Abstract
Objective:
We compare here the principal characteristics of over-the-counter moisturizers with physiologic lipid-based barrier repair therapy.
Data Sources:
An extended literature revealed that moisturizers are considered standard ancillary therapy for anti-inflammatory skin disorders, like atopic dermatitis (AD). Additional studies have shown that physiologic lipid-based barrier repair therapy can comprise effective, stand-alone therapy for pediatric AD.
Results:
Not all moisturizers are beneficial - some negatively impact skin function, and in doing so, they risk inducing or exacerbating inflammation in patients with AD. The frequent self-reported occurrences of ‘sensitive skin’ in atopics could reflect the potential toxicity of such formulations. A still unanswered question is whether improper formulations could also prove to be counterproductive in other types of sensitive skin, such as rosacea. In contrast, we show how physiologic lipid-based barrier repair therapy (BRT), if comprised of the three, key stratum corneum lipids in sufficient quantities and at an appropriate molar ratio, can correct the barrier abnormality thereby reducing inflammation in AD, and possibly in other inflammatory dermatoses, such as the ‘adult’ eczemas and possibly even psoriasis.
Conclusion:
We provide guidelines for the appropriate dispensation of moisturizers and physiologic lipid-based, barrier repair therapy for the treatment of AD. Both OTC (AtopalmÒ) and Rx (EpiCeramÒ) products are available in the USA with these characteristics.
Keywords: Antihistamines, atopic dermatitis, barrier function, barrier response, ceramides, cytokines, epidermal lipids, kallikreins, moisturizers, PPAR2, pH
Introduction
Atopic dermatitis (AD) can be considered a ‘waste basket’ of inherited, inflammatory skin disorders, characterized by localized-to-generalized cutaneous inflammation. With a prevalence of up to 20% in Western and Asian pediatric populations it is considered the most common of inflammatory skin diseases 1, 2. Due to chronic pruritus, with disturbed sleep patterns, it can severely impact quality of life 3, while also imposing a considerable economic burden 4. Because AD is often triggered and amplified by S. aureus colonization, secondary infections are common, and impose an additional therapeutic challenge. AD is often the harbinger of the so-called ‘atopic march’, in which the characteristic skin disease, appearing as early as 1–3 months of age, can progress to asthma and seasonal rhinitis, a pathogenic sequence that has been questioned by some authorities 5, 6.
Two opposing, though not necessarily mutually exclusive views of disease pathogenesis hold sway. According to the ‘inside-outside’ perspective, it is the TH2-dominant immunophenotype of AD that provokes an overlying permeability barrier abnormality, allowing antigens to enter the skin, and amplify disease expression 7–9. Yet, the discovery of underlying, inherited mutations 2, 10 and TH2-driven down-regulation in the expression of the structural protein, filaggrin 11, illuminate an alternate, ‘outside-to-inside’ view of disease pathogenesis 12–14.
To fully comprehend the latter paradigm, one must first embrace the concept that the principal role of the epidermis it to construct a permeability barrier that allows life in a desiccating, terrestrial environment 15, 16. Accordingly, the epidermis undertakes a strong commitment to maintaining barrier competence, involving the expenditure of substantial resources to compensate for any external stressors that threaten permeability barrier competence. Although the regulatory mechanisms that sustain barrier function have not been fully characterized, they include transcriptional control of both epidermal structural protein and lipid production/secretion via peroxisomal proliferator activating receptors (PPARs) 17; Nrf-2 activators (resveratrol) 18; as well as unstable, soluble mediators, such as nitric oxide 19.
When the barrier is abrogated; e.g., with solvents, mechanical forces, or sequential tape stripping, multiple repair sequences respond with alacrity, including a cytokine cascade 20, and pH-initiated, serine protease (Kallikrein, KLK) activation 21, each regulating different components of the repair response. In considering different strategies to restore and optimize barrier competence, a key goal of topical therapy should be to support such compensatory responses. In the paragraphs that follow, we will describe different categories of topical therapy, and compare their impact on AD skin.
Structural Basis for the Permeability Barrier
Though much attention in recent years has focused on filaggrin, an AD phenotype can occur in diverse inherited mutations in structural and enzymatic proteins that interfere with either the loading, delivery, or post-secretory processing of the lipid and enzymatic contents of lamellar bodies within the extracellular spaces of the stratum corneum (SC) 14. These secreted lipids form stacks of lamellar bilayers that fill the extracellular spaces, accounting for ≈10% of the mass of the SC in normal skin. In AD, the failure to deliver a full complement of lipids to the SC interstices results in reduced amounts of extracellular lipids, producing a ‘leaky’ extracellular matrix that permits excessive loss of body water, quantitated as rates of transepidermal water loss (TEWL) 22. An immediate consequence of a flawed delivery mechanism is a decline in the total lipid content needed to generate a fully competent permeability barrier. Yet, because the permeability and antimicrobial barriers are both closely linked and interdependent 23, the permeability barrier defect results in parallel flaws in antimicrobial defense that facilitate penetration of microbial pathogens and allergens into the skin.
Problems with the barrier are not confined to patients with AD. Human neonates fail to develop fully competent permeability barriers until approximately six months of post-natal life, a problem that is amplified in premature infants 24, 25. Now, consider the other end of the human timeline - aging skin, which emerges initially as a reversible pH-induced barrier abnormality around the age of fifty 26, which is further amplified by defects in lipid synthesis still later in life 27, 28.
Then, there is another wastebasket of diagnoses, which include the afore-mentioned seasonally aggravated, adult eczemas, as well as seborrheic and stasis dermatitis. The next category includes rosacea, often mis-termed acne rosacea, while the final category is more nebulous – the multitudes who suffer from ‘sensitive skin’, which we suggest is often initiated or provoked by improperly formulated moisturizers. From a purely commercial perspective, these products can promote a perverse vicious circle that demands evermore frequent product applications. We turn next to the stated goal of this narrative, which is to provide updated guidelines for appropriate deployment of topical skin care
Moisturizer Ingredients and Their Mechanisms of Action
Classic moisturizing products, like Aquaphor and Eucerin, are water-in-oil formulations, enriched in occlusive ingredients, such as petrolatum or lanolin 29. Their hydrophobic nature allows them to coat the surface of the skin with a water-repellent layer that impedes the bidirectional movement of water across the skin. By blocking the movement of water out of the skin, these agents effectively, though temporarily, trap water within the stratum corneum, ameliorating the xerosis that is characteristic of AD and other, age- and seasonally associated eczematous disorders. Moreover, by improving the hydration of the stratum corneum (SC), they can dampen inflammation 30. Yet, it is important to note that these occlusive moisturizers are inert ingredients that do not address the underlying biochemical abnormalities in AD.
Many moisturizers also contain one or more humectants, such as glycerin, which imbibe water from the surrounding atmosphere. Yet, because AD typically flares during winter months, when indoor humidities typically decline drastically due to forced-air and radiant heating, humectants are often paired with an occlusive agent, such as petrolatum, to protect against further drying of the skin, which could otherwise exacerbate AD symptoms.
Many moisturizers also incorporate emollient vegetable oils, such as coconut, jojoba or avocado oils. While these agents can impart an elegant texture to such formulations, they provide no scientifically proven benefits, with one key exception - certain vegetable oils, such as sunflower, safflower, borage or corn oil, sea buckthorn oil, which are enriched in the essential fatty acid, linoleic acid, and/or gamma-linolenic acid. Components of these oils can: i) improve barrier function 31, 32; ii) enhance barrier function and reduce inflammation via activation of peroxisome proliferator-activated receptors (PPARs) 33; and/or iii) even provide nutritional benefits in mice and human neonates 32, 34. Yet, allergic sensitization can occur not only in patients treated with peanut oil, but also with sunflower seed oil 35. Finally, botanical ingredients are increasingly being added to moisturizers, and some of these can be beneficial. For example, chamomile contains anti-inflammatory substances, such as apigenin, which improve symptoms in AD animal models 36.
It should be noted that a few popular over-the-counter moisturizers also include a skin-identical or synthetic ceramide, or a ceramide mimetic (‘pseudoceramide’) 37. Although topical ceramides, when provided at sufficient concentrations, can improve both permeability barrier function and stratum corneum hydration 38, their concentration levels in most formulations is usually too low to impart measurable benefits. It seems likely that the term ‘ceramide’ often appears to be included in such preparations for marketing purposes. Most importantly, as described below, if the ceramide is provided without the addition of the other two key physiologic lipids at an appropriate ratio (i.e., with cholesterol and one or more free fatty acids), barrier function deteriorates rather than improves 39. Studies have shown that all three constituents must be provided together in an equimolar ratio to restore barrier function after disruption of normal skin 39.
Improperly Formulated Moisturizers Can Harm Individuals with Flawed Barriers
In a ‘sensitive skin’ animal model, we recently identified a serious flaw with most moisturizers that are currently on the market 40. While they may appear harmless when applied to normal skin that displays a robust barrier, many of these products could prove to be toxic when and if they are applied to the skin of individuals with self-reported ‘sensitive’ skin, likely also including subjects with a history of AD 40. Yet, these products rarely are tested in such ‘at-risk’ individuals - instead, such subjects typically are specifically excluded from any such investigations. Bottom line - while short-term relief may be obtained with these agents, if they further disrupt the skin barrier, they can initiate a vicious cycle that requires repeated applications of the same or alternate products.
Link between the Barrier Abnormality and Inflammatory Phenotype in AD
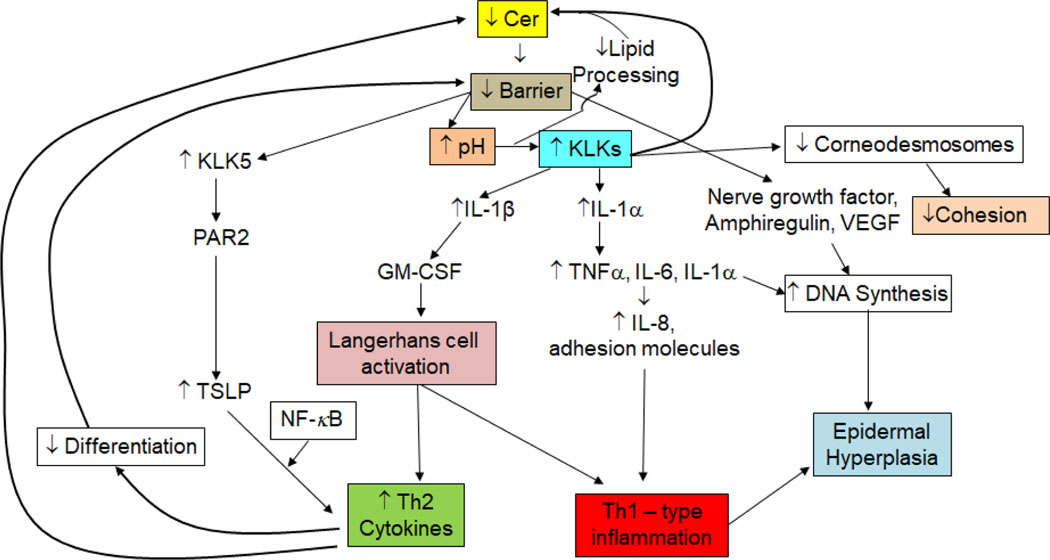
One inevitable consequence of the flawed barrier in AD links the barrier defect to a pro-inflammatory cytokine cascade, which in turn recruits the characteristic immunophenotype in AD, comprising the ‘outside-to-inside’ concept of disease pathogenesis in AD 41. In response to the sustained barrier defect in AD, a host of cytokines and growth factors is generated, in an inherently unsuccessful attempt to restore normal function in AD epidermis 42. Yet, due to the underlying biochemical abnormalities in AD, regardless of etiology, normal function cannot be restored (the authors compare the situation to being dealt a faulty set of cards!). Hence, the epidermis continues to send out these signals, designed to promote barrier repair, until a Th2-, Th-17, IL-33-dominant inflammatory milieu develops 9. It is this ‘outside-inside’ paradigm of AD pathogenesis, due to an underlying, inherited barrier abnormality that sustains this pro-inflammatory cytokine cascade 43 (Fig. 1).
Figure 1:
Barrier-initiated Cytokine Cascade Leads to Multiple Downstream Consequences in Atopic Dermatitis (modified from 14). Abbreviations: GM-CSF, granulocyte-macrophage colony- stimulating factor ; IL-1, interleukin-1; KLK, kallikrein; NF-κB, nuclear factor kappa B; TNFα, tumor necrosis factor alpha; TSLP, thymic stromal lymphopoietin
Pathogenic Role of an Elevated pH in AD
An inevitable consequence both of the flawed barrier and inflammation in AD is an elevation in the pH of the skin surface 44.The deleterious consequences of an elevated pH in AD include activation of yet another outside-to-inside cytokine cascade that begins with the activation of the serine protease (kallikrein, KLK), KLK5, followed by the generation of the pro-Th-2 cytokine, TSLP, which in turn recruits the Th2 and Th17 cells that secrete the ‘bad’ cytokines (i.e., IL-4, IL-5, IL-13, IL-17A, and IL-33) 45 (Fig. 1). Th2 cytokines further compromise the barrier by down-regulating the synthesis of: i) epidermal structural proteins 46; ii) tight junction proteins 47; iii) ceramides 48; iv) fatty acid elongases 49, and v) a key antimicrobial peptide; i.e., LL-37 50. Hence, the initial ‘outside-to-inside’ cytokine cascade in AD quickly morphs into an ‘outside-to-inside- back-to-outside’ vicious circle 51. Furthermore, KLKs exhibit a neutral-to-alkaline pH optimum, and their activation at the neutral pH of AD further compromises a set of other critical functions (Fig. 1). Finally, while the low pH of normal SC (i.e., 4.5 – 5) inhibits the growth of S. aureus and S. pyogenesis, the normal flora (e.g., S. epidermidis and Corynebacterium) instead thrive at a lower pH 44, 52. In contrast, the elevated pH of inflamed skin in AD further favors pathogen colonization and growth.
Barrier Restorative Therapy in AD
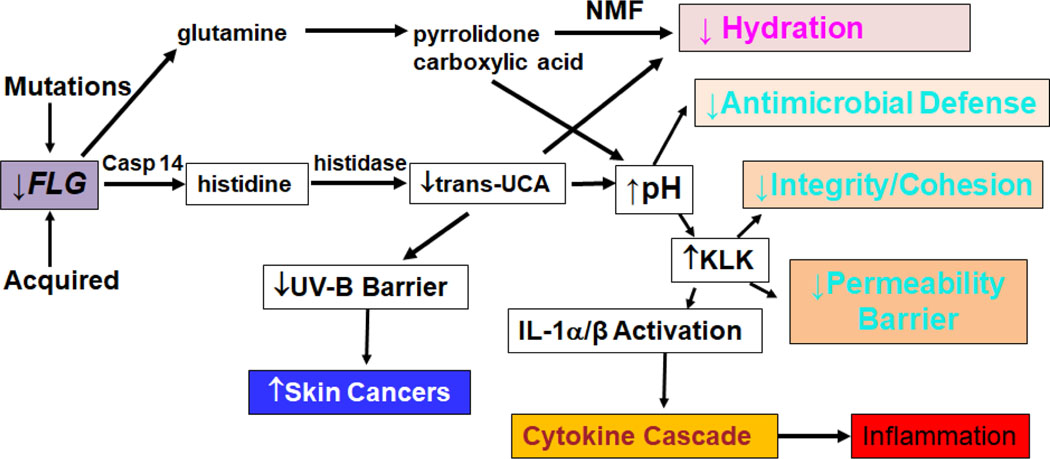
A substantial literature supports the deployment of moisturizers along with anti-inflammatory agents in AD 53. This approach seems prudent, since co-applications of moisturizers under nursing supervision have been shown to reduce reliance upon topical steroids in AD management 54, 55. Yet, our recent studies have shown that some commonly employed moisturizers can harm the skin, particularly when they are deployed in settings where the barrier already is compromised 55, as in the case in AD, but also in rosacea and in individuals with self-reporting ‘sensitive skin’. Here, we will compare the key differences between ubiquitous, over-the-counter moisturizers and preparations formulated specifically to correct the inherited abnormalities in AD. Although these mutations typically delete or reduce the expression of structural proteins, most notably filaggrin, their net effect is to compromise either the synthesis, loading, or secretion of lamellar body contents (Fig. 2) 14. The result of these aberrant mechanisms is both a global reduction in all three key barrier lipids, along with a further TH2-driven decline in ceramide content and fatty acid chain length (see above and 56). By hydrating the SC, moisturizers can alleviate the xerosis that is such a prominent feature of AD, but they have not yet been shown to provide stand-alone therapy for even mild cases of AD. Moreover, whether they really prevent the initial development of AD, as suggested in several recent studies 57, 58, is debatable, because another recent study failed to show any preventive benefits of moisturizer therapy alone 59.
Figure 2:
Multiple Downstream Consequences of Filaggrin Deficiency in Atopic Dermatitis (modified from 14) Abbreviations: Casp 14, Caspase 14; KLK, Kallikrien, NMF, natural moisturizing factor
Physiologic Lipid-Based Therapy of AD
Topically applied physiologic lipids, in contrast to moisturizers, do not form an occlusive layer on the SC surface. Instead, they are quickly absorbed into the underlying nucleated cell layers, where they incorporate into nascent lamellar bodies as they form in the trans-Golgi apparatus of stratum spinosum and granulosum cells 39. There, the absorbed lipids join with de novo synthesized lipids, immediately prior to their secretion into the extracellular spaces. But as shown in Figure 1, not only the synthesis, but also secretion of lipids is impaired in AD, resulting in a global reduction in the ‘big three’ physiologic lipids (ceramides, cholesterol. and free fatty acids). Therefore, in addressing first, the reduced lipid content of the SC in AD (i.e., from ≈10% to ≈5% of the weight of normal SC), these physiologic lipids ideally should be provided at a high, final concentration of at least 5%. Then, because of the further Th2-cytokine-induced reduction in ceramide content of the SC 48, the three lipids ideally should be provided as a ceramide-dominant mixture (i.e., at about a 3:1:1 molar ratio) 39, with a natural or synthetic ceramide as the dominant species.
In light of the deleterious pH abnormality in AD, this final formulation should ideally be adjusted to a pH of ≤5 in order to compensate for the elevated pH of inflamed skin in AD. As noted above, lowering the pH of the SC alone provides numerous potential benefits, including reductions in inflammation, while also enhancing the permeability barrier, stratum corneum cohesion, and antimicrobial defense. The free fatty acids in the formulation not only are critical for the barrier and as acidifying agents, but some also activate PPARα and PPARβ/δ, improving epidermal function and further reducing inflammation 33. In addition, fatty acid activators of PPARs can: i) prevent the emergence of steroid side effects 60; ii) override the negative effects of calcineurin inhibitors on barrier function 61; and iii) prevent rebound flares following withdrawal of topical steroids 51. Finally, several topical ingredients, including the triple lipids and even petrolatum, have been shown to enhance epidermal production of the key antimicrobial peptide, LL-37 62.
Alternative Approaches Can Boost Ceramide Production
Other approaches can also enhance ceramide synthesis by alternate mechanisms. For example, eucalyptus oil extracts, when added to cultured keratinocytes, increases ceramide synthesis, and have been forwarded as a potential cosmetic ingredient 63, though topical eucalyptus oil can cause allergic contact dermatitis. Residents of the cutaneous microbiome generate metabolites, notably a commensal bacteria metabolite, N-palmitoyl serinol (NPS) (NEUROMIDE® has been registered with the US FDA as DMF Type IV), stimulate epidermal ceramide synthesis by activating endocannabinoid receptors 64. Activation of one such receptor, CB-1, results in benefits for the barrier 65, 66. NPS (NEUROMIDE®) has demonstrated remarkable efficacy as a pro-barrier 66, anti-inflammatory 64, 67, as well as anti-aging ingredient 68 in both in vitro and in vivo models.
Efficacy of Triple Physiologic Lipid-Based, Barrier Repair Therapy in AD
Unlike moisturizers, topical ceramide-dominant, triple lipid products amplify lipid production and delivery to the SC intercellular spaces, replenishing the lamellar bilayers that are critical for normal barrier function and antimicrobial defense. Chamlin et al. 69 evaluated 24 pediatric patients with recalcitrant AD. While all of these patients continued to use standard therapy (including potent topical steroids and/or tacrolimus), the sole intervention was substitution of a ceramide-dominant, triple-lipid product for each patient’s prior moisturizer. Follow-up SCORAD scores showed a rapid improvement in clinical scores in 22/24 patients. Not only did clinical scores improve, but both epidermal barrier function and SC cohesion also improved. Ultrastructure of lipid-treated human epidermis revealed enhanced lamellar membrane production, a change that was absent from patients who had been previously treated with common moisturizers 69. More recently, a ceramide-dominant, triple-lipid prescription formulation (EpiCeram® emulsion) also improved skin barrier function in comparison to conventional moisturizers in AD patients 70. This ceramide-dominant product was then assessed in a multicenter, investigator-blinded, comparative study of 121 pediatric patients, 6 months to 12 years, with moderate-to-severe AD 71. Patients were randomized to either EpiCeram alone or a mid-potency, fluorinated steroid, fluticasone (Cutivate®) cream. By 28 days, patients treated with EpiCeram alone demonstrated reductions in SCORAD scores that were comparable to fluticasone. Moreover, EpiCeram treatment not only reduced disease severity, but also pruritus AD, while improving sleep quality with an efficacy comparable to fluticasone. Although not yet formally evaluated, I recommend applications of EpiCeram prior to steroid delivery, thereby providing a more hydrophobic environment for drug delivery. Together, this information provides guidelines for the utilization of a physiologic lipid-based, barrier repair approach as therapy in the treatment of AD.
How Barrier Repair Therapy Is Anti-Inflammatory in AD
Managing AD often requires the use of topical anti-inflammatory agents (topical corticosteroids, topical calcineurin inhibitors or PDE4 inhibitors), and in adults with recalcitrant, moderate-to-severe AD, systemic biologics (e.g., IL-4, IL13, IL17, or IL-33 inhibitors). But in clinical settings, management should not lose focus on the skin barrier. Clinicians are presented with many choices for managing the compromised barrier that is a central participant in AD pathogenesis. Though parsing through these choices can be difficult, many moisturizers appear to provide little or no benefit, and as noted above, some could even be harmful 40.
Animal studies suggest that moisturizers alone, by restoring SC hydration, reduce cytokine production, mast cell hypertrophy and degranulation, as well as epidermal hyperplasia 30, 72. To the extent that occlusive ingredients like petrolatum improve permeability barrier function, they too can dampen cytokine production. However, the anti-inflammatory activity of the triple physiologic lipid-based formulation can be attributed to several additional characteristics, which include: i) inactivation of kallikreins that compromise SC structural integrity at a low pH; ii) inhibition of pathogen colonization with reductions in attendant, superantigen-initiated inflammation; and iii) activation of two key lipid-processing enzymes, β-glucocerebrosidase and acidic sphingomyelinase, which generate ceramides required to form the extracellular lamellar bilayers 73. Finally, iv) as noted above, certain free fatty acids in these formulations can activate PPARs, which in turn can reduce inflammation by several parallel mechanisms 74.
Conclusion (Table 1)
Table 1:
Standard and More Recent Approaches to Fix Barrier Abnormalities in Atopic Dermatitis
| Long-Standing: |
|---|
|
|
| • Educate (soaps, hydration, ↓ϕstress) |
| • Hydrate (emollients → ↓steroid usage) |
| • ↓S. aureus carriage |
| • Interrupt Itch-Scratch cycle * |
| More Recent: |
| • Topical barrier repair (Cer-dominant [3:1:1] ratio of Cer, FFA, Chol); Cer precursors (e.g., serinol); ↑Cer synthesis (e.g., eucalyptus oil) |
| • Suberythemogenic UVB (benefits the barrier) |
| • Reduce SC pH (↓inflammation and ↑Cer production) |
| • Serine protease (kallikrein) inhibitors (↓inflammation) |
| • PAR2 inhibitors (↓pruritis) |
| • Enhance innate immunity (↑AMP expression with barrier repair) |
| • Stimulate filaggrin production (PPAR or LXR activators; naturally occurring bioflavonoids [e.g., hesperidin]) |
| • Topical Nrf-2 activators (e.g., resveratrol) can improve barrier and fight inflammation |
Note: Antihistamines benefit the barrier.74
The recent emphasis on anti-inflammatory therapy, and particularly new biologics, has overshadowed efforts to bolster barrier function as primary or ancillary therapy for AD. Yet, these potent agents are not indicated for use in most children, and not for patients with relatively limited disease, particularly if they can be managed effectively with currently available therapy, with well-known side effect profiles. Among the available options to further enhance barrier function in AD are antihistamines 75, which though not particularly effective at controlling pruritus in AD, have been shown to both enhance barrier function and reduce inflammation in an AD mouse model 40 (Table 1). Lowering the pH alone is highly effective 76; hence, any formulation developed for AD, should be deployed at a reduced pH. It would be logical to deploy KLK inhibitors, many of which are naturally occurring, such as α1-anti-trypsin inhibitor or soybean trypsin inhibitor. KLKs not only are directly destructive, but they also bind to and activate plasminogen activator type 2 receptors (PAR2), which in turn block lamellar body secretion 77 and provoke pruritus 78. Hence, small peptide inhibitors of PAR2 could yet enter the therapeutic armamentarium for AD. Because reduced exposure to the benefits of suberythemogenic UV-B has been proposed as a key factor in the recent, urban resurgence of AD 79, it would seem prudent to recommend moderate amounts of exposure to ambient UV-B as a part of the management plan for AD patients. Finally, not only PPAR activators 17, but also several bioflavonoid ingredients, such as hesperidin and apigenin 80, 81, have been shown to boost filaggrin production, and could therefore prove useful in those AD patients who do not exhibit double-allele mutations in filaggrin gene expression.
KEY MESSAGES.
Optimal barrier function is required for mammalian life/survival in a desiccating, terrestrial environment.
Loss-of-function mutations in filaggrin and other genes provoke similar, atopic dermatitis (AD)-like phenotypes.
Therapy with most over-the-counter moisturizers compromise barrier function further in atopic dermatitis mouse models.
Triple lipid-based therapies, comprising an optimized ratio of ceramides (3), cholesterol (1), and free fatty acids (1), normalize barrier function in atopic dermatitis.
Topical applications of an optimized-ratio formulation have proven as effective as a mid-potency, fluorinated steroid (fluticasone) in pediatric atopic dermatitis.
Studies are underway to determine whether this form of therapy will prevent the atopic march.
Acknowledgments
Excellent editorial assistance was provided by Joan S. Wakefield. Research reported in this publication was supported by the National Institute of Arthritis, Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R01AR061106, administered by the Northern California Institute for Research and Education, with additional resources provided by the Veterans Affairs Medical Center, San Francisco. This content is solely the responsibility of the authors and does not necessarily represent the official views of either the National Institutes of Health or the Department of Veterans Affairs.
Funding Source:
Research reported in this publication was supported by the National Institute of Arthritis, Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R01 AR061106, administered by the Northern California Institute for Research and Education, with additional resources provided by the Veterans Affairs Medical Center, San Francisco.
Abbreviations:
- AD
atopic dermatitis
- BRT
barrier repair therapy
- KLK
kallikrein
- NPS
N-palmitoyl serinol
- PPAR
peroxisome proliferator-activated receptor
- SC
stratum corneum
Footnotes
Trial Registration: Not applicable
Conflicts of Interest: Dr. Elias is a co-inventor of EpiCeram®, licensed from the University of California to Primus Pharmaceuticals, LLC, Scottsdale, AZ
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Asher MI, Montefort S, Bjorksten B, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet 2006; 368: 733–43. [DOI] [PubMed] [Google Scholar]
- 2.Weidinger S, Beck LA, Bieber T, et al. Atopic dermatitis. Nat Rev Dis Primers 2018; 4: 1. [DOI] [PubMed] [Google Scholar]
- 3.Chamlin SL, Mattson CL, Frieden IJ, et al. The price of pruritus: sleep disturbance and cosleeping in atopic dermatitis. Arch Pediatr Adolesc Med 2005; 159: 745–50. [DOI] [PubMed] [Google Scholar]
- 4.Chung J, Simpson EL. The socioeconomics of atopic dermatitis. Ann Allergy Asthma Immunol 2019; 122: 360–366. [DOI] [PubMed] [Google Scholar]
- 5.Gustafsson D, Sjoberg O, Foucard T. Development of allergies and asthma in infants and young children with atopic dermatitis--a prospective follow-up to 7 years of age. Allergy 2000; 55: 240–5. [DOI] [PubMed] [Google Scholar]
- 6.Dharmage SC, Lowe AJ, Matheson MC, et al. Atopic dermatitis and the atopic march revisited. Allergy 2014; 69: 17–27. [DOI] [PubMed] [Google Scholar]
- 7.De Benedetto A, Kubo A, Beck LA. Skin barrier disruption: a requirement for allergen sensitization? J Invest Dermatol 2012; 132: 949–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vestergaard C, Yoneyama H, Murai M, et al. Overproduction of Th2-specific chemokines in NC/Nga mice exhibiting atopic dermatitis-like lesions. J Clin Invest 1999; 104: 1097–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leung DY. Pathogenesis of atopic dermatitis. J Allergy Clin Immunol 1999; 104: S99–108. [DOI] [PubMed] [Google Scholar]
- 10.Palmer CN, Irvine AD, Terron-Kwiatkowski A, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet 2006; 38: 441–6. [DOI] [PubMed] [Google Scholar]
- 11.Howell MD, Kim BE, Gao P, et al. Cytokine modulation of atopic dermatitis filaggrin skin expression. J Allergy Clin Immunol 2009; 124: R7–R12. [DOI] [PubMed] [Google Scholar]
- 12.Proksch E, Folster-Holst R, Jensen JM. Skin barrier function, epidermal proliferation and differentiation in eczema. J Dermatol Sci 2006; 43: 159–69. [DOI] [PubMed] [Google Scholar]
- 13.Elias PM. Skin barrier function. Curr Allergy Asthma Rep 2008; 8: 299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elias PM, Wakefield JS. Mechanisms of abnormal lamellar body secretion and the dysfunctional skin barrier in patients with atopic dermatitis. J Allergy Clin Immunol 2014; 134: 781–791 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madison KC. Barrier function of the skin: “la raison d’etre” of the epidermis. J Invest Dermatol 2003; 121: 231–41. [DOI] [PubMed] [Google Scholar]
- 16.Elias PM, Friend DS. The permeability barrier in mammalian epidermis. J Cell Biol 1975; 65: 180–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmuth M, Jiang YJ, Dubrac S, et al. Thematic Review Series: Skin Lipids. Peroxisome proliferator-activated receptors and liver X receptors in epidermal biology. J Lipid Res 2008; 49: 499–509. [DOI] [PubMed] [Google Scholar]
- 18.Park K, Elias PM, Hupe M, et al. Resveratrol stimulates sphingosine-1-phosphate signaling of cathelicidin production. J Invest Dermatol 2013; 133: 1942–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Man MQ, Wakefield JS, Mauro TM, et al. Role of nitric oxide in regulating epidermal permeability barrier function. Exp Dermatol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wood LC, Jackson SM, Elias PM, et al. Cutaneous barrier perturbation stimulates cytokine production in the epidermis of mice. J Clin Invest 1992; 90: 482–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hachem JP, Houben E, Crumrine D, et al. Serine protease signaling of epidermal permeability barrier homeostasis. J Invest Dermatol 2006; 126: 2074–86. [DOI] [PubMed] [Google Scholar]
- 22.Scharschmidt TC, Man MQ, Hatano Y, et al. Filaggrin deficiency confers a paracellular barrier abnormality that reduces inflammatory thresholds to irritants and haptens. J Allergy Clin Immunol 2009; 124: 496–506, 506 e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aberg KM, Man MQ, Gallo RL, et al. Co-regulation and interdependence of the mammalian epidermal permeability and antimicrobial barriers. J Invest Dermatol 2008; 128: 917–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams M, Elias P, Feingold K. Regulation of differentiation in newborn human keratinocytes by endogenous ligands of nuclear hormone receptors. J Skin Barrier Res 2000; 2: 3–21. [Google Scholar]
- 25.Yosipovitch G, Maayan-Metzger A, Merlob P, et al. Skin barrier properties in different body areas in neonates. Pediatrics 2000; 106: 105–8. [DOI] [PubMed] [Google Scholar]
- 26.Choi EH, Man MQ, Xu P, et al. Stratum corneum acidification is impaired in moderately aged human and murine skin. J Invest Dermatol 2007; 127: 2847–56. [DOI] [PubMed] [Google Scholar]
- 27.Ghadially R, Brown BE, Sequeira-Martin SM, et al. The aged epidermal permeability barrier. Structural, functional, and lipid biochemical abnormalities in humans and a senescent murine model. J Clin Invest 1995; 95: 2281–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghadially R, Brown BE, Hanley K, et al. Decreased epidermal lipid synthesis accounts for altered barrier function in aged mice. J Invest Dermatol 1996; 106: 1064–9. [DOI] [PubMed] [Google Scholar]
- 29.Elias PM, Man MQ, Thornfeldt CR, et al. The epidermal permeability barrier: effects of physiologic and non-physiological lipids. In: Hoppe U, editors. The Lanolin Book. Hamburg, Germany: Beieersdorf AG; 1999: pp. 253–278. [Google Scholar]
- 30.Denda M, Sato J, Tsuchiya T, et al. Low humidity stimulates epidermal DNA synthesis and amplifies the hyperproliferative response to barrier disruption: implication for seasonal exacerbations of inflammatory dermatoses. J Invest Dermatol 1998; 111: 873–8. [DOI] [PubMed] [Google Scholar]
- 31.Hou DD, Di ZH, Qi RQ, et al. Sea Buckthorn (Hippophae rhamnoides L.) Oil Improves Atopic Dermatitis-Like Skin Lesions via Inhibition of NF-kappaB and STAT1 Activation. Skin Pharmacol Physiol 2017; 30: 268–276. [DOI] [PubMed] [Google Scholar]
- 32.Darmstadt GL, Mao-Qiang M, Chi E, et al. Impact of topical oils on the skin barrier: possible implications for neonatal health in developing countries. Acta Paediatr 2002; 91: 546–54. [DOI] [PubMed] [Google Scholar]
- 33.Schmuth M, Haqq CM, Cairns WJ, et al. Peroxisome proliferator-activated receptor (PPAR)-beta/delta stimulates differentiation and lipid accumulation in keratinocytes. J Invest Dermatol 2004; 122: 971–83. [DOI] [PubMed] [Google Scholar]
- 34.Darmstadt GL, Badrawi N, Law PA, et al. Topically applied sunflower seed oil prevents invasive bacterial infections in preterm infants in Egypt: a randomized, controlled clinical trial. Pediatr Infect Dis J 2004; 23: 719–25. [DOI] [PubMed] [Google Scholar]
- 35.Lack G, Fox D, Northstone K, et al. Factors associated with the development of peanut allergy in childhood. N Engl J Med 2003; 348: 977–85. [DOI] [PubMed] [Google Scholar]
- 36.Man MQ, Hupe M, Man G, et al. Topical apigenin alleviates cutaneous inflammation in murine models. Evidence-Based Compl & Alt Medicine 2012: Online [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tessema EN, Gebre-Mariam T, Neubert RHH, et al. Potential Applications of Phyto-Derived Ceramides in Improving Epidermal Barrier Function. Skin Pharmacol Physiol 2017; 30: 115–138. [DOI] [PubMed] [Google Scholar]
- 38.Danby SG, Brown K, Higgs-Bayliss T, et al. The Effect of an Emollient Containing Urea, Ceramide NP, and Lactate on Skin Barrier Structure and Function in Older People with Dry Skin. Skin Pharmacol Physiol 2016; 29: 135–47. [DOI] [PubMed] [Google Scholar]
- 39.Man MQ, Feingold KR, Thornfeldt CR, et al. Optimization of physiological lipid mixtures for barrier repair. J Invest Dermatol 1996; 106: 1096–101. [DOI] [PubMed] [Google Scholar]
- 40.Li Z, Hu L, Elias PM, et al. Skin care products can aggravate epidermal function: studies in a murine model suggest a pathogenic role in sensitive skin. Contact Dermatitis 2018; 78: 151–158. [DOI] [PubMed] [Google Scholar]
- 41.Brunner PM, Leung DYM, Guttman-Yassky E. Immunologic, microbial, and epithelial interactions in atopic dermatitis. Ann Allergy Asthma Immunol 2018; 120: 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feingold KR, Schmuth M, Elias PM. The regulation of permeability barrier homeostasis. J Invest Dermatol 2007; 127: 1574–6. [DOI] [PubMed] [Google Scholar]
- 43.Elias PM, Hatano Y, Williams ML. Basis for the barrier abnormality in atopic dermatitis: outside-inside-outside pathogenic mechanisms. J Allergy Clin Immunol 2008; 121: 1337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Elias PM. The how, why and clinical importance of stratum corneum acidification. Exp Dermatol 2017; 26: 999–1003. [DOI] [PubMed] [Google Scholar]
- 45.Jang H, Matsuda A, Jung K, et al. Skin pH Is the Master Switch of Kallikrein 5-Mediated Skin Barrier Destruction in a Murine Atopic Dermatitis Model. J Invest Dermatol 2016; 136: 127–35. [DOI] [PubMed] [Google Scholar]
- 46.Howell MD, Novak N, Bieber T, et al. Interleukin-10 downregulates anti-microbial peptide expression in atopic dermatitis. J Invest Dermatol 2005; 125: 738–45. [DOI] [PubMed] [Google Scholar]
- 47.Gruber R, Elias PM, Crumrine D, et al. Filaggrin genotype in ichthyosis vulgaris predicts abnormalities in epidermal structure and function. Am J Pathol 2011; 178: 2252–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hatano Y, Adachi Y, Elias PM, et al. The Th2 cytokine, interleukin-4, abrogates the cohesion of normal stratum corneum in mice: implications for pathogenesis of atopic dermatitis. Exp Dermatol 2013; 22: 30–5. [DOI] [PubMed] [Google Scholar]
- 49.Berdyshev E, Goleva E, Bronova I, et al. Lipid abnormalities in atopic skin are driven by type 2 cytokines. JCI Insight 2018; 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ong PY, Ohtake T, Brandt C, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med 2002; 347: 1151–60. [DOI] [PubMed] [Google Scholar]
- 51.Hatano Y, Man MQ, Uchida Y, et al. Murine atopic dermatitis responds to peroxisome proliferator-activated receptors alpha and beta/delta (but not gamma) and liver X receptor activators. J Allergy Clin Immunol 2010; 125: 160–9 e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Korting HC, Kober M, Mueller M, et al. Influence of repeated washings with soap and synthetic detergents on pH and resident flora of the skin of forehead and forearm. Results of a cross-over trial in health probationers. Acta Derm Venereol 1987; 67: 41–7. [PubMed] [Google Scholar]
- 53.Boguniewicz M, Fonacier L, Guttman-Yassky E, et al. Atopic dermatitis yardstick: Practical recommendations for an evolving therapeutic landscape. Ann Allergy Asthma Immunol 2018; 120: 10–22 e2. [DOI] [PubMed] [Google Scholar]
- 54.Cork MJ, Britton J, Butler L, et al. Comparison of parent knowledge, therapy utilization and severity of atopic eczema before and after explanation and demonstration of topical therapies by a specialist dermatology nurse. Br J Dermatol 2003; 149: 582–9. [DOI] [PubMed] [Google Scholar]
- 55.Lowe AJ, Su JC, Allen KJ, et al. A randomized trial of a barrier lipid replacement strategy for the prevention of atopic dermatitis and allergic sensitization: the PEBBLES pilot study. Br J Dermatol 2018; 178: e19–e21. [DOI] [PubMed] [Google Scholar]
- 56.Janssens M, van Smeden J, Gooris GS, et al. Increase in short-chain ceramides correlates with an altered lipid organization and decreased barrier function in atopic eczema patients. J Lipid Res 2012; 53: 2755–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Simpson EL, Chalmers JR, Hanifin JM, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol 2014; 134: 818–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lowe AJ, Leung DYM, Tang MLK, et al. The skin as a target for prevention of the atopic march. Ann Allergy Asthma Immunol 2018; 120: 145–151. [DOI] [PubMed] [Google Scholar]
- 59.Horimukai K, Morita K, Narita M, et al. Transepidermal water loss measurement during infancy can predict the subsequent development of atopic dermatitis regardless of filaggrin mutations. Allergol Int 2016; 65: 103–8. [DOI] [PubMed] [Google Scholar]
- 60.Demerjian M, Choi EH, Man MQ, et al. Activators of PPARs and LXR decrease the adverse effects of exogenous glucocorticoids on the epidermis. Exp Dermatol 2009; 18: 643–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim M, Jung M, Hong S, et al. Topical calcineurin inhibitors compromise stratum corneum integrity and epidermal and antimicrobial barrier function. Exp Dermatol 2010; 19: 501–510. [DOI] [PubMed] [Google Scholar]
- 62.Rodriguez-Martin M, Martin-Ezquerra G, Man MQ, et al. Expression of epidermal CAMP changes in parallel with permeability barrier status. J Invest Dermatol 2011; 131: 2263–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ishikawa J, Shimotoyodome Y, Chen S, et al. Eucalyptus increases ceramide levels in keratinocytes and improves stratum corneum function. Int J Cosmet Sci 2012; 34: 17–22. [DOI] [PubMed] [Google Scholar]
- 64.Shin KO, Kim S, Park BD, et al. N-Palmitoyl Serinol Stimulates Ceramide Production through a CB1-Dependent Mechanism in In Vitro Model of Skin Inflammation. Int J Mol Sci 2021; 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roelandt T, Heughebaert C, Bredif S, et al. Cannabinoid receptors 1 and 2 oppositely regulate epidermal permeability barrier status and differentiation. Exp Dermatol 2012; 21: 688–93. [DOI] [PubMed] [Google Scholar]
- 66.Jeong S, Kim M, Lee S, et al. Epidermal endocannabinoid system (EES) and its cosmetic application. Cosmetics 2019; 6: 1–10. [Google Scholar]
- 67.Wen S, Ye L, Liu D, et al. Topical N-palmitoyl serinol, a commensal bacterial metabolite, prevents the development of epidermal permeability barrier dysfunction in a murine model of atopic dermatitis-like skin. Can J Vet Res 2021; 85: 201–204. [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao JQ, Zhang YL, Li YJ, et al. Topical applications of a novel formulation improve epidermal permeability barrier in chronologically aged humans. J Cosmet Dermatol 2021. [DOI] [PubMed] [Google Scholar]
- 69.Chamlin SL, Kao J, Frieden IJ, et al. Ceramide-dominant barrier repair lipids alleviate childhood atopic dermatitis: changes in barrier function provide a sensitive indicator of disease activity. J Am Acad Dermatol 2002; 47: 198–208. [DOI] [PubMed] [Google Scholar]
- 70.Kircik LH. Effect of skin barrier emulsion cream vs a conventional moisturizer on transepidermal water loss and corneometry in atopic dermatitis: a pilot study. J Drugs Dermatol 2014; 13: 1482–4. [PubMed] [Google Scholar]
- 71.Sugarman J, Parish L. Efficacy of a lipid-based, barrier repair formulation in moderate-to-severe pediatric atopic dermatitis. J Drugs Dermatol 2009; 8: 1106–1111. [PubMed] [Google Scholar]
- 72.Denda M, Wood LC, Emami S, et al. The epidermal hyperplasia associated with repeated barrier disruption by acetone treatment or tape stripping cannot be attributed to increased water loss. Arch Dermatol Res 1996; 288: 230–8. [DOI] [PubMed] [Google Scholar]
- 73.Elias PM. The skin barrier as an innate immune element. Sem Immunopath 2007; 29: 3–14. [DOI] [PubMed] [Google Scholar]
- 74.Man MQ, Fowler AJ, Schmuth M, et al. Peroxisome-proliferator-activated receptor (PPAR)-gamma activation stimulates keratinocyte differentiation. J Invest Dermatol 2004; 123: 305–12. [DOI] [PubMed] [Google Scholar]
- 75.Lin TK, Man MQ, Santiago JL, et al. Topical antihistamines display potent anti-inflammatory activity linked in part to enhanced permeability barrier function. J Invest Dermatol 2013; 133: 469–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hatano Y, Man MQ, Uchida Y, et al. Maintenance of an acidic stratum corneum prevents emergence of murine atopic dermatitis. J Invest Dermatol 2009; 129: 1824–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Demerjian M, Hachem JP, Tschachler E, et al. Acute modulations in permeability barrier function regulate epidermal cornification: role of caspase-14 and the protease-activated receptor type 2. Am J Pathol 2008; 172: 86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Elias PM, Steinhoff M. “Outside-to-inside” (and now back to “outside”) pathogenic mechanisms in atopic dermatitis. J Invest Dermatol 2008; 128: 1067–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Thyssen JP, Zirwas MJ, Elias PM. Potential role of reduced environmental UV exposure as a driver of the current epidemic of atopic dermatitis. J Allergy Clin Immunol 2015; 136: 1163–9. [DOI] [PubMed] [Google Scholar]
- 80.Hou M, Sun R, Hupe M, et al. Topical apigenin improves epidermal permeability barrier homoeostasis in normal murine skin by divergent mechanisms. Exp Dermatol 2013; 22: 210–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Man G, Mauro TM, Kim PL, et al. Topical hesperidin prevents glucocorticoid-induced abnormalities in epidermal barrier function in murine skin. Exp Dermatol 2014; 23: 645–651. [DOI] [PMC free article] [PubMed] [Google Scholar]