Abstract
Using 2 global postmarketing surveillance databases, Goldman and colleagues report that progressive multifocal leukoencephalopathy (PML), a viral disease associated with profound immunosuppression, occurs in approximately 0.9 cases per 1000 recipients of CD19-directed CAR T-cell therapy. The risk of PML appears higher with CAR T-cell therapy than other cancer therapies, but its precise role cannot be distinguished from antecedent therapies that these patients receive.
TO THE EDITOR:
Progressive multifocal leukoencephalopathy (PML) is a rare, devastating, and fatal demyelinating disease of the central nervous system (CNS) caused by the JC virus (JCV). It typically affects patients with profound immunosuppressive states secondary to AIDS or iatrogenic exposures.1 CD19-directed chimeric antigen receptor T-cell (CAR-T) therapy is an effective treatment for B-cell malignancies.2,3 However, complications such as cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) are common. Furthermore, CAR-T is associated with immunosuppression secondary to off-target effects of CAR-T, lymphodepleting agents, cytopenia, and hypogammaglobulinemia.4, 5, 6 We hypothesized that CD19-CAR-T therapy increases the risk of PML. Descriptions of post−CD19-CAR-T PML cases have been reported in anecdotal case reports and in a case series from the US Food and Drug Administration’s FDA Adverse Event Reporting System (FAERS).7, 8, 9, 10 However, the incidence of PML after CD19-CAR-T and whether CD19-CAR-T is associated with PML risk remain to be determined. We address these questions in this retrospective pharmacovigilance study using 2 global postmarketing surveillance databases.
To estimate increased reporting of PML following commercial CD19-CAR-T therapy, we compared the reporting of CD19-CAR-T−related PML with that of other drugs in the entire FAERS database. Patient-level data were obtained via a Freedom of Information Act request. To identify a significant disproportionality signal, we used the reporting odds ratio (ROR) and the lower bound of the information component 95% credibility interval (IC025).11,12 To increase signal detection specificity, we restricted the analysis to reports in which CD19-CAR-T was the primary suspect.
To estimate the incidence of PML, we queried the Center for International Blood and Marrow Transplant Research (CIBMTR) registry, which captures approximately 65% of all commercial CAR-T therapy performed in the United States (see supplemental Methods for additional details, available on the Blood website).13
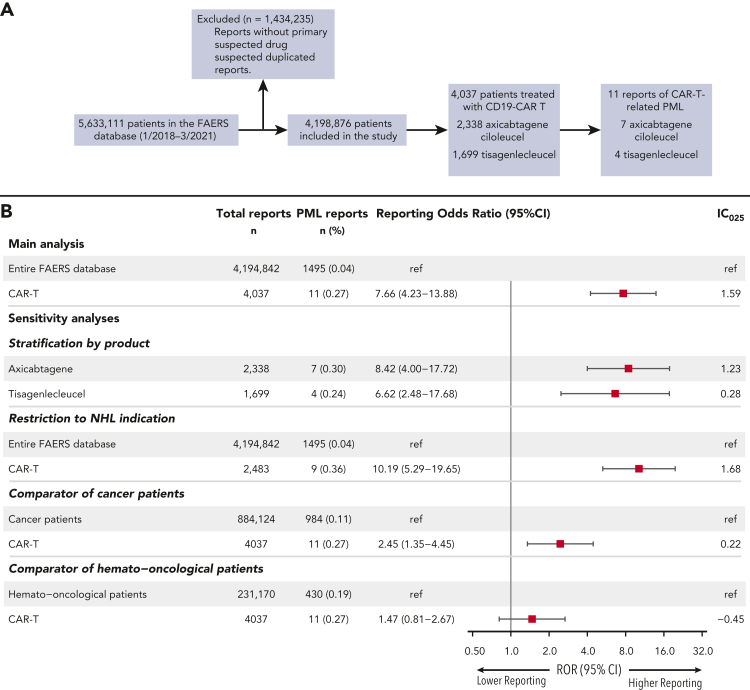
Of 4,198,876 eligible patients in the full FAERS database, 4037 patients received CD19-CAR-T (axicabtagene ciloleucel, n = 2338; tisagenlecleucel, n = 1699) between 1 January 2018 and 31 March 2021. We identified 11 reports of PML in which CD19-CAR-T therapy (axicabtagene ciloleucel, n = 7; tisagenlecleucel, n = 4) was considered the primary suspect (Figure 1A). PML cases were reported mostly by healthcare professionals (n = 10 of 11 [91%]) and the events occurred in non-Hodgkin lymphoma (NHL) patients (n = 9 of 10 [90%]), apart from 1 patient with acute lymphoblastic leukemia (ALL) (Table 1; supplemental Table 1). The median age of PML patients was 63 (IQR = 58-67) years, slightly higher than for other CD19-CAR-T−treated patients reported to FAERS (57 [IQR = 28-67], P = .163). Female sex was enriched in the PML cohort compared to other FAERS patients (70.0% vs 39%, P = .052). A history of HIV or of natalizumab use was not reported in the patients with available data. However, other PML risk factors, including chemotherapy and rituximab exposure, were common. At the time of PML diagnosis, 5 patients had active hematological disease, and 4 patients were in complete remission after CAR-T. The CAR-T recipients who developed PML presented with varied neurological symptoms, most commonly motor and cognitive disorders (supplemental Table 1). Time to PML onset was variable, ranging between 6 and 31 months from CD19-CAR-T administration, with a median of 8 months (IQR = 8-15 months). In 9 patients with available data, PML diagnosis was confirmed in 5 and 3 patients by JCV presence in the cerebrospinal fluid and a brain biopsy, respectively. Notably, the reported case fatality rate of PML was 78% (n = 7 of 9) at last follow-up. PML treatment was described in 2 patients, 1 patient with pembrolizumab and the other with mefloquine, IVIG, and mirtazapine; both were alive at last follow-up.
Figure 1.
Study flowchart and disproportionality analysis of CAR-T−related PML adverse events (AEs). (A) Reports without a defined primary suspected drug for a given AE and suspected duplicated reports were excluded. (B) Disproportionality analysis of CAR-T products compared to the full FAERS database (ie, “main analysis”) and sensitivity analyses using restricted comparator groups of cancer patients and hemato-oncological patients. Results of disproportionality analysis by CAR-T product and following restriction to NHL patients are also presented. A positive lower bound of the information component 95% credibility interval (IC₀₂₅ > 0) and a lower limit of the ROR 95% confidence interval above 1 are the conventional thresholds for significant signal detection.
Table 1.
Population characteristics by PML reporting status among patients treated with CD19-CAR-T and reported to FAERS
| PML reports (n = 11) | Non-PML reports (n = 4026) | P value | |
|---|---|---|---|
| Reporting region | |||
| Americas | 5/11 (45.5) | 3291/4025 | .010 |
| Europe | 5/11 (45.5) | 618/4025 | |
| Asia | 1/11 (9.0) | 60/4025 | |
| Australia | 0/11 (0.00) | 56/4025 | |
| Reporter | |||
| Health professional | 10/11 (90.9) | 3559/3914 (90.9) | >.99 |
| Consumer/lawyer | 1/11 (9.1) | 355/3914 (9.1) | |
| Event year | |||
| 2018 | 0/8 (0.0) | 729/2429 (29.9) | .040 |
| 2019 | 2/8 (25.0) | 934/2429 (38.5) | |
| 2020 | 6/8 (75.0) | 705/2429 (29.0) | |
| 2021∗ | 0/8 (0.0) | 61/2429 (2.5) | |
| Age, y | |||
| Median (IQR) | 63 (58-67) n = 10 | 57 (28-67) n = 2882 | .163 |
| Sex | |||
| Female | 7/10 (70.0) | 1254/3255 (38.5) | .052 |
| Male | 3/10 (30.0) | 2001/3255 (61.5) | |
| Product | |||
| Axicabtagene-ciloleucel | 7/11 (63.6) | 2331/4026 (57.9) | .770 |
| Tisagenlecleucel | 4/11 (36.4) | 1695/4026 (42.1) | |
| Indication | |||
| NHL | 9/10 (90.0) | 2474/3316 (74.6) | .544 |
| ALL | 1/10 (10.0) | 791/3316 (23.9) | |
| Other | 0/10 (0.0) | 51/3316 (1.5) | |
| Time to AE onset, mo | |||
| Median (IQR) | 8 (8-15) n = 10 | 0.13 (0.03-0.33) n = 1983 | <.001 |
| Min-max | 6-31 n = 10 | 0-82 n = 1983 | |
| Reported fatality rate | 7/9 (77.8) | 906/3831 (23.6) | <.001 |
All values are n (%) unless otherwise indicated.
IQR, interquartile range.
Only cases reported before 31 March 2021 were included.
To determine whether CD19-CAR-T therapy is associated with increased PML reporting, we performed a disproportionality analysis. PML reporting was higher in patients treated with CD19-CAR-T compared to unexposed patients (ie, remaining FAERS database) (ROR = 7.66 [95% CI, 4.23-13.88], IC025 = 1.59). We further conducted several sensitivity analyses. First, in a stratified analysis by CAR-T product, both axicabtagene ciloleucel and tisagenlecleucel were significantly associated with increased PML reporting (n = 7, ROR = 8.42 [95% CI, 4.00-17.72], and n = 4, ROR= 6.62 [95% CI, 2.48-17.68], respectively; both IC025 >0) (Figure 1B). Second, restricting the analysis to patients receiving CAR-T for NHL yielded consistent results (n = 9, ROR = 10.19 [95% CI, 5.29-19.65], IC025 = 1.68). Finally, when restricted comparator groups were selected, increased reporting of CAR-T−related PML remained significant compared to that in cancer patients (ROR = 2.45 [95% CI, 1.35-4.45], IC025 = 0.22), whereas compared to hemato-oncological patients, a numerically, non−statistically significant, higher ROR was observed (ROR = 1.47 [95% CI, 0.81-2.67], IC025 = −0.45) (Figure 1B). Overall, these findings support an association between CD19-CAR-T therapy and greater PML risk.
The CIBMTR registry included 3239 patients who received commercial CD19-CAR-T cells between 1 January 2018 and 31 Decemeber 2020. We identified 2 CD19-CAR-T−related PML cases in NHL patients, reported 7.5 and 16 months after axicabtagene ciloleucel therapy (supplemental Table 2). At last follow-up, 1 patient was alive and the other deceased. Another case of a 12-year-old ALL patient presenting with neurological symptoms and radiological findings of leukoencephalopathy 9 months after tisagenlecleucel was reported. Although PML is most likely, a definitive diagnosis was not reported to the registry. Considering all 3 reported cases, the estimated incidence of PML following CD19-CAR-T is 0.9 (95% CI = 0.2-2.7) cases per 1000 CD19-CAR-T−treated patients.
The use of CD19-CAR-T therapy in hematological malignancies is rapidly increasing, with a growing number of indications and introduction at earlier stages of the disease. Thus, characterization of the full spectrum of CAR-T toxicity is a clinical priority. This post-marketing surveillance study suggests PML as a potentially rare and fatal complication of CD19-CAR-T therapy. The increased reporting of PML following CD19-CAR-T treatment was consistent across different statistical methods (ROR and IC025) and multiple sensitivity analyses. PML case patients had a high fatality proportion (78%), consistent with previous studies that observed 52% to 83% mortality rates depending on the underlying cause.14 The highly variable time of PML onset is in line with previous studies of natalizumab and rituximab.15,16 It may reflect variations in the host immune response and a pre-symptomatic stage, as well as potentially delayed diagnosis.16 Using the CIBMTR registry, we deduce an incidence of 0.9 cases per 1000 CAR-T recipients, similar to the incidence of PML in patients treated with natalizumab (1 per 1000), which is 1 of the most notable drug-PML associations.16,17 As early PML diagnosis is associated with favorable outcomes,18 and as novel immune-based strategies for PML treatment have early promising results (eg, immune checkpoint blockade, recombinant human interleukin-7, and BK virus−specific T lymphocytes, which cross-react with JCV),1,19, 20, 21 clinicians should be conscious of this potential complication.
PML develops almost exclusively in immunocompromised patients due to the reactivation of a dormant JCV.1 Lymphopenia, neutropenia, B-cell aplasia, lymphodepletion regimens, and CRS treatment with interleukin-6 inhibitors or corticosteroids may all contribute to the immunosuppressive state in CAR-T recipients.4,5 Cellular immunity is crucial in protecting against JVC reactivation, and PML is most common in patients with CD4+ T-cell deficiencies, as in HIV infection. Thus, prolonged impairment of cellular immunity in CAR-T recipients,4,5 which may be precipitated by CAR-associated B-cell depletion,22 provides a biological rationale for an increased PML risk.1,16
This study has several limitations. Causality between CAR-T and PML occurrence cannot be established due to the inherent nature of the FAERS. Causes for JCV reactivation and PML are likely multifactorial and reflect the cumulative exposure to immunosuppressive interventions. Indeed, prior administration of rituximab and intensive chemotherapies, which are associated with increased PML risk,15,23 were common in our cohort. Quantifying and comparing the attribution of each of these exposures is challenging. Nonetheless, the established immunosuppressive effect of CAR-T therapy,4,5,24 coupled with increased PML reporting likelihood in the primary and most sensitivity analyses, support a meaningful contribution of CAR-T to PML risk.
In conclusion, post-marketing surveillance data suggest PML as a rare and potentially fatal complication of CAR-T therapy that may develop months to years later. Clinicians should be conscious of this complication, which is likely the result of prolonged B-cell aplasia and immunosuppression rather than direct neurological toxicity of CAR-T cells.
Conflict-of-interest disclosure: R.S. has a consulting or advisory role with Medexus, MyBiotics. M.-A.P. has stock and other ownership interests in NexImmune; honoraria from MorphoSys; a consulting or advisory role with Incyte, Merck, Servier/Pfizer, NexImmune, Novartis, MolMed, Medigene, Takeda, Nektar, AbbVie, Cidara Therapeutics, Celgene, Kite/Gilead, Bristol Myers Squibb, Omeros, and Vor Biopharma; and has received research funding from Incyte (to institution for clinical trial support), Miltenyi Biotec (to institution for clinical trial support), Novartis (to institution for clinical trial support), Kite, a Gilead company (to institution for clinical trial support), and Nektar (to institution for clinical trial support). E.R. has a consulting role with Novartis (outside the submitted work).
Acknowledgments
The authors thank the Cellular Immunotherapy Data Resource (CIDR) from the Center for International Blood and Marrow Transplant Research (CIBMTR) for providing registry data on patients treated with chimeric antigen receptor T cells.
This work was supported in part by National Institutes of Health/National Cancer Institute Cancer Center Support Grant P30 CA008748. R.S. was supported by the American Society of Transplantation and Cellular Therapy New Investigator Award, the American Society of Hematology Fellow Scholar Award, a grant from the Long Island Sound Chapter, Swim Across America, the Robert Hirschhorn Award, and the Memorial Sloan Kettering Steven Greenberg Lymphoma Research Award.
Authorship
Contribution: A.G. and R.S. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis; A.G., R.S., and M.-A.P. conceptualized and designed the study; A.G., R.S., M.-A.P., E.R., J.C., B.D.S., and M.C.P. acquired, analyzed, or interpreted data; A.G. and R.S. drafted the manuscript; A.G., R.S., M.-A.P., E.R., J.C., B.D.S., and M.C.P. critically revised the manuscript; A.G., E.R., and R.S. provided statistical analysis; and R.S. provided supervision.
Footnotes
The FAERS is a publicly available and anonymized database.
The online version of this article contains a data supplement.
Supplementary Material
References
- 1.Cortese I, Reich DS, Nath A. Progressive multifocal leukoencephalopathy and the spectrum of JC virus-related disease. Nat Rev Neurol. 2021;17(1):37–51. doi: 10.1038/s41582-020-00427-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45–56. doi: 10.1056/NEJMoa1804980. [DOI] [PubMed] [Google Scholar]
- 4.Strati P, Varma A, Adkins S, et al. Hematopoietic recovery and immune reconstitution after axicabtagene ciloleucel in patients with large B-cell lymphoma. Haematologica. 2021;106(10):2667–2672. doi: 10.3324/haematol.2020.254045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baird JH, Epstein DJ, Tamaresis JS, et al. Immune reconstitution and infectious complications following axicabtagene ciloleucel therapy for large B-cell lymphoma. Blood Adv. 2021;5(1):143–155. doi: 10.1182/bloodadvances.2020002732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rejeski K, Perez A, Sesques P, et al. CAR-HEMATOTOX: a model for CAR T-cell-related hematologic toxicity in relapsed/refractory large B-cell lymphoma. Blood. 2021;138(24):2499–2513. doi: 10.1182/blood.2020010543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sdrimas K, Diaz-Paez M, Camargo JF, Lekakis LJ. Progressive multifocal leukoencephalopathy after CAR T therapy. Int J Hematol. 2020;112(1):118–121. doi: 10.1007/s12185-020-02840-x. [DOI] [PubMed] [Google Scholar]
- 8.Mackenzie S, Shafat M, Roddy H, et al. Pembrolizumab for the treatment of progressive multifocal leukoencephalopathy following anti-CD19 CAR-T therapy: a case report. eJHaem. 2021;2(4):848–853. doi: 10.1002/jha2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mian A, Andrapalliyal N, Weathers AL, Pohlman B, Hill BT. Late occurrence of progressive multifocal leukoencephalopathy after anti-CD19 chimeric antigen receptor T-cell therapy. Eur J Haematol. 2021;106(4):584–588. doi: 10.1111/ejh.13583. [DOI] [PubMed] [Google Scholar]
- 10.Mackenzie S, Laurence A, O’Reilly M, Peggs KS, Roddie C. Progressive multifocal leukoencephalopathy in the era of chimeric antigen receptor T-cell therapy. Lancet Haematol. 2021;8(12):e870–e873. doi: 10.1016/S2352-3026(21)00316-1. [DOI] [PubMed] [Google Scholar]
- 11.Bate A, Evans SJW. Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol Drug Saf. 2009;18(6):427–436. doi: 10.1002/pds.1742. [DOI] [PubMed] [Google Scholar]
- 12.Harpaz R, Dumouchel W, Lependu P, et al. Performance of pharmacovigilance signal-detection algorithms for the FDA Adverse Event Reporting System. Clin Pharmacol Ther. 2013;93(6):539–546. doi: 10.1038/clpt.2013.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moskop A, Hu Z-H, Pasquini MC. Center for International Blood and Marrow Transplant Research; Milwaukee: 2020. Current uses of CAR T-cell therapies in the US: CIDR summary slides. [Google Scholar]
- 14.Bartsch T, Rempe T, Leypoldt F, et al. The spectrum of progressive multifocal leukoencephalopathy: a practical approach. Eur J Neurol. 2019;26(4) doi: 10.1111/ene.13906. 566-e41. [DOI] [PubMed] [Google Scholar]
- 15.Carson KR, Evens AM, Richey EA, et al. Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the Research on Adverse Drug Events and Reports project. Blood. 2009;113(20):4834–4840. doi: 10.1182/blood-2008-10-186999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Major EO, Yousry TA, Clifford DB. Pathogenesis of progressive multifocal leukoencephalopathy and risks associated with treatments for multiple sclerosis: a decade of lessons learned. Lancet Neurol. 2018;17(5):467–480. doi: 10.1016/S1474-4422(18)30040-1. [DOI] [PubMed] [Google Scholar]
- 17.Bloomgren G, Richman S, Hotermans C, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med. 2012;366(20):1870–1880. doi: 10.1056/NEJMoa1107829. [DOI] [PubMed] [Google Scholar]
- 18.Vermersch P, Kappos L, Gold R, et al. Clinical outcomes of natalizumab-associated progressive multifocal leukoencephalopathy. Neurology. 2011;76(20):1697–1704. doi: 10.1212/WNL.0b013e31821a446b. [DOI] [PubMed] [Google Scholar]
- 19.Cortese I, Beck ES, Al-Louzi O, et al. BK virus-specific T cells for immunotherapy of progressive multifocal leukoencephalopathy: an open-label, single-cohort pilot study. Lancet Neurol. 2021;20(8):639–652. doi: 10.1016/S1474-4422(21)00174-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cortese I, Muranski P, Enose-Akahata Y, et al. Pembrolizumab treatment for progressive multifocal leukoencephalopathy. N Engl J Med. 2019;380(17):1597–1605. doi: 10.1056/NEJMoa1815039. [DOI] [PubMed] [Google Scholar]
- 21.Alstadhaug KB, Croughs T, Henriksen S, et al. Treatment of progressive multifocal leukoencephalopathy with interleukin 7. JAMA Neurol. 2014;71(8):1030–1035. doi: 10.1001/jamaneurol.2014.825. [DOI] [PubMed] [Google Scholar]
- 22.Durali D, De Goër De Herve MG, Gasnault J, Taoufik Y. B cells and progressive multifocal leukoencephalopathy: search for the missing link. Front Immunol. 2015;6 doi: 10.3389/fimmu.2015.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bennett CL, Focosi D, Socal MP, et al. Progressive multifocal leukoencephalopathy in patients treated with rituximab: a 20-year review from the Southern Network on Adverse Reactions. Lancet Haematol. 2021;8(8):e593–e604. doi: 10.1016/S2352-3026(21)00167-8. [DOI] [PubMed] [Google Scholar]
- 24.Fried S, Avigdor A, Bielorai B, et al. Early and late hematologic toxicity following CD19 CAR-T cells. Bone Marrow Transplant. 2019;54(10):1643–1650. doi: 10.1038/s41409-019-0487-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.