Abstract
We investigated self-rating of cognitive task performance (self-appraisal) and the difference between self-rating and actual task performance (appraisal discrepancy) in cognitively healthy older adults and their relationship with cortical thickness and Alzheimer’s disease (AD) biomarkers, amyloid and tau. All participants (N = 151) underwent neuropsychological testing and 1.5T structural magnetic resonance imaging. A subset (N = 66) received amyloid-PET with [11C] PiB and tau-PET with [18F] Flortaucipir. We found that worse performers had lower self-appraisal ratings, but still overestimated their performance, consistent with the Dunning-Kruger effect. Self-appraisal rating and appraisal discrepancy revealed distinct relationships with cortical thickness and AD pathology. Greater appraisal discrepancy, indicating overestimation, was related to thinning of inferior-lateral temporal, fusiform, and rostral anterior cingulate cortices. Lower self-appraisal was associated with higher entorhinal and inferior temporal tau. These results suggest that overestimation could implicate structural atrophy beyond AD pathology, while lower self-appraisal could indicate early behavioral alteration due to AD pathology, supporting the notion of subjective cognitive decline prior to objective deficits.
Keywords: Metacognition, Subjective cognitive decline, PET, Tau, Cortical thickness
1. Introduction
The awareness and monitoring of one’s own cognitive ability or thought processes (Flavell, 1976; Brown, 1978; Cosentino and Stern, 2005) is often impaired in patients with Alzheimer’s disease (AD) (Starkstein, 2014; Rosen et al., 2014; Hallam et al., 2020). Two pathologic features of AD are β-amyloid (Aβ) accumulation and hyperphosphorylated tau aggregation. Both pathologies typically occur decades before the onset of symptoms (Jack et al., 2013). In addition, cortical atrophy in regions affected by AD may be identified almost a decade before reaching the symptomatic stage (Dickerson et al., 2011). Understanding an individual’s perception of cognitive ability and their metacognitive accuracy might provide insights into the underlying mechanisms of cognitive change in aging and age-related pathology.
The self-perceived concerns of reduced cognition or memory in individuals without objective cognitive impairment is termed subjective cognitive decline (SCD) (Jessen et al., 2014). Although SCD is more extensively discussed in the context of early AD (Jessen et al., 2020) and is proposed as a feature of the earliest detectable behavioral alteration in the AD continuum according to the National Institute of Aging-Alzheimer’s Association research framework (Jack et al., 2018), it is common in the aging population (van Harten et al., 2018). It is often determined with questionnaires qualitatively assessing general cognition and overall cognitive or memory complaints (Jessen et al., 2014; La Joie et al., 2016; Perrotin et al., 2017; Chen et al., 2019; Morrison et al., 2021). Previous literature has shown that SCD is associated with altered brain structure (Fan et al., 2018) and function (Chen et al., 2021) as well as Aβ (Perrotin et al., 2017) and tau (Swinford et al., 2018) deposition and hypometabolism (Mosconi et al., 2008; for review, see Wang et al., 2020). However, not all clinically normal elderly whose memory and cognitive functions are actually declining report SCD, and not all individuals with SCD show signs of AD. Traditional neuropsychological methods are not very sensitive to detecting early behavioral deficits in preclinical AD due to individual variability in performance (Saxton et al., 2004), and quantitative measures of metacognition have not been widely applied to investigations of SCD (Molinuevo et al., 2017). Therefore, quantitatively examining metacognitive ability and accuracy might give us a new way of identifying and understanding SCD in cognitively healthy individuals.
To quantitatively measure self-perception of cognitive performance, previous studies have used self-appraisal percentile rating of performance on specific neuropsychological tasks, which allows for a calculation of an appraisal discrepancy score from actual performance to assess metacognition. This has been applied to studies of neurodegeneration (Eslinger et al., 2005; Massimo et al., 2013; Rosen et al., 2010), adults with attention deficit hyperactivity disorder (Butzbach et al., 2021), healthy and neuropsychologically at risk adults (Rothlind et al., 2016), and in children (Krueger et al., 2011). This method of examining task-specific self-appraisal of cognition offers not only insights to immediate, task-related subjective judgment processes that might not be accessed by questionnaires, but also the discrepancy between subjective and objective performance indexing metacognitive precision. The present study utilizes this method of assessing self-appraisal and appraisal discrepancy and investigates their relationships with cortical thickness and AD biomarkers. We aimed to explore how disease pathology might be manifested in the subjective assessment of an individual’s ability at a time when objective impairment was not apparent.
The goals of the present study were to examine (1) the behavioral characteristics related to metacognition, (2) the structural correlates of metacognition, and (3) the relationship between AD biomarkers of Aβ and tau and metacognition in a sample of cognitively healthy older adults. Given that the self-appraisal rating is a subjective judgment of cognitive function and measures similar constructs as what underlie SCD (Rabin et al., 2015), we hypothesized that we would find a relationship between self-appraisal and AD biomarker pathology. On the other hand, as previous literature has demonstrated individual differences in brain correlates of metacognition in other neurological and psychiatric disorders (David et al., 2012; Fleming and Dolan, 2012; Quattrini et al., 2019; Alkan et al., 2020), we hypothesize that appraisal discrepancy, measuring metacognitive accuracy, would be related to structural differences in cortical thickness. In addition, since lower self-appraisal may reflect depression besides memory complaints (Buckley et al., 2013; Grambaite et al., 2013; Hollands et al., 2015), we controlled for subclinical depressive symptoms to further de-lineate the relationships.
2. Material and methods
2.1. Participants
A total of 151 cognitively healthy older adults from the Berkeley Aging Cohort Study (BACS) were included in the study. The BACS is an ongoing longitudinal investigation of cognitive and brain changes in cognitively healthy individuals. All participants included in this study were over age 65, had mini mental state examination (MMSE) score ≥ 25, had neuropsychological testing data including subjective ratings of their performance, and received 1.5T structural magnetic resonance (MRI) imaging. Sixty six of the 151 participants later received positron emission tomography (PET) imaging for Aβ with 11C-Pittsburgh compound B (PiB) and tau with 18F-Flortaucipir (FTP). In our study, all participants at the time of enrolment were living independently, had no major systemic disease, neurologic or psychiatric disorder, or history of substance abuse, and perform within the normal range. The study was approved by the Institutional Review Board at Lawrence Berkeley National Laboratory (LBNL) and the University of California, Berkeley. Written, informed consent was obtained from all study participants.
2.2. Neuropsychological assessment
All participants underwent a standard neuropsychological testing session that assessed episodic memory, working memory, visuospatial ability, executive function, and language. Subclinical depressive symptoms were assessed with the geriatric depression scale (GDS) (Yesavage et al., 1983). For analyses using MRI data to examine cortical thickness and metacognition, the neuropsychological testing session was within 6 months of the MRI scan. For analyses using PET data to examine AD biomarkers and metacognition, the neuropsychological testing session was within 6 months of the tau PET scan.
For the present study, we included 6 cognitive measures that had corresponding online self-appraisal ratings: long delay free recall of the California Verbal Learning Test (CVLT) (Delis et al., 2000), delayed recall of Visual Reproduction (Wechsler, 1997), Digit Span (Wechsler, 1997), number correct in 60 seconds of the Stroop test (Golden, 1978), Verbal Fluency (Spreen and Benton, 1977), and Category Fluency. For each task, raw scores were converted to z-scores and then percentile frequencies on a normal distribution. An average objective performance score (in percentile) was calculated by taking the mean of all 6 task performance percentiles for each participant.
Two subjective variables were included in the study: self-appraisal rating and appraisal discrepancy. We used an online performance monitoring paradigm (Perrotin et al., 2012) to assess participants’ metacognitive ability and accuracy. A post-diction self-appraisal rating was recorded after each of the above 6 tasks. The participant was asked to estimate their task performance on a percentile scale relative to their peers of the same age, sex, and education. An average self-appraisal rating (in percentile) was computed for each participant by taking the mean of all 6 task self-appraisal ratings. A higher self-appraisal rating indicates better self-perceived performance.
An appraisal discrepancy score was calculated for each task by subtracting the task objective performance score from the participant’s self-appraisal rating. An average appraisal discrepancy score was calculated by taking the mean of all appraisal discrepancy scores of the 6 tasks. A positive appraisal discrepancy score indicates an overestimation of performance; a negative score indicates underestimation of performance. A score of 0 represents perfect accuracy.
2.3. MRI acquisition and preprocessing
T1-weighted magnetization-prepared rapid gradient acquisition with gradient echo structural MRI scans were acquired for all participants with a 1.5T Siemens Magnetom Avanto scanner at LBNL (voxel size = 1 mm isotropic, repetition time = 2110 milli seconds, echo time = 3.58 milli seconds, flip angle = 15°). All T1 magnetization-prepared rapid gradient acquisition with gradient echo scans were processed using FreeSurfer version 5.3 (https://surfer.nmr.mgh.harvard.edu). Separate MRIs were obtained for the measurement of cortical thickness, and for parcellating the brain for PET data analysis.
2.4. PET acquisition and preprocessing
Details of PiB and FTP-PET acquisition were published previously (Ossenkoppele et al., 2016; Schöll et al., 2016). PiB and FTP were synthesized at the Biomedical Isotope Facility at LBNL and all PET scans were acquired on a Biograph 6 Truepoint PET/ computed tomography scanner in 3D acquisition mode. Prior to PET scanning, a computed tomography scan was collected for attenuation correction. Participants were injected with 15 mCi of PiB. 90 minutes of dynamic emission data were acquired and then binned into 35 frames (4 × 15 seconds, 8 × 30 seconds, 9 × 60 seconds, 2 × 180 seconds, 10 × 300 seconds, and 2 × 600 seconds). For tau PET, participants were injected with 10 mCi of FTP and data acquired from 80 to 100 minutes post-injection were binned as 4 × 5 minute frames. PET images were reconstructed using an ordered subset expectation maximization algorithm with weighted attenuation and scatter correction and smoothed with a 4 mm Gaussian kernel. PIB and FTP PET were usually performed on the same day.
For PiB-PET data processing, distribution volume ratio (DVR) values were generated with Logan graphical analysis by calculating the slope from 35 to 90 minutes post-injection and normalized using the cerebellar gray matter as reference region (Logan et al., 1996; Price et al., 2005). A global PiB index was calculated using multiple FreeSurfer derived regions of interests (ROIs) as previously described (Mormino et al., 2012), averaging the frontal, temporal, parietal, and posterior cingulate ROIs. A global PiB DVR threshold for this composite ROI of 1.065 was used to determine PiB positivity (Villeneuve et al., 2015).
For FTP-PET data processing, standardized uptake value ratio (SUVR) images were created based on the mean tracer retention from 80 to 100 minutes post-injection and normalized by the mean tracer retention in the inferior cerebellar gray matter. SUVR images were partial volume corrected using the Rousset Geometric Transfer Matrix approach as previously described (Rousset et al., 1998; Baker et al., 2017). We focused on 2 FreeSurfer parcellated ROIs, entorhinal cortex and inferior temporal cortex, and took the average of the regional FTP SUVR of the left and right hemispheres to create the mean regional FTP SUVR used in the analyses.
2.5. Statistical analyses
Statistical analyses were conducted using R (https://www.R-project.org) for cognitive and regional PET data, and FreeSurfer was used for analysis of whole-brain MRI data. First, associations between objective performance and subjective measures (self-appraisal, appraisal discrepancy) were explored using Pearson’s correlation. Then, for self-appraisal, appraisal discrepancy, as well as objective cognitive performance, 3 whole brain vertex-wise analyses were conducted to examine their relationship to cortical thickness, using general linear models in FreeSurfer and adjusting for age, sex, education, and GDS. Multiple comparison correction was applied using Monte Carlo Simulation, with both a liberal p value set at p < 0.01 and a stricter p value set at p < 0.001. Finally, multiple regressions were used to investigate the relationship between AD biomarker pathology and measures of self-appraisal/appraisal discrepancy as well as objective cognitive performance. All models were adjusted for age, sex, education, and GDS, and all predictors were mean centered to minimize multi-collinearity; in these models PiB × FTP interactions were also included because of the well-known acceleration of Aβ on tau effects (Hanseeuw et al., 2019). For models with significant PiB × FTP interactions, we used the Johnson-Neyman procedure to identify the range of global PiB DVR values where regional FTP SUVR had a significant effect on the dependent measures (Johnson and Fay, 1950; Aiken et al., 1991).
3. Results
3.1. Demographics
Participants’ demographic characteristics are presented in Table 1. A total of 151 participants (89 female, 62 male) were included in the study, with a mean age of 76.09 years (SD 5.60) and an average of 16.79 years of education (SD 1.92). In the subset of 66 participants who received PET scans, 34 were PiB− and 32 were PiB+, reflecting our efforts to retain PIB+ participants in the study. The PiB+ group was significantly younger than the PiB− group (p = 0.029) but there were no significant group differences in sex, years of education, GDS, MMSE, and neuropsychological tasks performance. There were no significant differences in any variables between the MRI only group and the PET group (all p > 0.1)
Table 1.
Demographics
| MRI sample, N = 151 | Total N = 66 | PET sample | ||||
|---|---|---|---|---|---|---|
| PiB-negative, N = 34 | PiB-positive, N = 32 | Effect size | p value (PiB+ vs. PiB–) | |||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |||
| Sex = male (%) | 62 (41.1) | 29 (43.9) | 13 (38.2) | 16 (50.0) | V = 0.09 | 0.475 |
| Age (y) | 76.09 (5.60) | 78.36 (5.29) | 79.74 (6.06) | 76.91 (3.90) | d = 0.55 | 0.029 |
| APOE ε4+ (n [%]) | 43/149 (29%) | 20/66 (30%) | 3/34 (8.8) | 17/32 (53.1) | V = 0.45 | <0.001 |
| Education (y) | 16.79 (1.92) | 16.83 (1.78) | 17.15 (1.62) | 16.50 (1.90) | d = 0.37 | 0.14 |
| GDS | 3.32 (2.78) | 3.26 (3.15) | 3.65 (3.42) | 2.84 (2.83) | d = 0.26 | 0.304 |
| MMSE | 28.69 (1.35) | 28.73 (1.25) | 29.00 (0.89) | 28.44 (1.50) | d = 0.46 | 0.067 |
| CVLT LDFR | 10.42 (3.27) | 10.09 (3.60) | 10.56 (3.72) | 9.59 (3.46) | d = 0.27 | 0.28 |
| VRII recall | 53.52 (21.84) | 53.21 (20.02) | 52.74 (21.35) | 53.72 (18.84) | d = −0.05 | 0.844 |
| Digit span | 16.11 (3.81) | 15.56 (3.93) | 16.24 (4.40) | 14.84 (3.29) | d = 0.36 | 0.152 |
| Stroop correct in 60 s | 46.49 (11.43) | 47.88 (10.06) | 48.68 (10.73) | 47.03 (9.39) | d = 0.16 | 0.511 |
| Verbal fluency | 46.04 (12.28) | 47.55 (11.27) | 49.38 (11.09) | 45.59 (11.31) | d = 0.34 | 0.174 |
| Category fluency | 34.05 (8.40) | 33.09 (8.05) | 33.71 (8.45) | 32.44 (7.69) | d = 0.16 | 0.527 |
| COG-MRI interval (d) | 89.84 (44.10) | |||||
| COG-FTP interval (d) | 87.17 (48.35) | 84.88 (48.02) | 89.59 (49.34) | d = −0.10 | 0.696 | |
| MRI-FTP interval (d)* | 1030.94 (1020.37) | 1016.74 (1083.98) | 1046.03 (965.29) | d = −0.03 | 0.908 | |
| PiB-positive (%) | 32 (48.5) | |||||
Key: APOE, apolipoprotein E; GDS, geriatric depression scale; MMSE, mini-mental state examination; MRI, magnetic resonance imaging; CVLT LDFR, California verbal learning test long delay free recall; VRII, visual reproduction long delay; FTP, Flortaucipir; V, Cramér’s V; d, Cohen’s d.
Time between MRI obtained for cortical thickness and FTP PET scan.
3.2. Self-appraisal, appraisal discrepancy, and neuropsychological task performance
First, we assessed the relationship between self-appraisal, appraisal discrepancy, and objective neuropsychological task performance. Average task performance had a significant positive association with average self-appraisal ratings (r = 0.323, p < 0.001) and a significant negative association with average appraisal discrepancy (r = −0.621, p < 0.001) (Fig. 1). Thus, individuals with better overall task performance had higher self-appraisal but nevertheless underestimated their performance, whereas those with worse overall task performance had lower self-appraisal but overestimated their performance. In addition, while there were no significant sex differences in self-appraisal rating, we found that male participants had significantly worse performance than female participants in CVLT long delay free recall (p < 0.001), verbal fluency (p = 0.002), and category fluency (p < 0.001) tasks. As a result, male participants had significantly greater overestimation in CVLT long delay free recall (p < 0.001), verbal fluency (p = 0.002), and category fluency (p = 0.013) tasks.
Fig. 1.
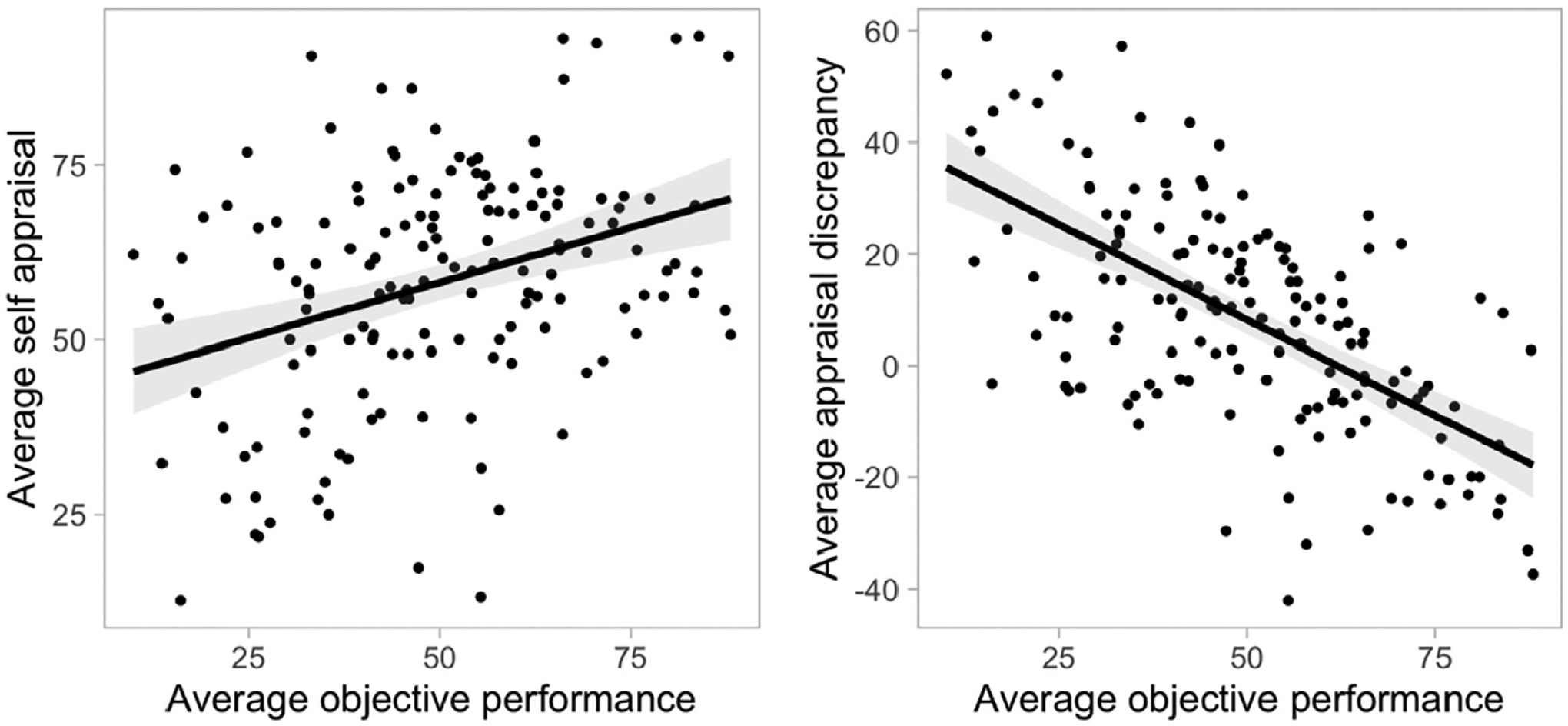
Relationship between self-appraisal/appraisal discrepancy and neuropsychological task performance. Better objective performance was related to higher self-appraisal and lower appraisal discrepancy.
3.3. Self-appraisal, appraisal discrepancy, and cortical thickness
Next, we explored the relationship between self-appraisal rating, appraisal discrepancy, and cortical thickness, while controlling for age, sex, education, and GDS. The vertex-wise whole-brain cortical thickness analysis revealed a significant negative relationship between average appraisal discrepancy and cortical thickness (Table 2, Fig. 2A, Supplementary Fig. 1A). More overestimation of performance was associated with thinner cortex predominantly in temporal lobe regions, specifically including bilateral inferior temporal, parahippocampal, fusiform, and right superior temporal, as well as inferior parietal cortices and anterior cingulate (p < 0.01, Monte-Carlo simulation). After applying a stricter threshold of Monte-Carlo simulation at p < 0.001, remaining significant clusters include bilateral parahippocampal, left inferior temporal and inferior parietal, and right superior temporal cortices (Table 2). There was no positive association between appraisal discrepancy and cortical thickness. No association was found between self-appraisal ratings and cortical thickness.
Table 2.
Results of vertex-wise whole brain cortical thickness analyses examining the relationship between cortical thickness and appraisal discrepancy at a liberal and a conservative threshold of Monte Carlo simulation correction for multiple comparisons
| Max | VtxMax | Size(mm^2) | TalX | TalY | TalZ | CWP | CWPLow | CWPHi | NVtxs | WghtVtx | Region |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Liberal threshold, Monte Carlo simulation at p < 0.01 | |||||||||||
| Left hemisphere | |||||||||||
| −3.924 | 130,706 | 1106.81 | −52.1 | −26.4 | −17.5 | 0.0001 | 0 | 0.0002 | 1626 | −4654.2 | Inferior temporal |
| −3.741 | 53,302 | 926.14 | −38.7 | −47.3 | −13.5 | 0.0001 | 0 | 0.0002 | 1491 | −3760.65 | Fusiform |
| −4.918 | 9460 | 715.14 | −39.6 | −60.8 | 23.5 | 0.0014 | 0.0009 | 0.0019 | 1334 | −3681.99 | Inferior parietal |
| −5.641 | 120,176 | 665.22 | −47.5 | −47.7 | 18 | 0.0029 | 0.0022 | 0.0036 | 1474 | −4500.41 | Inferior parietal |
| −5.17 | 31,775 | 448.68 | −23 | −19.1 | −23.7 | 0.034 | 0.0317 | 0.0363 | 928 | −2887.39 | Parahippocampal |
| Right hemisphere | |||||||||||
| −3.561 | 70,560 | 1570.56 | 52.8 | −27.8 | −22.1 | 0.0001 | 0 | 0.0002 | 2448 | −5992.8 | Inferior temporal |
| −5.039 | 88,831 | 927.27 | 36.3 | −32.7 | 17.5 | 0.0001 | 0 | 0.0002 | 2099 | −5713.16 | Superior temporal |
| −3.421 | 149,270 | 707.79 | 13.1 | 27.7 | 28 | 0.0021 | 0.0015 | 0.0027 | 1494 | −3651.81 | Anterior cingulate |
| −4.799 | 127,730 | 451.04 | 24.5 | −18.2 | −23.9 | 0.0361 | 0.0337 | 0.0385 | 847 | −2650.38 | Parahippocampal |
| −2.729 | 53,317 | 444.37 | 28.2 | −51.5 | −10.7 | 0.0394 | 0.0369 | 0.0419 | 859 | −1937 | Fusiform |
| Conservative threshold, Monte Carlo simulation at p < 0.001 | |||||||||||
| Left hemisphere | |||||||||||
| −3.924 | 130,706 | 480.62 | −52.1 | −26.4 | −17.5 | 0.0001 | 0 | 0.0002 | 707 | −2359 | Inferior temporal |
| −5.17 | 31,775 | 249.43 | −23 | −19.1 | −23.7 | 0.0077 | 0.0066 | 0.0088 | 513 | −1851.03 | Parahippocampal |
| −5.641 | 120,176 | 214.52 | −47.5 | −47.7 | 18 | 0.0156 | 0.014 | 0.0172 | 447 | −1862.46 | Inferior parietal |
| Right hemisphere | |||||||||||
| −4.799 | 127,730 | 252.7 | 24.5 | −18.2 | −23.9 | 0.0077 | 0.0066 | 0.0088 | 474 | −1725.98 | Parahippocampal |
| −5.039 | 88,831 | 175.43 | 36.3 | −32.7 | 17.5 | 0.0361 | 0.0337 | 0.0385 | 461 | −1661.23 | Superior temporal |
Key: Max, maximum −log10(p value) in the cluster; VtxMax, vertex number at the maximum; size, surface area (mm2) of cluster; Tal(XYZ), the talairach coordinate of the maximum; CWP, cluster-wise p value; CWPLow and CWPHi, 90% confidence interval for CWP; NVtxs, number of vertices in cluster; WghtVtx, weight of cluster (size × intensity).
Fig. 2.
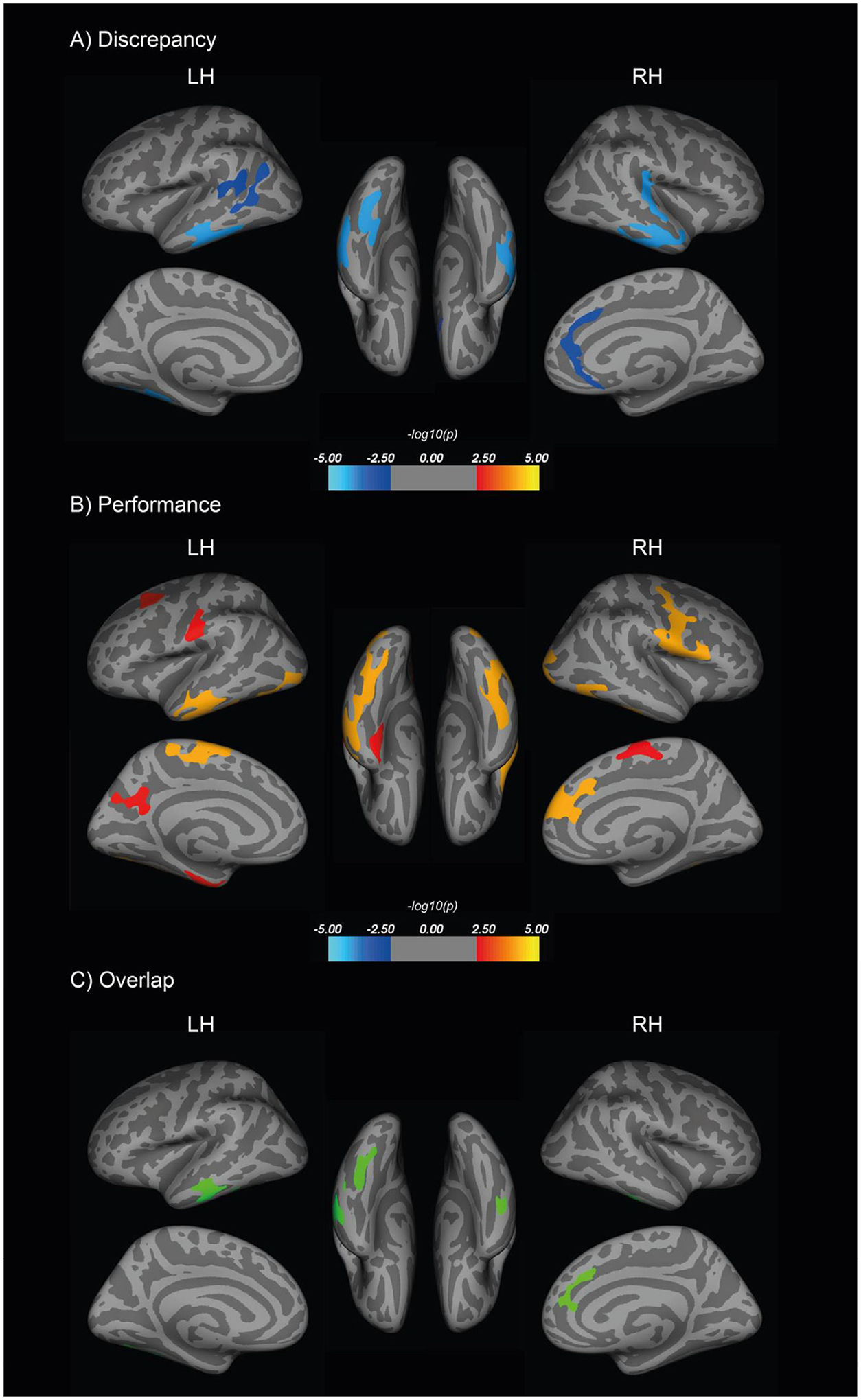
Vertex-wise whole brain analyses were conducted to examine the relationship between cortical thickness and (A) average appraisal discrepancy (overestimation correlated with thinner cortices) and (B) average objective performance, after adjusting for age, sex, education, and GDS (worse objective performance correlated with thinner cortices). The maps shown here used a liberal threshold of cluster-wise Monte Carlo simulation correction at p < 0.01. Overlapping regions are presented in (C). GDS, geriatric depression scale.
We also found a significant relationship between better objective cognitive performance and greater thickness in a wide range of cortices, including bilateral precuneus, left middle temporal, middle frontal, fusiform, superior parietal, paracentral and postcentral, and right inferior temporal, superior frontal, lateral occipital, paracentral, and precentral regions (Fig. 2B, Supplementary Fig. 1B). Next, we applied the discrepancy cortical thickness map (after Monte Carlo simulation correction at p < 0.01) onto the objective cognitive performance cortical thickness map (after Monte Carlo simulation correction at p < 0.01) to generate an overlap of identified cortical regions in common, including bilateral inferior temporal and fusiform, left middle temporal, and right superior frontal and rostral anterior cingulate cortices (Fig. 2C). Cortical thinning in these areas was related to worse objective performance and more overestimation of performance.
3.4. Self-appraisal, appraisal discrepancy, global Aβ, and regional tau
We investigated the relationship between self-appraisal rating, global PiB DVR, and entorhinal or inferior temporal FTP SUVR, while controlling for age, sex, education, and GDS (Table 3, models 1 and 2). Higher entorhinal FTP SUVR was associated with lower self-appraisal (p = 0.018). There was a significant interaction between global PiB DVR and entorhinal FTP SUVR (p = 0.019) (model 1, Fig. 3A and B), such that higher global Aβ level increased the effect of higher entorhinal tau on lower average self-appraisal ratings. The effect of entorhinal FTP SUVR on self-appraisal became significant after global PiB DVR reached a value of 1.11 (corresponding to 16 Centiloids). Higher GDS (p < 0.001) and lower global PiB DVR (p = 0.003) were also significantly associated with lower self-appraisal ratings. After adjusting for objective performance in the model, which was significantly related to self-appraisal (p = 0.021), the main effect of entorhinal tau on self-appraisal was not significant (p = 0.191), but there remained a significant PiB × FTP interaction (p = 0.009) such that in individuals with higher PiB DVR, those with = higher EC tau had lower self-appraisal (Supplementary Table 1, model 1). There was also a significant negative association between average self-appraisal rating and inferior temporal FTP SUVR (p 0.045; model 2, Fig. 3C). In this model, higher GDS (p < 0.001) = and lower PiB DVR (p = 0.037) were significantly related to lower self-appraisal, but there was no significant interaction between global PiB DVR and inferior temporal FTP SUVR on average self-appraisal rating. After adjusting for objective performance, there was no significant effect of IT tau (p = 0.116), but a significant effect of objective performance (p = 0.010; Supplementary Table 1, model 2).
Table 3.
Regression statistics estimating appraisal discrepancy and self-appraisal from EC and IFT FTP and covariates
| Covariates | Estimate | Std. error | t Value | p Value | Sig |
|---|---|---|---|---|---|
| Model 1. Self-appraisal and EC FTP | |||||
| (Intercept) | 0.537 | 2.225 | 0.241 | 0.810 | |
| EC FTP | −20.138 | 8.265 | −2.437 | 0.018 | * |
| Age | 0.653 | 0.335 | 1.950 | 0.056 | † |
| Sex: male | 1.943 | 3.353 | 0.580 | 0.564 | |
| GDS | −1.990 | 0.513 | −3.882 | 0.000 | ‡ |
| Education | −1.300 | 0.918 | −1.415 | 0.162 | |
| PiB DVR | 31.915 | 10.462 | 3.051 | 0.003 | § |
| PiB × FTP | −64.738 | 26.714 | −2.423 | 0.019 | * |
| Model 2. Self-appraisal and IFT FTP | |||||
| (Intercept) | 0.003 | 2.397 | 0.001 | 0.999 | |
| IFT FTP | −24.421 | 11.939 | −2.046 | 0.045 | * |
| Age | 0.548 | 0.342 | 1.603 | 0.114 | |
| Sex: male | 2.292 | 3.582 | 0.640 | 0.525 | |
| GDS | −2.044 | 0.539 | −3.792 | 0.000 | ‡ |
| Education | −1.711 | 0.978 | −1.749 | 0.086 | † |
| PiB DVR | 22.487 | 10.554 | 2.131 | 0.037 | * |
| PiB × FTP | −74.157 | 45.029 | −1.647 | 0.105 | |
| Model 3. Appraisal discrepancy and EC FTP | |||||
| (Intercept) | −4.912 | 2.971 | −1.654 | 0.104 | |
| EC FTP | 15.102 | 11.035 | 1.369 | 0.176 | |
| Age | 0.464 | 0.447 | 1.039 | 0.303 | |
| Sex: male | 15.422 | 4.477 | 3.445 | 0.001 | § |
| GDS | −1.934 | 0.685 | −2.825 | 0.006 | § |
| Education | −3.497 | 1.226 | −2.852 | 0.006 | § |
| PiB DVR | 21.578 | 13.969 | 1.545 | 0.128 | |
| PiB × FTP | −86.759 | 35.667 | −2.432 | 0.018 | * |
| Model 4. Appraisal discrepancy and IFT FTP | |||||
| (Intercept) | −6.070 | 3.159 | −1.922 | 0.060 | † |
| IFT FTP | −1.992 | 15.733 | −0.127 | 0.900 | |
| Age | 0.684 | 0.451 | 1.517 | 0.135 | |
| Sex: male | 15.555 | 4.721 | 3.295 | 0.002 | § |
| GDS | −2.097 | 0.710 | −2.953 | 0.005 | § |
| Education | −3.364 | 1.289 | −2.611 | 0.011 | * |
| PiB DVR | 17.878 | 13.908 | 1.285 | 0.204 | |
| PiB × FTP | −56.129 | 59.339 | −0.946 | 0.348 | |
Key: EC, entorhinal cortex; IT, inferior temporal; FTP, Flortaucipir; SUVR, standardized update value ratio; GDS, geriatrics depression scale; PiB, Pittsburg compound B; DVR, distribution volume ratio.
p < 0.05.
p < 0.1.
p < 0.001.
p < 0.01.
Fig. 3.
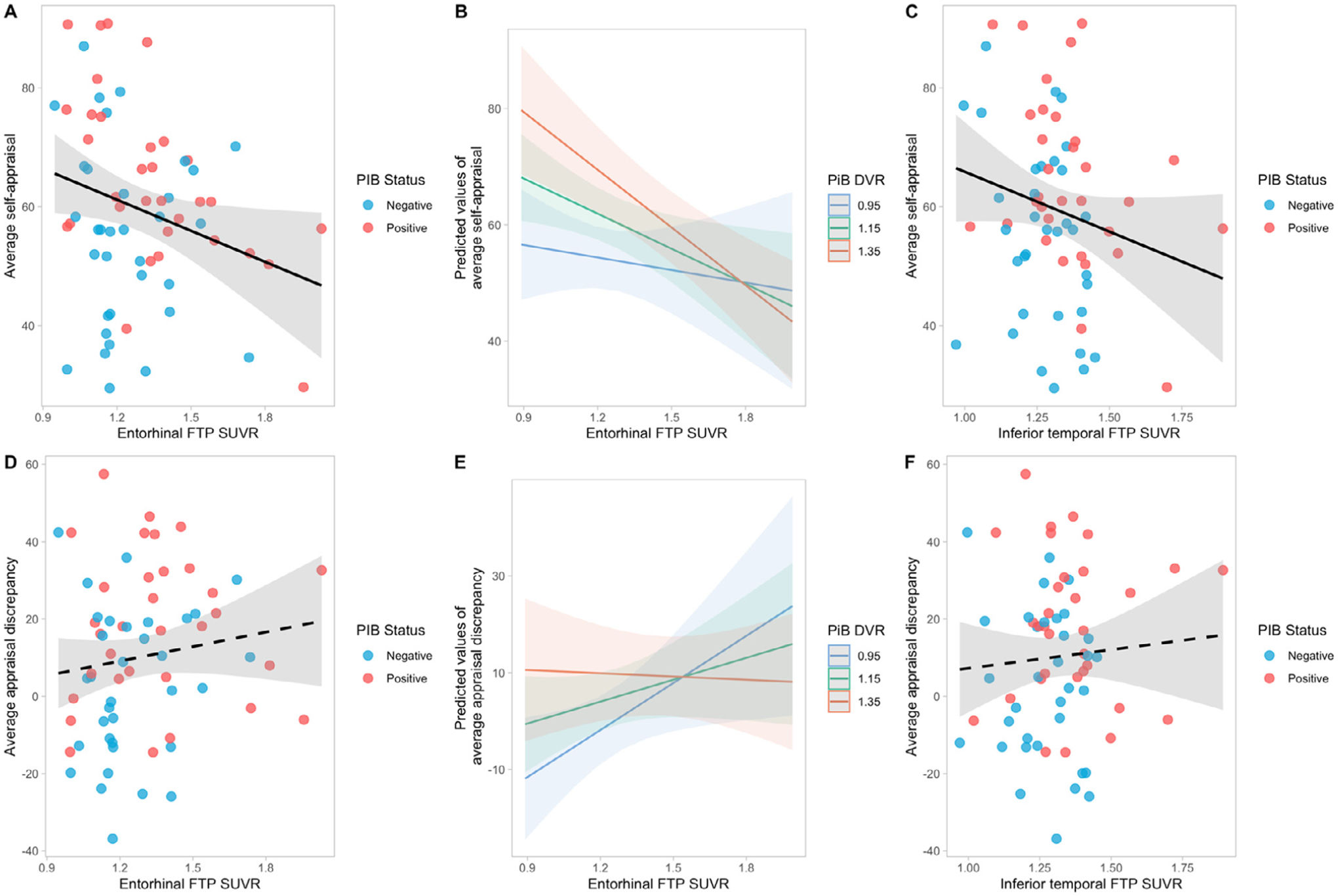
Relationship between self-appraisal/appraisal discrepancy and AD biomarkers, adjusting for age, sex, education, and GDS. Higher entorhinal FTP SUVR was significantly associated with lower average self-appraisal ratings (A), especially in individuals with higher global PiB DVR (B). Higher inferior temporal FTP SUVR was related to lower average self-appraisal ratings (C) but there was no significant interaction between global PiB DVR and inferior temporal FTP SUVR. (D) shows the relationship between entorhinal FTP SUVR and average appraisal discrepancy. There was a significant interaction between global PiB DVR and entorhinal FTP SUVR: higher entorhinal tau was related to greater average appraisal discrepancy (overestimation) but in individuals with lower amyloid level; this interaction is unlikely to be biologically significant (see text) (E). There was no significant association between inferior temporal FTP SUVR and average appraisal discrepancy (F). FTP. Flortaucipir; SUVR, standardized update value ratio; GDS, geriatrics depression scale; PiB, Pittsburg compound B; PiB positivity DVR threshold, 1.065.
Next, we examined the association of appraisal discrepancy, global PiB DVR, and entorhinal/inferior temporal FTP SUVR, while controlling for the same covariates (Table 3, models 3 and 4). There was no significant main effect of entorhinal FTP SUVR on appraisal discrepancy, but a statistically significant interactive effect between global PiB DVR and entorhinal FTP SUVR (p = 0.018) (Fig. 3D and E). We found that the effect of entorhinal FTP SUVR on appraisal discrepancy was predicted to become significant only if global PiB DVR was lower than 1.02 or greater than 1.94. Given the global PiB DVR range 0.98–1.89 in our sample, it appears that this interaction was likely to be irrelevant across the majority of PiB values. In addition, being a male (p = 0.002), lower GDS score (p = 0.005), and lower education (p = 0.006) were also found to be significantly associated with a tendency towards overestimation. In the inferior temporal tau model, no significant associations were found between average appraisal discrepancy and inferior temporal FTP SUVR, and there was no interactive effect between global PiB DVR and inferior temporal FTP SUVR on average appraisal discrepancy. However, the significant effects of sex (p = 0.002), GDS (p = 0.005), and education (p = 0.011) remained (model 4, Fig. 3F).
Finally, we examined the relationship between average objective performance and AD biomarkers while adjusting for the same covariates (Supplementary Table 2). Higher entorhinal FTP SUVR was significantly related to lower average objective performance (p = 0.001, Supplementary Fig. 2A) but no significant interaction between global PiB DVR and entorhinal FTP SUVR was found. There was a negative, but statistically insignificant relationship between inferior temporal FTP SUVR and performance (p = 0.140, Supplementary Fig. 2B).
4. Discussion
In this study, we investigated the relationships between metacognition and objective performance, brain structure, and AD pathology in a community sample of cognitively healthy older individuals. We used an online self-appraisal rating directly recording participants’ estimation of task performance as a measure of subjective judgment and an appraisal discrepancy calculated to quantify the extent of over- and underestimation of task performance as a measure of metacognitive accuracy. We found that worse performers tended to have lower self-appraisal yet still overestimated their cognitive performance, consistent with the no-table Dunning-Kruger effect where individuals with worse abilities tended to overestimate their performance and ability (Kruger and Dunning, 1999). While male and female participants did not differ in self-appraisal ratings, male participants had greater overestimation in verbal tasks due to low performance, consistent with previous reports (Colvin et al., 2018; Asperholm et al., 2019). The 2 measures revealed distinct associations with brain morphology and AD biomarkers. Greater appraisal discrepancy, indicating overestimation of performance, was related to cortical thinning in temporal, anterior cingulate, and parietal cortices. These regions overlapped, to some extent, with brain regions where cortical thinning was related to worse objective performance. However, appraisal discrepancy did not have apparent relationships with regional tau or global Aβ. On the other hand, self-appraisal as a measure of subjective awareness was sensitive to AD pathology, but not cortical atrophy. Specifically, lower self-appraisal ratings related to higher inferior temporal tau, as well as higher entorhinal tau in individuals with higher global Aβ deposition. This suggests that even in cognitively healthy older adults, structural differences in temporal, parietal, and medial regions may underlie inaccurate metacognitive appraisal, and that higher AD pathology could be manifested by lower self-appraisal ratings. Furthermore, affective features do not appear to be driving our results for self-appraisal and appraisal discrepancy because these results persist despite GDS adjustment.
Previous research has shown that impaired metacognitive ability is associated with reduced right posterior cingulate and medial prefrontal cortical thickness in a cognitively diverse sample of older adults (Bertrand et al., 2018). Here, we report that in cognitively healthy older individuals, worse objective performance and greater overestimation of performance were related to cortical thinning in inferior-lateral temporal and fusiform and rostral anterior cingulate cortices. Young and older adults with cortical thinning in these areas show reduced memory performance (Busovaca et al., 2016), and may also have worse metacognitive monitoring (Kruger and Dunning, 1999). Decreased cortical thickness of medial and inferior temporal gyri has been shown to be related to increased risk and progression towards AD (Dickerson et al., 2009). Moreover, inferior temporal and fusiform regions form part of the anterior-temporal network, which is particularly vulnerable to early tau deposition (Lowe et al., 2018; Maass et al., 2019), and inferior temporal tauopathy is associated with cognitive impairment (Johnson et al., 2016). We also found greater overestimation to be related to parahippocampal thinning, an area involved in retrospective metamemory judgements (Vaccaro and Fleming, 2018). However, areas where reduced cortical thickness was associated with appraisal discrepancy in our study are not limited to the memory domain. Fusiform morphology is implicated in metacognitive assessment of motor functions. A previous study examining metacognitive ability with a visuomotor task in healthy adults found that higher grey matter volume in the right fusiform gyrus was associated with greater metacognitive sensitivity (Sinanaj et al., 2015). A recent study found that in young adults, greater left fusiform grey matter volume was related to greater overestimation of handwriting quality, another visuomotor function (Li et al., 2021). These findings suggest that while appraisal discrepancy is related to frontal and temporal lobe cortical atrophy, this measure is not specific to AD pathology but rather reflects overall decreased metacognition and functions beyond memory.
The right anterior cingulate has been demonstrated to be involved in error detection (Carter, 1998) and error information processing (Holroyd et al., 2004). A functional study of healthy young adults found that the anterior cingulate cortex is implicated in the metacognition mismatch of a person’s confidence and actual performance, similar to the appraisal discrepancy measure in the present study (Metcalfe et al., 2012). The prefrontal and posterior cingulate cortex are involved in self-reflective thought processes (Johnson et al., 2002). Several studies have found the right midline structures to be involved in self-awareness processes (Bertrand et al., 2018; Muñoz-Neira et al., 2019). Moreover, higher levels of anosognosia correlated with greater atrophy and hypometabolism in the left dorsal anterior cingulate in AD patients (Guerrier et al., 2018). Decreased grey matter volume in anterior cingulate and fusiform is related to anosognosia in memory and non-memory domains in mild cognitive impairment (MCI) and AD patients (Valera-Bermejo et al., 2020). Together, these structural findings suggest that as these brain regions become more atrophic in aging, objective performance deteriorates, self-appraisal ability worsens, and error monitoring capability declines.
In contrast to appraisal discrepancy, we found effects of AD pathology on self-appraisal rating: both higher entorhinal and inferior temporal tau were associated with lower self-appraisal ratings. Consistent with a previous finding (Buckley et al., 2017), decreased self-appraisal of performance was related to increased entorhinal tau burden. We also found an interaction between entorhinal tauopathy and global Aβ level on self-appraisal with higher Aβ exacerbating the tau effect, an effect not observed in Buckley et al (2017). One possible explanation is that even though both studies included cognitively healthy older adults and investigated cognitive deficit awareness, the main variable of interest in our study tapped into the online monitoring and appraisal process, rather than global subjective cognitive concerns, which may be more sensitive to more progressed tau pathology. This is partly supported by the non-significant interactive effect in the inferior tau model: high tau buildup in the inferior temporal lobe is already associated with positive global Aβ deposition (Sanchez et al., 2021) and that Aβ threshold might not play a significant role in this relationship. Controlling for objective performance in the models revealed that lower objective performance played a role in the effect of tau on lower self-appraisal. However, higher EC tau in high-amyloid individuals was still related to lower self-appraisal, suggesting that the self-appraisal, independent of objective performance, was related to tauopathy in EC. This suggests that lower self-perceived cognition could be an early indicator of more pronounced AD pathology in the cognitively healthy. While the self-appraisal, online measure in the current study differs from traditional general ratings of subjective cognition usually incorporated into SCD definitions, it appears to provide a similar measure that may tap into an individual’s self-perception of decline that might not be available to outside observers.
Our findings add to the previous studies investigating AD biomarkers and memory awareness in individuals with MCI (Therriault et al., 2018), anosognosia (Guerrier et al., 2018), and unimpaired individuals (Vogel et al., 2017; Buckley et al., 2017; Chen et al., 2019; Vannini et al., 2019; Gagliardi et al., 2020; d’Oleire Uquillas et al., 2020), by quantitively examining subjective appraisal, appraisal accuracy, and their relations to AD pathology and cortical structure. They appear to reveal a double dissociation where appraisal discrepancy was related to cortical atrophy, but not AD pathology, while self-appraisal was associated with AD pathology, but not cortical atrophy. The intricate relationships between metacognition and pathology suggest a potential timeframe with regard to the differences in the sensitivity of subjective cognitive awareness measures in the detection of cognitive impairment in preclinical AD. Overestimation was related to cortical thinning in temporal, parietal, and anterior cingulate regions, reflecting a metacognitive deficit accompanying lower objective performance. However, the missing relationship with AD biomarkers suggests that this measure is not specific to AD, but likely reflects individual differences and age-related changes in higher order cognitive processes. In contrast, lower self-appraisal ratings were more sensitive to AD pathology. Aβ+ and high tau individuals had lower individual appraisal ratings, which may reflect an early awareness in preclinical AD when behavioral deficits begin to emerge, further suggesting the validity of subjective complaints of worse cognition in early AD. Together, these findings suggest that in the practice of examining subjective cognitive concerns of older adults, using a variety of metacognitive measures might help track SCD status in cognitively healthy individuals and better identify those who may or may not be developing AD pathology.
This study has its limitations. First, while our study highlights the use of online subjective measures, the self-appraisal rating of specific neuropsychological tasks involves specific, task-related self-perception processes that are not the same as SCD which reflects general cognitive or memory concerns. Having more comprehensive offline questionnaires examining metacognitive abilities as well as SCD would be beneficial to further examine the relationship between metacognitive precision and cognition along with AD biomarkers. Second, the convenience sampling nature of the study resulted in the study demographics being highly educated, affluent, and mostly of white European descent, which does not reflect the demographic profile of the general aging population. In addition, there are few participants in this study sample of cognitively healthy older individuals who fall in the category of having both high amyloid and high tau. Future research could investigate metacognitive abilities and accuracy in different subgroups of cognitively healthy individuals with varying levels of amyloid and tau deposition to clarify and add onto this relationship. Furthermore, our study used a liberal MMSE score ≥25 as a cut off criterion to define a cognitively unimpaired cohort; while such a threshold is low, none of the participants with scores of 25 or 26 had performance below the normal range (≤1.5 SD on 2 tests in the same domain based on the BACS norms) and have no cognitive or symptomatic features of MCI. We also re-conducted analyses with only those whose MMSE were ≥27. The vertex analyses revealed smaller but similar networks of regions, and the primary PET results were similar with marginally significant results. We also acknowledge that the use of average measures of self-appraisal and appraisal discrepancy across tasks has limitations. Although it offers a quick measure that captures the overall, global status of SCD and metacognitive precision, it is not sensitive to domain-specific inaccuracy, which was noted in previous literature (Ryals et al., 2019). Lastly, we are limited in making inferences about the direct relationships between tauopathy and cortical atrophy with metacognition because we do not have tau PET at the same time of the cortical thickness analyses. Cross-sectional snapshots of metacognitive appraisal, performance, and neuropathology are insufficient to understand the intricacy of self-awareness in aging. Future studies should examine longitudinal online metacognitive measures and further explore their relationship to the progression of AD pathology and structural atrophy.
In conclusion, our findings demonstrated that overestimation of performance was associated with cortical thinning in a range of temporal and frontal regions which were also related to poorer objective performance in cognitively healthy older adults. Having higher tau deposition was related to lower self-appraisal in performance which may reflect an increased awareness of performance deficits in individuals with AD pathology.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants AG034570 and AG062542. Avid Radiopharmaceuticals enabled the use of the 18F-Flortaucipir tracer but did not provide direct funding and were not involved in data analysis or interpretation.
Footnotes
Disclosure statement
Dr. Jagust has served as a consultant to Biogen, Bioclinica, and Genentech. All other authors report no conflicts of interests.
CRediT authorship contribution statement
Kailin Zhuang: Conceptualization, Investigation, Data curation, Methodology, Formal analysis, Visualization, Writing – original draft, Writing – review & editing. Xi Chen: Methodology, Investigation, Validation, Writing – review & editing. Kaitlin E. Cassady: Writing – review & editing, Writing – review & editing. Suzanne L. Baker: Methodology, Validation, Writing – review & editing. William J. Jagust: Funding acquisition, Project administration, Validation, Writing – review & editing.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.neurobiolaging.2022.06.007.
References
- Aiken LS, West SG, Reno RR, 1991. Multiple Regression: Testing and Interpreting Interactions. SAGE, New York. [Google Scholar]
- Alkan E, Davies G, Greenwood K, Evans SLH, 2020. Brain structural correlates of metacognition in first-episode psychosis. Schizophrenia Bull. 46, 552–561. doi: 10.1093/schbul/sbz116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asperholm M, Högman N, Rafi J, Herlitz A, 2019. What did you do yesterday? A meta-analysis of sex differences in episodic memory. Psychol. Bull 145, 785–821. doi: 10.1037/bul0000197. [DOI] [PubMed] [Google Scholar]
- Baker SL, Maass A, Jagust WJ, 2017. Considerations and code for partial volume correcting [18 F]-AV-1451 tau PET data. Data Brief 15, 648–657. doi: 10.1016/j.dib.2017.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand E, Azar M, Rizvi B, Brickman AM, Huey ED, Habeck C, Landeira-Fernandez J, Mograbi DC, Cosentino S, 2018. Cortical thickness and metacognition in cognitively diverse older adults. Neuropsychology 32, 700–710. doi: 10.1037/neu0000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AL, 1978. Knowing when, where, and how to remember: a problem of metacognition. Adv. Instructional Psychol 1, 77–165. [Google Scholar]
- Buckley R, Saling MM, Ames D, Rowe CC, Lautenschlager NT, Macaulay SL, Martins RN, Masters CL, O’Meara T, Savage G, Szoeke C, Villemagne VL, Ellis KA Australian Imaging Biomarkers and Lifestyle Study of Aging (AIBL) Research Group, 2013. Factors affecting subjective memory complaints in the AIBL aging study: biomarkers, memory, affect, and age. Int. Psychogeriatr 25, 1307–1315. doi: 10.1017/S1041610213000665. [DOI] [PubMed] [Google Scholar]
- Buckley RF, Hanseeuw B, Schultz AP, Vannini P, Aghjayan SL, Properzi MJ, Jackson JD, Mormino EC, Rentz DM, Sperling RA, Johnson KA, Amariglio RE, 2017. Region-specific association of subjective cognitive decline with tauopathy independent of global β-amyloid burden. JAMA Neurol 74, 1455. doi: 10.1001/jamaneurol.2017.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busovaca E, Zimmerman ME, Meier IB, Griffith EY, Grieve SM, Kor-gaonkar MS, Williams LM, Brickman AM, 2016. Is the Alzheimer’s disease cortical thickness signature a biological marker for memory? Brain Imaging Be-hav 10, 517–523. doi: 10.1007/s11682-015-9413-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butzbach M, Fuermaier ABM, Aschenbrenner S, Weisbrod M, Tucha L, Tucha O, 2021. Metacognition in adult ADHD: subjective and objective perspectives on self-awareness of cognitive functioning. J Neural Transm 128, 939–955. doi: 10.1007/s00702-020-02293-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, 1998. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science 280, 747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Chen X, Farrell ME, Moore W, Park DC, 2019. Actual memory as a mediator of the amyloid-subjective cognitive decline relationship. Alzheimer’s Dementia 11, 151–160. doi: 10.1016/j.dadm.2018.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Farrell ME, Rundle MM, Chan MY, Moore W, Wig GS, Park DC, 2021. The relationship of functional hippocampal activity, amyloid deposition, and longitudinal memory decline to memory complaints in cognitively healthy older adults. Neurobiol. Aging 105, 318–326. doi: 10.1016/j.neurobiolaging.2021.04.020. [DOI] [PubMed] [Google Scholar]
- Colvin LE, Malgaroli M, Chapman S, MacKay-Brandt A, Cosentino S, 2018. Mood and personality characteristics are associated with metamemory knowledge accuracy in community-based cohort of older adults. J. Int. Neuropsychol. Soc 24, 498–510. doi: 10.1017/S1355617717001345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosentino S, Stern Y, 2005. Metacognitive theory and assessment in dementia: Do we recognize our areas of weakness? J. Int. Neuropsychol. Soc 11, 910–919. doi: 10.1017/S1355617705050964. [DOI] [PubMed] [Google Scholar]
- David AS, Bedford N, Wiffen B, Gilleen J, 2012. Failures of metacognition and lack of insight in neuropsychiatric disorders. Phil. Trans. R. Soc. B 367, 1379–1390. doi: 10.1098/rstb.2012.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA, 2000. California Verbal Learning Test, second edition Psychological Corporation, San Antonio, TX. [Google Scholar]
- Dickerson BC, Feczko E, Augustinack JC, Pacheco J, Morris JC, Fischl B, Buckner RL, 2009. Differential effects of aging and Alzheimer’s disease on medial temporal lobe cortical thickness and surface area. Neurobiol. Aging 30, 432–440. doi: 10.1016/j.neurobiolaging.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Stoub TR, Shah RC, Sperling RA, Killiany RJ, Albert MS, Hyman BT, Blacker D, deToledo-Morrell L, 2011. Alzheimer-signature MRI biomarker predicts AD dementia in cognitively normal adults. Neurology 76, 1395–1402. doi: 10.1212/WNL.0b013e3182166e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Oleire Uquillas F, Jacobs HIL, Schultz AP, Hanseeuw BJ, Buckley RF, Sepulcre J, Pascual-Leone A, Donovan NJ, Johnson KA, Sperling RA, Vannini P, 2020. Functional and pathological correlates of judgments of learning in cognitively unimpaired older adults. Cerebral Cortex 30, 1974–1983. doi: 10.1093/cercor/bhz217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eslinger PJ, Dennis K, Moore P, Antani S, Hauck R, Grossman M, 2005. Metacognitive deficits in frontotemporal dementia. J. Neurol. Neurosurg. Psychiatry 76, 1630–1635. doi: 10.1136/jnnp.2004.053157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L-Y, Lai Y-M, Chen T-F, Hsu Y-C, Chen P-Y, Huang K-Z, Cheng T-W, Tseng W-YI, Hua M-S, Chen Y-F, Chiu M-J, 2018. Diminution of context association memory structure in subjects with subjective cognitive decline. Hum. Brain Mapp 39, 2549–2562. doi: 10.1002/hbm.24022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming SM, Dolan RJ, 2012. The neural basis of metacognitive ability. Philos. Trans. Royal Soc. B 367, 1338–1349. doi: 10.1098/rstb.2011.0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliardi Geoffroy, Houot Marion, Cacciamani Federica, Habert M-O, Dubois Bruno, Epelbaum Stéphane, Audrain C, Auffret A, Bakardjian H, Baldacci F, Batrancourt B, Benakki I, Benali H, Bertin H, Bertrand A, Boukadida L, Cacciamani F, Causse V, Cavedo E, Cherif Touil S, Chiesa PA, Colliot O, Dalla Barba G, Depaulis M, Dos Santos A, Dubois B, Dubois M, Epelbaum S, Fontaine B, Francisque H, Gagliardi G, Genin A, Genthon R, Glasman P, Gombert F, Habert MO, Hampel H, Hewa H, Houot M, Jungalee N, Kas A, Kilani M, La Corte V, Le Roy F, Lehericy S, Letondor C, Levy M, Lista S, Lowrey M, Ly J, Makiese O, Masetti I, Mendes A, Metzinger C, Michon A, Mochel F, Nait Arab R, Nyasse F, Perrin C, Poirier F, Poisson C, Potier MC, Ratovohery S, Revillon M, Rojkova K, Santos-Andrade K, Schindler R, Servera MC, Seux L, Simon V, Skovronsky D, Thiebaut M, Uspenskaya O, Vlaincu M, Lamari F for the INSIGHT-preAD study group, 2020. The meta-memory ratio: a new cohort-independent way to measure cognitive awareness in asymptomatic individuals at risk for Alzheimer’s disease. Alzheimer’s Res. Ther 12, 57. doi: 10.1186/s13195-020-00626-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden CJ, 1978. Stroop Color and Word Test: A Manual for Clinical and Experimental Uses. Stoelting Co, Chicago, IL, pp. 1–32. [Google Scholar]
- Grambaite R, Hessen E, Auning E, Aarsland D, Selnes P, Fladby T, 2013. Correlates of subjective and mild cognitive impairment: depressive symptoms and CSF biomarkers. Dement. Geriatr. Cogn. Dis. Extra 3, 291–300. doi: 10.1159/000354188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrier L, Le Men J, Gane A, Planton M, Salabert A-S, Payoux P, Dumas H, Bonneville F, Péran P, Pariente J, 2018. Involvement of the cingulate cortex in anosognosia: a multimodal neuroimaging study in alzheimer’s disease patients. JAD 65, 443–453. doi: 10.3233/JAD-180324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallam B, Chan J, Gonzalez Costafreda S, Bhome R, Huntley J, 2020. What are the neural correlates of meta-cognition and anosognosia in Alzheimer’s disease? A systematic review. Neurobiol. Aging 94, 250–264. doi: 10.1016/j.neurobiolaging.2020.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanseeuw BJ, Betensky RA, Jacobs HIL, Schultz AP, Sepulcre J, Becker JA, Cosio DMO, Farrell M, Quiroz YT, Mormino EC, Buckley RF, Papp KV, Amariglio RA, Dewachter I, Ivanoiu A, Huijbers W, Hedden T, Marshall GA, Chhatwal JP, Rentz DM, Sperling RA, Johnson K, 2019. Association of amyloid and tau with cognition in preclinical alzheimer disease: a longitudinal study. JAMA Neurol 76, 915. doi: 10.1001/jamaneurol.2019.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollands S, Lim YY, Buckley R, Pietrzak RH, Snyder PJ, Ames D, Ellis KA, Harrington K, Lautenschlager N, Martins RN, Masters CL, Villemagne VL, Rowe CC, Maruff P, 2015. Amyloid-β related memory decline is not associated with subjective or informant rated cognitive impairment in healthy adults. J. Alzheimers Dis 43, 677–686. doi: 10.3233/JAD-140678. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Nieuwenhuis S, Yeung N, Nystrom L, Mars RB, Coles MGH, Cohen JD, 2004. Dorsal anterior cingulate cortex shows fMRI response to internal and external error signals. Nat. Neurosci 7, 497–498. doi: 10.1038/nn1238. [DOI] [PubMed] [Google Scholar]
- Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtz-man DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R, Elliott C, Masliah E, Ryan L, Silverberg N, 2018. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimer’s Dementia 14, 535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, Shaw LM, Vemuri P, Wiste HJ, Weigand SD, Lesnick TG, Pankratz VS, Donohue MC, Trojanowski JQ, 2013. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol 12, 207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen F, Amariglio RE, Buckley RF, van der Flier WM, Han Y, Molinuevo JL, Rabin L, Rentz DM, Rodriguez-Gomez O, Saykin AJ, Sikkes SAM, Smart CM, Wolfsgruber S, Wagner M, 2020. The characterisation of subjective cognitive decline. Lancet Neurol 19, 271–278. doi: 10.1016/S1474-4422(19)30368-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen F, Amariglio RE, van Boxtel M, Breteler M, Ceccaldi M, Chételat G, Dubois B, Dufouil C, Ellis KA, van der Flier WM, Glodzik L, van Harten AC, de Leon MJ, McHugh P, Mielke MM, Molinuevo JL, Mosconi L, Osorio RS, Perrotin A, Petersen RC, Rabin LA, Rami L, Reisberg B, Rentz DM, Sachdev PS, de la Sayette V, Saykin AJ, Scheltens P, Shulman MB, Slavin MJ, Sperling RA, Stewart R, Uspenskaya O, Vellas B, Visser PJ, Wagner M Subjective Cognitive Decline Initiative (SCD-I) Working Group, 2014. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimer’s Dementia 10, 844–852. doi: 10.1016/j.jalz.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell John H, 1976. Metacognitive aspects of problem solving. The Nature of Intelligence [Google Scholar]
- Johnson KA, Schultz A, Betensky RA, Becker JA, Sepulcre J, Rentz D, Mormino E, Chhatwal J, Amariglio R, Papp K, Marshall G, Albers M, Mauro S, Pepin L, Alverio J, Judge K, Philiossaint M, Shoup T, Yokell D, Dickerson B, Gomez-Isla T, Hyman B, Vasdev N, Sperling R, 2016. Tau positron emission tomographic imaging in aging and early Alzheimer disease: Tau PET in Aging and Early AD. Ann. Neurol 79, 110–119. doi: 10.1002/ana.24546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PO, Fay LC, 1950. The Johnson-Neyman technique, its theory and application. Psychometrika 15, 349–367. doi: 10.1007/BF02288864. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP, 2002. Neural correlates of self-reflection. Brain 125, 1808–1814. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- Krueger CE, Rosen HJ, Taylor HG, Espy KA, Schatz J, Rey-Casserly C, Kramer JH, 2011. Know thyself: real-world behavioral correlates of self-appraisal accuracy. Clin. Neuropsychol 25, 741–756. doi: 10.1080/13854046.2011.569759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger J, Dunning D, 1999. Unskilled and unaware of it: how difficulties in recognizing one’s own incompetence lead to inflated self-assessments. J. Personality Soc. Psychol 77, 1121–1134. [DOI] [PubMed] [Google Scholar]
- La Joie R, Perrotin A, Egret S, Pasquier F, Tomadesso C, Mézenge F, Desgranges B, La Sayette V, Chételat G, 2016. Qualitative and quantitative assessment of self-reported cognitive difficulties in nondemented elders: Association with medical help seeking, cognitive deficits, and β-amyloid imaging. Alzheimer’s Dementia 23–34. doi: 10.1016/j.dadm.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Lin Z, Tao R, Xu M, Kong S, Bi H-Y, Yang Y, 2021. Neuroanatomical correlates of self-awareness of highly practiced visuomotor skills. Brain Struct. Funct 226, 2295–2306. doi: 10.1007/s00429-021-02328-2. [DOI] [PubMed] [Google Scholar]
- Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL, 1996. Distribution volume ratios without blood sampling from graphical analysis of PET data. J. Cereb. Blood Flow Metab 16, 834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- Lowe VJ, Wiste HJ, Senjem ML, Weigand SD, Therneau TM, Boeve BF, Josephs KA, Fang P, Pandey MK, Murray ME, Kantarci K, Jones DT, Vemuri P, Graff-Radford J, Schwarz CG, Machulda MM, Mielke MM, Roberts RO, Knopman DS, Petersen RC, Jack CR, 2018. Widespread brain tau and its association with ageing, Braak stage and Alzheimer’s dementia. Brain 141, 271–287. doi: 10.1093/brain/awx320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maass A, Berron D, Harrison TM, Adams JN, La Joie R, Baker S, Mellinger T, Bell RK, Swinnerton K, Inglis B, Rabinovici GD, Düzel E, Jagust WJ, 2019. Alzheimer’s pathology targets distinct memory networks in the ageing brain. Brain 142, 2492–2509. doi: 10.1093/brain/awz154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massimo L, Libon DJ, Chandrasekaran K, Dreyfuss M, McMillan CT, Rascov-sky K, Boller A, Grossman M, 2013. Self-appraisal in behavioural variant frontotemporal degeneration. J. Neurol. Neurosurg. Psychiatry 84, 148–153. doi: 10.1136/jnnp-2012-303153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe J, Butterfield B, Habeck C, Stern Y, 2012. Neural correlates of people’s hypercorrection of their false beliefs. J. Cognit. Neurosci 24, 1571–1583. doi: 10.1162/jocn_a_00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinuevo JL, Rabin LA, Amariglio R, Buckley R, Dubois B, Ellis KA, Ewers M, Hampel H, Klöppel S, Rami L, Reisberg B, Saykin AJ, Sikkes S, Smart CM, Snitz BE, Sperling R, van der Flier WM, Wagner M, Jessen F Subjective Cognitive Decline Initiative (SCD-I) Working Group, 2017. Implementation of subjective cognitive decline criteria in research studies. Alzheimer’s Dementia 13, 296–311. doi: 10.1016/j.jalz.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormino EC, Brandel MG, Madison CM, Rabinovici GD, Marks S, Baker SL, Jagust WJ, 2012. Not quite PIB-positive, not quite PIB-negative: Slight PIB elevations in elderly normal control subjects are biologically relevant. NeuroImage 59, 1152–1160. doi: 10.1016/j.neuroimage.2011.07.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison C, Dadar M, Shafiee N, Villeneuve S, Louis Collins D, 2021. Regional brain atrophy and cognitive decline depend on definition of subjective cognitive decline. Neuroimage Clin 33, 102923. doi: 10.1016/j.nicl.2021.102923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi L, De Santi S, Brys M, Tsui WH, Pirraglia E, Glodzik-Sobanska L, Rich KE, Switalski R, Mehta PD, Pratico D, Zinkowski R, Blennow K, de Leon MJ, 2008. Hypometabolism and altered cerebrospinal fluid markers in normal apolipoprotein E E4 carriers with subjective memory complaints. Biol. Psychiatry 63, 609–618. doi: 10.1016/j.biopsych.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Neira C, Tedde A, Coulthard E, Thai NJ, Pennington C, 2019. Neural correlates of altered insight in frontotemporal dementia: a systematic review. Neuroimage Clin 24, 102066. doi: 10.1016/j.nicl.2019.102066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossenkoppele R, Schonhaut DR, Schöll M, Lockhart SN, Ayakta N, Baker SL, O’Neil JP, Janabi M, Lazaris A, Cantwell A, Vogel J, Santos M, Miller ZA, Bettcher BM, Vossel KA, Kramer JH, Gorno-Tempini ML, Miller BL, Jagust WJ, Rabinovici GD, 2016. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer’s disease. Brain 139, 1551–1567. doi: 10.1093/brain/aww027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrotin A, La Joie R, de La Sayette V, Barré L, Mézenge F, Mutlu J, Guilloteau D, Egret S, Eustache F, Chételat G, 2017. Subjective cognitive decline in cognitively normal elders from the community or from a memory clinic: Differential affective and imaging correlates. Alzheimers Dement 13, 550–560. doi: 10.1016/j.jalz.2016.08.011. [DOI] [PubMed] [Google Scholar]
- Perrotin A, Mormino EC, Madison CM, Hayenga AO, 2012. Subjective cognition and amyloid deposition imaging: a Pittsburgh compound B positron emission tomography study in normal elderly individuals. Arch. Neurol 69, 223. doi: 10.1001/archneurol.2011.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JC, Klunk WE, Lopresti BJ, Lu X, Hoge JA, Ziolko SK, Holt DP, Meltzer CC, DeKosky ST, Mathis CA, 2005. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J. Cereb. Blood Flow Metab 25, 1528–1547. doi: 10.1038/sj.jcbfm.9600146. [DOI] [PubMed] [Google Scholar]
- Quattrini G, Pini L, Pievani M, Magni LR, Lanfredi M, Ferrari C, Boccardi M, Bignotti S, Magnaldi S, Cobelli M, Rillosi L, Beneduce R, Rossi G, Frisoni GB, Rossi R, 2019. Abnormalities in functional connectivity in border-line personality disorder: correlations with metacognition and emotion dysregulation. Psychiatry Res 283, 118–124. doi: 10.1016/j.pscychresns.2018.12.010. [DOI] [PubMed] [Google Scholar]
- Rabin LA, Smart CM, Crane PK, Amariglio RE, Berman LM, Boada M, Buckley RF, Chételat G, Dubois B, Ellis KA, Gifford KA, Jefferson AL, Jessen F, Katz MJ, Lipton RB, Luck T, Maruff P, Mielke MM, Molinuevo JL, Naeem F, Perrotin A, Petersen RC, Rami L, Reisberg B, Rentz DM, Riedel-Heller SG, Risacher SL, Rodriguez O, Sachdev PS, Saykin AJ, Slavin MJ, Snitz BE, Sperling RA, Tandetnik C, van der Flier WM, Wagner M, Wolfsgruber S, Sikkes SAM, 2015. Subjective cognitive decline in older adults: an overview of self-report measures used across 19 international research studies. J. Alzheimers Dis 48 (Suppl 1), S63–S86. doi: 10.3233/JAD-150154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen HJ, Alcantar O, Rothlind J, Sturm V, Kramer JH, Weiner M, Miller BL, 2010. Neuroanatomical correlates of cognitive self-appraisal in neurodegenerative disease. NeuroImage 49, 3358–3364. doi: 10.1016/j.neuroimage.2009.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen HJ, Alcantar O, Zakrzewski J, Shimamura AP, Neuhaus J, Miller BL, 2014. Metacognition in the behavioral variant of frontotemporal dementia and Alzheimer’s disease. Neuropsychology 28, 436–447. doi: 10.1037/neu0000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothlind J, Dukarm P, Kraybill M, 2016. Assessment of self-awareness of cognitive function: correlations of self-ratings with actual performance ranks for tests of processing speed, memory and executive function in non-clinical samples. Arch. Clin. Neuropsychol doi: 10.1093/arclin/acw109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousset OG, Ma Y, Evans AC, 1998. Correction for partial volume effects in PET: principle and validation. J. Nucl. Med 39, 904–911. [PubMed] [Google Scholar]
- Ryals AJ, O’Neil JT, Mesulam M-M, Weintraub S, Voss JL, 2019. Memory awareness disruptions in amnestic mild cognitive impairment: comparison of multiple awareness types for verbal and visuospatial material. Aging Neuropsychol. Cognit 26, 577–598. doi: 10.1080/13825585.2018.1503994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez JS, Becker JA, Jacobs HIL, Hanseeuw BJ, Jiang S, Schultz AP, Properzi MJ, Katz SR, Beiser A, Satizabal CL, O’Donnell A, DeCarli C, Killiany R, El Fakhri G, Normandin MD, Gómez-Isla T, Quiroz YT, Rentz DM, Sperling RA, Seshadri S, Augustinack J, Price JC, Johnson KA, 2021. The cortical origin and initial spread of medial temporal tauopathy in Alzheimer’s disease assessed with positron emission tomography. Sci. Transl. Med 13, eabc0655. doi: 10.1126/scitranslmed.abc0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton J, Lopez OL, Ratcliff G, Dulberg C, Fried LP, Carlson MC, Newman AB, Kuller L, 2004. Preclinical Alzheimer disease: neuropsychological test performance 1.5 to 8 years prior to onset. Neurology 63, 2341–2347. doi: 10.1212/01.WNL.0000147470.58328.50. [DOI] [PubMed] [Google Scholar]
- Schöll M, Lockhart SN, Schonhaut DR, O’Neil JP, Janabi M, Ossenkoppele R, Baker SL, Vogel JW, Faria J, Schwimmer HD, Rabinovici GD, Jagust WJ, 2016. PET imaging of tau deposition in the aging human brain. Neuron 89, 971–982. doi: 10.1016/j.neuron.2016.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinanaj I, Cojan Y, Vuilleumier P, 2015. Inter-individual variability in metacognitive ability for visuomotor performance and underlying brain structures. Conscious. Cognit 36, 327–337. doi: 10.1016/j.concog.2015.07.012. [DOI] [PubMed] [Google Scholar]
- Spreen O, Benton AL, 1977. Neurosensory Center Comprehensive Examination for Aphasia: Manual of instructions (NCCEA) (rev. ed.). University of Victoria, Victoria, BC. [Google Scholar]
- Starkstein SE, 2014. Anosognosia in Alzheimer’s disease: Diagnosis, frequency, mechanism and clinical correlates. Cortex 61, 64–73. doi: 10.1016/j.cortex.2014.07.019. [DOI] [PubMed] [Google Scholar]
- Swinford CG, Risacher SL, Charil A, Schwarz AJ, Saykin AJ, 2018. Memory concerns in the early Alzheimer’s disease prodrome: regional association with tau deposition. Alzheimers Dement. (Amst) 10, 322–331. doi: 10.1016/j.dadm.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therriault J, Ng KP, Pascoal TA, Mathotaarachchi S, Kang MS, Struyfs H, Shin M, Benedet AL, Walpola IC, Nair V, Gauthier S, Rosa-Neto P For the Alzheimer’s Disease Neuroimaging Initiative, 2018. Anosognosia predicts default mode network hypometabolism and clinical progression to dementia. Neurology 90, e932–e939. doi: 10.1212/WNL.0000000000005120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccaro AG, Fleming SM, 2018. Thinking about thinking: a coordinate-based meta-analysis of neuroimaging studies of metacognitive judgements. Brain Neurosci. Adv 2. doi: 10.1177/2398212818810591, 239821281881059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera-Bermejo JM, De Marco M, Mitolo M, McGeown WJ, Venneri A, 2020. Neuroanatomical and cognitive correlates of domain-specific anosognosia in early Alzheimer’s disease. Cortex 129, 236–246. doi: 10.1016/j.cortex.2020.04.026. [DOI] [PubMed] [Google Scholar]
- van Harten AC, Mielke MM, Swenson-Dravis DM, Hagen CE, Edwards KK, Roberts RO, Geda YE, Knopman DS, Petersen RC, 2018. Subjective cognitive decline and risk of MCI: the mayo clinic study of aging. Neurology 91, e300–e312. doi: 10.1212/WNL.0000000000005863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannini P, d’Oleire Uquillas F, Jacobs HIL, Sepulcre J, Gatchel J, Amariglio RE, Hanseeuw B, Papp KV, Hedden T, Rentz DM, Pascual-Leone A, Johnson KA, Sperling Reisa.A., 2019. Decreased meta-memory is associated with early tauopathy in cognitively unimpaired older adults. Neuroimage Clin 24, 102097. doi: 10.1016/j.nicl.2019.102097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve S, Rabinovici GD, Cohn-Sheehy BI, Madison C, Ayakta N, Ghosh PM, La Joie R, Arthur-Bentil SK, Vogel JW, Marks SM, Lehmann M, Rosen HJ, Reed B, Olichney J, Boxer AL, Miller BL, Borys E, Jin L-W, Huang EJ, Grinberg LT, DeCarli C, Seeley WW, Jagust W, 2015. Existing Pittsburgh Compound-B positron emission tomography thresholds are too high: statistical and pathological evaluation. Brain 138, 2020–2033. doi: 10.1093/brain/awv112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel JW, Dolealová MV, Joie RL, Marks SM, Schwimmer HD, Landau SM, Jagust WJ, 2017. Subjective cognitive decline and β-amyloid burden predict cognitive change in healthy elderly. Neurology 89, 2002–2009. doi: 10.1212/wnl.0000000000004627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Huang W, Su L, Xing Y, Jessen F, Sun Y, Shu N, Han Y, 2020. Neuroimaging advances regarding subjective cognitive decline in preclinical Alzheimer’s disease. Mol. Neurodegeneration 15, 55. doi: 10.1186/s13024-020-00395-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D, 1997. The Wechsler Memory Scale - Third Edition: Administration & Scoring Manual The Psychological Corporation, San Antonio, TX. [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO, 1983. Development and validation of a geriatric depression screening scale: a preliminary report. J. Psychiatr. Res 17, 37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


