Abstract
To induce cell fate changes, do transcription factors engage open domains of chromatin or elicit chromatin opening in a pioneering fashion? In this issue of Developmental Cell, Delás et al. (2023) show that the same sonic hedgehog inducing signal can yield different neural tube fates by either modality.
The cell-fate world seems to be divided into two camps. There are those who believe that each developmental step is already prepatterned, perhaps from the pluripotent or totipotent stages, or even the egg, by histone marks and open chromatin sites that enable newly expressed transcription factors to bind and activate an alternative gene regulatory network. The model evades the chicken-and-egg question of how the chromatin patterns are set, but nature doesn’t always operate in simple ways. Another camp observes that certain transcription factors have the inherent capacity to target “closed chromatin,” or nucleosomal DNA, and initiate secondary events that lead to chromatin opening and an alternative genetic network. Which is it? In this issue of Developmental Cell, the Briscoe laboratory (1) shows that multiple different cell types emerging from a common progenitor can use different gene induction modalities and, curiously enough, in response to different levels of the same inducing signal, sonic hedgehog.
For the moment, let’s focus on the ultimate cell fate change: that of the germ cells to the zygote. Work on fruit fly, zebrafish, and mouse embryos has assessed chromatin states before and after developmental transcription factors are induced and the zygotic genome is activated (2–5). In these cases, nucleosome-binding, pioneer transcription factors target closed chromatin and initiate opening events that lead to zygotic genome activation. Pioneer factors also initiate cell reprogramming from fibroblasts to pluripotency and trans-differentiation from one somatic fate to another (6). Consistent with these observations, pioneer factors have distinctive molecular dynamics in chromatin (7), in some cases having longer residence times (3) while, in others, an ability to scan highly compacted chromatin that other factors lack (8).
But embryonic development entails many cell fate changes and it appears that there is “more than one way to skin a cat,” so to speak, when it comes to activating a new genetic network. For example, different cell types are specified in the ventral neural tube in response to graded levels of sonic hedgehog (Shh). Shh initially emanates from the notochord, below the developing neural tube, and later from the floor plate, at the bottom of the neural tube (Figure 1). Shh acts via Gli proteins upon target sites in neural progenitors that are also bound by Sox2 and different combinations of transcriptional repressors, thereby creating windows, of sorts, in the chromatin of progenitor cells (9, 10). In the new study by Delás et al. 2023 (1), the authors first establish an embryonic stem cell-based system to generate neural progenitors that can be appropriately induced by a Shh agonist (here, called Shh) to yield p3, pMN, p2, and p0–1 neural subtypes, using high to low concentrations of Shh, respectively. To accommodate variation in neural subtype induction at each Shh concentration, the authors then applied CaTS-ATAC, wherein ATAC-Seq is used to map open sites in chromatin on formaldehyde-fixed cells and enabling different neural subtypes to be isolated by flow cytometry. The approach was used at different time points of Shh treatment and serves as a paradigm for future studies of other mixed cell types emerging from a common progenitor population.
Figure 1.
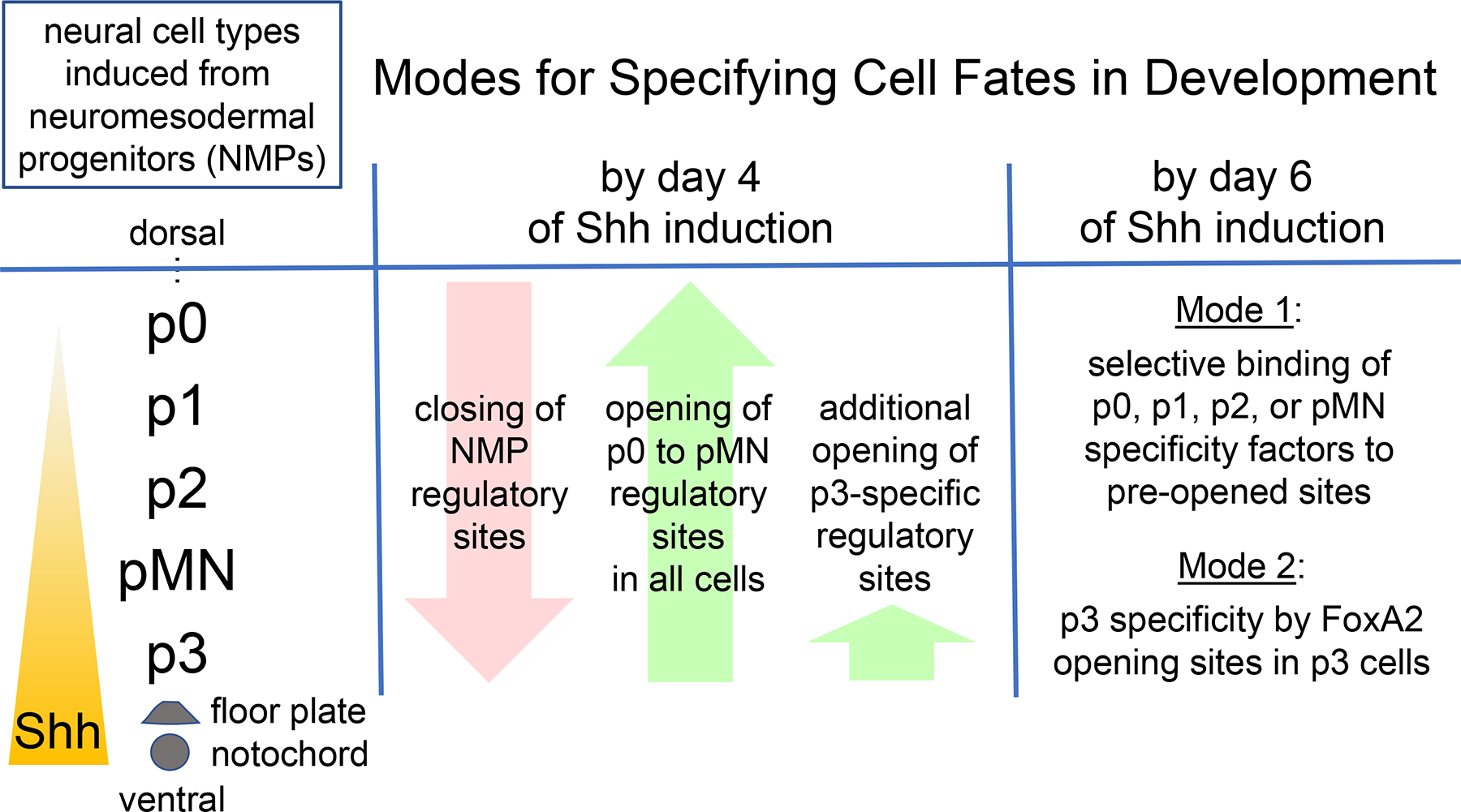
Two modes for cell fate induction in chromatin. Common neuromesodermal progenitors (NMPs) give rise to different neural tube fates, p0, p1, p2, pMN, and p3, in response to ventral Shh signaling from the notochord and the floor plate of the neural tube. In the embryonic stem cell-derived model of Melas et al. (2023), by four days of Shh induction, NMP regulatory sequences in chromatin become transposase-resistant, or “closed,” while regulatory sites for most of the neural fates become sensitive, or “opened.” By two days later, in Mode 1, different pre-open sites become targeted by newly induced factors that specify either the p0-p1, p2, or pMN fates. In Mode 2, additional regulatory sites become opened by FoxA2, which enables other factors to bind and activate the p3 neural fate.
Surprisingly, different concentrations of Shh did not change the distinct pattern of open chromatin sites in each of the neural subtypes that emerged. That is, each new cell type acquired a particular pattern of open chromatin regardless of the Shh concentrations used, indicating that a pre-existing landscape of factors enables the Shh morphogen to be interpreted for cell fate determination. On the other hand, there was a marked difference in how the p0–1, p2, and pMN cell types were induced, compared to the p3 cell type. For the p0–1, p2, and pMN cells, by four days of Shh they exhibited a general closure of regulatory sites of the neuromesodermal progenitors (here, called pre-neural) and opening of most new sites in common, but even after resolution of the different cell fates by day six, the cells still shared most open sites in common (Figure 1). Footprinting analysis of the ATAC-Seq data and other studies revealed differential transcription factor occupancy at the shared open sites between the three different cell types. Thus, after an initial chromatin opening event, the p0–1, p2, and pMN cell fates are determined by differential activities of transcription factors acting on open target regions. By contrast, Shh induced an additional, different pattern of open chromatin sites in p3 cells, which was tracked back to the Shh induction of the pioneer transcription factor FOXA2. FOXA2 was then shown to be required for the p3 fate and its ectopic expression in neural progenitors was sufficient to open p3 target site chromatin and initiate p3 cell specification.
To summarize, induction of all the ventral neural cell types involve an initial suppression of the pre-neural progenitor program and an opening of new sites that are active across the ventral neural tube (Figure 1). Yet the p0–1, p2, and pMN cell types become specified by transcription factors acting upon a common set of the newly opened regulatory sites and the p3 cell type becomes specified by a pioneer factor opening chromatin for a different regulatory network. The well-controlled experimental system firmly establishes the different modes of gene network induction.
The Delas et al. study (1) also provided insight into an evolutionary relationship between the neural and endoderm lineages. FOXA2 is a paradigmatic pioneer factor that was originally discovered to enable liver specification from the endoderm (6). Interestingly, Delas et al. found that FOXA2 targets various regulatory genes in common between p3 neural cells and endoderm lineages. While it may be the result of convergent evolution, early diploblastic organisms derived from ectoderm and mesoderm may have evolved via a common, FOXA2-dependent gene regulatory network, a vestige of which is seen in the p3 cells.
As with any definitive study, the system raises questions that go deeper into the underlying mechanisms. How does the pre-neural network get shut down? What collectively opens the p0–1, p2, and pMN regions to allow for differentiating transcription factors to bind? How are the differentiating transcription factors’ activities confined to the p0–1, p2, and pMN domains of the neural tube? Do other graded responses to morphogens similarly use different modalities to induce different cell fates? Delás et al. give us a road map for answering many of these issues.
Footnotes
Declaration of Interests
I am on the advisory board for Developmental Cell.
References
- 1.Delás et al. (2023) Developmental Cell, in press. [Google Scholar]
- 2.McDaniel et al. (2019) Molecular Cell 74, 1–11.30951649 [Google Scholar]
- 3.Tang et al. (2022) NSMB 20, 665–676. [Google Scholar]
- 4.Miao et al. (2022) Molecular Cell 82, 986–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gassler et al. (2022) Science 10.1126/science.abn7478. [DOI] [Google Scholar]
- 6.Zaret KS (2020) Ann. Rev. Genetics 54, 367–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michael AK, and Thoma NH (2021) Cell 184, 1–13. [DOI] [PubMed] [Google Scholar]
- 8.Lerner et al. (2020) Molecular Cell 79, 1–12.32619466 [Google Scholar]
- 9.Peterson et al. (2012) Genes Dev 26, 2802–2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kutejova et al. (2016) Developmental Cell 36, 639–653. [DOI] [PMC free article] [PubMed] [Google Scholar]


