Abstract
Background
COVID − 19 vaccine can lead to various local and systemic side effects, including menstrual irregularities in women. There is no robust quantitative evidence of the association between the COVID − 19 vaccine and menstrual irregularities. A meta-analysis was performed to estimate the pooled prevalence of a range of menstrual disorders that may occur in women following COVID − 19 vaccination.
Methods
After searching for epidemiological studies, we systematically performed a meta-analysis on PubMed/Medline, EMBASE, and Science Direct. Sixteen studies were finally included in the study. We estimated the pooled prevalence and corresponding 95 % confidence intervals (CIs) for a group of menstrual disorders, including menorrhagia, polymenorrhea, abnormal cycle length, and oligomenorrhea. Heterogeneity was assessed using the I2 statistic and the Q test.
Results
Overall, the pooled prevalence of menorrhagia was 24.24 % (pooled prevalence 24.24 %; 95 % CI: 12.8–35.6 %). The pooled prevalence of polymenorrhea was 16.2 % (pooled prevalence: 16.2 %; 95 % CI: 10.7–21.6 %). The pooled prevalence of abnormal cycle length was relatively lower than that of the other disorders (pooled prevalence: 6.6 %; 95 % CI: 5.0–8.2 %). The pooled prevalence of oligomenorrhea was 22.7 % (95 % CI: 13.5–32.0 %).
Conclusion
The findings indicate that menorrhagia, oligomenorrhea, and polymenorrhea were the most common menstrual irregularities after vaccination. The findings also suggest that a relatively high proportion of women suffer from menstrual irregularities. Further longitudinal studies are needed to confirm the causal relationship between COVID-19 vaccination and menstrual irregularities.
Keywords: COVID-19 vaccine, Menstrual irregularities, Meta-analysis
Introduction
Since the outbreak of the COVID − 19 pandemic, researchers and public health professionals have attempted to vaccinate populations worldwide [1]. As a result, a number of vaccines have been developed, including mRNA-based vaccines (Pfizer and Moderna), inactivated whole-virus vaccines (Sinopharm and Sinovac), or recombinant vaccines (e.g., Johnson & Johnson and Oxford-AstraZeneca) [2]. Governments have supported COVID − 19 mass vaccination campaigns, but these vaccines are not without a range of side effects, including both local and systemic side effects [3], [4]. Many women reported that their menstrual cycle changed after COVID − 19 vaccination [5], [6]. In addition, women who experienced headaches after COVID − 19 vaccination had a higher rate of menstrual disorders [7]. More specifically, women reported disturbances such as altered cycle length, heavier bleeding, and heavy and painful menstruation after COVID − 19 vaccination [5], [6], [7], [8]. A significant number of adverse events have been reported regardless of the type of vaccine used, and menstrual irregularities are independent of the type of vaccine used [5], [6], [7], [8]. After vaccination, menstrual irregularities normalize within two months in about half of cases [5]. Concerns about a possible link between COVID − 19 vaccination and menstrual irregularities have led to hesitation to get vaccinated. This concern has been reported on social media more than any other side effect [9]. However, social media reports cannot be relied upon because they are falsifiable and may not reflect the true association between COVID − 19 vaccination and menstrual irregularities [10]. Therefore, more evidence from population-based studies is needed to support the hypothesis. Researchers have conducted observational studies to estimate the prevalence of menstrual irregularities in women after COVID-19 vaccination. These studies have been conducted in both developing and developed countries [12]. In addition, the results of these studies were summarized in a recent systematic review that provides a qualitative overview of the association between COVID − 19 vaccination and menstrual irregularities in women [13]. However, there is no quantitative synthesis of the literature that can provide a pooled prevalence with precision limits for different types of menstrual irregularities. Therefore, we performed a meta-analysis of all studies conducted during the pandemic COVID-19 and estimated the pooled prevalence of a range of menstrual irregularities that may occur in women after vaccination. The results of this meta-analysis provide useful insights into the impact of COVID − 19 vaccination on menstrual irregularities in women.
Materials and methods
We performed a meta-analysis to quantitatively summarize available data from epidemiologic studies that investigated the effect of COVID-19 vaccines on menstrual disorders in women.
Inclusion and exclusion criteria
Studies were included in the meta-analysis if they met the following criteria: 1) They were epidemiologic studies that examined the association between the COVID − 19 vaccine and a range of menstrual disorders in women; 2) They were exclusively quantitative studies published during the period of the COVID-19 pandemic; 3) They examined a range of menstrual disorders including menorrhagia (heavy menstrual bleeding), oligomenorrhea (infrequent periods, usually less than 6–8 periods per year), polymenorrhea (a menstrual cycle shorter than 21 days in which the amount of blood flow is normal), and abnormal cycle length (cycles outside the range of 21–35 days or periods lasted longer than 7 days).
Studies were excluded if: 1) They were studies without full text; 2) They were randomized controlled trials because the objective of the study was to estimate the pooled prevalence of menstrual irregularities due to COVID-19 vaccine; 3) They were opinions, critiques of previous research studies, qualitative studies, secondary data, or editorials.
Sources of information and search strategy
We systematically searched PubMed/MEDLINE, EMBASE and Science Direct databases in September 2022. In addition, we examined the references of the selected articles for other related publications based on the selection criteria. We performed an independent search to scan the results for potentially relevant studies, followed by retrieval of the full-text articles. The primary outcome of interest was all types of menstrual irregularities. We identified a mixture of keywords and text words for Medical Subject Heading (MeSH). The most common search terms in the abstracts and titles were “COVID-19 vaccine”, “menorrhagia”, “oligomenorrhea”, “dysmenorrhea”, “polymenorrhea” and “abnormal menstrual cycle length”. Then these main terms were combined by combinations (AND, OR). We also used the truncation (*) with the same root word to find more research articles. We used truncation to ensure that all possible variants of the search term were found. We also used filters for language (English) and publication period (2020 - September 2022) to include appropriate studies in the search.
Data abstraction
We imported all relevant research studies into an EndnoteTM file in which each study was evaluated, and we also used this software to check titles for duplicates. Full-text publication was not considered for abstracts that did not explicitly address the aim of the study. Finally, the full-text articles of the remaining relevant research papers were read and evaluated against the selection criteria. Eligible articles were then abstracted and summarized using a standardized form. We then removed articles after eliminating duplicates, reviewing titles and abstracts, and removing articles that did not fit the scope of this study according to the inclusion criteria. In addition, the bibliographies of the remaining studies were reviewed to ensure that no important research articles were overlooked. Reviewers independently screened the articles, and compared their judgments and extracted abstracts to identify and resolve inconsistencies. Independent reviewers extracted study characteristics from eligible research articles using a standardized data extraction form. The reviewers reviewed the data extraction tables before beginning data extraction to ensure that key findings from the relevant studies were included. Additionally, recent research articles on the selected topic were reviewed to describe the items on the data extraction form. Discrepancies were handled by mutual agreement. We extracted the following elements: (a) title; (b) year published; (c) sample size or population; (d) country or study setting; (e) type of menstrual irregularity; and (f) prevalence of menstrual irregularities.
Statistical analysis
The prevalence, and corresponding 95 % confidence intervals (95 % CI) were used to examine the effect of the COVID-19 vaccine and a range of menstrual irregularities in women. Because the outcome was binary (yes/no), percentages were used to estimate the pooled prevalence of a given disorder and its 95 % CI. Open Meta[analyst] software was used to perform this meta-analysis. Heterogeneity was assessed using the I2 statistic and the Q test [14]. The cut-off values for the I2 statistic were used to classify heterogeneity as very low (0–25 %), low (25–50 %), moderate (50–75 %), and high (>75 %). Although we originally used a commonly used fixed effects model, this model may underestimate the uncertainty in our results [15]. In addition, the fixed effects model produced narrow confidence intervals that did not account for the actual variance between studies [15]. The fixed effects model assumes that studies are from populations with the same effect size, which may not be the case with real-world data. The random effects model, on the other hand, assumes that the studies come from populations with different effect sizes, which seems to be a reasonable assumption. However, in a sensitivity analysis, we used both fixed-effects and random-effects models, but both models showed similar heterogeneity.
The inverse variance method and a random effects model were used for this meta-analysis because of differences between studies in sample size, outcome assessment, and measurement tools. This was also confirmed by a higher degree of heterogeneity as measured by the I2 statistic and the Q test. Finally, a random effects model was used to calculate the overall effect size and its respective 95 % CI to assess the impact of the COVID-19 vaccine on various menstrual disorders [16]. To assess statistical heterogeneity, forest plots were created to calculate individual and pooled prevalence for different types of menstrual disorders after the vaccination. We created four forest plots for common menstrual disorders such as polymenorrhea, menorrhagia, abnormal menstrual cycle length, and oligomenorrhea.
Results
Results of the search strategy
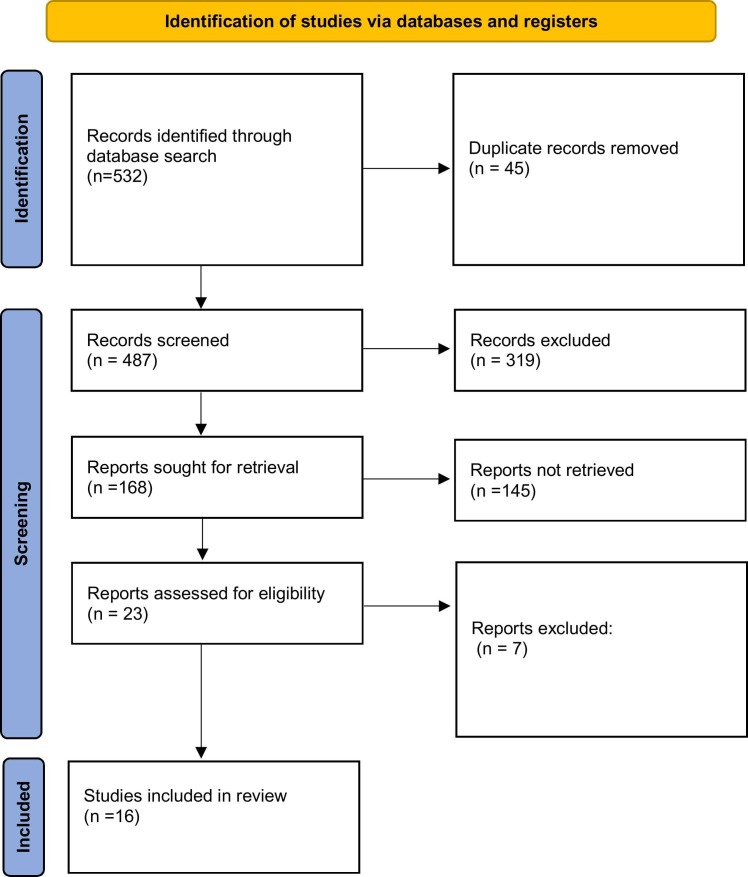
The selected articles were searched first by title, then by abstract and finally by full-text article. Our preliminary search identified 532 citations in different databases, and after removing 45 duplicates, 487 unique observational studies were searched for their titles and abstracts. We then excluded 319 studies because these studies did not meet the eligibility criteria. We then reviewed 23 studies, and seven additional studies were removed. Finally, we included 16 studies in the quantitative analysis as shown in the PRISMA flowchart for screening studies ( Fig. 1).
Fig. 1.
PRISMA flowchart.
Characteristics of the eligible studies
Regarding the study design, all studies were cross-sectional studies conducted in different countries. Two of the studies were conducted in China [17], [18], two in the United States [19], [20], and two in Saudi Arabia [21], [22], three in the United Kingdom [23], [24], [25], and one each in Pakistan [26], Jordan and Saudi Arabia [27], Norway [28], Africa [29], and Italy [5]. Finally, one study was multi-centric as it was conducted in different countries worldwide [30]. The total sample size of all eligible studies ranged from 164 to 84943.
Key findings on the effect of COVID-19 vaccination on menstrual disorders
The studies did not provide information on the overall prevalence of menstrual disorders; instead, the authors provided information on the specific type of menstrual disorder. Therefore, we created several forest plots by type of menstrual disorder such as menorrhagia, polymenorrhea, abnormal cycle length, and oligomenorrhea.
Findings regarding the prevalence of menorrhagia after COVID-19 vaccination
Quantitative synthesis and assessment of heterogeneity.
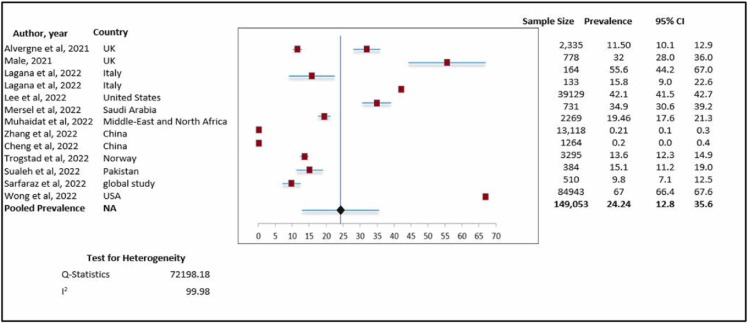
( Fig. 2) shows the forest plot for the pooled prevalence of menorrhagia after COVID-19 vaccination.
Fig. 2.
Prevalence of menorrhagia. Meta-analysis of 13 research studies.
We included thirteen studies to analyze the data for the pooled prevalence of menorrhagia after COVID-19 vaccination. Overall, the pooled prevalence was 24.24 % (pooled prevalence 24.24 %; 95 % CI: 12.8–35.6 %). This indicates that approximately one quarter of women complained of menorrhagia after COVID-19 vaccination. However, when we assessed heterogeneity, we found relatively high heterogeneity with an I2 value of 99.9 % (chi-square = 72198.18).
Findings regarding the prevalence of polymenorrhea after COVID-19 vaccination
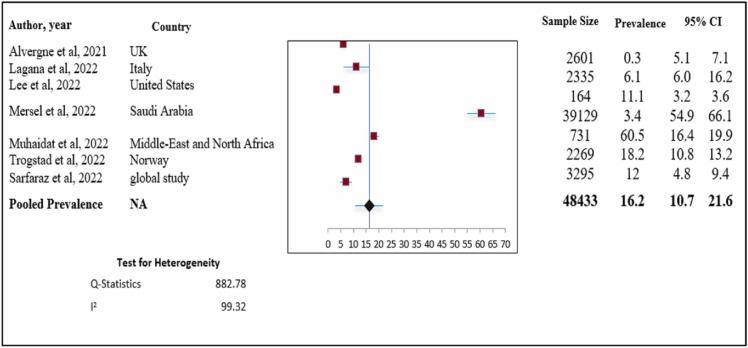
Quantitative synthesis and assessment of heterogeneity ( Fig. 3) shows the forest plot for the pooled prevalence of polymenorrhea after COVID-19 vaccination.
Fig. 3.
Total prevalence of polymenorrhea. Meta-analysis of 7 research studies.
We included seven studies to analyze data on the pooled prevalence of polymenorrhea after COVID-19 vaccination. Overall, the pooled prevalence was 16.2 % (pooled prevalence: 16.2 %; 95 % CI: 10.7–21.6 %). This indicates that approximately 16 % of women complained of menorrhagia after COVID-19 vaccination. When we assessed heterogeneity, we found relatively high heterogeneity with an I2 value of 99.9 % (chi-square = 882.78).
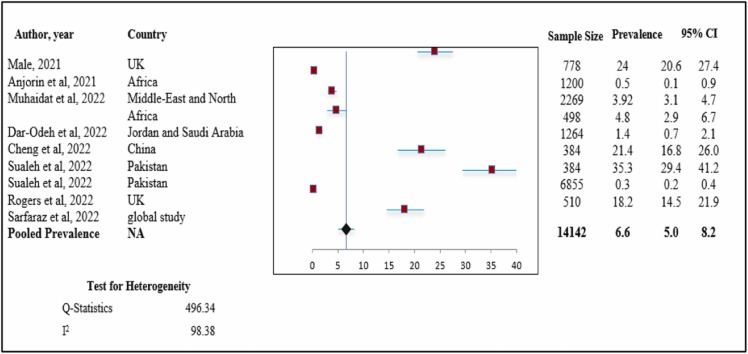
Findings regarding the prevalence of abnormal menstrual cycle length after COVID-19 vaccination
Quantitative synthesis and assessment of heterogeneity ( Fig. 4) shows the forest plot for the pooled prevalence of abnormal menstrual cycle length after COVID-19 vaccination.
Fig. 4.
Prevalence of abnormal menstrual cycle length. Meta-analysis of 9 research studies.
We included nine studies to analyze the data for the pooled prevalence of abnormal menstrual cycle length after COVID-19 vaccination. Overall, the pooled prevalence was 6.6 % (pooled prevalence: 6.6 %; 95 % CI: 5.0–8.2 %). When we assessed heterogeneity, we found relatively high heterogeneity with an I2 value of 98.38 % (chi-square= 496.34).
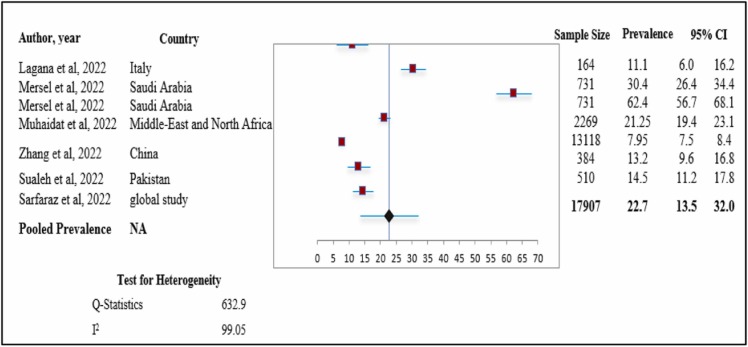
Findings regarding the prevalence of oligomenorrhea after COVID-19 vaccination
Quantitative synthesis and assessment of heterogeneity ( Fig. 5) shows the forest plot for the pooled prevalence of oligomenorrhea after COVID-19 vaccination.
Fig. 5.
Prevalence of oligomenorrhea. Meta-analysis of 7 research studies.
We included seven studies to analyze data for the pooled prevalence of oligomenorrhea after COVID-19 vaccination. Overall, the pooled prevalence was 22.7 % (pooled prevalence: 22.7 %; 95 % CI: 13.5–32.0 %). This suggests that approximately one quarter of women experienced symptoms of oligomenorrhea after COVID-19 vaccination. Heterogeneity was higher with an I2 value of 99.05 % (chi-square = 632.9).
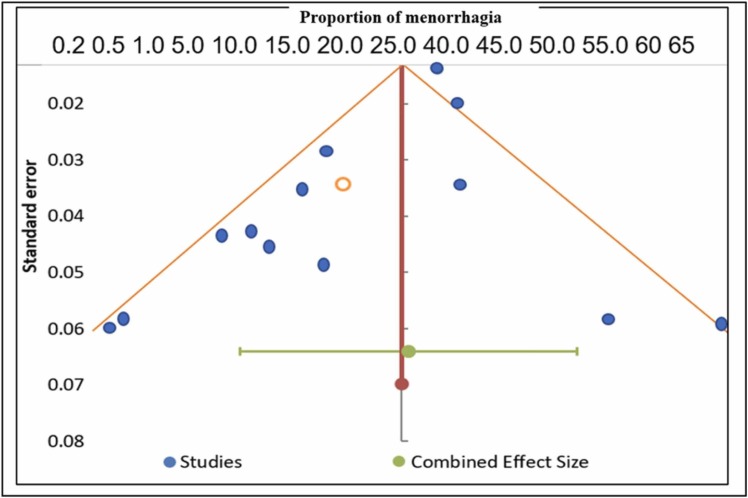
Publication bias assessment
We examined the potential for publication bias using a funnel plot – asymmetry in the funnel plot would tell us that larger samples with null findings are underrepresented. The funnel plot in Fig. 6 does not appear to be asymmetric, as a number of the included studies (n = 13) lie on either side of the overall effect size and thus have a symmetrical shape. This suggests that the probability of publication bias is low. In addition, Egger’s test was used to assess publication bias. Egger’s test tests the null hypothesis that there are no small study effects. A significant p-value indicates that small study effects may be driving our findings. However, the p-value for Egger’s test was 0.45, indicating that small study effects cannot drive our findings. Thus, there seems to be a low risk of publication bias.
Fig. 6.
Funnel plot showing publication bias (Prevalence of menorrhagia. Meta-analysis of 13 research studies).
Discussion
This meta-analysis was performed to estimate the pooled prevalence of various menstrual disorders in women after COVID-19 vaccination. We found that approximately one quarter of women suffered from menorrhagia, followed by oligomenorrhea, polymenorrhea, and abnormal menstrual cycle length. We found that the proportion of women who developed polymenorrhea was lower than those who developed menorrhagia Comparing the prevalence of these menstrual irregularities with the existing literature, we found that the results of a systematic review indicated that 6.3 % of women had oligomenorrhea, 9.94 % of women had polymenorrhea, and approximately one quarter of women had abnormal cycle length [31]. Similarly, a meta-analysis conducted in Iran found that the burden of oligomenorrhea was 13.1 %, while about 9.9 % and 19.24 % of women suffered from polymenorrhea and menorrhagia, respectively [32]. In general, we found a relatively higher burden of these menstrual disorders than in the existing literature [31], [32]. These differences in results could be due to differences in the population, as our meta-analysis was conducted on women after COVID-19 vaccination, and it appears that the vaccination increases the burden of menstrual irregularities. Furthermore, these differences could also be due to differences in the characteristics or demographic profile of the women. These findings suggest that women should be screened for various menstrual abnormalities before vaccination or that they should monitor changes in menstrual cycles after vaccination. Pre-vaccination and post-vaccination menstrual cycles in women should be tracked over time. Counseling about menstrual irregularities also needs to be included in the vaccination counseling plan.
Our findings are consistent with a recent systematic review that reported that menstrual disorders such as menorrhagia and polymenorrhea are common in women after COVID-19 vaccination [13]. Although there are consistent results for the effect of the COVID-19 vaccine on menstrual cycle irregularities, it is difficult to draw a firm conclusion about the relationship between these irregularities and COVID-19 vaccine. Other cofounding factors could be related to menstrual irregularities, and several factors of the COVID-19 pandemic need to be considered, particularly stress and the COVID-19 disease. COVID-19 control strategies such as lockdown have led to high levels of stress associated with menstrual irregularities [33]. According to Li et al., psychological factors could play a role in such conditions; however, a case of a 27-year-old woman who developed amenorrhea after recovering from SARS-CoV-2 infection had no psychological symptoms. SARS-CoV-2 might attack ovarian tissue and granulosa cells and decrease ovarian function and oocyte quality. The exact effects of SARS-CoV-2 on the ovaries and its potential mechanisms are unclear [34]. Various environmental and biological stressors can affect the hormones released by the pituitary gland, which may eventually affect the menstrual cycle [35], [36], [37]. The COVID-19 pandemic and vaccination may all play a role, and one cannot exclude the other because there is no solid evidence. It is more likely that the menstrual changes are due to the immune response to vaccination than to a specific type of vaccine [38]. It is likely that women experience biological stress due to the increased immune response after vaccination, which leads to a change in hormones, thereby affecting the menstrual cycle [39], [40]. In addition, vaccines can lead to the production of antibodies and activate immune cells [41]. These immune cells can lead to changes in the menstrual cycle and affect the endometrium, resulting in altered bleeding [42]. However, these biological mechanisms by which vaccines may alter the menstrual cycle need to be further explored in future studies.
Strengths and limitations
This study provides an estimated pooled prevalence of various menstrual irregularities in women after COVID-19 vaccination. This meta-analysis also included studies from around the world with appropriate sample sizes, providing a way to assess the prevalence of menstrual irregularities in women from diverse backgrounds.
This meta-analysis has some limitations, and its results must be interpreted with caution. First, all included studies were cross-sectional; therefore, there is a potential threat to internal validity, and a temporal association between the COVID-19 vaccine and menstrual irregularities cannot be established. Second, the women included in each study may not represent the underlying target population because the samples were not randomly drawn. Most studies were from several high-income countries, so their results may not be generalizable to other populations, particularly low-income countries. In addition, we found a large heterogeneity, which could be due to various factors such as sample size, sampling strategy, differences in the nature of the populations, or different settings. Third, the psychological status of women during the COVID-19 pandemic was not assessed. The COVID-19 pandemic has the potential to increase stress, which may lead to menstrual irregularities.Given these limitations, we hope that this meta-analysis will provide useful insights to both physicians and policy makers to further explore the adverse effects of the COVID-19 vaccine on women’s menstrual cycles. Because the COVID-19 vaccine may reduce morbidity and mortality associated with COVID-19, it may be premature to draw firm conclusions about its association with menstrual irregularities. Therefore, more robust epidemiologic studies are needed to evaluate the effects of the COVID-19 vaccine on menstruation in women.
Conclusion
The results of the current meta-analysis show that after COVID-19 vaccination, approximately one quarter of women developed menorrhagia, followed by oligomenorrhoea and polymenorrhoea. Before firm conclusions are drawn and without robust evidence, it may be unethical to deny women the benefits of the COVID-19 vaccine. Therefore, a risk-benefit analysis must be performed before prescribing the COVID-19 vaccine to women. Such a risk-benefit assessment can be individualized, and the COVID-19 vaccine can be prescribed after assessing the risk profile of the women. These women must be closely monitored to determine if they develop severe menstrual irregularities following COVID-19 vaccination.
Ethical approval
Not require.
Funding
No funding sources.
Conflict of Interest
The authors declare that they have no competing interests.
References
- 1.Holder J. Tracking Coronavirus Vaccinations Around The World. The New York Times; 2021. p. 30. [Google Scholar]
- 2.Han X., Xu P., Ye Q. Analysis of Covid‐19 vaccines: types, thoughts, and application. J Clin Lab Anal. 2021;35 doi: 10.1002/jcla.23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salah H.M., Mehta J.L. Covid-19 vaccine and myocarditis. Am J Cardiol. 2021;157 doi: 10.1016/j.amjcard.2021.07.009. 164-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beatty A.L., Peyser N.D., Butcher X.E., Cocohoba J.M., Lin F., Olgin J.E., Pletcher M.J., Marcus G.M. Analysis of Covid-19 vaccine type and adverse effects following vaccination. Jama Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.40364. E2140364-E2140364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laganà A.S., Veronesi G., Ghezzi F., Ferrario M.M., Cromi A., Bizzarri M., Garzon S., Cosentino M. Evaluation of menstrual irregularities after Covid-19 vaccination: results of the mecovac survey. Open Med. 2022;17:475–484. doi: 10.1515/med-2022-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdollahi A., Naseh I., Kalroozi F., Kazemi-Galougahi M.H., Nezamzadeh M., Sabeti Billandi S., Yousefi Zoshk M., Sterility Comparison of side effects of Covid-19 vaccines: Sinopharm, Astrazeneca, Sputnik V, And Covaxin in women in terms of menstruation disturbances, hirsutism, and metrorrhagia: a descriptive-analytical cross-sectional study. Int J Fertil. 2022;16:237–243. doi: 10.22074/IJFS.2022.544706.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dabbousi Aa, El Masri J., El Ayoubi Lm, Ismail O., Zreika B., Salameh P. Menstrual Abnormalities Post-Covid Vaccination: A Cross-Sectional Study On Adult Lebanese Women. Ir J Med Sci. [DOI] [PMC free article] [PubMed]
- 8.Kurdoğlu Z. Do the Covid-19 vaccines cause menstrual irregularities. Inte J Women’s Health Reprod Sci. 2021;9:158–159. [Google Scholar]
- 9.Brumfiel G. Why reports of menstrual changes after covid vaccine are tough to study. Npr August. 2021;9 [Google Scholar]
- 10.Katz A., Tepper Y., Eran A., Birk O. Web and social media searches highlight menstrual irregularities as a global concern in Covid-19 vaccinations. Medrxiv. 2022 doi: 10.1038/s41598-022-20844-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hallberg E., Sundström A., Larsson M., Arthurson V., Ljung R. Association between menstrual cycle length and coronavirus disease 2019 (Covid-19) vaccination: A U.S. Cohort. Obstet Gynecol. 2022;139:940–941. doi: 10.1097/AOG.0000000000004781. [DOI] [PubMed] [Google Scholar]
- 13.Nazir M., Asghar S., Rathore M.A., Shahzad A., Shahid A., Ashraf Khan A., Malik A., Fakhar T., Kausar H., Malik J. Menstrual abnormalities after Covid-19 vaccines: a systematic review. Vacunas. 2022;23:S77–S87. doi: 10.1016/j.vacun.2022.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 15.Overton R.C. A comparison of fixed-effects and mixed (random-effects) models for meta-analysis tests of moderator variable effects. Psychol Methods. 1998;3:354–379. [Google Scholar]
- 16.Barili F., Parolari A., Kappetein P.A., Freemantle N. Statistical primer: heterogeneity, random- or fixed-effects model analyses? Inter Cardiovasc Thorac Surg. 2018;27:317–321. doi: 10.1093/icvts/ivy163. [DOI] [PubMed] [Google Scholar]
- 17.Zhang, B., Yu, X., Liu, J. & Liu, P. 2022. Covid-19 Vaccine And Menstrual Conditions In Female: Data Analysis Of The Vaccine Adverse Event Reporting System. [DOI] [PMC free article] [PubMed]
- 18.Cheng Y., Li T., Zheng Y., Xu B., Bi Y., Hu Y., Zhou Y.-H. Self-reported adverse events among chinese healthcare workers immunized with covid-19 vaccines composed of inactivated Sars-Cov-2. Hum Vaccin Immunother. 2022:1–7. doi: 10.1080/21645515.2022.2064134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong K.K., Heilig C.M., Hause A., Myers T.R., Olson C.K., Gee J., Marquez P., Strid P., Shay D.K. Menstrual irregularities and vaginal bleeding after Covid-19 vaccination reported to v-safe active surveillance, USA In December, 2020–January, 2022: an observational cohort study. Lancet Digit Health. 2022;4 doi: 10.1016/S2589-7500(22)00125-X. E667-E675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee K.M., Junkins E.J., Luo C., Fatima U.A., Cox M.L., Clancy K.B. Investigating trends in those who experience menstrual bleeding changes after Sars-Cov-2 vaccination. Sci Adv. 2022;8 doi: 10.1126/sciadv.abm7201. Eabm7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morsi A.A., Mersal E.A., Hassanein A.M., Alshammri A., Alshammari A., Alkahmous N., Alhuwayji F., Elfawal R.G. The association between Covid-19 Pfizer vaccine and the reported post-vaccination menstrual changesi citizen and resident women In Ksa: results of Riyadh survey study. Egypt J Hosp Med. 2022;87:1442–1448. [Google Scholar]
- 22.Alghamdi A.N., Alotaibi M.I., Alqahtani A.S., Al Aboud D., Abdel-Moneim A.S. Bnt162b2 and Chadox1 Sars-Cov-2 post-vaccination side-effects among saudi vaccinees. Front Med. 2021;1796 doi: 10.3389/fmed.2021.760047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Male V. Effect of Covid-19 vaccination on menstrual periods in a retrospectively recruited cohort. Medrxiv. 2021 [Google Scholar]
- 24.Rogers A., Rooke E., Morant S., Guthrie G., Doney A., Duncan A., Mackenzie I., Barr R., Pigazzani F., Zutis K. Adverse events and overall health and well-being after Covid-19 vaccination: interim results from the Vac4covid cohort safety study. BMJ Open. 2022;12 doi: 10.1136/bmjopen-2021-060583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alvergne, A., Kountourides, G., Argentieri, A., Agyen, L., Rogers, N., Knight, D., Sharp, G.C., Maybin, J.A. & Olszewska, Z. 2021. [DOI] [PMC free article] [PubMed]
- 26.Sualeh, M., Uddin, M.R., Junaid, N., Khan, M. & Pario, A. 2022. Impact Of Covid-19 Vaccination On The Menstrual Cycle: A Cross-Sectional Study From Karachi, Pakistan. [DOI] [PMC free article] [PubMed]
- 27.Dar-Odeh N., Abu-Hammad O., Qasem F., Jambi S., Alhodhodi A., Othman A., Abu-Hammad A., Al-Shorman H., Ryalat S., Abu-Hammad S. Long-term adverse events of three Covid-19 vaccines as reported by vaccinated physicians and dentists, a study from Jordan and Saudi Arabia. Hum Vaccin Immunother. 2022;18 doi: 10.1080/21645515.2022.2039017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trogstad, L. 2022. Increased Occurrence Of Menstrual Disturbances In 18-To 30-Year-Old Women After Covid-19 Vaccination. Available At Ssrn 3998180.
- 29.Anjorin A.A., Odetokun I.A., Nyandwi J.B., Elnadi H., Awiagah K.S., Eyedo J., Abioye A.I., Gachara G., Maisara A.M., Razouqi Y. Public health surveillance for adverse events following Covid-19 vaccination in Africa. Vaccines. 2022;10:546. doi: 10.3390/vaccines10040546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarfraz A., Sarfraz Z., Sarfraz M., Nadeem Z., Felix M., Cherrez-Ojeda I. Menstrual irregularities following Covid-19 vaccination: a global cross-sectional survey. Ann Med Surg (Lond) 2022;81 doi: 10.1016/j.amsu.2022.104220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sani, K., Lachyan, A.S. & Simon, N.H. 2021. Systematic Review On Prevalence Of Menstrual Disorders Among Women.
- 32.Samani R.O., Hashiani A.A., Razavi M., Vesali S., Rezaeinejad M., Maroufizadeh S., Sepidarkish M. The Prevalence Of Menstrual Disorders In Iran: A Systematic Review And Meta-Analysis. 2018;16:665. [PMC free article] [PubMed] [Google Scholar]
- 33.Lebar V., Laganà A.S., Chiantera V., Kunič T., Lukanović D. The effect of Covid-19 on the menstrual cycle: a systematic review. J Clin Med. 2022;11(13):3800. doi: 10.3390/Jcm11133800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Puca E., Puca E. Premature ovarian failure related to SARS-CoV-2 Infection. J Med Cases. 2022;13(4):155–158. doi: 10.14740/jmc3791. Epub 2022 Mar 25. PMID: 35464328; PMCID: PMC8993449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suzuki S., Hosono A. No association between Hpv vaccine and reported post-vaccination symptoms in Japanese young women: results of the Nagoya study. Papillomavirus Res. 2018;5:96–103. doi: 10.1016/j.pvr.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagma S., Kapoor G., Bharti R., Batra A., Batra A., Aggarwal A., Sablok A. To evaluate the effect of perceived stress on menstrual function. J Clin Diagn Res. 2015;9 doi: 10.7860/JCDR/2015/6906.5611. Qc01-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valsamakis G., Chrousos G., Mastorakos G. Stress, female reproduction and pregnancy. Psychoneuroendocrinology. 2019;100:48–57. doi: 10.1016/j.psyneuen.2018.09.031. [DOI] [PubMed] [Google Scholar]
- 38.Male V. Menstrual changes after Covid-19 vaccination. Bmj. 2021;374:N2211. doi: 10.1136/Bmj.N2211. [DOI] [PubMed] [Google Scholar]
- 39.Girardi G., Bremer A.A. Scientific evidence supporting coronavirus disease 2019 (Covid-19) vaccine efficacy and safety in people planning to conceive or who are pregnant or lactating. Obstet Gynecol. 2022;139:3–8. doi: 10.1097/AOG.0000000000004636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Skelly D.T., Harding A.C., Gilbert-Jaramillo J., Knight M.L., Longet S., Brown A., Adele S., Adland E., Brown H., Tipton T., Stafford L., Mentzer A.J., Johnson S.A., Amini A., Tan T.K., Schimanski L., Huang K.A., Rijal P., Frater J., Goulder P., Conlon C.P., Jeffery K., Dold C., Pollard A.J., Sigal A., De Oliveira T., Townsend A.R., Klenerman P., Dunachie S.J., Barnes E., Carroll M.W., James W.S. Two doses Of Sars-Cov-2 vaccination induce robust immune responses to emerging Sars-Cov-2 variants of concern. Nat Commun. 2021;12:5061. doi: 10.1038/s41467-021-25167-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heinz F.X., Stiasny K. Distinguishing features of current Covid-19 vaccines: knowns and unknowns of antigen presentation and modes of action. NPJVaccin. 2021;6:104. doi: 10.1038/s41541-021-00369-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monin L., Whettlock E.M., Male V. Immune responses in the human female reproductive tract. Immunology. 2020;160:106–115. doi: 10.1111/imm.13136. [DOI] [PMC free article] [PubMed] [Google Scholar]