Abstract
We describe the creation of a pluripotent ubiquitin-conjugating enzyme (E2) generated through a single amino acid substitution within the catalytic domain of RAD6 (UBC2). This RAD6 derivative carries out the stress-related function of UBC4 and the cell cycle function of CDC34 while maintaining its own DNA repair function. Furthermore, it carries out CDC34's function in the absence of the CDC34 carboxy-terminal extension. By using sequence and structural comparisons, the residues that define the unique functions of these three E2s were found on the E2 catalytic face partitioned to either side by a conserved divide. One of these patches corresponds to a binding site for both HECT and RING domain proteins, suggesting that a single substitution in the catalytic domain of RAD6 confers upon it the ability to interact with multiple ubiquitin protein ligases (E3s). Other amino acid substitutions made within the catalytic domain of RAD6 either caused loss of its DNA repair function or modified its ability to carry out multiple E2 functions. These observations suggest that while HECT and RING domain binding may generally be localized to a specific patch on the E2 surface, other regions of the functional E2 face also play a role in specificity. Finally, these data also indicate that RAD6 uses a different functional region than either UBC4 or CDC34, allowing it to acquire the functions of these E2s while maintaining its own. The pluripotent RAD6 derivative, coupled with sequence, structural, and phylogenetic data, suggests that E2s have diverged from a common multifunctional progenitor.
Ubiquitin-conjugating enzymes (E2s) comprise a class of eukaryotic enzymes that function at an intermediate step in the reaction pathway leading to protein ubiquitination. Initially, E2s accept ubiquitin from the active-site cysteine of a ubiquitin-activating enzyme (E1) to their own active-site cysteine via a transthiolation reaction. Subsequently, thiol ester-linked ubiquitin is donated by E2s in one of two possible ways. E2s may transfer ubiquitin directly to a lysine side chain found either on a protein substrate or on a ubiquitin moiety in a growing multiubiquitin chain through the formation of an isopeptide bond. Alternatively, E2s transfer ubiquitin to a cysteine of a ubiquitin protein ligase (E3) through a second transthiolation reaction. In the former case, an E3 functions to assist the E2 in the recognition of a protein substrate, while in the latter case, it is believed that the E3 directly links ubiquitin to either the protein substrate or a growing multiubiquitin chain. Assembly of a multiubiquitin chain on a protein substrate by either an E2 or an E3 targets the protein for degradation by the 26S proteosome (35, 39, 43).
E2s have been shown to consist of a conserved catalytic domain that contains the active-site cysteine. This conservation is observed in both sequence and structural comparisons of E2s. For example, the 11 E2s and two ubiquitin-like conjugating enzymes (UBC9 and UBC12) from the yeast Saccharomyces cerevisiae exhibit between 20 and 92% identity. Superimposition of the six solved E2 crystal structures also makes it apparent that the three-dimensional folding of different E2s shows little variation (3–5, 16, 54, 58). It is thought that the requirement of E2s to interact with E1 and ubiquitin (or their cognates) during E2-ubiquitin thiol ester formation placed considerable evolutionary constraints on the E2 structure, resulting in the observed conservation (4, 50).
Although E2s have a function in common in ubiquitin thiol ester formation, they have also been shown to function within a wide range of cellular pathways resulting in the ubiquitination of protein substrates particular to those pathways. This requires that downstream of E2-ubiquitin thiol ester formation, an E2 must interact with proteins that are specific to that pathway, such as an E3 or a target, through unique points of contact other than those employed with ubiquitin and E1.
To acquire these individual functional characteristics, E2s have exploited tolerable levels of structural variation. One source of structural variety comes in the form of carboxy- and amino-terminal extensions to the conserved catalytic domain, the type II, III, and IV E2s (15). In addition, loop structures can result from amino acid insertions within the conserved catalytic domain. These additions to the catalytic domain have been reported to contribute to the unique functional characteristics of several E2s. For example, CDC34 (UBC3) from S. cerevisiae is required for the G1 to S phase transition of the cell cycle (10) and is responsible for the degradation of several cell cycle-related proteins (7). CDC34 possesses both a carboxy-terminal extension and an insertion near its active-site cysteine, both of which play a role in its cell cycle function (26, 31, 50). Furthermore, the carboxy-terminal extension has been shown to help mediate the interaction between CDC34 and its E3, namely, the SCF complex (32).
The type I E2s (15), however, are capable of performing their unique cellular functions without the requirement of extensions or insertions. In these cases, protein interactions unique to an E2 must be determined solely by the distribution of specific amino acid side chains on the conserved catalytic domain surface. In the present work, we demonstrate that a single amino acid replacement within an E2 catalytic domain results in the acquisition of two other E2 functions in addition to its own. These results shed light on the nature of E2-E3 interactions and suggest that E2s may have evolved from a pluripotent progenitor.
MATERIALS AND METHODS
Plasmids and yeast strains.
The S. cerevisiae high-copy-number, TRP1-based E2 expression plasmids used in growth and UV sensitivity studies are identical to YEP96 (8), except that the rad6ΔC, ubc1ΔC, and UBC4 coding sequences replace the ubiquitin coding sequence. The specific codon changes and the corresponding residue changes for each of these E2 sequences are listed in Table 1. Group A (plasmids 1 to 3) are the parental E2 constructs that were used as positive controls throughout this study. rad6ΔC encodes a truncated version of RAD6 that contains the functionally active catalytic domain (residues 1 to 153) but lacks the C-terminal acidic tail (residues 154 to 172). Additional codon changes within rad6ΔC reflect the creation of new restriction sites within the coding sequence to facilitate subsequent gene manipulation. Changes at codon positions 2 and 3, 51 and 52, 98 and 99, and 153 create the restriction sites SacI, KasI, SmaI, and EcoRV, respectively. Changes at codons 2, 51, 98, and 99 have no effect on the polypeptide sequence, whereas changes at codons 3, 52, and 153 result in conservative amino acid replacements (Table 1). The rad6ΔC plasmid fully complements the UV sensitivity of a rad6Δ-null strain in either the low-copy-number or the high-copy-number form.
TABLE 1.
Yeast E2 expression plasmidsa
| Group | rad6ΔC
|
ubc1ΔC/UBC4
|
||
|---|---|---|---|---|
| Substitution type | Substitutions | Substitution type | Substitutions | |
| A | 1 | C-terminal truncation (residues 154–172), S(TCC)2S(AGC), T(ACA)3S(TCA), G(GGA)51G(GGC), T(ACT)52A(GCC), P(CCA)/98P(CCC), M(ATG)153I(ATC) | 2 | ubc1ΔC; C-terminal truncation (residues 151–215), S(TCT)2S(AGC), R(AGG)3S(TCA)∗ |
| 3 | UBC4; S(TCT)2S(AGT)∗ | |||
| B | 4 | E(GAA)61T(ACA) | ||
| 5 | N(AAT)65F(TTT)∗ | |||
| 6 | Y(TAT)82N(AAC)∗ | |||
| 7 | Q(CAG)93K(AAG) | |||
| 8 | S(TCG)120D(GAT) | |||
| 9 | N(AAC)123V(GTC) | |||
| 10 | D(AAC)132T(ACT) | |||
| 11 | N(AAC)32D(GAC), N(AAC)37Q(CAG) | |||
| 12 | N65F, Y82N∗ | |||
| 13 | S120D, N123V | |||
| 14 | F(TTT)13L(TTG),R(CGT)15D(GAT), V(GTA)24C(TGT) | |||
| 15 | Y(TAT)100L(TTG), D(GAT)101T(ACT); T(ACA)107L(TTA), Q(CAA)110C(TGT) | |||
| 16 | R(AGG)54F(TTC), L(TTG)73T(ACT), E(GAA)75K(AAA), T(ACG)144W(TGG), V(GTA)145T(ACA), S(TCT)148Y(TAT), W(TGG)149A(GCA) | 18 | ubc4; F(TTC)63N(AAC), N(AAT)80Y(TAT), D(GAT)118S(TCT), V(GTA)121N(AAT)∗ | |
| 17 | R(AGG)8Q(CAG) | |||
| C | 19 | rad6ΔC1–10 (rad6 residues 1–10 are replaced by residues 1–8 of Ubc4) | 22 | ubc41–8 (Ubc4 residues 1–8 replaced by residues 1–10 of RAD6) |
| 20 | rad6ΔCCdc34a (Cdc34 residues 101–112 between rad6Δ Q93 and N94) | 23 | ubc41–50 (Ubc4 residues 1–50 replaced by residues 1–52 of Rad6) | |
| 21 | rad6ΔCCdc34b (Cdc34 tail residues 171–295 after rad6Δ), N65F | |||
| D | 24 | ubc1ΔC; R(AGG)6A(GCA) | ||
| 25 | ubc1ΔC; S(TCG)97R(AGG) | |||
| 26 | ubc1ΔC; A(GCG)111R(AGG) | |||
| 27 | ubc1ΔC; E(GAA)125A(GCC) | |||
| 28 | ubc1ΔC; E(GAA)125A(GCT), H(CAC)129A(GCC), L(TTA)131A(GCA), R(CGG)132A(GCG), E(GAA)135A(GCA) | |||
| 29 | ubc1ΔC; R(AGA)134A(ACG) | |||
Shown are the codon and residue changes for each of the yeast-high copy E2 expression plasmids used in the present study. Plasmids are grouped (A to D) and numbered (1 to 29) according to substitution type. A, E2 catalytic domains used as positive controls; B, reciprocal amino acid substitutions made between rad6ΔC and UBC4. C, transposition of amino acid stretches; D, selected nonreciprocal substitutions. Asterisks indicate plasmids created and tested in both high-copy and low-copy forms.
Nucleotide substitutions at codon 2 of UBC4 and codons 2 and 3 of ubc1ΔC introduced a SacI restriction site without alteration of the UBC4 polypeptide sequence, whereas an R-to-S substitution is introduced at codon 3 of ubc1ΔC (Table 1, plasmids 2 and 3). In ubc1ΔC, the codons specifying its 64-residue C-terminal tail have also been deleted. Both low- and high-copy-number ubc1ΔC and UBC4 plasmids restore UBC4 function to a ubc4Δ ubc5Δ-null strain.
All subsequent substitutions (plasmids 4 to 29) were made within the context of the three parental plasmids described above. Plasmids in group B each have substitutions that resulted in the transposition of selected amino acid residues from one E2 to their corresponding positions in another E2. Plasmids 4 to 16 encode substitutions in RAD6ΔC that replace selected residues with their UBC4 counterparts, whereas substitutions in plasmid 18 replace selected residues within UBC4 with their RAD6 counterparts. Plasmid 17 was constructed to test whether the previously reported role for residues R6, R7, and R8 of RAD6 in DNA repair could be ascribed to residue R8 (33). The residue positions chosen for replacement were selected because they are strongly conserved within either the RAD6 or the UBC4 E2 family but exhibit poor conservation between families. E2 derivatives expressed from plasmids in group C (19 to 23) each contain a contiguous stretch of residues that have been transposed from one E2 into the corresponding position of the other E2. Plasmids listed in group D (24 to 29) encode amino acid substitutions in ubc1ΔC whose phenotypic behavior was used in the construction of Fig. 4 and 5. All of the substitutions described in Table 1 were verified by DNA sequencing.
FIG. 4.
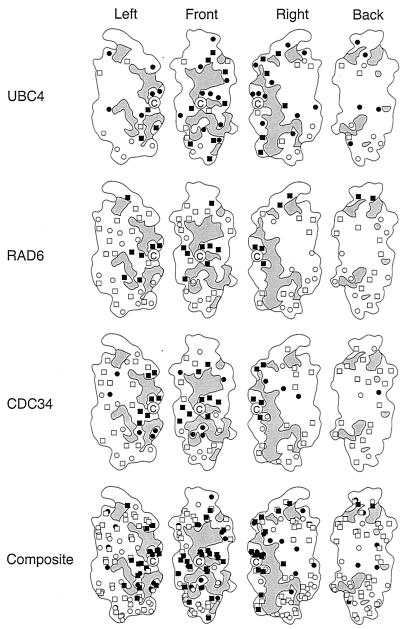
Surface map of three E2 catalytic domains based on function. For each E2, the catalytic domain of S. cerevisiae RAD6 has been used as the map template rotated through increments of 90°. Gray areas represent regions that are structurally conserved. The active-site cysteine is marked by the letter C. Amino acid substitutions with no effect on function are indicated with open boxes. Substitutions that affect functions that are specific to each E2 are indicated with filled boxes. Open circles denote residues that are unconserved within each E2 family indicated, while closed circles denote residues that exhibit conservation within an E2 family but variation between families.
FIG. 5.
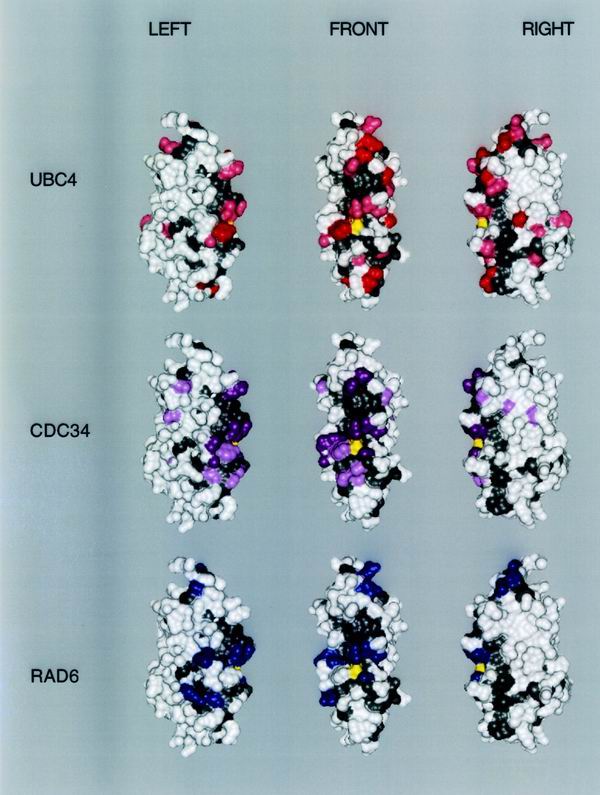
Three-dimensional map of functionally important surfaces. The three-dimensional surface of S. cerevisiae RAD6 is used as the map template rotated to the left or right by 60°. The amino terminus is found at the top of the structures. The active-site cysteine is yellow. Conserved solvent-accessible regions are gray. Residues of clear specific functional importance for each E2 based on mutation studies are highlighted in dark green (UBC4), dark magenta (CDC34), or blue (RAD6). Residues of potential importance to E2 specificity according to the criteria described in Results are highlighted in light green (UBC4) or pink (CDC34).
Low-copy-number TRP1 S. cerevisiae expression plasmids (encoding a selected set of rad6ΔC, ubc1ΔC, or UBC4 derivatives) were constructed by positioning the BamHI/ClaI fragment (containing the CUP1 promoter, the E2 coding sequence, and the CYC1 terminator) of the plasmids described above between the BamHI and ClaI sites of CEN/ARS plasmid pRS314 (49).
High-copy-number, TRP1-based plasmids encoding CDC34 or the C-terminal truncation of cdc34Δ170 have been previously described (50).
The pET3a-based rad6ΔC and ubc1ΔC plasmids used for the expression and purification of recombinant protein from Escherichia coli are identical in sequence to the pET3a-ubc1ΔC plasmid described elsewhere (12), except for the coding sequences for each of the rad6ΔC and ubc1ΔC derivatives described in Table 2. pET3a-rad6ΔC and its derivatives also contain the following Arg codon substitutions, which were found to dramatically increase their levels of expression: Arg-6, -7, and -11 (AGA to CGA) and Arg-8 (AGG to CGG). Details pertaining to the construction of all plasmids are available on request.
TABLE 2.
Phenotypes of rad6ΔC, ubc1ΔC, and UBC4a
| Group and substitution type | E2 | Substitution(s) | Viability at 30°C (ubc4Δ ubc5Δ)b | % Viability in canavanine (ubc4Δ ubc5Δ)c | % Viability under UV light (rad6Δ)d | Thiolester formatione |
|---|---|---|---|---|---|---|
| A | ||||||
| 1 | rad6ΔC | None (parental) | + | − | 98 | 100 |
| 2 | ubc1ΔC | None (parental) | +++++ | 22 | − | 100 |
| 3 | UBC4 | None (parental) | ++++++ | 33 | − | NDf |
| B | ||||||
| 5 | rad6ΔC | N65F | +++ | 4 | 71 | ND |
| 6 | rad6ΔC | Y82N | ++ | − | 98 | ND |
| 8 | rad6ΔC | S120D | + | − | − | ND |
| 9 | rad6ΔC | N123V | + | − | 6 | ND |
| 12 | rad6ΔC | N65F, Y82N | ++++ | 13 | 47 | ND |
| 13 | rad6ΔC | S120D, N123V | + | − | − | 84 |
| 17 | rad6ΔC | R8Q | + | − | − | 102 |
| 18 | UBC4 | F63N, N80Y, D118S, V121N | +++++ | 25 | − | ND |
| C | ||||||
| 19 | rad6ΔC1–10 | Residues 1–10 from Ubc4 | + | − | 70 | ND |
| 20 | rad6ΔCCdc34 | Residues 101–112 from Cdc34 | ND | ND | − | ND |
| 22 | ubc41–8 | Residues 1–8 from Rad6 | ++++++ | 42 | − | ND |
| 23 | ubc41–50 | Residues 1–52 from Rad6 | ++++ | − | ND | |
| D | ||||||
| 24 | ubc1ΔC | R6A | + | − | ND | 99 |
| 25 | ubc1ΔC | S97R | + | − | ND | 112 |
| 26 | ubc1ΔC | A111R | + | − | ND | 105 |
| 28 | ubc1ΔC | E125A, H129A, L131A, R132A, E135A | +++++ | 14 | ND | 122 |
Plasmids are numbered and grouped as in Table I. Plasmids with phenotypes that do not differ significantly from the parental control were excluded (4, 7, 10, 11, 14 to 16, 28, and 30). Growth and canavanine sensitivity were measured in the ubc4Δ ubc5Δ-null strain (MHY508).
Growth was scored relative to the colony size of the null strain (+) and the null strain carrying the UBC4 positive control plasmid (++++++).
Canavanine sensitivity was measured as the percentage of colonies formed on canavanine-containing plates relative to colony formation on plates without canavanine. A minus sign denotes a level of viability of less than 0.1%.
UV sensitivity was measured in rad6Δ-null strain KMY20. The percentage of viable cells following irradiation was calculated as the number of colonies formed relative to the number formed by an unirradiated control. A minus sign denotes a level of survival of less than 0.1%.
Ubiquitin thiolester formation was measured in vitro by using purified components as described in Materials and Methods. Values are expressed as the percentage of thiol ester formed relative to that formed by the appropriate parental E2.
ND, not determined.
For growth and canavanine sensitivity studies, plasmids were introduced into ubc4Δ ubc5Δ-null strain MHY508 (obtained from M. Hochstrasser). MHY508 (2) has the genotype MATα his3Δ200 leu2-3,112 ura3-52 lys2-801 trp1-1 ubc4Δ1::HIS3 ubc5Δ1::LEU2. For UV sensitivity studies, plasmids were introduced into rad6Δ-null strain KMY20 (obtained from K. Madura). KMY20 has the genotype MATa ade2-1 his3-832 trp1-289 ura3-52 rad6Δ::URA3.
To test for 5-fluoroorotic acid (FOA) sensitivity, plasmids were introduced into cdc34Δ-null strain YES71 (MATα ura3-52 trp1-63 leu2Δ1 his3Δ cdc34-2Δ::HIS3). Viability of YES71 is maintained by a high-copy-number URA3 expression plasmid that encodes CDC34.
Phenotype analysis.
For growth studies, strains MHY508 and KMY20 containing TRP1 vectors were grown in fully supplemented SD liquid medium lacking tryptophan (48) to exponential growth phase. Cells (105) from each culture were then plated onto fully supplemented SD plates (lacking tryptophan) and grown at 30°C. Growth was scored on the basis of colony size. Colonies were given designations of + to ++++++ based on their sizes relative to colonies produced by cells expressing either rad6ΔC (+) or UBC4 (++++++). For stress sensitivity experiments, MHY508 strains were grown at 30°C on plates in the presence or absence of canavanine sulfate (1.5 μg/ml) or at the temperatures indicated. Colonies were counted after 7 days. For UV sensitivity experiments, plates containing KMY20 strains were irradiated with 72 J of short-wavelength UV light (254 nm) per m2. Plasmid rescue of the cdc34Δ-null defect associated with YES71 was assessed by determining the persistence of cell viability upon loss of the URA3-CDC34 maintenance plasmid in the presence of FOA. Cells were grown to early exponential phase in fully supplemented SD liquid medium lacking uracil and tryptophan. Cells (106) were then spread onto fully supplemented SD plates containing FOA (1 mg/ml) and then incubated at 25°C for 5 days. Colony formation indicated loss of the URA3-CDC34 maintenance plasmid and complementation of the cdc34Δ-null defect by the E2 gene of the TRP1-based plasmid.
Protein expression and purification.
Expression of protein from pET3a plasmids, cell harvesting, and lysis were performed as previously described for CDC34 (15). For the purification of rad6ΔC and its derivatives, clarified supernatants were passed over a MonoQ HR 5/10 ion-exchange column (Pharmacia) equilibrated with 50 mM Tris-HCl (pH 7.5)–1 mM EDTA–1 mM dithiothreitol (DTT) and the proteins were eluted with an NaCl gradient of 0 to 1 M. rad6ΔC eluted at approximately 240 mM NaCl. The Mono Q fractions were concentrated by Centricon (Amicon) filtration and then loaded onto a Superdex 75 HR 10/30 size exclusion column (Pharmacia) equilibrated with 50 mM HEPES (pH 7.5)–150 mM NaCl–1 mM EDTA–1 mM DTT. Peak fractions were collected, pooled, and concentrated to 500 μl. Glycerol was added to a final concentration of 5%, and the protein samples were stored at −80°C. Expression of 35S-radiolabeled ubiquitin and subsequent cell lysis were carried out as previously described for 35S-radiolabeled CDC34 (38). For the purification of ubc1ΔC, its derivatives, and 35S-radiolabeled ubiquitin, clarified supernatants were passed over a MonoQ HR5/10 ion-exchange column equilibrated with 50 mM Tris-HCl (pH 7.5)–1 mM EDTA–1 mM DTT. The flowthrough was concentrated to 2 ml by Centricon filtration and then run over a MonoS HR5/10 ion-exchange column (Pharmacia) equilibrated with 50 mM HEPES (pH 7.5)–1 mM EDTA–1 mM DTT. The flowthrough was again collected and concentrated to 500 μl and then passed over a Superdex 75 HR10/30 size exclusion column equilibrated with 50 mM HEPES (pH 7.5)–150 mM NaCl–1 mM EDTA–1 mM DTT. Peak fractions were collected, pooled, and concentrated to 500 μl. Glycerol was added to a final concentration of 5%, and the protein samples were stored at −80°C. Each of these protocols resulted in essentially pure protein with typical yields of 0.25 to 0.5 mg/100 ml of culture. Purified poly-His-tagged E1 from S. cerevisiae (Uba1–6His) was a gift from S. Sadis and D. Finley (Harvard Medical School).
E2-ubiquitin thiol ester formation.
Thiol ester reactions were performed in 0.5 ml of buffer A (10 mM HEPES, 40 mM NaCl, 5 mM MgCl2, 5 mM ATP, 50 μg of bovine serum albumin per ml [final pH 7.5]) supplemented with the protease inhibitors antipain, aprotinin, chymostatin, leupeptin, and pepstatin A (each at 20 μg/ml), phenylmethylsulfonyl fluoride (180 μg/ml), and an ATP regeneration system (5 μg of creatine kinase per ml, 0.6 U of inorganic pyrophosphatase per ml, 3.3 mg of phosphocreatine per ml). Also present in each reaction mixture were E2 (100 nM) and [35S]ubiquitin (100 nM with a specific activity of 1.5 × 105 cpm/μg). The reaction was initiated by the addition of ubiquitin-activating enzyme (Uba1–6His at 25 nM), followed by a 5-min incubation at 30°C prior to subsequent analysis. With these reaction conditions, E2-ubiquitin thiol ester yields varied linearly with respect to the E2 concentrations of the positive controls rad6ΔC and ubc1ΔC.
Following incubation, each reaction mixture was immediately loaded onto a Superdex 75 HR 10/30 gel filtration column (Pharmacia) equilibrated with 50 mM HEPES (pH 7.5)–150 mM NaCl–1 mM EDTA–50 μg of bovine serum albumin per ml. Reaction components were eluted from the column in 0.5-ml fractions by using buffer B at 0.5 ml/min. Counts per minute fell into three well-resolved peaks corresponding to either [35S]ubiquitin or [35S]ubiquitin that had been incorporated into E2-ubiquitin thiol ester or E1. Thiol ester yields were calculated by using the specific radioactivity of incorporated [35S]ubiquitin and were expressed as percentages of the total E2 in the reaction.
E2 structural alignment.
Three-dimensional structures of S. cerevisiae UBC1 (unpublished data), RAD6 (UBC2; 58), UBC4, and UBC7 (4, 5) and Homo sapiens UBC9 (54) were aligned by using the Superimpose function contained within the Viewer module of Insight II, version 95.0 (Molecular Simulations Inc.). Alignments were performed by using nine structurally conserved amino acid positions distributed throughout the length of the polypeptide (positions 17, 34, 49, 66, 77, 86, 96, 110, and 135 according to the numbering of S. cerevisiae UBC4). The root mean square deviation for these four superimposed structures at the nine positions indicated equaled 0.83 Å. The resulting alignment served as the guide for the computer-aided alignment of the remaining E2 catalytic domains shown in Fig. 3 (1, 10, 11, 14, 20, 21, 30, 45–47, 52, 57). The evolutionary analysis shown in Fig. 2 was generated by the Clustal method (contained in MegAlign-DNA Star) using the multiple-protein sequence alignment described above.
FIG. 3.
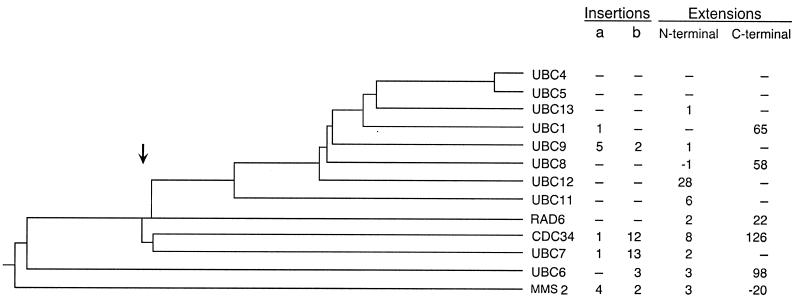
Structure-based sequence alignment of the E2 catalytic domains from S. cerevisiae (see Materials and Methods). The two positions that accommodate insertions are labeled a and b.
FIG. 2.
Evolutionary relationships among E2s from S. cerevisiae (see Materials and Methods). Also indicated are the lengths of major sequence landmarks for each E2. The letters a and b refer to the insertion positions highlighted in Fig. 3.
Amino acid substitutions used for E2 surface maps (see Fig. 4).
For rad6ΔC, UBC4, and ubc1ΔC, amino acid substitutions with and without phenotypic effects were taken from Table 2. Characterized substitutions from other sources were as follows (square brackets denote multiple substitutions) for RAD6: no effect on RAD6 DNA repair function, [K17A, E18A, D19A], [E58A, D60A, E61A, E62A], [K132A, D132A, K134A], and [K139A, R140A, K142A, E143A]; negative effect on RAD6 DNA repair function, [R6A, R7A, R8A], [K71A, E75A], and [E86A, D90A] (33). Those for CDC34 were as follows: no effect on CDC34 function, [R4A, K5A], [R14A, R17A, E18A], [D21A, K23A, K24A], [E32A, E34A, D35A, D36A], [E51A, D52A], [K61A, R65A], [E68A, D69A], R78A, E122A, [E133A, D134A], [D144A, D148A], [R150A, K151A, E154A], [K157A, R159A, K161A], [E163A, E165A, R166A], [K168A, D170A], [R14A, R17A, E18A, D21A, K23A, K24], and [D144A, D148A, R150A, K151A, E154A]; negative effect on CDC34 function, [R90A, D91A, R93A], [E109A, D111A, E113A] (36), [S97A or D], [S139A or D], and [G103 to T114 deleted] (31).
RESULTS
We approached the problem of E2 specificity with the simple assumption that the unique determinants of specificity for each E2 catalytic domain could be identified by searching for amino acids whose transposition from one E2 to another resulted in a corresponding transposition of function. Such an approach has previously been used to make a single amino acid substitution in NEDD8, making it a substrate for the ubiquitin pathway (56). The ubiquitin-conjugating enzymes UBC4 and RAD6 (UBC2) from S. cerevisiae were employed, as each carries out a distinct, nonoverlapping function and their respective biological properties can be easily measured by determining cell viability in simple plating experiments.
Initially, comparisons of the sequence similarities and differences both within and among the six members of the RAD6 and UBC4 families from S. cerevisiae, Schizosaccharomyces pombe, Drosophila melanogaster, Caenorhabditis elegans, Arabidopsis thaliana, and H. sapiens were carried out (6, 9, 24, 25, 28, 40, 41, 42, 45, 53, 55, 59). From these comparisons, we identified 21 positions that displayed strong conservation within each family but differed between families. These positions were distributed more or less uniformly throughout the E2 polypeptide, and notably, only one of these positions was not found on the protein surface (UBC4 A147, RAD6 W149).
Using this information, we attempted to convert the DNA repair function of S. cerevisiae RAD6 (14) to the growth and stress resistance functions of S. cerevisiae UBC4 (45) by replacing the amino acids at these 21 positions in RAD6, either singly or in combination, with their UBC4 counterparts (Table 1, plasmids 4 to 16). For these experiments, a derivative of RAD6 lacking its C-terminal polyaspartate tail (rad6ΔC) was used because of both the absence of the tail in other members of the RAD6 family and the fact that the tail is not essential for its DNA repair function in S. cerevisiae (34).
Contrary to our initial expectation, substitutions at 18 of these 21 positions displayed no transposition of the UBC4 growth or stress resistance functions to rad6ΔC. Furthermore, these 18 substitutions had no adverse effect on the DNA repair function of rad6ΔC, even when present together in the various combinations listed in Table 1 (plasmids 4, 7, 10, 11, and 14 to 16). Thus, none of these 18 positions plays a significant role in determining the unique characteristics of RAD6 or UBC4.
On the other hand, two substitutions in rad6ΔC resulted in the elimination of (S120D) or a strong reduction in (N123V) the DNA repair activity of RAD6 without affecting the efficiency of E2-ubiquitin thiol ester formation (Table 2, rows 8, 9, and 13). Neither of these substitutions imparted any UBC4 character to rad6ΔC. Conversely, the transposition of their RAD6 counterparts into UBC4 had little, if any, effect on UBC4 function and failed to produce any degree of RAD6 function (Table 2, row 17). Thus, positions 120 and 123 are necessary, if not sufficient, determinants of RAD6 function whereas their UBC4 counterparts (positions 118 and 121) are not essential to the UBC4 functions tested.
The DNA repair function of RAD6 has also been previously shown to be dependent upon at least one of three arginines (residues R6, R7, and R8) at its amino terminus (33). It was expected that by replacing the amino-terminal residues of RAD6 with those of UBC4 (plasmid 19), the DNA repair function of RAD6 would be lost, as UBC4 has only one arginine residue (R6) in this region, corresponding to position R8 in RAD6. Contrary to this expectation, minimal loss of DNA repair function was observed (Table 2, row 19), indicating that of the three amino-terminal arginine residues in RAD6, R8 plays the pivotal role in its DNA repair function. We confirmed this by replacing R8 in RAD6 with Q (the residue found at the analogous position in UBC9), which resulted in complete loss of the DNA repair function (Table 2, row 17).
The most striking observation that emerged from this mutational analysis was that the single transposition of F63 from UBC4 to the N65 position of rad6ΔC resulted in a functionally hybrid E2 that had acquired the growth and stress properties of UBC4 with minimal loss of its original DNA repair properties (Table 2, row 5). The N65F derivative of rad6ΔC also acquired UBC4's ability to degrade a noncleaved ubiquitin–β-galactosidase fusion (data not shown), as originally reported by Johnson et al. (17, 18). A similar but weaker effect was observed when N80 of UBC4 was transposed to the Y82 position of rad6ΔC, which also augmented the UBC4 character of rad6ΔC when combined with the N65F transposition with minimal loss of its DNA repair function (Table 2, rows 6 and 12). Notably, the reciprocal F63N and N80Y transpositions into UBC4 had little, if any, effect on UBC4 function (Table 2, row 17) and these transpositions did not impart any RAD6 characteristics to UBC4.
Two conclusions can be drawn from this analysis. (i) The functional determinants of RAD6 and UBC4 are not spatially equivalent but map to different regions on the E2 surface. This accounts for the ability of one E2 to acquire the properties of the other while retaining its own characteristics. (ii) RAD6 has more functionally relevant surface properties in common with UBC4 than would be predicted on the basis of function or sequence comparisons.
A pluripotent E2.
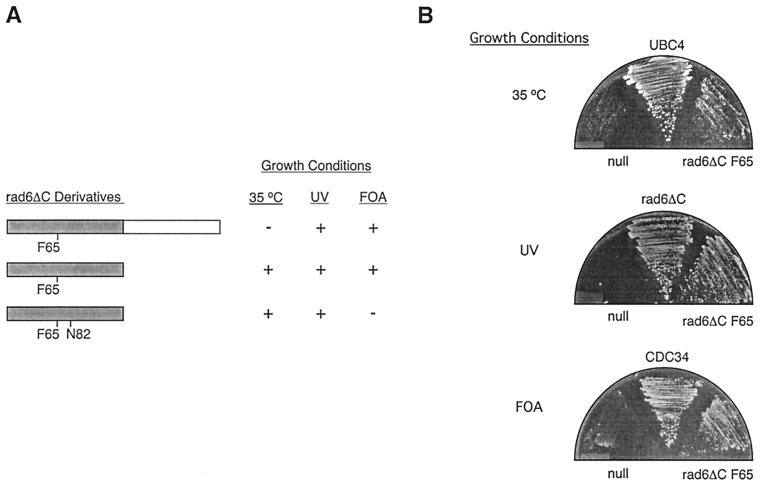
Previous studies have shown that appending the CDC34 tail to the catalytic domain of RAD6 (but not UBC4) creates an E2 chimera capable of fully substituting for the cell cycle function of CDC34 without loss of RAD6 DNA repair activity (26, 50). Given the observations cited above, it seemed reasonable to expect that by appending the CDC34 tail to the bifunctional rad6ΔC F65 derivative, a pluripotent E2 could be created that incorporates the UBC4, RAD6, and CDC34 activities in a single polypeptide. Contrary to our expectation, we observed that a chimeric E2 consisting of rad6ΔC F65 and the CDC34 tail could rescue the cdc34Δ-null strain and the UV sensitivity of the rad6Δ-null strain (Fig. 1) but severely exacerbated the growth phenotype of the ubc4Δ ubc5Δ-null strain (data not shown). Thus, the CDC34 tail appears to interfere with an essential function, likely that of the remaining UBC4 family member, UBC1. This observation raises the possibility that one role of an E2s carboxy-terminal extension is to isolate a particular function to that E2.
FIG. 1.
A pluripotent E2. (A) Complementation of defects associated with the ubc4Δ ubc5Δ (35°C), rad6Δ (UV), or cdc34Δ (FOA) mutant strains by various rad6ΔC derivatives. The positions of point substitutions introduced into the rad6ΔC catalytic domain (gray) are indicated, as is the addition of the CDC34 tail domain (white) to the carboxy terminus of rad6ΔC. The ability (+) or inability (−) of each derivative to allow colony formation by each strain under the conditions given was determined. (B) The ability of a plasmid expressing rad6ΔC F65 to complement each disruption strain is compared to that of a negative control plasmid containing no E2 gene (null) or the appropriate positive control plasmid expressing either UBC4, CDC34, or rad6ΔC (see Materials and Methods).
Neither RAD6 nor the cdc34Δ170 catalytic domain can substitute for CDC34 in the absence of the CDC34 tail (26, 50); therefore, it was a surprise to find that the rad6ΔC F65 catalytic domain was able to restore significant partial function to the CDC34-null strain (Fig. 1). In contrast, the rad6ΔC [F65 N82] derivative is inactive with respect to CDC34 function (Fig. 1). The CDC34 character of rad6ΔC F65, relative to rad6ΔC and rad6ΔC [F65, N82], can be partially explained by the fact that F65 and Y82 of rad6ΔC F65 are analogs of F72 and Y89 in CDC34. The failure of rad6ΔC [F65, N82] and the RAD6 catalytic domain, [N65, Y82], to function as CDC34 establishes these two residues as critical determinants of CDC34 function within the context of rad6ΔC. What makes this result particularly striking, however, is that a single amino acid replacement not only bypasses the CDC34 tail as an obligatory cell cycle determinant but also blends the functional properties of the three E2s within a single catalytic domain.
Evolutionary relationships.
The ability of the RAD6 catalytic domain to accommodate the functions of UBC4 and CDC34 in addition to its own suggested the possibility that these three E2s diverged from a common RAD6-like E2 progenitor that embodied the functions related to stress response, cell cycle, and DNA repair. In light of the fundamental importance of these processes to cell viability, such a divergence may have been among the first in E2 evolution.
These contentions are supported by the S. cerevisiae E2 phylogenetic analysis shown in Fig. 2. Typically, the precision of these sorts of analyses depends on the accuracy of the multiple-peptide sequence alignment from which they are derived. In turn, the precision of this alignment is strongly influenced by arbitrarily established penalties associated with the positions of gaps and insertions within a given sequence. To minimize alignment uncertainty, we first produced an alignment of five structurally determined E2s based on their structural superimposition (see Materials and Methods). This alignment served as a reliable basis for the fitting of the remaining E2s from S. cerevisiae (Fig. 3). The phylogenetic relationships that arise from this analysis indicate that E2s diverged from a common ancestor at a similar time into three branches, leading to separate lineages for modern RAD6, UBC4, and CDC34.
The RAD6-like ancestry of UBC4, CDC34, and indeed all other contemporary E2s (with the possible exception of UBC6) is suggested by the similarity between RAD6 and the E2-like protein Mms2. Although distantly related, Mms2 shares more similarity with RAD6 than with any other E2. Furthermore, Mms2 participates in the same DNA repair pathway as RAD6 (1). Thus, the fact that Mms2 was the first to diverge from the common E2 lineage supports the possibility that all other E2s from S. cerevisiae had an ancestor in common that most resembled RAD6.
Mapping of the determinants of specificity.
A picture of the structural relationships among RAD6, UBC4, and CDC34 emerges when the mutational analyses derived from this work and elsewhere (see Materials and Methods) are combined with protein sequence comparisons and viewed in three dimensions. In Fig. 4, a structurally based schematic of the RAD6 catalytic domain is used as a common template to highlight three different surface attributes of the three E2s. Gray areas highlight surface features that are common to all previously identified E2s and include the solvent-exposed backbone and residue side chains that are identical from protein to protein. These regions are expected to include sites of interactions for ubiquitin and E1 that are required for the universally shared process of E2-ubiquitin thiol ester formation. Ubc9 and Ubc12 were naturally excluded from this analysis because of their involvement in non-ubiquitin conjugation processes involving the ubiquitin homologues Smt3 (Sumo-1) and Rub1, respectively (19, 27). Open symbols correspond to the positions of individual side chains that are presumed to be functionally inessential based on their lack of conservation within a given E2 family (circles) or directly demonstrated to be inessential by site-specific mutagenesis (squares). Closed symbols denote side chains that have been shown to play a role in functional properties that are unique to a given E2 without affecting E2-ubiquitin thiol ester formation (squares) or side chains that are candidates for unique functional determinants based on their conservation within a given E2 family, coupled with their lack of conservation between E2 families, and which could not be excluded on the basis of existing mutational data (circles).
In the case of UBC4 and CDC34, the latter candidates were further selected by exploiting the observation that less-related homologues could complement the loss of E2 activity in vivo (ubc1ΔC in the case of UBC4; tx61 [37] and rad6ΔC F65 in the case of CDC34). Thus, many of the conserved residues that are less important to the specific properties of UBC4 or CDC34 can be filtered out by selecting only surface residues that are conserved within each of their complementation groups.
A three-dimensional representation of these data is presented in Fig. 5. As in Fig. 4, gray residues highlight regions that are common to all E2s. Colored residues correspond to determinants that are specific to the UBC4 function (green), the CDC34 function (magenta), or the RAD6 function (blue). In each E2 example, the darker color highlights residues that have been established as determinants by mutational analyses. The lighter color corresponds to determinants that have been implicated both by their conservation within the UBC4 or CDC34 complementation groups, coupled with their lack of conservation in functionally unrelated E2s. What emerges from Fig. 4 and 5 is a general relationship between the surface structure of three E2 catalytic domains and the functional properties associated with each. The portion of the catalytic domain that appears to be most important to E2 function exists largely as a continuous surface that surrounds the active site. This surface is actually a patchwork that is formed from the intimate association of patches involved in the generic functions of all E2s, such as ubiquitin and E1 interactions, and patches associated with the unique functional properties of each E2. In some cases, the boundaries that demarcate patches are similar between E2s. In other cases, boundaries vary between E2s. The observation that RAD6 has preserved the determinants of other E2s in addition to its own underscores the structural interpretation that not all of these determinants are spatially coincident.
DISCUSSION
Two opposing evolutionary forces appear to have shaped the forms of contemporary E2s. On the one hand, the evolutionary inertia that preserved the key catalytic elements common to all E2s resisted changes to their structures. On the other hand, the tendency toward structural variation provided a source for the diversity of functions observed among E2s today.
The generation of E2 diversity appears to have occurred in two possible ways: (i) changes in the distribution of amino acids on a common E2 frame through amino acid substitutions and (ii) the introduction of novel peptide elements through insertions and extensions.
We propose that E2s evolved from an ancestral E2 capable of carrying out multiple E2 functions. Phylogenetic evidence and the pluripotent nature of rad6ΔC F65 suggest that RAD6 most closely resembles this ancestral E2 and that it consisted solely of the conserved E2 catalytic domain. Subsequent gene duplication, coupled with changes in the distribution of amino acids within the conserved catalytic domain, may have led to E2 diversity and functional specialization. In some instances, such changes appear to have been coupled with insertions and extensions, such as in the case of CDC34. Curiously, these additional peptide elements are not required for the cell cycle function of CDC34 based on the pluripotent nature of rad6ΔC F65. Similarly, other work has shown that deletion of CDC34's catalytic domain insertion, in combination with proper amino acid substitutions, generates a fully functional derivative of CDC34 (31). Furthermore, these changes convert these positions to the residues found in RAD6, possibly accounting for the ability of rad6ΔC F65 to carry out this cell cycle function in the absence of the catalytic domain insertion.
These examples highlight the fact that, even though insertions and extensions may have been acquired to define functional specificity, these functions remain an inherent part of the catalytic domain. These insertions and extensions appear to be dispensable with respect to E2 function and were likely acquired at some point after E2 functions had been established within the catalytic domain.
This interpretation is supported by sequence comparisons and phylogenetic analysis. With respect to insertions, this analysis suggests that the short and variable lengths of E2 insertions did not appear abruptly, carrying with them new functional attributes, but rather developed incrementally with time. Interestingly, the phylogenetic analysis presented in Fig. 2 indicates that most of the loops in present-day E2s arose fairly infrequently and independently from one another after the divergence of UBC4 and CDC34 from RAD6. The notable exceptions to this conclusion are the loops of UBC7 and CDC34, which were clearly acquired from a common ancestor. The observation that these loops are not absolutely required for the in vivo activity of these E2s is consistent with this phylogenetic interpretation (31). The infrequent and independent appearance of loops at only two positions within the catalytic domain attests to the tight evolutionary constraint placed on its modification.
Phylogenetic evidence also suggests that while extensions play a role in E2 specificity, they too were acquired relatively late in the chronology of E2, after many of their functions had been established (Fig. 2). The tails of CDC34, RAD6, and UBC1, for example, were all acquired following the divergences that led to their final forms. This conclusion is also supported by E2 comparisons across species. The tail of CDC34 shares only 9% identity with the tail of its human counterpart, tx61 (37), and the two are therefore unlikely to have the same evolutionary origin. The fact that tx61 can substitute for CDC34 in vivo argues against the notion that the tail plays a central role in substrate specificity. Similarly, the polyaspartate tail of RAD6 is not found on any of the other RAD6 homologues identified from other organisms and is not required for the DNA repair function of RAD6 (34). Finally, the tail of UBC1 exhibits only 13% identity with its bovine homologue, E2-25K, indicating that these tails are likely of independent origin. As in the case of RAD6, the tail of UBC1 is not required for its stress response activity (Table 2, row 2). The phylogenetic evidence therefore suggests that several essential E2 functions were fixed in a primordial E2 prior to the acquisition of inserts and extensions.
E2 structure and function.
From the standpoint of structure, the most striking feature of the E2 surface is the distribution of residues that are conserved among all E2s, relative to residues that probably contributed to the functional divergence of UBC4, RAD6, and CDC34 (Fig. 5). Both classes of residues are found as a largely contiguous patchwork that surrounds the active-site cysteine. When combined, the determinants of UBC4, RAD6, and CDC34 form two clusters that are separated from one another by a conserved partition (Fig. 6). Residues that are important to the functions of each of these E2s can be found in both clusters (Fig. 5).
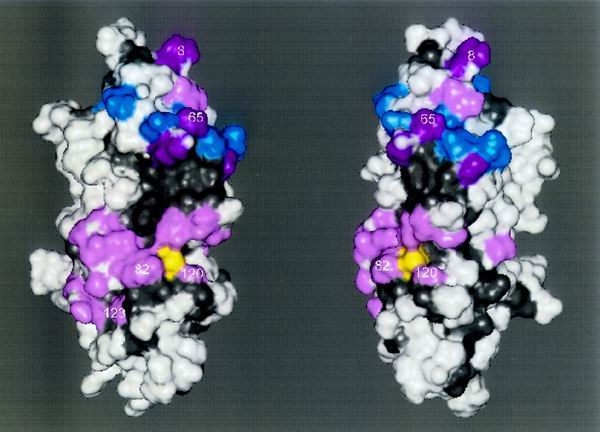
FIG. 6.
Surface determinants of E2 function are concentrated in two patches. Mapped onto the S. cerevisiae RAD6 crystal structure are all of the residues which play a role in the functional specificity of either UBC4, CDC34, or RAD6. The two views of RAD6 vary by a rotation of 30°, and the amino terminus is found at the top of the structures. Shown on the surface are (i) residues from UBCH7 which form contacts with the HECT domain of E6-AP (blue), (ii) residues identified as determinants of UBC4, CDC34, or RAD6 function (Fig. 5) that coincide with UBCH7/E6-AP HECT contact points (magenta), and (iii) residues identified as determinants of UBC4, CDC34, or RAD6 function (Fig. 5) that do not coincide with UBCH7/E6-AP HECT contact points (pink). The active-site cysteine is shown (yellow), as are conserved E2 residues (gray). Numbers indicate amino acid positions within rad6ΔC that either play a role in the DNA repair function of RAD6 or allow it to acquire the UBC4 and CDC34 functions.
Recent crystallographic evidence immediately points to a role for the upper cluster as a site of interaction for both HECT and RING domain E3s. For example, Fig. 6 shows that residues in this upper cluster overlap residues of the human E2 UBCH7 that make contacts with the HECT domain of E6-AP (13). It should be noted that many of these contact points also overlap those that UBCH7 makes with the RING domain of c-CBL (60). In particular, the interaction of UBCH7 with either the HECT domain or the RING domain reveals a pivotal role for F63, the analogue of F65, which is discussed here. Based on these observations, it is likely that F65 adapts rad6ΔC to E3s that are specific to either UBC4 (such as the HECT domain protein UFD4 [17, 23] or the RING domain protein APC11 [29]) or CDC34 (such as the RING domain protein HRT1 [22, 44, 51]).
While this position plays a role as a general site for E3 binding, it appears that other elements on the functional E2 face are required to define the specificity of the interaction of any given E2-E3 pair. For example, a substitution at position 65 of RAD6 has little effect on its ability to participate in the DNA repair pathway (Table 2, row 5). Thus, position 65 may play a role in RAD6-E3 interactions, such as with RING domain protein RAD18, but other elements must define the specificity of these interactions. Such elements might include positions 8, 120, and 123, given that amino acid substitutions at these positions dramatically affect its DNA repair function.
Similarly, replacement of the phenylalanine at the analogous position in UBC4 (F63N) has virtually no effect on UBC4 function (Table 2, row 18). In this case, the interaction of UBC4 with an E3 probably places a greater emphasis on residues that lie adjacent to or near F63.
Finally, while the F65 substitution appears to adapt the RAD6 catalytic domain to multiple E3s, the efficacy of this adaptation in terms of gain of function is dependent on other positions within the catalytic domain, in particular, on position 82 of rad6ΔC. In the pluripotent rad6ΔC F65 derivative, position 82 carries a Y residue. In this configuration, all three E2 functions are carried out by a single E2, albeit to various degrees (Table 2; Fig. 1). When the Y82N substitution is made, however, the UBC4 function is enhanced while the RAD6 function is again partially affected and the CDC34 function is completely eliminated. This observation highlights the need not only for the correct residue at the general E3 binding site but the need for residues outside of this site.
Throughout most of its evolution, RAD6 has apparently preserved the capacity to interact with several structurally unrelated E3s. It is unlikely that these different E3-dependent mechanisms of target selection were all rooted by a common evolutionary origin. Rather, the dissimilarity among these E3s suggests that different proteins independently evolved an affinity for the catalytic face of the E2. The simplest conclusion that can be drawn from these findings is that the mechanistic variations that are employed in target selection owe their existence to the multivalency of RAD6's antecedent.
ACKNOWLEDGMENTS
This work was made possible by grants from the Medical Research Council of Canada (MRC), the National Cancer Institute of Canada, and the Alberta Heritage Foundation for Medical Research (AHFMR). M.J.E. is an MRC Scientist and an AHFMR Senior Scholar.
REFERENCES
- 1.Broomfield S, Chow B L, Xiao W. MMS2 encoding a ubiquitin-conjugating-enzyme-like protein is a member of the yeast error-free postreplication repair pathway. Proc Natl Acad Sci USA. 1998;95:5678–5683. doi: 10.1073/pnas.95.10.5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen P, Johnson P, Sommer T, Jentsch S, Hochstrasser M. Multiple ubiquitin-conjugating enzymes participate in the in vivo degradation of the yeast MATα2 repressor. Cell. 1993;74:357–369. doi: 10.1016/0092-8674(93)90426-q. [DOI] [PubMed] [Google Scholar]
- 3.Cook W J, Jeffery L C, Sullivan M L, Vierstra R D. Three-dimensional structure of a ubiquitin-conjugating enzyme E2. J Biol Chem. 1992;267:15116–15121. doi: 10.2210/pdb1aak/pdb. [DOI] [PubMed] [Google Scholar]
- 4.Cook W J, Jeffery L C, Xu Y, Chau V. Tertiary structures of class I ubiquitin-conjugating enzymes are highly conserved: crystal structure of yeast UBC4. Biochemistry. 1993;32:13809–13817. doi: 10.1021/bi00213a009. [DOI] [PubMed] [Google Scholar]
- 5.Cook W J, Martin P D, Edwards B F, Yamazaki R K, Chau V. Crystal structure of a class I ubiquitin conjugating enzyme (UBC7) from Saccharomyces cerevisiae at 2.9 angstroms resolution. Biochemistry. 1997;36:1621–1627. doi: 10.1021/bi962639e. [DOI] [PubMed] [Google Scholar]
- 6.Damagnez V, Rolfe M, Cottarel G. Schizosaccharomyces pombe and Candida albicans cDNA homologues of the Saccharomyces cerevisiae UBC4 gene. Gene. 1995;155:137–138. doi: 10.1016/0378-1119(94)00926-j. [DOI] [PubMed] [Google Scholar]
- 7.Deshaies R J. SCF and Cullin/RING H2-based ubiquitin ligases. Annu Rev Cell Dev Biol. 1999;15:435–467. doi: 10.1146/annurev.cellbio.15.1.435. [DOI] [PubMed] [Google Scholar]
- 8.Ellison M J, Hochstrasser M. Epitope tagged ubiquitin. A new probe for analyzing ubiquitin function. J Biol Chem. 1991;266:21150–21157. [PubMed] [Google Scholar]
- 9.Girod P A, Carpenter T B, van Nocker S, Sullivan M L, Vierstra R D. Homologs of the essential ubiquitin conjugating enzymes UBC1, 4, and 5 in yeast are encoded by a multigene family in Arabidopsis thaliana. Plant J. 1993;3:545–552. doi: 10.1046/j.1365-313x.1993.03040545.x. [DOI] [PubMed] [Google Scholar]
- 10.Goebl M G, Yochem J, Jentsch S, McGrath J P, Varshavsky A, Byers B. The yeast cell cycle gene CDC34 encodes a ubiquitin-conjugating enzyme. Science. 1988;241:1331–1335. doi: 10.1126/science.2842867. [DOI] [PubMed] [Google Scholar]
- 11.Goffeau A, Barrell B G, Bussey H, Davis R W, Dujon B, Feldmann H, Galibert F, Hoheisel J D, Jacq C, Johnston M, Louis E J, Mewes H W, Murakami Y, Philippsen P, Tettelin H, Oliver S G. Life with 6000 genes. Science. 1996;274:563–567. doi: 10.1126/science.274.5287.546. [DOI] [PubMed] [Google Scholar]
- 12.Hodgins R, Gwozd C, Arnason T, Cummings M, Ellison E M. The tail of ubiquitin-conjugating enzyme redirects multi-ubiquitin chain synthesis from the lysine 48-linked configuration to a novel nonlysine-linked form. J Biol Chem. 1996;271:28766–28771. doi: 10.1074/jbc.271.46.28766. [DOI] [PubMed] [Google Scholar]
- 13.Huang L, Kinnucan E, Wang G, Beaudenon S, Howley P M, Huibregtse J M, Pavletich N P. Structure of an E6AP-UbcH7 complex: insights into ubiquitination by the E2–E3 enzyme cascade. Science. 1999;286:1321–1326. doi: 10.1126/science.286.5443.1321. [DOI] [PubMed] [Google Scholar]
- 14.Jentsch S, McGrath J P, Varshavsky A. The yeast DNA repair gene RAD6 encodes a ubiquitin-conjugating enzyme. Nature. 1987;329:131–134. doi: 10.1038/329131a0. [DOI] [PubMed] [Google Scholar]
- 15.Jentsch S. The ubiquitin-conjugation system. Annu Rev Genet. 1992;26:179–207. doi: 10.1146/annurev.ge.26.120192.001143. [DOI] [PubMed] [Google Scholar]
- 16.Jiang F, Basavappa R. Crystal structure of the cyclin-specific ubiquitin-conjugating enzyme from clam, E2-C, at 2.0 A resolution. Biochemistry. 1999;38:6471–6478. doi: 10.1021/bi9901329. [DOI] [PubMed] [Google Scholar]
- 17.Johnson E S, Bartel B, Seufert W, Varshavsky A. Ubiquitin as a degradation signal. EMBO J. 1992;11:497–505. doi: 10.1002/j.1460-2075.1992.tb05080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson E S, Ma P C, Ota I M, Varshavsky A. A proteolytic pathway that recognizes ubiquitin as a degradation signal. J Biol Chem. 1995;270:17442–17456. doi: 10.1074/jbc.270.29.17442. [DOI] [PubMed] [Google Scholar]
- 19.Johnson E S, Blobel G. Ubc9p is the conjugating enzyme for the ubiquitin-like protein Smt3p. J Biol Chem. 1997;272:26799–26802. doi: 10.1074/jbc.272.43.26799. [DOI] [PubMed] [Google Scholar]
- 20.Jungmann J, Reins H A, Schobert C, Jentsch S. Resistance to cadmium mediated by ubiquitin-dependent proteolysis. Nature. 1993;361:369–371. doi: 10.1038/361369a0. [DOI] [PubMed] [Google Scholar]
- 21.Kaiser P, Seufert W, Hofferer L, Kofler B, Sachsenmaier C, Herzog H, Jentsch S, Schweiger M, Schneider R. A human ubiquitin-conjugating enzyme homologous to yeast UBC8. J Biol Chem. 1994;269:8797–8802. [PubMed] [Google Scholar]
- 22.Kamura T, Koepp D M, Conrad M N, Skowyra D, Moreland R J, Iliopoulos O, Lane W S, Kaelin W G, Jr, Elledge S J, Conaway R C, Harper J W, Conaway J W. RBX1, a component of the VHL tumor suppressor complex and SCF ubiquitin ligase. Science. 1999;284:657–661. doi: 10.1126/science.284.5414.657. [DOI] [PubMed] [Google Scholar]
- 23.Koegl M, Hoppe T, Schlenker S, Ulrich H D, Mayer T U, Jentsch S. A novel ubiquitination factor, E4, is involved in multiubiquitin chain assembly. Cell. 1999;96:635–644. doi: 10.1016/s0092-8674(00)80574-7. [DOI] [PubMed] [Google Scholar]
- 24.Koken M, Reynolds P, Bootsma D, Hoeijmakers J, Prakash S, Prakash L. DHR6, a Drosophila homolog of the yeast DNA-repair gene RAD6. Proc Natl Acad Sci USA. 1991;88:3832–3836. doi: 10.1073/pnas.88.9.3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koken M H, Reynolds P, Jaspers-Dekker I, Prakash L, Prakash S, Bootsma D, Hoeijmakers J H. Structural and functional conservation of two human homologs of the yeast DNA repair gene RAD6. Proc Natl Acad Sci USA. 1991;88:8865–8869. doi: 10.1073/pnas.88.20.8865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kolman C J, Toth J, Gonda D K. Identification of a portable determinant of cell cycle function within the carboxy-terminal domain of the yeast CDC34 (UBC3) ubiquitin (E2) conjugating enzyme. EMBO J. 1992;11:3081–3090. doi: 10.1002/j.1460-2075.1992.tb05380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lammer D, Mathias N, Laplaza J M, Jiang W, Liu Y, Callis J, Goebl M, Estelle M. Modification of yeast CDC53p by the ubiquitin-related protein RUB1p affects function of the SCF-CDC4 complex. Genes Dev. 1998;12:914–926. doi: 10.1101/gad.12.7.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leggett D S, Jones D, Candido E P. Caenorhabditis elegans UBC-1, a ubiquitin-conjugating enzyme homologous to yeast RAD6/UBC2, contains a novel carboxy-terminal extension that is conserved in nematodes. DNA Cell Biol. 1995;14:883–891. doi: 10.1089/dna.1995.14.883. [DOI] [PubMed] [Google Scholar]
- 29.Leverson, J. D., C. Joazeiro, A. M. Page, H.-K. Huang, P. Hieter, and T. Hunter. The Apc11 RING-H2 finger mediates E2-dependent ubiquitination. Mol. Biol. Cell 11:2315–2325. [DOI] [PMC free article] [PubMed]
- 30.Liakopoulos D, Doenges G, Matuschewski K, Jentsch S. A novel protein modification pathway related to the ubiquitin system. EMBO J. 1998;17:2208–2214. doi: 10.1093/emboj/17.8.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y, Mathias N, Steussy N, Goebl M G. Intragenic suppression among CDC34 (UBC3) mutations defines a class of ubiquitin-conjugating catalytic domains. Mol Cell Biol. 1995;15:5635–5644. doi: 10.1128/mcb.15.10.5635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mathias N, Steussy N C, Goebl M G. An essential domain within CDC34p is required for binding to a complex containing CDC34p and CDC53p in Saccharomyces cerevisiae. J Biol Chem. 1998;273:4040–4045. doi: 10.1074/jbc.273.7.4040. [DOI] [PubMed] [Google Scholar]
- 33.McDonough M, Sangan P, Gonda D K. Characterization of novel yeast RAD6 (UBC2) ubiquitin-conjugating enzyme mutants constructed by charge-to-alanine scanning mutagenesis. J Bacteriol. 1995;177:580–585. doi: 10.1128/jb.177.3.580-585.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morrison A, Miller E J, Prakash L. Domain structure and functional analysis of the carboxyl-terminal polyacidic sequence of the RAD6 protein of Saccharomyces cerevisiae. Mol Cell Biol. 1988;8:1179–1185. doi: 10.1128/mcb.8.3.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pickart C M. Polyubiquitin chains. In: Peters J-M, Harris J R, Finley D, editors. Ubiquitin and the biology of the cell. New York, N.Y: Plenum Press, Inc.; 1998. pp. 19–63. [Google Scholar]
- 36.Pitluk Z W, McDonough M, Sangan P, Gonda D K. Novel CDC34 (UBC3) ubiquitin-conjugating enzyme mutants obtained by charge-to-alanine scanning mutagenesis. Mol Cell Biol. 1995;15:1210–1219. doi: 10.1128/mcb.15.3.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Plon S E, Leppig K A, Do H N, Groudine M. Cloning of the human homolog of the CDC34 cell cycle gene by complementation in yeast. Proc Natl Acad Sci USA. 1993;90:10484–10488. doi: 10.1073/pnas.90.22.10484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ptak C, Prendergast J A, Hodgins R, Kay C M, Chau V, Ellison M J. Functional and physical characterization of the cell cycle ubiquitin-conjugating enzyme CDC34 (UBC3): identification of a functional determinant within the tail that facilitates CDC34 self-association. J Biol Chem. 1994;42:26539–26545. [PubMed] [Google Scholar]
- 39.Rechsteiner M. The 26S proteosome. In: Peters J-M, Harris J R, Finley D, editors. Ubiquitin and the biology of the cell. New York, N.Y: Plenum Press, Inc.; 1998. pp. 147–189. [Google Scholar]
- 40.Reynolds P, Weber S, Prakash L. RAD6 gene of Saccharomyces cerevisiae encodes a protein containing a tract of 13 consecutive aspartates. Proc Natl Acad Sci USA. 1985;82:168–172. doi: 10.1073/pnas.82.1.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reynolds P, Koken M H, Hoeijmakers J H, Prakash S, Prakash L. The rhp6+ gene of Schizosaccharomyces pombe: a structural and functional homolog of the RAD6 gene from the distantly related yeast Saccharomyces cerevisiae. EMBO J. 1990;9:1423–1430. doi: 10.1002/j.1460-2075.1990.tb08258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scheffner M, Huibregtse J M, Howley P M. Identification of a human ubiquitin-conjugating enzyme that mediates the E6-AP dependent ubiquitination of p53. Proc Natl Acad Sci USA. 1993;91:8797–8801. doi: 10.1073/pnas.91.19.8797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scheffner M, Smith S, Jentsch S. The ubiquitin-conjugation system. In: Peters J-M, Harris J R, Finley D, editors. Ubiquitin and the biology of the cell. New York, N.Y: Plenum Press, Inc.; 1998. pp. 65–98. [Google Scholar]
- 44.Seol J H, Feldman R M, Zachariae W, Shevchenko A, Correll C C, Lyapina S, Chi Y, Galova M, Claypool J, Sandmeyer S, Nasmyth K, Deshaies R J. Cdc53/cullin and the essential hrt1 RING-H2 subunit of SCF define a ubiquitin ligase module that activates the E2 enzyme cdc34. Genes Dev. 1999;13:1614–1626. doi: 10.1101/gad.13.12.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seufert W, Jentsch S. Ubiquitin-conjugating enzymes UBC4 and UBC5 mediate selective degradation of short-lived and abnormal proteins. EMBO J. 1990;9:543–550. doi: 10.1002/j.1460-2075.1990.tb08141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seufert W, McGrath J P, Jentsch S. UBC1 encodes a novel member of an essential subfamily of yeast ubiquitin-conjugating enzymes involved in protein degradation. EMBO J. 1990;9:4535–4541. doi: 10.1002/j.1460-2075.1990.tb07905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seufert W, Futcher B, Jentsch S. Role of a ubiquitin-conjugating enzyme in degradation of S- and M-phase cyclins. Science. 1995;373:78–81. doi: 10.1038/373078a0. [DOI] [PubMed] [Google Scholar]
- 48.Sherman F, Fink G R, Hicks J B. Methods in yeast genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1986. [Google Scholar]
- 49.Sikorski R S, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Silver E T, Gwozd T J, Ptak C, Goebl M, Ellison M J. A chimeric ubiquitin conjugating enzyme that combines the cell cycle properties of CDC34 (UBC3) and the DNA repair properties of RAD6 (UBC2): implications for the structure, function, and evolution of the E2s. EMBO J. 1992;11:3091–3098. doi: 10.1002/j.1460-2075.1992.tb05381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Skowyra D, Koepp D M, Kamura T, Conrad M N, Conaway R C, Conaway J W, Elledge S J, Harper J W. Reconstitution of G1 cyclin ubiquitination with complexes containing SCFGRR1 and RBX1. Science. 1999;284:662–665. doi: 10.1126/science.284.5414.662. [DOI] [PubMed] [Google Scholar]
- 52.Sommer T, Jentsch S. A protein translocation defect linked to ubiquitin conjugation at the endoplasmic reticulum. Nature. 1993;365:176–179. doi: 10.1038/365176a0. [DOI] [PubMed] [Google Scholar]
- 53.Sullivan M L, Vierstra R D. Cloning of a 16-kDa ubiquitin carrier protein from wheat and Arabidopsis thaliana. Identification of functional domains by in vitro mutagenesis. J Biol Chem. 1991;266:23878–23885. [PubMed] [Google Scholar]
- 54.Tong H, Hateboer G, Perrakis A, Bernards R, Sixma T K. Crystal structure of murine/human Ubc9 provides insight into the variability of the ubiquitin conjugating system. J Biol Chem. 1997;272:21381–21387. doi: 10.1074/jbc.272.34.21381. [DOI] [PubMed] [Google Scholar]
- 55.Treier M, Seufert W, Jentsch S. Drosophila UbcD1 encodes a highly conserved ubiquitin-conjugating enzyme involved in selective protein degradation. EMBO J. 1992;11:367–372. doi: 10.1002/j.1460-2075.1992.tb05059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Whitby F G, Xia G, Pickart C M, Hill C P. Crystal structure of the human ubiquitin-like protein NEDD8 and interactions with ubiquitin pathway enzymes. J Biol Chem. 1998;273:34983–34991. doi: 10.1074/jbc.273.52.34983. [DOI] [PubMed] [Google Scholar]
- 57.Wiebel F F, Kunau W-H. The Pas2 protein essential for peroxisome biogenesis is related to ubiquitin-conjugating enzymes. Nature. 1992;359:73–76. doi: 10.1038/359073a0. [DOI] [PubMed] [Google Scholar]
- 58.Worthylake D K, Prakash S, Hill C P. Crystal structure of the Saccharomyces cerevisiae ubiquitin-conjugating enzyme Rad6 at 2.6 A resolution. J Biol Chem. 1998;273:6271–6276. doi: 10.1074/jbc.273.11.6271. [DOI] [PubMed] [Google Scholar]
- 59.Zhen M, Heinlein R, Jones D, Jentsch S, Candido E P M. The ubc-2 gene of Caenorhabditis elegans encodes a ubiquitin-conjugating enzyme involved in selective protein degradation. Mol Cell Biol. 1993;13:1371–1377. doi: 10.1128/mcb.13.3.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zheng N, Wang P, Jeffrey P D, Pavletich P. Structure of a c-Cbl-UbcH7 complex: RING domain function in ubiquitin-protein ligases. Cell. 2000;102:533–539. doi: 10.1016/s0092-8674(00)00057-x. [DOI] [PubMed] [Google Scholar]