Abstract
Increased intraventricular pressure gradients due to dynamic left ventricular outflow tract obstruction during exercise have long been known to cause different symptoms. Exercise stress echocardiography is fundamental in the diagnostic approach of symptoms presenting during exercise. We hypothesize on the possible pathophysiological mechanisms responsible for our patient's syncopal episodes.
Keywords: exercise stress echocardiography, intraventricular gradients, syncope, systolic anterior motion of the mitral valve
Increased intraventricular pressure gradients due to dynamic left ventricular outflow tract obstruction during exercise may cause different symptoms. Exercise stress echocardiography is fundamental in the evaluation of these symptoms.

1. INTRODUCTION
Syncope is defined as a transient loss of consciousness due to cerebral hypoperfusion, characterized by a rapid onset, short duration, and spontaneous complete recovery. 1 The most common etiology is vasovagal syncope (also called neurally mediated) 2 , 3 but other etiologies such as orthostatic hypotension, cardiac arrhythmias, or cardiac structural abnormalities should also be considered. When approaching a patient with syncope, the challenge is the identification of those etiologies that might have life‐threatening consequences. Ominous risk markers include, among others, abnormal electrocardiographic findings, 4 syncope with marked exertion, and the presence of structural heart disease. 5 The development of significant intraventricular gradients during exercise is considered rare and usually is in association with left ventricular hypertrophy. 6 , 7 In the last years, a number of other conditions have been related to the presence of intraventricular gradients even in the absence of left ventricular hypertrophy. 8 , 9 In the case presented here, we describe an adult man with vagal symptoms since adolescence that unexpectedly developed an intraventricular gradient during exercise.
2. CASE PRESENTATION
A 57‐year‐old man was observed in the outpatient clinic due to occasional chest discomfort on light exercise and syncope on strenuous exercise. He had long‐lasting complaints of dizziness and presyncope/syncope related to exercise which began when he was 17 years of age. Other than the referred symptoms, there was no relevant personal or family history of sudden death or heart disease. He underwent medical evaluation uncountable times and since at least 20 years of age was labeled as depressive and treated with mirtazapine. Physical examination including neurological assessment was unremarkable. The resting twelve‐lead electrocardiogram (ECG) was normal (Figure 1). The resting transthoracic echocardiogram was also normal, with no left ventricular hypertrophy, abnormalities in the mitral valve or the subvalvular apparatus. The various Holter ECG recordings done throughout his life were normal. A treadmill exercise test was performed as the symptoms were related to exercise. During the treadmill test (Bruce protocol), the patient developed silent effort‐induced ST‐segment depression (1.5 mm in the inferior leads, DI and V4‐V6) (Figure 2). The patient underwent an exercise stress echocardiography, and during the exam developed an intraventricular gradient of 150 mmHg with an end‐systolic peak and systolic anterior motion (SAM) of the mitral valve after exercise in the upright position (Figure 3 and Movie S1) which disappeared in the first minute of the recovery period in left lateral decubitus as the patient had severe symptoms at that moment. There was a fall in blood pressure at the end of the exercise test accompanying the symptoms. The exercise stress echocardiogram was negative for obstructive epicardial coronary artery disease since no regional wall motion abnormalities were found. The patient was treated with bisoprolol 5 mg daily and has not suffered from any symptoms after initiating treatment. Another exercise stress echocardiogram was later performed during which the patient was asymptomatic and only a residual intraventricular gradient was detected.
FIGURE 1.

Normal twelve‐lead electrocardiogram, at rest.
FIGURE 2.

Effort‐induced ST‐segment depression in twelve‐lead electrocardiogram during exercise (1.5 mm in the inferior leads, DI and V4‐V6).
FIGURE 3.
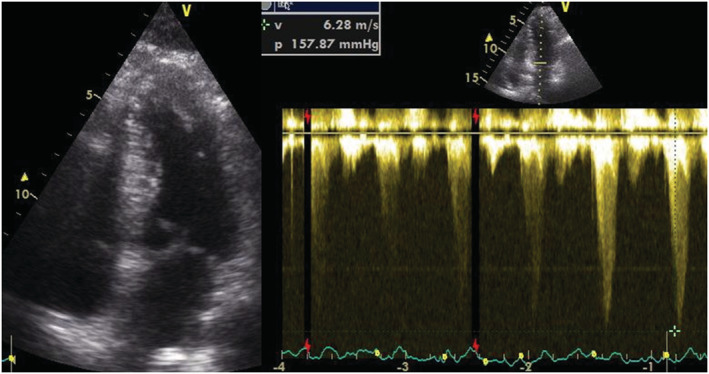
Intraventricular gradient of 150 mmHg with an end‐systolic peak measured with continuous‐wave doppler and systolic anterior motion (SAM) of the mitral valve after exercise in the upright position.
3. DISCUSSION AND CONCLUSION
It has long been known that small intraventricular gradients are a common phenomenon. Three mechanisms have been proposed 10 as causes for the gradients to increase drastically during exercise: (1) increase in nonobstructive physiological gradients; (2) end‐systolic obstruction secondary to ventricular cavitary obliteration; and (3) mid‐systolic obstruction caused by SAM of the mitral valve restricting ejection flow. However, for SAM to occur, it is thought that there must be present some modification of the geometry of the ventricular chamber or the mitral valve apparatus. 11 This was not the case with our patient. It has been demonstrated that intraventricular gradients can be caused by maneuvers that change loading conditions in structurally normal hearts, 12 and it is known that exercise can elicit such changes. During physical activity myocardial contractility, stroke volume and ejection fraction are increased. These lead to reduction in end‐systolic dimensions and volume and accelerates trans‐left ventricular outflow tract (LVOT) flow with subsequent possible SAM of the anterior mitral leaflet, moving toward the proximal part of interventricular septum causing flow obstruction and significant pressure gradients and symptoms. Therefore, during exercise, dynamic left ventricular outflow tract obstruction (DLVOTO) may occur in the absence of left ventricle (LV) hypertrophy and be associated with high gradients and cardiac symptoms.
In this case, the fact that the patient had a normal echocardiogram means that the morphological study of this heart would probably reveal no abnormalities. The phenomenon that we detected during and immediately after exercise testing—mitral valve SAM and intraventricular gradient associated with ST alterations associated with DLVOTO 13 —could well have been responsible for his symptoms. The electrocardiogram changes found in our patient, ST‐T wave abnormalities on exercise, can be related to transient dynamic left ventricular outflow tract and midcavity obstruction. The DLVOTO is associated with an increase in myocardial work and demand for oxygen supply causing angina secondary to enhanced demand–supply mismatch with subsequent subendocardial ischemia, which, ultimately, is manifested as ST segment depression on surface ECG. 13 , 14 Betablocker therapy was used in this patient, following the rationale that it has a negative inotropic and chronotropic effect, and hence antagonizes LVOT and midcavity obstructions. 13 , 15
In our opinion, the case described, in which significant abnormalities in cardiac function were found only during and after exercise, suggests that exercise stress echocardiography should be applied to other patients that have dizziness or syncope related to exercise but no structural abnormalities. We hypothesize that exercise‐induced intraventricular gradients may be involved—at least in some patients—in the pathophysiology of the so‐called vasovagal syncope related to exercise, as was the case of our patient.
The symptoms triggered by the exercise stress echocardiography occurred predominantly after the beginning of recovery, at this moment, there is a decrease in venous return, which leads to a partially empty left ventricular chamber, reduced cardiac output and hypotension. 16 As a normal response to these changes, there is the activation of afferent stimuli from baroreceptors in the carotid sinus, aortic arch, and left ventricle which ultimately leads to the activation of efferent stimuli that increase the sympathetic tonus and catecholamine secretion and, on the contrary, decrease the parasympathetic tonus. Consequently, this results in vasoconstriction and positive inotropic and chronotropic effects. The resultant diminished end‐diastolic left ventricular volume and LV hypercontractility create the increased intraventricular gradients associated with the SAM of the mitral valve. The increased pressure on the ventricular walls causes the activation of baroreceptors (high‐pressure C‐fiber afferent nerves), stimulating paradoxically the vagal response, also known as the Bezold–Jarish Reflex. This reflex may be purely vasodepressor (hypotensive), cardioinhibitory (bradycardia) or mixed, as described in the literature. Finally, exercise‐induced dizziness or syncope may be also explained by reduced stroke volume and hypotension secondary to midcavity or LVOT obstruction. 17 , 18 As explained here, we may infer that the etiology of syncope is primarily cardiac in origin and would introduce the new classification of functional/dynamic cardiogenic syncope, apart from the two already existing ones: arrhythmic and structural.
In summary, we may infer that the syncopal episodes suffered by the patient presented here have a dual etiology: a vasovagal and cardiac functional obstructive component. The European Society of Cardiology (ESC) Syncope Guidelines (2018) recommends a Two‐dimensional and Doppler echocardiography during exercise to detect provocable left ventricular outflow tract obstruction in patients with hypertrophic cardiomyopathy (HCM), a history of syncope, and a resting or provoked peak instantaneous left ventricular outflow tract gradient <50 mmHg (Evidence Class I, Level B). 1 Taking into account our case, we suggest the realization of Exercise Stress Echocardiography in patients who suffer a syncopal or presyncopal episode during and right after the termination of exercise, even though they may not present a history of HCM or other structural abnormalities. However, this would require a large‐scale prospective randomized trial to verify.
AUTHOR CONTRIBUTIONS
Nuno Alexandre Simões Cotrim: Writing – original draft; writing – review and editing. Pedro Silva Cunha: Resources; supervision; validation; writing – original draft. Jorge Guardado: Supervision; validation; writing – review and editing. Carlos Cotrim: Conceptualization; resources; supervision; validation; writing – original draft; writing – review and editing. Luís Baquero: Supervision; validation; writing – review and editing.
FUNDING INFORMATION
No funding.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interests.
ETHICAL APPROVAL
Not applicable.
CONSENT
Written informed consent for publication of the clinical details and images was obtained from the patient.
Supporting information
Movie S1.
ACKNOWLEDGMENTS
Not applicable.
Cotrim N, Cunha PS, Guardado J, Cotrim C, Baquero L. Intraventricular gradients—When we have to “exercise” our minds: A case report. Clin Case Rep. 2023;11:e7010. doi: 10.1002/ccr3.7010
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
REFERENCES
- 1. Brignole M, Moya A, de Lange FJ, et al. 2018 ESC guidelines for the diagnosis and management of syncope. Eur Heart J. 2018;39(21):1883‐1948. doi: 10.1093/eurheartj/ehy037 [DOI] [PubMed] [Google Scholar]
- 2. Hatoum T, Sheldon R. A practical approach to investigation of syncope. Can J Cardiol. 2014;30(6):671‐674. doi: 10.1016/j.cjca.2014.03.043 [DOI] [PubMed] [Google Scholar]
- 3. van Dijk JG, Wieling W. Pathophysiological basis of syncope and neurological conditions that mimic syncope. Prog Cardiovasc Dis. 2013;55(4):345‐356. doi: 10.1016/j.pcad.2012.10.016 [DOI] [PubMed] [Google Scholar]
- 4. Ungar A, Del Rosso A, Giada F, et al. Early and late outcome of treated patients referred for syncope to emergency department: the EGSYS 2 follow‐up study. Eur Heart J. 2010;31(16):2021‐2026. doi: 10.1093/eurheartj/ehq017 [DOI] [PubMed] [Google Scholar]
- 5. Akdemir B, Krishnan B, Senturk T, Benditt DG. Syncope: assessment of risk and an approach to evaluation in the emergency department and urgent care clinic. Indian Pacing Electrophysiol J. 2015;15(2):103‐109. doi: 10.1016/j.ipej.2015.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peteiro J, Montserrat L, Castro‐Beiras A. Labil subaortic obstruction during exercise stress echocardiography. Am J Cardiol. 1999;84(9):1119‐1123, A10‐11. doi: 10.1016/s0002-9149(99)00517-2 [DOI] [PubMed] [Google Scholar]
- 7. Lau TK, Navarijo J, Stainback R. Pseudo‐false‐positive exercise treadmill testing caused by systolic anterior motion of the anterior mitral valve leaflet. Tex Heart Inst J. 2001;28(4):308‐311. [PMC free article] [PubMed] [Google Scholar]
- 8. Dimitrow PP, Cheng TO . Standing position alone or in combination with exercise as a stress test to provoke left ventricular outflow tract gradient in hypertrophic cardiomyopathy and other conditions. Int J Cardiol. 2010;143(3):219‐222. doi: 10.1016/j.ijcard.2010.04.026 [DOI] [PubMed] [Google Scholar]
- 9. Cotrim C, João I, Fazendas P, et al. Clinical applications of exercise stress echocardiography in the treadmill with upright evaluation during and after exercise. Cardiovasc Ultrasound. 2013;11:26. doi: 10.1186/1476-7120-11-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yotti R. What is the relevance of an intraventricular ejection pressure gradient induced by exercise? Rev Esp Cardiol. 2004;57(12):1139‐1142. [PubMed] [Google Scholar]
- 11. Luckie M, Khattar RS. Systolic anterior motion of the mitral valve–beyond hypertrophic cardiomyopathy. Heart. 2008;94(11):1383‐1385. doi: 10.1136/hrt.2007.122069 [DOI] [PubMed] [Google Scholar]
- 12. Grose R, Maskin C, Spindola‐Franco H, Yipintsoi T. Production of left ventricular cavitary obliteration in normal man. Circulation. 1981;64(3):448‐455. doi: 10.1161/01.cir.64.3.448 [DOI] [PubMed] [Google Scholar]
- 13. Saeed S, Vegsundvåg J. Usefulness of stress echocardiography in assessment of dynamic left ventricular obstructions: case series and review of the literature. Cardiology. 2021;146(4):441‐450. doi: 10.1159/000516188 [DOI] [PubMed] [Google Scholar]
- 14. Cabrera Bueno F, Rodríguez Bailón I, López Salguero R, et al. Dynamic left ventricular outflow tract obstruction induced by exercise. Rev Esp Cardiol. 2004;57(12):1179‐1187. [PubMed] [Google Scholar]
- 15. Cotrim C, Lopes LR, Almeida AR, et al. Efficacy of beta‐blocker therapy in symptomatic athletes with exercise‐induced intra‐ventricular gradients. Cardiovasc Ultrasound. 2010;8:38. doi: 10.1186/1476-7120-8-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Seeley AD, Giersch GEW, Charkoudian N. Post‐exercise body cooling: skin blood flow, venous pooling, and orthostatic intolerance. Front Sports Act Living. 2021;3:658410. doi: 10.3389/fspor.2021.658410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rocha BML, Gomes RV, Cunha GJL, et al. Diagnostic and therapeutic approach to cardioinhibitory reflex syncope: a complex and controversial issue. Rev Port Cardiol (Engl Ed). 2019;38(9):661‐673. doi: 10.1016/j.repc.2018.11.007 [DOI] [PubMed] [Google Scholar]
- 18. Arthur W, Kaye GC. The pathophysiology of common causes of syncope. Postgrad Med J. 2000;76(902):750‐753. doi: 10.1136/pmj.76.902.750 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie S1.
Data Availability Statement
All data generated or analyzed during this study are included in this published article [and its supplementary information files].


