TO THE EDITOR:
Tisagenlecleucel (tisa-cel) is a CD19 chimeric antigen receptor (CAR) T-cell therapy that has shown exceptional efficacy in young individuals with relapsed/refractory B-cell acute lymphoblastic leukemia (ALL), leading to an overall remission rate of more than 80%.1 Among complications of CAR T-cell therapy, macrophage activation syndrome (MAS)/hemophagocytic lymphohistiocytosis (HLH) is a life-threatening toxicity characterized by hyperinflammation and manifesting as persistent fevers, splenomegaly, end-organ damage, cytopenias, coagulopathy, and hypofibrinogenemia. MAS/HLH can be considered a distinct variant of cytokine release syndrome (CRS) that commonly occurs after CAR T-cell therapy.
After CAR T-cell therapy, MAS/HLH has been reported in as many as 7 of 201 cases (3.48%).2 HLH as a complication of malignancy itself is most commonly seen with lymphomas and is rarely associated with leukemia (6.4%).3 The mortality rate is high in individuals with hematologic malignancies, although the precise mortality rate of CAR T-cell therapy–associated MAS/HLH is unknown because of limited data.4 MAS/HLH after tisa-cel therapy in B-cell ALL has been described in the literature, and various anticytokine therapies including anakinra, etoposide, and dexamethasone have been used as treatment.5 Interferon-γ (IFN-γ) is thought to play a central role in the pathogenesis of HLH.6,7 Emapalumab is an anti–IFN-γ antibody that is approved for the treatment of primary HLH.8
We report a case of CAR T-cell therapy–associated MAS/HLH that was successfully treated with emapalumab in combination with anakinra and corticosteroids.
A 19-year-old Hispanic man with relapsed/refractory Philadelphia-like B-cell ALL with P2RY8-CRLF2 fusion had brief response to frontline hyperfractionated-cyclophosphamide, vincristine, doxorubicin, and dexamethasone. He was refractory to second-line blinatumomab and subsequently to venetoclax and salvage chemotherapy combined with peg-asparaginase. Prelymphodepletion bone marrow biopsy was hypercellular (>95%), with persistent B-lymphoblastic leukemia (97% blasts). He was lymphodepleted with fludarabine and cyclophosphamide, and tisa-cel was administered. The study was approved by the City of Hope Institutional Review Board in accordance with the Declaration of Helsinki.
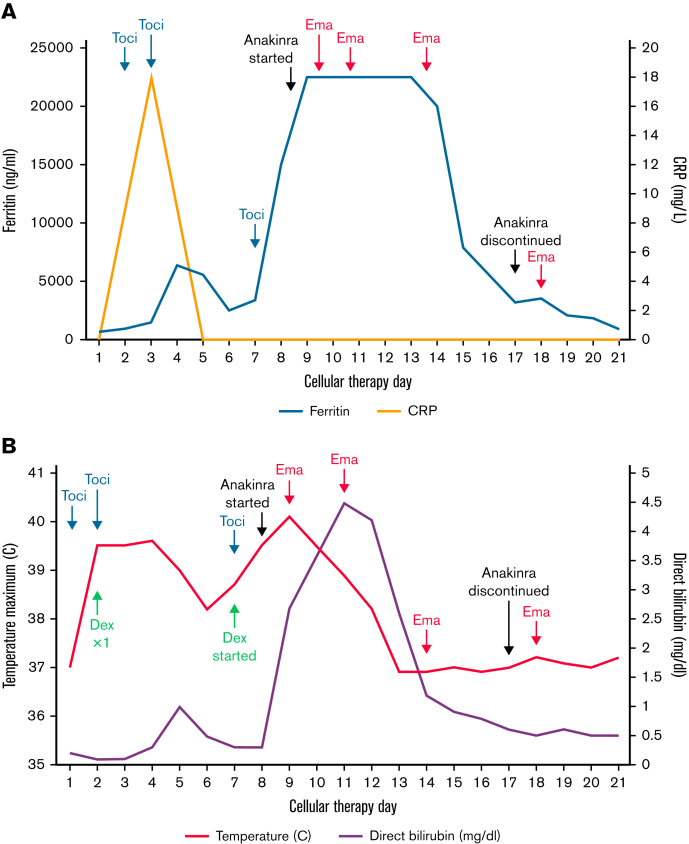
Within 12 hours of tisa-cel, he developed grade 1 CRS that quickly progressed to grade 2, manifesting as fevers and hypotension. He received IV fluids, tocilizumab on days 1 and 2, and dexamethasone on day 2 with clinical response. On day +7, he developed persistent fevers, transaminitis, ferritin elevation from 3282 ng/mL on day +6 to 15 195 ng/mL, and MAS/HLH was suspected. A computerized tomography scan revealed splenomegaly. He was given a third dose of tocilizumab, dexamethasone, and acetaminophen but without improvement. His fever peaked at 40.1°C on day +8, his ferritin level rose above the level of detection by our laboratory, and IFN-γ level was 300 pg/mL. Fibrinogen dropped below 100 mg/dL, and his fasting triglyceride level peaked at 435 mg/dL on day +13, direct bilirubin level peaked at 4.5 mg/dL on day +10, and lactate dehydrogenase level peaked at 1677 U/L on day +11. He had persistent cytopenias. On day +8, dexamethasone was increased to 10 mg every 6 hours, and we initiated anakinra 200 mg subcutaneously every 8 hours and further increased the frequency to 200 mg every 6 hours on day +9. Although this stabilized his blood pressure, he was still experiencing persistent high-grade fevers. He received the first dose of emapalumab at 1 mg/kg on day +9 with rapid defervescence and improvement in clinical and laboratory parameters. He became much more stable from this point. The most significant clinical response was noted within a few hours of the first emapalumab infusion, with resolution of persistent fevers and improvement in heart rate and blood pressure. His response is plotted in Figure 1. Figure 1A shows the response of inflammatory markers to therapies used. Fibrinogen was the last of the HLH markers to improve. We did not observe any unanticipated adverse events attributed to emapalumab use, including infectious complications in this patient. He received 3 additional doses at 1 mg/kg on days +11, +14, and +18, during which time we were successfully able to taper off his steroids and anakinra. Throughout, we transfused cryoprecipitate and platelets to meet a fibrinogen goal of 100 mg/dL and platelet count of 50 000/μL, and transfusion independence was eventually achieved. He also received empiric antibiotics, posaconazole, and acyclovir. Bone marrow evaluation on day 29 was consistent with a minimal residual disease (MRD)-negative complete remission (CR).
Figure 1.
Response of clinical and laboratory parameters to therapy. (A) Ferritin, CRP levels, and key interventions. (B) Temperature and direct bilirubin. Maximum ferritin value detectable in our laboratory was 22 500 ng/mL. Toci, tocilzumab; Dex, dexamethasone; Ema, emapalumab.
IFN-γ, a mediator of systemic inflammation and macrophage activation, is thought to play a key role in development of MAS/HLH, which is supported by the high serum level observed in this case. Although criteria like HLH2004 exist for diagnosis of primary HLH, there is no consensus on the diagnostic criteria for MAS/HLH secondary to CAR T-cell therapy. Some criteria used for primary HLH diagnosis like cytopenias and hypofibrinogenemia are common in patients undergoing CAR T-cell therapy and therefore are not useful. Moreover, hemophagocytosis, another HLH criterion, is difficult to assess in suboptimal marrow specimens and those with active leukemia. Neelapu et al9 have proposed a ferritin level > 10 000 ng/mL within the CRS window of 5 days after CAR T-cell therapy along with 2 of the following: ≥grade 3 hepatic or renal impairment, ≥grade 3 pulmonary edema, and hemophagocytosis by hematopathology to be diagnostic of MAS/HLH, and they also suggested that CRS and MAS/HLH may belong to the same disease spectrum. Although we agree with this definition, a more practical definition that does not include bone marrow evaluation may be useful. We suggest that clinicians should suspect underlying MAS/HLH if the following criteria are met: persistent fever with ferritin level > 10 000 ng/mL despite C-reactive protein (CRP) response after tociluzimab, ≥grade 2 elevation in transaminases or direct bilirubin, and hypofibrinogenemia (≤150 mg/dL). An elevated IFN-γ level is most useful if available. A useful criterion appears to be the relative levels of inflammatory markers, ferritin, and CRP after the administration of tocilizumab. CRP is predominantly a surrogate marker for interleukin-6 (IL-6), and if tocilizumab administration leads to improvement in CRP but clinically the patient continues to decline with fevers and rising ferritin, an alternative driver of CRS (ie, IFN-γ) should be considered, and in this setting, MAS/HLH is likely.10
Emapalumab is an anti–IFN-γ antibody recently approved for the treatment of primary HLH, and here, we illustrated a potential role in managing in secondary HLH/MAS caused by CAR T-cell therapy. Notably, a case of refractory grade 4 CRS was managed successfully with emapalumab in combination with other immunomodulatory drugs.11 We made the decision to try this drug based on evidence of its effectiveness in primary HLH and the limited benefit observed from treatment of CAR T-cell therapy–associated HLH with tocilizumab, anakinra, and steroids.8 The temporal profile of response suggests that emapalumab is effective in quickly improving MAS/HLH. We empirically administered 4 doses, but given the long half-life of emapalumab (25 days in healthy volunteers), a lesser number of doses may be sufficient. A recent pharmacometric study suggests that dosing based on initial IFN-γ level is feasible. Because IFN-γ level is not detectable after emapalumab administration, measurement of CXCL9, a chemokine induced by IFN-γ, could reflect HLH activity and aid additional dosing and duration of therapy.12 Given the high cost of emapalumab, such tailored dosing strategies and early use may limit the cost associated with its use in the setting of CAR T therapy. Anakinra, which is an IL-1 receptor blocker, and dexamethasone were also used as adjunctive agents and may have potentially contributed to our patient’s recovery. Although high doses of anakinra, up to 10 mg/kg daily, have been shown to be effective in cases of MAS,13, 14, 15, 16 there is limited evidence available to support its use in this setting.
Preclinical data suggest that CAR T-cell therapy efficacy against hematologic malignancies is not impaired by IFN-γ blockade.17 The efficacy of emapalumab in this case and the preserved function of the CAR T-cell therapy based on the day +29 bone marrow biopsy that revealed an MRD-negative CR suggest that emapalumab use with steroids and anakinra did not have deleterious effects on CAR T-cell therapy efficacy; however, early remissions may not be predictive of durable response. Based on this encouraging outcome with emapalumab use in severe HLH/MAS secondary to CAR T-cell therapy, a prospective study investigating its efficacy and safety is warranted. This is crucial given the increasing number of approved and investigational CAR T-cell therapies in hematologic malignancies and the anticipated increase in incidence of secondary HLH/MAS related to this therapy.
Conflict-of-interest disclosure: V.P. has received consulting fees from Novartis. I.A. reported consulting/advisory roles with KiTE pharmaceuticals. The remaining authors declare no competing financial interests.
Acknowledgments
Contribution: M.R., D.N., I.A., and V.P. provided concept and design; M.R., J.H.B., L.E.B., M.H., I.A., and V.P. wrote, reviewed, and/or revised the manuscript; M.R. acquired data; and M.R., I.A., and V.P. provided administrative, technical, or material support.
Footnotes
Deidentified participant data will be shared upon request.
References
- 1.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sandler RD, Tattersall RS, Schoemans H, et al. Diagnosis and management of secondary HLH/MAS following HSCT and CAR-T cell therapy in adults: a review of the literature and a survey of practice within EBMT centres on behalf of the Autoimmune Diseases Working Party (ADWP) and Transplant Complications Working Party (TCWP) Front Immunol. 2020;11:524. doi: 10.3389/fimmu.2020.00524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang H, Xiong L, Tang W, Zhou Y, Li F. A systematic review of malignancy-associated hemophagocytic lymphohistiocytosis that needs more attentions. Oncotarget. 2017;8(35):59977–59985. doi: 10.18632/oncotarget.19230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alblooshi RM, Deotare U, Michelis FV, et al. My jamais vu in post allogeneic hematopoietic cell transplant: a review on secondary hemophagocytosis in adults. Bone Marrow Transplant. 2020;55(5):867–872. doi: 10.1038/s41409-019-0711-1. [DOI] [PubMed] [Google Scholar]
- 5.Martín-Rojas RM, Gómez-Centurión I, Bailén R, et al. Hemophagocytic lymphohistiocytosis/macrophage activation syndrome (HLH/MAS) following treatment with tisagenlecleucel. Clin Case Rep. 2022;10(1) doi: 10.1002/ccr3.5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jordan MB, Hildeman D, Kappler J, Marrack P. An animal model of hemophagocytic lymphohistiocytosis (HLH): CD8+ T cells and interferon gamma are essential for the disorder. Blood. 2004;104(3):735–743. doi: 10.1182/blood-2003-10-3413. [DOI] [PubMed] [Google Scholar]
- 7.Sepulveda FE, de Saint Basile G. Hemophagocytic syndrome: primary forms and predisposing conditions. Curr Opin Immunol. 2017;49:20–26. doi: 10.1016/j.coi.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Locatelli F, Jordan MB, Allen C, et al. Emapalumab in children with primary hemophagocytic lymphohistiocytosis. N Engl J Med. 2020;382(19):1811–1822. doi: 10.1056/NEJMoa1911326. [DOI] [PubMed] [Google Scholar]
- 9.Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15(1):47–62. doi: 10.1038/nrclinonc.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cosenza M, Sacchi S, Pozzi S. Cytokine release syndrome associated with T-cell-based therapies for hematological malignancies: pathophysiology, clinical presentation, and treatment. Int J Mol Sci. 2021;22(14):7652. doi: 10.3390/ijms22147652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McNerney KO, DiNofia AM, Teachey DT, Grupp SA, Maude SL. Potential role of IFNγ inhibition in refractory cytokine release syndrome associated with CAR T-cell therapy. Blood Cancer Discov. 2022;3(2):90–94. doi: 10.1158/2643-3230.BCD-21-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacqmin P, Snoeck E, Jordan MB, et al. Emapalumab in primary hemophagocytic lymphohistiocytosis and the pathogenic role of interferon gamma: a pharmacometric model-based approach. Br J Clin Pharmacol. 2022;88(5):2128–2139. doi: 10.1111/bcp.15133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grom AA, Horne A, De Benedetti F. Macrophage activation syndrome in the era of biologic therapy. Nat Rev Rheumatol. 2016;12(5):259–268. doi: 10.1038/nrrheum.2015.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah NN, Highfill SL, Shalabi H, et al. CD4/CD8 T-cell selection affects chimeric antigen receptor (CAR) T-cell potency and toxicity: updated results from a phase I anti-CD22 CAR T-cell trial. J Clin Oncol. 2020;38(17):1938–1950. doi: 10.1200/JCO.19.03279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ajeganova S, De Becker A, Schots R. Efficacy of high-dose anakinra in refractory macrophage activation syndrome in adult-onset Still’s disease: when dosage matters in overcoming secondary therapy resistance. Ther Adv Musculoskelet Dis. 2020;12:1–9. doi: 10.1177/1759720X20974858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bami S, Vagrecha A, Soberman D, et al. The use of anakinra in the treatment of secondary hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2020;67(11) doi: 10.1002/pbc.28581. [DOI] [PubMed] [Google Scholar]
- 17.Bailey SR, Vatsa S, Larson RC, et al. Blockade or deletion of IFNγ reduces macrophage activation without compromising CAR T-cell function in hematologic malignancies. Blood Cancer Discov. 2022;3(2):136–153. doi: 10.1158/2643-3230.BCD-21-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]