27):e20973. doi: 10.1097/MD.0000000000020973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blumenthaler AN, Allen CJ, Ikoma N, Blum M, Das P, Minsky BD, Mansfield PF, Ajani JA, Badgwell BD. Laparoscopic HIPEC for low-volume peritoneal metastasis in gastric and gastroesophageal adenocarcinoma. Ann Surg Oncol. 2020;27(13):5047–5056. doi: 10.1245/s10434-020-08968-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kitai T. The role of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the treatment of peritoneal carcinomatosis: a systematic review including evidence from Japan. Surg Today. 2021;51(7):1085–1098. doi: 10.1007/s00595-020-02180-7. [DOI] [PubMed] [Google Scholar]
- 10.Asmar AE, Bendavides M, Moreau M, Hendlisz A, Deleporte A, Khalife M, Donckier V, Liberale G. Postoperative C-reactive protein kinetics predict postoperative complications in patients treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis. World J Surg Oncol. 2020;18(1):311. doi: 10.1186/s12957-020-02081-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leebmann H, Piso P. Hypertherme intraperitoneale Chemotherapie [Hyperthermic intraperitoneal chemotherapy] Chirurg. 2019;90(7):593–604. doi: 10.1007/s00104-019-0982-5. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka M, Hattori Y, Ishii T, Tohnai R, Itoh S, Kawa Y, Kono Y, Urata Y, Satouchi M. The efficacy of carboplatin plus nanoparticle albumin-bound paclitaxel after cisplatin plus pemetrexed in non-squamous non-small-cell lung cancer patients. Respir Investig. 2020;58(4):269–274. doi: 10.1016/j.resinv.2019.12.008. [DOI] [PubMed] [Google Scholar]
- 13.Hassan MS, Awasthi N, Li J, Williams F, Schwarz MA, Schwarz RE, von Holzen U. Superior therapeutic efficacy of nanoparticle albumin bound paclitaxel over Cremophor-bound paclitaxel in experimental esophageal adenocarcinoma. Transl Oncol. 2018;11(2):426–435. doi: 10.1016/j.tranon.2018.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ota D, Akatsuka S, Nishi T, Kato T, Takeuchi M, Tsuji M, Fukuuchi A. Phase I study of combination therapy with weekly nanoparticle albumin-bound paclitaxel and cyclophosphamide in metastatic breast Cancer patients. Anticancer Res. 2019;39(12):6903–6907. doi: 10.21873/anticanres.13910. [DOI] [PubMed] [Google Scholar]
- 15.Zong Y, Wu J, Shen K. Nanoparticle albumin-bound paclitaxel as neoadjuvant chemotherapy of breast cancer: a systematic review and meta-analysis. Oncotarget. 2017;8(10):17360–17372. doi: 10.18632/oncotarget.14477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sato S, Kunisaki C, Tanaka Y, Sato K, Miyamoto H, Yukawa N, Fujii Y, Kimura J, Takagawa R, Takahashi M, Kosaka T, Akiyama H, Saigusa Y, Taguri M, Yamanaka T, Endo I. A phase II study of tri-weekly low-dose nab-paclitaxel chemotherapy for patients with advanced gastric Cancer. Anticancer Res. 2018;38(12):6911–6917. doi: 10.21873/anticanres.13068. [DOI] [PubMed] [Google Scholar]
- 17.Kashiwada T, Saito Y, Terasaki Y, Hisakane K, Takeuchi S, Sugano T, Miyanaga A, Noro R, Minegishi Y, Seike M, Kubota K, Gemma A. Interstitial lung disease associated with nanoparticle albumin-bound paclitaxel treatment in patients with lung cancer. Jpn J Clin Oncol. 2019;49(2):165–173. doi: 10.1093/jjco/hyy180. [DOI] [PubMed] [Google Scholar]
- 18.Xie F, Chen R, Zhang L, Yin Z, Zhu Q, You S, Jiang C, Li Y, Li S, Zha X, Wang J. Efficacy of two-weekly nanoparticle albumin-bound paclitaxel as neoadjuvant chemotherapy for breast cancer. Nanomedicine (Lond) 2019;14(12):1595–1603. doi: 10.2217/nnm-2018-0485. [DOI] [PubMed] [Google Scholar]
- 19.Lee H, Park S, Kang JE, Lee HM, Kim SA, Rhie SJ. Efficacy and safety of nanoparticle-albumin-bound paclitaxel compared with solvent-based taxanes for metastatic breast cancer: a meta-analysis. Sci Rep. 2020;10(1):530. doi: 10.1038/s41598-019-57380-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsurutani J, Hara F, Kitada M, Takahashi M, Kikawa Y, Kato H, Sakata E, Naito Y, Hasegawa Y, Saito T, Iwasa T, Taira N, Takashima T, Kashiwabara K, Aihara T, Mukai H. Randomized phase II study to determine the optimal dose of 3-week cycle nab-paclitaxel in patients with metastatic breast cancer. Breast. 2021;55:63–68. doi: 10.1016/j.breast.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan H, Hu J, Liu S. Efficacy and safety of nanoparticle albumin-bound paclitaxel in non-small cell lung cancer: a systematic review and meta-analysis. Artif Cells Nanomed Biotechnol. 2019;47(1):268–277. doi: 10.1080/21691401.2018.1552595. [DOI] [PubMed] [Google Scholar]
- 22.Kato Y, Okuma Y, Watanabe K, Yomota M, Kawai S, Hosomi Y, Okamura T. A single-arm phase II trial of weekly nanoparticle albumin-bound paclitaxel (nab-paclitaxel) monotherapy after standard of chemotherapy for previously treated advanced non-small cell lung cancer. Cancer Chemother Pharmacol. 2019;84(2):351–358. doi: 10.1007/s00280-019-03843-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang S, Liang Q, Chi Y, Zhuo M, An T, Duan J, Wang Z, Wang Y, Zhong J, Yang X, Chen H, Wang J, Zhao J. Retrospective analysis of the effectiveness and tolerability of nab-paclitaxel in Chinese elderly patients with advanced non-small-cell lung carcinoma. Thorac Cancer. 2020;11(5):1149–1159. doi: 10.1111/1759-7714.13356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tian Z, Zhang F, Li P, Wang J, Yang J, Zhang P, Yao W, Wang X. Albumin-bound paclitaxel and gemcitabine combination therapy in soft tissue sarcoma. BMC Cancer. 2020;20(1):698. doi: 10.1186/s12885-020-07199-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoffman RM, Bouvet M. Nanoparticle albumin-bound-paclitaxel: a limited improvement under the current therapeutic paradigm of pancreatic cancer. Expert Opin Pharmacother. 2015;16(7):943–947. doi: 10.1517/14656566.2015.1016912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Luca R, Blasi L, Alù M, Gristina V, Cicero G. Clinical efficacy of nab-paclitaxel in patients with metastatic pancreatic cancer. Drug Des Devel Ther. 2018;12:1769–1775. doi: 10.2147/DDDT.S165851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koizumi W, Morita S, Sakata Y. A randomized phase III trial of weekly or 3-weekly doses of nab-paclitaxel versus weekly doses of Cremophor-based paclitaxel in patients with previously treated advanced gastric cancer (ABSOLUTE trial) Jpn J Clin Oncol. 2015;45(3):303–306. doi: 10.1093/jjco/hyu205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okunaka M, Kotani D, Demachi K, Kawazoe A, Yoshino T, Kawasaki T, Shitara K. Retrospective cohort study of nanoparticle albumin-bound paclitaxel plus ramucirumab versus paclitaxel plus ramucirumab as second-line treatment in patients with advanced gastric cancer. BMC Cancer. 2020;20(1):1111. doi: 10.1186/s12885-020-07614-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sasaki Y, Nishina T, Yasui H, Goto M, Muro K, Tsuji A, Koizumi W, Toh Y, Hara T, Miyata Y. Phase II trial of nanoparticle albumin-bound paclitaxel as second-line chemotherapy for unresectable or recurrent gastric cancer. Cancer Sci. 2014;105(7):812–817. doi: 10.1111/cas.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He MM, Wang F, Jin Y, Yuan SQ, Ren C, Luo HY, Wang ZQ, Qiu MZ, Wang ZX, Zeng ZL, Li YH, Wang FH, Zhang DS, Xu RH. Phase II clinical trial of S-1 plus nanoparticle albumin-bound paclitaxel in untreated patients with metastatic gastric cancer. Cancer Sci. 2018;109(11):3575–3582. doi: 10.1111/cas.13813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roviello G, Conter FU, Mini E, Generali D, Traversini M, Lavacchi D, Nobili S, Sobhani N. Nanoparticle albumin-bound paclitaxel: a big nano for the treatment of gastric cancer. Cancer Chemother Pharmacol. 2019;84(4):669–677. doi: 10.1007/s00280-019-03887-2. [DOI] [PubMed] [Google Scholar]
- 32.Zhang C, Awasthi N, Schwarz MA, Hinz S, Schwarz RE. Superior antitumor activity of nanoparticle albumin-bound paclitaxel in experimental gastric cancer. PLoS One. 2013;8(2):e58037. doi: 10.1371/journal.pone.0058037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiong J, Han S, Ding S, He J, Zhang H. Antibody-nanoparticle conjugate constructed with trastuzumab and nanoparticle albumin-bound paclitaxel for targeted therapy of human epidermal growth factor receptor 2-positive gastric cancer. Oncol Rep. 2018;39(3):1396–1404. doi: 10.3892/or.2018.6201. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura M, Ojima T, Katsuda M, Hayata K, Kitadani J, Nakamori M, Yamaue H. Phase 1 study of combined chemotherapy of nab-paclitaxel, S-1, and Oxaliplatin for gastric Cancer with peritoneal metastasis (NSOX study) Oncology. 2021;99(1):57–61. doi: 10.1159/000509396. [DOI] [PubMed] [Google Scholar]
- 35.Sawasaki M, Tsubamoto H, Nakamoto Y, Kakuno A, Sonoda T. S-1, Oxaliplatin, nab-paclitaxel and Itraconazole for conversion surgery for advanced or recurrent gastric Cancer. Anticancer Res. 2020;40(2):991–997. doi: 10.21873/anticanres.14033. [DOI] [PubMed] [Google Scholar]
- 36.Lin JL, Lin JX, Lin JP, Zheng CH, Li P, Xie JW, Wang JB, Lu J, Chen QY, Huang CM. Safety and efficacy of Camrelizumab in combination with nab-paclitaxel plus S-1 for the treatment of gastric Cancer with serosal invasion. Front Immunol. 2022;12:783243. doi: 10.3389/fimmu.2021.783243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Markman M, Rowinsky E, Hakes T, Reichman B, Jones W, Lewis JL, Jr, Rubin S, Curtin J, Barakat R, Phillips M, et al. Phase I trial of intraperitoneal taxol: a Gynecoloic oncology group study. J Clin Oncol. 1992;10(9):1485–1491. doi: 10.1200/JCO.1992.10.9.1485. [DOI] [PubMed] [Google Scholar]
- 38.Manzanedo I, Pereira F, Serrano Á, Pérez-Viejo E, Martínez-Torres B, Carrión L, Calzas J. The use of cisplatin plus doxorubicin or paclitaxel in hyperthermic intraperitoneal chemotherapy (HIPEC) for stage IIIC or IV epithelial ovarian cancer: a comparative study. Clin Transl Oncol. 2019;21(10):1357–1363. doi: 10.1007/s12094-019-02065-3. [DOI] [PubMed] [Google Scholar]
- 39.Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396(10251):635–648. doi: 10.1016/S0140-6736(20)31288-5. [DOI] [PubMed] [Google Scholar]
- 40.Machlowska J, Baj J, Sitarz M, Maciejewski R, Sitarz R. Gastric Cancer: epidemiology, risk factors, classification, genomic characteristics and treatment strategies. Int J Mol Sci. 2020;21(11):4012. doi: 10.3390/ijms21114012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data analyzed and used for this manuscript are available within the manuscript.