Abstract
Background:
People globally are turning to herbal products to reconnect with nature. Cost efficacy and minimal side effects are the reasons for this changeover. This study assessed the effect of Amorphophallus paeoniifolius as an antimicrobial agent against Porphyromonas gingivalis, Prevotella intermedia, and Fusobacterium nucleatum.
Aim:
To determine and compare the antimicrobial activity of aqueous and ethanolic extracts of A. paeoniifolius on periodontal pathogens.
Materials and Methods:
Aqueous and ethanolic extracts of A. paeoniifolius were tested against the standard strains of the selected bacteria. Minimum inhibitory concentrations (MIC) and minimum bactericidal concentration (MBC) were used. These tests assessed the lowest concentrations of test agent, either by showing a lack of turbidity or by no or few bacterial growth colonies, respectively. In this study, tetracycline hydrochloride was used as the control group.
Results:
Aqueous and ethanolic extracts of A. paeoniifolius showed antibacterial activity at various concentrations against the selected organisms. While assessing the MBC, the aqueous and ethanolic extracts of A. paeoniifolius and tetracycline hydrochloride exhibited bactericidal activity against F. nucleatum at all concentrations. The ethanolic extract of Amorphophallus paeoniifolius and tetracycline hydrochloride showed bactericidal action, whereas the aqueous extract exhibited bacteriostatic action against P. gingivalis. The aqueous and ethanolic extracts of A. paeoniifolius showed bacteriostatic action, whereas tetracycline hydrochloride showed bactericidal action against P. intermedia.
Conclusion:
Both aqueous and ethanolic extracts of A. paeoniifolius showed antibacterial activity against standard strains of P. gingivalis, P. intermedia, and F. nucleatum. The ethanolic extract showed a significant antibacterial effect against the selected microorganisms when compared to the aqueous extract of A. paeoniifolius.
Key words: Amorphophallus paeoniifolius extracts, Fusobacterium nucleatum, herbaceous medicinal plants, periodontal pathogens, Porphyromonas gingivalis, Prevotella intermedia
INTRODUCTION
The oral cavity is inhabited by a diverse microflora. Numerous bacterial species have been involved in various oral diseases. Multiple factors such as microbial challenge and host immune response usually lead to the destruction of the periodontium. Despite the existence of over 700 different kinds of bacteria in the oral cavity, only a small number - primarily the Gram negative bacteria, are truly involved in the development and progression of periodontal disease.[1]
Periodontal treatment through the ages has focused on the removal of the etiological factors (plaque and calculus) by scaling and root planing. Host modulatory therapies and risk factor modification like smoking cessation therapy constitute a comprehensive treatment strategy for periodontitis. Systemic antibiotics can also be used along or in conjunction with conventional therapy to retard bacterial proliferation.[2]
Number of microorganisms is sensitive to tetracycline, a broad-spectrum antibiotic. Tetracyclines are considered to be bacteriostatic but may have a bactericidal effect at high concentrations. Staining of teeth, enamel hypoplasia, and photosensitivity reactions are some of the drawbacks of using tetracyclines. To overcome the drawbacks, in recent days, naturally occurring herbs such as Allium sativum,[3] Azadirachta indica,[2] and Aloe vera[2] are being tested for their antimicrobial potential.
Amorphophallus paeoniifolius (Araceae family) is herbaceous medicinal plant commonly known as elephant foot yam, used in the field of traditional medicine. It has been studied for its antibacterial, antifungal, cytotoxic, as well as analgesic activities. Extracts of A. paeoniifolius have been evaluated for its antibacterial property against Bacillus subtilis, Bacillus megaterium, and many more. However, on reviewing literature, its potential antibacterial activity against periodontal pathogens seems yet to be determined.
In view of the above facts, the study was designed to determine and compare the antimicrobial activity of aqueous and ethanolic extracts of A. paeoniifolius on periodontal pathogens.
MATERIALS AND METHODS
Standard strains of Porphyromonas gingivalis (ATCC 33227), Prevotella intermedia (JCM 11150), and Fusobacterium nucleatum (ATCC 25886) were obtained from our Central Research Laboratory, and a commercially available pure-based tetracycline hydrochloride® was used.
A. paeoniifolius tubers were procured locally, washed thoroughly, sliced into small pieces, shade dried, and milled to powder form using a mechanical grinder. Then, the obtained powder was stored in airtight bottles. The aqueous and ethanolic extracts of A. paeoniifolius were prepared using the Soxhlet extraction method.[4]
The serial broth dilution method was carried out to check the minimum inhibitory concentrations of extracts of A. paeoniifolius and tetracycline hydrochloride. Stock solutions for our study were constituted by mixing 200 mg of prepared aqueous and ethanolic extracts in 1 ml of distilled water, respectively. To study the minimal inhibitory concentrations (MIC) of aqueous extract of A. paeoniifolius, a set of 12 sterile vials were labeled serially from 1 to 12 and placed on a rack. Tube number 1 consisted of 400 ml of working dilution of aqueous extract of A. paeoniifolius (stock solution). 200 ml of thioglycolate broth was added in all tubes numbered from 2 to 12. Followed by this, 10 ml of bacterial suspension was added from tube number 1 to 10 and tube number 12. To tube number 11, no organism was added. From tube number 1, 200 ml of A. paeoniifolius extract was transferred to tube number 2 and mixed well. Now, from the mixed solution in the tube number 2, 200 ml of solution was transferred to the tube number 3 and was mixed well. This was continued till tube number 11 and at last 200 ml of the mixture was discarded from tube number 11. Tube number 11 consisted of broth and extract but no organism, and this was considered as broth control (to ascertain sterility check). Tube number 12 consisted of broth and organism but no extract and was considered as organism control (to check organism growth). By following this serial dilution, the concentrations of the aqueous extract of A. paeoniifolius achieved were 100, 50, 25, 12.5, 6.25, 3.1, 1.6, 0.8, 0.4, and 0.2 mg/ml, respectively. The tubes were then incubated for 48 h at 37°C. After the incubation, a visual inspection was carried out to determine the MIC values. Turbidity in the MIC tube indicated growth of the bacteria implying that the bacteria were resistant to the aqueous extract of A. paeoniifolius. The same procedure was carried out for ethanolic extract of A. paeoniifolius and tetracycline hydrochloride. Tetracycline hydrochloride acts as a standard control group in the present study.
From the MIC dilution tubes, the tubes which were found sensitive to MIC were plated and the colony count was noted after incubating it for 24 h. MBC was done to see whether there was bactericidal or bacteriostatic effect of the extracts and tetracycline hydrochloride against the selected organisms. If there was no growth, it was considered to have bactericidal effect. If there was growth, it was concluded to be bacteriostatic.
Data analysis
The data collected were entered into the MS Office Excel worksheet and analyzed using the SPSS software version 20.0 (IBM Corp., Armonk, N.Y., USA). Descriptive statistics such as mean standard deviation of numerical data was depicted. Group comparison was done using Mann–Whitney test. At P < 0.05, level of significance was set.
RESULTS
Table 1 and Graph 1 illustrate minimum inhibitory concentration (MIC) values of aqueous and ethanolic extracts of A. paeoniifolius and tetracycline hydrochloride on P. gingivalis, P. intermedia, and F. nucleatum. All the organisms tested showed sensitivity to an aqueous extract of A. paeoniifolius at 0.4 mg/ml. P. intermedia was found to be sensitive to ethanolic extract of A. paeoniifolius at 0.4 mg/ml, whereas P. gingivalis and F. nucleatum were sensitive at 0.2 mg/ml. P. gingivalis and P. intermedia were found sensitive to tetracycline hydrochloride at 0.2 mg/ml, whereas F. nucleatum was found sensitive at 0.4 mg/ml.
Table 1.
Minimum inhibitory concentration of Amorphophallus paeoniifolius extracts (aqueous and ethanolic) and tetracycline hydrochloride in mg/ml against Porphyromonas gingivalis, Prevotella intermedia, and Fusobacterium nucleatum
| 100 mg/ml | 50 mg/ml | 25 mg/ml | 12.5 mg/ml | 6.25 mg/ml | 3.12 mg/ml | 1.6 mg/ml | 0.8 mg/ml | 0.4 mg/ml | 0.2 mg/ml | |
|---|---|---|---|---|---|---|---|---|---|---|
| Pg | ||||||||||
| AEAP | Sensitive | Sensitive | Sensitive | Sensitive | Sensitive | Sensitive | Sensitive | Sensitive | Sensitive | Resistant |
| EEAP | Sensitive | Sensitive | Sensitive | Sensitive | Sensitive | Sensitive | Sensitive | Sensitive | Sensitive | Sensitive |
| TCH | Sensitive | Sensitive | Sensitive | Sensitive | Sensitive | Sensitive | Sensitive | Sensitive | Sensitive | Sensitive |
| Pi | ||||||||||
| AEAP | Sensitive | Sensitive | Sensitive | Sensitive | Sensitive | Sensitive | Sensitive | Sensitive | Sensitive | Resistant |
| EEAP | Sensitive | Sensitive | Sensitive | Sensitive | Sensitive | Sensitive | Sensitive | Sensitive | Sensitive | Resistant |
| TCH | Sensitive | Sensitive | Sensitive | Sensitive | Sensitive | Sensitive | Sensitive | Sensitive | Sensitive | Sensitive |
| Fn | ||||||||||
| AEAP | Sensitive | Sensitive | Sensitive | Sensitive | Sensitive | Sensitive | Sensitive | Sensitive | Sensitive | Resistant |
| EEAP | Sensitive | Sensitive | Sensitive | Sensitive | Sensitive | Sensitive | Sensitive | Sensitive | Sensitive | Sensitive |
| TCH | Sensitive | Sensitive | Sensitive | Sensitive | Sensitive | Sensitive | Sensitive | Sensitive | Sensitive | Resistant |
A. paeoniifolius – Amorphophallus paeoniifolius; AEAP – Aqueous extract of A. paeoniifolius; EEAP – Ethanolic extract of A. paeoniifolius; TCH – Tetracycline hydrochloride; Pg – Porphyromonas gingivalis; Pi – Prevotella intermedia; Fn – Fusobacterium nucleatum
Graph 1.
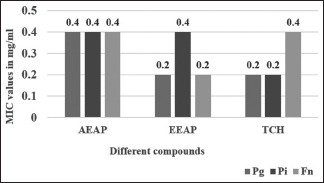
Minimum inhibitory concentration of Amorphophallus paeoniifolius extracts (aqueous and ethanolic) and tetracycline hydrochloride in mg/ml against Porphyromonas gingivalis (Pg), Prevotella intermedia (Pi), and Fusobacterium nucleatum (Fn). AEAP – Aqueous extract of Amorphophallus paeoniifolius; EEAP – Ethanolic extract of Amorphophallus paeoniifolius; TCH – Tetracycline hydrochloride
Table 2 and Graph 2 illustrate minimum bactericidal concentration (MBC) values of aqueous and ethanolic extracts of A. paeoniifolius and tetracycline hydrochloride on P. gingivalis, P. intermedia, and F. nucleatum. The bactericidal effect means there is no growth of microorganisms, whereas the bacteriostatic effect means there is a growth of microorganisms. The aqueous and ethanolic extracts of A. paeoniifolius and tetracycline hydrochloride exhibited bactericidal activity against F. nucleatum at all concentrations. The bacteriostatic activity was exhibited by aqueous extract of A. paeoniifolius (15 colonies were formed), whereas the bactericidal activity was exhibited by ethanolic extract of A. paeoniifolius and tetracycline hydrochloride against P. gingivalis at 0.2 mg/ml. The aqueous extract of A. paeoniifolius exhibited bacteriostatic activity against P. intermedia at 0.2 mg/ml (834 colonies were formed) and the ethanolic extract of A. paeoniifolius exhibited bacteriostatic activity against P. intermedia at 0.2 mg/ml (100 colonies were formed) and at 0.4 mg/ml (70 colonies were formed), respectively. Tetracycline hydrochloride exhibited bactericidal activity against tested organisms at all concentrations.
Table 2.
Minimum bactericidal concentration of Amorphophallus paeoniifolius extracts (aqueous and ethanolic) and tetracycline hydrochloride in mg/ml against Porphyromonas gingivalis, Prevotella intermedia, and Fusobacterium nucleatum
| 100 mg/ml | 50 mg/ml | 25 mg/ml | 12.5 mg/ml | 6.25 mg/ml | 3.12 mg/ml | 1.6 mg/ml | 0.8 mg/ml | 0.4 mg/ml | 0.2 mg/ml | |
|---|---|---|---|---|---|---|---|---|---|---|
| Pg | ||||||||||
| AEAP | NG | NG | NG | NG | NG | NG | NG | NG | NG | 15* |
| EEAP | NG | NG | NG | NG | NG | NG | NG | NG | NG | NG |
| TCH | NG | NG | NG | NG | NG | NG | NG | NG | NG | NG |
| Pi | ||||||||||
| AEAP | NG | NG | NG | NG | NG | NG | NG | NG | NG | 834* |
| EEAP | NG | NG | NG | NG | NG | NG | NG | NG | 70* | 100* |
| TCH | NG | NG | NG | NG | NG | NG | NG | NG | NG | NG |
| Fn | ||||||||||
| AEAP | NG | NG | NG | NG | NG | NG | NG | NG | NG | NG |
| EEAP | NG | NG | NG | NG | NG | NG | NG | NG | NG | NG |
| TCH | NG | NG | NG | NG | NG | NG | NG | NG | NG | NG |
*Indicates number of colonies formed. NG – No growth; A. paeoniifolius – Amorphophallus paeoniifolius; AEAP – Aqueous extract of A. paeoniifolius; EEAP – Ethanolic extract of A. paeoniifolius; TCH – Tetracycline hydrochloride; Pg – Porphyromonas gingivalis; Pi–Prevotella intermedia; Fn – Fusobacterium nucleatum
Graph 2.
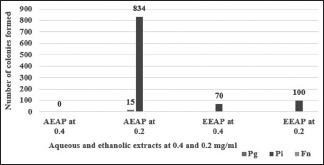
Minimum bactericidal concentration of Amorphophallus paeoniifolius extracts (aqueous and ethanolic) at 0.2 and 0.4 mg/ml concentration against Porphyromonas gingivalis (Pg), Prevotella intermedia (Pi), and Fusobacterium nucleatum (Fn). AEAP – Aqueous extract of Amorphophallus paeoniifolius; EEAP – Ethanolic extract of Amorphophallus paeoniifolius
DISCUSSION
A. paeoniifolius commonly called “Elephant foot yam” is a highly potential tropical tuber of Araceae family. A. paeoniifolius tuber having a good source of protein as well as starch and is very popularly used vegetable in various Indian cuisines. The tuberous roots of the plant have blood purifier properties and have been used traditionally for the treatment of various diseases. A. paeoniifolius has been studied for its analgesic activity, anticonvulsant activity, anthelmintic activity, anti-inflammatory activity, antioxidant activity, antitumor activity, central nervous system depressant activity,[5] as well as cytotoxic activities.[6] It is also mentioned in the literature that aqueous and ethanolic extracts of A. paeoniifolius have a significant antibacterial activity.
Singh and Wadhwa[5] published a review on multiple potential of aroid A. paeoniifolius. They had stated that both Gram-positive and Gram-negative bacteria were inhibited to certain extent by both aqueous and organic solvent extracts of A. paeoniifolius.
Kadali et al.[7] conducted a study and assessed the antibacterial activity of both A. paeoniifolius tuber and its peel extracts. In this study, the antibacterial activity was measured by well-diffusion method. The ethanolic tuber extract of the plant showed an inhibitory effect on four bacterial species such as Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, and Streptococcus mutans. The ethanolic tuber extract displayed no effect on B. subtilis. The ethanolic extract of tuber showed a diameter of inhibition zones ranging from 6 mm to 18 mm. The ethanolic extract of peel showed inhibitory effect only on two bacterial species such as S. aureus and P. aeruginosa. The ethanolic extract of peel exhibited an inhibition zone ranging from 7 mm to 16 mm. Water extracts of tuber (inhibition zone ranging from 7 mm to 9 mm) and peel (inhibition zone ranging from 6 mm to 9 mm) inhibited only one bacterial species such as S. aureus and S. mutans, respectively. The ethanolic extract exhibited a significant antibacterial activity than the aqueous extract on selected microorganisms.
Miyake et al.[8] conducted a study where they had assessed the killing and inhibitory activity of many antibiotics against periopathogens and concluded that broad-spectrum antibiotics such as tetracyclines showed inhibitory activity against P. gingivalis and P. intermedia.
Saquib et al.[9] conducted a study to evaluate and compare the antibacterial efficiency of herbal extracts in combination with antibiotics on organisms associated with periodontitis. They stated that antibacterial activity was shown from Cinnamomum zeylanicum and S. persica plants, mainly ethanolic extract against all the experimented periodontal pathogens. The results of this study revealed that S. persica in combination with tetracycline exhibited activity against the majority of periodontal pathogens. In contrast, C. zeylanium and amoxicillin combination worked well against Treponema denticola.
To the best of our knowledge, this is the first in vitro study to assess the antimicrobial activity of A. paeoniifolius extracts (aqueous and ethanolic) against periodontal pathogens. In the present in vitro study, we focused on determining and comparing antimicrobial activity of aqueous and ethanolic extracts of A. paeoniifolius against standard strains of Gram-negative anaerobes such as P. gingivalis, P. intermedia, and F. nucleatum which are associated with periodontal disease.
In our study, we determined the minimum inhibitory concentration (MIC) and minimum bacterial concentration (MBC) of aqueous and ethanolic extracts of A. paeoniifolius and tetracycline hydrochloride to detect antibacterial activity. In MIC, all the organisms tested showed sensitivity to an aqueous extract of A. paeoniifolius at 0.4 mg/ml. P. intermedia was found to be sensitive to ethanolic extract of A. paeoniifolius at 0.4 mg/ml, whereas P. gingivalis and F. nucleatum were sensitive at 0.2 mg/ml. P. gingivalis and P. intermedia were found sensitive to tetracycline hydrochloride at 0.2 mg/ml, whereas F. nucleatum was found sensitive at 0.4 mg/ml. Statistically significant difference was observed between aqueous and ethanolic extracts of A. paeoniifolius against P. gingivalis at 0.2 mg/ml concentration (P = 0.03). Statistically significant difference was observed between aqueous and ethanolic extracts of A. paeoniifolius against P. intermedia at 0.2 mg/ml concentration (P = 0.04).
In MBC, the aqueous [Figure 1a] and ethanolic [Figure 1b] extracts of A. paeoniifolius and tetracycline hydrochloride [Figure 1c] exhibited bactericidal activity against F.nucleatum at all concentrations. The aqueous extract of A. paeoniifolius exhibited bacteriostatic activity against P. gingivalis and P. intermedia [Figures 2a and 3a]. Ethanolic extract of A. paeoniifolius exhibited bactericidal activity against P. gingivalis and bacteriostatic activity against P. intermedia [Figures 2b and 3b]. The bactericidal activity was exhibited by tetracycline hydrochloride against P. gingivalis and P. intermedia [Figures 2c and 3c].
Figure 1.
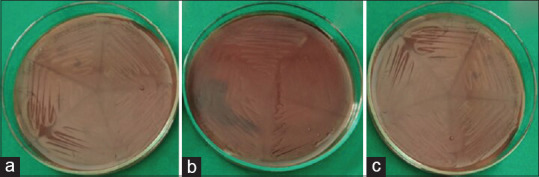
In Minimum Bactericidal Concentration (MBC) the Amorphophallus paeoniifolius extracts (aqueous and ethanolic) and tetracycline hydrochloride exhibited bacteriostatic or bactericidal activity against F.nucleatum. (a) Aqueous extract exhibited bactericidal activity at all concentrations. (b) Ethanolic extract exhibited bactericidal activity at all concentrations. (c) Tetracycline hydrochloride exhibited bactericidal activity at all concentrations
Figure 2.
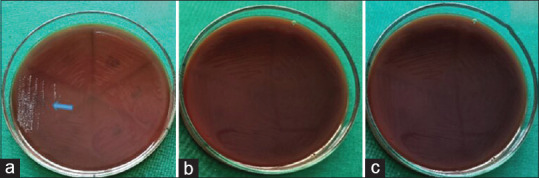
In Minimum Bactericidal Concentration (MBC) the Amorphophallus paeoniifolius extracts (aqueous and ethanolic) and tetracycline hydrochloride exhibited bacteriostatic or bactericidal activity against P.gingivalis. (a) Aqueous extract exhibited bacteriostatic activity at 0.2 mg/ml concentration. (b) Ethanolic extract exhibited bactericidal activity at all concentrations. (c) Tetracycline hydrochloride exhibited bactericidal activity at all concentrations
Figure 3.
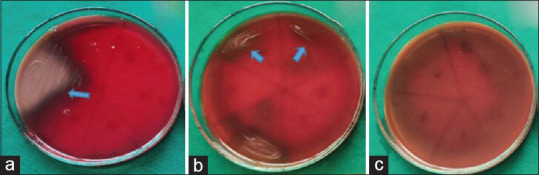
In Minimum Bactericidal Concentration (MBC) the Amorphophallus paeoniifolius extracts (aqueous and ethanolic) and tetracycline hydrochloride exhibited bacteriostatic or bactericidal activity against P.intermedia (a) Aqueous extract exhibited bacteriostatic activity at 0.2 mg/ml concentration. (b) Ethanolic extract exhibited bacteriostatic activity at 0.4 and 0.2 mg/ml concentration. (c)Tetracycline hydrochloride exhibited bactericidal activity at all concentrations
Biologically active compounds usually occur in low concentration in plants. An extraction technique is that which is able to obtain extracts with a high yield of active constituents and with minimal changes to their functional properties.[10] Ehiowemwenguan et al.[11] conducted a study, where a conclusion was drawn that, ethanolic extract of banana peel contains glycosides, alkaloids, flavonoids, and tannins, while aqueous extracts contain only glycosides and alkaloids. This could be attributed to the ethanolic extract having a capacity to dissolve more active compounds than water. Moreover, this could be the responsible for an enhanced antibacterial effect of ethanolic extracts compared to aqueous. Drawing reference to the same,[11] in our current study, the ethanolic extract could have dissolved more active compounds than the aqueous extract. Our study results point to ethanolic extract exhibiting a significant antibacterial effect than aqueous extract of A. paeoniifolius.
CONCLUSION
The results suggest that aqueous and ethanolic extracts of A. paeoniifolius showed antibacterial activity against P. gingivalis, P. intermedia, and F. nucleatum. In comparison between aqueous and ethanolic extracts, the ethanolic extract showed a significant antibacterial effect against the selected microorganisms. However, clinical studies will be required to confirm the antibacterial activity of aqueous and ethanolic extracts of A. paeoniifolius for its probable use in treating periodontal diseases.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no Conflicts of interest.
Acknowledgment
The authors express heartfelt gratitude to Dr. Kishore G Bhat (Director), Central Research Lab, Maratha Mandal’s Nathajirao G. Halgekar Institute of Dental Sciences and Research Centre, Belgaum, for the support provided in conducting microbiological assays, and Dr. Anuradha Bandiwadekar (Reader), Department of Public Health Dentistry, Maratha Mandal’s Nathajirao G. Halgekar Institute of Dental Sciences and Research Centre, Belgaum, for providing statistical analysis for the study.
REFERENCES
- 1.Caranzza F. 10th ed. St. Louis: Elsevier Publication; 2000. A Textbook of Clinical Periodontics and Implantology. [Google Scholar]
- 2.Kudalkar MD, Nayak A, Bhat KS, Nayak RN. Effect of Azadirachta indica (Neem) and Aloe vera as compared to subantimicrobial dose doxycycline on matrix metalloproteinases (MMP)-2 and MMP-9: An in-vitro study. Ayu. 2014;35:85–9. doi: 10.4103/0974-8520.141947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shetty S, Thomas B, Shetty V, Bhandary R, Shetty RM. An in-vitro evaluation of the efficacy of garlic extract as an antimicrobial agent on periodontal pathogens: A microbiological study. Ayu. 2013;34:445–51. doi: 10.4103/0974-8520.127732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ansil PN, Wills PJ, Varun R, Latha MS. Cytotoxic and apoptotic activities of Amorphophallus campanulatus (Roxb.). Bl. Tuber extracts against human colon carcinoma cell line HCT-15. Saudi J Biol Sci. 2014;21:524–31. doi: 10.1016/j.sjbs.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh A, Wadhwa N. A review on multiple potential of aroid:Amorphophallus paeoniifolius . Int J Pharm Sci Rev Res. 2014;24:55–60. [Google Scholar]
- 6.Dey YN, Ota S, Srikanth N, Jamal M, Wanjari M. A phytopharmacological review on an important medicinal plant –Amorphophallus paeoniifolius . Ayu. 2012;33:27–32. doi: 10.4103/0974-8520.100303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kadali VN, Ramesh T, Pola SR, Sandeep BV. Assessment of antibacterial activity of Amorphophallus paeoniifolius tuber and its peel extracts. Trop Plant Res. 2016;3:172–5. [Google Scholar]
- 8.Miyake Y, Tsuruda K, Okuda K, Widowati, Iwamoto Y, Suginaka H. In vitro activity of tetracyclines, macrolides, quinolones, clindamycin and metronidazole against periodontopathic bacteria. J Periodontal Res. 1995;30:290–3. doi: 10.1111/j.1600-0765.1995.tb02136.x. [DOI] [PubMed] [Google Scholar]
- 9.Saquib SA, AlQahtani NA, Ahmad I, Kader MA, Al Shahrani SS, Asiri EA. Evaluation and comparison of antibacterial efficacy of herbal extracts in combination with antibiotics on periodontal pathobionts: An in vitro microbiological study. Antibiotics (Basel) 2019;8:E89. doi: 10.3390/antibiotics8030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Condori SQ, Foglio MA, Rosaa PT, Meireles MA. Obtaining –Caryophyllene from Cordia verbenacea de Candolle by supercritical fluid extraction. Journal of Supercritical Fluids. 2008;46:27–32. [Google Scholar]
- 11.Ehiowemwenguan G, Emoghene AO, Inetianbor JE. Aantibacterial and phytochemical analysis of banana fruit peel. IOSR J Pharm. 2014;4:18–25. [Google Scholar]


