Abstract
We recently reported an economic choice task in which squirrel monkeys chose between differing amounts of remifentanil, a fast-acting opioid, or a food reward to develop a preclinical screen for evaluating potential pharmacotherapies for opioid dependence. Herein, two known opioid addiction treatments are evaluated using this task, as well as a potential new agent, cariprazine, a dopamine D2/D3 receptor partial agonist currently used to treat bipolar disorder and schizophrenia. Preclinical rodent studies suggest this class of compounds may reduce opiate self-administration. Squirrel monkeys were pretreated daily with clinically relevant doses of each compound during the five days of treatment evaluation using the economic choice task. Shifts in drug preference were measured as changes in subjects’ indifference values, where the probability of drug and milk choice are equivalent. Buprenorphine produced a significant shift in indifference value between baseline and treatment weeks, indicating a decrease in drug preference. Subjects treated with methadone and cariprazine did not show any significant shift in drug preference. Differences between the buprenorphine and methadone results likely reflect a lack of opioid dependence in the subjects. The cariprazine results suggest that it does not alter opioid reward in non-dependent primates over a five day period.
Keywords: opioid, opioid maintenance therapy, remifentanil, economic choice task, buprenorphine, methadone, cariprazine
1. Introduction
For pharmacotherapeutic treatment of opioid use disorder, opioid maintenance therapy is the most common approach. Evidence has shown it to be effective in reducing opioid use and associated withdrawal symptoms (1–3). Currently, the only FDA approved treatments for opioid maintenance therapy are buprenorphine and methadone. Buprenorphine is a partial mu-opioid agonist, and methadone is a full mu-opioid agonist. Both drugs have much longer half-lives than opiates such as heroin, allowing them to decrease symptoms of withdrawal with once-daily treatment (4). However, chronic use of either opioid can lead to unpleasant physical side effects, with patients regularly reporting constipation, nausea, and respiratory system depression (5, 6). Like all opioid agonists, these drugs have potential for abuse and overdose (7); therefore, there is a need to research alternative mechanisms that can be targeted.
One potential target is the dopamine D3 receptor (8). A recent study in rats found that a high affinity D3 partial agonist attenuated heroin-enhanced hyperactivity, heroin self-administration, heroin-induced reinstatement, and cue-induced reinstatement of heroin-seeking (9). In humans, studies have shown that partial D2/D3 agonists can reduce stimulant use, specifically with methamphetamine (10). Cariprazine, an FDA approved antipsychotic and D3-preferring D2/D3 partial agonist (11), has also been shown to reduce comorbid stimulant use when studying its effects in pilot studies of patients with psychosis (10, 12). Based on these results in both animal and human models, cariprazine may be a viable treatment for opioid use disorder. Because cariprazine is an existing treatment, it could be quickly examined in clinical trials if promising results were found in a preclinical study looking strictly at its effects on opioid use.
There is a growing recognition of the inadequacy of many preclinical approaches when evaluating novel treatments for drug abuse. Common approaches, such as drug self-administration and reinstatement, bear little resemblance to real-world situations, reducing their predictive validity (13). Drug vs. non-drug choice procedures offer advantages in avoiding any rate-altering effects of agents being evaluated, instead evaluating the allocation of choices toward alternative reinforcers. In this regard, these approaches can be considered models for contingency management, a highly effective behavioral approach to treating addiction (14). To increase confidence in the reliability of a given preclinical approach to therapeutic development, it has been proposed that the reverse translation of existing treatments can help to validate the current task (13).
Using an economic choice task with the rapidly metabolized mu-opioid receptor agonist remifentanil (15), the aim of this study was to: 1) reverse translate existing opioid-based therapies, methadone and buprenorphine, to evaluate the ability of the task to identify previously established, clinically useful treatments; and 2) evaluate the non-opioid treatment cariprazine to alter drug choice on an economic choice task.
2. Materials and Methods
2.1. Subjects:
All procedures in animal subjects were carried out in accordance with the Guide for the Care and Use of Laboratory Animals (8th ed.). This study utilized eleven male squirrel monkeys (Saimiri sciureus; body weight 700-1200 g) housed individually in a temperature and humidity-controlled environment with a 12:12 hour light cycle, beginning with lights on at 7:00 am. Subjects were provided with water ad libitum, as well as a diet consisting of Teklad biscuits (8794; Envigo; Indianapolis, IN) given once daily, with additional daily fruit and vegetable enrichment. Each monkey was equipped with either a polyvinyl chloride (TYGON®; inner diameter, 0.38mm; outer diameter, 0.89mm) or polyurethane catheter (inner diameter, 0.6mm; outer diameter, 0.9mm) inserted into a jugular or femoral vein, externalized at their back. Catheters were protected by a jacket (Ludomed Inc.; Canada) fitted to each monkey with a zipper in the back for easy access. Catheters were flushed with saline between experiments, and “locked” on Fridays with 0.3 ml of either a 100 UI/ml heparin dextrose solution or a taurolidine solution (TCS; Norfolk Access Technologies).
2.2. Drug-Milk Choice Task:
As previously described (15), subjects were placed in a custom-made acrylic chair in an enclosed chamber fitted with a fan for ventilation, speaker for white-noise, and a touchscreen (Elo TouchSystems; Menlo Park, CA). Two syringe pumps (Harvard Apparatus; South Natick, MA) were located outside the chamber, one of which delivered remifentanil hydrochloride dissolved in 0.9% saline (0.5μg/ml), while the other delivered 30% sweetened condensed milk (food reward). Remifentanil has a 2-3 min half-life in humans (16) permitting repeated administration with minimal accumulation that might alter behavior on subsequent trials. It was administered intravenously through the catheter and the condensed milk was delivered into a milk well located directly in front of the subjects. All data collection and programming were done using either E-Prime Professional 3.0 or MonkeyLogic, a NIMH MATLAB-based software.
The study utilized the drug-milk choice task previously published in Brown et al. (15). Briefly, subjects chose between two stimuli corresponding to either a remifentanil or a milk reward. Remifentanil reward magnitude was represented by 1-4 green triangles which corresponded to different unit doses of remifentanil (0.08-0.32 μg/kg/infusion). Milk rewards were indicated with 1-4 red circles, which corresponded to different amounts of milk (75-300 μl/kg). For the methadone and buprenorphine studies, the options remained on the screen until one stimulus was selected. In subsequent cariprazine studies, a limited hold (5 sec) was added, followed by a 2 sec blank screen and repetition of the same stimuli if no choice was made. In all experiments, the response latency was calculated as the time that the stimuli were present on the screen, from stimulus onset until the selection of a stimulus. Once the subject made a choice, it received its selected reinforcer, and a 60 second inter-trial interval (ITI) was initiated. A completed session consisted of 108 completed trials, with each drug-milk reward choice being presented randomly in a counterbalanced manner, 8 times. Every third trial was a choice between two different milk quantities (1:2, 1:3, 1:4, 2:3) to provide additional time for clearance of remifentanil and to provide a trial type in which drug was not an option. For all studies, drug effects were determined by comparison to a contemporaneous baseline from the week prior.
As previously described (15), choice allocation (probability of drug choice) was plotted vs. a “reward contrast” defined as (# drug stimuli - # milk stimuli)/(# drug stimuli + # milk stimuli). This created a non-categorical representation of choice options that permitted logistical regression to describe drug choice as a function of offer type. Using this approach, an indifference value (IndV) could be calculated at which the probability of drug and milk choice are equivalent. Change in IndV between weeks with and without drug pretreatment was the primary outcome measure.
2.3. Pretreatments:
Doses of methadone (1.0 mg/kg, n = 9), buprenorphine (0.1 mg/kg, n = 11; 0.32 mg/kg, n = 8), and cariprazine (10μg/kg, n = 7; 30 μg/kg, n = 8; 100 μg/kg, n = 6) were administered intravenously, twenty-two hours prior to the task, for five consecutive days. Each buprenorphine pretreatment dose was followed by at least a two-week washout period, and cariprazine doses were administered following a minimum three-week washout. Methadone and buprenorphine dosing was based on clinical studies (3, 17, 18), while cariprazine dosing was based on preclinical PET studies of D3 receptor occupancy in NHPs and clinically recommended doses (19, 20).
2.4. Analysis:
Treatment and baseline weeks were analyzed by using the last three days of each week to allow time for subjects to adjust to experimental manipulations. Paired t-tests were used to assess the significance of the shifts in IndV’s from baseline to treatment weeks. The 2-way repeated measure ANOVAs analyzed the differences between baseline and treatment weeks, looking at each subject’s average indifference value and response latencies, with subject and reward contrasts as the repeated factors. To limit the impact of attentional lapses on response latencies, the reported values reflect the average of latencies below the 90th percentile.
3. Results
Administration of 0.1 mg/kg buprenorphine produced an increase in the mean IndV by 29 +/− 0.09, indicating that the subjects’ preferences significantly shifted away from remifentanil and towards milk (t(10) = 2.23, p = 0.01) (figure 1A). Similarly, following 0.32 mg/kg buprenorphine pretreatment, IndV increased by 0.35 +/− 0.07 (t(7) = 2.36, p = 0.002) (figure 1B). While the higher dose did have a larger increase in IndV, it was not significantly different from the change following the lower dose. Response latencies were not affected by either dose, with no differences between baseline or treatment weeks observed. There were no systematic day by day progressions of effect across the five days of treatment, as can be seen in supplemental fig. 1.
Figure 1:
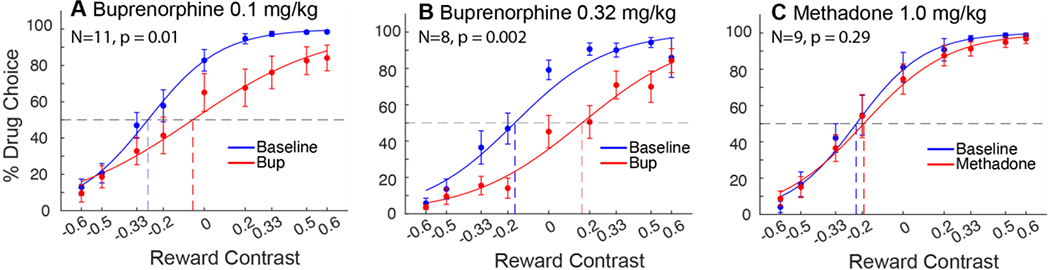
Choice allocation curves of buprenorphine and methadone pretreatments compared to contemporaneous baseline. Curves represent the group average choice over the last 3 days of each respective week. A) Buprenorphine 0.1 mg/kg produced a significant shift in IndV, p = 0.01. B) Buprenorphine 0.32 mg/kg also produced a significant shift in IndV, p = 0.002. C) Methadone at 1.0 mg/kg did not change choice allocation between remifentanil and milk.
Methadone administered at 1.0 mg/kg had no significant effect on IndV (figure 1C). There was also no difference in response latencies between baseline and treatment weeks. Administration of a higher dose of methadone (2.0 mg/kg) was found to cause acute lethargy and impaired motor function in pilot subjects and was not pursued further.
Cariprazine was administered in three doses (10 mg/kg, 30 mg/kg, & 100 mg/kg). No dose examined produced a significant change in IndV (figure 2A–C). Response latencies were also not affected. A higher dose of 300 mg/kg revealed lethargy and impaired motor function as well as decreased appetite.
Figure 2:
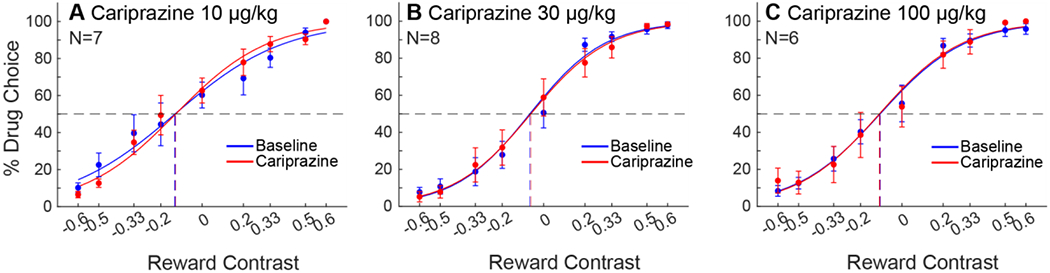
Choice allocation curves of cariprazine pretreatments for baseline and treatment weeks, as presented in Fig. 1 A) Cariprazine at 10 μg/kg, B) 30 μg/kg, and C) 100 μg/kg did not change choice allocation between remifentanil and milk.
4. Discussion
In the present study, we used a novel approach, drug vs. non-drug economic choice in squirrel monkeys to measure shifts in choice allocation after pre-treatment with buprenorphine, methadone, and cariprazine. Given the efficacy of the two opioids as clinical treatments, this is a “reverse-translation” to evaluate the choice procedure’s ability to track shifts in drug choice allocation. Cariprazine was also examined in order to determine its potential therapeutic efficacy. Buprenorphine significantly reduced drug choice, whereas methadone and cariprazine had no effect.
Our reverse-translation of two clinically effective opioid agonist approaches provide a mixed review of the economic choice procedure. Results from the study are congruent with what is observed in clinical settings. Clinically, methadone treatment at higher dosages (60-100 mg/day) is more effective for those dependent on opioids (21, 22), validating the dose used in this study. Being a full agonist rather than a partial agonist, its lack of effect is consistent with previous studies, which suggest that full agonists have greatest efficacy in drug dependent subjects (23). The current study used remifentanil for opioid reward, and due to its short half-life, remifentanil is quickly metabolized and has no active metabolites (24), thereby reducing the likelihood of dependence. The subjects in this study did not show signs of withdrawal when treated with naltrexone (unpublished observations, Brown et al), and in our previous study (15), morphine, another full agonist, had no effect on choice allocation. This is also consistent with previous literature in non-dependent rats (25) and primates (23, 26, 27). In dependent animals, there is an effect of a full agonist to reduce drug choice [25], presumably through a negative reinforcement mechanism in which the need to increase drug choice to relieve withdrawal is obviated by the agonist pretreatment (28). The lack of dependence must be viewed as a limitation of our approach to adequately model relief of withdrawal. Buprenorphine’s ability to shift drug choice is likely a consequence of its partial agonist nature. We previously demonstrated that opiate antagonists dramatically shift drug preference (15), and buprenorphine can clearly partially block agonist effects as shown in rat choice procedure (29), and given that in clinical use, care must be taken not to precipitate withdrawal in highly dependent individuals. Thus, overall, we consider our economic choice approach to be most informative with respect to the acute rewarding effects of the agonist remifentanil.
Given that there were no significant differences in response latencies, these results are consistent with our dosing regimen being below a point that would produce non-specific effects on motor function (30–32). It could be argued that our 22-hour pretreatment regimen might be too long prior to testing; however, clinical evidence suggests methadone is cleared as slowly as buprenorphine, with an average half-life of approximately 38 hours (4, 33). We do not have pharmacokinetic information on these drugs in squirrel monkeys; but given that buprenorphine was effective, and each test compound was administered at doses lower than those that cause non-selective effects, we do not believe clearance is the reason methadone had no effect.
Cariprazine also showed no effects on choice allocation. While there is less clear dependence of opiate reward on dopaminergic mechanisms, D3 receptor partial agonists and antagonists have helped decrease oxycodone and heroin taking and seeking behaviors in rodents (9, 34, 35), and there is an ongoing phase 2 clinical trial (https://clinicaltrials.gov/ct2/show/NCT05063201) investigating cariprazine and its effects on those with opioid use disorder. It is important to note, however, that opioid reward mechanisms are not limited to interactions between mu-opioid receptors in the mesolimbic dopamine pathway, a region where D3 receptors are highly concentrated (36). There are three important differences between our current study and prior studies with rodents suggesting possible efficacy of a D3 partial agonist. First, those previous studies used oxycodone, which has a longer half-life and is abused in humans. The shorter half-life of remifentanil would not prevent a mu-agonist mediated reward, and extensive studies in macaques have utilized remifentanil as a mu-agonist in evaluating potential treatments for opioid abuse (37, 38). Second, ours utilized a choice allocation procedure, while the rodent studies used more traditional rate-dependent approaches. Third, and most important, there are often differences in drug effects and predictive accuracy of potential treatments between rodents and primates. Locomotor and neurochemical sensitization with repeated treatment is a widely observed phenomenon in rodents that is not observed in human (39) or non-human (40) primates. Potential addiction treatments that look promising in rodents, such as CRF antagonists, fail in primate choice studies (41) or in the clinic (42). Our opinion is that a species difference is the most likely explanation. Given the sensitivity of our choice procedure to shifts in mu-opioid reward, our interpretation of the cariprazine results is that it does not alter acute opiate reward.
In conclusion, we were able to further confirm the predictive validity of an economic choice paradigm for identifying potential therapeutic approaches that alter acute opiate reward, which is reflected in shifts in drug choice allocation. Our results for cariprazine were not consistent with previous rodent results, which suggested cariprazine’s ability to acutely reduce opiate reward. Further clinical trials in populations with other psychiatric disorders with comorbid opiate dependence will potentially reveal therapeutic uses our approach does not speak to.
Supplementary Material
Acknowledgements
We would like to thank Eric Thorndike for his technical assistance, as well as Nabil Daddaoua for assistance with data analysis.
Funding
This research was supported by the Intramural Research Program of the NIH, NIDA ( ZIA DA000622 - CWB; Z1A DA000424 – AHN).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest: None.
References
- 1.Ward J, Hall W, Mattick RP (1999): Role of maintenance treatment in opioid dependence. The Lancet 353:221–226. [DOI] [PubMed] [Google Scholar]
- 2.Mattick RP, Breen C, Kimber J, Davoli M (2009): Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database of Systematic Reviews. [Google Scholar]
- 3.Ling W, Charuvastra C, Collins JF, Batki S, Brown LS Jr., Kintaudi P, et al. (1998): Buprenorphine maintenance treatment of opiate dependence: a multicenter, randomized clinical trial. Addiction 93: 475–486. [DOI] [PubMed] [Google Scholar]
- 4.Balyan R, Hahn D, Huang H, Chidambaran V (2020): Pharmacokinetic and pharmacodynamic considerations in developing a response to the opioid epidemic. Expert Opin Drug Metab Toxicol 16: 125–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Porreca F, Ossipov MH (2009): Nausea and Vomiting Side Effects with Opioid Analgesics during Treatment of Chronic Pain: Mechanisms, Implications, and Management Options. Pain Medicine 10: 654–662. [DOI] [PubMed] [Google Scholar]
- 6.Boom M, Niesters M, Sarton E, Aarts L, Smith TW, Dahan A (2012): Non-analgesic effects of opioids: opioid-induced respiratory depression. Curr Pharm Des 18: 5994–6004. [DOI] [PubMed] [Google Scholar]
- 7.Whelan PJ, Remski K (2012): Buprenorphine vs methadone treatment: A review of evidence in both developed and developing worlds. J Neurosci Rural Pract 3: 45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rasmussen K, White DA, Acri JB (2019): NIDA’s medication development priorities in response to the Opioid Crisis: ten most wanted. Neuropsychopharmacology 44: 657–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galaj E, Bi G-H, Klein B, Hempel B, Shaik AB, Gogarnoiu ES, et al. (2022): A highly D3R-selective and efficacious partial agonist (S)-ABS01-113 compared to its D3R-selective antagonist enantiomer (R)-ABS01-113 as potential treatments for opioid use disorder. Neuropsychopharmacology 47: 2309–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Truong TT, Li B (2022): Case Series: Cariprazine for treatment of methamphetamine use disorder. The American Journal on Addictions 31: 85–88. [DOI] [PubMed] [Google Scholar]
- 11.Kiss B, Horváth A, Némethy Z, Schmidt E, Laszlovszky I, Bugovics G, et al. (2010): Cariprazine (RGH-188), a dopamine D(3) receptor-preferring, D(3)/D(2) dopamine receptor antagonist-partial agonist antipsychotic candidate: in vitro and neurochemical profile. J Pharmacol Exp Ther 333: 328–340. [DOI] [PubMed] [Google Scholar]
- 12.Csehi R, Dombi ZB, Sebe B, Molnár MJ (2022): Real-Life Clinical Experience With Cariprazine: A Systematic Review of Case Studies. Front Psychiatry 13: 827744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Venniro M, Banks ML, Heilig M, Epstein DH, Shaham Y (2020): Improving translation of animal models of addiction and relapse by reverse translation. Nature Reviews Neuroscience 21: 625–643. [DOI] [PubMed] [Google Scholar]
- 14.Banks ML, Negus SS (2012): Preclinical Determinants of Drug Choice under Concurrent Schedules of Drug Self-Administration. Adv Pharmacol Sci 2012:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown SO, Effinger DP, Montoro RA, Daddaoua N, Justinova Z, Moerke MJ, et al. (2022): Economic choice between remifentanil and food in squirrel monkeys. Neuropsychopharmacology 47: 1398–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kapila A, Glass PSA, Jacobs JR, Muir KT, Hermann DJ, Shiraishi M, et al. (1995): Measured Context-sensitive Half-times of Remifentanil and Alfentanil. Anesthesiology 83: 968–975. [DOI] [PubMed] [Google Scholar]
- 17.Bao Y-p, Liu Z-m, Epstein DH, Du C, Shi J, Lu L (2009): A Meta-Analysis of Retention in Methadone Maintenance by Dose and Dosing Strategy. The American Journal of Drug and Alcohol Abuse 35: 28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson RE, Chutuape MA, Strain EC, Walsh SL, Stitzer ML, Bigelow GE (2000): A Comparison of Levomethadyl Acetate, Buprenorphine, and Methadone for Opioid Dependence. New England Journal of Medicine 343: 1290–1297. [DOI] [PubMed] [Google Scholar]
- 19.Periclou A, Phillips L, Ghahramani P, Kapás M, Carrothers T, Khariton T (2021): Population Pharmacokinetics of Cariprazine and its Major Metabolites. Eur J Drug Metab Pharmacokinet 46: 53–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seneca N, Finnema SJ, Laszlovszky I, Kiss B, Horváth A, Pásztor G, et al. (2011): Occupancy of dopamine D2 and D3 and serotonin 5-HT1A receptors by the novel antipsychotic drug candidate, cariprazine (RGH-188), in monkey brain measured using positron emission tomography. Psychopharmacology 218: 579–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faggiano F, Vigna-Taglianti F, Versino E, Lemma P (2003): Methadone maintenance at different dosages for opioid dependence. Cochrane Database of Systematic Reviews. [DOI] [PubMed] [Google Scholar]
- 22.Trafton JA, Minkel J, Humphreys K (2006): Determining effective methadone doses for individual opioid-dependent patients. PLoS Med 3: 380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Negus SS (2006): Choice between heroin and food in nondependent and heroin-dependent rhesus monkeys: effects of naloxone, buprenorphine, and methadone. J Pharmacol Exp Ther 317: 711–723. [DOI] [PubMed] [Google Scholar]
- 24.Panlilio LV, Schindler CW (2000): Self-administration of remifentanil, an ultra-short acting opioid, under continuous and progressive-ratio schedules of reinforcement in rats. Psychopharmacology (Berl) 150: 61–66. [DOI] [PubMed] [Google Scholar]
- 25.Townsend EA, Blough BE, Epstein DH, Negus SS, Shaham Y, Banks ML (2022): Effect of TRV130 and methadone on fentanyl-vs.-food choice and somatic withdrawal signs in opioid-dependent and post-opioid-dependent rats. Neuropsychopharmacology 47: 2132–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Banks ML, Blough BE (2015): Effects of Environmental Manipulations and Treatment with Bupropion and Risperidone on Choice between Methamphetamine and Food in Rhesus Monkeys. Neuropsychopharmacology 40: 2198–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Withey SL, Spealman RD, Bergman J, Paronis CA (2019): Behavioral Effects of Opioid Full and Partial Agonists During Chronic Buprenorphine Treatment. J Pharmacol Exp Ther 371: 544–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pathan H, Williams J (2012): Basic opioid pharmacology: an update. Br J Pain 6:11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Townsend EA, Schwienteck KL, Robinson HL, Lawson ST, Banks ML (2021): A drug-vs-food “choice” self-administration procedure in rats to investigate pharmacological and environmental mechanisms of substance use disorders. Journal of Neuroscience Methods 354: 109110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burke NN, Ferdousi M, Deaver DR, Finn DP, Roche M, Kelly JP (2019): Locomotor and anti-immobility effects of buprenorphine in combination with the opioid receptor modulator samidorphan in rats. Neuropharmacology 146: 327–336. [DOI] [PubMed] [Google Scholar]
- 31.Sankararaman A, Masiulis I, Richardson DR, Andersen JM, Mørland J, Eisch AJ (2012): Methadone does not alter key parameters of adult hippocampal neurogenesis in the heroin-naïve rat. Neuroscience Letters 516: 99–104. [DOI] [PubMed] [Google Scholar]
- 32.Zimnisky R, Chang G, Gyertyán I, Kiss B, Adham N, Schmauss C (2013): Cariprazine, a dopamine D(3)-receptor-preferring partial agonist, blocks phencyclidine-induced impairments of working memory, attention set-shifting, and recognition memory in the mouse. Psychopharmacology (Berl) 226: 91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Auvity S, Goutal S, Caillé F, Vodovar D, Pruvost A, Wimberley C, et al. (2021): Pharmacokinetic neuroimaging to study the dose-related brain kinetics and target engagement of buprenorphine in vivo. Neuropsychopharmacology 46: 1220–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Guglielmo G, Kallupi M, Sedighim S, Newman AH, George O (2019): Dopamine D(3) Receptor Antagonism Reverses the Escalation of Oxycodone Self-administration and Decreases Withdrawal-Induced Hyperalgesia and Irritability-Like Behavior in Oxycodone-Dependent Heterogeneous Stock Rats. Front Behav Neurosci 13: 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.You Z-B, Gao J-T, Bi G-H, He Y, Boateng C, Cao J, et al. (2017): The novel dopamine D3 receptor antagonists/partial agonists CAB2-015 and BAK4-54 inhibit oxycodone-taking and oxycodone-seeking behavior in rats. Neuropharmacology 126: 190–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Galaj E, Newman AH, Xi Z-X (2020): Dopamine D3 receptor-based medication development for the treatment of opioid use disorder: Rationale, progress, and challenges. Neuroscience & Biobehavioral Reviews 114: 38–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maguire DR, France CP (2020): Interactions between opioids and cannabinoids: Economic demand for opioid/cannabinoid mixtures. Drug and Alcohol Dependence 212: 108043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maguire DR, Gerak LR, Woods JH, Husbands SM, Disney A, France CP (2018): Long-lasting effects of methocinnamox on heroin self-administration in rhesus monkeys. Journal of Pharmacology and Experimental Therapeutics 368: 88–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Narendran R, Martinez D (2008): Cocaine abuse and sensitization of striatal dopamine transmission: A critical review of the preclinical and clinical imaging literature. Synapse (New York, NY) 62: 851–869. [DOI] [PubMed] [Google Scholar]
- 40.Bradberry CW (2007): Cocaine sensitization and dopamine mediation of cue effects in rodents, monkeys, and humans: areas of agreement, disagreement, and implications for addiction. Psychopharmacology 191: 705–717. [DOI] [PubMed] [Google Scholar]
- 41.Negus SS, Rice KC (2009): Mechanisms of Withdrawal-Associated Increases in Heroin Self-Administration: Pharmacologic Modulation of Heroin vs Food Choice in Heroin-Dependent Rhesus Monkeys. Neuropsychopharmacology 34: 899–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwandt ML, Cortes CR, Kwako LE, George DT, Momenan R, Sinha R, et al. (2016): The CRF1 Antagonist Verucerfont in Anxious Alcohol-Dependent Women: Translation of Neuroendocrine, But not of Anti-Craving Effects. Neuropsychopharmacology 41: 2818–2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


