Abstract
Objectives
To describe the clinical presentation, management, and outcome of cases treated for septic peritonitis secondary to intra-peritoneal grass awn migration.
Animals
Six client-owned dogs and 1 client-owned cat.
Procedures
Clinical data of dogs and cats treated surgically for septic peritonitis secondary to an intra-peritoneal grass awn identified during surgery between January 2014 and December 2021 were retrospectively reviewed. Data included signalment, clinical presentation, blood test results, diagnostic imaging findings, surgical procedure, postoperative complications, and outcome. Telephone interviews were conducted for long-term follow-up.
Results
Six dogs and 1 cat met the inclusion criteria. The most common reported clinical signs were lethargy (n = 7), anorexia/dysorexia (n = 4), and pyrexia (n = 4). The vegetal foreign body was not identified in any case with ultrasound (0/5) and only suspected for one case with a computed tomography scanner (1/4). A grass awn was identified within an omental abscess for each case during surgery. Abscess resection for each case resulted in partial pancreatectomy and a splenectomy for 1 case, and partial gastrectomy for another case. All cases survived to discharge. Only one minor post-operative complication was identified, and no other complication was reported at the long-term telephone interview.
Conclusion and clinical significance
Septic peritonitis secondary to omental grass awn foreign body is an uncommon condition that has a good to excellent prognosis after surgical treatment. Identification of omental grass awn with ultrasound and computed tomography is rare. Therefore, particular care should be given to omental exploration during surgery for septic peritonitis with no underlying cause identified.
Résumé
Péritonite septique secondaires à la migration de barbes de graminées : 7 cas (2014-2021)
Objectifs
Décrire la présentation clinique, la prise en charge et pronostic des chiens et chats traité pour une péritonite septique secondaire à la migration intrapéritonéale d’un corps étranger végétal.
Animaux
Six chiens et un chat traités.
Protocole
Les informations des dossiers des chiens et chats pris en charge chirurgicalement pour une péritonite septique pour lesquelles un corps étranger végétal intrapéritonéal a été identifié entre janvier 2014 et décembre 2021 ont été obtenues. Les données concernant le signalement, la présentation clinique, les analyses sanguines, les examens d’imagerie, les procédures chirurgicales, les complications post-opératoires et l’évolution post-opératoire ont été collectées. Le suivi à long terme a été réalisé par téléphone.
Résultats
Six chiens et un chat ont été inclus dans l’étude. Les signes cliniques les plus fréquents rapportés sont l’apathie (n = 7), l’anorexie/dysorexie (n = 4) et l’hyperthermie (n = 4). Les corps étrangers végétaux n’ont été identifiés dans aucun cas par échographie (0/5) et suspectés dans un cas à l’examen tomodensitométrique (1/4). Les corps étrangers ont tous été identifiés au sein d’un abcès dans l’omentum en chirurgie. Une résection de l’abcès a été réalisée dans chaque cas associé à une pancréatectomie partielle et une splénectomie pour un cas et une gastrectomie partielle pour un autre cas. Tous les animaux ont survécu. Seule une complication mineure a été rapportée en post-opératoire et aucune autre complication n’a été rapportée lors des entretiens téléphoniques.
Conclusion et portée clinique
Les péritonites septiques secondaires à des migrations intra-péritonéale de corps étrangers végétaux sont des affections rares qui semblent être associées à un pronostic de survie bon à excellent après prise en charge chirurgicale. L’identification des corps étrangers dans l’omentum est difficile par échographie ou examen tomodensitométrique. Par conséquent, une attention particulière doit être portée à l’exploration de l’omentum lors de prise en charge chirurgicale de péritonite septique sans cause sous-jacente identifiée.
(Traduit par les auteurs)
Introduction
Grass awn migration related diseases are frequently encountered in some regions of the world, especially southern Europe, Oceania, and some parts of the United States (1–3). Various clinical signs can be encountered depending on the location of the migration. The most frequent locations for grass awn migration include the thoracic, subcutaneous, and retroperitoneal spaces (1–5). Migration into the peritoneal cavity is possible and results in clinical signs related to the affected organs. Pancreatic, prostatic, uterine, vesical, and splenic migrations have been described (6–9) and secondary septic peritonitis can occur.
Septic peritonitis is a life-threatening condition defined as an inflammation of the peritoneum secondary to bacterial contamination and infection. History and clinical signs are not specific and include lethargy, anorexia, vomiting, abdominal discomfort, polyuria, and polydipsia (10–12). Diagnosis is based on identification of intracellular bacteria and degenerative neutrophils within abdominal fluid (10). Comparison of glucose level and lactatemia between a blood sample and abdominal fluid can also be used. Elevation of lactate and a decrease of at least 20 mg/dL in glucose levels inside abdominal fluid point to septic peritonitis (10,11). Emergency care and rapid surgical exploration after stabilization are necessary to give the patient the highest chance of recovery. Survival rate is similar for dogs and cats and ranges from 32 to 80% depending on the underlying cause (10–12). Intestinal perforations secondary to intestinal foreign body, neoplasia, surgical suture dehiscence, or trauma are reported most frequently (10,11). Some cases of septic peritonitis secondary to wood stick migration from the gastrointestinal tract have been described (13) but none have been reported due to grass awn migration.
The purpose of this case series is to describe the clinical presentation, management, and outcome of dogs and cats treated for septic peritonitis secondary to intraperitoneal grass awn migration.
Materials and methods
Electronic medical records of our hospital were reviewed to identify all cases of dogs and cats treated for septic peritonitis secondary to grass awn migration between January 2014 and December 2021. Search words included: septic peritonitis, grass awn migration, and omental, hepatic, pancreatic, urinary bladder, retroperitoneal, kidney, and splenic abscesses. Only cases with septic peritonitis treated surgically and ≥ 1 intraabdominal grass awns identified during surgery were included. Septic peritonitis was diagnosed if intracellular bacteria were identified inside polynuclear neutrophils at fluid cytology or if glycemia was lower and lactatemia higher in the fluid than in the blood. Cases were excluded if no or only local peritonitis was identified. All records were reviewed by 2 authors. Signalment, clinical signs, duration of clinical signs prior to presentation, complete blood (cell) count, plasma biochemical analysis results, imaging findings, surgical techniques and findings, duration of hospitalization, bacteriologic culture results, postoperative treatments, and complications were recorded. Short-term follow-up information (until discharge from the hospital or at the time of suture removal) was also obtained from the medical records. Imaging findings were collected and reviewed by the same author including ultrasonographic findings and computed tomography (CT) findings. Ultrasonographic examinations were performed by an ACVR or ECVDI diplomate with experience in diagnostic ultrasound and migrating grass awns that are frequently encountered in our geographic area. Surgeries were performed by an ECVS diplomate or a resident under direct supervision. Location of grass awns, lesions encountered, and surgical techniques were noted. Minor postoperative complications were defined as all events related to the surgery that were managed medically or resolved without treatment. Major complications included those that required a second surgery or that resulted in the dog’s death or euthanasia. For the long-term follow-up, telephone interviews were conducted with owners or referring veterinarians by the same author at least 2 mo after discharge. Questions were asked about wound healing, resolution of clinical signs, treatment observance, and recurrence.
Results
Six dogs and 1 cat met the inclusion criteria. Case signalment, clinical presentation, blood tests results, imaging findings, surgical findings, and follow-up are summarized in Table 1. Dog breeds included Lhasa apso (n = 1), English setter (n = 1), Basset fauve de Bretagne (n = 1), German shepherd (n = 1), Brittany spaniel (n = 1), and Belgian shepherd (n = 1). There were 3 intact males and 3 intact females. The median age was 38 mo (range: 14 to 64 mo) and median body weight was 22.75 kg (range: 7.8 to 30.5 kg). The only cat was a 29-month-old intact female domestic shorthair, weighing 3.3 kg.
Table 1.
Summary of case signalment and clinical presentation.
| Case | Breed | Age (y) | Sex | Body weight (kg) | History | Clinical examination |
|---|---|---|---|---|---|---|
| 1 | Lhasa apso | 5 | M | 7.8 | Anorexia, lethargy (3 d). Septic peritonitis without response to medical treatment. Grass awn removal from penis. |
↑T°C 39.2, abdominal distension, ↑HR |
| 2 | English setter | 1 | M | 22 | Lethargy, weight loss (1 mo). | ↑T°C 39.5 abdominal distension, ↑HR & RR |
| 3 | Basset fauve de Bretagne | 2 | F | 14.2 | Lethargy, fluctuating hyperthermia (5 mo). Laparotomy: no foreign body. No response to broad spectrum ATB. |
↑T°C 39.3, abdominal and paralumbar pain, ↓HR |
| 4 | German shepherd | 3 | M | 26.6 | Vomiting, lethargy, hyperthermia, abdominal distension (15 d). No response to broad spectrum ATB. | ↑T°C 39.5 ↑HR & RR, congestive mucous membranes, abdominal distension |
| 5 | Belgian shepherd | 3 | F | 30.5 | Hyperthermia, lethargy, and abdominal distension, PUPD (6 d). | ↑T°C 39.5, abdominal distension, ↑HR |
| 6 | Brittany spaniel | 4 | F | 23.5 | Lethargy, vomiting, tenesme (1 wk). Abdominal septic effusion, no response to broad spectrum ATB. | ↑T°C 39.8 ↑HR & RR, abdominal distension |
| 7 | Domestic shorthair cat | 2 | F | 3.3 | Lethargy, back pain, vulva licking (2 mo). Prostration, hyperthermia, abdominal pain (1 wk). No response to NSAID. | Abdominal pain |
T°C — Corporeal temperature; HR — Heart rate; RR — Respiratory rate; ↑ — Increase; ↓ — Decrease.
The most often reported clinical signs were lethargy (n = 7), anorexia/dysorexia (n = 4), pyrexia (n = 4), vomiting (n = 2), abdominal pain (n = 1), and abdominal distension (n = 1). The other clinical signs reported were weight loss, polyuria/polydipsia, and tenesmus. One dog had a history of grass awn removal from the penis a few months before (Case 1) and the cat had a 2-month history of vulva licking. The median time between the onset of clinical signs and presentation to our hospital was 58 d (range: 7 to 150 d). Five animals (4 dogs and the cat) were initially presented to their referring veterinarian, with no response to the initial treatment including a laparotomy for Case 3 (Table 1). Clinical findings at the time of admission to our hospital included hyperthermia in all dogs (n = 6), abdominal distension (n = 5), tachycardia (n = 4), abdominal discomfort (n = 3), and bradycardia (n = 1).
Hematological results were available for all dogs (Table 2). The most common hematological findings included neutrophilia with a left shift (n = 6) and anemia (n = 2). The most common biochemical findings included hypoalbuminemia (n = 5), hyperlactatemia (n = 4), and hypoglycaemia (n = 2). C-reactive protein dosage was only available for Case 3 and was elevated. Abdominal effusion was reported in all cases and was consistent with septic effusion in 5 cases with intracellular bacteria identified within polynuclear neutrophils on cytologic examination. In 2 cases, segmented neutrophils with no bacteria were identified on cytologic examination of the abdominal fluid. Those cases had received antibiotics prior to presentation. For these 2 cases glucose was lower in the fluid than in the blood and lactate was higher.
Table 2.
Summary of complementary examination results.
| Case | Laboratory findings | Imaging findings |
|---|---|---|
| 1 | Left shift neutrophilia, ↓ albuminemia 21 g/L, ↑ lactatemia 4.4 mmol/L | US: severe peritoneal effusion, severe generalized steatitis, pancreatitis, hypoechogenic hepatopathy |
| 2 | Left shift neutrophilia, anemia, ↓ albuminemia 23 g/L | US: severe peritoneal effusion, severe generalized steatitis, ill-delineated hypoechoic patchy nodular peritoneal foci |
| 3 | Left shift neutrophilia, ↑CRP 92.9 mg/L | CT: severe peritoneal effusion, diffuse hypoenhancing foci within steatitis |
| 4 | Left shift neutrophilia, anemia, ↓ albuminemia 17 g/L, ↑ lactatemia 7.3 mmol/L, ↓ glycemia 0.6 g/L | US: severe peritoneal effusion, severe localized steatitis, ill-delineated hypoechoic patchy nodular focus in left cranial abdomen, heterogenous hepatopathy |
| 5 | Left shift neutrophilia, ↓ albuminemia 22 g/L, ↑ lactatemia 4.3 mmol/L | US: moderate peritoneal effusion, severe diffuse steatitis, abdominal lymphadenomegaly, hypoechoic hepatopathy, paralytic ileus |
| CT: moderate peritoneal effusion, generalized steatitis, abdominal lymphadenomegaly | ||
| 6 | Left shift neutrophilia, ↓ albuminemia 23 g/L, ↑ lactatemia 3 mmol/L, ↓ glycemia 0,6 g/L | US: severe peritoneal effusion, focal left cranial abdominal steatitis, abdominal lymphadenomegaly, hypoechoic hepatopathy |
| CT: severe peritoneal effusion, several hypoenhancing foci in localized steatitis in left cranial abdomen, left caudal bronchiectasis, abdominal lymphadenomegaly | ||
| 7 | No abnormality | CT: moderate peritoneal effusion, suspicion of peritoneal foreign body, cavitary nodule in right caudal abdomen, moderate localized steatitis |
US — Ultrasound; CT — Computed tomography; ↑ — Increase; ↓ — Decrease.
Diagnostic imaging reports were available for all cases: ultrasound (n = 5), CT (n = 4), or both (n = 2). Ultrasonographic examination failed to identify grass awn in all 5 cases. Peritoneal effusion was identified in all cases in variable amounts [moderate (n = 2) or severe (n = 3)] and variable echogenicity [hypoechoic fluid (n = 2) and echoic fluid (n = 3)]. Severe localized or generalized peritoneal was also identified in all cases; described as illdefined heterogenous and hyperechoic thickening of the peritoneal fat. An ill-delineated hypoechoic patchy nodular focus was observed in 2 dogs, but no abscess was clearly identified. Other abdominal lesions identified are summarized in Table 2. The most frequent lesions identified on CT were similar to ultrasonographic examination including peritoneal effusion (n = 4) and focal to diffuse heterogenous fat to soft tissue attenuating partially enhancing areas within the peritoneal fat indicating steatitis (n = 3) (Figure 1). A migrating foreign body was suspected in only one case (Case 7) in a caudal and right-sided cavitary lesion (Figure 2). Two other cases had cavitary lesions described as diffuse hypoenhancing foci within steatitis in Case 3 and as several hypoenhancing foci in localized steatitis in the cranial and left lateral part of the abdomen in Case 6. In that case, mild increase in bronchus diameter in the caudal left pulmonary lobe was observed, without changes of the bronchial wall or lumen. Surgical exploration with coeliotomy was performed in all cases. Surgical findings and surgical techniques are summarized in Table 3. In all procedures, a single grass awn was identified within an omental abscess without any sign of gastrointestinal perforation, organ disruption, or draining tract (Figure 3). In Case 2, a grass awn was located in an abscess at the surface of the greater curvature of the stomach. In Case 4, the abscess involved the extremity of the left arm of the pancreas with adhesions to the spleen, stomach, and jejunum. En-bloc resection of the omental abscesses were performed with thermofusion (Ligasure; Medtronic, Brampton, Ontario) for all 7 cases. Partial pancreatectomy and splenectomy were performed in Case 4, and partial gastrectomy in Case 2. A Jackson-Pratt abdominal drain was placed in all cases. Bacterial culture (aerobic and anerobic) was performed and was positive for all cases except Case 1. Aerobic bacteria identified were Staphylococcus pseudintermedius in 1 case and Pasteurella multocida in 2 cases. Anaerobic bacteria were Porphyromonas sp. in 2 cases, Porphyromonas gingivalis in 1 case, and Peptostreptococcus canis in association with Porphyromonas sp. in Case 3. Postoperative analgesia was provided with morphine chlorhydrate (0.1 to 0.2 mg/kg, IV, q4h) or methadone (0.1 to 0.2 mg/kg, IV, q4h) for 24 to 72 h.
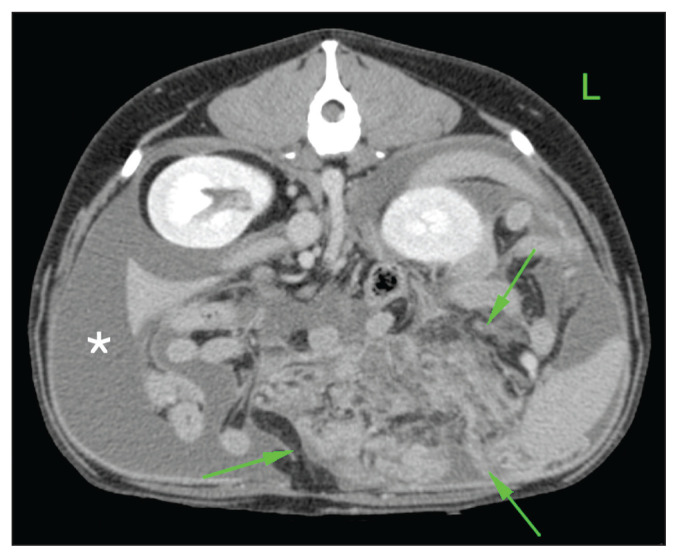
Figure 1.
Computed tomographic view of the cranial abdomen of Case 5 displayed in soft tissue window. A localized and severe steatitis is seen in the left cranial abdomen caudal to the gastric fundus (arrows). Some hypoenhancing foci indicating cavitary lesions are identified, without evidence of peritoneal foreign body. Peritoneal fluid is also noted (asterisk).
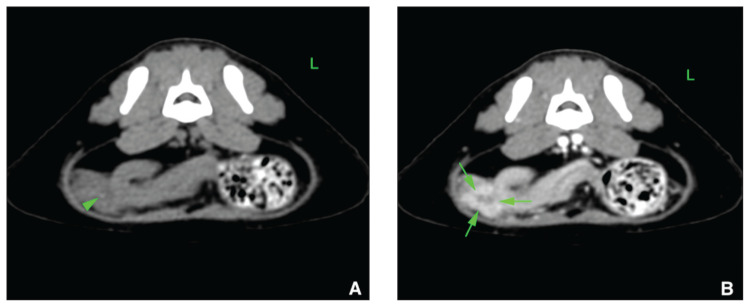
Figure 2.
Computed tomographic view of the caudal abdomen of Case 7 displayed in a soft tissue window in transverse plane (A — No contrast medium injection; B — After contrast medium injection). A 3-mm hyperattenuating linear structure (arrowhead) is seen in the caudoventral, right aspect of the peritoneal space. This structure is located in a slightly hypoattenuating non-enhancing cavitation (arrows), whereas the surrounding soft tissue structures exhibit mild hyperattenuation and enhancement. This structure was interpreted to represent a possible foreign body.
Table 3.
Summary surgical findings, surgical treatment, and follow-up.
| Case | Surgical findings | Surgical treatment | During hospital (d) | Follow-up |
|---|---|---|---|---|
| 1 | Severe peritoneal effusion, omental abscess with a grass awn | Omental abscess resection | 8 | Survived to discharge. No post-operative complications. Alive 1382 d after surgery. |
| 2 | Severe peritoneal effusion, omental abscess near the great stomach curvature with a grass awn | Omental abscess resection and partial gastrectomy | 7 | Survived to discharge. Subcutaneous seroma. Alive 1035 d after surgery. |
| 3 | Severe peritoneal effusion, multiple omental abscesses with a grass awn inside one cavity | Omental resection of abscesses | 5 | Survived to discharge. No post-operative complications. Alive 746 d after surgery. |
| 4 | Severe peritoneal effusion, omental and left pancreatic abscess with a grass awn, Adhesion between spleen, stomach, and jejunum | Omental abscess resection, partial pancreatectomy, and splenectomy | 4 | Survived to discharge. No post-operative complications. Alive 498 d after surgery. |
| 5 | Moderate peritoneal effusion, numerous omental abscesses with adhesion to the stomach without draining tract, grass awn inside omental abscess | Omental resection of abscesses | 5 | Survived to discharge. No post-operative complications. Alive at 81 d after surgery. |
| 6 | Severe peritoneal effusion, omental abscess with a grass awn | Omental abscess resection | 6 | Survived to discharge. No post-operative complications. Alive at 102 d after surgery. |
| 7 | Moderate peritoneal effusion, omental abscess with a grass awn in the right caudal abdomen | Omental abscess resection | 3 | Survived to discharged. No post-operative complications. Alive 213 d after surgery. |
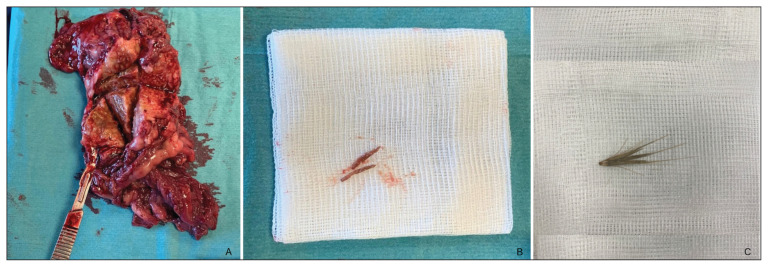
Figure 3.
A — Postoperative photograph of an omental abscess resected (Case 3). B — After dissection of the different lesions retrieved, a grass awn was identified inside one of them. C — A normal aspect of a grass awn.
Peri- and postoperative amoxicillin and clavulanic acid (12.5 to 20 mg/kg, PO, q12h) was administered in all cases for a median of 10 d (range: 8 to 15 d). When necessary, treatment was readjusted according to antibiogram results. Nonsteroidal antiinflammatory drugs were administered in 3 dogs and the cat.
All animals survived to discharge after a median time of hospitalization of 5 d (range: 3 to 8 d). Only 1 short-term complication was reported in Case 2 with development of a subcutaneous seroma. Median time between discharge and long-term telephone follow-up was 622 d (range: 81 to 1382 d). All animals were still alive, and all treatments prescribed had been correctly administered. No other complication or recurrence was reported.
Discussion
This case series is, to our knowledge, the first concerning dogs and a cat treated for septic peritonitis secondary to a migrating grass awn.
Cases of migrating vegetal foreign bodies causing disease are frequent in some regions of the world, especially southern Europe, Oceania, and some parts of the USA (1,2,3,5). Grass awn foreign bodies may affect animals of any age or breed and seem to be more frequent in dogs than in cats (2). The inclusion of 3 hunting dogs and 2 working dogs in this series is consistent with previous reports in which hunting and sporting breeds were overrepresented (1,2,14,15). A large spectrum of clinical signs and lesions can be encountered depending on the location of the migration and the entrance path. Most of the migrations reported in the different studies are within the respiratory tract, subcutaneous tissues, or sublumbar muscles. In all our cases, a grass awn was located in an omental abscess without any signs of a draining tract and no lesions were identified in the surrounded organs indicating a migration through them. Although the presence of a grass awn within the abdomen does not allow elucidation of a precise pathway of entry, theories can be considered based upon the clinical information. Case 7 had a 2-month history of vulva licking reported by his owner associated with back pain. Uterine migration of grass seeds in dogs and cats have been described (16,17). Migration from the vulva and then vagina and uterus was suspected in our cat. Case 1 had an history of grass awn removal from the penis a few days before initial examination by the attending veterinarian. Migrations of grass seeds through the urinary tract (bladder, urethra, prostate) have been described in dogs and cats (2,7,8) and should be included in the differential diagnosis of intramural bladder lesions or urethral obstruction. However, this migration pathway seems unlikely herein as no micturition problem was identified. Ingestion of grass awn and transmural migration through the gastric wall was suspected in Cases 2 and 4 because of the close relationship between omental abscess and the gastric wall even if no gastric lesion was detected, as previously described (15). However, Hopper et al (18) described a case of pneumothorax and focal peritonitis in a dog due to a migration of an inhaled grass awn localized in an omental abscess. Thus, migration through the lungs can be considered for Cases 5, 6, and 7. The presence of an enlarged bronchus on the CT scan for Case 6 and culture of P. multocida for Cases 5 and 7 lend some support to this theory; however, these findings do not provide definitive evidence of pulmonary migration. Pasteurella multocida is a bacterium commonly located in the respiratory tract, whereas S. pseudointermedius was cultured in Case 2, which may favor transcutaneous migration.
In our case series, ultrasound and CT imaging were used for abdominal scanning, which is consistent with previous studies reporting these as preferred imaging techniques for etiologic evaluation of lethargy and pyrexia. Radiologists who performed and interpreted the examinations had solid experience in detection of migrating foreign bodies because of the high incidence in this geographic area. Nevertheless, a foreign body was suspected in only 1 case on CT examination, and the detection of a peritoneal foreign body was missed in all cases on ultrasonographic examinations. This is not consistent with a previous study that showed good sensitivity using ultrasound, which facilitates foreign body diagnosis in the abdominal cavity (6). Several factors could be responsible for the failure to detect peritoneal foreign bodies with ultrasonographic examination in the current case series as air in digestive tract or body weight and size of patient. In ultrasonographic cases, diffuse or focal hyperechoic and heterogenous thickened of the peritoneal fat referred as steatitis probably impaired identification of abscesses or vegetal foreign bodies. Our findings are also not consistent with the detection of vegetal foreign bodies by CT examination according to previous reports (3,13) that indicated a sensitivity between 84 and 100% in diagnosis of foreign bodies in sublumbar, abdominal wall, or thoracic locations. It could be explained by CT attenuation of vegetal foreign bodies as sometimes similar to surrounding tissue, thus making identification more difficult (8,19), with false negatives from 10 to 100% (14,20). Major imaging abnormalities in our case series were abdominal effusion and generalized or focal steatitis resulting from inflammation of fat tissue, primary or secondary to inflammation of surrounding structure. These imaging findings are consistent with previous studies on ultrasonographic and CT examinations in septic peritonitis (6,21–23). Although diagnostic imaging failed to identify the foreign body, for several cases a probable location of the cause of inflammation or infection was identified; this increased our suspicion of grass awn foreign bodies. There was a good correlation between diagnostic imaging lesions and surgical findings, in line with other studies reporting an agreement between 77 and 78% for CT examination (3,20). This was also the case for the cat with suspected foreign body on CT examination, which was located in the right-sided and caudal abdomen. Finally, according to this study, ultrasound and CT remain reliable techniques to detect peritoneal lesions and are useful for exploratory surgery. These techniques allow the exclusion of numerous underlying causes such as gastrointestinal leakage or pyometra for ultrasound and CT, and possible concomitant lesions such as discospondylitis or pulmonary lesions for CT.
Prognosis after surgical treatment in our case series was excellent, with all animals being discharged and alive without recurrence at the time of the telephone interview up to 45 mo later. Survival rates for dogs and cats treated surgically for septic peritonitis is similar and ranges from 32 to 80% depending on the underlying causes (10–13), with septic biliary peritonitis carrying the poorest prognosis. Multiple preoperative factors have been identified as negative prognostic factors for dogs and cats treated for septic peritonitis, notably preoperative multiple organ dysfunction syndrome, hypotension, hypoalbuminemia, hypoglycemia, or high hematocrit (19,24,25). Five of our cases had at least 1 of these factors. Moreover, hospitalization time here was comparable to other studies describing septic peritonitis (19,22,25,26). Thus, the difference in survival rate between our cases and the literature may be related to the underlying cause. Omental abscesses are easy to treat if their location allows a complete resection, as in our cases. Bacterial contamination could also be more limited compared to other sources of infection such as intestinal leakage. Post-operative care such as early feeding, continuous rate infusion, drugs during hospitalization or time of drain removal could also have influenced recovery. Unfortunately, all those data were not available. Finally, this difference may be related to our small number of cases, which may not be representative of a larger population. As no underlying causes have been identified prior to surgery, all those cases could have been first classified has primary septic peritonitis. However, one study has shown a better survival rate for dogs treated medically for primary septic peritonitis than those treated surgically (22). Thus, in a geographic area where grass awns migrations are frequent, our results should support the systematic methodology for surgical exploration for septic peritonitis when possible, even if primary septic peritonitis is suspected. For such cases, complete omental exploration should be carried out if no underlying cause is identified during surgery.
This series had some limitations, several being related to its retrospective nature. There was no standardization on diagnostic imaging techniques, which could have led to bias in preoperative detection of grass awns. There was also no standardization of surgical and postoperative treatments. Moreover, some data may have been missed if not reported in the patient’s medical records, especially in the case history. Data about postoperative evolution and treatment during hospitalization were also missing for every case. Finally, the small number of cases in this series precluded statistical analyses and limited robust comparisons to larger studies.
In conclusion, septic peritonitis secondary to free omental grass awn foreign body is an uncommon condition that has a good to excellent prognosis following surgical treatment, although these findings need to be validated with larger studies. In our series, identification of an omental grass awn with preoperative ultrasound and CT was difficult. Therefore, omental exploration is recommended for cases of septic peritonitis in which no underlying cause was identified prior to surgery and no other abnormalities are determined, especially in areas with a high incidence of grass awn migration. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Brennan KE, Ihrke PJ. Grass awn migration in dogs and cats: A retrospective study of 182 cases. J Am Vet Med Assoc. 1983;182:1201–1204. [PubMed] [Google Scholar]
- 2.Combs M, Decker A, Young P, et al. Grass seed foreign body-related disease in dogs and cats: A wide spectrum of clinical presentations. Aust Vet Pract. 2017;47:13–24. [Google Scholar]
- 3.Griffeuille E, Seriot P, Baudin-Tréhiou C, et al. Comparison of computed tomography and surgical findings and investigation of their associations with outcomes for dogs with sublumbar abscesses. J Am Vet Med Assoc. 2021;259:1300–1308. doi: 10.2460/javma.20.07.0403. [DOI] [PubMed] [Google Scholar]
- 4.Baudin-Tréhiou C, Gibert S, Sériot P, Dunié-Mérigot A, Blond L. CT is helpful for the detection and presurgical planning of lung perforation in dogs with spontaneous pneumothorax induced by grass awn migration: 22 cases. Vet Radiol Ultrasound. 2020;61:157–166. doi: 10.1111/vru.12831. [DOI] [PubMed] [Google Scholar]
- 5.Sériot P, Dunié-Mérigot A, Baudin-Tréhiou C, et al. Treatment and outcome of spontaneous pneumothorax secondary to suspected migrating vegetal foreign body in 37 dogs. Vet Rec. 2021;189:e22. doi: 10.1002/vetr.22. [DOI] [PubMed] [Google Scholar]
- 6.Citi S, Mannucci T, Pedala F, Vannozzi I, Vignoli M. Acute pancreatitis associated with peritoneal migration of grass awn in two dogs. Acta Veterinaria. 2017;67:587–592. [Google Scholar]
- 7.Lomax KG. Mean seeds, migrating plant awns embedded in a miniature poodle’s bladder wall. Open Vet J. 2021;11:418–421. doi: 10.5455/OVJ.2021.v11.i3.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marchesi MC, Moretti G, Angeli G, et al. Prostatic localization of a migrating grass awn foreign body in a dog. Vet Sci. 2020;7:192. doi: 10.3390/vetsci7040192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song JH, Jang HM, Lee HC, et al. Grain foreign body embedded in the spleen and immune-mediated hemolytic anemia in a Maltese dog. J Vet Clin. 2017;34:39–42. [Google Scholar]
- 10.Parsons KJ, Owen LJ, Lee K, Tivers MS, Gregory SP. A retrospective study of surgically treated cases of septic peritonitis in the cat (2000–2007) J Small Anim Pract. 2009;50:518–524. doi: 10.1111/j.1748-5827.2009.00790.x. [DOI] [PubMed] [Google Scholar]
- 11.Ragetly GR, Bennett RA, Ragetly CA. Septic peritonitis: Etiology, pathophysiology, and diagnosis. Compen Contin Educ Vet. 2011;33:1–6. [PubMed] [Google Scholar]
- 12.Swann H, Hughes D. Diagnosis and management of peritonitis. Vet Clin North Am: Small Anim Pract. 2000;30:603–615. doi: 10.1016/s0195-5616(00)50041-2. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Pertierra S, Das S, Burton C, et al. Surgical management of intrathoracic wooden skewers migrating from the stomach and duodenum in dogs: 11 cases (2014–2020) J Small Anim Pract. 2022;63:403–411. doi: 10.1111/jsap.13474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vansteenkiste DP, Lee KCL, Lamb CR. Computed tomographic findings in 44 dogs and 10 cats with grass seed foreign bodies. J Small Anim Pract. 2014;55:579–584. doi: 10.1111/jsap.12278. [DOI] [PubMed] [Google Scholar]
- 15.Gnudi G, Volta A, Bonazzi M, Gazzola M, Bertoni G. Ultrasonographic features of grass awn migration in the dog. Vet Radiol Ultrasound. 2005;46:423–426. doi: 10.1111/j.1740-8261.2005.00077.x. [DOI] [PubMed] [Google Scholar]
- 16.Gatel L, Gory G, De Pauw B, Rault DN. Diagnosis and ultrasound-guided retrieval of a vaginal foreign body in a dog and a cat. Vlaams Diergeneeskd Tijdschr. 2014;83:55–59. [Google Scholar]
- 17.Benzimra C, Couturier L, Gatel L, et al. Ultrasonographic findings associated with uterine migrating grass seeds in eleven dogs. Vlaams Diergeneeskd Tijdschr. 2021;90:17–21. [Google Scholar]
- 18.Hoper JH, Lester VL, Irwin PJ, Eger CE, Richardson JL. Imaging diagnosis: Pneumothorax and focal peritonitis in a dog due to migration of an inhaled grass awn. Vet Radiol Ultrasound. 2004;45:136–138. doi: 10.1111/j.1740-8261.2004.04022.x. [DOI] [PubMed] [Google Scholar]
- 19.Fink O, Buysse A, Drobatz KJ, Bentley A. Identification of risk factors for recurrent secondary septic peritonitis following initial surgical treatment of secondary septic peritonitis in dogs. J Vet Emerg Crit Care. 2020;30:213–220. doi: 10.1111/vec.12939. [DOI] [PubMed] [Google Scholar]
- 20.Bouabdallah R, Moissonnier P, Delisle F, et al. Use of preoperative computed tomography for surgical treatment of recurrent draining tracts. J Small Anim Pract. 2014;55:89–94. doi: 10.1111/jsap.12163. [DOI] [PubMed] [Google Scholar]
- 21.Lamb CR, Pope EHW, Lee KCL. Results of computed tomography in dogs with suspected wooden foreign bodies. Vet Radiol Ultrasound. 2017;58:144–150. doi: 10.1111/vru.12457. [DOI] [PubMed] [Google Scholar]
- 22.Culp WTN, Zeldis TE, Reese MS, Drobatz KJ. Primary bacterial peritonitis in dogs and cats: 24 cases (1990–2006) J Am Vet Med Assoc. 2009;234:906–913. doi: 10.2460/javma.234.7.906. [DOI] [PubMed] [Google Scholar]
- 23.Boysen SR, Tidwell AS, Penninck DG. Ultrasonographic findings in dogs and cats with gastrointestinal perforation. Vet Radiol Ultrasound. 2003;44:556–564. doi: 10.1111/j.1740-8261.2003.tb00507.x. [DOI] [PubMed] [Google Scholar]
- 24.Kenney EM, Rozanski EA, Rush JE, et al. Association between outcome and organ system dysfunction in dogs with sepsis: 114 cases (2003–2007) J Am Vet Med Assoc. 2010;236:83–87. doi: 10.2460/javma.236.1.83. [DOI] [PubMed] [Google Scholar]
- 25.Scotti KM, Koenigshof A, Sri-Jayantha LSH, et al. Prognostic indicators in cats with septic peritonitis (2002–2015): 83 cases. J Vet Emerg Crit Care. 2019;29:647–652. doi: 10.1111/vec.12896. [DOI] [PubMed] [Google Scholar]
- 26.Ruthrauff CM, Smith J, Glerum L. Primary bacterial septic peritonitis in cats: 13 cases. J Am Anim Hosp Assoc. 2009;45:268–276. doi: 10.5326/0450268. [DOI] [PubMed] [Google Scholar]