Abstract
SIGNIFICANCE:
Alcohol drinking and nicotine vaping often co-occur and dependence on both substances is common. However, the impact of nicotine vaping on alcohol consumption is not fully understood.
METHODS:
We examined the effects of nicotine vaping on ethanol drinking in female and male C57BL/6J mice using an electronic nicotine delivery system and intermittent access two-bottle choice (IA-2BC) drinking. Mice were exposed to electronic nicotine vapor (3%) or propylene glycol/vegetable glycerol (PG/VG) control for 3 h sessions daily for 4 weeks and voluntary alcohol consumption was monitored. Nicotine vapor exposure was stopped and voluntary alcohol drinking was measured for a 2 week abstinence period. We also examined the effects of alcohol and nicotine on locomotion, temperature, and nicotine metabolism.
RESULTS:
Following acute nicotine vapor exposure, alcohol drinking was increased in males but not in females. Thermoregulation was disrupted following nicotine vapor exposure and voluntary drinking. Male and female mice displayed increased locomotor activity immediately following chronic nicotine vapor exposure, and an anxiolytic effect was seen in males. In nicotine vapor abstinence, female mice displayed increased alcohol consumption. Locomotor activity and anxiolytic effects remained elevated in male but not female mice. Female mice displayed higher levels of serum nicotine and hydroxycotinine, suggesting impaired metabolism following chronic drinking and nicotine vapor exposure.
CONCLUSION:
Collectively, these results suggest that while both male and female ethanol-drinking mice experience the stimulatory effects of nicotine vapor, only in males is there a parallel increase in ethanol drinking and only females display impairments in nicotine metabolism after drinking.
1. INTRODUCTION
Historically, nicotine consumption occurred through tobacco products like cigarettes (Dani & Balfour, 2011). Although smoking has declined (Jebai et al., 2022; Wang et al., 2018), Electronic Nicotine Delivery system (ENDs) use has increased (Chaffee et al., 2017; Jebai et al., 2022; Palazzolo, 2013). The perception of fewer associated health risks and increased social use has contributed to increased co-consumption with alcohol (Palazzolo, 2013). The prevalence and popularity of ENDs continues to increase compared to cigarettes (Chaffee et al., 2017; Jebai et al., 2022; Palazzolo, 2013; Wang et al., 2018) and is perceived as a more socially-acceptable form of nicotine consumption, particularly among populations who often engage in combined drinking/vaping behavior. Previous studies employed methods of nicotine delivery including ingestion, mini-pumps or injections which do not mimic human consumption (Caldarone et al., 2008; DeBaker, Moen, et al., 2020; Kasten et al., 2016; LeSage et al., 2002). With increasing alcohol/ENDs co-use, an improved understanding of the effects of combined vaping and drinking is imperative. Previous studies examined nicotine use in treatment-seeking alcohol use disorder patients and found that alcohol contributed to higher nicotine metabolite ratios in men but not women (Dermody et al., 2020), and that non-smoking days correlated with longer periods of abstinence from drug and alcohol use, such that starting or resuming smoking could be an indicator of likelihood to relapse (Kohn et al., 2003).
Studies specifically investigating rodent nicotine vapor exposure report effects on temperature regulation and locomotor function (Garrett et al., 2021; Honeycutt et al., 2020; Javadi-Paydar et al., 2019; Lallai et al., 2021). We previously reported that nicotine vapor exposure altered amygdala activity, thermoregulation, and locomotion (Zhu et al., 2021) using 12% (120 mg) nicotine vapor, however this concentration is higher than that found in commercial products (Zhu et al., 2021). Importantly, route of administration can affect nicotine metabolism, with inhalation resulting in higher immediate nicotine serum levels compared to oral routes which produce increases over more prolonged time periods (Benowitz et al., 2009; Henderson & Cooper, 2021). Research on the role of rodent drug and alcohol interactions is advancing, with studies investigating the effects of ethanol drinking and nicotine exposure yielding variable results. One study found that chronic nicotine exposure increased alcohol consumption regardless of whether drinking occurred with or without interruption early in development (Montanari et al., 2021). Another study found that nicotine increased alcohol consumption or had no effect on drinking depending on dose (Le et al., 2000). One study reported that nicotine and alcohol co-use in adolescent rats decreased freezing and contextual fear memory in males, suggesting sex-specific vulnerability (Ruffolo et al., 2021). Despite growing literature on alcohol and nicotine co-use, the interaction of electronic nicotine vapor and alcohol remains unclear. As method, route, and dosage can contribute to variations in alcohol consumption, a clinically-relevant method of nicotine delivery is important so results can be better translated to human co-consumption.
We examined the effects of electronic nicotine delivery and nicotine abstinence on voluntary ethanol drinking in female and male C57BL/6J mice. After baseline drinking, mice received electronic nicotine vapor exposure for 4 weeks while voluntary alcohol consumption was monitored followed by a 2-week nicotine abstinence period. We also examined the effects of alcohol and nicotine on locomotion, thermoregulation, and nicotine metabolite levels to determine the effects of co-use on behavior and nicotine metabolism.
2. MATERIALS & METHODS
2.1. Animals
Adult (>P60) male and female C57BL/6J mice (n=12/sex) (Jackson Laboratory) were used for all experiments. Mice were single-housed in a temperature- and humidity-controlled environment with 12/12h light/dark cycle (7AM/7PM lights on/off) with ad libitum access to food and water, except during vapor exposure. All experimental procedures were approved by the Institutional Animal Care and Use Committee.
2.2. Drugs
Ethanol (19% v/v) was prepared from 95% ethanol (Pharmaco Aaper) diluted in tap or bottled water. (−)- nicotine free base (Sigma Aldrich) was diluted to a 3% nicotine concentration in 1:1 propylene glycol (Sigma Aldrich) and vegetable glycerol (Fisher).
2.3. Voluntary Ethanol (EtOH) Drinking
Following one week acclimation to single-housing, enrichment removal, and bottle drinking, mice underwent intermittent access two bottle choice (IA2BC) drinking as previously described (Agoglia et al., 2022). Drinking tubes containing 19% (v/v) unsweetened EtOH or water were left on for 18h on Monday, Wednesday, and Friday, with 2 water drinking tubes available between drinking sessions. Drinking tubes were alternated to avoid side preference. Drinking tubes in empty cages controlled for spillage during measurements.
2.4. Electronic Nicotine Vapor Delivery
One hour prior to vapor sessions, mice were grouped-housed and transferred to vapor chambers (LJARI Inc). Mice received vaporized delivery of 30 mg/ml (3%) (−)-nicotine free base diluted in a 1:1 (v/v) PG /VG solution or a 1:1 PG/VG control solution as previously described (Zhu et al., 2021). E-vape tanks (Baby Beast Brother, Smok) were attached to vapor generators (95 W, Model SVS200). Vapor controllers (Model SSV-1) determined duration and frequency of vapor delivery to air-tighy vacuum-controlled chambers. Sessions consisted of 3s vapor deliveries with 10min intervals between vapor deliveries (chamber clearance ~1 minute) for a total of 18 deliveries. Vapor sessions began between 12 and 1PM and lasted 3h with lights off. Once sessions ended animals were single-housed and returned to housing facility. Vapor sessions were carried out daily M-F.
2.5. Body Temperature
Core body temperatures were measured immediately following vapor on day 1 and 5 using a digital thermometer (Stoelting Co) with a mouse rectal probe (Braintree Scientific).
2.6. Open Field Study
Mice were placed in a square plexiglass chamber (27.9 cm2; ENV-510, Med Associates) with two sets of 16 pulse-modulated infrared photobeams (located on opposite walls) recording X-Y ambulatory movements. Distance traveled, velocity, and time spent in the center were recorded for a period of 10min.
2.7. Serum Analysis
Trunk blood was collected immediately following a final vaping session at the end of the experiment. For acute exposure mice after a single vapor session, for chronic EtOH drinking mice, following 4 weeks of vapor exposure and 2 weeks of abstinence. Blood was collected via rapid decapitation without anesthesia. Nicotine, cotinine, and hydroxycotinine levels were analyzed through liquid chromatography-tandem mass spectrometry (LC-MS/MS) as in previous studies (Ghosh et al., 2019; Zhu et al., 2021).
2.8. Statistical Analysis
Statistical analyses were conducted (Prism 9.0, Graph Pad) with threshold for significance set at p<0.05. Female and male cohorts were run separately over close but different time periods (1 month between cohorts), precluding the ability to directly compare males and females and explicitly examine sex differences. Serum analysis was processed and analyzed by mass spectrometry at the same time, so the levels of nicotine metabolites could be directly compared. Drinking data were analyzed using mixed 2-way ANOVA (factors: nicotine, time). Locomotor activity was analyzed by unpaired Student’s t tests (total distance traveled, time in center) or mixed 2-way ANOVAs (distance traveled; Factors: nicotine, time). Metabolite levels were analyzed by an unpaired Student’s t test and compared to drinking levels using Pearson’s correlation. Body temperature was analyzed with a mixed 2-way ANOVA (factors: nicotine, time). When applicable, Bonferroni’s test was used for post hoc analysis comparisons. Unless otherwise noted, all data are presented as mean ± standard error. All statistical tests performed are listed in supplemental materials.
3. RESULTS
3.1. Baseline EtOH and H2O drinking
Male and female mice were allowed five sessions of baseline drinking before vaping initiation (Fig 1A). Females began drinking EtOH at higher levels than males on session 1 and their consumption remained stable on sessions 3 and 5 (Fig 1B). Males began drinking at lower levels than females, however their intake increased through drinking sessions (Fig 1C; Day: F (2.782, 27.82) = 43.79, p<0.0001). By the fifth day of drinking both females and males drank ~20 g/kg of EtOH over 18 hours. Throughout the baseline drinking period females maintained relatively steady H2O intake (Fig 1D; Day: F (4, 40) = 2.590, p=0.0511). Males maintained steady H2O intake with some decreases over time (Fig 1E; Day: F (2.229, 22.29) = 4.537; p=0.0193).
Figure 1. Baseline EtOH and H2O intake in female and male mice prior to vape.
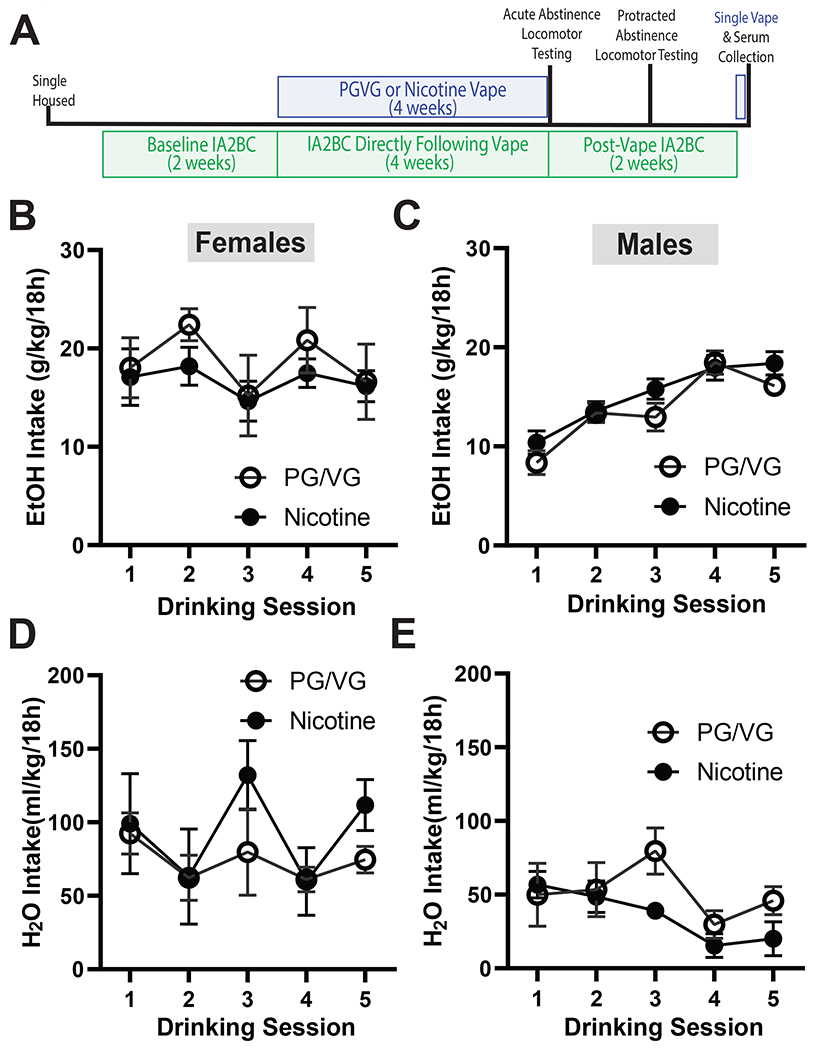
(A) Experimental timeline of intermittent access two bottle choice (IA2BC) and electronic nicotine vaping (vape) followed by locomotor testing. There were no baseline differences in EtOH intake in female mice (B) or male (C) mice. Similarly, there were no baseline differences in H2O intake in female mice (D) or male (E) mice.
Data are expressed as mean ± SEM (B-E). Data were analyzed by a 2-way ANOVAs in each sex. N=5-6 per group
3.2. Immediate effects of vaping on voluntary EtOH and H2O drinking
Passive vaping exposure was performed using commercially available chambers (La Jolla Alcohol Research) (Fig 2A), as previously described (Zhu et al., 2021). Following each session, alcohol bottles were replaced onto home cages two hours before the dark cycle and then removed to measure drinking levels 4 hours into the light cycle (Fig 2B).
Figure 2. EtOH and H2O intake directly following vape in female and male mice.
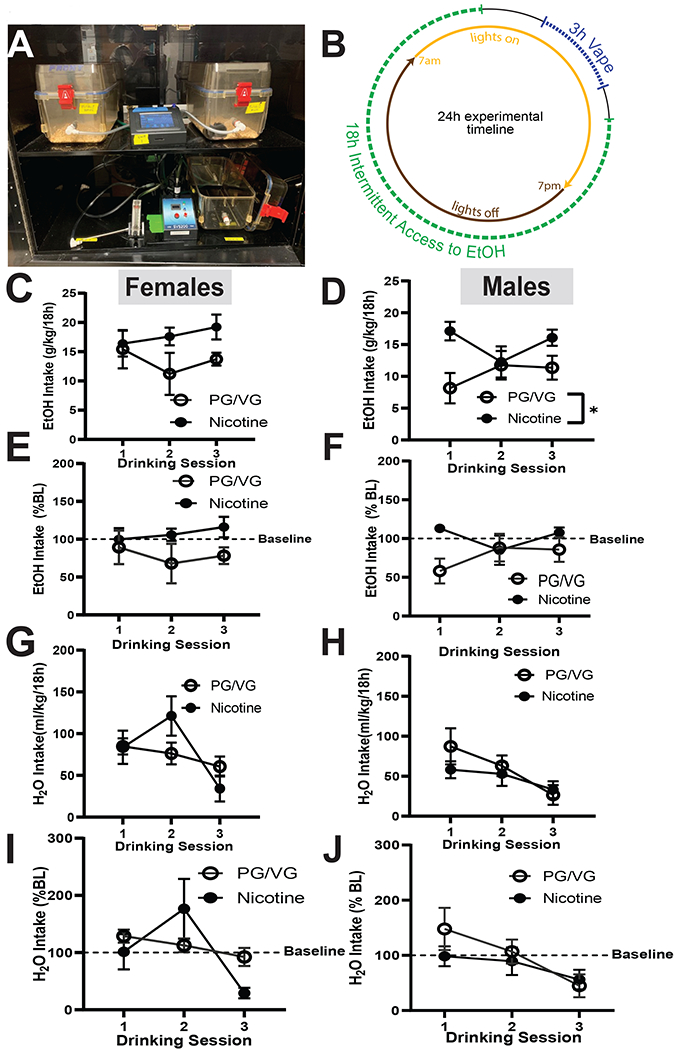
(A) Electronic vapor delivery system. (B) 24hr experimental timeline of daily vapor exposure and alcohol drinking. No significant differences in EtOH consumption in the PG/VG and nicotine groups were observed immediately following vapor exposure in females (C). In males (D), nicotine group drank significantly more than the PG/VG mice (p< 0.05). When compared to baseline drinking, there were no differences in either female (E) or male (F) mice. No significant differences were observed in H2O intake in between the PG/VG and nicotine groups in both sexes, regardless of whether we examined raw intake (G,H) or normalized it to baseline (I,J).
Data are expressed as mean ± SEM (C-J). Data were analyzed by a 2-way ANOVAs in each sex. N=5-6 per group. * p<0.05
Following the first vapor inhalation session, both Nicotine and PG/VG females showed no difference in alcohol intake. On sessions 2 and 3 of drinking, however, PG/VG mice consumed less EtOH while nicotine mice consumed more EtOH (Fig 2C,). Following the first vape session, the male PG/VG mice drank less EtOH than the Nicotine group. On sessions 2 and 3 there was a slight increase in EtOH drinking in the male PG/VG group, but intake was still significantly less than the nicotine group (Fig 2D, Nicotine: F (1, 10) =13.94, p=0.0039).
When drinking levels were normalized to baseline, EtOH intake in the female Nicotine group remained similar to the baseline levels and the PG/VG group drinking decreased over sessions 2 and 3. (Fig 2E). When male drinking levels were normalized to baseline, the decrease in the PGVG group on session 1 remained, however drinking levels were not significantly different between groups. (Fig 2F).
In females, a significant effect of day was observed, but no effect of nicotine or an interaction (Fig 2G, Day: F (1. 712, 15.41) =3.950, p=0.0465). In males, both groups drank similar levels of H2O immediately following vaping, and both groups consumed less H2O on Day 3 (Fig 2H, Day: F (1.757, 17.57) =3.568, p=0.0550). When pre-nicotine H2O intake was normalized to baseline, female Nicotine mice drank more H2O than PG/VG mice on session 2, and drinking levels decreased on session 3, but no differences reached significance (2I). The male cohort steadily decreased their H2O intake through the first week of vaping with no differences between Nicotine and PG/VG groups (Fig 2J). Together these data suggest that immediate exposure to nicotine vapor influences alcohol drinking, but only in male mice.
3.3. Effects of nicotine vaping on body temperature
We measured core body temperature in female and male mice immediately following the first day of vapor and again after 5 vapor sessions. In females, nicotine-exposed mice had significantly higher core body temperatures at both day 1 and 5 of vaping compared to the PG/VG group. (Fig 3A, Day x Nicotine: F (1, 9) = 22.02, p= 0.0011; Day: F(1, 9)= 38.74, p=0.0002; Nicotine: F(1, 9)= 162.3, p< 0.0001). The male Nicotine group also had a significantly higher core body temperatures as compared to the PG/VG group, with that difference increasing after 5 vaping sessions (Fig 3B, Day x Nicotine: F(1, 10)= 29.01, p= 0.0003; Day: F(1,10)= 31.56, p= 0.0002; Nicotine: F(10,10)= 152.4, p<0.0001). These results suggest that nicotine vapor exposure and voluntary drinking disrupt thermoregulation in a non-sex specific manner.
Figure 3. Effects of nicotine on body temperature in female and male mice.
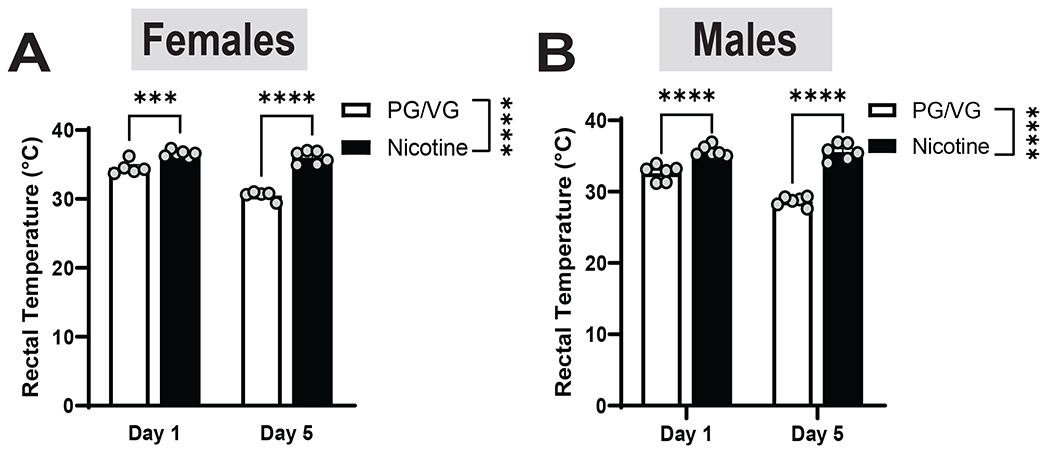
Nicotine increased body temperature in both females (A; p< 0.0001) and males (B; p< 0.0001) at both time points.
Data are expressed as mean ± SEM. Data were analyzed by a 2-way ANOVAs in each sex. N=5-6 per group. *** p<0.001, **** p<0.00001.
3.4. Prolonged effects of vaping on voluntary EtOH and H2O drinking
We next measured drinking levels in female and male mice following 4 weeks of vapor exposure. In female and male mice, drinking remained stable during weeks 2 and 3. Both the female Nicotine and PG/VG groups consumed similar levels of EtOH on week 4, however there was a significant main effect of time in the nicotine group (Fig 4A, Day: F(1.526, 13.73)=9.809, p= 0.00037). Similar results were observed in the males, however, when comparing drinking levels immediately following vapor exposure, the difference between Nicotine and PG/VG drinking levels disappeared (Fig 4B; Nicotine: F(1, 10)=1.211, p=0.2968). These results suggest that immediate effects of nicotine on EtOH drinking dissipate after a prolonged period. We also compared EtOH drinking levels with previously-established baselines and saw that in the females there was a main effect of day and a significant difference between the Nicotine and PG/VG group with the PG/VG group drinking less overall (Fig 4C; Day: F(1.451, 13.06)= 8.351, p= 0.0076; Nicotine: F(1, 9)= 7.520, p= 0.0228). In the males we saw a significant difference between the Nicotine and PG/VG groups likely driven by lower drinking in the PG/VG group on Day 1 (Fig 4D; Nicotine: F(1, 10)= 7.713, p= 0.0195). While overall EtOH drinking remained relatively stable in both sexes, nicotine-exposed mice maintained higher EtOH intake than PG/VG mice.
Figure 4. EtOH and H2O intake directly following 4 weeks of vape and drinking in female and male mice.
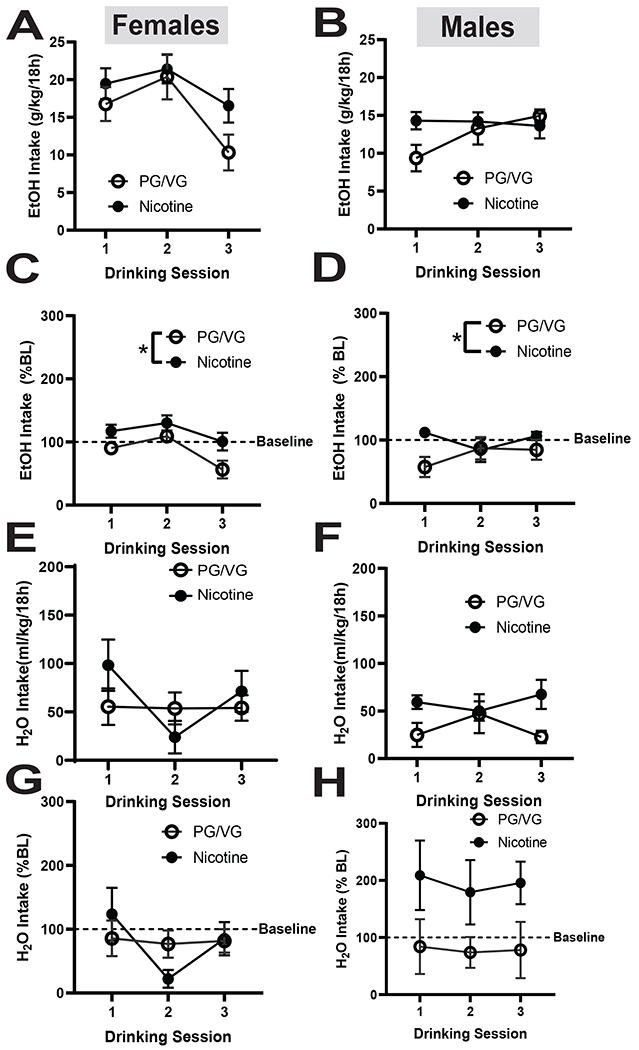
(A) In females, the nicotine group drank more than the PG/VG mice, but no statistical significance was found. In males there was no statistical difference in between the nicotine and the PG/VG groups (B). When compared to baseline drinking, there was a difference in female (C) and male (D) mice (p< 0.05). No significant differences were observed in H2O intake in between the PG/VG and nicotine groups in both sexes, regardless of whether we examined raw intake (E,F) or normalized it to baseline (G,H).
Data are expressed as mean ± SEM (A-H). Data were analyzed by a 2-way ANOVAs in each sex. N=5-6 per group. * p<0.05
We also looked at the H2O intake following prolonged vapor exposure and noted that in females, the Nicotine group exhibited fluctuations in drinking levels while the PG/VG group maintained stable intake. There was a significant main effect of time and an interaction between time and treatment, but no significant differences between groups (Fig 4E; Day x Nicotine: F(2, 18)= 4.4345, p= 0.0289; Day: F(1.267, 11.40)= 5.777, p= 0.0284; Nicotine: F(1, 9)= 0.3647, p= 0.5608). In males, both Nicotine and PG/VG groups maintained stable H2O intake, with no differences between groups (Fig 4F). When compared to baseline H2O drinking, females from both Nicotine and PG/VG groups drank less than original baseline levels (Fig 4G). In males, we saw increased H2O intake in the Nicotine group, but the PG/VG group continued to drink below the baseline. There were no significant interactions between vapor exposure and drinking levels (Fig 4H). While overall H2O drinking remained relatively stable in both sexes, nicotine-exposed mice maintained higher H2O intake than PG/VG mice.
3.5. Impact of nicotine vaping on locomotor activity
We next determine the effects of nicotine and alcohol on locomotion following 4 weeks of chronic vape exposure and EtOH drinking. In females, the Nicotine group travelled greater distances than the PG/VG group (Fig 5A, t= 6.393, df= 10, p<0.0001 by unpaired t-test) as did males, (Fig 5B, t= 10.75, df = 10, p<0.0001). In the female cohort, we saw main effects of time and treatment, where the nicotine group consistently travelled more than the PG/VG group, however, we observed a steady decrease in movement from both groups, consistent with habituation to the testing chamber (Fig 5C; Time: F (4.366, 43.66) =14.62, p< 0.0001; Nicotine: F(1, 10)= 42.94 , p< 0.0001). In males, we also observed significant main effects of time and treatment but no interaction (Fig 5D, Time: F (4.046, 40.46) = 8.149, p< 0.0001; Nicotine: F(1, 10)= 115.6 , p< 0.0001). Male mice also decreased distance travelled over time, however, Nicotine males displayed less habituation compared to PG/VG males or nicotine females. We measured time spent in the center as a proxy for anxiety-like behavior. In the female cohort, we saw no significant difference in center time between Nicotine and PG/VG groups (Fig 5E). In the male cohort the nicotine group spent significantly greater center time as compared to the PG/VG group (Fig 5F, t= 5.982, df= 10, p= 0.0001). These results suggest that nicotine increases locomotion in both female and male mice and has anxiolytic-like effects in males.
Figure 5. Locomotor activity directly following the last vape session in female and male mice.
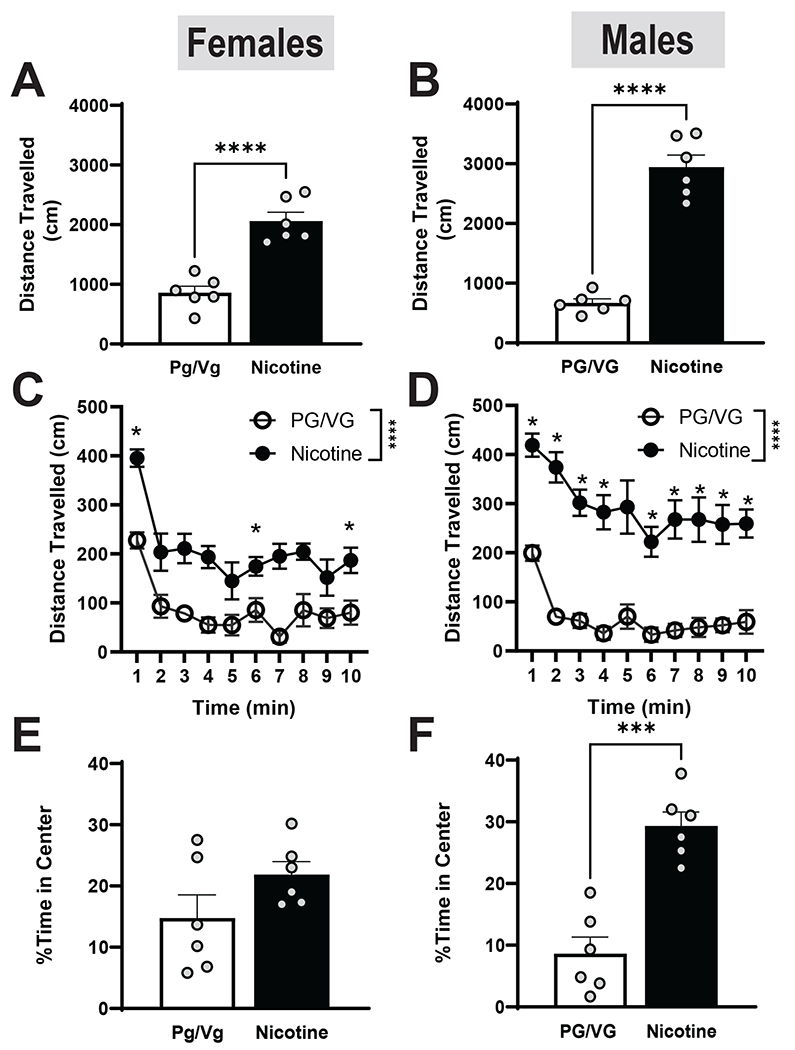
Compared to the PG/VG group, the nicotine group travelled significantly faster in both female (A; p< 0.0001) and male (B; p< 0.0001) mice. When separated into 1 min time bins, the nicotine group traveled significantly more distance in both females (C; p<0.0001) and males (D; p<0.0001). In females (E), there was no effect of nicotine on time spent in the center. However, in males (F), the nicotine group spent significantly more time in the center of the arena (p<0.001).
Data are expressed as mean ± SEM. Panels A-B and E-F utilized an unpaired a t test, whereas panels C-D were analyzed with a 2-way ANOVA. N=5-6 per group. *p< 0.05, *** p< 0.001, **** p< 0.0001
3.6. Nicotine metabolite levels after acute vapor exposure and no EtOH drinking
To examine differences in nicotine metabolism following a single Nicotine vapor exposure session we collected serum from female and male mice. Nicotine is metabolized into cotinine which is broken down into hydroxycotinine. We measured all three nicotine metabolites to obtain a better understanding of metabolic breakdown. Females and males exhibited similar serum nicotine levels (Fig 6A, t= 0.2824, df= 6, p= 0.7871), but males had significantly higher serum cotinine levels (6B, t= 6.741, df= 6, p= 0.0005). No differences were observed in serum hydroxycotinine levels in between females and males (6C, t=0.6894, df= 6, p= 0.4181).
Figure 6. Nicotine metabolite levels following a vape session.
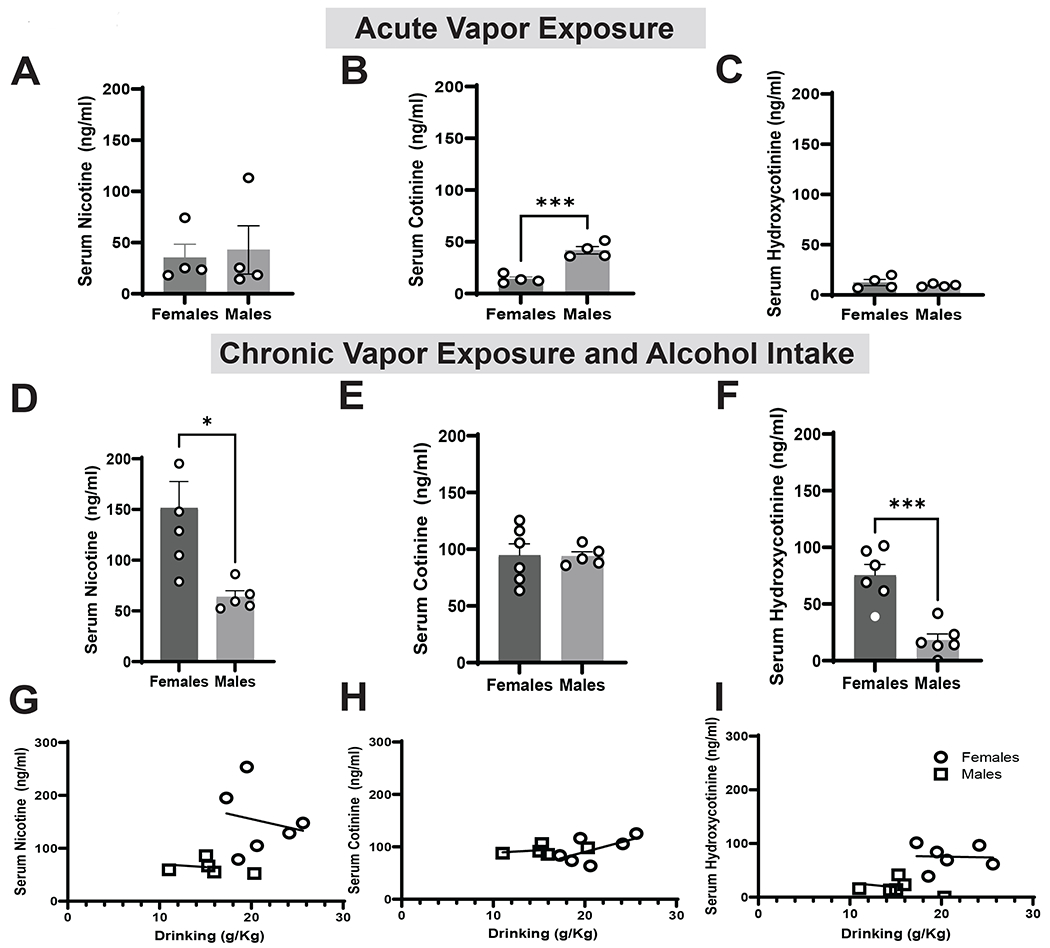
(A) There were no differences in serum nicotine levels in female and male mice following a single vapor exposure session. (B) Compared to females, males had significantly higher serum cotinine levels (p<0.0005). (C) There were no differences in serum Hydroxycotinine levels in both female and male mice. (D) Compared to females, males showed significantly lower serum nicotine levels (p<0.05). (E) There were no differences in serum cotinine levels in female and male mice. (F) Compared to females, males showed significantly lower serum hydroxycotenine levels (p<0.001). There were no significant associations in between EtOH intake and nicotine metabolite levels (G-I)
Data are expressed as mean ± SEM (A-F) or individual data points with lines of best fit (D-F). Panels A-F utilized an unpaired t test, whereas panels G-I utilized Pearson’s correlation. N=5-6 per group. * p<0.05, *** p<0.001.
3.7. Nicotine metabolite levels after vapor exposure and chronic voluntary EtOH drinking
To examine differences in nicotine metabolism following chronic EtOH drinking we collected serum from male and female mice following a final vape session. Females had significantly higher serum nicotine levels than males (Fig 6D, t= 2.992, df= 9, p=0.0152). Females and males exhibited similar serum cotinine levels (Fig 6E, t= 0.06517, df= 9, p=0.9495), but females had significantly higher serum hydroxycotinine than males (Fig 6F, t= 5.150, df= 10, p=0.0004).
To further examine the impact of alcohol on nicotine metabolism, we correlated serum metabolite levels with drinking. There was no correlation between nicotine serum levels and alcohol intake during the previous two weeks (Fig 6G, females: r(9)=0.212, p=0.7022; males: r(10)= −0.2772, p= 0.6516), no correlation between serum cotinine and alcohol intake (Fig 6H) or between serum hydroxycotinine levels and alcohol intake (Fig 6I).
3.8. Effects of vaping abstinence on voluntary EtOH and H2O drinking
We next measured EtOH and H2O intake during two weeks of abstinence from nicotine vapor. During abstinence, Nicotine females continued to drink similar amounts of EtOH as in weeks 2 and 4 of vaping, however the PG/VG group increased drinking to levels similar to the Nicotine group with no significant difference between groups (Fig 7A) In males, the PG/VG group drank more than the Nicotine group during abstinence, but the difference was not significant (Fig 7B). We also examined H2O intake during abstinence from nicotine vapor. During the 2 weeks of abstinence, the female PG/VG group drank more H2O than the Nicotine group, but no significant difference was observed between groups (Fig 7C). In males, H2O intake remained stable with both groups drinking a similar amount. While an initial 2-way ANOVA test revealed significant interactions, further statistical testing showed only an effect on day 3, possibly due to locomotor testing (Fig 7D, Day x Nicotine: F (4,40) = 2.795, p= 0.0388; Day: F (2.583, 25.83) = 3.179, p= 0.0471). Overall, abstinence from nicotine vapor exposure did not significantly alter alcohol or water drinking behavior.
Figure 7. EtOH intake in female and male mice during two weeks of vape abstinence.
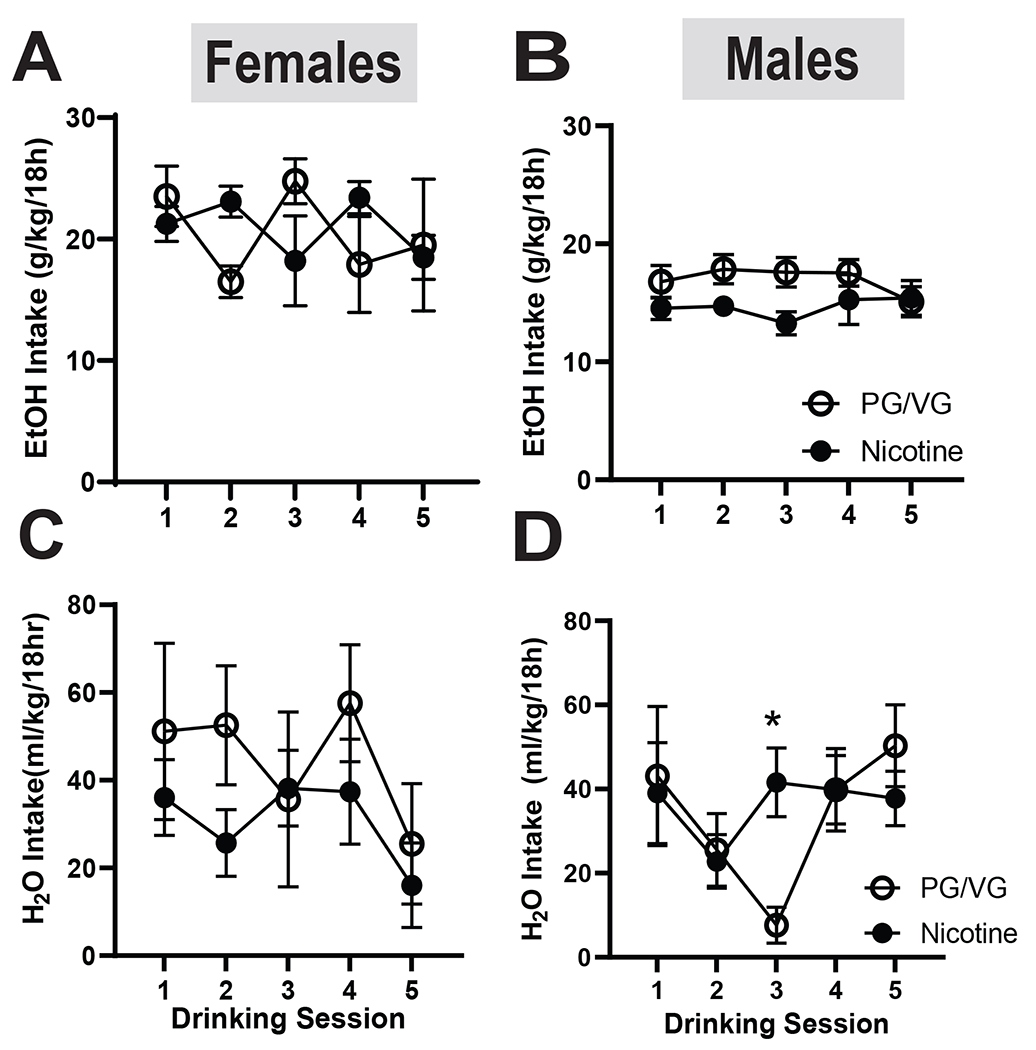
(A) In females, the nicotine group drank significantly less EtOH on day 3 compared to the PG/VG group (p<0.05) (B) In males, there was no effect of nicotine abstinence on EtOH intake. (C) In females, there was no effect of nicotine abstinence on H2O intake. (D) In males, the nicotine group drank significantly more H2O on day 3 compared to the PG/VG group (p<0.05).
Data are expressed as mean ± SEM. Data were analyzed by a 2-way ANOVAs in each sex. N=5-6 per group. * p<0.05
3.9. Impact of nicotine vaping abstinence on locomotor activity
To determine the effects of nicotine abstinence and alcohol consumption on activity, we measured locomotion in mice during abstinence from vapor exposure. Following one week of abstinence, the previously-observed difference in distance travelled between female PG/VG and the Nicotine groups disappeared (Fig 8A, t= 0.8100, df= 10, =0.4368). In males, the Nicotine group continued to move significantly more than the PG/VG group (Fig 8B, t=2.445, df= 10, p=0.0345), although the difference was of a lesser magnitude than previously observed. A 2-way ANOVA revealed a main effect of Time but no interaction between Time and treatment in female mice (Fig 8C, Time: F (1.296, 12.96) = 10.37, p=0.0044). Similar results were observed in male mice where there was a main effect of Time but no effect of treatment (Fig 8D, Time: F (2.973, 29.73) = 18.49, p< 0.0001). We also analyzed the time spent in the center of the arena as a proxy for anxiety-like behavior. In females we continued to observe no difference in center time between Nicotine and PG/VG groups (Fig 8E, t= 0.4195, df= 10, =0.6837), However, in males, the Nicotine group continued to spend significantly more time in the center than the PG/VG group (Fig 8F, t= 2.507, df= 10, =0.0311), suggesting a prolonged anxiolytic effect. Overall, these results suggest that nicotine has a prolonged effect on locomotion in male but not female mice, and persistent anxiolytic effects in males.
Figure 8. Locomotor activity following one week of vape abstinence.
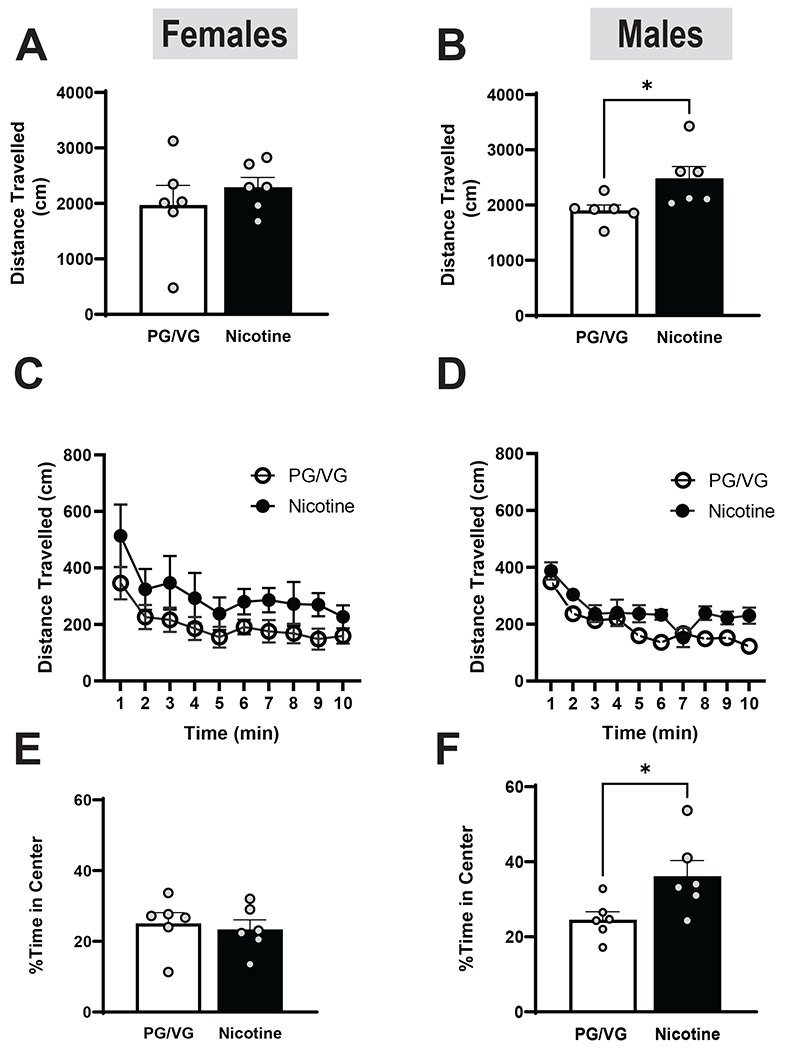
In females (A) there was no significant difference in velocity in between the PG/VG and nicotine groups. However, in males the nicotine group traveled significantly faster than the PG/VG group (B; p<0.05). No significant differences were found in distance travelled in both female (C) and male (D) mice when separated into 1 min time bins. In females (E), there was no effect of nicotine on time spent in the center. However, in males (F), the nicotine group spent significantly more time in the center of the arena (p<0.05).
Data are expressed as mean ± SEM. Panels A-B and E-F utilized an unpaired a t test, whereas panels C-D were analyzed with a 2-way ANOVA. N=5-6 per group. *p< 0.05.
4. Discussion
Nicotine and alcohol use remain some of the leading causes of preventable death (Frie et al., 2021) and the effects of co-use are not fully understood. Thus, we investigated electronic nicotine delivery and voluntary alcohol consumption in female and male mice. In the first week of vaping alcohol consumption was increased in male mice but not in female mice. We also observed differences in core body temperature between nicotine and PG/VG groups in both male and female mice. Immediately following the final vapor session after chronic exposure, both male and female nicotine mice had significantly increased locomotor activity and male mice displayed decreased anxiety-like behavior. After 1 week of nicotine abstinence, locomotor activity in male nicotine mice was elevated compared to PG/VG controls and anxiolytic effects persisted, with no significant differences in female mice. Collectively, these results suggest that nicotine vapor exposure increases locomotor activity in ethanol-drinking males and females, but only increases voluntary ethanol drinking in males. We also found sex differences in nicotine metabolism, with females showing impairments potentially related to alcohol drinking. Altogether these results suggest that while both male and female ethanol-drinking mice experience the stimulatory effects of nicotine vapor inhalation, only males display parallel increases in ethanol drinking. This is consistent with clinical literature suggesting that e-cigarette users engage in more problematic alcohol use behavior than non-e-cigarette users (Hershberger et al., 2016; Rothrock et al., 2020). Another study reported that human male adolescents consumed more nicotine than females, but both groups engaged in problematic alcohol use behavior (Cannizzaro et al., 2022). Collectively this work suggests that males could be more susceptible to the effects of alcohol and nicotine co-use and thus are more prone to increased use of either substance.
Consistent with previous reports (Ruffolo et al., 2021) female mice consumed significantly more alcohol as compared to males and female drinking was not altered by vapor exposure (Nicotine or PG/VG). Females maintained higher EtOH consumption than males, however there was no difference in overall alcohol intake between PG/VG and nicotine groups. In males an immediate increase in EtOH consumption in the nicotine group was observed within the first week of vapor inhalation, however as vapor inhalation sessions progressed that difference disappeared. These findings are consistent with previous research indicating that nicotine does not influence EtOH intake (DeBaker, Robinson, et al., 2020). While the previous studies consisted of nicotine dissolved in water, our findings also suggest that chronic ENDS also do not affect long-term alcohol drinking in mice.
Current studies employ various forms of nicotine administration including subcutaneous delivery or ingestion (Caldarone et al., 2008; Kasten et al., 2016; LeSage et al., 2002). However, these nicotine administration paradigms are incongruent with human electronic nicotine vapor usage. We used a passive form of nicotine vapor delivery with a vapor exposure paradigm designed to mimic human vapor intake. Although this method has good face validity, there are some limitations. Notably, the vapor administration is passive, so a volitional component is lacking and there is no ability to measure preference or motivational aspects of nicotine vapor exposure. As previously mentioned, current studies administer nicotine through drinking water or via injections (Caldarone et al., 2008; Kasten et al., 2016; LeSage et al., 2002). While these methods allow for precise experimental concentrations of nicotine, they introduce additional confounds like injection stress or issues of taste preference when paired with alcohol drinking.
Previous studies in alcohol-drinking humans have found that elevated nicotine metabolite ratios (NMR) led to males breaking down nicotine faster than females, who exhibited no changes in NMR.As well as continuous use of nicotine following alcohol use recovery increases likelihood of relapse (Dermody et al., 2020). As nicotine metabolism and pharmacokinetics are dose- and exposure-dependent, the absence of a history of alcohol exposure could yield different serum nicotine metabolite levels than mice with a history of alcohol exposure. We analyzed nicotine metabolite levels in non-drinking mice following a single vape session. There were no differences in serum nicotine or hydroxycotinine levels, however males had higher cotinine levels. In the alcohol-drinking mice and chronic vapor-exposed mice, females showed higher nicotine and hydroxycotinine levels than the males, suggesting that alcohol may affect nicotine metabolism but only in female mice. Other papers have found that following 0.05% vapor exposure, male rats showed higher cotinine levels than females following chronic vapor exposure. While this paper was done at a lower concentration and in a rat model, the blood serum results are concurrent with our acute serum levels which showed that males have higher cotinine levels than females following a single vapor exposure. Additional studies are required to determine the effects of alcohol on nicotine metabolism.
Both nicotine and alcohol have been shown to produce dose- and exposure-dependent effects on thermoregulation and activity, however the effects of nicotine vaping in a drinking population have not been examined. We previously reported that acute and repeated exposure to a higher concentration of nicotine vapor (12%) produced hypothermic effects in mice as compared to PG/VG counterparts. Reductions in body temperature occurred in parallel with reduced locomotor activity following acute exposure and increased locomotor activity after repeated (5 day) exposure (Zhu et al., 2021). Another study found that vapor exposure using nicotine tartrate salt produced dose-dependent reductions in body temperature and increased locomotor activity in rats (Javadi-Paydar et al., 2019) This study, using a lower concentration of nicotine freebase vapor (3%), found that alcohol-drinking and nicotine vapor-exposed mice had higher core temperatures and increased locomotor activity as compared to the PG/VG controls. This is consistent with previous rat and mouse studies that report increased locomotor activity following EtOH and nicotine administration (Cross & Leslie, 2021; Gulick & Gould, 2009). These findings are in contrast with our previous work (Zhu et al., 2021) however, the nicotine concentrations and exposure parameters used were different. Our thermoregulatory findings are also in contrast to earlier work in rats reporting decreases in body temperature, potentially due to differences in species or exposure paradigm, however locomotor effects are consistent (Javadi-Paydar et al., 2019), As changes in nicotine metabolism can influence behavioral effects, this paper, in conjunction with previous studies highlights the importance of considering pharmacokinetics in behavioral outcome following nicotine exposure.
The increased activity following chronic alcohol drinking and nicotine vapor exposure was seen in both males and females but was only observed in males after a period of abstinence from nicotine vapor exposure. In addition, male nicotine-exposed mice also displayed an anxiolytic effect, spending more time in the center of the arena as compared to females or PG/VG controls. This behavior is consistent with previous studies that reported anxiolytic effects of nicotine (Honeycutt et al., 2020; McGranahan et al., 2011; Torres et al., 2013). These results suggest that alcohol and nicotine co-use disrupt thermoregulation, increase locomotion, and enhance anxiolytic properties but in a sex-specific manner.
5. Conclusions
The results of this study provide important information on how nicotine and alcohol interactions may alter physiological processes and behavior in a sex-specific manner. These findings have implications for the consequences of alcohol and nicotine co-use in a real-world context. Further study of these interactions is needed to fully understand how co-use affects the growing population of adults that often drink and vape, and if said interactions play any role in reinforcement of substance seeking behavior.
Supplementary Material
Highlights:
Acute nicotine vaping increases short-term alcohol drinking in male mice
Chronic nicotine vaping does not affect long-term drinking in male and female mice
Nicotine vapor promotes locomotion in males and females and is anxiolytic in males
Female mice displayed higher levels of serum nicotine and hydroxycotinine
Nicotine vapor abstinence does not impact drinking or locomotor effects in males
Funding:
P30-ES10126 (CRE), P01-HL-108808 (CRE), R01-AA028782 (CWH), P30-DK-065988 (CRE), R01-AA-026858 (MAH), P60-AA-011605 (MAH), T32-AA-007573 (SGQ), and F32-AA-030493 (SGQ).
Footnotes
Conflict of interest: No conflicts were reported (double check with other authors)
References
- Agoglia AE, Zhu M, Quadir SG, Bluitt MN, Douglass E, Hanback T, … Herman MA (2022). Sex-specific plasticity in CRF regulation of inhibitory control in central amygdala CRF1 neurons after chronic voluntary alcohol drinking. Addict Biol, 27(1), e13067. doi: 10.1111/adb.13067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Hukkanen J, & Jacob P (2009). Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol (192), 29–60. doi: 10.1007/978-3-540-69248-5_2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldarone BJ, King SL, & Picciotto MR (2008). Sex differences in anxiety-like behavior and locomotor activity following chronic nicotine exposure in mice. Neurosci Lett, 439(2), 187–191. doi: 10.1016/j.neulet.2008.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannizzaro E, Lavanco G, Castelli V, Cirrincione L, Di Majo D, Martines F, … Plescia F (2022). Alcohol and Nicotine Use among Adolescents: An Observational Study in a Sicilian Cohort of High School Students. Int J Environ Res Public Health, 19(10). doi: 10.3390/ijerph19106152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffee BW, Couch ET, & Gansky SA (2017). Trends in characteristics and multi-product use among adolescents who use electronic cigarettes, United States 2011-2015. PLoS One, 12(5), e0177073. doi: 10.1371/journal.pone.0177073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross SJ, & Leslie FM (2021). Combined nicotine and ethanol age-dependently alter neural and behavioral responses in male rats. Behav Pharmacol, 32(4), 321–334. doi: 10.1097/FBP.0000000000000622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani JA, & Balfour DJ (2011). Historical and current perspective on tobacco use and nicotine addiction. Trends Neurosci, 34(7), 383–392. doi: 10.1016/j.tins.2011.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBaker MC, Moen JK, Robinson JM, Wickman K, & Lee AM (2020). Unequal interactions between alcohol and nicotine co-consumption: suppression and enhancement of concurrent drug intake. Psychopharmacology (Berl), 237(4), 967–978. doi: 10.1007/s00213-019-05426-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBaker MC, Robinson JM, Moen JK, Wickman K, & Lee AM (2020). Differential patterns of alcohol and nicotine intake: Combined alcohol and nicotine binge consumption behaviors in mice. Alcohol, 85, 57–64. doi: 10.1016/j.alcohol.2019.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dermody SS, Hendershot CS, Andrade AK, Novalen M, & Tyndale RF (2020). Changes in Nicotine Metabolite Ratio Among Daily Smokers Receiving Treatment for Alcohol Use Disorder. Nicotine Tob Res, 22(2), 256–263. doi: 10.1093/ntr/nty265 [DOI] [PubMed] [Google Scholar]
- Frie JA, Nolan CJ, Murray JE, & Khokhar JY (2021). Addiction-Related Outcomes of Nicotine and Alcohol Co-use: New Insights Following the Rise in Vaping. Nicotine Tob Res. doi: 10.1093/ntr/ntab231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett PI, Honeycutt SC, Marston C, Allen N, Barraza AG, Dewey M, … Hillhouse TM (2021). Nicotine-free vapor inhalation produces behavioral disruptions and anxiety-like behaviors in mice: Effects of puff duration, session length, sex, and flavor. Pharmacol Biochem Behav, 206, 173207. doi: 10.1016/j.pbb.2021.173207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Coakley RD, Ghio AJ, Muhlebach MS, Esther CR Jr., Alexis NE, & Tarran R (2019). Chronic E-Cigarette Use Increases Neutrophil Elastase and Matrix Metalloprotease Levels in the Lung. Am J Respir Crit Care Med, 200(11), 1392–1401. doi: 10.1164/rccm.201903-0615OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulick D, & Gould TJ (2009). Interactive effects of ethanol and nicotine on learning, anxiety, and locomotion in C57BL/6 mice in the plus-maze discriminative avoidance task. Neuropharmacology, 57(3), 302–310. doi: 10.1016/j.neuropharm.2009.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson BJ, & Cooper SY (2021). Nicotine formulations impact reinforcement-related behaviors in a mouse model of vapor self-administration. Drug Alcohol Depend, 224, 108732. doi: 10.1016/j.drugalcdep.2021.108732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershberger AR, Karyadi KA, VanderVeen JD, & Cyders MA (2016). Combined expectancies of alcohol and e-cigarette use relate to higher alcohol use. Addict Behav, 52, 13–21. doi: 10.1016/j.addbeh.2015.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honeycutt SC, Garrett PI, Barraza AG, Maloy AN, & Hillhouse TM (2020). Repeated nicotine vapor inhalation induces behavioral sensitization in male and female C57BL/6 mice. Behav Pharmacol, 31(6), 583–590. doi: 10.1097/FBP.0000000000000562 [DOI] [PubMed] [Google Scholar]
- Javadi-Paydar M, Kerr TM, Harvey EL, Cole M, & Taffe MA (2019). Effects of nicotine and THC vapor inhalation administered by an electronic nicotine delivery system (ENDS) in male rats. Drug Alcohol Depend, 198, 54–62. doi: 10.1016/j.drugalcdep.2019.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jebai R, Osibogun O, Li W, Gautam P, Bursac Z, Ward KD, & Maziak W (2022). Temporal Trends in Tobacco Product Use Among US Middle and High School Students: National Youth Tobacco Survey, 2011-2020. Public Health Rep, 333549221103812. doi: 10.1177/00333549221103812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasten CR, Frazee AM, & Boehm SL (2016). Developing a model of limited-access nicotine consumption in C57Bl/6J mice. Pharmacol Biochem Behav, 148, 28–37. doi: 10.1016/j.pbb.2016.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn CS, Tsoh JY, & Weisner CM (2003). Changes in smoking status among substance abusers: baseline characteristics and abstinence from alcohol and drugs at 12-month follow-up. Drug Alcohol Depend, 69(1), 61–71. doi: 10.1016/s0376-8716(02)00256-9 [DOI] [PubMed] [Google Scholar]
- Lallai V, Chen YC, Roybal MM, Kotha ER, Fowler JP, Staben A, … Fowler CD (2021). Nicotine e-cigarette vapor inhalation and self-administration in a rodent model: Sex- and nicotine delivery-specific effects on metabolism and behavior. Addict Biol, 26(6), e13024. doi: 10.1111/adb.13024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Corrigall WA, Harding JW, Juzytsch W, & Li TK (2000). Involvement of nicotinic receptors in alcohol self-administration. Alcohol Clin Exp Res, 24(2), 155–163. doi: 10.1111/j.1530-0277.2000.tb04585.x [DOI] [PubMed] [Google Scholar]
- LeSage MG, Keyler DE, Shoeman D, Raphael D, Collins G, & Pentel PR (2002). Continuous nicotine infusion reduces nicotine self-administration in rats with 23-h/day access to nicotine. Pharmacol Biochem Behav, 72(1-2), 279–289. doi: 10.1016/s0091-3057(01)00775-4 [DOI] [PubMed] [Google Scholar]
- McGranahan TM, Patzlaff NE, Grady SR, Heinemann SF, & Booker TK (2011). alpha4beta2 nicotinic acetylcholine receptors on dopaminergic neurons mediate nicotine reward and anxiety relief. J Neurosci, 31(30), 10891–10902. doi: 10.1523/JNEUROSCI.0937-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montanari C, Secci ME, Driskell A, McDonald KO, Schratz CL, & Gilpin NW (2021). Chronic nicotine increases alcohol self-administration in adult male Wistar rats. Psychopharmacology (Berl), 238(1), 201–213. doi: 10.1007/s00213-020-05669-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazzolo DL (2013). Electronic cigarettes and vaping: a new challenge in clinical medicine and public health. A literature review. Front Public Health, 1, 56. doi: 10.3389/fpubh.2013.00056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothrock AN, Andris H, Swetland SB, Chavez V, Isaak S, Pagane M, … Rothrock SG (2020). Association of E-cigarettes with adolescent alcohol use and binge drinking-drunkenness: A systematic review and meta-analysis. Am J Drug Alcohol Abuse, 46(6), 684–698. doi: 10.1080/00952990.2020.1771723 [DOI] [PubMed] [Google Scholar]
- Ruffolo J, Frie JA, Thorpe HHA, Talhat MA, & Khokhar JY (2021). Alcohol and Vaporized Nicotine Co-Exposure During Adolescence Contribute Differentially to Sex-Specific Behavioral Effects in Adulthood. Nicotine Tob Res. doi: 10.1093/ntr/ntab250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres OV, Gentil LG, Natividad LA, Carcoba LM, & O’Dell LE (2013). Behavioral, Biochemical, and Molecular Indices of Stress are Enhanced in Female Versus Male Rats Experiencing Nicotine Withdrawal. Front Psychiatry, 4, 38. doi: 10.3389/fpsyt.2013.00038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TW, Asman K, Gentzke AS, Cullen KA, Holder-Hayes E, Reyes-Guzman C, … King BA (2018). Tobacco Product Use Among Adults - United States, 2017. MMWR Morb Mortal Wkly Rep, 67(44), 1225–1232. doi: 10.15585/mmwr.mm6744a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Echeveste Sanchez M, Douglass EA, Jahad JV, Hanback TD, Guhr Lee TN, … Herman MA (2021). Electronic Nicotine Vapor Exposure Produces Differential Changes in Central Amygdala Neuronal Activity, Thermoregulation and Locomotor Behavior in Male Mice. eNeuro, 8(4). doi: 10.1523/ENEURO.0189-21.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


