Abstract
Resveratrol is a polyphenol with diverse pharmacological activities, but its clinical efficacy is limited due to low solubility/permeability, light-induced isomerization, auto-oxidation, and rapid metabolism. Nanodelivery systems, such as liposomes, polymeric nanoparticles, lipid nanocarriers, micelles, nanocrystals, inorganic nanoparticles, nanoemulsions, protein-based nanoparticles, exosomes, macrophages, and red blood cells (RBCs) have shown great potential for improving the solubility, biocompatibility, and therapeutic efficacy of resveratrol. This review comprehensively summarizes the recent advances in resveratrol nanoencapsulation and describes potential strategies to improve the pharmacokinetics of existing nanoformulations, enhance targeting, reduce toxicity, and increase drug release and encapsulation efficiency. The article also suggests that in order to avoid potential safety issues, resveratrol nanoformulations must be tested in vivo in a wide range of diseases.
Keywords: Resveratrol, nanocarriers, nanoencapsulation, drug delivery system, bioavailability
1. Introduction
1.1. Pharmacological effects of RES
Resveratrol (RES) (Figure 1) is a natural polyphenolic antitoxin secreted by at least 100 different plants after fungal infection or pathogen attack (Nam, 2006; Vestergaard & Ingmer, 2019; Ahmed et al., 2017; Huang & Mazza, 2011; Tome-Carneiro et al., 2013). RES has been used for the treatment of various diseases (Bhullar & Hubbard, 2015), as it can effectively scavenge free radicals (Prysyazhna et al., 2019), regulate the expression and activity of antioxidant enzymes (Gal et al., 2021), as well as exert anti-inflammatory (Nunes et al., 2018), anti-aging (Grilc et al., 2021), antidiabetic (Rocha et al., 2021), and cardioprotective effects (Gal et al., 2021). RES also exhibits significant neuroprotective effects in central nervous system diseases, such as Alzheimer’s disease (Huang et al., 2021), by inhibiting microglial activation and modulating neuroinflammation (Moradi et al., 2020). It can protect against cancers of the breast (Vargas et al., 2020), prostate (Khusbu et al., 2020), lung (Yousef et al., 2017), colon (Yuan et al., 2019), liver (Zhao et al., 2021), gastrointestinal tract (Xu et al., 2017), pancreas (Srivani et al., 2020), ovary (Guo et al., 2015), and skin (Iqubal et al., 2021).
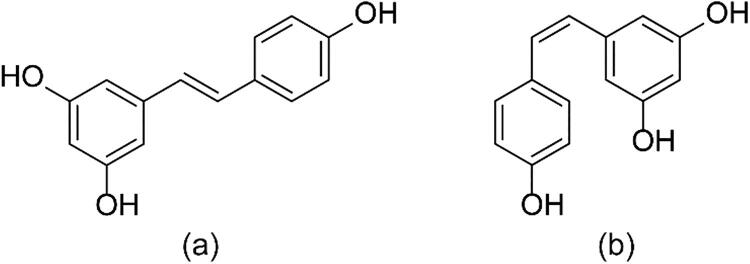
Figure 1.
Configuration of (a) trans- and (b) cis-RES.
1.2. Pharmacokinetics issues with RES
RES must overcome many pharmacokinetic hurdles before it can be considered clinically useful in chemotherapy. To comprehensively investigate the bioavailability of RES, 14 C-labeled RES was administered orally and intravenously 5–6 and five healthy subjects with doses of 25 and 0.2 mg, respectively (Walle et al., 2004). Despite the fact that it is well absorbed when taken orally, with a bioavailability of around 70%, the bioavailability of RES itself is close to zero due to extensive metabolism in the liver and intestines, including glucuronidation and sulfation, which produces metabolites with lower biological activity than RES. After ingestion of RES, two maximum peaks in RES plasmatic levels are obtained: one is found 30–60 min following ingestion, and a second peak is found after 6 h. These findings suggest that an enteric recircularization of RES metabolites takes place. In addition, peak plasma levels of RES and metabolites of 491 ± 90 ng/mL (about 2 microM) and a plasma half-life of 9.2 ± 0.6 h. RES can be rapidly absorbed, yielding peak plasma concentration (Cmax) between 0.83 and 1.5 h post-dose. However, only trace amounts of unchanged RES (<5 ng/mL) could be detected in plasma (Cottart et al., 2010). Investigations of RES metabolism in vivo in rodent models showed that the liver is a major accumulation site for RES and its metabolites (Yu et al., 2002). Systemic in vivo distribution in rodents is characterized by a peak concentration at 30 min (Soleas et al., 2001), with metabolites becoming detectable 3 h post-administration (Sale et al., 2004). Compounding more to the problem is RES low water solubility, which is around 0.03 mg/mL, hence affecting the compound’s absorption and bioavailability (Summerlin et al., 2015). Moreover, it exerts certain therapeutic effects only at low concentrations, implying that even a modest increase in RES bioavailability may have strong therapeutic effect (Calabrese et al., 2010; Calabrese et al., 2010).
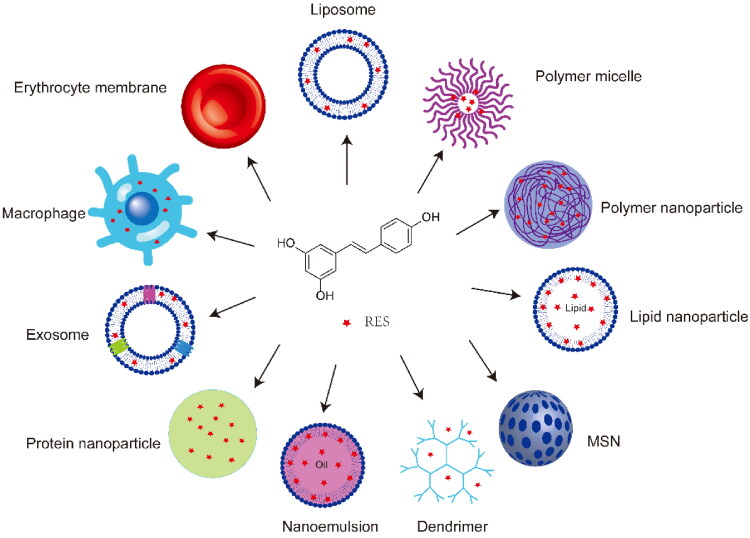
To improve the solubility, stability and bioavailability of RES, enhance its permeability and therapeutic efficacy, and reduce its toxicity, the drug has been loaded into several natural, semi-synthetic, and synthetic nanodelivery systems (Fang & Bhandari, 2010; Ghalandarlaki et al., 2014), including liposomes (Abu Lila & Ishida, 2017), polymer nanoparticles (George et al., 2019), micelles (Lu et al., 2018), lipid nanocarriers (Garces et al., 2018), nanocrystals (Jermain et al., 2018), inorganic nanoparticles (Yang et al., 2019), dendrimers (Fischer & Vogtle, 1999), nanoemulsions (Gupta et al., 2016), and bionic drug delivery systems (Chen et al., 2016) (Figure 2). The RES bioavailability effects after RES loading with distinct types of nanotechnology-based carriers administered orally, intravenously, which are discussed next, are summarized in Table 1.
Figure 2.
RES-loaded nanoformulations. MSN, mesoporous silica nanoparticles.
Table 1.
List of the most relevant in vivo studies concerning RES bioavailability upon oral, i.v. administration of different RES-loaded nanotechnology-based carriers.
| Nanocarriers | In vivo model | Via | RES dose | Outcomes (comparatively to free RES) | Ref |
|---|---|---|---|---|---|
| liposomes | Charles Foster rats | i.v. | 2 mg/kg dose. | AUC: 30-fold increased; t1/2: 29.7-fold increased; CL: 33-fold decreased; MRT: 29.5-fold decreased |
Vijayakumar et al. (2016) |
| Polymeric nanoparticles (PLGA) | Wistar rats | Oral | 20.0 mg/kg | AUC0→∞ : 10.6-fold increased; Cmax: 1.2-fold increased; Tmax: 28.0-fold increased; Absorption rate: Ka was 7.2-fold increased); |
Singh & Pai (2014) |
| Lipid nanocarrier | Wistar rats | i.v. | 2 mg/kg | AUC0→∞:8.7-fold increased; Cmax: 1.4-fold increased; CL: 13.4-fold decreased; t1/2: 15-fold increased. |
Poonia et al. (2020) |
| Micelles (TPGS) | Sprague-Dawley rats | Oral | 20.0 mg/kg | AUC0→∞: 3.5-fold increased; Cmax: 2.2-fold increased; MRT: 1.2-fold increased. |
Singh et al. (2017) |
| Nanocrystals | Wistar rats | Oral | 40 mg/kg | AUC: 6.3-fold increased; Tmax: 2-fold decreased; Cmax: 3-fold increased; MRT: 3-fold increased. |
Argenziano et al. (2022) |
| Nanoemulsions | Wistar rats | Oral | 120 mg/kg | AUC0 →∞: 1.3-fold increased; Cmax: 3.4-fold increased; CL: 1.2-fold decreased; MRT: 1.1-fold decreased; Vd: 1.5-fold decreased. |
Hao et al. (2015) |
| Protein-based nanoparticles | Kunming mice | i.v. | 1.5 mg/kg | Targeting efficiency increased; RES accumulation in the liver, kidney, heart, and ovaries; |
Guo et al. (2010) |
AUC: area under the concentration-time curve (plasma exposure); t1/2: plasma half life; CL: clearance; MRT: mean residence time; AUC0 →∞: area under the concentration time-curve from time zero to infinity; Cmax: peak plasma concentration; i.v.: intravenous; Ka: absorption rate constant; PLGA: Poly (lactic-co-glycolic acid); RES: Resveratrol; tmax: time to achieve the maximum concentration; TPGS: D-α-tocopherol polyethylene glycol 400 succinate
In this review, we summarize recent advances in RES nanoencapsulation and the resulting benefits, and we discuss current limitations affecting the in vivo behavior and therapeutic efficacy of RES-loaded nanocarriers.
2. Nanocarriers used for RES delivery
2.1. Exogenous nanoparticles
2.1.1. Liposomes
Liposomes are stable spherical vesicles made up of cholesterol and nontoxic phospholipids. Due to their amphiphilic nature, biocompatibility, biodegradability and easy surface modification, liposomes have been extensively used as carriers for hydrophilic drugs and lipophilic molecules (Table 2) (Li et al., 2019). For example, the encapsulation of free RES into liposomes (Lip-RES) at 37 °C under light conditions improved its chemical stability and bioavailability, while also increasing RES uptake by white adipocytes by 25% (Zu et al., 2018). Lip-RES showed superior effectiveness relative to free RES in against DXR-induced renal toxicity in rats (Alhusaini et al., 2022). In addition, Lip-RES increased the uptake of RES by cardiomyocytes and thus significantly activated the maximum cellular respiratory capacity (Tsujioka et al., 2022).
Table 2.
RES-loaded liposomes used for the treatment of various diseases.
| Composition | Targeting moiety | Preparation method | Physicochemical characteristics | Cell line/animal model | Disease | Major outcome | Ref. |
|---|---|---|---|---|---|---|---|
| Soy PC; cholesterol | None | Film dispersion | PS, ∼110 nm; PI, 0.140; ZP, ∼-28 mV; DL, 25.3%; EE, 96% | Murine 3T3-L1 fibroblasts | Obesity | Increased water solubility and stability; enhanced browning of white fat cells | Zu et al. (2018) |
| 1,2-Bis-myristyloxyamidopropyl ornithine; sucrose laurate L126 | Tumor microenvironment | Thin-film hydration | PS, ∼140 nm; ZP, ∼40 mV; EE, >90% | Human breast cancer MCF-7 and MCR-5 cells; male BALB/c nude mice | Breast cancer | Enhanced bioavailability and anti-tumor activity | Zhao et al. (2020) |
| Pluronic® L64; tocopherol-PEG-succinate; phospholipon 90 G | None | Ice-bath sonication | PS, ∼85 nm; PI, ∼0.2; ZP, −20 mV; DL, 5.3%; EE, 94.9% | Erythrocyte cells | Oxidative stress | Extension of half-life in the blood circulation | Caddeo et al. (2018) |
| PT-98T; cholesterol; DSPE-PEG2000 | None | Thin-film hydration | PS, ∼136 nm; ZP, −11 mV; DL, 3.9%; EE, 81.3% | Mouse macrophages; L929 mouse fibroblasts; human umbilical vein endothelial cells; BALB/c female mice (7–8 weeks old) | Periodontitis | High anti-inflammatory activity | Shi et al. (2021) |
| Chitosan; Au; SPC | Tumor microenvironment | None | PS, ∼140 nm; ZP, ∼7 mV | HeLa cells | Cervical cancer | Improved drug cellular uptake; synergistic antitumor effect | Wang et al. (2017) |
| 1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine; cholesterol; PEG-PE; TPP-DSPE-PEG | Mitochondria | Thin-film hydration | PS, ∼115 nm; PI, 0.22; ZP, ∼10.46 mV | B16F-10 cells | Melanoma | Increased antitumor activity | Kang & Ko (2019) |
DL: drug loading efficiency; DSPE: 1,2-distearoyl-sn-glycero-3-phosphoethanolamine; EE: entrapment efficiency; PC: phosphatidylcholine; PE: phosphatidylethanolamine; PEG: poly(ethylene glycol); PI: polydispersity index; PS: particle size; SPC: soybean phosphatidylcholine; TPP, 4-carboxybutyl triphenylphosphonium bromide; ZP: zeta potential
Engineering liposomes to release their cargo only under specific conditions can prevent premature leakage of drugs into the circulation (Abri Aghdam et al., 2019). For example, a previous study prepared RES-loaded liposomes using 1,2-bis-myristyloxyamidopropyl ornithine and sucrose laurate L126 to achieve controlled drug release. The carbamate bond in the lipid structure was stable under neutral conditions, but acidic conditions triggered RES release, indicating that this nanoplatform can enhance RES accumulation in the acidic tumor microenvironment and improve its antitumor efficacy (Zhao et al., 2020). However, conventional liposomes are unstable during storage (Caddeo et al., 2018) and have low targeting ability (Laginha et al., 2005). Therefore, RES-loaded liposomes were modified with poly(ethylene glycol) (PEG), resulting in a nanoplatform (PEG-lip-RES) with better stability and biocompatibility; low toxicity against murine macrophage cells (RAW264.7), mouse fibroblast cells (L929), and human vascular endothelial cells (HUVEC); prolonged half-life; and good anti-oxidant effects (Shi et al., 2021). In another study, the surface of liposomes was modified with chitosan and then coated with gold nanoshells to construct multifunctional liposomes responsive to pH and near-infrared light that released RES in a controlled manner, leading to synergistic antitumor effects against HeLa cells (Wang et al., 2017). RES induces cell death through the mitochondrial apoptotic pathway, where mitochondria play a central role in the release of pro-apoptotic factors. The surface of RES-loaded liposomes was modified with cationically charged compounds such as 4-carboxybutyltriphenylphosphine bromide and dequinoline, which cross the mitochondrial membrane to promote drug accumulation in mitochondria of tumor cells, leading to greater cytotoxicity against tumor cells (Maherani et al., 2011; Kang & Ko, 2019).
2.1.2. Polymer nanoparticles
Polymer nanoparticles are submicron-sized colloidal particles made from natural or synthetic polymers (Zu et al., 2021), including polylactic acid, poly(lactic-co-glycolic acid) (PLGA), polycaprolactone, gelatin, and polysaccharides (Lu & Park, 2013) (Table 3). Polymer nanoparticles can adsorb, wrap, or chemically bind to target compounds (Masood, 2016), enhancing the stability of drugs, especially protein drugs, prolonging their circulation in vivo, and releasing them in a controlled manner (Langer, 2000). Changing the composition and structure of polymer nanoparticles can also tune their behavior under different conditions (Masood, 2016; Zhang et al., 2021).
Table 3.
RES-loaded polymer nanoparticles used for the treatment of various diseases.
| Composition | Targeting moiety | Preparation method | Physicochemical characteristics | Cell line/animal model | Disease | Major outcome | Ref. |
|---|---|---|---|---|---|---|---|
| PLGA | None | Solvent displacement | PS, ∼ 202.8 nm; PI, ∼0.17; EE, 89.32 ± 3.51% | Prostate cancer LNCaP cells | Prostate cancer | Increased cytotoxicity in LNCaP cells | Nassir et al. (2018) |
| PLGA; polyvinyl alcohol | None | None | PS, ∼ 257.9 nm; PI, ∼0.26; ZP, 27.99 mV; EE, 20% | Rats | Isoproterenol-induced myocardial infarction | Improved bioactivity | Sun etal. (2020) |
| PLGA; N-oleoyl-d-galactosamine Tween 80 | None | Solvent diffusion | PS, ∼108.4 nm; PI, ∼0.217; ZP, −46.3 mV; EE, 97.22 ± 2.31% | RAW264.7 cells; rats | Myocardial injury | Improved oral bioavailability | Siu et al. (2018) |
| Folic acid-conjugated PLGA; CTAB | Enterocytes | None | PS, 131 ± 9 nm; PI, 0.181; ZP, −10.7 mV; EE, 59.1 ± 3.3% | Caco-2 cells; rats with intestinal inflammation | Colonic inflammation | Protection under acidic conditions; inflammation suppression | Naserifar et al. (2020) |
| PLGA; chitosan; alginate | None | O/W emulsion technology | PS, ∼255 nm; PI, 0.097 ± 0.095; ZP, ∼13.5 mV; EE, 87.26% | DSS-induced ulcerative colitis mice | Colonic inflammation | Enhanced colon-targeting ability; improved inflammation indicators | Jin et al. (2021) |
| PLGA; polyvinyl alcohol; lactoferrin | Brain capillaries | Emulsion solvent evaporation | PS, 148.2 ± 4.2 nm; PI, 0.12 ± 0.18; ZP, −23.1 ± 3.0 mV; DL, 6.1 ± 0.3%; EE, 75.2 ± 4.1% | SH-SY5Y cells; mice with Parkinson’s disease | Colonic inflammation | Increased blood-brain barrier permeability; enhanced neuroprotective effect | Katila et al. (2022) |
| Sulfobutylether-β-cyclodextrin (4% w/v); polyvinyl alcohol; polyethyleneimine | None | Solvent evaporation | PS, 264.2 ± 0.03 nm; PI, 0.16 ± 0.03; ZP, −1.46 ± 1.47 mV; DL, 0.72 ± 0.09%; EE, 29.1 ± 2.0% | A549, H157, H460, H4006, H358, HEK-293 cells | Non-small cell lung cancer | Increased water solubility; enhanced cytotoxicity | Wang et al. (2020) |
| mPEG750-PLA1000 | None | None | PS, 162.2 ± 2.9 nm; PI, 0.062 ± 0.024; ZP, −11.0 ± 0.4 mV; DL, 8.7%; EE, 95.1 ± 0.1% | B16-F10 cells; C57BL/6J mouse model | Melanoma | Reduced degradation and metabolism; increased antitumor activity | Yee et al. (2022) |
CTAB: cetyltrimethylammonium bromide; DL: drug loading efficiency; DSS: dextran sodium sulfate; EE: entrapment efficiency; mPEG: methoxy poly (ethylene glycol); PI: polydispersity index; PLGA: poly(lactic-co-glycolic acid); PS: particle size; ZP: zeta potential
PLGA is the polymer most commonly used for the synthesis of nanocarriers. For example, loading RES into PLGA nanoparticles (15.6 μM) improved its IC50 value from 29.7 to 15.6 μM and induced apoptosis in LNCaP prostate cancer cells, without adverse effects on normal macrophages (Nassir et al., 2018). In another study, RES-loaded PLGA nanoparticles showed better anti-inflammatory, anti-oxidant, and cardioprotective effects than free RES in the treatment of myocardial injury in vivo (Sun et al., 2020). However, polymer nanoparticles do not enhance the efficiency of passive targeting (Zhang et al., 2014; Shahnaz et al., 2017). Therefore, their surface has been modified with ligands or macromolecules to enhance their ability to target disease sites. For example, modifying RES-loaded PLGA nanoparticles with the monosaccharide galactose improved intestinal uptake, antitumor effects, and oral bioavailability of RES in rats (Siu et al., 2018). In another study, modifying RES-loaded PLGA nanoparticles with folic acid enhanced their ability to enter colon cells and suppress colon inflammation (Naserifar et al., 2020).
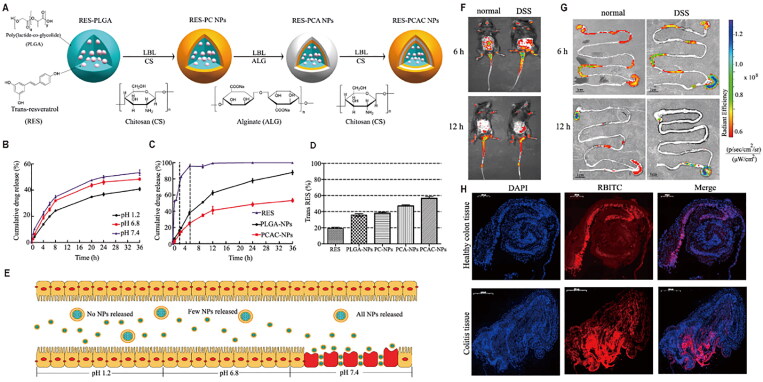
The kinetics of cargo release from PLGA nanoparticles, especially the initial burst, is difficult to control (Reinhold & Schwendeman, 2013). To address this drawback, the surface of RES-loaded PLGA nanoparticles was modified with chitosan and alginate to form a polymer membrane (PCAC nanoparticles) (Figure 3) (Jin et al., 2021). Following oral administration to mice, the electrostatic interactions between chitosan and alginate were enhanced under acidic conditions in the stomach, reducing pore size and slowing drug release, while alkaline pH similar to that in the intestines enlarged the pores and accelerated drug release. Thus, PCAC nanoparticles not only protected the drug from degradation but also released it selectively in the simulated intestinal fluid. By labeling PCAC nanoparticles with rhodamine B isothiocyanate, investigators showed that the nanoplatform penetrated deep into the mucosa through the enhanced permeability and retention effect, targeting inflammatory cells and enhancing the therapeutic effects of RES. In another study, RES-loaded PLGA nanoparticles were modified with lactoferrin, a natural iron-binding cationic glycoprotein that targets brain capillaries, helping them cross the blood–brain barrier (Katila et al., 2022). In addition, the modification of PLGA nanoparticles with sulfobutylether-β-cyclodextrin significantly increased the water solubility of RES and enhanced its antitumor activity against non-small cell lung cancer (Wang et al., 2020). Similarly, methoxy poly (ethylene glycol)-poly(lactide) nanoparticles improved the liver accumulation and plasma stability of free RES, leading to good therapeutic effects in a mouse model of melanoma (Yee et al., 2022).
Figure 3.
(A) Preparation of resveratrol (RES)-loaded PLGA nanoparticles (NPs) modified with chitosan and alginate (PCAC-NPs) by the layer-by-layer (LBL) assembly method. (B) Release curves of PCAC-NPs at pH 1.2, 6.8, and 7.4. (C) Release curves of RES, PLGA-NPs, and PCAC-NPs in simulated digestive fluid. (D) Retention of different formulations exposed to ultraviolet light for 120 min. (E) Schematic illustration of the path of PCAC-NPs after entering simulated gastric fluid. (F) Fluorescence distribution in mice at 6 and 12 h after oral administration of RES-PCAC-NPs. (G) Near-infrared fluorescence images showing the accumulation of NPs in the colon at 6 and 12 h post-administration. (H) Uptake of NPs by colon tissues after 6 h of co-incubation. Reprinted from (Shahnaz et al., 2017) with permission. DAPI: 4′,6-diamidino-2-phenylindole; DSS: dextran sodium sulfate; PLA: polylactic acid; PLGA: poly(lactic-co-glycolic acid); RBITC: rhodamine B isothiocyanate.
2.1.3. Nanomicelles
Nanomicelles are macromolecules with a hydrophobic core and a hydrophilic shell that form from block or graft copolymers in aqueous solution when the micelle concentration exceeds the critical micelle concentration (Table 4) (Xu et al., 2020; Feng et al., 2020). Nanomicelles can encapsulate hydrophobic drugs through covalent binding or physical trapping (Lu & Park, 2013), protecting them from the external environment, improving their pharmacokinetic properties, and reducing toxicity (Kataoka et al., 2001). The hydrophilic shell also enables them to escape clearance by the reticuloendothelial system (Sawant & Torchilin, 2010).
Table 4.
RES-loaded nanoparticles used for the treatment of various diseases.
| Classification | Composition | Preparation method | Physicochemical characteristics | Cell line/animal model | Disease | Major outcome | Ref. |
|---|---|---|---|---|---|---|---|
| Polymer micelles | Stearic acid; inulin; Pluronic F68 | Solvent evaporation | PS, 172 nm; PI, 0.237; ZP, −18 mV; EE, 56.03 ± 1.0% | Human colorectal cancer cells (HCT116); Sprague-Dawley rats | Colon cancer | pH-sensitive release; enhanced cytotoxicity in HCT116 cells; improved pharmacokinetics | Jangid et al. (2020) |
| Pluronic F68; Pluronic F127; Polylactic acid | Thin-film hydration | PS, ∼52.97 nm; PI, ∼0.684; ZP, −32.5 ± 4.10 mV; DL, 2.56 ± 0.25%; EE, 76.20 ± 4.51% | Adult male Wistar rats (150 ± 20 g) | Arthritis | Improved cartilage lesions and synovial inflammation | Kamel et al. (2019) | |
| Solid lipid nanoparticles | Soy PC; Kolliphor® HS15; (+)-Alpha (α)-tocopherol acetate | Solvent evaporation | PS, ∼140 nm; PI, 0.084; ZP, −19 mV; DL, 96.5%; EE, 28.5% | Murine 3T3-L2 fibroblasts | Obesity | Increased water solubility and stability; enhanced browning of white fat cells | Zu et al. (2018) |
| Egg yolk lecithin; molten glycerol monostearate; poloxamer 188 | Emulsification/diffusion; sonication | PS, 271.13 nm; ZP, −25.8 ± 0.33 mV; EE, 23.98% | Sprague-Dawley rats | Doxorubicin-induced cardiotoxicity | Alleviation of doxorubicin-induced cardiotoxicity | Zhang et al. (2019) | |
| D-α-Tocopheryl polyethylene glycol 1000 succinate; stearic acid; lecithin; Tween80 | Emulsification and low-temperature solidification | PS, 271.13 nm; ZP, −25.6 ± 1.3 mV; EE, 32.4 ± 2.6% | SKBR3/PR cells; SKBR3/PR tumor-bearing mice | Breast cancer | Increased cellular uptake; improved antitumor effects | Wang et al. (2021) | |
| Capmul MCMC10; CTAB; Tween 80 | Hot high-pressure homogenization | PS, 139 nm; PI, 0.271; ZP, 50.25 mV; DL, 24.3%; EE, 83.8% | HepG2 cells; rats with hepatocellular carcinoma | Hepatocellular carcinoma | Improved antitumor activity | Rahman et al. (2020) | |
| Nanostructured lipid carriers | Poly(ε-caprolactone); capric/caprylic triglyceride; sorbitan monostearate; polysorbate 80 | None | PS, 250 ± 10 nm; PI, 0.15 ± 0.04; ZP, −15.8 ± 3.0 mV; DL, 0.964 ± 0.037%; EE, 99.89 ± 1.3% | A/J mice | Acute lung injury | Improved anti-acute lung injury activity | de Oliveira et al. (2019) |
| Sodium cholate; Tween-80; trimyristin; triolein; phosphatidylcholines; span-80 | Hot melt emulsification | PS, 55.78 nm; PI, 0.244; ZP, −25.6 mV | Human coronary artery endothelial cells | Hypertension | Restored vasodilator responses | Astley et al. (2021) | |
| Glyceryl monostearate; 1,2-dipalmitoyl-sn-glycero-3-phosphocholine; polysorbate 80 | Thin-film hydration/ultrasonic dispersion | PS, 123.7 nm; PI, 0.082; ZP, −19.4 mV; EE, 94.4% | Pulmonary artery smooth muscle cells; male Sprague–Dawley rats | Pulmonary arterial hypertension | Improved drug delivery to the lungs | Li et al. (2020) | |
| Oleic acid; stearic acid; phospholipon; poloxamer; folic acid | Solvent injection | PS, 82.44 ± 2.78 nm; PI, 0.375 ± 0.124; ZP, −42.15 ± 3.76 mV; DL, 8.05 ± 0.75%; EE, 86.34 ± 1.98% | MCF-7 cells | Breast cancer | Enhanced anticancer activity; higher accumulation | Poonia et al. (2019) | |
| Capric triglyceride; shea butter; olivem 1000 and 800; vitamin E; red myrrh; allantoin; EDTA-2Na; sodium hyaluronate; CMC-Na; methylisothiazolinone; glycerol | Ultrasonic emulsification | PS, 175.6 ± 11.2 nm; PI, 0.179 ± 0.007; ZP, −54.3 ± 3.0 mV; EE, 97.76% | HaCaT cells; rabbits | Improved anti-ultraviolet radiation and antioxidant activity in vivo | Miao et al. (2021) | ||
| Nanocrystals | Pluronic F127 | Wet media milling | PS, 270 ± 7.2 nm; PI, 0.310 ± 0.005 | Ehrlich ascites tumor cells; Swiss albino inbred mice | Ehrlich ascites carcinoma | Reduced tumor cell proliferation | Xiong et al. (2020) |
| Polyvinylpyrrolidone K90 | Anti-solvent precipitation | PS, 222.54 ± 1.66 nm; PI, 0.125 ± 0.035; ZP, −9.41 ± 0.37 mV; EE, 21.74% | Madin–Darby canine kidney cells; SH-SY5Y cells; zebrafish embryos at 6 h post-fertilization; Sprague-Dawley rats | Parkinson’s disease | Improved oral bioavailability and brain accumulation | Ancic et al. (2022) | |
| Gold nanoparticles | NaAuCl4; gum arabic | None | PS, 16.7 ± 4.6 nm | MDAMB-231, PANC-1, PC-3 cells | Breast, pancreatic, prostate cancer | Synergistic anti-tumor effects | Thipe et al. (2019) |
| Dendrimers | Glycosylated maize dendrimer dextran | None | ZP, −9.5 mV; DL, 1.4% | Caco-2 cells; HaCaT cells | Enhanced drug solubility | Shi et al. (2020) | |
| Nanoemulsions | Coconut oil; Pluronic P107; Cremophor EL | Simple vortexing | PS, 110.37 ± 2.16 nm; PI, 0.194 ± 0.003; ZP, −21.13 ± 1.628 mV | Wistar rats | Alzheimer’s disease | Improved brain-targeting efficacy | Kotta et al. (2021) |
| Tween 20; Neem seed oil; HEPES buffer | Simple vortexing | PS, 137.8 ± 0.5 nm; PI, 0.22 ± 0.1; ZP, −23.0 ± 0.7 mV | Human T24 bladder cancer cells | Bladder cancer | High loading capacity; enhanced solubility | Rinaldi et al. (2021) | |
| Miglyol 812; polysorbate 80; ethanol | None | PS, 24 ± 7 nm; PI, 0.291 ± 0.062; ZP, −15.8 ± 2.6 mV | Human immortalized T/C28a2 chondrocytes | Osteoarthritis | Improved protection against oxidative stress-mediated T/C28a2 cell death | Le Clanche et al. (2018) | |
| Isopropyl myristate; polysorbate 80; ethanol | None | PS, 103 ± 14 nm; PI, 0.389 ± 0.051; ZP, −14.7 ± 2.0 mV | Human immortalized T/C28a2 chondrocytes | Osteoarthritis | Improved protection against oxidative stress-mediated death in T/C28a2 cells | Le Clanche et al. (2018) |
CTAB: cetyltrimethylammonium bromide; DL: drug loading efficiency; EE: entrapment efficiency; PC: phosphatidylcholine; PI: polydispersity index; PS: particle size; ZP: zeta potential
Pluronic F68 is widely used for the preparation of nanomicelles due to its low cost and good biocompatibility, but its high critical micelle concentration significantly reduces drug encapsulation efficiency (Chaudhari & Patil, 2014; Kim et al., 2021). Therefore, a recent study conjugated the two ends of Pluronic F68 with stearic acid and inulin, respectively, to increase the hydrophobic segment, reduce the critical micelle concentration, and protect the drug-loaded nanocapsules from the gastric environment while improving the oral bioavailability of RES and achieving controlled drug release for colon cancer treatment (Jangid et al., 2020). In another study, a mixed micellar system prepared using poloxamers 188 and 407 was loaded with RES and coated with bioresorbable polylactic acid to form hybrid nanomicelles with better biocompatibility and anti-arthritic effects than free RES (Kamel et al., 2019).
2.1.4. Solid lipid nanoparticles
Solid lipid nanoparticles (SLNs) are nanosized materials that can be loaded with hydrophilic and lipophilic drugs and easily modified with ligands due to their functional surface groups (Garces et al., 2018). SLNs are considered effective carriers, as they can encapsulate or disperse drugs in natural or synthetic solid lipids, giving rise to solid colloidal structures that can store lipophilic molecules (Table 4). For example, loading RES onto SLNs significantly increased the plasma concentration and area under the curve of the drug, reduced the time needed to reach the maximum plasma concentration, and promoted oral absorption, thereby improving the ability of RES to protect against doxorubicin-induced cardiac toxicity in mice (Zhang et al., 2019). SLNs have also been identified as a promising delivery platform for therapeutic agents against tumor drug resistance (Majidinia et al., 2020). For instance, in comparison with free resveratrol, RES-loaded SLNs promoted the absorption of drugs by down-regulating the expression of overexpression of drug efflux transporters P-gp and breast cancer drug resistance protein (Wang et al., 2021). Another study reported that cationic RES-loaded SLNs showed significantly stronger anti-hepatocellular carcinoma activity in vitro and in vivo than conventional RES-loaded SLNs because of their stronger affinity for negatively charged tumor cell membranes (Rahman et al., 2020). Nevertheless, single solid lipids may form a perfect lattice structure during the preparation of SLNs; in this case, the lattice squeezes out the drug during storage, leading to low drug loading efficiency and leakage (Chen et al., 2010; Das et al., 2012).
2.1.5. Nanostructured lipid carriers
Nanostructured lipid carriers (NLCs) are a new generation of lipid nanoparticles developed to overcome the limitations of SLNs (Khosa et al., 2018). To prepare NLCs, a certain proportion of liquid lipids is introduced into solid lipid carriers, disrupting the perfect lattice structure of SLNs; this reduces drug leakage during storage, stabilizes the nanoparticles (Tapeinos et al., 2017), and increases their capacity for hydrophobic drugs, which are highly soluble in liquid lipids (Table 4) (Alam et al., 2015). Recent studies have shown that RES-loaded NLCs prepared by interfacial polymer deposition can improve acute lung injury (de Oliveira et al., 2019), protect blood vessels, and enhance the antihypertensive effects of RES (Astley et al., 2021; Li et al., 2021). Lecithin is naturally present in plant and animal tissue, with a combination of glycerophospholipids. A nanoplatform based on the lipid structure of lecithin to encapsulate RES was designed. This nanoparticle is stable at ambient temperature as well as at 4 °C for up to 12 months, with inherent anti-oxidant and anti-cancer properties; indicating the feasibility of using this system as an cost-effective, and low side-effect anti-cancer therapeutic (Liang et al., 2022). To improve the tumor targeting ability of such nanoparticles, RES-loaded NLCs were modified with folic acid, improving cytotoxicity in MCF-7 cells overexpressing folate receptor (Poonia et al., 2019). NLCs have also shown the ability to protect skin by delivering RES to the stratum corneum and epidermis, but their fluidity limited their stability on the skin surface. To improve the stability and accumulation of RES on the epidermis, NLCs were modified with a hydrogel with good viscosity and ductility, resulting in a nanoplatform with promising protective effects against ultraviolet radiation and oxidants (Miao et al., 2021).
2.1.6. Nanocrystals
Drug nanocrystals are usually formed as nanosuspensions in the presence of surfactants, polymers, or their mixture as stabilizers (Lu et al., 2019; Li et al., 2021). Nanocrystals can improve the dissolution and absorption of insoluble drugs by increasing the specific surface area and saturation solubility, showing higher drug loading capacity than other nanoformulations (Fontana et al., 2018) (Table 4). The use of nanocrystals also reduces the potential toxicity of excipients and promotes drug accumulation at target sites (Lu et al., 2017). However, their interactions with biologically tissues should be investigated in detail, as they can persist for a long time in biological environments. To enhance the solubility and bioavailability of RES, another study developed RES-loaded nanocrystals by the spontaneous conjugation of RES with hydroxypropyl methyl cellulose through van der Waals forces. The prepared nano-system entered cells through lattice-protein-mediated endocytosis, significantly enhancing the cellular uptake of RES, and protecting neurons from chemically induced cytotoxicity. Moreover, they showed negligible toxicity toward zebrafish embryos and larvae and exhibited more favorable pharmacokinetics and oral bioavailability in rats. Similar results were observed in mice with Parkinson’s disease, suggesting that nanocrystals may be a promising formulation for both oral and systemic delivery of RES (Xiong et al., 2020). Loading RES into nanocrystals significantly strengthened the drug’s ability to inhibit the proliferation of peritoneal tumor cells in Ehrlich ascites tumor (EAT)-bearing mice. RES-loaded nanocrystals ameliorated free RES-induced hepatocyte necrosis and apoptosis and liver fibrosis; however, as with free RES, RES-loaded nanocrystals resulted in inflammation of proximal tubular necrosis and glomerular swelling, as well as a slight elevation of several biochemical parameters that did not prolong the life span of EAT bearing mice (Ancic et al., 2022). In order to increase the beneficial effects and reduce risks associated with resveratrol nanocrystals, additional factors such as dose, genetics, health status, and the nature of the target cells should also be considered.
2.1.7. Inorganic nanoparticles
Inorganic nanoparticles (Fan et al., 2020), such as mesoporous silica nanoparticles (MSNs), gold nanoparticles (GNPs), silver nanoparticles (AgNPs), and quantum dots (QDs), have been used as delivery carriers for therapeutic cargos (Sperling & Parak, 2010; Pearce & O’Reilly, 2019) due to their unique magnetic and optical properties, which distinguish them from their organic and polymeric counterparts (Huang et al., 2011) (Table 4).
GNPs exhibit good stability, high thermal, optical, and electrical activity, high surface area, and multifunctionality (Amina and Guo, 2020), RES was biocoupled with GNPs via the cross-linking agent polyvinylpyrrolidone (RES@PVP-GNPs) to enhance the delivery performance and anti-tumor efficacy of RES (Lee et al., 2022). However, GNPs are usually synthesized by physical or chemical methods that may be toxic to humans. In contrast, synthesizing GNPs with plant-derived secondary metabolites such as RES is environmentally friendlier and may be safer for subsequent in vivo use (Bharadwaj et al., 2021; Akintelu et al., 2021). For example, RES-loaded GNPs were synthesized at room temperature through the RES-mediated reduction of Au3+ into Au0, and the nanoparticle surface was then wrapped with highly branched gum Arabic to improve drug loading efficiency and overall stability. The modified GNPs had optimal cellular uptake at 24 h post-incubation and exhibited good synergistic antitumor effects (Thipe et al., 2019). The same method was used to prepare AgNPs (Kup et al., 2020).
MSNs are also widely used in drug delivery and biomedicine due to their large surface area and pore volume. It has been reported that encapsulating RES in colloidal MSNs with high loading capacity (20% w/w) and excellent encapsulation efficiency (100%) can enhance its solubility by 95% and improve in vitro release kinetics, leading to stronger anti-inflammatory and anti-tumor activities than free RES (Summerlin et al., 2016).
2.1.8. Dendrimers
Dendrimers are highly branched, star-shaped macromolecules with nanometer-scale dimensions (Svenson, 2009). Unlike conventional polymers, the molecular weight and chemical composition of dendrimers can be controlled by modulating their synthesis, resulting in higher loading capacity (Menjoge et al., 2010) and improved biocompatibility, pharmacokinetics (Lee et al., 2005), and polydispersity (Boas & Heegaard, 2004). In a recent study, RES was conjugated to the amino terminus of glycosylated maize dendrimer dextran, affording a nano-delivery system with higher solubility and anti-oxidant activity that improved the cellular uptake of RES and protected against oxidative cell damage in Caco-2 cells (Shi et al., 2020) (Table 4).
2.1.9. Nanoemulsions
Nanoemulsions are a biphasic dispersion of two immiscible liquids, one in the dispersed phase and the other in the continuous phase, which are generally stabilized using surfactants and co-surfactants as emulsifiers (Bonferoni et al., 2019). Nanoemulsions are usually formed using high pressure homogenizers, high shear stirring, or ultrasound generators as external forces to promote the release and absorption of the drug after digestion (Choradiya & Patil, 2021), while enhancing targeted drug delivery and minimizing adverse and toxic reactions (Jaiswal et al., 2015) (Table 4). For example, a RES-loaded nanoemulsion was prepared using coconut oil as the oil phase and Pluronic-107 and Cremophor EL as surfactants (Kotta et al., 2021). The optimized preparation showed better drug release properties than an RES suspension in 0.5% (w/v) sodium carboxymethyl cellulose and exhibited a good brain-targeting effect after intranasal administration in rats. The nanoemulsion was also stable at room temperature for 3 months (Kotta et al., 2021).
Among such nanomaterials, oil-in-water (O/W) nanoemulsions are considered ideal for encapsulating RES, as they can be easily prepared through high-energy processes using natural ingredients and low emulsifier concentrations. For example, an RES-based O/W nanoemulsion significantly reduced cell viability in bladder T24 cancer cells and enhanced the cytotoxic activity of RES through fast intracellular drug uptake (Rinaldi et al., 2021), suggesting that O/W nanoemulsions can effectively improve RES bioavailability. In another study, two RES self-emulsifying systems increased the tolerance of human immortalized chondrocytes toward RES, reduced its cytotoxicity at high concentrations, promoted drug uptake by membranes and cells, and improved the anti-oxidant activity of the free drug (Le Clanche et al., 2018).
2.2. Bionic drug delivery systems
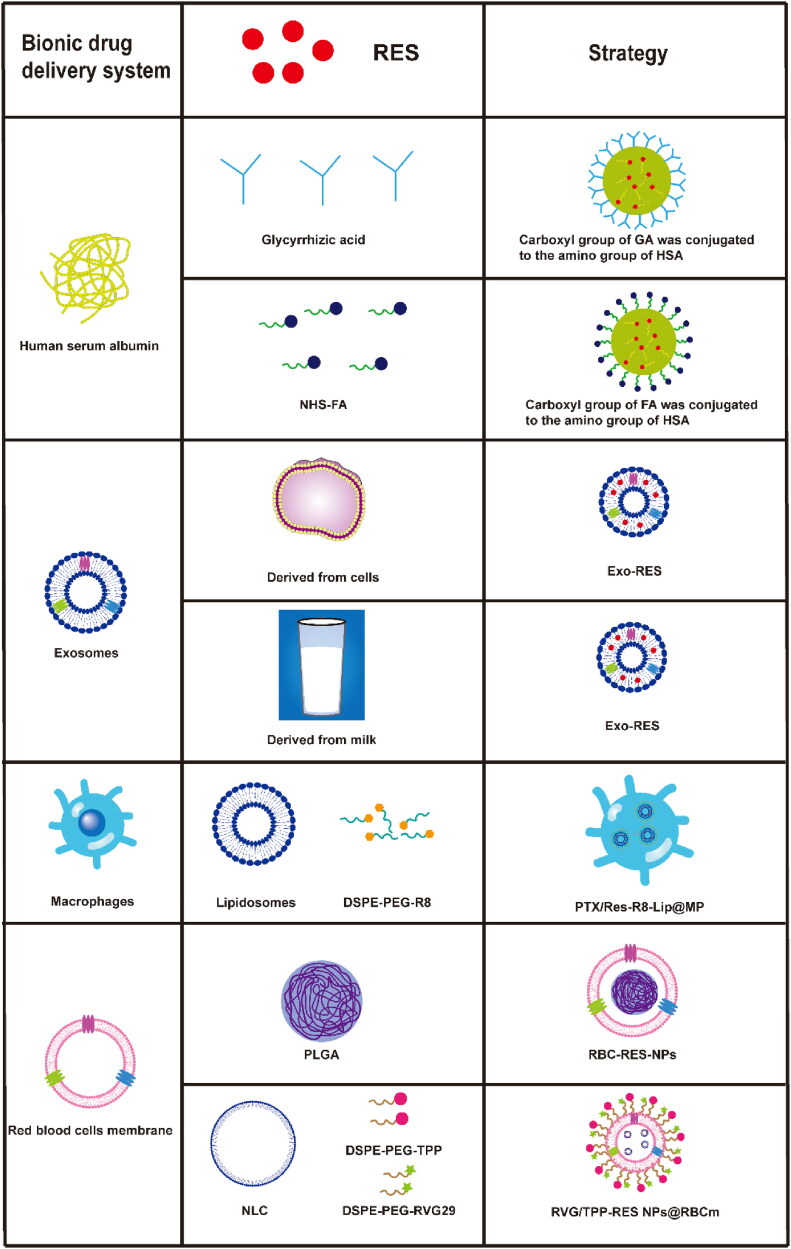
Synthetic nanocarriers protect drugs from degradation and improve their oral bioavailability and therapeutic effects, but they suffer from weak targeting, toxicity, and easy elimination by the immune system (Torchilin, 2005). To avoid these problems, bionic drug delivery systems resemble natural particles such as cells (Chen et al., 2022), pathogens (Yin et al., 2022), and endogenous proteins (Wu et al., 2020), mimicking their in vivo activity and selectively delivering drugs to target sites. The result is lower immunogenicity, fewer side effects, and stronger therapeutic effects than with conventional nanoparticles (Li et al., 2021) (Figure 4).
Figure 4.
Bionic drug delivery systems loaded with resveratrol (RES). DSPE: 1,2-distearoyl-sn-glycero-3-phosphoethanolamine; Exo: exosomes; FA: folic acid; GA: glycyrrhizic acid; HSA: human serum albumin; MP: macrophage; NHS: N-hydroxysuccinimide; NLC: nanostructured lipid carrier; PEG: poly(ethylene glycol); PTX: paclitaxel; R8: octaarginine; PLGA: poly (lactic acid)-glycolic acid; RBCm: red blood cell membrane; RVG: rabies virus glycoprotein; TPP: 4-carboxybutyl triphenylphosphonium bromide.
2.2.1. Protein-based nanoparticles
Albumin, the most abundant plasma protein, plays a key role in the metabolism, transfer, and distribution of nutrients in cells. Albumin has been used extensively in nanotechnology as carrier due to its good drug binding ability, high stability, biodegradability, low toxicity, non-immunogenicity, and biocompatibility (Iqbal et al., 2021). Hydrophobic drugs such as RES can be easily encapsulated into albumin nanoparticles, while carboxyl and amino groups on the surface of albumin facilitate surface functionalization of its nanoparticles (Zhu et al., 2017). For example, encapsulating RES into glycyrrhizin acid-conjugated human serum albumin nanoparticles significantly improved its pharmacokinetic properties, bioavailability, and targeting of the liver (Wu et al., 2020). In another study, folate-modified RES-loaded human serum albumin nanoparticles selectively delivered RES to tumor sites and induced apoptosis in HepG2 cells more effectively than free RES (Lian et al., 2019).
2.2.2. Exosomes
Exosomes are cell-derived vesicles with a particle size of 40–160 nm that can transfer chemical or genomic contents from parental to daughter cells (Yan & Jiang, 2020). Their membrane also contains several integrin-interacting proteins and antigens, allowing exosomes to overcome various biological barriers and achieve specific recognition and long circulation in blood as well as escape clearance by the immune system (Wang et al., 2019; Wang et al., 2022). Thus, exosomes have been extensively used as drug carriers to improve therapeutic outcomes. For example, loading RES into exosomes derived from primary microglia allowed the drug to penetrate the blood–brain barrier and stabilized it, prolonging its therapeutic efficacy. In addition, the exosomes activated neuronal autophagy via PI3K signaling, significantly promoting neuronal repair after central nervous system injury (Fan et al., 2020).
However, the extraction of exosomes from biological fluids and cell culture media is inefficient (Haney et al., 2015). In contrast, milk has been identified as a cost-efficient source of large amounts of exosomes that show cross-species biocompatibility, lack toxicity, encapsulate hydrophilic and lipophilic macromolecules, and efficiently cross the blood–brain barrier (Munagala et al., 2016). RES was loaded passively into milk-derived exosomes, which then delivered the drug selectively to rat mammary tissue, inhibiting the proliferation of MCF-7 and MDA-MB-231 breast cancer cells more strongly than free RES (Gonzalez-Sarrias et al., 2022).
2.2.3. Macrophages
Macrophages enter the tumor microenvironment after surgical resection by recruiting monocyte chemoattractant protein-1 (CCL-2) and pro-inflammatory factors, suggesting that macrophage-derived carriers may enhance drug delivery and accumulation in scattered tumor cells that escape resection. In one approach, liposomes were modified with octa-arginine, a cell-penetrating peptide, and loaded simultaneously with RES and paclitaxel. The obtained liposomes were then internalized by macrophages, affording a cell-mediated carrier with high drug-loading capacity as well as the ability to target sites of inflammation and tumors. The liposomes entered tumor cells, inhibiting their growth and postoperative recurrence in a 4T1 orthotopic mouse model (Qiu et al., 2021).
2.2.4. Red blood cells
Red blood cells (RBCs) are responsible for the transport of oxygen to tissues or organs and have a lifespan of about 115 d in the human body, as some of their membrane glycoproteins protect them from immune system clearance (Dupire et al., 2012; Franco, 2012). For example, the transmembrane protein CD47 on the RBC membrane prevents their uptake by macrophages by selectively binding to the signal regulatory protein-α on macrophages, which acts as a ‘don’t eat me’ marker (Muzykantov, 2010). Due to their high drug-loading capacity and easy collection, RBCs are considered ideal drug carriers for prolonged circulation with good biocompatibility and low immunogenicity (Gutierrez Millan et al., 2012). For example, coating RES-loaded PLGA nanoparticles with RBC membrane prolonged the circulation of RES and released the drug in a sustained manner after systemic injection in rats, leading to a half-life significantly longer than that of free RES or uncoated nanoparticles (Li et al., 2019). However, in the treatment of brain diseases such as Alzheimer’s disease, the use of toxic organic reagents in the preparation of PLGA nanoparticles and the acidic by-products of PLGA during degradation may make it unsuitable for long-term use in the brain (Yang, 2010; Fuhrmann et al., 2015). NLCs based on natural lipids with better biocompatibility may be more suitable for brain formulations than PLGA nanoparticles (Fu et al., 2019). To enhance the efficacy of anti-Alzheimer’s disease treatment, RBC membrane-encapsulated nanostructured lipid particles (NPs@RBCm) were prepared and rabies virus glycoprotein (RVG29) targeting the brain and triphenylphosphine cation (TPP) targeting the mitochondria were introduced using a green lipid insertion method to the RBC membrane surface (RVG/TPP NPs@RBCm), allowing RES delivery across the blood-brain barrier and subsequent targeting of neuronal mitochondria (Han et al., 2020). The experimental nanoformulations did not cause significant damage to normal cells or organs in experimental mice, and the erythrocyte membranes were able to persist a long time in circulation. In addition, co-culture models and in vivo imaging showed that RVG/TPP NPs@RBCm penetrated the blood–brain barrier better than NPs@RBCm, and it targeted neuronal cells, where it localized to mitochondria. These results suggest that polymer-based bionic drug delivery systems camouflaged with RBC membranes can effectively prolong the efficacy of RES.
3. Discussion and future perspectives
RES has a wide range of pharmaceutical activities and promising applications in natural medicine, but its unstable pharmacokinetics undermine its therapeutic efficacy and hinder clinical application. To overcome these drawbacks, RES has been encapsulated into specific nanocarriers, including liposomes, polymeric nanoparticles, SLNs, protein-based nanoparticles, and inorganic nanoparticles, which can modulate drug release to reach significant therapeutic concentrations in plasma and improve bioavailability. Among these nanocarriers, polymer nanoparticles are most widely used due to their high encapsulation efficiency, which significantly reduces the amount of nanocarriers required to achieve the desired bioactivity and to reduce potential toxic and side effects (Santos et al., 2014). The behavior of polymer nanoparticles under different conditions or in response to specific stimuli can easily be tuned by changing their composition and structure, while their surface can be functionalized with ligands that bind to specific cell receptors for targeted RES delivery. Despite their advantages, synthetic nanoparticles such as inorganic nanoparticles and nano-emulsions have low encapsulation capacity and present several toxicity and safety issues that limit their therapeutic efficacy (Rezaei et al., 2019; Roy et al., 2019).
Compared to synthetic nano-systems, biologically derived carriers can greatly improve the biological distribution, cellular uptake, and controlled release of encapsulated drugs, while showing higher biocompatibility and lower toxicity (Bu et al., 2019). Bionic drug delivery systems have strong affinity for cells and can easily escape phagocytosis by endothelial reticulocytes, stabilizing drugs in the circulation. However, proteins, exosomes, and other bionic nanocarriers cannot easily be obtained on a large scale, highlighting the need to discover novel delivery systems for naturally derived drugs like RES that show low water solubility, weak ability to penetrate cells, and poor bioavailability. Additional studies are also needed to extend our knowledge on the pharmacokinetics, biodistribution, toxicity, and biocompatibility of RES-loaded nano-formulations and validate their performance in vivo.
4. Conclusion
Our review illustrates how the encapsulation of RES into synthetic or natural nanocarriers can improve its physicochemical properties and targeted delivery, offering an effective approach for custom-made treatments. However, the therapeutic efficacy of RES-loaded nanoparticles should be further investigated with in vivo studies and clinical trials to ensure their suitability for the clinic.
Funding Statement
This work was supported by the [Science and Technology Department of Sichuan Province #1] under Grant [number 23NSFSC0589]; [Xuzhou District People’s Government - Science and Technology Project of Southwest Medical University #2] under grant [number 2019-YBXNYD-4]; and [Scientific and technological achievements Transformation Project of Southwest Medical University #3] under Grant [number 2020-6].
Ethical approval statement
NA.
Author contributions
Chunhong Li and Zhen Wang contributed equally. All authors have given approval to the final version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors
References
- Abri Aghdam M, Bagheri R, Mosafer J, et al. (2019). Recent advances on thermosensitive and ph-sensitive liposomes employed in controlled release. J Control Release 315:1–15. [DOI] [PubMed] [Google Scholar]
- Abu Lila AS, Ishida T. (2017). Liposomal delivery systems: design optimization and current applications. Biol Pharm Bull 40:1–10. [DOI] [PubMed] [Google Scholar]
- Ahmed T, Javed S, Javed S, et al. (2017). Resveratrol and Alzheimer’s disease: mechanistic insights. Mol Neurobiol 54:2622–35. [DOI] [PubMed] [Google Scholar]
- Akintelu SA, Yao B, Folorunso AS. (2021). Bioremediation and pharmacological applications of gold nanoparticles synthesized from plant materials. Heliyon 7:e06591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam T, Pandit J, Vohora D, et al. (2015). Optimization of nanostructured lipid carriers of lamotrigine for brain delivery: in vitro characterization and in vivo efficacy in epilepsy. Expert Opin Drug Deliv 12:181–94. [DOI] [PubMed] [Google Scholar]
- Alhusaini AM, Fadda LM, Alanazi AM, et al. (2022). Nano-resveratrol: a promising candidate for the treatment of renal toxicity induced by doxorubicin in rats through modulation of beclin-1 and mtor. Front Pharmacol 13:826908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amina SJ, Guo B. (2020). A review on the synthesis and functionalization of gold nanoparticles as a drug delivery vehicle. Int J Nanomed 15:9823–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancic D, Orsolic N, Odeh D, et al. (2022). Resveratrol and its nanocrystals: a promising approach for cancer therapy? Toxicol Appl Pharmacol 435:115851. [DOI] [PubMed] [Google Scholar]
- Argenziano M, Ansari IA, Muntoni E, et al. (2022). Lipid-coated nanocrystals as a tool for improving the antioxidant activity of resveratrol. Antioxidants 11:1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astley C, Houacine C, Zaabalawi A, et al. (2021). Nanostructured lipid carriers deliver resveratrol, restoring attenuated dilation in small coronary arteries, via the ampk pathway. Biomedicines 9:1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharadwaj KK, Rabha B, Pati S, et al. (2021). Green synthesis of gold nanoparticles using plant extracts as beneficial prospect for cancer theranostics. Molecules 26:6389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhullar KS, Hubbard BP. (2015). Lifespan and healthspan extension by resveratrol. Biochim Biophys Acta 1852:1209–18. [DOI] [PubMed] [Google Scholar]
- Boas U, Heegaard PM. (2004). Dendrimers in drug research. Chem Soc Rev 33:43–63. [DOI] [PubMed] [Google Scholar]
- Bonferoni MC, Rossi S, Sandri G, et al. (2019). Nanoemulsions for "nose-to-brain" drug delivery. Pharmaceutics 11:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu LL, Rao L, Yu GT, et al. (2019). Cancer stem cell-platelet hybrid membrane-coated magnetic nanoparticles for enhanced photothermal therapy of head and neck squamous cell carcinoma. Adv Funct Mater 29:1807733. [Google Scholar]
- Caddeo C, Pucci L, Gabriele M, et al. (2018). Stability, biocompatibility and antioxidant activity of peg-modified liposomes containing resveratrol. Int J Pharm 538:40–7. [DOI] [PubMed] [Google Scholar]
- Calabrese E, Mattson M, Calabrese V. (2010). Dose response biology: the case of resveratrol. Hum Exp Toxicol 29:1034–7. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Mattson MP, Calabrese V. (2010). Resveratrol commonly displays hormesis: occurrence and biomedical significance. Hum Exp Toxicol 29:980–1015. [DOI] [PubMed] [Google Scholar]
- Chaudhari SP, Patil JR. (2014). Study of block copolymer micelles as vehicles for hydrophobic drug lamotrigine. IJPER 48:55–66. [Google Scholar]
- Chen CC, Tsai TH, Huang ZR, Fang JY. (2010). Effects of lipophilic emulsifiers on the oral administration of lovastatin from nanostructured lipid carriers: physicochemical characterization and pharmacokinetics. Eur J Pharm Biopharm 74:474–82. [DOI] [PubMed] [Google Scholar]
- Chen J, Jin J, Li K, et al. (2022). Progresses and prospects of neuroprotective agents-loaded nanoparticles and biomimetic material in ischemic stroke. Front Cell Neurosci 16:868323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Zhao PF, Luo ZY, et al. (2016). Cancer cell membrane-biomimetic nanoparticles for homologous-targeting dual-modal imaging and photothermal therapy. Acs Nano 10:10049–57. [DOI] [PubMed] [Google Scholar]
- Choradiya BR, Patil SB. (2021). A comprehensive review on nanoemulsion as an ophthalmic drug delivery system. J Mol Liq 339:116751. [Google Scholar]
- Cottart CH, Nivet-Antoine V, Laguillier-Morizot C, Beaudeux JL. (2010). Resveratrol bioavailability and toxicity in humans. Mol Nutr Food Res 54:7–16. [DOI] [PubMed] [Google Scholar]
- Das S, Ng WK, Tan RB. (2012). Are nanostructured lipid carriers (nlcs) better than solid lipid nanoparticles (slns): development, characterizations and comparative evaluations of clotrimazole-loaded slns and nlcs? Eur J Pharm Sci 47:139–51. [DOI] [PubMed] [Google Scholar]
- de Oliveira MTP, de Sa Coutinho D, Tenorio de Souza E, et al. (2019). Orally delivered resveratrol-loaded lipid-core nanocapsules ameliorate lps-induced acute lung injury via the erk and pi3k/akt pathways. Int J Nanomedicine 14:5215–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupire J, Socol M, Viallat A. (2012). Full dynamics of a red blood cell in shear flow. Proc Natl Acad Sci USA 109:20808–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Li Y, Huang S, et al. (2020). Resveratrol-primed exosomes strongly promote the recovery of motor function in sci rats by activating autophagy and inhibiting apoptosis via the pi3k signaling pathway. Neurosci Lett 736:135262. [DOI] [PubMed] [Google Scholar]
- Fan Z, Jiang B, Zhu Q, et al. (2020). Tumor-specific endogenous fe(ii)-activated, mri-guided self-targeting gadolinium-coordinated theranostic nanoplatforms for amplification of ros and enhanced chemodynamic chemotherapy. ACS Appl Mater Interfaces 12:14884–904. [DOI] [PubMed] [Google Scholar]
- Fang ZX, Bhandari B. (2010). Encapsulation of polyphenols - a review. Trend Food Sci Technol 21:510–23. [Google Scholar]
- Feng X, Chen YT, Li LY, et al. (2020). Preparation, evaluation and metabolites study in rats of novel isoginkgetin-loaded tpgs/soluplus mixed nanomicelles. J Food Drug Anal 28:309–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer M, Vogtle F. (1999). Dendrimers: from design to application—A progress report. Angew Chem Int Ed 38:884–905. [DOI] [PubMed] [Google Scholar]
- Fontana F, Figueiredo P, Zhang P, et al. (2018). Production of pure drug nanocrystals and nano co-crystals by confinement methods. Adv Drug Deliv Rev 131:3–21. [DOI] [PubMed] [Google Scholar]
- Franco RS. (2012). Measurement of red cell lifespan and aging. Transfus Med Hemother 39:302–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu SY, Liang M, Wang YL, et al. (2019). Dual-modified novel biomimetic nanocarriers improve targeting and therapeutic efficacy in glioma. ACS Appl Mater Interfaces 11:1841–54. [DOI] [PubMed] [Google Scholar]
- Fuhrmann T, Ghosh M, Otero A, et al. (2015). Peptide-functionalized polymeric nanoparticles for active targeting of damaged tissue in animals with experimental autoimmune encephalomyelitis. Neurosci Lett 602:126–32. [DOI] [PubMed] [Google Scholar]
- Gal R, Deres L, Toth K, et al. (2021). The effect of resveratrol on the cardiovascular system from molecular mechanisms to clinical results. Int J Mol Sci 22:10152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garces A, Amaral MH, Lobo JMS, Silva AC. (2018). Formulations based on solid lipid nanoparticles (sln) and nanostructured lipid carriers (nlc) for cutaneous use: a review. Eur J Pharm Sci 112:159–67. [DOI] [PubMed] [Google Scholar]
- George A, Shah PA, Shrivastav PS. (2019). Natural biodegradable polymers based nano-formulations for drug delivery: a review. Int J Pharm 561:244–64. [DOI] [PubMed] [Google Scholar]
- Ghalandarlaki N, Alizadeh AM, Ashkani-Esfahani S. (2014). Nanotechnology-applied curcumin for different diseases therapy. Biomed Res Int 2014:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Sarrias A, Iglesias-Aguirre CE, Cortes-Martin A, et al. (2022). Milk-derived exosomes as nanocarriers to deliver curcumin and resveratrol in breast tissue and enhance their anticancer activity. Int J Mol Sci 23:2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilc NK, Sova M, Kristl J. (2021). Drug delivery strategies for curcumin and other natural nrf2 modulators of oxidative stress-related diseases. Pharmaceutics 13:2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Peng Y, Li Y, et al. (2015). Cell death pathway induced by resveratrol-bovine serum albumin nanoparticles in a human ovarian cell line. Oncol Lett 9:1359–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo LY, Peng Y, Yao JP, et al. (2010). Anticancer activity and molecular mechanism of resveratrol-bovine serum albumin nanoparticles on subcutaneously implanted human primary ovarian carcinoma cells in nude mice. Cancer Biother Radiopharm 25:471–7. [DOI] [PubMed] [Google Scholar]
- Gupta A, Eral HB, Hatton TA, Doyle PS. (2016). Nanoemulsions: formation, properties and applications. Soft Matter 12:2826–41. [DOI] [PubMed] [Google Scholar]
- Gutierrez Millan C, Colino Gandarillas CI, Sayalero Marinero ML, Lanao JM. (2012). Cell-based drug-delivery platforms. Ther Deliv 3:25–41. [DOI] [PubMed] [Google Scholar]
- Han Y, Chu XY, Cui L, et al. (2020). Neuronal mitochondria-targeted therapy for Alzheimer’s disease by systemic delivery of resveratrol using dual-modified novel biomimetic nanosystems. Drug Deliv 27:502–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney MJ, Klyachko NL, Zhaoa YL, et al. (2015). Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J Controlled Release 207:18–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao JF, Gao Y, Zhao J, et al. (2015). Preparation and optimization of resveratrol nanosuspensions by antisolvent precipitation using box-behnken design. Aaps Pharmscitech 16:118–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HC, Barua S, Sharma G, et al. (2011). Inorganic nanoparticles for cancer imaging and therapy. J Control Release 155:344–57. [DOI] [PubMed] [Google Scholar]
- Huang J, Huang N, Xu S, et al. (2021). Signaling mechanisms underlying inhibition of neuroinflammation by resveratrol in neurodegenerative diseases. J Nutr Biochem 88:108552. [DOI] [PubMed] [Google Scholar]
- Huang X, Mazza G. (2011). Simultaneous analysis of serotonin, melatonin, piceid and resveratrol in fruits using liquid chromatography tandem mass spectrometry. J Chromatogr A 1218:3890–9. [DOI] [PubMed] [Google Scholar]
- Iqbal H, Yang T, Li T, et al. (2021). Serum protein-based nanoparticles for cancer diagnosis and treatment. J Control Release 329:997–1022. [DOI] [PubMed] [Google Scholar]
- Iqubal MK, Iqubal A, Imtiyaz K, et al. (2021). Combinatorial lipid-nanosystem for dermal delivery of 5-fluorouracil and resveratrol against skin cancer: delineation of improved dermatokinetics and epidermal drug deposition enhancement analysis. Eur J Pharm Biopharm 163:223–39. [DOI] [PubMed] [Google Scholar]
- Jaiswal M, Dudhe R, Sharma PK. (2015). Nanoemulsion: an advanced mode of drug delivery system. 3 Biotech 5:123–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jangid AK, Patel K, Jain P, et al. (2020). Inulin-pluronic-stearic acid based double folded nanomicelles for ph-responsive delivery of resveratrol. Carbohydr Polym 247:116730. [DOI] [PubMed] [Google Scholar]
- Jermain SV, Brough C, Williams RO. (2018). Amorphous solid dispersions and nanocrystal technologies for poorly water-soluble drug delivery - an update. Int J Pharm 535:379–92. [DOI] [PubMed] [Google Scholar]
- Jin M, Li S, Wu Y, et al. (2021). Construction of chitosan/alginate nano-drug delivery system for improving dextran sodium sulfate-induced colitis in mice. Nanomaterials (Basel 11:1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamel R, Abbas H, Shaffie NM. (2019). Development and evaluation of pla-coated co-micellar nanosystem of resveratrol for the intra-articular treatment of arthritis. Int J Pharm 569:118560. [DOI] [PubMed] [Google Scholar]
- Kang JH, Ko YT. (2019). Enhanced subcellular trafficking of resveratrol using mitochondriotropic liposomes in cancer cells. Pharmaceutics 11:423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka K, Harada A, Nagasaki Y. (2001). Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv Drug Deliv Rev 47:113–31. [DOI] [PubMed] [Google Scholar]
- Katila N, Duwa R, Bhurtel S, et al. (2022). Enhancement of blood-brain barrier penetration and the neuroprotective effect of resveratrol. J Control Release 346:1–19. [DOI] [PubMed] [Google Scholar]
- Khosa A, Reddi S, Saha RN. (2018). Nanostructured lipid carriers for site-specific drug delivery. Biomed Pharmacother 103:598–613. [DOI] [PubMed] [Google Scholar]
- Khusbu FY, Zhou X, Roy M, et al. (2020). Resveratrol induces depletion of traf6 and suppresses prostate cancer cell proliferation and migration. Int J Biochem Cell Biol 118:105644. [DOI] [PubMed] [Google Scholar]
- Kim J, Shim MK, Yang S, et al. (2021). Combination of cancer-specific prodrug nanoparticle with bcl-2 inhibitor to overcome acquired drug resistance. J Control Release 330:920–32. [DOI] [PubMed] [Google Scholar]
- Kotta S, Mubarak Aldawsari H, Badr-Eldin SM, et al. (2021). Coconut oil-based resveratrol nanoemulsion: optimization using response surface methodology, stability assessment and pharmacokinetic evaluation. Food Chem 357:129721. [DOI] [PubMed] [Google Scholar]
- Kup FO, Coskuncay S, Duman F. (2020). Biosynthesis of silver nanoparticles using leaf extract of aesculus hippocastanum (horse chestnut): evaluation of their antibacterial, antioxidant and drug release system activities. Mater Sci Eng C-Mater Biol Appl 107:110207. [DOI] [PubMed] [Google Scholar]
- Laginha K, Mumbengegwi D, Allen T. (2005). Liposomes targeted via two different antibodies: assay, b-cell binding and cytotoxicity. Biochim Biophys Acta 1711:25–32. [DOI] [PubMed] [Google Scholar]
- Langer R. (2000). Biomaterials in drug delivery and tissue engineering: one laboratory’s experience. Acc Chem Res 33:94–101. [DOI] [PubMed] [Google Scholar]
- Le Clanche S, Cheminel T, Rannou F, et al. (2018). Use of resveratrol self-emulsifying systems in t/c28a2 cell line as beneficial effectors in cellular uptake and protection against oxidative stress-mediated death. Front Pharmacol 9:538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, MacKay JA, Frechet JM, Szoka FC. (2005). Designing dendrimers for biological applications. Nat Biotechnol 23:1517–26. [DOI] [PubMed] [Google Scholar]
- Lee DG, Lee M, Go EB, Chung N. (2022). Resveratrol-loaded gold nanoparticles enhance caspase-mediated apoptosis in panc-1 pancreatic cells via mitochondrial intrinsic apoptotic pathway. Cancer Nanotechnol 13:34. [Google Scholar]
- Li A, Zhao Y, Li Y, et al. (2021). Cell-derived biomimetic nanocarriers for targeted cancer therapy: cell membranes and extracellular vesicles. Drug Deliv 28:1237–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Wang X, Li R, et al. (2019). Resveratrol-loaded plga nanoparticles functionalized with red blood cell membranes as a biomimetic delivery system for prolonged circulation time. J Drug Delivery Sci Technol 54:101369. [Google Scholar]
- Li J, Wang Z, Zhang H, et al. (2021). Progress in the development of stabilization strategies for nanocrystal preparations. Drug Deliv 28:19–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Du C, Guo N, et al. (2019). Composition design and medical application of liposomes. Eur J Med Chem 164:640–53. [DOI] [PubMed] [Google Scholar]
- Li Z, Qiao W, Wang C, et al. (2020). Dppc-coated lipid nanoparticles as an inhalable carrier for accumulation of resveratrol in the pulmonary vasculature, a new strategy for pulmonary arterial hypertension treatment. Drug Deliv 27:736–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian B, Wu M, Feng Z, et al. (2019). Folate-conjugated human serum albumin-encapsulated resveratrol nanoparticles: preparation, characterization, bioavailability and targeting of liver tumors. Artif Cells Nanomed Biotechnol 47:154–65. [DOI] [PubMed] [Google Scholar]
- Liang MY, Guo MY, Saw PE, Yao YD. (2022). Fully natural lecithin encapsulated nano-resveratrol for anti-cancer therapy. Int J Nanomedicine 17:2069–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Lv Y, Li T. (2019). Hybrid drug nanocrystals. Adv Drug Deliv Rev 143:115–33. [DOI] [PubMed] [Google Scholar]
- Lu Y, Park K. (2013). Polymeric micelles and alternative nanonized delivery vehicles for poorly soluble drugs. Int J Pharm 453:198–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Park K. (2013). Polymeric micelles and alternative nanonized delivery vehicles for poorly soluble drugs. Int J Pharm 453:198–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Qi J, Dong X, et al. (2017). The in vivo fate of nanocrystals. Drug Discov Today 22:744–50. [DOI] [PubMed] [Google Scholar]
- Lu Y, Zhang ES, Yang JH, Cao ZQ. (2018). Strategies to improve micelle stability for drug delivery. Nano Res 11:4985–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maherani B, Arab-Tehrany E, Mozafari MR, et al. (2011). Liposomes: a review of manufacturing techniques and targeting strategies. Curr Nanosci 7:436–52. [Google Scholar]
- Majidinia M, Mirza-Aghazadeh-Attari M, Rahimi M, et al. (2020). Overcoming multidrug resistance in cancer: recent progress in nanotechnology and new horizons. IUBMB Life 72:855–71. [DOI] [PubMed] [Google Scholar]
- Masood F. (2016). Polymeric nanoparticles for targeted drug delivery system for cancer therapy. Mater Sci Eng C Mater Biol Appl 60:569–78. [DOI] [PubMed] [Google Scholar]
- Menjoge AR, Kannan RM, Tomalia DA. (2010). Dendrimer-based drug and imaging conjugates: design considerations for nanomedical applications. Drug Discov Today 15:171–85. [DOI] [PubMed] [Google Scholar]
- Miao L, Daozhou L, Ying C, et al. (2021). A resveratrol-loaded nanostructured lipid carrier hydrogel to enhance the anti-uv irradiation and anti-oxidant efficacy. Colloids Surf B Biointerfaces 204:111786. [DOI] [PubMed] [Google Scholar]
- Moradi SZ, Momtaz S, Bayrami Z, et al. (2020). Nanoformulations of herbal extracts in treatment of neurodegenerative disorders. Front Bioeng Biotechnol 8:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munagala R, Aqil F, Jeyabalan J, Gupta RC. (2016). Bovine milk-derived exosomes for drug delivery. Cancer Lett 371:48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzykantov VR. (2010). Drug delivery by red blood cells: vascular carriers designed by mother nature. Expert Opin Drug Deliv 7:403–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam N. (2006). Naturally occurring nf-kappab inhibitors. Mini Rev Med Chem 6:945–51. [DOI] [PubMed] [Google Scholar]
- Naserifar M, Hosseinzadeh H, Abnous K, et al. (2020). Oral delivery of folate-targeted resveratrol-loaded nanoparticles for inflammatory bowel disease therapy in rats. Life Sci 262:118555. [DOI] [PubMed] [Google Scholar]
- Nassir AM, Shahzad N, Ibrahim IAA, et al. (2018). Resveratrol-loaded plga nanoparticles mediated programmed cell death in prostate cancer cells. Saudi Pharm J 26:876–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes S, Danesi F, Del Rio D, Silva P. (2018). Resveratrol and inflammatory bowel disease: the evidence so far. Nutr Res Rev 31:85–97. [DOI] [PubMed] [Google Scholar]
- Pearce AK, O’Reilly RK. (2019). Insights into active targeting of nanoparticles in drug delivery: advances in clinical studies and design considerations for cancer nanomedicine. Bioconjug Chem 30:2300–11. [DOI] [PubMed] [Google Scholar]
- Poonia N, Kaur Narang J, Lather V, et al. (2019). Resveratrol loaded functionalized nanostructured lipid carriers for breast cancer targeting: systematic development, characterization and pharmacokinetic evaluation. Colloids Surf B Biointerfaces 181:756–66. [DOI] [PubMed] [Google Scholar]
- Poonia N, Lather V, Narang JK, et al. (2020). Resveratrol-loaded folate targeted lipoprotein-mimetic nanoparticles with improved cytotoxicity, antioxidant activity and pharmacokinetic profile. Mater Sci Eng C-Mater Biol Appl 114:111016. [DOI] [PubMed] [Google Scholar]
- Prysyazhna O, Wolhuter K, Switzer C, et al. (2019). Blood pressure-lowering by the antioxidant resveratrol is counterintuitively mediated by oxidation of cgmp-dependent protein kinase. Circulation 140:126–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y, Ren K, Zhao W, et al. (2021). A "dual-guide" bioinspired drug delivery strategy of a macrophage-based carrier against postoperative triple-negative breast cancer recurrence. J Control Release 329:191–204. [DOI] [PubMed] [Google Scholar]
- Rahman M, Almalki WH, Afzal O, et al. (2020). Cationic solid lipid nanoparticles of resveratrol for hepatocellular carcinoma treatment: systematic optimization, in vitro characterization and preclinical investigation. Int J Nanomedicine 15:9283–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhold SE, Schwendeman SP. (2013). Effect of polymer porosity on aqueous self-healing encapsulation of proteins in plga microspheres. Macromol Biosci 13:1700–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaei A, Fathi M, Jafari SM. (2019). Nanoencapsulation of hydrophobic and low-soluble food bioactive compounds within different nanocarriers. Food Hydrocolloids 88:146–62. [Google Scholar]
- Rinaldi F, Maurizi L, Forte J, et al. (2021). Resveratrol-loaded nanoemulsions: in vitro activity on human t24 bladder cancer cells. Nanomaterials (Basel) 11:1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha S, Lucas M, Ribeiro D, et al. (2021). Nano-based drug delivery systems used as vehicles to enhance polyphenols therapeutic effect for diabetes mellitus treatment. Pharmacol Res 169:105604. [DOI] [PubMed] [Google Scholar]
- Roy H, Nayak BS, Abdul Rahaman S. (2019). Characterization and biology of nanomaterials for drug delivery. Amsterdam, Netherlands: Elsevier Science, 445–75. [Google Scholar]
- Sale S, Verschoyle RD, Boocock D, et al. (2004). Pharmacokinetics in mice and growth-inhibitory properties of the putative cancer chemopreventive agent resveratrol and the synthetic analogue trans 3,4,5,4’-tetramethoxystilbene. Br J Cancer 90:736–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos AC, Costa G, Veiga F, et al. (2014). Advance in methods studying the pharmacokinetics of polyphenols. Curr Drug Metab 15:96–115. [DOI] [PubMed] [Google Scholar]
- Sawant RR, Torchilin VP. (2010). Multifunctionality of lipid-core micelles for drug delivery and tumour targeting. Mol Membr Biol 27:232–46. [DOI] [PubMed] [Google Scholar]
- Shahnaz G, Edagwa BJ, McMillan J, et al. (2017). Development of mannose-anchored thiolated amphotericin b nanocarriers for treatment of visceral leishmaniasis. Nanomedicine (Lond) 12:99–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Zhang Y, Zhang X, et al. (2021). Remodeling immune microenvironment in periodontitis using resveratrol liposomes as an antibiotic-free therapeutic strategy. J Nanobiotechnol 19:429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Ye F, Lu K, et al. (2020). Characterizations and bioavailability of dendrimer-like glucan nanoparticulate system containing resveratrol. J Agric Food Chem 68:6420–9. [DOI] [PubMed] [Google Scholar]
- Singh G, Pai RS. (2014). Optimized plga nanoparticle platform for orally dosed trans-resveratrol with enhanced bioavailability potential. Expert Opin Drug Deliv 11:647–59. [DOI] [PubMed] [Google Scholar]
- Singh SK, Makadia V, Sharma S, et al. (2017). Preparation and in-vitro/in-vivo characterization of trans-resveratrol nanocrystals for oral administration. Drug Deliv Transl Res 7:395–407. [DOI] [PubMed] [Google Scholar]
- Siu FY, Ye S, Lin H, Li S. (2018). Galactosylated plga nanoparticles for the oral delivery of resveratrol: enhanced bioavailability and in vitro anti-inflammatory activity. Int J Nanomedicine 13:4133–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleas GJ, Angelini M, Grass L, et al. (2001). Absorption of trans-resveratrol in rats. Methods Enzymol 335:145–54. [DOI] [PubMed] [Google Scholar]
- Sperling RA, Parak WJ. (2010). Surface modification, functionalization and bioconjugation of colloidal inorganic nanoparticles. Philos Trans A Math Phys Eng Sci 368:1333–83. [DOI] [PubMed] [Google Scholar]
- Srivani G, Behera SK, Dariya B, et al. (2020). Resveratrol binds and inhibits transcription factor hif-1alpha in pancreatic cancer. Exp Cell Res 394:112126. [DOI] [PubMed] [Google Scholar]
- Summerlin N, Qu Z, Pujara N, et al. (2016). Colloidal mesoporous silica nanoparticles enhance the biological activity of resveratrol. Colloids Surf B Biointerfaces 144:1–7. [DOI] [PubMed] [Google Scholar]
- Summerlin N, Soo E, Thakur S, et al. (2015). Resveratrol nanoformulations: challenges and opportunities. Int J Pharm 479:282–90. [DOI] [PubMed] [Google Scholar]
- Sun L, Hu Y, Mishra A, et al. (2020). Protective role of poly(lactic-co-glycolic) acid nanoparticle loaded with resveratrol against isoproterenol-induced myocardial infarction. Biofactors 46:421–31. [DOI] [PubMed] [Google Scholar]
- Svenson S. (2009). Dendrimers as versatile platform in drug delivery applications. Eur J Pharm Biopharm 71:445–62. [DOI] [PubMed] [Google Scholar]
- Tapeinos C, Battaglini M, Ciofani G. (2017). Advances in the design of solid lipid nanoparticles and nanostructured lipid carriers for targeting brain diseases. J Control Release 264:306–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thipe VC, Amiri KP, Bloebaum P, et al. (2019). Development of resveratrol-conjugated gold nanoparticles: interrelationship of increased resveratrol corona on anti-tumor efficacy against breast, pancreatic and prostate cancers. Int J Nanomed 14:4413–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tome-Carneiro J, Larrosa M, Gonzalez-Sarrias A, et al. (2013). Resveratrol and clinical trials: the crossroad from in vitro studies to human evidence. Curr Pharm Des 19:6064–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torchilin VP. (2005). Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov 4:145–60. [DOI] [PubMed] [Google Scholar]
- Tsujioka T, Sasaki D, Takeda A, et al. (2022). Resveratrol-encapsulated mitochondria-targeting liposome enhances mitochondrial respiratory capacity in myocardial cells. Int J Mol Sci 23:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas JE, Puga R, Lenz G, et al. (2020). Cellular mechanisms triggered by the cotreatment of resveratrol and doxorubicin in breast cancer: a translational in vitro-in silico model. Oxid Med Cell Longev 2020:5432651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestergaard M, Ingmer H. (2019). Antibacterial and antifungal properties of resveratrol. Int J Antimicrob Agents 53:716–23. [DOI] [PubMed] [Google Scholar]
- Vijayakumar MR, Vajanthri KY, Balavigneswaran CK, et al. (2016). Pharmacokinetics, biodistribution, in vitro cytotoxicity and biocompatibility of vitamin e tpgs coated trans resveratrol liposomes. Colloids Surf B Biointerfaces 145:479–91. [DOI] [PubMed] [Google Scholar]
- Walle T, Hsieh F, DeLegge MH, et al. (2004). High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos 32:1377–82. [DOI] [PubMed] [Google Scholar]
- Wang H, Sui H, Zheng Y, et al. (2019). Curcumin-primed exosomes potently ameliorate cognitive function in ad mice by inhibiting hyperphosphorylation of the tau protein through the akt/gsk-3beta pathway. Nanoscale 11:7481–96. [DOI] [PubMed] [Google Scholar]
- Wang M, Liu Y, Zhang X, et al. (2017). Gold nanoshell coated thermo-ph dual responsive liposomes for resveratrol delivery and chemo-photothermal synergistic cancer therapy. J Mater Chem B 5:2161–71. [DOI] [PubMed] [Google Scholar]
- Wang W, Zhou M, Xu Y, et al. (2021). Resveratrol-loaded tpgs-resveratrol-solid lipid nanoparticles for multidrug-resistant therapy of breast cancer: in vivo and in vitro study. Front Bioeng Biotechnol 9:762489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Parvathaneni V, Shukla SK, et al. (2020). Inhalable resveratrol-cyclodextrin complex loaded biodegradable nanoparticles for enhanced efficacy against non-small cell lung cancer. Int J Biol Macromol 164:638–50. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhao X, Zhong Y, et al. (2022). Biomimetic exosomes: a new generation of drug delivery system. Front Bioeng Biotechnol 10:865682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Zhong C, Deng Y, et al. (2020). Resveratrol loaded glycyrrhizic acid-conjugated human serum albumin nanoparticles for tail vein injection ii: pharmacokinetics, tissue distribution and bioavailability. Drug Deliv 27:81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong S, Liu W, Zhou Y, et al. (2020). Enhancement of oral bioavailability and anti-parkinsonian efficacy of resveratrol through a nanocrystal formulation. Asian J Pharm Sci 15:518–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Jia F, Singh PK, et al. (2017). Synergistic anti-glioma effect of a coloaded nano-drug delivery system. Int J Nanomedicine 12:29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XY, Sun LP, Zhou L, et al. (2020). Functional chitosan oligosaccharide nanomicelles for topical ocular drug delivery of dexamethasone. Carbohydr Polym 227:115356. [DOI] [PubMed] [Google Scholar]
- Yan W, Jiang S. (2020). Immune cell-derived exosomes in the cancer-immunity cycle. Trends Cancer 6:506–17. [DOI] [PubMed] [Google Scholar]
- Yang GB, Phua SZF, Bindra AK, Zhao YL. (2019). Degradability and clearance of inorganic nanoparticles for biomedical applications. Adv Mater 31:1805730. [DOI] [PubMed] [Google Scholar]
- Yang H. (2010). Nanoparticle-mediated brain-specific drug delivery, imaging, and diagnosis. Pharm Res 27:1759–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee YJ, Benson HAE, Dass CR, Chen Y. (2022). Evaluation of novel conjugated resveratrol polymeric nanoparticles in reduction of plasma degradation, hepatic metabolism and its augmentation of anticancer activity in vitro and in vivo. Int J Pharm 615:121499. [DOI] [PubMed] [Google Scholar]
- Yin T, Diao Z, Blum NT, et al. (2022). Engineering bacteria and bionic bacterial derivatives with nanoparticles for cancer therapy. Small 18:e2104643. [DOI] [PubMed] [Google Scholar]
- Yousef M, Vlachogiannis IA, Tsiani E. (2017). Effects of resveratrol against lung cancer: in vitro and in vivo studies. Nutrients 9:1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Shin YG, Chow A, et al. (2002). Human, rat, and mouse metabolism of resveratrol. Pharmaceutical Research 19:1907–14. [DOI] [PubMed] [Google Scholar]
- Yuan L, Zhou M, Huang D, et al. (2019). Resveratrol inhibits the invasion and metastasis of colon cancer through reversal of epithelial mesenchymal transition via the akt/gsk3beta/snail signaling pathway. Mol Med Rep 20:2783–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zhu K, Zeng H, et al. (2019). Resveratrol solid lipid nanoparticles to trigger credible inhibition of doxorubicin cardiotoxicity. Int J Nanomedicine 14:6061–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Mehta A, Tong Z, et al. (2021). Development of polymeric nanoparticles for blood-brain barrier transfer-strategies and challenges. Adv Sci (Weinh) 8:2003937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XW, Chen GJ, Zhang TP, et al. (2014). Effects of pegylated lipid nanoparticles on the oral absorption of one bcs ii drug: a mechanistic investigation. Int J Nanomed 9:5503–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F, Qin J, Liang Y, Zhou R. (2021). Exploring anti-liver cancer targets and mechanisms of oxyresveratrol: in silico and verified findings. Bioengineered 12:9939–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YN, Cao YN, Sun J, et al. (2020). Anti-breast cancer activity of resveratrol encapsulated in liposomes. J Mater Chem B 8:27–37. [DOI] [PubMed] [Google Scholar]
- Zhu XY, Wu CH, Qiu SH, et al. (2017). Effects of resveratrol on glucose control and insulin sensitivity in subjects with type 2 diabetes: systematic review and meta-analysis. Nutr Metab 14:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zu M, Ma Y, Cannup B, et al. (2021). Oral delivery of natural active small molecules by polymeric nanoparticles for the treatment of inflammatory bowel diseases. Adv Drug Deliv Rev 176:113887. [DOI] [PubMed] [Google Scholar]
- Zu Y, Overby H, Ren G, et al. (2018). Resveratrol liposomes and lipid nanocarriers: comparison of characteristics and inducing browning of white adipocytes. Colloids Surf B Biointerfaces 164:414–23. [DOI] [PMC free article] [PubMed] [Google Scholar]