Abstract
Background
Infants with SVHD experience morbidity related to pulmonary vascular inadequacy. Metabolomic analysis involves a systems biology approach to identifying novel biomarkers and pathways in complex diseases. The metabolome of infants with SVHD is not well understood and no prior study has evaluated the relationship between serum metabolite patterns and pulmonary vascular readiness for staged SVHD palliation.
Objectives
The purpose of this study was to evaluate the circulating metabolome of interstage infants with single ventricle heart disease (SVHD) and determine whether metabolite levels were associated with pulmonary vascular inadequacy.
Methods
This was a prospective cohort study of 52 infants with SVHD undergoing Stage 2 palliation and 48 healthy infants. Targeted metabolomic phenotyping (175 metabolites) was performed by tandem mass spectrometry on SVHD pre-Stage 2, post-Stage 2, and control serum samples. Clinical variables were extracted from the medical record.
Results
Random forest analysis readily distinguished between cases and controls and preoperative and postoperative samples. Seventy-four of 175 metabolites differed between SVHD and controls. Twenty-seven of 39 metabolic pathways were altered including pentose phosphate and arginine metabolism. Seventy-one metabolites differed in SVHD patients between timepoints. Thirty-three of 39 pathways were altered postoperatively including arginine and tryptophan metabolism. We found trends toward increased preoperative methionine metabolites in patients with higher pulmonary vascular resistance and higher postoperative tryptophan metabolites in patients with greater postoperative hypoxemia.
Conclusions
The circulating metabolome of interstage SVHD infants differs significantly from controls and is further disrupted after Stage 2. Several metabolites showed trends toward association with adverse outcomes. Metabolic dysregulation may be an important factor in early SVHD pathobiology.
Key words: arginine, congenital heart disease, Glenn operation, methionine, pulmonary hypertension, tryptophan
Central Illustration
Single ventricle heart disease (SVHD) is a severe type of congenital heart disease characterized by patients having only one functional pumping chamber. SVHD affects between 2 and 8 infants per 10,000 live births, is typically fatal without intervention, and has no available cure.1 However, 3-staged surgical palliation allows many children with SVHD to survive into adulthood.2 From the first week of life (following completion of a Stage 1 operation for most patients) until the Stage 2 operation, subjects are described as “interstage.” Despite variability patient-specific cardiac and vascular anatomy, interstage SVHD children share a common physiology that involves complete intracardiac mixing of systemic and pulmonary venous returns, an unobstructed connection from the single ventricle to systemic arteries, and a pressure-restrictive source of pulmonary blood flow (typically a shunt between the aorta, innominate artery, or right ventricle and the pulmonary arteries). The second of the staged SVHD operations, the superior cavo-pulmonary anastomosis (Stage 2, bidirectional Glenn or hemi-Fontan), is most often performed between 4 and 6 months of age.2 During a patient’s Stage 2 surgery, the mechanism of blood delivery to the lungs is converted from an active process driven by ventricular systole to a passive process via a direct connection between the superior vena cava and the central pulmonary arteries.
In order for the lungs to passively accept sufficient blood flow for oxygenation with post-Stage 2 anatomy, infants with SVHD must experience significant growth and development of the pulmonary vasculature during the interstage period.3 However, many affected children do not (ie, experience pulmonary vascular inadequacy) and therefore struggle to tolerate Stage 2 physiology.4 In historical cohorts, among infants undergoing Stage 2 palliation, as many as 25% may experience complications in the immediate postsurgical period directly linked to insufficient pulmonary blood flow including severe hypoxemia, respiratory failure, and persistent pleural effusions.4 Current standard of care preoperative testing to determine Stage 2 readiness is limited to serial oxygen saturation measurements, echocardiograms, cardiac magnetic resonance imaging, computed tomography angiography, and cardiac catheterization.5
Despite the significant morbidity that can result from imprecise assessment of Stage 2 readiness, there are no validated biomarkers or personalized medicine strategies to identify subjects in this population at risk for failure to tolerate Stage 2 physiology. Prior work from our group demonstrated shifts in both the global circulating proteome and individual targeted proteins between SVHD infants and healthy controls and an association between altered protein levels and pulmonary vascular tolerance of the transition to Stage 2 physiology.6,7 These findings suggest a profound biochemical disruption associated with SVHD physiology that may offer both prognostic value and insight into the mechanisms underlying the postoperative and long-term morbidity experienced by SVHD patients.
Metabolomic analyses represent a new systems biology approach to identify novel biomarkers and pathways in complex diseases. Metabolites are the small molecule (<1.5 kDa) ultimate products of gene expression and represent the primary determinants of cellular phenotype.8,9 Prior studies have demonstrated a distinct circulating metabolic phenotype that is associated with adverse clinical outcomes in children undergoing cardiac surgery with cardiopulmonary bypass.10,11 Further work identified changes in metabolic pathways associated with pulmonary vascular tone and growth in adult patients with pulmonary hypertension.12 Small studies have also suggested changes in the circulating metabolome of adult and older pediatric patients with SVHD who have completed Stage 3 palliation compared to healthy controls.13,14 However, no prior study has measured the metabolome of young infants with SVHD compared to similar aged healthy children or evaluated whether circulating metabolite levels could offer insight into pulmonary vascular readiness for proceeding through staged SVHD palliation.
Here, we report the results of a prospective cohort study of the circulating metabolome in infants with SVHD undergoing Stage 2 palliation. We hypothesized that interstage SVHD infants would have a distinct circulating metabolome compared to healthy controls and that the metabolome would shift significantly following Stage 2 palliation. We further hypothesized that patients with greater derangement in metabolite levels both preoperatively and in response to Stage 2 palliation would have a larger burden of post-Stage 2 complications related to insufficient pulmonary blood flow.
Methods
The Colorado Multiple Institutional Review Board approved this study. Written informed consent was obtained from the study subjects’ parents in all cases.
Subjects
Inclusion criteria for this cohort have been previously described.6 Briefly, we prospectively enrolled consecutive subjects aged 1 month to 2 years with SVHD at Children’s Hospital Colorado who were either undergoing a pre-Stage 2 cardiac catheterization or a Stage 2 surgical palliation without plan for catheterization. Subjects who underwent Stage 2 palliation without sample collection at cardiac catheterization did so because either cardiac catheterization was performed prior to obtaining informed consent or because catheterization was not deemed clinically necessary by the treatment team. We considered Stage 2 palliation to include any form of superior cavo-pulmonary anastomosis (Glenn or Hemi-Fontan operations) regardless of whether a patient had a prior Stage 1 palliation. Children with a persistent, additional pulsatile source of pulmonary blood flow after Stage 2 (so-called 1.5 ventricle repair) were excluded.
We identified similar-aged control subjects from the surgical schedule at Children’s Hospital Colorado. Control subject inclusion criteria included weight >4 kg and age 3 to 12 months. All controls were undergoing anesthesia for an elective, noncardiac procedure with a clinical need for intravenous access. We excluded potential control subjects for any known or suspected cardiac, pulmonary, infectious, or genetic abnormalities.
Clinical data
We extracted clinical data from the clinical electronic medical record (Epic Systems, Verona, WI). The research team also downloaded postoperative oxygen saturation measurements at 1-minute intervals (∼1,400 per 24 hours) from the BedMaster monitoring system in the cardiac intensive care unit (Anandic Medical Systems, Feuerthalen, Switzerland). All study data were managed using REDCap electronic data capture tools hosted at University of Colorado.
Prior to data collection, we identified clinical variables of interest to evaluate the relationship between classic clinical predictors of Stage 2 readiness, metabolite levels, and clinical outcomes. The primary clinical variable of interest was percent of time in the first 48 postoperative hours with clinically significant hypoxemia, defined as an oxygen saturation below 70% (48h low sat%). We computed this variable as the ratio of the number of recorded saturation levels <70% to the total number of saturation recordings in the first 48 postoperative hours, times 100. Secondary variables included hemodynamic measurements obtained during pre-Stage 2 catheterization, mean saturation levels, endotracheal intubation time, chest tube days, and volume of chest tube drainage.
Sample collection
All preoperative samples were obtained under general anesthesia. For SVHD subjects enrolled at the time of pre-Stage 2 catheterization, we obtained systemic venous samples from either the femoral vein sheath or a catheter located in the superior vena cava prior to any procedural interventions. We then obtained an additional pulmonary venous sample during catheterization for comparison to the systemic venous samples. For subjects enrolled at other times, a systemic venous sample was obtained from a clinically placed central venous line on the day of Stage 2 palliation before initiation of cardiopulmonary bypass. We obtained a postoperative systemic venous sample at 2 hours after arrival in the cardiac intensive care unit from a clinically placed central venous catheter. We obtained control subject samples from a systemic vein after induction of anesthesia at the time of IV placement.
Metabolomics analysis
We processed all whole blood samples for serum at the time of collection. Serum samples were then aliquoted, frozen at −80 °C, and analyzed in 2 batches. Sample analysis was performed using a previously validated approach based on liquid chromatography-tandem mass spectrometry.15 Samples were analyzed using an Agilent 1200 series high-performance liquid chromatography system (Agilent Technologies) interfaced with an AB Sciex 5500 hybrid triple quadrupole/linear ion trap mass spectrometer (Concord) equipped with an electrospray ionization source operating in the positive/negative switch mode. A total of 175 metabolite relative abundances were measured across the 4 time points for cases and 1 time point for controls.
Statistical analysis
Prior to analysis, we batch corrected the metabolite abundances by regressing out the batch effect using simple linear regression. We then log transformed the metabolite abundances for all univariate linear regression analyses. Each of the a priori identified metabolites was detectable in the patient samples. The metabolite sorbitol was excluded from preoperation vs postoperation analyses due to a similar fragmentation pattern on liquid chromatography-tandem mass spectrometry with mannitol, an osmotic diuretic routinely given during cardiopulmonary bypass.
A random forest classifier was fit to distinguish between groups of interest. The random forest was fit with metabolite relative abundances as the features and the group membership (eg, case vs control) as the outcome. The models were evaluated using leave-one-out cross-validation. Multidimensional scaling (principal coordinate analysis) was then employed to reduce the dimension of the proximity distance matrix, generated by the random forest classifier, to 2 dimensions. We then plotted the reduced data dimensions to visualize group clusters. Permutation-based feature importance was used to describe the individual metabolites that were most impactful in classifying the subjects.
To compare metabolite levels between the cases and controls as well as between preoperation vs postoperation samples, we fit multiple univariate linear regressions where the dependent variable was the metabolite, and the independent variable was the group. To test the association between metabolite levels and outcomes, we fit multiple univariate linear regressions where the dependent variable was the clinical outcome, and the independent variable was the metabolite. We adjusted for multiple testing using false discovery rate (FDR) with FDR <0.05 considered significant. To identify individual metabolites that most significantly differed between groups of interest, we computed permutation-based feature importance scores and normalized them to be between 0 and 100, with 100 being the feature of greatest importance.
We used MetaboAnalystR version 3.2.0 to register the individual metabolites according to KEGG ID and perform a pathway analysis. We used quantitative enrichment scores computed from the relative-betweenness centrality for the pathway topology. Global tests were used to determine significant pathways and impact scores. We adjusted for multiple testing using FDR.
Results
Study population
Fifty-two SVHD cases and 48 healthy controls were enrolled. Preoperative systemic vein samples were obtained for all cases and controls. A preoperative pulmonary vein sample was collected for 38 cases. Four (4) cases ultimately did not undergo Stage 2 palliation (3 heart transplant, 1 biventricular repair). Therefore, postoperative samples were available for 48 cases. Demographics and clinical features are shown in Table 1. Despite similar weights, the control patients were slightly older than the SVHD cases. Controls were undergoing surgery with urology (eg, hypospadias, chordee, n = 37), orthopedics (eg, supernumerary digit, hip dysplasia, n = 5), neurosurgery (eg, craniosynostosis, n = 5), ophthalmology (eg, strabismus, n = 3), or other surgical teams (eg, inguinal hernia, n = 8). Pulmonary vascular hemodynamics, measured preoperatively, were highly variable within the cohort. Although the median values for mean pulmonary artery pressure (12 mm Hg) and indexed pulmonary vascular resistance (PVRi) (2.0 WU/m2) are within normal limits, 10 of 52 subjects had calculated PVRi >3.0, the accepted upper limit of normal.
Table 1.
Demographics
| Control (n = 48) | Case (n = 52) | P Value | |
|---|---|---|---|
| Weight, kg | |||
| Mean ± SD | 7.86 ± 1.26 | 6.85 ± 7.36 | 0.335 |
| Median [min, max] | 7.68 [5.54, 11.2] | 5.60 [4.20, 58.0] | |
| Age, y | |||
| Mean ± SD | 0.625 ± 0.207 | 0.376 ± 0.220 | <0.0001 |
| Median [min, max] | 0.564 [0.277, 0.992] | 0.348 [0.123, 1.78] | |
| Sex | |||
| Female | 18 (37.5) | 22 (42.3) | 0.775 |
| Male | 30 (62.5) | 30 (57.7) | |
| Ventricle morphology | |||
| LV | 10 (19.2) | ||
| RV | 42 (80.8) | ||
| Stage 1 operation | |||
| Norwood | 35 (67.3) | ||
| Other | 5 (9.6) | ||
| PA band | 5 (9.6) | ||
| Shunt only | 6 (11.5) | ||
| Mean PA pressure, mm Hg | |||
| Mean ± SD | 13.3 ± 3.22 | ||
| Median [min, max] | 12.0 [8.00, 23.0] | ||
| PVRi, U × m2 | |||
| Mean ± SD | 2.16 ± 0.923 | ||
| Median [min, max] | 2.00 [1.00, 5.60] | ||
| Ventricle EDP [mmHg] | |||
| Mean ± SD | 6.22 ± 2.12 | ||
| Median [min, max] | 6.00 [3.00, 12.0] | ||
| Qp/Qs | |||
| Mean ± SD | 1.18 ± 0.617 | ||
| Median [min, max] | 1.10 [0.300, 3.30] | ||
| CPB time, min | |||
| Mean ± SD | 132 ± 62.7 | ||
| Median [min, max] | 130 [50.0, 398] | ||
| ETT duration, h | |||
| Mean ± SD | 39.0 ± 78.6 | ||
| Median [min, max] | 16.0 [1.00, 504] | ||
| Chest tube duration, d | |||
| Mean ± SD | 3.15 ± 2.45 | ||
| Median [min, max] | 2.00 [1.00, 11.0] | ||
| Pleural drainage, mL | |||
| Mean ± SD | 180 ± 153 | ||
| Median [min, max] | 146 [0, 701] | ||
| Discharge PDE5i therapy | |||
| No | 30 (57.7) | ||
| Yes | 17 (32.7) | ||
| 48 h mean sat, % | |||
| Mean ± SD | 78.2 ± 4.61 | ||
| Median [min, max] | 79.0 [68.3, 86.8] | ||
| 48 h mean sat below 70%, % | |||
| Mean ± SD | 11.3 ± 15.5 | ||
| Median [min, max] | 4.10 [0.400, 57.4] |
Values are n (%) unless otherwise indicated.
CPB = cardiopulmonary bypass; EDP = end diastolic pressure; ETT = endotracheal tube; LV = left ventricle; PA = pulmonary artery; PDE5i = phosphodiesterase type 5 inhibitor; PVRi = indexed pulmonary vascular resistance; Qp/Qs = ratio of pulmonary to systemic blood flow; RV = right ventricle.
Postoperative outcomes
The subset of the SVHD cohort who ultimately underwent Stage 2 palliation (n = 48) experienced moderate morbidity. The median time spent with saturations below 70% in the first 48 hours was 4.1%. Median postoperative endotracheal intubation time was 16 hours and chest tube duration was 2 days. No patients required a second bypass operation during their admission and 47 of 48 patients survived to hospital discharge, with the one fatality occurring after a complex course several months after Stage 2 palliation. All surviving patients received oxygen during their post-Stage 2 course and were discharged home on supplemental oxygen (1/4-1/2 L/min by nasal cannula) according to our local protocol. Seventeen patients were discharged home on sildenafil therapy with the typical indication being persistent postoperative hypoxemia. Preoperative PVRi did not differ significantly between those subjects who were discharged on sildenafil and those who were not (P = 0.5238).
Case-control metabolomics analysis
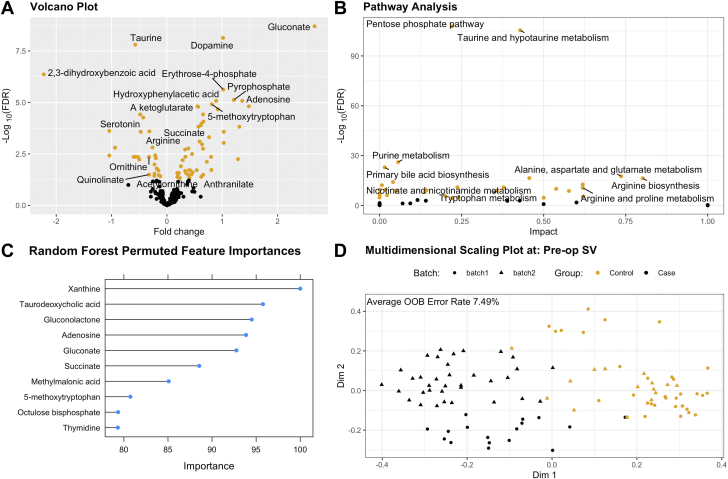
We first sought to evaluate how the individual metabolites measured varied between the SVHD cases (preoperation systemic vein sample) and healthy controls (Figure 1). After adjusting for multiple comparisons, 74 of the 175 measured targets differed between groups (Table 2). Individual metabolites found to have highly different levels between cases and controls are highlighted in the volcano plot in Figure 1A. On pathway analysis, 27 of 39 assessed pathways were found to be significantly altered between the groups (Supplemental Table 1). Individual pathways with the most impactful metabolites measured and the most significant differences are highlighted in Figure 1B, including the pentose phosphate pathway, arginine biosynthesis and metabolism, tryptophan metabolism, nicotinamide metabolism, and glutamate metabolism.
Figure 1.
Metabolomic Changes Between Infants With Single Ventricle Heart Disease and Controls
(A) Volcano plot demonstrating individual metabolites compared between cases and controls. Metabolites with false discovery rate (FDR) <0.05 are labeled in orange, while those with FDR ≥0.05 are black. Positive fold change indicates higher levels in cases than controls. (B) Pathway analysis delineates metabolic pathways that are altered in cases compared to controls. Larger values on Y axis indicated a greater degree of statistical significance in each pathway’s metabolite levels. Larger values on the X axis indicate that the measured metabolites are more impactful to that particular pathway. (C) Individual metabolites identified as the most important features distinguishing cases from controls on random forest analysis. (D) Multidimensional scaling plot depicts the results of random forest analysis. With high accuracy, the circulating metabolome distinguishes cases from controls after correction for batch effect.
Table 2.
Metabolites With Different Circulating Levels Between Cases and Controls
| Metabolite | Estimate | P Value | FDR |
|---|---|---|---|
| Gluconate | 2.66747587 | 1.14E-11 | 2.00E-09 |
| Dopamine | 1.01363688 | 8.38E-11 | 7.33E-09 |
| Taurine | −0.5717815 | 2.71E-10 | 1.58E-08 |
| 2,3-dihydroxybenzoic acid | −2.225194 | 9.98E-09 | 4.37E-07 |
| Erythrose-4-phosphate | 1.01849256 | 6.79E-08 | 2.38E-06 |
| Pyrophosphate | 1.20855353 | 2.61E-07 | 7.62E-06 |
| Adenosine | 1.36025793 | 3.86E-07 | 8.45E-06 |
| Hydroxyphenylacetic acid | 0.88909785 | 3.46E-07 | 8.45E-06 |
| 5-methoxytryptophan | 0.810373 | 6.32E-07 | 1.23E-05 |
| A ketoglutarate | 0.55324666 | 9.01E-07 | 1.55E-05 |
| Shikimate-3-phosphate | 1.48037051 | 9.75E-07 | 1.55E-05 |
| Aconitate | 0.56568156 | 1.12E-06 | 1.64E-05 |
| 4-pyridoxic acid | 0.9195702 | 1.59E-06 | 2.14E-05 |
| Hypoxanthine | −0.4831705 | 3.30E-06 | 3.85E-05 |
| Pantothenate | 0.65398624 | 3.30E-06 | 3.85E-05 |
| 3-methylphenylacetic acid | −0.4276407 | 4.94E-06 | 5.40E-05 |
| Aminoadipic acid | 0.65990748 | 8.23E-06 | 8.47E-05 |
| Octulose monophosphate | 0.62271187 | 1.13E-05 | 0.00011031 |
| Indole-3-carboxylic acid | 0.61269529 | 1.42E-05 | 0.00013091 |
| Octulose bisphosphate | 1.30912393 | 1.73E-05 | 0.00015172 |
| Succinate | 0.56736036 | 1.83E-05 | 0.00015291 |
| Serotonin | −1.0395698 | 3.05E-05 | 0.00024293 |
| Aspartate | −0.3161954 | 3.38E-05 | 0.00025683 |
| Thymidine | 1.02763767 | 3.80E-05 | 0.00026634 |
| Xanthine | −0.4739582 | 3.77E-05 | 0.00026634 |
| Fructose-6-phosphate | 0.76242755 | 7.25E-05 | 0.00048793 |
| Carbamoyl aspartate | 0.59037281 | 0.00012236 | 0.0007931 |
| S adenosylmethionine | 1.02660927 | 0.00014785 | 0.00092409 |
| Myoinositol | 0.63564208 | 0.00018023 | 0.00108761 |
| Arginine | −0.2605929 | 0.00026296 | 0.00153392 |
| 2-aminooctanoic acid | −0.9384351 | 0.00027875 | 0.0015736 |
| Lysine | 0.38732799 | 0.00031446 | 0.00171973 |
| Xanthurenic acid | 0.65231665 | 0.00033341 | 0.00176811 |
| Urea | 0.32746064 | 0.0003756 | 0.00193322 |
| Methylmalonic acid | 0.45498984 | 0.00064226 | 0.00321132 |
| Betaine aldehyde | −0.2105196 | 0.00077135 | 0.00374963 |
| Taurodeoxycholic acid | −1.0385855 | 0.00080398 | 0.00380259 |
| Biotin | −0.5237922 | 0.00097045 | 0.0044051 |
| Glucose phosphate | −0.601172 | 0.00108239 | 0.0044051 |
| Homoserine | 0.33503686 | 0.0010485 | 0.0044051 |
| Inosine | 0.79613981 | 0.00101624 | 0.0044051 |
| Ornithine | −0.3242812 | 0.00106063 | 0.0044051 |
| Trans trans farnesyl diphosphate | −0.5710305 | 0.00104028 | 0.0044051 |
| Methionine | −0.2002328 | 0.00128644 | 0.00511654 |
| Gluconolactone | 1.28515174 | 0.00145808 | 0.00567031 |
| NHPA | −0.4996616 | 0.00156164 | 0.00594101 |
| Homocysteine | 0.28447036 | 0.00171163 | 0.0063731 |
| Hippurate | 0.72047476 | 0.00208369 | 0.0075968 |
| Malate | −0.2241675 | 0.00288115 | 0.01028981 |
| Acetyllysine | 0.25333186 | 0.00357986 | 0.0125295 |
| Betaine | −0.182563 | 0.00473113 | 0.01623428 |
| Glycolate | 0.80395031 | 0.00565154 | 0.01901961 |
| P hydroxybenzoate | 0.53691887 | 0.00611515 | 0.01945731 |
| Spermidine | 0.4017803 | 0.00601525 | 0.01945731 |
| S ribosylhomocysteine | 0.41665447 | 0.00605647 | 0.01945731 |
| Sarcosine | −0.1527065 | 0.00650581 | 0.02033065 |
| Acetylserine | 0.33016983 | 0.00692363 | 0.02125675 |
| Sedoheptulose bisphosphate | 0.46422657 | 0.00815374 | 0.0246018 |
| Glutamine | 0.18844567 | 0.00880506 | 0.02611669 |
| Methylcysteine | 0.21396102 | 0.00921404 | 0.02687428 |
| Glyceraldehyde-3-phosphate | 0.34577313 | 0.0095401 | 0.02736913 |
| Indole | −0.2225697 | 0.01013444 | 0.02860528 |
| Deoxyinosine | 0.44508149 | 0.01119631 | 0.03110086 |
| Quinolinate | −0.3198984 | 0.01168931 | 0.03196295 |
| Guanosine | 0.65425323 | 0.01254155 | 0.03277328 |
| Hydroxyproline | 0.2005175 | 0.01219926 | 0.03277328 |
| Methylnicotinate | 0.38376537 | 0.01254748 | 0.03277328 |
| Serine | −0.1460517 | 0.01326298 | 0.03413267 |
| Dimethylglycine | −0.2279696 | 0.01455331 | 0.03691058 |
| Alanine | −0.1189982 | 0.01528423 | 0.03821057 |
| Anthranilate | 0.61960364 | 0.01664484 | 0.04102601 |
| Acetylornithine | 0.19112507 | 0.01723761 | 0.04189696 |
| Glycine | 0.23303764 | 0.01963704 | 0.0470751 |
| Ethanolamine | 0.40661168 | 0.0200186 | 0.04734129 |
Positive estimate values reflect metabolites with higher circulating levels in cases than controls.
FDR = false discovery rate.
We then analyzed how the measured metabolome taken as a whole was able to distinguish between SVHD cases and healthy controls. On random forest analysis, the metabolome was readily able to distinguish between groups (out of box error rate = 7.49%; <10% is considered good classification of results, Figure 1D). Leave-one-out cross validation demonstrated sensitivity of 95.8% and specificity of 98.1% to distinguish the groups. The individual features of greatest importance in the random forest classification are shown in Figure 1C. We did not note any sex-based differences in the metabolome either among SVHD cases or healthy controls. Using random forest analysis, we also did not note a global shift in the metabolome between systemic vein and pulmonary vein samples for the SVHD cases (data not shown). On single testing, 19 metabolites differed between systemic vein and pulmonary vein with a raw P value <0.05 (glutamate, alpha ketoglutarate, carbamoyl aspartate, hippurate, taurine, dihydroxyacetone phosphate, dimethyl glycine, S-ribosylhomocysteine, carbamoyl phosphate, glyceraldehyde 3-phosphate, asparagine, serotonin, sorbitol, trigonelline, 3-methyl thioproprionate, atrolactic acid, CDP ethanolamine, hydroxyphenyl acetic acid). Only glutamate remained significant after accounting for multiple comparisons (P = 8.39 × 10−5, FDR = 0.014).
Preoperation vs postoperation metabolomics analysis
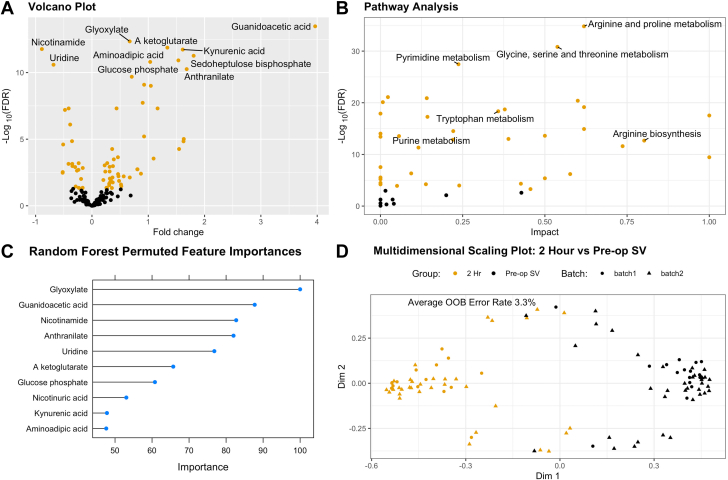
Next, we sought to evaluate how individual metabolites measured in a systemic vein changed between preoperation and 2 hours postoperation among SVHD cases (Figure 2). After removal of sorbitol and correction for multiple comparisons, 71 of 174 individual metabolites differed between groups (Table 3). Metabolites with highly different levels between preoperation and postoperation are highlighted in Figure 2A with the majority of targets showing higher levels postoperation than preoperation. On pathway analysis, 33 of 39 assessed pathways differed significantly between groups (Supplemental Table 2). Individual pathways with the most impactful metabolites measured and the most significant differences are highlighted in Figure 2B, including arginine metabolism and biosynthesis, tryptophan metabolism, glycine metabolism, and pyrimidine metabolism.
Figure 2.
Metabolomic Changes Between Infants With SVHD Pre-Stage 2 and Post-Stage 2
(A) Volcano plot demonstrating individual metabolites compared between pre-stage 2 and post-stage 2. Metabolites with FDR <0.05 are labeled in orange, while those with FDR ≥0.05 are black. Positive fold change indicates higher levels in postoperative samples than preoperative. (B) Pathway analysis delineates metabolic pathways that are altered in postoperative samples compared to preoperative. Larger values on Y axis indicated a greater degree of statistical significance in each pathway’s metabolite levels. Larger values on the X axis indicate that the measured metabolites are more impactful to that particular pathway. (C) Individual metabolites identified as the most important features distinguishing preoperative from postoperative samples on random forest analysis. (D) Multidimensional scaling plot depicts the results of random forest analysis. With high accuracy, the circulating metabolome distinguishes pre-stage 2 from post-Stage 2 samples after correction for batch effect.
Table 3.
Metabolites With Different Circulating Levels Between Pre-Stage 2 and Post-Stage 2
| Metabolite | Estimate | P Value | FDR |
|---|---|---|---|
| Guanidoacetic acid | 3.96388723 | 1.92E-16 | 3.34E-14 |
| Glyoxylate | 0.67028293 | 5.18E-15 | 4.51E-13 |
| A ketoglutarate | 1.3391801 | 2.30E-14 | 1.33E-12 |
| Nicotinamide | −0.8896103 | 3.93E-14 | 1.71E-12 |
| Kynurenic acid | 1.61176258 | 5.36E-14 | 1.86E-12 |
| Sedoheptulose bisphosphate | 1.80597162 | 1.87E-13 | 5.43E-12 |
| Glucose phosphate | 1.53463991 | 4.86E-13 | 1.21E-11 |
| Aminoadipic acid | 1.03409255 | 7.26E-13 | 1.58E-11 |
| Uridine | −0.6825379 | 1.33E-12 | 2.57E-11 |
| Anthranilate | 1.68226864 | 3.15E-12 | 5.48E-11 |
| Imidazoleacetic acid | 0.70679679 | 1.30E-11 | 2.05E-10 |
| Myoinositol | 0.92639458 | 5.61E-11 | 8.14E-10 |
| Nicotinuric acid | 1.04545271 | 7.36E-11 | 9.85E-10 |
| 3-phosphoglycerate | 0.90102717 | 1.49E-09 | 1.85E-08 |
| Acetoacetate | 0.43677675 | 4.20E-09 | 4.88E-08 |
| Serine | −0.4136587 | 4.78E-09 | 4.89E-08 |
| Spermidine | 1.16537087 | 4.53E-09 | 4.89E-08 |
| Hydroxyproline | −0.4794446 | 6.56E-09 | 6.34E-08 |
| Methionine sulfoxide | −0.3870272 | 8.69E-08 | 7.96E-07 |
| Putrescine | 0.92555747 | 7.17E-07 | 6.24E-06 |
| 2,3-dihydroxybenzoic acid | 1.63040798 | 1.16E-06 | 9.64E-06 |
| Threonine | −0.3542004 | 1.78E-06 | 1.41E-05 |
| Indoleacrylic acid | 1.62603299 | 1.91E-06 | 1.45E-05 |
| Dimethylglycine | −0.5114178 | 3.45E-06 | 2.50E-05 |
| Guanine | 1.54735022 | 8.18E-06 | 5.48E-05 |
| Hydroxyphenylpyruvate | 0.35887583 | 8.09E-06 | 5.48E-05 |
| S adenosylhomocysteine | 0.4610034 | 3.51E-05 | 0.00022647 |
| Phenylpyruvate | 1.09446116 | 4.58E-05 | 0.00028458 |
| 3-methylphenylacetic acid | 0.38769621 | 5.46E-05 | 0.00032738 |
| Valine | 0.27125558 | 0.00010827 | 0.00062799 |
| Betaine aldehyde | −0.2360638 | 0.00011283 | 0.00063332 |
| Choline | −0.3498066 | 0.00013546 | 0.00071427 |
| Glutamate | −0.4163346 | 0.00013501 | 0.00071427 |
| Uric acid | 0.22564083 | 0.00018039 | 0.00092319 |
| Citrulline | −0.3479013 | 0.00020886 | 0.00103834 |
| Phenyllactic acid | 0.37251494 | 0.00021878 | 0.00105743 |
| Homocysteine | 0.31361818 | 0.00025227 | 0.00118637 |
| Acetyllysine | −0.2983923 | 0.00027 | 0.00123632 |
| Carnitine | −0.1633644 | 0.00032602 | 0.00145454 |
| Gluconolactone | 0.80866842 | 0.00042906 | 0.00182089 |
| Hippurate | 0.80525763 | 0.00042159 | 0.00182089 |
| Glutamine | −0.2403642 | 0.00048247 | 0.0019988 |
| 2-hydroxyglutarate | −0.2662634 | 0.00056856 | 0.0023007 |
| Homoserine | 0.31372092 | 0.00067203 | 0.00265758 |
| Purine | 0.2367815 | 0.00069872 | 0.00270171 |
| 4-pyridoxic acid | −0.5190204 | 0.00077853 | 0.00294489 |
| Trehalose-6-phosphate | −0.5207173 | 0.00089558 | 0.00331556 |
| Gluconate | 0.8699759 | 0.00107208 | 0.00388629 |
| Glucosamine | 0.46373143 | 0.00117904 | 0.00418678 |
| NHPA | 0.47098274 | 0.00174239 | 0.00606352 |
| Xanthurenic acid | 0.56378956 | 0.00181578 | 0.006195 |
| Octulose bisphosphate | 0.67158031 | 0.00223552 | 0.00748039 |
| 5-hydroxyindole acetic acid | 0.36449342 | 0.00229011 | 0.00751847 |
| Indole-3-carboxylic acid | 0.31737311 | 0.00282295 | 0.00909616 |
| Acetylglutamate | −0.1933624 | 0.00305454 | 0.00966345 |
| Phenylalanine | 0.20270217 | 0.0037437 | 0.01163221 |
| Xanthosine | 0.33699258 | 0.00425846 | 0.01299951 |
| Pipecolic acid | 0.48112517 | 0.00453736 | 0.01361209 |
| Kynurenine | 0.32462081 | 0.0055518 | 0.01637311 |
| 5-methoxytryptophan | 0.3689803 | 0.00607694 | 0.01762312 |
| Riboflavin | 0.30632102 | 0.00768402 | 0.02191835 |
| P hydroxybenzoate | 0.5154541 | 0.00894627 | 0.02510727 |
| Atrolactic acid | 0.30674787 | 0.01024698 | 0.02830118 |
| Acetylalanine | 0.52083539 | 0.0124781 | 0.03392484 |
| Pantothenate | −0.2887177 | 0.01327982 | 0.03554907 |
| Methionine | −0.1658002 | 0.01704513 | 0.04455557 |
| Nicotinate | 0.25720192 | 0.01715646 | 0.04455557 |
| Glycine | −0.2325545 | 0.01760595 | 0.04505052 |
| Cholesteryl sulfate | 0.35401046 | 0.01834505 | 0.04626143 |
| Glycerate | −0.169139 | 0.01900421 | 0.04723904 |
| Orotate | 0.26852315 | 0.02018164 | 0.04945923 |
Positive estimate values reflect metabolites with higher circulating levels in post-Stage 2 samples than pre-Stage 2.
FDR = false discovery rate.
We then evaluated the extent to which the measured metabolome could be used to distinguish preoperative from 2 h postoperative samples. Multidimensional scaling plot based on random forest analysis demonstrated an out of box error rate of 3.3%, indicating excellent discrimination of groups (Figure 2D). Leave-one-out cross-validation demonstrated sensitivity of 97.9% and specificity of 96.2% to distinguish the groups. The individual features of greatest importance in the random forest classification are shown in Figure 2C.
Outcome analysis
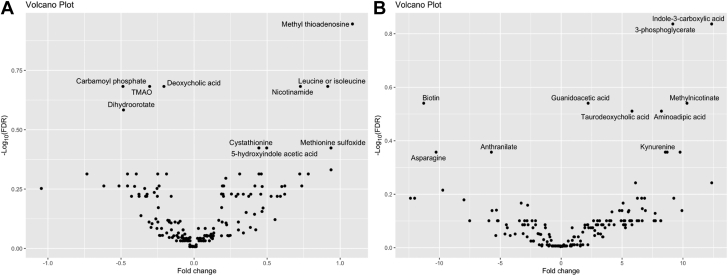
We then evaluated whether any individual metabolites differed between SVHD subjects with favorable or unfavorable clinical outcomes. First, we evaluated whether any metabolites measured preoperatively were significantly associated with adverse hemodynamics as measured at the pre-Stage 2 catheterization. Dividing the SVHD population into those with elevated PVRi (>3 WU/m2) and those with normal PVRi (≤3 WU/m2), a random forest analysis based on the preoperation systemic venous metabolome was able to distinguish the groups with high accuracy (out of box error rate 4.6%) (Supplemental Figure 1). Twelve of 175 metabolites showed an uncorrected association with PVRi (raw P < 0.05): methyl thioadenosine, carbamoyl phosphate, deoxycholic acid, leucine, nicotinamide, trimethylamine oxide, dihydroorotate, 5-hydroxyindole acetic acid, cystathionine, methionine sulfoxide, methionine, and 3-hydroxyisovaleric acid (Figure 3A). None of these differences remained significant after adjustment for multiple comparisons (FDR >0.05) (Supplemental Table 3). When compared against preoperative outcomes, metabolites measured in a systemic vein and those measured in a pulmonary vein behaved similarly (data not shown).
Figure 3.
Association Between Metabolites and Outcomes
(A) Volcano plot depicting the association between metabolites measured in the preoperation systemic vein and indexed pulmonary vascular resistance. Positive fold change suggests higher metabolite levels in subjects with higher PVRi. (B) Volcano plot depicting the association between metabolites measured in the systemic vein at 2 hours postoperation and percent of the first 48 postoperative hours spent with oxygen saturation below 70%. Positive fold change suggests higher metabolite levels in subjects with greater hypoxemia burden.
Then we evaluated whether any of the measured metabolites were associated with post-Stage 2 outcomes. There was no appreciable association between either systemic vein or pulmonary vein preoperation metabolite levels and postoperation outcomes. When measured at 2 hours postoperation, 14 of 174 metabolites showed an uncorrected association with 48h low sat% (raw P < 0.05): 3-phosphoglycerate, indole-3-carboxylic acid, biotin, guanidoacetic acid, methylnicotinate, aminoadipic acid, taurodeoxycholic acid, anthranilate, asparagine, kynurenine, s-adenosylhomocysteine, trigonelline, cystathionine, and methylsulfoxide (Figure 3B). None of these differences remained significant after adjustment for multiple comparisons (FDR >0.05) (Supplemental Table 4).
Discussion
In this study, we report the novel finding that infants with SVHD undergoing evaluation for Stage 2 palliation have a distinct metabolomic fingerprint from similar aged healthy infants (Central Illustration). Twenty-seven of 39 tested metabolic pathways were affected in this population, with the pentose phosphate pathway, arginine biosynthesis and metabolism, tryptophan metabolism, nicotinamide metabolism, and glutamate metabolism pathways among the most altered. We further found a shift in the metabolome between the preoperative and immediate recovery periods from Stage 2 palliation including alterations in 33 of 39 evaluated pathways and highly significant changes in the arginine metabolism and biosynthesis, glycine metabolism, pyrimidine metabolism, and tryptophan metabolism pathways. Although not fully powered after adjustment for multiple comparisons, we find preliminary evidence to suggest an association between both preoperative metabolites and elevated pulmonary vascular resistance as well as postoperative metabolites and post-Stage 2 hypoxemia.
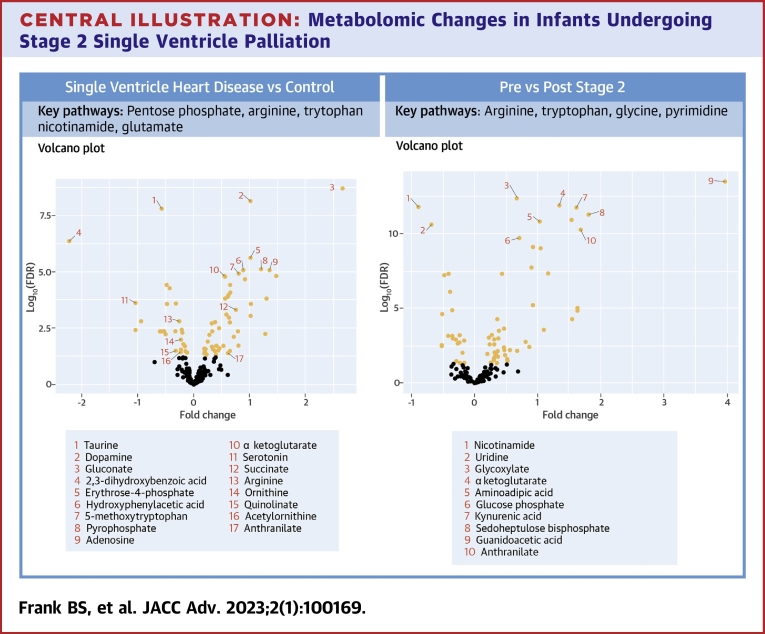
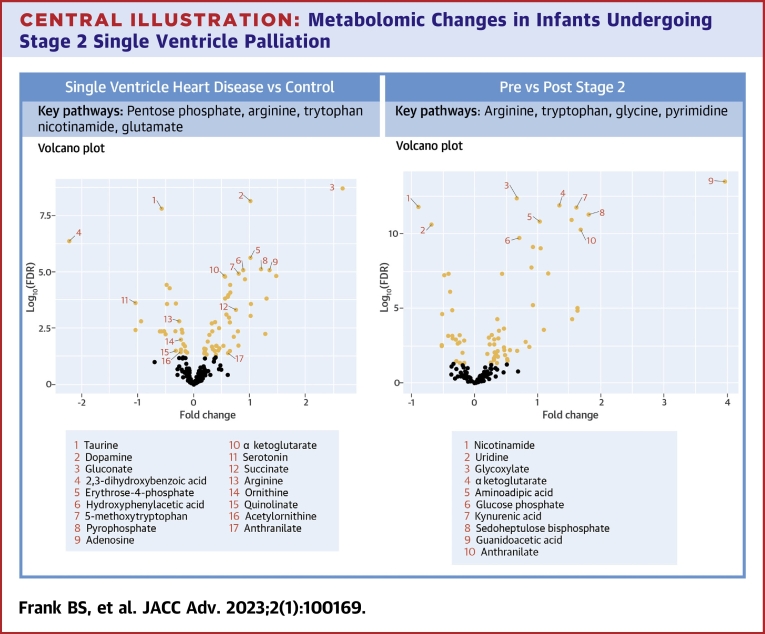
Central Illustration.
Metabolomic Changes in Infants Undergoing Stage 2 Single Ventricle Palliation
(Left) Volcano plot demonstrating individual metabolites compared between cases and controls. (Right) Volcano plot demonstrating individual metabolites compared between pre-Stage 2 and post-Stage 2. Metabolites with FDR <0.05 are labeled in orange. Select altered metabolites of interest and metabolic pathways are noted.
Interstage Single Ventricle Heart Disease cases vs healthy controls
Random forest analysis, a supervised machine learning algorithm, is a powerful ensemble learning method for classification used to evaluate the capacity of the data contained in a large -omics data set to distinguish between 2 groups.16 Previous studies have used dimension reduction techniques such as multidimensional scaling or others to help visualize clusters thereby demonstrating distinct metabolic phenotypes between healthy adults and those with coronary artery disease as well as those with and without asthma.17,18 Two prior small studies used similar techniques in identifying a shift in the metabolome between older children with SVHD and healthy controls, including alterations in many of the same metabolites we describe here.14,19 Our finding that the groups were readily and accurately distinguished by random forest analysis with high leave-one-out cross-validation supports the conclusion that the global metabolome of infants with SVHD is distinct from that of healthy children.
Of the 27 pathways that statistically differed between SVHD cases and controls, the 2 most significantly altered were arginine metabolism and the pentose phosphate pathway. Arginine metabolism to citrulline through the nitric oxide synthase family of enzymes is the dominant endogenous source of nitric oxide, a potent pulmonary vasodilator.20 Activity in this pathway is known to be a major modulator of pulmonary vascular tone and remodeling in patients with hypoxemia and pulmonary hypertension. 21,22 Clinically, this pathway is frequently modulated to augment pulmonary blood flow via inhaled nitric oxide delivery, phosphodiesterase type 5 inhibitors, and soluble guanylate cyclase stimulators.23, 24, 25 In our cohort, we find decreased circulating levels of arginine and increased levels of citrulline in SVHD infants compared to healthy controls. This shift could reflect increased nitric oxide synthase activity in interstage SVHD infants, which would serve to endogenously induce pulmonary vasodilation but also deplete arginine availability. Future studies including comprehensive pathway mapping and enzymatic activity assays will be important to clarify the mechanisms underlying these changes.
The pentose phosphate pathway was also heavily modulated in our SVHD patients compared to controls. The pentose phosphate pathway is a fundamental component of cellular metabolism providing precursors for nucleotide and amino acid biosynthesis as well as NADPH to maintain redox balance during times of cellular stress.26 Changes in pentose phosphate intermediates have previously been described in the polycythemia response to altitude as well as the response to hypoxemia, providing one possible explanation for its modulation in our population.27 Increased pentose phosphate activity modulated through increased expression and activity of its rate-limiting enzyme, glucose-6-phosphate dehydrogenase, is known to cause smooth muscle cell proliferation and worsening of pulmonary hypertension in animal models of chronic hypoxia-induced pulmonary hypertension and bronchopulmonary dysplasia.28,29 Studies have also shown that inhibition of this pathway may be helpful with ameliorating these pathologic changes.30 We found that levels of the 4 measured pentose phosphate-associated metabolites (glyceraldehyde 3-P, gluconate, glucono-1,5-lactone, and erythrose 4-P) were all increased in SVHD cases compared to controls. As activity of this pathway is typically regulated upstream, increased levels of the intermediates suggest that SVHD patients may experience increased pentose phosphate throughput.26 We hypothesize that this increased pentose phosphate activity may represent a pathologic response to altered pulmonary vascular flow in interstage SVHD patients that puts them at risk of pulmonary arterial remodeling.
In analyzing the preoperative samples, we also evaluated whether circulating levels of any individual metabolites were associated with preoperative markers of cardiopulmonary pathology. Although our study was not sufficiently powered to detect this difference after adjusting for multiple comparisons, we noted that on single variable testing there were 3 members of the methionine metabolism pathway (cystathionine, methionine sulfoxide, methionine) that showed a positive correlation with pulmonary vascular resistance. Prior studies have shown higher levels of methionine metabolites in both adult SVHD patients and in pulmonary hypertension patients compared to controls.31,32 Preclinical studies have also shown elevated methionine intermediates in a rat model of pulmonary hypertension.33 A targeted evaluation of the mechanistic role of derangements in methionine metabolism in pulmonary vascular growth, tone, and development among infants and young children with SVHD is an important area for future study.
SVHD cases post-stage 2 compared to interstage
Comparing the preoperation and postoperation samples, the random forest analysis was able to distinguish the groups with a high degree of accuracy. This finding is aligned with prior studies demonstrating a distinct postoperative metabolic fingerprint in infants undergoing surgery with cardiopulmonary bypass.10,11 Among the pathways that showed significant differences between pre- and post-Stage 2, 2 of the most altered were arginine metabolism and tryptophan metabolism (via both the kynurenine and serotonin pathways). Prior work from our group has shown similar postoperative changes in these metabolic pathways in a mixed population of children undergoing cardiopulmonary bypass.11 In a preliminary analysis from a subset of this cohort, we previously reported changes in kynurenine pathway intermediates between SVHD infants and healthy controls.34 In the current cohort, we also found evidence to support a possible relationship between higher levels of 2 members of the tryptophan-kynurenine metabolism pathway, kynurenine and anthranilate, and post-Stage 2 hypoxemia burden. A prior study of adult patients with pulmonary hypertension demonstrated a similar positive correlation between both kynurenine and anthranilate levels and pulmonary vascular resistance.12 Furthermore, there is known crosstalk between the kynurenine metabolism pathway and nitric oxide synthesis via arginine metabolism.35 Together, this suggests that pulmonary arterial vasoconstriction may be the mechanistic link between tryptophan intermediates and post-Stage 2 hypoxemia. The extent to which changes in tryptophan metabolism may play a role as either biomarker or mediator of disease in pulmonary vascular recovery from Stage 2 palliation for SVHD patients is an important area for future study.
Study Limitations
This study has important limitations. As a single-center study focusing on a rare disease, the sample size is modest. Because of this and the large number of targets identified, the power of the clinical outcome analyses is limited. Variations in clinical practice between subjects including preoperation medication regimen, vasoactive medication, pulmonary vasodilator use, supplemental oxygen support, and blood product exposure may confound our results, including analysis of the postoperative samples. As the analyses reported here include relative circulating concentration only, studies using absolute quantification methodologies in larger populations will be important to validate these results. As this study is focused entirely on the Stage 2 period, longitudinal studies evaluating the relationship between metabolomic changes over time and clinical outcomes will be necessary. Case systemic venous samples were drawn from central venous access whereas control samples were typically drawn from peripheral venous access, introducing the possibility for confounding related to the precise site of sampling. Despite similar inclusion criteria, the enrolled control subjects were slightly older than SVHD patients. Given the biologic plausibility of the differences in the case-control analysis, we think it is unlikely that the small age difference significantly affected the results. However, future studies of the effect of age on the metabolome will be important. All the subjects live and were evaluated at our center at approximately 5,000 feet of elevation, potentially limiting generalizability for centers closer to sea level.
Conclusions
We report the novel finding that the circulating metabolome of interstage infants with SVHD differs significantly from healthy controls including notable shifts in arginine and pentose phosphate metabolism. There was a further shift in the circulating metabolome between preoperative and postoperative samples with arginine and tryptophan intermediates among the most significantly altered. Preliminary findings showed a possible association between preoperative methionine intermediates and pulmonary vascular resistance as well as between postoperative tryptophan intermediates and hypoxemia burden.
Funding support and author disclosures
This study was supported by the American Heart Association (AHA 20CDA35310498 and AHA18IPA34170070) and the National Institutes of Health (NIH/NCATS Colorado CTSA, No. UL1 TR001082 and NIH/NHLBI K23HL123634). All authors have reported that they have no relationships relevant to the contents of this paper to disclose.
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE: The global circulating metabolome of interstage SVHD patients is readily distinguishable from that of healthy infants suggesting significant metabolic derangement in affected infants. We find preliminary evidence to suggest an association between increased circulating methionine and tryptophan metabolite levels and adverse peri-Stage 2 clinical outcomes.
TRANSLATIONAL OUTLOOK: Metabolic pathway analysis may be useful to identify both biomarkers of disease and potential therapeutic targets in SVHD.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables and a figure, please see the online version of this paper.
Supplementary data
References
- 1.Hoffman J.I.E., Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–1900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- 2.Ohye R.G., Schranz D., D'Udekem Y. Current therapy for hypoplastic left heart syndrome and related single ventricle lesions. Circulation. 2016;134:1265–1279. doi: 10.1161/CIRCULATIONAHA.116.022816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caspi J., Pettitt T.W., Mulder T., Stopa A. Development of the pulmonary arteries after the Norwood procedure: comparison between Blalock-Taussig shunt and right ventricular-pulmonary artery conduit. Ann Thorac Surg. 2008;86:1299–1304. doi: 10.1016/j.athoracsur.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 4.Kogon B.E., Plattner C., Leong T., Simsic J., Kirshbom P.M., Kanter K.R. The bidirectional Glenn operation: a risk factor analysis for morbidity and mortality. J Thorac Cardiovasc Surg. 2008;136:1237–1242. doi: 10.1016/j.jtcvs.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 5.Brown D.W., Gauvreau K., Powell A.J., et al. Cardiac magnetic resonance versus routine cardiac catheterization before bidirectional Glenn anastomosis: long-term follow-up of a prospective randomized trial. J Thorac Cardiovasc Surg. 2013;146:1172–1178. doi: 10.1016/j.jtcvs.2012.12.079. [DOI] [PubMed] [Google Scholar]
- 6.Frank B.S., Khailova L., Silveira L., et al. Proteomic profiling identifies key differences between inter-stage infants with single ventricle heart disease and healthy controls. Transl Res. 2021;229:24–37. doi: 10.1016/j.trsl.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frank B.S., Khailova L., Silveira L., et al. Increased circulating endothelin 1 is associated with postoperative hypoxemia in infants with single-ventricle heart disease undergoing superior cavopulmonary anastomosis. J Am Heart Assoc. 2022;11 doi: 10.1161/JAHA.121.024007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christians U., Klawitter J., Klawitter J. Biomarkers and transplantation - proteomics and metabolomics. Ther Drug Monit. 2016;38:S70–S74. doi: 10.1097/FTD.0000000000000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xia J., Broadhurst D.I., Wilson M., Wishart D.S. Translational biomarker discovery in clinical metabolomics: an introductory tutorial. Metabolomics. 2013;9:280–299. doi: 10.1007/s11306-012-0482-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Correia G.D., Wooi Ng K., Wijeyesekera A., et al. Metabolic profiling of children undergoing surgery for congenital heart disease. Crit Care Med. 2015;43:1467–1476. doi: 10.1097/CCM.0000000000000982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davidson J.A., Pfeifer Z., Frank B., et al. Metabolomic fingerprinting of infants undergoing cardiopulmonary bypass: changes in metabolic pathways and association with mortality and cardiac intensive care unit length of stay. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.118.010711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis G.D., Ngo D., Hemnes A.R., et al. Metabolic profiling of right ventricular-pulmonary vascular function reveals circulating biomarkers of pulmonary hypertension. J Am Coll Cardiol. 2016;67:174–189. doi: 10.1016/j.jacc.2015.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Motoki N., Motoki H., Utsumi M., et al. Identification of metabolomic profile related to adult Fontan pathophysiology. Int J Cardiol Heart Vasc. 2021;37 doi: 10.1016/j.ijcha.2021.100921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pires da Silva J., Pietra A.E., Baybayon-Grandgeorge A.N., Garcia A.M. Serum metabolic profiling identifies key differences between patients with single-ventricle heart disease and healthy controls. Int J Transl Med. 2022;2:78–96. [Google Scholar]
- 15.Klepacki J., Klawitter J., Klawitter J., Thurman J.M., Christians U. A high-performance liquid chromatography-tandem mass spectrometry-based targeted metabolomics kidney dysfunction marker panel in human urine. Clin Chim Acta. 2015;446:43–53. doi: 10.1016/j.cca.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schonlau M., Zou R.Y. The random forest algorithm for statistical learning. Stata J. 2020;20:3–29. [Google Scholar]
- 17.Jung J., Kim S.H., Lee H.S., et al. Serum metabolomics reveals pathways and biomarkers associated with asthma pathogenesis. Clin Exp Allergy. 2013;43:425–433. doi: 10.1111/cea.12089. [DOI] [PubMed] [Google Scholar]
- 18.Deidda M., Piras C., Cadeddu Dessalvi C., et al. Blood metabolomic fingerprint is distinct in healthy coronary and in stenosing or microvascular ischemic heart disease. J Transl Med. 2017;15:112. doi: 10.1186/s12967-017-1215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Connell T.M., Logsdon D.L., Mitscher G., Payne R.M. Metabolic profiles identify circulating biomarkers associated with heart failure in young single ventricle patients. Metabolomics. 2021;17:95. doi: 10.1007/s11306-021-01846-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luneburg N., Harbaum L., Hennigs J.K. The endothelial ADMA/NO pathway in hypoxia-related chronic respiratory diseases. Biomed Res Int. 2014;2014:501612. doi: 10.1155/2014/501612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Howell K., Costello C.M., Sands M., Dooley I., McLoughlin P. L-Arginine promotes angiogenesis in the chronically hypoxic lung: a novel mechanism ameliorating pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2009;296:L1042–L1050. doi: 10.1152/ajplung.90327.2008. [DOI] [PubMed] [Google Scholar]
- 22.Zuckerbraun B.S., George P., Gladwin M.T. Nitrite in pulmonary arterial hypertension: therapeutic avenues in the setting of dysregulated arginine/nitric oxide synthase signalling. Cardiovasc Res. 2011;89:542–552. doi: 10.1093/cvr/cvq370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kinsella J.P., Steinhorn R.H., Krishnan U.S., et al. Recommendations for the use of inhaled nitric oxide therapy in premature newborns with severe pulmonary hypertension. J Pediatr. 2016;170:312–314. doi: 10.1016/j.jpeds.2015.11.050. [DOI] [PubMed] [Google Scholar]
- 24.Rosenkranz S., Ghofrani H.A., Beghetti M., et al. Riociguat for pulmonary arterial hypertension associated with congenital heart disease. Heart. 2015;101:1792–1799. doi: 10.1136/heartjnl-2015-307832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barst R.J., Ivy D.D., Gaitan G., et al. A randomized, double-blind, placebo-controlled, dose-ranging study of oral sildenafil citrate in treatment-naive children with pulmonary arterial hypertension. Circulation. 2012;125:324–334. doi: 10.1161/CIRCULATIONAHA.110.016667. [DOI] [PubMed] [Google Scholar]
- 26.Stincone A., Prigione A., Cramer T., et al. The return of metabolism: biochemistry and physiology of the pentose phosphate pathway. Biol Rev Camb Philos Soc. 2015;90:927–963. doi: 10.1111/brv.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu C., Liu B., Zhang E.L., et al. Elevated pentose phosphate pathway is involved in the recovery of hypoxiainduced erythrocytosis. Mol Med Rep. 2017;16:9441–9448. doi: 10.3892/mmr.2017.7801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chettimada S., Gupte R., Rawat D., Gebb S.A., McMurtry I.F., Gupte S.A. Hypoxia-induced glucose-6-phosphate dehydrogenase overexpression and -activation in pulmonary artery smooth muscle cells: implication in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2015;308:L287–L300. doi: 10.1152/ajplung.00229.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gong J., Feng Z., Peterson A.L., et al. The pentose phosphate pathway mediates hyperoxia-induced lung vascular dysgenesis and alveolar simplification in neonates. JCI Insight. 2021;6(5) doi: 10.1172/jci.insight.137594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kitagawa A., Jacob C., Jordan A., Waddell I., McMurtry I.F., Gupte S.A. Inhibition of glucose-6-phosphate dehydrogenase activity attenuates right ventricle pressure and hypertrophy elicited by VEGFR inhibitor + hypoxia. J Pharmacol Exp Ther. 2021;377:284–292. doi: 10.1124/jpet.120.000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michel M., Dubowy K.O., Zlamy M., et al. Targeted metabolomic analysis of serum phospholipid and acylcarnitine in the adult Fontan patient with a dominant left ventricle. Ther Adv Chronic Dis. 2020;11 doi: 10.1177/2040622320916031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heresi G.A., Mey J.T., Bartholomew J.R., et al. Plasma metabolomic profile in chronic thromboembolic pulmonary hypertension. Pulm Circ. 2020;10:1–11. doi: 10.1177/2045894019890553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao J.H., He Y.Y., Guo S.S., et al. Circulating plasma metabolomic profiles differentiate rodent models of pulmonary hypertension and idiopathic pulmonary arterial hypertension patients. Am J Hypertens. 2019;32:1109–1117. doi: 10.1093/ajh/hpz121. [DOI] [PubMed] [Google Scholar]
- 34.Sabapathy D., Klawitter J., Silveira L., et al. Activation of kynurenine pathway of tryptophan metabolism after infant cardiac surgery with cardiopulmonary bypass: a prospective cohort study. Metabolomics. 2020;16:93. doi: 10.1007/s11306-020-01714-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stone T.W., Darlington L.G. Endogenous kynurenines as targets for drug discovery and development. Nat Rev Drug Discov. 2002;1:609–620. doi: 10.1038/nrd870. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.