Abstract
Background:
Ultrasonic scaling is a potential source of aerosol contamination in dental clinics. The two primary sources of microbial load in aerosols are from the oral cavity and dental unit water line. Literature evidence suggest that the use of preprocedural mouth rinse reduce the bacterial load in aerosol generated during ultrasonic scaling.
Aim:
The aim of the study is to assess the comparative efficacy of reduction in viable bacteria in the aerosol at patient’s chest area, doctor’s mask area and two feet beside the patient following use of chlorhexidine/herbal formulation diluted in the water source by a randomized controlled clinical trial.
Materials and Methods:
Forty-five subjects (with chronic gingivitis) were matched for age, gender, and gingival index score. The subjects were randomized and received ultrasonic scaling with distilled water (control)/chlorhexidine (tTest)/herbal formulation (test). Aerosol produced during scaling was collected at patient’s chest area, doctor’s mask area, two feet beside the patient on blood agar plates, which were incubated at 37°C for 48 h and total colony forming units (CFUs) were counted.
Results:
A significant reduction in the total CFUs’ counts was observed at all the three sites sampled in test groups (chlorhexidine group and herbal formulation group) as compared to control (P < 0.01).
Conclusion:
The addition of antiseptic agents to the water source contributed to a significant reduction of the cultivable microbial counts in the aerosol and hence can be used to reduce the risk of cross-infection during ultrasonic scaling.
Key words: Aerosols, chlorhexidine, colony-forming units assay, cross infection, periodontal debridement
INTRODUCTION
Aerosol is a suspension of solid or liquid particles containing bacteria or viruses. Particle size may vary from 0.001 to >100 μm.[1] Aerosol with smaller particle size has been found to have greatest potential to penetrate and lodge in smaller passages of lungs and thereby transmit infection.[2] Aerosols and splatter pose a main concern in dental community due to possible spread of infectious and potentially harmful agents among patient and dental personnel.[2]
Dental plaque, oral fluids, and respiratory tract are the source of numerous bacteria and viruses in oral cavity. Over six million microorganisms have been reported to be present in one ml of saliva.[3]
The use of ultrasonic scaler is a potential source of aerosol production in a dental clinic.[4] Aerosols generated from patient’s mouth contain up to a million bacteria per cubic foot of air.[5] Association of these aerosols with respiratory, ophthalmic and skin infections, tuberculosis, and hepatitis B has been established.[5–7]
The current literature suggests that having patients use an antimicrobial rinse before treatment will decrease microbial content in aerosols. Chlorhexidine, a broad-spectrum antimicrobial agent, is considered the gold standard and has substantivity of eight to 12 h.[8] Preprocedural rinsing with the chlorhexidine mouth wash produced a 94.1% reduction in recoverable colony forming units (CFUs) compared to the control (no prerinsing).[9] Chlorhexidine gluconate was found to be effective even at a dilution of 1/320, which was suggested as the maximum dilution at which inhibition can be achieved.[10]
Aerosol-generated during ultrasonic scaling contains complex of microbes originating from patient’s oral cavity and from dental unit water line (DUWL). Preprocedural mouth rinsing has been proved to be effective in reducing the microbial contamination in aerosol primarily originating from oral cavity. With this background, the objective of this study was to assess the reduction in viable bacteria in the aerosol at patient chest area, doctor’s mask area, and two feet beside the patient following use of chlorhexidine/herbal formulation dilutions in the water source.
MATERIALS AND METHODS
This study was designed as a randomized controlled double-blinded clinical trial. One hundred and fifty patients attending the out-patient department were screened, and 45 subjects were selected for the study based on certain specific inclusion and exclusion criteria. Ethical approval for the study was obtained from Institutional Ethics Committee and the trial was registered in the Clinical Trials Registry of India (CTRI/2017/06/008926).
Written informed consent was obtained from study participants before inclusion in the study. Systemically healthy subjects above 18 years of age with moderate/severe gingival inflammation which was assessed based on gingival index score (1.1–3.0),[11] subjects with fair or poor oral hygiene based on oral hygiene index–simplified score (1.3–6.0)[12] and subjects with ≥20 teeth were included in the study. Current smokers, pregnant women, patients on antibiotics/anti-inflammatory/any other drugs in the past 6 months, subjects with healthy gingiva (with no clinical signs of gingival inflammation) were excluded from the study. Patient enrollment and elicitation of detailed history and clinical examination were performed using a prepared proforma by a single examiner (L. A). Intra-examiner variability was assessed and the kappa value was >90%.
Sample size calculation was done based on the study by Gupta et al.,[13] the power of the study being β = 80% and Type I error a = 5%. The sample size required to show the difference between the control and test group was 15 per group. Patients were divided into three groups (Group I and Group II and Group III) with 15 patients each. Group I subjects received ultrasonic scaling with distilled water (placebo) to which food color (green) was added to match the test group. Group II subjects received ultrasonic scaling with chlorhexidine diluted water. Two ml of chlorhexidine gluconate solution was diluted in one liter of water to obtain a dilution of one in 320 dilution (maximum inhibitory dilution) as per the study by Nascimento et al.[10] (with an effective concentration of 0.0625% of chlorhexidine). Group III subjects received ultrasonic scaling with herbal formulation diluted water. Ten ml of herbal formulation (HiOra®) was diluted in one liter of water (1:100 dilution). HiOra®, a proprietary Ayurvedic medicine solution consisted of a mixture of herbal extracts, powders, and oils.
Randomization was performed using a computer generated random table. Randomization list was received by A. L and sealed envelopes containing the allotted code numbers were placed in a closed container in the operatory. Before the scaling procedure, the dilutions of chlorhexidine and herbal formulation were prepared and added to the water source as per the code available in the envelope, opened on the day of the procedure. The operator (L. V) who performed scaling was blinded regarding use of antiseptic in the water source and the microbiologist who performed the microbial colony counting remained blinded throughout the study.
An operatory containing single dental unit was selected to perform the study. Before the commencement of the trial, a new DUWL was installed. Only one patient was treated per day to avoid cross contamination. The operatory was fumigated, 8 h before the procedure to minimize air contamination. The baseline sample of the microbial count in the room was collected by exposing the blood agar plates 30 min before procedure. Blood agar is an enriched media which allows growth of all fastidious organisms when compared to nutrient agar. During ultrasonic scaling, aerosol was collected on blood agar plates kept at three different areas: patient’s chest area, operator mask level, and two feet to the left side of the patient. The blood agar plates were exposed for 20 min during the treatment. The clinical procedure of ultrasonic scaling was performed by the same operator (L. V) for all the study participants. The plates were sealed immediately and incubated at 37°C for 48 h in a sterile incubator. After 48 h, total number of CFUs of aerobic bacteria were counted on each agar plates under magnification.
ANOVA test was used for determining the significance of difference in reduction of CFU counts between the test and control group. Mean, frequency, and standard deviation were determined for all continuous variables. P < 0.05 was considered statistically significant.
RESULTS
A total of 45 individuals with an age range of 20–52 years of age were included in this study. The demographic data of the study population are summarized in Table 1. The demographic variables and clinical parameters were similar among the participants randomized into control and test groups [Table 1]. The total CFUs count at patient’s chest area, doctor’s mask area, and two feet beside the patient are summarized in Table 2.
Table 1.
The mean value of the demographic data and baseline clinical characteristics of Group I (placebo control), Group II (chlorhexidine), and Group III (herbal formulation)
| Group I (placebo) | Group II (CHX) | Group III (herbal formulation) | |
|---|---|---|---|
| Age (years) | 34.33±12.90 | 34.8±10.44 | 32.46±8.84 |
| Gender (male/female) | 7/8 | 8/7 | 7/8 |
| OHI-S score | 3.56±0.54 | 3.64±0.51 | 3.68±0.49 |
| GI score | 2.32±0.23 | 2.35±0.21 | 2.33±0.17 |
CHX - Chlorhexidine; OHI-S - Oral Hygiene Index-Simplified; GI - Gingival index
Table 2.
The mean and standard deviation of microbial counts (colony forming units) in three groups at different locations
| Groups | Mean±SD | ||
|---|---|---|---|
|
| |||
| Patient’s chest area (CFU/cm2) | Doctor’s mask area (CFU) | Two feet beside the patient (CFU) | |
| Group I - placebo | 102.13±73.37 | 77.86±49.61 | 41.40±18.87 |
| Group II - CHX | 11.60±5.11 | 12.33±7.45 | 11.13±7.15 |
| Group III - herbal formulation | 19.66±5.49 | 19.60±7.51 | 18.13±7.41 |
CHX - Chlorhexidine; CFU - Colony-forming unit; SD - Standard deviation
Intergroup comparison was made by one-way ANOVA. A significant difference in microbial CFUs (P < 0.001) was observed at the three sites evaluated in comparing the test groups – chlorhexidine (Group II)/herbal formulation (Group III) with placebo control [Table 3]. A substantial decrease in CFU was observed among both the test groups, however, the intergroup difference between the test groups (chlorhexidine and herbal formulation) was not statistically significant at patient’s chest area (P = 0.607), doctor’s mask area (P = 0.501), and two feet beside the patient (P = 0.130) [Table 3 and Figures 1–3]. The images of blood agar plates for the two interventions and one control group at three different sampling sites are provided in Supplementary Figures 1 (109KB, tif) –3 (92.2KB, tif) .
Table 3.
Comparison of mean microbial counts colony-forming unit between the three interventions (Group I - placebo control, Group II - chlorhexidine, Group III - herbal formulation) at patient’s chest area, doctor’s mask area, and two feet beside the patient area using one-way ANOVA analysis
| Groups | Mean difference | 95% CI | P | |
|---|---|---|---|---|
|
| ||||
| Lower bound | Upper bound | |||
| Patient’s chest area | ||||
| Placebo versus CHX | 90.53 | +59.15 | +121.91 | <0.001** |
| Placebo versus herbal formulation | 82.47 | +51.08 | +113.84 | <0.001** |
| Herbal formulation versus CHX | 8.07 | −23.31 | +39.44 | 0.607 |
| Doctor’s mask area | ||||
| Placebo versus CHX | 65.53 | +43.94 | +87.12 | <0.001** |
| Placebo versus herbal formulation | 58.27 | +36.68 | +79.85 | <0.001** |
| Herbal formulation versus CHX | 7.27 | −14.31 | +28.85 | 0.501 |
| 2 feet beside the patient area | ||||
| Placebo versus CHX | 30.27 | +21.11 | +39.41 | <0.001** |
| Placebo versus herbal formulation | 23.27 | +14.11 | +32.41 | <0.001** |
| Herbal formulation versus CHX | 7.00 | −2.14 | +16.14 | 0.130 |
**P value of <0.001 is considered statistically highly significant. CHX - Chlorhexidine; CI - Confidence interval; P - P value (Probability value)
Figure 1.
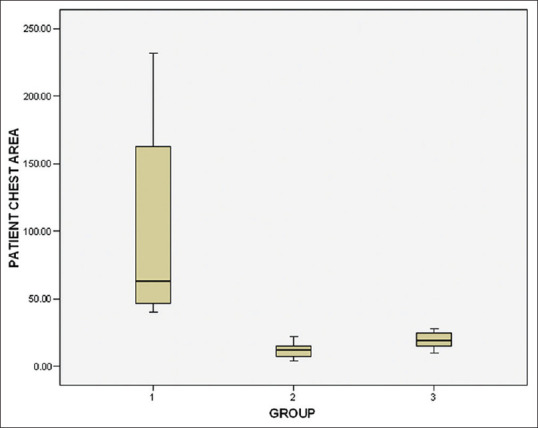
Box plot illustrating the colony-forming units at patient’s chest area with median and standard deviation
Figure 3.
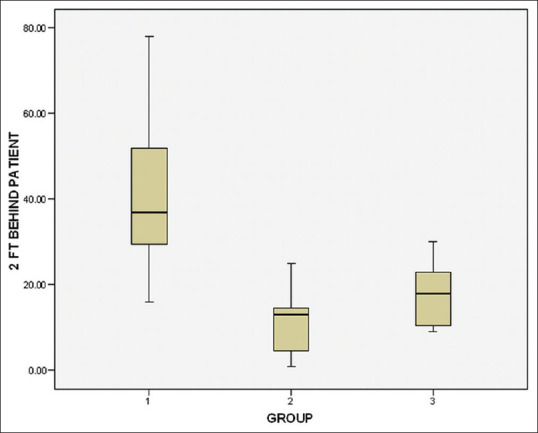
Box plot illustrating the total microbial counts colony-forming units at two feet beside the patient area showing the median and standard deviation
Figure 2.
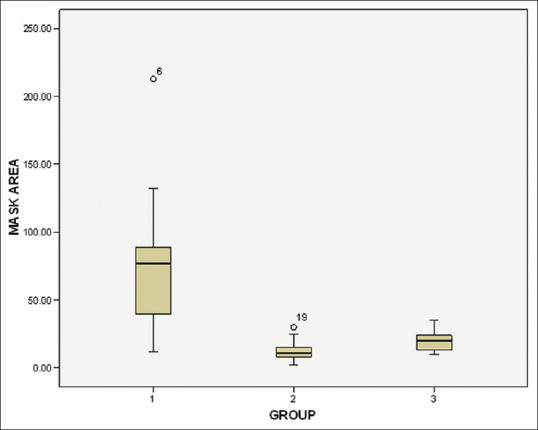
Box plot illustrating the total microbial count colony-forming units at doctor’s mask area showing median and standard deviation
DISCUSSION
The primary observations in this study were addition of chlorhexidine/herbal formulation to the water source resulted in a significant reduction of the microbial counts in the aerosol generated during ultrasonic scaling. The herbal formulation was found to be equally effective as chlorhexidine in reduction of microbial counts in the aerosol at the sites evaluated.
Pioneering work on aerobiology by Micik et al. used the terms “aerosol” and “splatter” in the dental environment. Particles <50 micrometers in diameter were defined as aerosols. The microbial content in aerosol poses a major threat for disease transmission through airborne route. The control and minimization of microbial content in aerosol are of great importance to the health of both the dentist and the patient. Aerosolized bacteria/viruses may remain suspended in the air for long periods of time and inhaled into the lungs of a susceptible person.[14–16]
Aerosols generated during dental procedure have gained importance in recent times, due to the risk of transmission of severe acute respiratory syndrome coronavirus 2 virus. In light of the COVID-19 pandemic, a recent review reported that respiratory bacterial pathogens and influenza viruses are present in saliva and viral shedding occurs in saliva during acute phases of all respiratory diseases. The microbial load in aerosol correlates with severity of respiratory diseases. Apart from the saliva being the primary source of microbial content in aerosol, the DUWL is also considered as the environmental source for aerosol microorganism.[17]
Acharya et al. in 2010 stated that dental handpieces, ultrasonic scalers, air polishing devices, and air abrasion units produce airborne particles by combined action of water sprays, compressed air, organic particles such as tissue, tooth dust, and organic fluids such as blood and saliva from the site where these instruments are used.[2] DUWL is another major source of microbes in aerosol. The microflora from the DUWL and the patient’s oral cavity in the form of aerosol mixes with the surrounding air, thus leading to change in the original composition of the environment. Eventually, it acts as a source of infection for both the dentist and the patients. Regular cleaning, disinfection, and sterilization of the unit water reservoir, filling it with distilled water, and application of chemicals to monitor the microbiological quality of DUWL water, assures effective microbiological control of water and safety of the unit users.[18]
Several infection control treatment strategies have been tried and implemented before and during the dental procedures, especially during ultrasonic scaling to minimize the microbial load in aerosol generated. Standard protective measures including gloves, goggles, shields, and masks are used universally during dental surgeries as an effective barrier against splatter thereby eliminating inherent danger in operative site.[19,20]
Literature evidence on studies to reduce the microbial content of aerosol from oral cavity have focused on use of preprocedural rinses and prevention/management of biofilm formation in DUWL. Commonly used agent to reduce the microbial content in aerosol includes chlorhexidine, povidone iodine, hydrogen peroxide. Among this chlorhexidine and povidone iodine has been used as preprocedural rinse to reduce the microbial contamination in aerosol and all the three has been used to disinfect DUWL.[21–24]
Chlorhexidine is considered an effective antiseptic against free-floating oral bacteria. Preprocedural mouth rinsing with antiseptic solutions has been found to be effective in reducing bacterial count in the air of operatory. Several studies have evaluated the efficacy of this protocol. Preoperative rinsing with 0.12% chlorhexidine gluconate diminished the quantity of aerobic and facultative flora of oral cavity.[25] The use of 0.2% chlorhexidine gluconate or essential oil containing mouthwashes as preprocedural mouth rinse can cause substantial reduction in bacterial counts in aerosol.[13]
Preprocedural rinses will be the primary target for reducing the microbiota in aerosol from oral cavity and does not affect the water source and water line. By adding antimicrobial agent in water source, the microbiota from water source, water line, and oral cavity will be reduced. Addition of 0.2% chlorhexidine into dental unit reservoir has shown to significantly reduce the bacterial and fungal contamination of the DUWL.[22]
Chlorhexidine, a broad-spectrum antibacterial agent, is considered the gold standard irrespective of its common side effects such as temporary loss of taste sensation, staining of teeth, restoration and mucosa, dryness and soreness of mucosa, and bitter taste.[8] Chlorhexidine gluconate was found effective even at a dilution of 1/320, which was suggested as its maximum inhibitory dilution.[10]
Herbal formulation (HiOra®) comprises components such as Miswak, nagavalli, gandhapurataila, ela, peppermint satva, yavani satva which have antimicrobial/antiplaque property.[26] The antigingivitis and antiplaque efficacy of the herbal formulation (HiOra®) have been found to be comparable to chlorhexidine as reported by Deshmukh et al.[27] The herbal formulation (HiOra®) had a minimum inhibitory concentration of 1/100 against commercially available strains of aerobic bacteria ([Streptococcus and Staphylococcus spp.] unpublished data from in vitro research performed by our team). Hence, in our present study, the same dilution of commercially available herbal formulation was prepared and utilized in the test group.
Dental unit water reservoir is the only source of water to the ultrasonic scaler unit and hence adding a antimicrobial agent to it will eliminate the DUWL biofilm formation and also minimize the microbial load from patient mouth when used during ultrasonic scaling.
To the best of our knowledge, this is the first study to evaluate the comparative efficacy in reducing microbial levels in aerosols produced during ultrasonic scaling following the addition of chlorhexidine and herbal formulation to the dental unit water reservoir.
In this study, chlorhexidine and herbal mouth wash were diluted in Dental Unit Water reservoir, and their efficacy in reducing the microbial level in aerosol produced during ultrasonic scaling was assessed using culture-based technique with blood agar plates. The results of this study show significant reduction in microbial levels in aerosol produced during ultrasonic scaling on addition of chlorhexidine and herbal formulation into Dental Unit Water reservoir.
The data in this study support the beneficial use of chlorhexidine or herbal formulation as disinfection agents in the booster water for scaling devices. This will reduce bacterial contamination in the aerosol generated and therefore enhance safety for the patient and the dental operator.
CONCLUSION
The addition of antiseptic agents to the water source contributed to a significant reduction of the cultivable microbial counts in the aerosol and hence can be used to reduce the risk of cross-infection during ultrasonic scaling.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Microbial colonies formed on blood agar plates exposed at patient's chest area in (a) Group I- Water/Placebo; (b) Group II- Chlorhexidine mouth wash; (c) Group III- Herbal mouth wash
Microbial colonies formed on blood agar plates exposed at doctor's mask area in (a) Group I- Water/Placebo; (b) Group II- Chlorhexidine mouth wash; (c) Group III- Herbal mouth wash
Microbial colonies formed on blood agar plates exposed at 2 feet beside patient in (a) Group I- Water/Placebo; (b) Group II- Chlorhexidine mouth wash; (c) Group III- Herbal mouth wash
REFERENCES
- 1.Tang JW, Li Y, Eames I, Chan PK, Ridgway GL. Factors involved in the aerosol transmission of infection and control of ventilation in healthcare premises. J Hosp Infect. 2006;64:100–14. doi: 10.1016/j.jhin.2006.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acharya S, Priya H, Purohit B, Bhat M. Aerosol contamination in a rural university dental clinic in south India. Int J Infect Control. 2010;6:1–7. [Google Scholar]
- 3.Cottone JA. Hepatitis B virus infection in the dental profession. J Am Dent Assoc. 1985;110:617–21. doi: 10.1016/s0002-8177(15)30011-8. [DOI] [PubMed] [Google Scholar]
- 4.Araujo MW, Andreana S. Risk and prevention of transmission of infectious diseases in dentistry. Quintessence Int. 2002;33:376–82. [PubMed] [Google Scholar]
- 5.Miller RL. Generation of airborne infection by high speed dental equipment. J Am Soc Prev Dent. 1976;6:14–7. [PubMed] [Google Scholar]
- 6.Shaw AB. Tuberculosis in medical and dental students;a study at Guy's hospital. Lancet. 1952;2:400–4. doi: 10.1016/s0140-6736(52)90218-3. [DOI] [PubMed] [Google Scholar]
- 7.Goldman HS, Hartman KS. Infectious diseases. Their disease, our unease: Infectious diseases and dental practice. Va Dent J. 1986;63:10–9. [PubMed] [Google Scholar]
- 8.Jones CG. Chlorhexidine: Is it still the gold standard? Periodontol 2000. 1997;15:55–62. doi: 10.1111/j.1600-0757.1997.tb00105.x. [DOI] [PubMed] [Google Scholar]
- 9.Fine DH, Mendieta C, Barnett ML, Furgang D, Meyers R, Olshan A, et al. Efficacy of preprocedural rinsing with an antiseptic in reducing viable bacteria in dental aerosols. J Periodontol. 1992;63:821–4. doi: 10.1902/jop.1992.63.10.821. [DOI] [PubMed] [Google Scholar]
- 10.Nascimento AP, Tanomaru JM, Matoba-Júnior F, Watanabe E, Tanomaru-Filho M, Ito IY. Maximum inhibitory dilution of mouthwashes containing chlorhexidine and polyhexamethylene biguanide against salivary Staphylococcus aureus . J Appl Oral Sci. 2008;16:336–9. doi: 10.1590/S1678-77572008000500006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Löe H. The gingival index, the plaque index and the retention index systems. J Periodontol. 1967;38:l610–6. doi: 10.1902/jop.1967.38.6.610. [DOI] [PubMed] [Google Scholar]
- 12.Greene JC, Vermillion JR. The simplified oral hygiene index. J Am Dent Assoc. 1964;68:7–13. doi: 10.14219/jada.archive.1964.0034. [DOI] [PubMed] [Google Scholar]
- 13.Gupta G, Mitra D, Ashok KP, Gupta A, Soni S, Ahmed S, et al. Efficacy of preprocedural mouth rinsing in reducing aerosol contamination produced by ultrasonic scaler: A pilot study. J Periodontol. 2014;85:562–8. doi: 10.1902/jop.2013.120616. [DOI] [PubMed] [Google Scholar]
- 14.Abel LC, Miller RL, Micik RE, Ryge G. Studies on dental aerobiology. IV. Bacterial contamination of water delivered by dental units. J Dent Res. 1971;50:1567–9. doi: 10.1177/00220345710500063601. [DOI] [PubMed] [Google Scholar]
- 15.Micik RE, Miller RL, Mazzarella MA, Ryge G. Studies on dental aerobiology. I. Bacterial aerosols generated during dental procedures. J Dent Res. 1969;48:49–56. doi: 10.1177/00220345690480012401. [DOI] [PubMed] [Google Scholar]
- 16.Miller RL, Micik RE, Abel C, Ryge G. Studies on dental aerobiology. II. Microbial splatter discharged from the oral cavity of dental patients. J Dent Res. 1971;50:621–5. doi: 10.1177/00220345710500031701. [DOI] [PubMed] [Google Scholar]
- 17.Kumar PS, Subramanian K. Demystifying the mist: Sources of microbial bioload in dental aerosols. J Periodontol. 2020;91:1113–22. doi: 10.1002/JPER.20-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murdoch-Kinch CA, Andrews NL, Atwan S, Jude R, Gleason MJ, Molinari JA. Comparison of dental water quality management procedures. J Am Dent Assoc. 1997;128:1235–43. doi: 10.14219/jada.archive.1997.0400. [DOI] [PubMed] [Google Scholar]
- 19.Bennett AM, Fulford MR, Walker JT, Bradshaw DJ, Martin MV, Marsh PD. Microbial aerosols in general dental practice. Br Dent J. 2000;189:664–7. doi: 10.1038/sj.bdj.4800859. [DOI] [PubMed] [Google Scholar]
- 20.Prospero E, Savini S, Annino I. Microbial aerosol contamination of dental healthcare workers'faces and other surfaces in dental practice. Infect Control Hosp Epidemiol. 2003;24:139–41. doi: 10.1086/502172. [DOI] [PubMed] [Google Scholar]
- 21.Logothetis DD, Martinez-Welles JM. Reducing bacterial aerosol contamination with a chlorhexidine gluconate pre-rinse. J Am Dent Assoc. 1995;126:1634–9. doi: 10.14219/jada.archive.1995.0111. [DOI] [PubMed] [Google Scholar]
- 22.Agahi RH, Hashemipour MA, Kalantari M, Ayatollah-Mosavi A, Aghassi H, Nassab AH. Effect of 0.2% chlorhexidine on microbial and fungal contamination of dental unit waterlines. Dent Res J (Isfahan) 2014;11:351–6. [PMC free article] [PubMed] [Google Scholar]
- 23.Douglas CW, Rothwell PS. Evaluation of a dental unit with a built-in decontamination system. Quintessence Int. 1991;22:721–6. [PubMed] [Google Scholar]
- 24.Mills SE, Lauderdale PW, Mayhew RB. Reduction of microbial contamination in dental units with povidone-iodine 10% J Am Dent Assoc. 1986;113:280–4. doi: 10.14219/jada.archive.1986.0178. [DOI] [PubMed] [Google Scholar]
- 25.Veksler AE, Kayrouz GA, Newman MG. Reduction of salivary bacteria by pre-procedural rinses with chlorhexidine 0.12% J Periodontol. 1991;62:649–51. doi: 10.1902/jop.1991.62.11.649. [DOI] [PubMed] [Google Scholar]
- 26.Singh A, Daing A, Dixit J. The effect of herbal, essential oil and chlorhexidine mouthrinse on de novo plaque formation. Int J Dent Hyg. 2013;11:48–52. doi: 10.1111/j.1601-5037.2012.00556.x. [DOI] [PubMed] [Google Scholar]
- 27.Deshmukh MA, Dodamani AS, Karibasappa G, Khairnar MR, Naik RG, Jadhav HC. Comparative evaluation of the efficacy of probiotic, herbal and chlorhexidine mouthwash on gingival health: A randomized clinical trial. J Clin Diagn Res. 2017;11:C13–6. doi: 10.7860/JCDR/2017/23891.9462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Microbial colonies formed on blood agar plates exposed at patient's chest area in (a) Group I- Water/Placebo; (b) Group II- Chlorhexidine mouth wash; (c) Group III- Herbal mouth wash
Microbial colonies formed on blood agar plates exposed at doctor's mask area in (a) Group I- Water/Placebo; (b) Group II- Chlorhexidine mouth wash; (c) Group III- Herbal mouth wash
Microbial colonies formed on blood agar plates exposed at 2 feet beside patient in (a) Group I- Water/Placebo; (b) Group II- Chlorhexidine mouth wash; (c) Group III- Herbal mouth wash


