Abstract
Background:
Due to the triplication of chromosome 21 and resultant extra copy of the amyloid precursor protein gene, most adults with Down syndrome (DS) develop Alzheimer disease (AD)-like pathology and dementia. Using positron emission tomography (PET) imaging of amyloid, we compared DS with another genetic form of AD, autosomal-dominant AD (ADAD).
Methods:
Participants with DS (n=192), ADAD mutation-carriers (MC) (n=265), and familial controls (n=202) with amyloid PET imaging from the Alzheimer’s Biomarker Consortium-Down Syndrome and the Dominantly Inherited Alzheimer Network were included. Global and regional amyloid burdens were compared by cognitive status, APOE ε4 status, sex, age, and estimated years to symptom onset (EYO). Finally, we evaluated the relationship between amyloid PET and CSF Aβ42/40 in a subset of participants.
Findings:
PET and CSF amyloid were inversely correlated in DS and MC participants (ρ = −0∙801 and −0.565 respectively; both p-values < 0∙001). There were no significant differences in global amyloid burden between MC and DS when grouped by cognitive status. We also did not observe a significant effect of APOE ε4-positivity or sex in either group. Amyloid accumulation occurred slightly later in participants with DS (EYO −17∙5) compared to MC (EYO −23∙0) (p-value < 0∙001). This difference was mainly driven by PSEN1 MCs. Early amyloid increases were seen in striatal and cortical regions in both MC and DS individuals. While widespread amyloid accumulation was seen in MC, occipital regions were spared in individuals with DS.
Interpretation:
This study demonstrates that, despite minor differences between DS and ADAD, the overall pathophysiology is similar between these two genetic forms of AD.
Funding:
National Institute on Aging, Riney and Brennan Funds, Eunice Kennedy Shriver National Institute of Child Health and Human Development, German Center for Neurodegenerative Diseases, and Research and Development Grants for Dementia from Japan Agency for Medical Research and Development.
INTRODUCTION
Down syndrome (DS), caused by full or partial triplication of chromosome 21, is the most common genetic disorder, with approximately 1 in 700 children born in the United States each year1. Due to this triplication, individuals with DS have an extra copy of the amyloid precursor protein (APP) gene on chromosome 21 and overproduce amyloid beta (Aβ). Consequently, almost all adults with DS develop amyloid plaques and tau neurofibrillary tangles, hallmarks of Alzheimer disease (AD)2. Given this fact and the dramatic rise of life expectancy, there is a growing population of adults with DS developing AD1,3.
Previous studies have utilized cognition, fluid biomarker, and imaging measures to understand the presentation and progression of AD in individuals with DS. Cognitive symptoms of AD develop at approximately 50–55 years, with cerebrospinal fluid (CSF) markers changing years prior to these symptoms3–5. Positron emission tomography (PET) imaging studies also identify amyloid accumulation in cortical and subcortical brain regions years before clinical symptoms6. However, questions remain regarding amyloid deposition in individuals with DS compared to other forms of AD, especially autosomal dominant AD (ADAD).
ADAD, another genetic form of AD, is caused by presenilin 1 (PSEN1), presenilin 2 (PSEN2), or APP gene mutations that lead to altered amyloid levels. Similar to DS, carriers of ADAD mutations develop AD at an earlier age (30–60 years old) than individuals with late-onset AD (LOAD) (≥65 years old)7. Studies assessing biomarker changes in DS and ADAD separately suggest similarities between these two genetic forms of AD2. To date, only two studies have directly compared DS and ADAD but in a limited number of individuals8,9. One study comparing amyloid PET found no differences between ADAD and DS but only included amyloid-positive, asymptomatic participants. The other study, which evaluated CSF biomarkers, reported greater CSF Aβ40 and Aβ42 levels in both asymptomatic and symptomatic DS individuals compared to ADAD counterparts. These studies suggest that, while AD pathology may be similar for DS and ADAD, differences may exist.
Here we evaluate amyloid deposition in two large cohorts of genetic causes of AD by comparing DS to ADAD. Amyloid PET was analyzed in relation to CSF amyloid levels to study the relationship between amyloid clearance and deposition. We then assessed global and regional amyloid PET as a function of cognitive performance and age for DS and ADAD individuals.
Given recent evidence of heterogeneity in amyloid accumulation between different ADAD mutations, we also compared amyloid PET measures between DS and ADAD mutation types10. These comparisons enhance our understanding of genetic forms of AD and may have important implications for more common forms of AD including LOAD. Given the development of novel therapeutics designed to reduce amyloid, this knowledge will be vital for providing these treatments to individuals with genetic forms of AD.
METHODS
Study Design and Participants
The Alzheimer’s Biomarker Consortium-Down Syndrome (ABC-DS) enrolls adults with DS (≥25 years old) and sibling-controls in a multi-site study that collects longitudinal clinical, cognitive, imaging, and fluid biomarker data. For this analysis, only participants from the first data release (January 2020) who had a magnetic resonance imaging (MRI) and amyloid PET scan were included (DS, n=192; sibling-controls, n=33).
The Dominantly Inherited Alzheimer Network (DIAN) Observational Study is a multi-site longitudinal study that enrolls individuals from families with an ADAD genetic mutation. Data from DIAN data freeze 15 (December 2020) containing mutation-carriers (MC; n=265) and non-carrier familial controls (NC; n=168) with MRI and amyloid PET imaging within a similar age range as ABC-DS participants (25–73 years old) were included.
Informed consent or assent, when appropriate, was obtained from all participants and from their legally authorized representative when necessary. Study protocols were approved by the local Institutional Review Boards of all ABC-DS and DIAN sites.
Procedures
Participants with DS are given a clinical dementia diagnosis by a committee with clinical training or extensive experience in evaluating dementia in individuals with DS. This committee considers several variables (Supplemental materials) to derive a consensus diagnosis of “cognitively stable”, “mild cognitive impairment” (MCI), or “dementia due to AD”. If no consensus is reached, a diagnosis of “no consensus” is given. For this analysis, individuals with a consensus diagnosis of cognitively stable were categorized as asymptomatic (aDS). Those with a consensus diagnosis of MCI or dementia due to AD were categorized as symptomatic (sDS). Individuals with a consensus diagnosis of “no consensus” were excluded from the comparisons by cognitive status.
Cognitive status for DIAN participants was determined using the Clinical Dementia Rating® (CDR®) scale. CDR = 0 indicates normal cognitive function, CDR = 0∙5 very mild dementia, CDR = 1 mild dementia, CDR = 2 moderate dementia, and CDR = 3 severe dementia. Only NC control participants with CDR = 0 were included. MC participants were categorized as asymptomatic (CDR=0; aMC) or symptomatic (CDR>0; sMC).
For participants with DS, karyotype was obtained from medical records or genetic testing. All DIAN study participants underwent genetic testing to determine PSEN1, PSEN2, or APP mutation status. For the analyses considering ADAD mutation type, mutation-carriers were categorized by location of mutation on PSEN1 before codon 200, PSEN1 after codon 200, PSEN2, or APP (Supplemental Material). Individuals carrying the APP Glu693Gln (Dutch) mutation were excluded due to evidence of inconsistent PET tracer uptake.
CSF samples were collected in a subset of ABC-DS and DIAN participants and processed at Washington University using a Lumipulse platform (Supplemental material). APOE genotype was determined from blood samples using KASP genotyping assays (LGC Genomics, Beverly, MA) for ABC-DS participants and a TaqMan assay (Applied Biosystems, Waltham, MA) for DIAN participants. Individuals were categorized as APOE4-positive if they had at least one ε4 allele.
T1-weighted MRI scans were collected for ABC-DS and DIAN participants on 3-Tesla MR scanners and segmented into regions of interest using FreeSurfer 5.3-HCP with identical quality control procedures. ABC-DS participants underwent amyloid PET imaging using [11C]-Pittsburgh Compound B (PiB) or [18F]-AV45 (Florbetapir) (Supplemental material). DIAN participants received PiB (Supplemental material). All PET images were processed and aligned to the FreeSurfer MR segmentation using an established processing pipeline (PET Unified Pipeline; https://github.com/ysu001/PUP). Regional standard uptake value ratios (SUVRs) were calculated using the cerebellar cortex as the reference region.
As ABC-DS used different tracers, SUVRs were transformed to the Centiloid scale11 (Supplemental material). For the regional analysis, we compared only the ABC-DS and DIAN participants with a PiB PET scan. PiB SUVR values were calculated from the 50–70 minute post-injection time window and underwent partial volume correction.
Statistical analysis
We evaluated differences in demographic characteristics between controls, MC, and DS using χ2-tests for categorical variables and Kruskal-Wallis rank sum tests for continuous variables after determining a non-normal distribution using the Kolmogorov-Smirnoff test. If differences were significant, post-hoc two-sample tests were performed. We used the Mann-Whitney U test to examine differences in amyloid accumulation between groups categorized by cognitive status: controls, aDS, sDS, aMC, and sMC. We also compared amyloid accumulation in these five groups with regards to APOE4 status and sex using the Mann-Whitney U test. The Benjamini-Hochberg procedure was used to correct for multiple comparisons.
We assessed the correlation between CSF measures of amyloid and amyloid PET levels using the Spearman Correlation test. Amyloid PET levels were assessed as a function of age. Since AD progression is typically evaluated in ADAD as a function of estimated years to symptom onset (EYO), we also compared amyloid levels over EYO. For DIAN participants, EYO was estimated by subtracting an individual’s current age from the age at which their parent began experiencing symptoms7. Since a method to calculate EYO in individuals with DS has not been established, we estimated EYO for ABC-DS participants by subtracting their age from an average age of symptom onset (AAO). Based the range of average age of AD symptom onset observed in prior studies, we calculated EYO in participants with DS using AAOs of 50, 52.5, and 55 years but focused on an AAO of 52.5 years for comparisons to MCs using EYO3,4,12–17. Amyloid was evaluated by age and EYO in controls, MC, and DS using a bootstrapping approach and generalized additive model (GAM) with a cubic regression spline (Supplemental Materials). We also assessed the regional pattern of amyloid accumulation in DS and MC participants (Supplemental Materials). All analyses used R (version 4.1.2) and the packages mgcv, tidymv, ggplot, and ggseg.
Role of the funding source
The funders of this study had no role in the study design, data collection, data analysis, data interpretation, or writing of this report.
RESULTS
Participant demographics
A total of 192 individuals with DS and 33 sibling controls from ABC-DS and 265 MC and 169 NC familial controls from DIAN were included. Controls from ABC-DS and DIAN were combined into a single group.
Control, DS, and MC groups did not differ by age or APOE ε4-positivity status (p>0∙05) (Table 1). There were fewer females in the DS group (44%) compared to control (61%) and MC groups (53%) (p=0∙003). A smaller percentage of individuals with DS identified as non-white compared to MCs (p=0∙027), although a large majority of all groups (>85%) identified as white. A higher percentage of MCs (38%) compared to DS (15%) were categorized as symptomatic (p<0∙001). Of the 101 symptomatic MCs, 57 (56%) were very mildly impaired (CDR=0∙5), 27 (27%) were mildly impaired (CDR=1), and 17 (17%) were moderately or severely impaired (CDR>1). Of the 28 symptomatic participants with DS, 16 (57%) had MCI, and 12 (43%) had dementia due to AD.
Table 1.
Participant demographics
| Controls (n = 202) | Down syndrome (DS) (n = 192) | Mutation-carrier (MC) (n=265) | p-value | |
|---|---|---|---|---|
| Age, years (median [IQR]) | 40 [33, 49] | 41 [35, 49] | 39 [33, 48] | 0∙137 |
|
| ||||
| Female | 123 (61%) | 84 (44%)* | 140 (53%) | 0∙003 |
|
| ||||
| Race | 0∙027 | |||
| White | 186 (92%) | 184 (96%)† | 232 (87%) | |
| Black or African American | < 3 (1%) | 2 (1%) | < 3 (1%) | |
| Unknown | 3 (1%) | 0 | < 3 (1%) | |
| Other | 12 (6%) | 6 (3%) | 29 (11%) | |
|
| ||||
| APOE ε4-positive | 57 (28%) | 38 (20%) | 78 (29%) | 0∙059 |
|
| ||||
| Cognitive status | < 0∙001 | |||
| Asymptomatic | 202 (100%) | 155 (81%)*† | 164 (62%)* | |
| Symptomatic | 0 | 28 (15%) | 101 (38%) | |
| No consensus | NA | 9 (4%) | NA | |
|
| ||||
| Down syndrome type | --- | |||
| Full trisomy 21 | -- | 168 (87∙5%) | -- | |
| Translocation | -- | 12 (6%) | -- | |
| Mosaicism | -- | 6 (3%) | -- | |
|
| ||||
| ADAD mutation type | --- | |||
| PSEN1 | -- | -- | 202 (76%) | |
| PSEN2 | -- | -- | 22 (8%) | |
| APP | -- | -- | 41 (15%) | |
|
| ||||
| Centiloid (median [IQR]) | -2∙93 [−5∙7, −0∙07] | 8∙45 [1∙3, 49∙8]*† | 31∙72 [4∙6, 67∙2]* | <0∙0001 |
Abbreviations: ADAD = autosomal dominant Alzheimer disease; APP = amyloid precursor protein; IQR = interquartile range; PSEN = presenilin; SD = standard deviation
Significantly different from control group (p < 0∙05 after Benjamini-Hochberg correction for multiple comparisons)
Significantly different from mutation-carriers (p < 0∙05 after Benjamini-Hochberg correction for multiple comparisons)
Comparison of PET and CSF amyloid
The subset of participants with DS with CSF data (n=32) was older (mean 49∙8 years [SD 5∙75 years]) than the MC group (n=216) (mean 39∙8 years [SD 9∙74 years]) but similar with regards to APOE4 status, sex, and race (Supplemental Table 2).
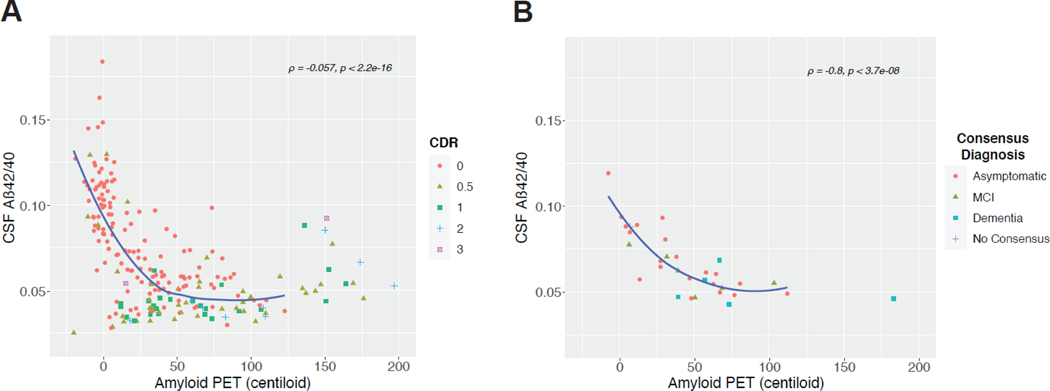
Amyloid PET (Centiloids) was plotted as a function of CSF Aβ42/40, Aβ42, and Aβ40 for MC and DS categorized by cognitive measure (CDR for MC, consensus diagnosis for DS) (Figure 1, Supplemental Figure 1). In MC participants, we measured a negative correlation between CSF Aβ42/40 and amyloid PET levels (ρ = −0∙565, p-value < 0∙001). CSF Aβ42/40 levels were diminished and Centiloids were elevated in symptomatic (CDR>0) compared to asymptomatic (CDR=0) MC participants (Figure 1A). We observed a similar relationship between CSF Aβ42/40 and Centiloids when we grouped MC participants by mutation type (Supplemental Figure 1). Similar results were also seen in participants with DS (Figure 1B). CSF Aβ42/40 levels were also negatively correlated with Centiloid levels (ρ = −0∙801, p-value < 0∙001). We also observed that “cognitively stable” participants have elevated Centiloids and reduced CSF Aβ42/40 versus participants with a consensus diagnosis of “dementia”. Similar results were observed when amyloid PET was plotted as a function of CSF Aβ42 in both MC and DS (ρ = −0∙53 and −0∙61, both p-values < 0∙001) (Supplemental Figure 2A and 2B), but no significant correlation was measured between CSF Aβ40 and amyloid PET in either group (Supplemental Figure 2C and 2D).
Figure 1:
Global amyloid deposition in Centiloids plotted against CSF levels of Aβ42/Aβ40 in A) ADAD MCs (n = 219) and B) participants with DS (n = 32). Plotted data points were categorized by participants’ cognitive status as measured by CDR in MCs and consensus diagnosis in DS. Correlation analysis between CSF Aβ42/Aβ40 and Centiloids was performed using Spearman’s rank correlation test. Abbreviations: Aβ = amyloid-beta; CDR = clinical diagnosis rating; CSF = cerebrospinal fluid; DS = Down syndrome; MC = mutation-carrier; MCI = mild cognitive impairment; PET = positron emission tomography
Amyloid PET as function of cognitive status, APOE4 status, and sex
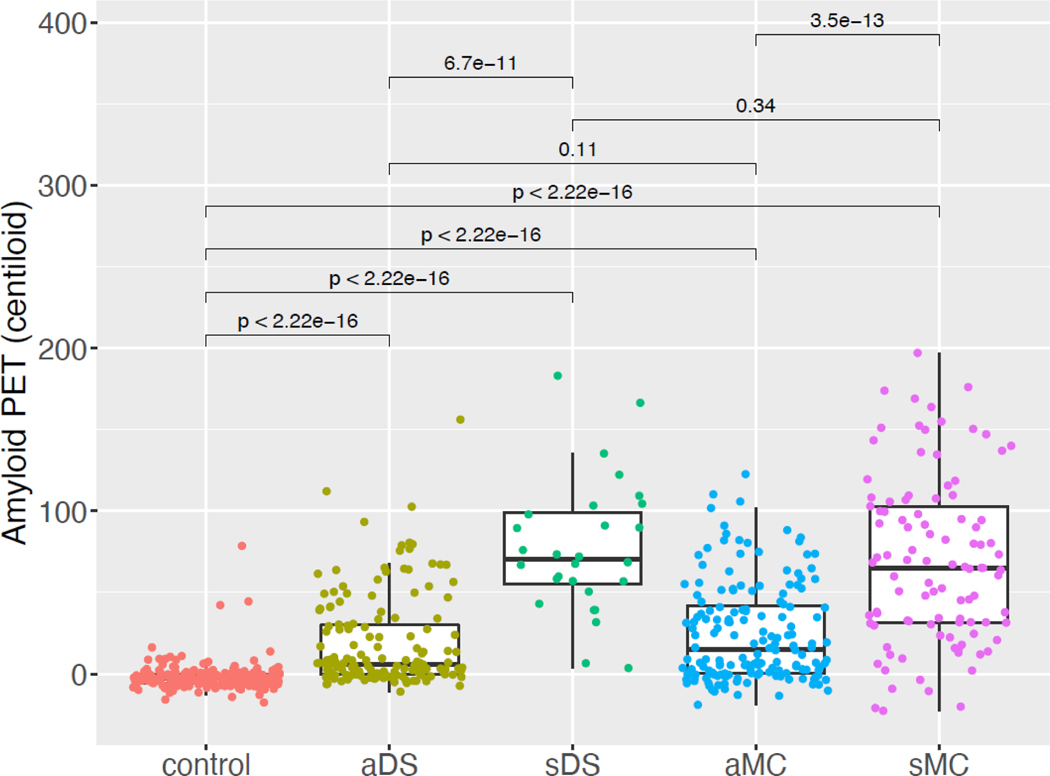
We compared amyloid levels in the control, DS, and MC groups categorized by cognitive status (control, asymptomatic DS [aDS], symptomatic DS [sDS], asymptomatic MC [aMC], and symptomatic MC [sMC]). As expected, Centiloids in controls clustered near zero (mean −1∙77 [SD 8∙77]) and were lower compared to aDS, sDS, aMC, and sMC (p-values<0∙001) (Figure 2). The sDS and sMC groups had higher levels compared to their respective asymptomatic groups (p-values<0∙001). Similar results were seen when sDS and sMC were further delineated into MCI and AD (CDR 0∙5–1 and CDR > 1 for MC, respectively) (data not shown). aDS and aMC were not different (p>0∙05) nor were sDS and sMC (p>0∙05).
Figure 2:
Global amyloid deposition for control, DS, and MC participants were grouped by cognitive status. P-values were calculated using the Mann-Whitney U test and adjusted for multiple comparisons using the Benjamini-Hochberg method. Abbreviations: aDS = asymptomatic DS; aMC = asymptomatic MC; sDS = symptomatic DS; sMC = symptomatic MC; DS = participants with Down syndrome; MC = autosomal dominant mutation carriers; PET = positron emission tomography
Since previous studies of LOAD have reported an effect of APOE4 status and sex on amyloid levels, we evaluated these variables in DS and MC individuals. No differences in amyloid PET were observed between APOE4-positive and APOE4-negative individuals or between males and females for all groups (aDS, sDS, aMC, or sMC) (p-values>0∙05) (Supplemental Figures 3A and 5A).
Amyloid PET as a function of EYO and age
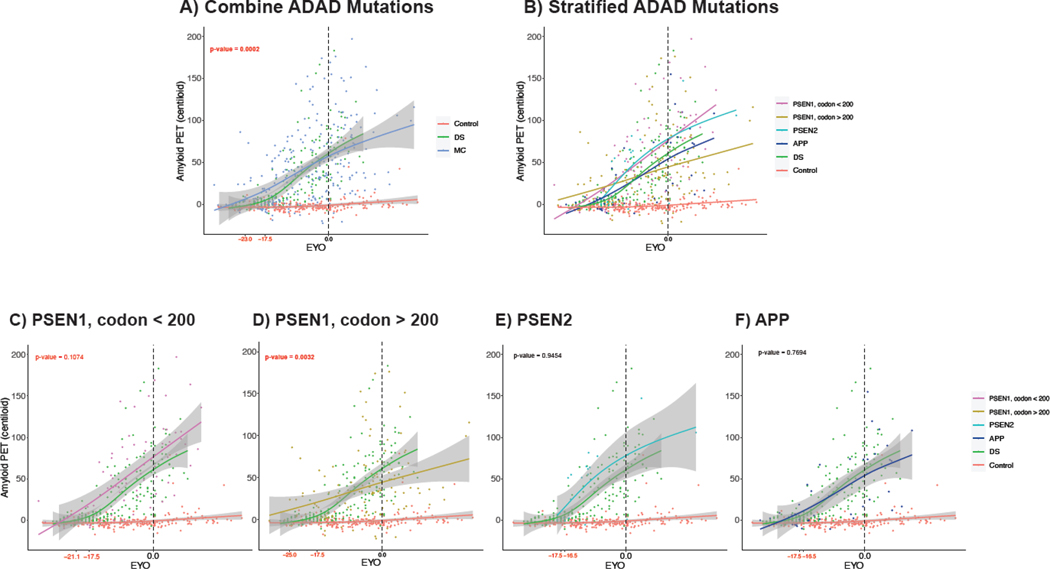
We evaluated trajectories of amyloid accumulation for controls, DS, and MC groups as measured by EYO using an average age of onset of 52.5 years for participants with DS (Figure 3). Centiloids were elevated in MCs at an earlier EYO compared to participants with DS (−23∙0 and −17∙5 years old, respectively; p-value < 0∙001). This result was similar when using an AAO of 50 and 55 years for participants with DS (p-value < 0∙001 and p-value = 0∙056, respectively; Supplemental Figure 6). When we compared DS to different ADAD mutation types using an AAO of 52.5 years of age, amyloid accumulation in participants with DS was elevated significantly later than PSEN1 mutations after codon 200 (p-value = 0∙0032; Figure 3D) and trended later than PSEN1 mutations before codon 200 (p-value = 0∙1074; Figure 3C). There were no significant difference between DS and PSEN2 or APP MCs (both p-values > 0∙05) (Figure 3E&F).
Figure 3:
Global amyloid deposition in Centiloids as a function of EYO for healthy controls, participants with DS, and A) all ADAD mutation type MCs B) MCs stratified by ADAD mutation type, C) PSEN1 before codon 200 MCs (n = 74), D) PSEN1 after codon 200 MCs (n = 128), E) PSEN2 MCs (n = 22), and F) APP MCs (n = 41). EYO was determined by parental AAO for MC and by AAO of 52.5 years for DS. Curves were generated using a generalized additive model and cubic regression spline. Using a bootstrapping approach, the median EYOs in red at which MC and DS Centiloids became significantly elevated compared to controls were calculated and compared to determine whether accumulation began significantly later in participants with DS compared to MCs. Abbreviations: AAO = age at onset; ADAD = autosomal dominant Alzheimer disease; APP = amyloid precursor protein; DS = Down syndrome; MC = mutation-carrier; PET = positron emission tomography; PSEN = presenilin
When we repeated the comparison to MC participants using age, Centiloids were elevated in MCs at an earlier age compared to participants with DS (25.4 and 34.8 years old, respectively; p-value < 0∙001) (Supplemental Figure 7A). When we compared DS to different ADAD mutations, PSEN1 mutations before or after codon 200 were elevated earlier than participants with DS (both p-values < 0∙001; Supplemental Figure 7C & D). Amyloid accumulation in participants with DS occurred at a similar age for PSEN2 and APP MCs (both p-values > 0∙05; Supplemental Figure 7E & F).
We evaluated if APOE4-positivity led to earlier changes in amyloid PET (left shift) in DS and MC individuals. When plotting Centiloids by EYO, we observed no temporal shift in APOE4-positive DS (Supplemental Figure 3B) or MC individuals (Supplemental Figure 3C). We also did not observe a significant difference between amyloid-positive and negative individuals when assessed by age range (Supplemental Figure 4). A similar analysis examining sex did not show a temporal shift in Centiloids between males and females for either DS (Supplemental Figure 5B) or MC individuals (Supplemental Figure 5C).
Regional amyloid PET group comparison
We were interested in the spatial pattern of amyloid distribution by EYO for DS compared to MC individuals. We examined SUVR values in 34 cortical and seven subcortical regions in the subset of DS (n=128), MC (n=265), and control (n=202) participants with a PiB PET scan. Within this subset, participants with DS were younger (p=0∙015) and a majority were asymptomatic (88%) (Supplemental Table 3). Fewer participants with DS were APOE4-positive (18%) compared to MC (29%) and controls (28%) (p=0.048).
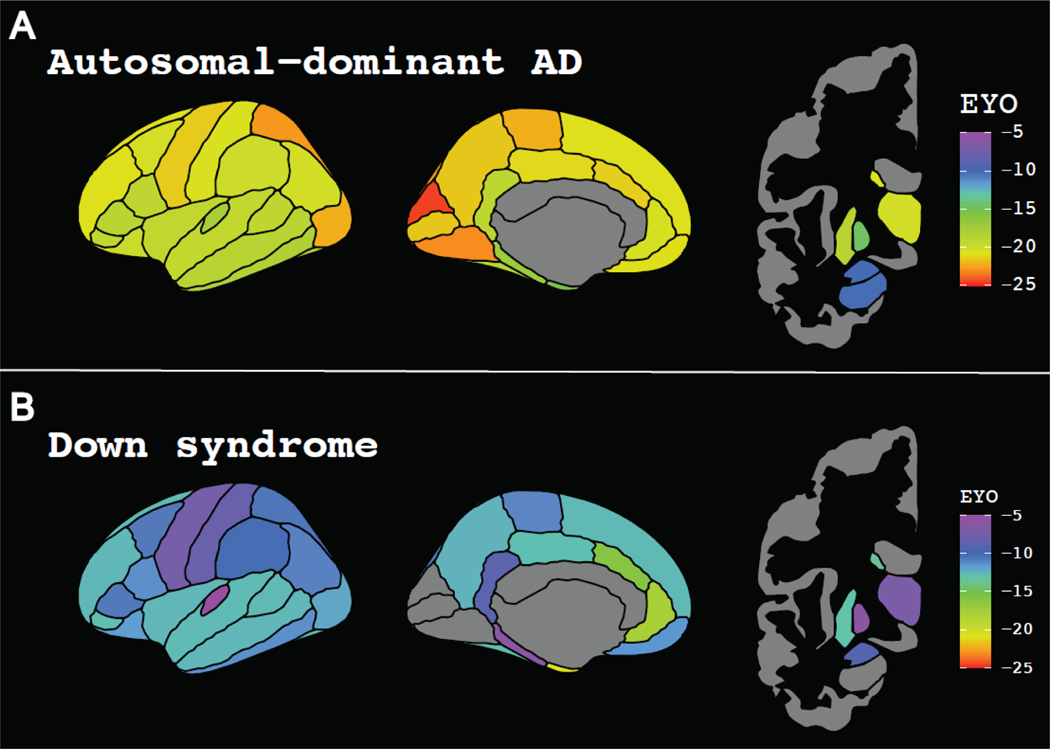
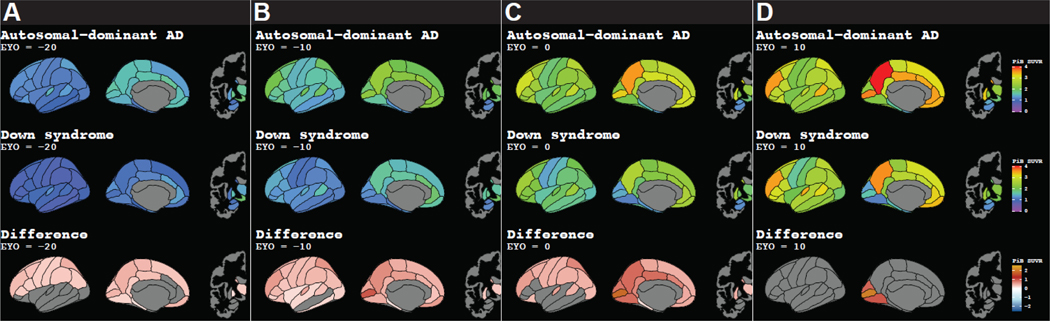
Earliest amyloid accumulation in MC occurred in the occipito-parietal cortices and was closely followed by the frontal lobe and striatum (Figure 4A, Figure 5, Video 1, Supplemental Figure 8, Supplemental Table 4). Early regional amyloid accumulation in participants with DS occurred in the frontal and striatum followed by regions in the parietal and temporal lobes (Figure 4B, Figure 5, Video 1). While we measured amyloid accumulation throughout all cortical regions in MC, amyloid SUVRs were not elevated in DS within the medial occipital regions (cuneus, pericalcarine, and lingual cortices).
Figure 4:
EYO at which regional amyloid accumulation in A) ADAD mutation-carriers and B) individuals with DS was significantly greater than controls. Using a bootstrapping approach, the EYO at which each region became significantly elevated was determined over 10,000 iterations. The median EYO for each region was calculated to assess the spatial pattern of amyloid accumulation. Abbreviations: ADAD = autosomal dominant Alzheimer disease; EYO = estimated years to symptom onset; DS = Down syndrome
Figure 5:
Regional PiB SUVRs at A) EYO = −20, B) EYO = −10, C) EYO = 0, and D) EYO = 10 in autosomal dominant AD MCs, participants with DS, and the significant difference between MC and DS SUVRs using a 99% confidence interval to adjust for multiple comparisons. Abbreviations: AD = Alzheimer’s disease; EYO = estimated years to symptom onset; DS = Down syndrome; MC = autosomal dominant AD mutation carrier; SUVR = standard uptake value ratio
DISCUSSION
In this study, we compared amyloid deposition within a large cohort of individuals with DS and MC for ADAD. Overall, we observed many similarities between individuals with DS and MC. This study is the first to assess the relationship between PET and CSF amyloid levels in DS. Our results showed an inverse relationship between the two and suggest amyloid levels decrease in the CSF prior to accumulation in the brain as measured by PET, similar to LOAD and ADAD18,19. When we assessed amyloid PET, Centiloid levels were similar between individuals with DS and MC when grouped by cognitive status. However, when we assessed amyloid levels by EYO and performed regional analyses, we noted slight differences between MC and individuals with DS. In particular, amyloid accumulation began earlier in MC compared to DS but approached similar levels by EYO 0. Our results suggest that this difference is driven primarily by PSEN1 mutations. The timing of initial amyloid accumulation in participants with DS was similar to APP MCs. We also observed early striatal and cortical amyloid increases in both MC and DS, but while significant amyloid accumulation was measured throughout the brain in MC, accumulation was spared in certain occipital regions for DS. Our findings suggest that, despite a few subtle differences, overall, cerebral amyloid deposition is similar between ADAD and DS.
When we assessed the relation to cognitive impairment, we observed similar results for DS and MC individuals. In both groups, asymptomatic participants had higher global amyloid levels compared to controls; symptomatic individuals with DS and ADAD had even higher amyloid deposition compared to their asymptomatic counterparts. This is supported by our results showing amyloid accumulation begins approximately two decades prior to symptom onset in both DS and ADAD. These results suggest amyloid accumulation begins in the early pre-clinical stages in individuals with DS and continues to increase as cognitive impairment progresses, similar to what is observed in LOAD and ADAD18,19.
While our results suggest amyloid begins accumulating at similar early stages of AD progression, we also observed subtle but significant differences between DS and MC in the timing of initial amyloid accumulation. Our results suggest amyloid accumulation begins slightly later in individuals with DS compared to MC. Upon differentiating MCs by ADAD mutation type, we saw that these changes were primarily driven by PSEN1 mutations. No significant differences were observed between individuals with DS and AAP MCs. One possible explanation for this temporal delay for DS is that other triplicated genes on chromosome 21, such as the gene for β-site APP cleaving enzyme 2 (BACE2), could be protective. Studies have suggested the θ-secretase activity of BACE2 cleaves amyloid into smaller non-amyloidogenic Aβ isomers instead of amyloidogenic Aβ4220. However, a study measured significantly higher levels of CSF Aβ42 in asymptomatic individuals with DS compared to asymptomatic MC, suggesting production of amyloidogenic Aβ42 in individuals with DS despite BACE2 triplication8. Another possible explanation for the delayed amyloid accumulation in individuals with DS is that they may clear amyloid more efficiently. A study of the DS critical region 1 (DSCR1) on chromosome 21 demonstrated improved amyloid clearance in mice with upregulated DSCR121. Future studies are needed to elucidate potential protective factors in individuals with DS as they could serve as a target for potential therapies.
With regards to spatial pattern of amyloid distribution, our results support previous work showing early amyloid accumulation in MC in the striatal, occipito-parietal, and frontal regions and was relatively consistent across mutation types9,22. Similar to previous studies, we measured early striatal amyloid accumulation in DS, in addition to early changes in the anterior cingulate and frontal cortex23. This early amyloid accumulation in the striatum in MC and DS supports previous studies and is a key deviation from LOAD9. However, while our results showed relatively early amyloid deposition in the medial occipital lobe in MC, we did not observe significant amyloid accumulation in this region for individuals with DS. Our work supports a previous amyloid PET study showing the occipital lobe is one of the last regions to develop amyloid in individuals with DS, a pattern more closely resembling LOAD23,24. These regional differences of amyloid deposition between DS, ADAD, and LOAD are important to consider for future clinical trials. Different regions may need to be included when determining amyloid-positivity for these groups as well as when evaluating the efficacy of anti-amyloid therapies.
The presence of at least one APOE ε4 allele is associated with earlier amyloid changes in LOAD25. However, both our work and prior ADAD studies did not observe an effect of APOE4 on the timing of amyloid accumulation in MC participants7,26,27. In DS, several studies have observed a significant effect of APOE4 on cognitive outcomes. With regards to the effect of APOE4 on amyloid PET measures in DS, mixed results have been observed14,28,29. Several studies reported no effect, but a recent study by Bejanin et al. observed higher amyloid PET deposition in subset of APOE4-positive participants aged 41–54 years30. In the present study of 192 participants with DS, amyloid PET was not elevated in APOE4-positive individuals, and amyloid accumulation did not occur earlier compared to APOE4-negative individuals. When we replicated the Bejanin et al. comparison by age range, we did not observe differences between amyloid-positive and negative participants with DS. Overall, our results suggest the presence of the APOE ε4 allele does not affect the magnitude or timing of amyloid accumulation in genetic forms of AD. These results suggest that genetic mutations in DS and MC individuals overshadow the effects of APOE genotype on amyloid. Observed changes in cognition seen with APOE ε4 positive with DS may be mediated by tau, but additional longitudinal studies of multiple biomarkers are needed.
Previous studies also identify a potential role of sex on the trajectory and development of LOAD31. Whether sex has an effect in individuals with DS is unclear. Some studies found no effect of sex on the prevalence or timing of dementia diagnosis in adults with DS. Other studies reported males with DS are more likely to develop dementia but at a later age than females14,28,32,33. We found no differences in the magnitude or timing of amyloid PET between males and females with DS. These results are supported by previous studies that also did not observe any effect of sex on amyloid PET23,34–37. These findings suggest that, while sex may affect cognitive presentation of AD, it does not affect amyloid deposition in adults with DS.
This large cohort of participants with DS and MC presents a notable strength of this study, but limitations should also be acknowledged. Although these two populations are genetically predisposed to develop AD, most ADAD mutations alter the processing of the APP protein by affecting γ-secretase activity. The closest direct comparison to DS would be individuals with a rare APP duplication. Only eight MC participants in our sample had an APP duplication. A future study with a larger sample of APP duplication carriers would be useful in assessing other genes on chromosome 21 and their effects on AD progression. Information on which ABC-DS participants with DS and sibling controls were related was unavailable, preventing us from adjusting for potential correlations between related participants. The cross-sectional nature of this study is another important limitation. Future longitudinal studies are necessary to better understand how amyloid accumulation for these two genetic forms of AD. Additionally, this study calculated EYO for individuals with DS using an average age of symptom onset based on the results of several prior studies of individuals with DS. However, using a fixed average age of onset in DS does not account for individual differences. Future analyses are necessary to improve our ability to predict symptom onset and calculate EYO for an individual with DS.
Despite these limitations, we used PET imaging to observe important similarities between two genetic forms of AD. Our results showed that levels of amyloid are similar between individuals with DS and ADAD. In both individuals with DS and ADAD, amyloid accumulation begins at the earliest stages of AD progression, around 20 years prior to the onset of cognitive symptoms. Our results still suggest subtle differences with amyloid accumulation beginning a few years later in individuals with DS and not occurring in all regions of the brain. The safety and efficacy of potential amyloid-lowering AD therapies has yet to be evaluated in individuals with DS. Based on our results, studying individuals with DS at least age 35 years or older would be ideal for potential anti-amyloid therapies. In conclusion, this study demonstrates that, while there are subtle differences between DS and ADAD, the overall pathophysiology is similar between these two genetic forms of AD.
Supplementary Material
RESEARCH IN CONTEXT.
Evidence before this study:
Between May 2021 and February 2022, we searched PubMed for articles relating to measures of cerebral amyloid in individuals with Down syndrome or autosomal dominant Alzheimer disease. Search terms included: “amyloid”, “Alzheimer disease”, “Alzheimer’s disease”, “autosomal dominant”, “cerebral”, “cerebrospinal fluid”, “Down syndrome”, “familial”, and “positron emission tomography”. While many studies have assessed amyloid biomarkers in individuals with Down syndrome (DS) and autosomal dominant Alzheimer disease (ADAD), we found only two that have directly compared these two genetic forms of Alzheimer disease and within a limited sample size. The first study comparing amyloid PET measures reported no differences between individuals with DS and ADAD but included only amyloid-positive, asymptomatic individuals. The other study compared only CSF biomarkers and measured significantly higher amyloid-β40 (Aβ40) and Aβ42 in individuals with DS.
Added value of this study:
To our knowledge, this is the first study to assess the relationship between CSF and PET amyloid measures in individuals with DS. It is also the largest study to date comparing amyloid accumulation as measured by PET imaging, processed in a similar manner, between individuals with DS and ADAD. We observed an inverse relationship between CSF and PET measures of amyloid in individuals with DS, which is similar to the relationship previously seen for ADAD and late-onset AD. Overall levels of amyloid accumulation were also similar between individuals with DS and ADAD with regard to degree of cognitive impairment. Amyloid accumulation began earlier in ADAD than in DS with regards to EYO, but this difference was primarily driven by carriers of PSEN1 mutations. Our analysis supported previous evidence of early striatal amyloid accumulation for both ADAD and DS. However, while amyloid accumulation occurred throughout the cerebral cortex in ADAD, we observed no accumulation in certain occipital regions in individuals with DS.
Implications of all the available evidence:
Our results support prior evidence suggesting similar levels of global amyloid accumulation in individuals with DS and ADAD. These findings also build on prior evidence that amyloid changes may occur in the CSF prior to cerebral accumulation measured by PET. Our findings suggest a potential protective factor delaying amyloid accumulation in individuals with DS, which may offer alternative targets for AD therapies. This timing and spatial distribution of amyloid accumulation are important to consider when designing and recruiting participants for clinical trials of amyloid-targeting therapies in DS and ADAD. The overwhelming similarities in the pattern of amyloid changes suggest potential overlap in the use of AD therapies for individuals with DS and ADAD.
ACKNOWLEDGEMENTS
Data collection and sharing for this project was supported by the Alzheimer’s Biomarker Consortium-Down Syndrome (ABC-DS, U01AG051406 and U01AG051412) funded by the National Institute on Aging (NIA) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development. We are grateful to the adults with Down syndrome, their siblings, and their families and care providers, as well as the ABC-DS research and support staff for their invaluable contributions to this study. Data collection and sharing for this project was also supported by The Dominantly Inherited Alzheimer Network (DIAN, U19AG032438) funded by the National Institute on Aging (NIA),the Alzheimer’s Association (SG-20-690363-DIAN), the German Center for Neurodegenerative Diseases (DZNE), Raul Carrea Institute for Neurological Research (FLENI), Partial support by the Research and Development Grants for Dementia from Japan Agency for Medical Research and Development, AMED, and the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), Spanish Institute of Health Carlos III (ISCIII), Canadian Institutes of Health Research (CIHR), Canadian Consortium of Neurodegeneration and Aging, Brain Canada Foundation, and Fonds de Recherche du Québec – Santé. This manuscript has been reviewed by DIAN Study investigators for scientific content and consistency of data interpretation with previous DIAN Study publications. We acknowledge the altruism of the participants and their families and contributions of the DIAN research and support staff at each of the participating sites for their contributions to this study. This research was also supported by the NIHR Cambridge Biomedical Research Centre (BRC-1215-20014*). The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care. We would also like to acknowledge the additional support provided by the Barnes-Jewish Hospital Foundation, the Charles F and Joanne Knight Alzheimer’s Research Initiative, the Hope Center for Neurological Disorders, the Mallinckrodt Institute of Radiology, the Paula and Rodger Riney fund, and the Daniel J. Brennan fund.
DECLARATION OF INTERESTS
TLSB has received support from the National Institute of Health and Seimens. She has a licensing agreement from Sora Neuroscience LLC but receives no financial compensation. She has received honoraria for lectures, presentations, speakers bureaus, or educational events from Biogen, Eisai Genetech and served on a Scientific Advisory Board for Biogen. She also holds a leadership role in other board, society, committee, or advocacy groups for the American Society for Neuroradiology (unpaid) and QIBA (unpaid). She has also participated in radiopharmaceuticals and technology transfers with Avid Radiopharmaceuticals, Cerveau, and LMI. EM received support from the National Institute on Aging, Anonymous Organization, GHR Foundation, DIAN-TU Pharma Consortium, Elie Lilly, and Hoffman La-Roche. He has received speaking fees from Eisai and Eli Lilly. He is also on the DSMB and Advisory boards of Elie Lilly, Alector, and Alzamend. WS has received support from the National Institute on Aging and the Eunice Kennedy Shriver National Institute of Child Health and Human Development. NS has received support from the National Institute on Aging and the Eunice Kennedy Shriver National Institute of Child Health and Human Development. He is also on the OSMB for the NIH Congenital Heart Defects Network. AMB reported receiving support from the National Institute of Health and consulting fees from Cognition Therapeutics, Inc. He also serves on a DSMB at Albert Einstein College of Medicine and is a chair of the International Neuropsychological Society Special Interest Group on Dementia. JPC serves as the chair of the American Neurological Association Dementia and Aging SIG and is on the medical advisory board of Humana Healthcare. CC has received consulting fees from GSK and Alector. GSD has received support from the National Institute on Aging, Alzheimer’s Association, and the Chan-Zuckerberg Initiative and is the clinical director for the Anti-NMDA Receptor Encephalitis Foundation (unpaid). He has also received consulting fees from Parabon Nanolabs Inc. and DynaMed (EBSCO) and holds stock in ANI Pharmaceuticals. He has provided expert testimony on cases pertaining to management of Wernicke encephalopathy. NCF is supported by the UK Dementia Research Institute and the NIHR UCLH Biomedical Research Centre. He has provided consultancy to Biogen, Ionis, Lilly and Roche (payment to UCL) and has served on a DSMB for Biogen. AMF reports personal fees from Roche Diagnostics, Araclon/Grifols, and Diadem Research and grants from Biogen, outside the submitted work. BH has received supported from Roche and Autism Speaks. He receives royalties from Oxford University Press for book publications and is the chair of the DSMB for the Department of Defense funded study “Comparative Effectiveness of EIBI and MABA.” AMG received support from the National Institutes of Health and royalty payments from Athena Diagnostics and Taconic Biosciences. She also consulted for AbbVie and Genetech and has stock in Cognition Therapeutics. BAG reports grants from National Institute of Health, during the conduct of the study. NRGR takes part in multicenter trials supported by Biogen, Eli Lilly, and AbbVie. SLH is supported by funding from the National Institute of Health and is the program chair for the Alzheimer’s Association ISTAART Down Syndrome and Alzheimer’s Disease PIA. BTC receives support from the National Institute of Health. EH receives support from the National Institute of Health and from the BrightFocus Foundation. WEK is supported by grants from the National Institute of Health. He is a co-inventor of PiB and thus has a financial interest in this license agreement and receives royalty payments from GE Healthcare. He also receives consulting fees from GE Healthcare and honorarium from Indiana University for grant consulting. He is a member of a data monitoring committee for Biogen. JCP receives support from the National Institute of Health and the National Institute on Aging. She is on the External Liason Committee of Center without Walls for Imaging Proteinopathies with PET, on the Advisory Council to the Director of the NIH Center for Scientific Review, and on the publications committee for the Journal of Cerebral Blood Flow and Metabolism. SS was the co-chair of the Investigator Steering Committee for the Aducanumab phase 3 program and he served as a site PI for the aducanumab and lecanemab phase 3 studies, the donanemab phase 2 trial and he was the Project Arm Leader for gantenerumab in DIAN-TU. He has received consulting income from Biogen, Lilly, Roche, Genentech and Eisai. He has no stock or royalties related to any medication in development. He serves on the planning committee for the National Disease Modifying Treatment and Diagnostic Registry Work Group and is a member of the ADRD Therapeutics Work Group. He is the first author for the report of ARIA in aducanumab phase 3 (Salloway, JAMA Neurology, 2022), the report of gantenerumab and solanezumab in DIAN-TU (Salloway, Nature Medicine, 2021). He is a co-author on the report of the donanemab phase 2 trial (Mintun, NEJM, 2021) and the Aducanumab Appropriate Use Recommendations (Cummings, Journal of the Prevention of Alzheimer’s Disease, 2021) SJKM receives support from the National Institute on Aging and funds from the New York State Office for People with Developmental Disabilities. She is also a consultant on a grand out of the University of Alabama and is a board member for the Research Foundation for Mental Hygiene, Inc. FL is supported by grants from the National Institute on Aging. CML is received grants from the National Institute of Health. JHL has received support from the National Institute of Health and the National Institute on Aging. JL receives support from the German Center for Neurodegenerative Diseases, German Research Foundation, and Verum Foundation. He has also received consulting fees from Axon Neuroscience and Biogen, author fees from Thieme medical publishers and W. Kohlhammer GmbH medical publishers, and speaker fees from Bayer Vital and Roche. He has received compensation from MODAG GmbH for duty as a part-time CMO and support from AbbVie and Biogen for attending meetings and/or travel. He also serves on the scientific advisory board of Axon Neurosciences. MM receives funding from the National Institute of Health and National Institute on Aging. He also receives royalties for intellectual property from Georgetown University and the University of Rochester and honoraria for public lectures/presentations from the Davis Phinney Foundation. He is on the scientific advisory board of Brain Neurotherapy Bio, LLC and thus receives stock options and support for attending meetings. HM receives support from the Japanese Agency for Medical Research and Development. MSR has received consulting fees from AC Immune and Keystone Bio. He is also the chair of data science monitoring board for Alzheon. HDR has received funding from the National Institute of Health. She is also on the scientific advisory committee for the Hereditary Disease Foundation. PRS has received funding from the National Institute of Health, an anonymous foundation paid through Washington University, Roth Charitable Foundation, the National Health and Medical Research Council (Australia), the Medical Research Future Fund (Australia), and NSW Health. He is the CEO of Neuroscience Research Australia and the Schizophrenia Research Institute and the president of the Australian Neuroscience Society. He is a company director for Neuroscience Research Australia Foundation, The Health-Science Alliance, Australian Association of Medical Research Institutes, Australian Dementia Network (ADNeT) Ltd, and StandingTall Pty Ltd. He is a member of the steering committee for Maridulu Budyari Gumal - Sydney Partnership for Health Education, Research and Enterprise (SPHERE), chair of the National Medical Advisory Panel for The Judith Jane Mason & Harold Stannett Williams Memorial Foundation, and an ambassador for Business Events Sydney. RJP receives support from the National Institute of Health and the National Institute on Aging. RJB is Director of DIAN–TU and Principal Investigator of DIAN–TU001. He receives research support from the NIA of the NIH, DIAN–TU trial pharmaceutical partners (Eli Lilly and Company, F. Hoffman La Roche Ltd, Janssen, Eisai, Biogen and Avid Radiopharmaceuticals), Alzheimer’s Association, GHR Foundation, Anonymous Organization, DIAN–TU Pharma Consortium (active: Biogen, Eisai, Eli Lilly and Company, Janssen, F. Hoffmann La Roche Ltd/Genentech; previous: AbbVie, Amgen, AstraZeneca, Forum, Mithridion, Novartis, Pfizer, Sanofi, United Neuroscience), NfL Consortium (Hoffman La-Roche, Biogen, AbbVie, and Bristol Meyer Squibb) and Tau SILK Consortium (Eli Lilly and Company, Biogen and AbbVie). He has been an invited speaker and consultant for AC Immune, F. Hoffman La Roche Ltd, the Korean Dementia Association, the American Neurological Association, and Janssen and has been a consultant for Amgen, Hoffman La-Roche and Eisai. He has submitted the US nonprovisional patent application “Methods for Measuring the Metabolism of CNS Derived Biomolecules In Vivo” and a provisional patent application “Plasma Based Methods for Detecting CNS Amyloid Deposition.” Dr. Holtzman is an inventor on patents for one of the treatments (solanezumab), which was tested in the DIAN clinical trials. If solanezumab is approved as a treatment for Alzheimer’s disease or Dominantly Inherited Alzheimer’s Disease, Washington University and Dr. Holtzman will receive part of the net sales of solanezumab from Eli Lilly that has licensed the patents related to solanezumab from Washington University.
Footnotes
AHB, RFA, BMA, SBB, OHB, CDC, SF, RLH, MJ, CMK, ITL, JHL, SOB, JW, CX, JH, NM, and SHZ declare no conflicts of interest.
DATA SHARING
The data used in this analysis are available upon request to the respective studies (ABC-DS and DIAN) provided data request applications are approved by the studies’ committees. The data request application is available for ABC-DS at https://pitt.co1.qualtrics.com/jfe/form/SV_cu0pNCZZlrdSxUN and for DIAN at https://dian.wustl.edu/our-research/for-investigators/dian-observational-study-investigator-resources/data-request-form/.
REFERENCES
- 1.Presson AP, Partyka G, Jensen KM, et al. Current estimate of Down Syndrome population prevalence in the United States. J Pediatr 2013; 163: 1163–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fortea J, Zaman SH, Hartley S, Rafii MS, Head E, Carmona-Iragui M. Alzheimer’s disease associated with Down syndrome: a genetic form of dementia. Lancet Neurol 2021; 20: 930–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fortea J, Vilaplana E, Carmona-Iragui M, et al. Clinical and biomarker changes of Alzheimer’s disease in adults with Down syndrome: a cross-sectional study. Lancet 2020; 395: 1988–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sinai A, Mokrysz C, Bernal J, et al. Predictors of Age of Diagnosis and Survival of Alzheimer’s Disease in Down Syndrome. J Alzheimer’s Dis 2017; 61: 717–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henson RL, Doran E, Christian BT, et al. Cerebrospinal fluid biomarkers of Alzheimer’s disease in a cohort of adults with Down syndrome. Alzheimer’s Dement Diagnosis, Assess Dis Monit 2020; 12. DOI: 10.1002/dad2.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hartley SL, Handen BL, Devenny D, et al. Cognitive decline and brain amyloid-β accumulation across 3 years in adults with Down syndrome. Neurobiol Aging 2017; 58: 68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryman DC, Acosta-Baena N, Aisen PS, et al. Symptom onset in autosomal dominant Alzheimer disease: A systematic review and meta-analysis. Neurology 2014; 83: 253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fagan AM, Henson RL, Li Y, et al. Comparison of CSF biomarkers in Down syndrome and autosomal dominant Alzheimer’s disease: a cross-sectional study. Lancet Neurol 2021; 20: 615–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen AD, McDade E, Christian B, et al. Early striatal amyloid deposition distinguishes Down syndrome and autosomal dominant Alzheimer’s disease from late‐ onset amyloid deposition. Alzheimer’s Dement 2018; 14: 743–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chhatwal JP, Schultz SA, McDade E, et al. Variant-dependent heterogeneity in amyloid β burden in autosomal dominant Alzheimer’s disease: cross-sectional and longitudinal analyses of an observational study. Lancet Neurol 2022; 21: 140–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Su Y, Flores S, Wang G, et al. Comparison of Pittsburgh compound B and florbetapir in cross‐ sectional and longitudinal studies. Alzheimer’s Dement Diagnosis, Assess Dis Monit 2019; 11: 180–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krinsky-McHale SJ, Zigman WB, Lee JH, et al. Promising outcome measures of early Alzheimer’s dementia in adults with Down syndrome. Alzheimer’s Dement (Amsterdam, Netherlands) 2020; 12: e12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCarron M, McCallion P, Reilly E, Dunne P, Carroll R, Mulryan N. A prospective 20-year longitudinal follow-up of dementia in persons with Down syndrome. J Intellect Disabil Res 2017; 61: 843–52. [DOI] [PubMed] [Google Scholar]
- 14.Hithersay R, Startin CM, Hamburg S, et al. Association of Dementia With Mortality Among Adults With Down Syndrome Older Than 35 Years. JAMA Neurol 2019; 76: 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lai F, Mercaldo N, Wang CM, Hersch GG, Rosas HD. Association between Inflammatory Conditions and Alzheimer’s Disease Age of Onset in Down Syndrome. J Clin Med 2021; 10: 3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coppus AMW, Schuur M, Vergeer J, et al. Plasma β amyloid and the risk of Alzheimer’s disease in Down syndrome. Neurobiol Aging 2012; 33: 1988–94. [DOI] [PubMed] [Google Scholar]
- 17.Iulita MF, Garzón Chavez D, Klitgaard Christensen M, et al. Association of Alzheimer Disease With Life Expectancy in People With Down Syndrome. JAMA Netw open 2022; 5: e2212910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fagan AM, Mintun MA, Mach RH, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann Neurol 2006; 59: 512–9. [DOI] [PubMed] [Google Scholar]
- 19.Fagan AM, Xiong C, Jasielec MS, et al. Longitudinal Change in CSF Biomarkers in Autosomal-Dominant Alzheimer’s Disease. Sci Transl Med 2014; 6. DOI: 10.1126/scitranslmed.3007901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alić I, Goh PA, Murray A, et al. Patient-specific Alzheimer-like pathology in trisomy 21 cerebral organoids reveals BACE2 as a gene dose-sensitive AD suppressor in human brain. Mol Psychiatry 2021; 26: 5766–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi C, Park J, Kim H, Chang KT, Park J, Min K. DSCR1 upregulation enhances dural meningeal lymphatic drainage to attenuate amyloid pathology of Alzheimer’s disease. J Pathol 2021; 255: 296–310. [DOI] [PubMed] [Google Scholar]
- 22.Gordon BA, Blazey TM, Su Y, et al. Spatial patterns of neuroimaging biomarker change in individuals from families with autosomal dominant Alzheimer’s disease: a longitudinal study. Lancet Neurol 2018; 17: 241–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Annus T, Wilson LR, Hong YT, et al. The pattern of amyloid accumulation in the brains of adults with Down syndrome. Alzheimer’s Dement 2016; 12: 538–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Villain N, Chételat G, Grassiot B, et al. Regional dynamics of amyloid-β deposition in healthy elderly, mild cognitive impairment and Alzheimer’s disease: a voxelwise PiB–PET longitudinal study. Brain 2012; 135: 2126–39. [DOI] [PubMed] [Google Scholar]
- 25.Mishra S, Blazey TM, Holtzman DM, et al. Longitudinal brain imaging in preclinical Alzheimer disease: impact of APOE ε4 genotype. Brain 2018; 141: 1828–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDade E, Wang G, Gordon BA, et al. Longitudinal cognitive and biomarker changes in dominantly inherited Alzheimer disease. Neurology 2018; 91: e1295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morenas-Rodríguez E, Li Y, Nuscher B, et al. Soluble TREM2 in CSF and its association with other biomarkers and cognition in autosomal-dominant Alzheimer’s disease: a longitudinal observational study. Lancet Neurol 2022; 21: 329–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lai F, Mhatre PG, Yang Y, Wang M, Schupf N, Rosas HD. Sex differences in risk of Alzheimer’s disease in adults with Down syndrome. Alzheimer’s Dement Diagnosis, Assess Dis Monit 2020; 12. DOI: 10.1002/dad2.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Startin CM, Hamburg S, Hithersay R, et al. Cognitive markers of preclinical and prodromal Alzheimer’s disease in Down syndrome. Alzheimer’s Dement 2019; 15: 245–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bejanin A, Iulita MF, Vilaplana E, et al. Association of Apolipoprotein E ɛ4 Allele With Clinical and Multimodal Biomarker Changes of Alzheimer Disease in Adults With Down Syndrome. JAMA Neurol 2021; 78: 937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wisch JK, Meeker KL, Gordon BA, et al. Sex-related Differences in Tau Positron Emission Tomography (PET) and the Effects of Hormone Therapy (HT). Alzheimer Dis Assoc Disord 2021; 35: 164–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mhatre PG, Lee JH, Pang D, et al. The Association between Sex and Risk of Alzheimer’s Disease in Adults with Down Syndrome. J Clin Med 2021; 10: 2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bayen E, Possin KL, Chen Y, Cleret de Langavant L, Yaffe K. Prevalence of Aging, Dementia, and Multimorbidity in Older Adults With Down Syndrome. JAMA Neurol 2018; 75: 1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lao PJ, Gutierrez J, Keator D, et al. Alzheimer-Related Cerebrovascular Disease in Down Syndrome. Ann Neurol 2020; 88: 1165–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hartley SL, Handen BL, Devenny D, et al. Cognitive indicators of transition to preclinical and prodromal stages of Alzheimer’s disease in Down syndrome. Alzheimer’s Dement Diagnosis, Assess Dis Monit 2020; 12. DOI: 10.1002/dad2.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cole JH, Annus T, Wilson LR, et al. Brain-predicted age in Down syndrome is associated with beta amyloid deposition and cognitive decline. Neurobiol Aging 2017; 56: 41–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lao PJ, Betthauser TJ, Hillmer AT, et al. The effects of normal aging on amyloid- β deposition in nondemented adults with Down syndrome as imaged by carbon 11–labeled Pittsburgh compound B. Alzheimer’s Dement 2016; 12: 380–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.