Abstract
We have previously identified a process in the yeast Saccharomyces cerevisiae that results in the contraction of elongated telomeres to wild-type length within a few generations. We have termed this process telomeric rapid deletion (TRD). In this study, we use a combination of physical and genetic assays to investigate the mechanism of TRD. First, to distinguish among several recombinational and nucleolytic pathways, we developed a novel physical assay in which HaeIII restriction sites are positioned within the telomeric tract. Specific telomeres were subsequently tested for HaeIII site movement between telomeres and for HaeIII site retention during TRD. Second, genetic analyses have demonstrated that mutations in RAD50 and MRE11 inhibit TRD. TRD, however, is independent of the Rap1p C-terminal domain, a central regulator of telomere size control. Our results provide evidence that TRD is an intrachromatid deletion process in which sequences near the extreme terminus invade end-distal sequences and excise the intervening sequences. We propose that the Mre11p-Rad50p-Xrs2p complex prepares the invading telomeric overhang for strand invasion, possibly through end processing or through alterations in chromatin structure.
The length of the simple sequence tracts present at the telomere is tightly regulated by coordinated mechanisms of telomere lengthening and shortening, leading to a steady-state equilibrium (20, 63, 66, 73). In the yeast Saccharomyces cerevisiae, individual telomeres consist of irregular TG1-3 sequences, with each complete telomeric tract maintained within 50 bp of a genetically determined mean size (63, 73). In yeast, as in most eukaryotes, the enzyme responsible for telomere lengthening is telomerase, a ribonucleoprotein reverse transcriptase consisting of a catalytic protein subunit, encoded by the EST2 gene (36, 37, 41), and an RNA species, encoded by the TLC1 gene (64). The RNA component of telomerase contains the template for the addition of telomeric repeats onto G-rich single-stranded substrates (20, 21, 64). In cells lacking telomerase, recombination among subtelomeric or telomeric sequences can also result in telomere lengthening (69, 70).
The mechanisms that counteract the possibly unlimited lengthening by telomerase are less well understood. Recent studies have provided evidence for several non-mutually exclusive mechanisms for maintaining a genetically set equilibrium of sizes. First, the major telomere binding protein, Rap1p, associating with the telomeric tract sequence motif GGTGTGTGGGTGT (14), is directly involved in achieving telomere length homeostasis. Rap1p activity is mediated in part through recruitment of positive and negative regulators of telomere addition to the Rap1p C-terminal 165 amino acids (10, 25, 30, 52, 77). The number of bound Rap1p C termini, or of factors associating with the C termini, is counted through an as-yet-unknown mechanism, until an optimal stable length is reached (44, 61). According to this model, shorter telomeres are elongated via telomerase to wild-type sizes, while overelongated telomeres gradually lose telomeric repeats until the wild-type size is regained. In this way, a homeostasis between addition and loss of telomeric sequences could be established (43, 44, 72). Second, Rap1p interacts directly or indirectly with telomerase to form a cap against the uncontrolled addition of telomeric tracts (29, 65). Third, the telomeric single-strand binding protein Cdc13p recruits multiple complexes to the telomeric 3′ single-stranded overhang, where they act to maintain a balance between telomere elongation and loss at the extreme terminus (9, 17, 18, 40, 56, 60).
In addition to these mechanisms, we have proposed that the rapid truncation of overelongated telomeres, termed telomeric rapid deletion (TRD), negatively regulates telomere length (35). This mechanism is distinct from the slow attrition of telomeric sequences observed after increases in telomere tract size (43). TRD is observed in wild-type RAP1 strains containing a subset of telomeres that range in size from 400 to 3,000 bp greater than that of wild type, with the vast majority (>80%) of deletion events at an individual telomere reducing telomeric tracts to wild-type size (termed complete deletions). For the remaining events (<20%), deletions lead to intermediate sizes (termed incomplete deletions). TRD occurs at the relatively high rate of 3 × 10−3 events/cell division/telomere (35).
Loss of the major recombination protein, RAD52, diminishes the TRD rate, implicating recombination in TRD (35). In addition, hpr1 cells, which display an elevated rate of recombination between direct repeats (1), also show an increase in TRD, raising the possibility that TRD may be an intrachromatid recombination event. However, no definitive mechanistic conclusions could be drawn from these initial genetic studies.
The frequency of complete rapid deletion events at an individual telomere depends upon the lengths of other telomeres in the cell (35). Cells that contain an increased number of wild-type length telomeres have a corresponding increase in complete deletions. Based on these results, we have proposed two components of TRD: a recombinational process between imperfect (homeologous) repeats and a “yardstick” that measures telomere lengths relative to one another.
The yeast and human Ku heterodimer, involved in nonhomologous end joining, also associate with telomeres in vivo and in vitro (3, 19, 32, 42). Interestingly, loss of either of the subunits of the yeast Ku heterodimer (yKu70 or yKu80) confers a large increase in the TRD rate (58), in nucleolytic degradation (58), and in the length of terminal 3′ single-stranded overhangs (19, 58). The yku70 and yku80 alleles also display a global decrease in telomere tract size (6, 59). These data suggest that the yKu heterodimer is part of a telomeric cap that guards against potentially deleterious processes such as promiscuous recombination and end degradation.
yKu acts together with a trimeric complex consisting of Mre11p, Rad50p, and Xrs2p (the MRX complex) in the nonhomologous end-joining pathway (8, 23, 42, 76). However, the role of the Mre11p-Rad50p-Xrs2p (MRX) complex is far more complicated and is additionally required for mitotic homologous recombination, induced by ionizing radiation (47), double-strand break formation (7, 27), and meiotic recombination (23). At telomeres, each MRX component plays a positive role in telomere addition (6, 50), which is mediated through the telomerase pathway (23, 34, 50). Further indicating a role of the MRX complex at telomeres, human Rad50, Mre11, and Nbs1 (the presumed Xrs2p homologue) associate with the telomere in vivo (78). However, the role of the MRX complex in TRD remains unknown.
We investigate here the mechanism of telomere rapid deletion. Through a combination of physical and genetic assays, we provide evidence that TRD is an intrachromatid recombination process, with sequences near or at the extreme terminus invading distal tract sequences. Our studies also indicate that the MRX complex plays a critical role in the TRD process.
MATERIALS AND METHODS
Plasmids.
Plasmid AD3ARUGT-IV [pVIIL URA3(−)tel] contains VIIL sequences, a “flipped” URA3 gene transcribing toward subtelomeric sequences [designated by (−)], and an 80-bp poly(TG1-3) seed sequence (64). pVR AD3ARUGT [pVR URA3(−)] was derived by ligating the 2.8-kb HindIII fragment of pVR URA3(−)tel (16) into the unique HindIII site of pVIIL URA3(−)tel. pVR URA3(−) in which the URA3 sequences are transcribed toward subtelomeric sequences was identified by Southern analysis. pADADE2 was used for labeling of VIIL telomeres with the URA3/ADE2 marker, as previously described (16).
p317/Tg1 (64), encoding the TLC1 telomerase RNA, was digested with EcoRI. The resulting 3.9-kb fragment was ligated into the EcoRI site of pRS314, giving rise to pRS314/TLC1. The NcoI/NciI fragment of pRS306/TLC1-1, encoding the mutant telomerase RNA, was ligated into NcoI/NciI-digested pRS314/TLC1, creating the centromeric plasmid pRS314/TLC1-1. TLC1-1 is a telomerase RNA that encodes a HaeIII site within its template. The RAP1-containing centromeric plasmid, pD130, has been previously described (35). pNKY83 contains a hisG-URA3-hisG disruption of the RAD50 gene cloned into pBR322 (2). pHO27, kindly provided by M. Resnick, is an integrating plasmid containing an mre11::URA3 disruption allele. pUC140, a kind gift of V. Lundblad, is an integrating plasmid that contains an est1::URA3 disruption allele on a 14-kb SphI fragment.
PstI digestion of pRS316 releases a 1.7-kb fragment containing the URA3 gene that was used as a probe for Southern analysis. pL909 contains the ADE2 gene and flanking sequences on a BamHI restriction fragment. Both the 3.6-kb BamHI fragment and the 1.1-kb NdeI/BamHI fragment, containing the terminal region ofADE2, were used as probes.
Yeast media and strains.
Rich (YPD [yeast extract-peptone-dextrose]), synthetic complete (SC), and SC omission media were prepared by standard methods. YPAD is identical to YPD except for supplementation with 30 mg of adenine/ml.
Haploid yeast strains used in this study are listed in Table 1. All strains were isogenic to the W303 background, with the exception of strains derived from DVL227–6c and TVL345. DVL227–6c is isogenic to the TVL345 background. W303-based strains carry a weak rad5 allele that does not influence most classes of recombination (46). All strains containing subtelomeric genes that are transcribed in the telomere-proximal to distal direction are termed the “(−)” derivative. Transformations that give rise to marked telomeres were performed as described previously (16). Each fragment used for telomere marking contained subtelomeric sequences, a selectable marker, and 80 bp of telomeric seed sequence.
TABLE 1.
Strains used in this study
| Strain(s) | Genotype |
|---|---|
| W303aa | MATa leu2-3,112 HIS3 ade2-1 trp1 ura3-1 |
| W303αa | MATα leu2-3,112 his3-11,15 ade2-1 trp1 ura3-1 |
| YDS/RAP1b | MATα rap1::LEU2 leu2-3,112 his3-11,15 ade2-1 trp1 ura3-1 pD130/RAP1 |
| CZY1/RAP1c | MATα rap1::LEU2 leu2-3,112 his3-11,15 ade2-1 trp1 ura3-1 pRS313/RAP1 VIIL::URA3/ADE2 |
| YDS/1-17/AD3ARUGT | MATα rap1::LEU2 leu2-3,112 his3-11,15 ade2-1 trp1 ura3-1 pRS313/rap1-17 VIIL::URA(−) |
| YDS/RAP1/AD3ARUGT | MATα rap1::LEU2 leu2-3,112 his3-11,15 ade2-1 trp1 ura3-1 pD130/RAP1 VIIL::URA(−) |
| WUF1 | MATa leu2-3,112 HIS3 ade2-1 trp1 ura3-1 VR::URA(−) |
| MUH4, MUH5, MUH 6, and MUH7 | MATα rap1::LEU2 leu2-3,112 his3-11,15 ade2-1 trp1 ura3-1 pRS313/rap1-17 VIIL::URA(−) |
| MUH15 | MATα rap1::LEU2 leu2-3,112 his3-11,15 ade2-1 trp1 ura3-1 pD130/RAP1 VIIL::URA(−) |
| MBH1 | MATα rap1::LEU2 leu2-3,112 his3-11,15 ade2-1 trp1 ura3-1 VIIL::URA3/ADE2(150 bp HaeIII site) pRS313/rap1-17 |
| MBH2 | MATα rap1::LEU2 leu2-3,112 his3-11,15 ade2-1 trp1 ura3-1 VIIL::URA3/ADE2(250-bp HaeIII site) pRS313/rap1-17 |
| MBH9 | MATα rap1::LEU2 leu2-3,112 his3-11,15 ade2-1 trp1 ura3-1 VIIL::URA3/ADE2(750-bp HaeIII site) pRS313/rap 1-17 |
| MBH9/ura3 | MATα rap1::LEU2 leu2-3,112 his3-11,15 ade2-1 trp1 ura3-1 VIIL::ura3/ADE2(750-bp HaeIII site) pRS313/rap1-17 |
| MEH9 | MATα rap1::LEU2 leu2-3,112 his3-11,15 ade2-1 trp1 ura3-1 VIIL::ura3/ADE2(750-bp HaeIII site) pRS313/rap1-17 est1::URA3 |
| MBH11-8b | MATα RAP1 leu2-3,112 his3-11,15 ade2-1 trp1 ura3-1 VIIL::URA3/ADE2(150-bp HaeIII site) |
| MBH21-20b | MATα RAP1 leu2-3,112 his3-11,15 ade2 trp1 ura3-1 VIIL::URA3/ADE2(250-bp HaeIII site) |
| MBH22-7b | MATα RAP1 leu2-3,112 his3-11,15 ade2-1 trp1 ura3-1 VIIL::URA3/ADE2(250-bp HaeIII site) |
| MBH91-3 | MATα RAP1 leu2-3,112 HIS3 ade2-1 trp1 ura3-1 VIIL::URA3/ADE2(750-bp HaeIII site) |
| MBH92-1a | MATα RAP1 leu2-3,112 his3-11,15 ade2-1 trp1 ura3-1 VIIL::URA3/ADE2(750-bp HaeIII site) |
| MBH93-6d | MATa RAP1 leu2-3,112 his3-11,15 ade2-1 trp1 ura3-1 VIIL::URA3/ADE2(750-bp HaeIII site) |
| BL27-11a | MATa RAP1 leu2-3,112 HIS3 ade2-1 trp1 ura3-1 VIIL::ura3/ADE2 |
| AJL437-1d | MATa RAP1 leu2-3,112 HIS3 ade2-1 trp1 ura3Δ::TRP1::ura3Δ VIIL::ura3/ADE2 |
| AJL528-1b | MATa RAP1 leu2-3,112 his3-11,15 ade2-1 trp1 ura3Δ::TRP1::ura3Δ VIIL::ura3/ADE2 |
| AJL528-2b | MATa RAP1 leu2-3,112 HIS3 ade2-1 trp1 ura3-1 VIIL::ura3/ADE2 |
| DJ204-10b | MATα RAP1 leu2-3,112 his3-11,15 ade2-1 trp1 ura3-1 VIIL::ura3/ADE2(750-bp HaeIII site) VR::URA3(−) |
| DJ309-1c | MATα rap1::LEU2 leu2-3,112 his3-11,15 ade2-1 trp1 ura3-1 pRS313/rap1-17 VIIL::ura3/ADE2(750-bp HaeIII site) |
| YP17-24c | MATα leu2-3,112 HIS3 ade2-1 trp1 ura3-1 VIIL::URA3/ADE2(250-bp HaeIII site) VR::URA3(−) |
| YPR50-3b | MATa leu2-3,112 his3-11,15 trp1 ura3-1 VIIL::ura3/ADE2 rad50::URA3 |
| YPR50-3d, -15c, and -16c | MATα leu2-3,112 HIS3 trp1 ura3-1 VIIL::ura3/ADE2 rad50::URA3 |
| YPR50-14b, -15a, and -16a | MATa leu2-3,112 HIS3 trp1 ura3-1 VIIL::ura3/ADE2 rad50::URA3 |
| YPR50-14c | MATa leu2-3,112 HIS3 trp1 ura3-1 VIIL::ura3/ADE2 RAD50 |
| DVL227-6cde | MATa leu2-Δ1 his3-Δ200 ade2-101 CF::TRP1 ura3-52 lys2-801 mre11::URA3 rad50::Kanr |
| MBR52-5b | MATα leu2-3,112 his3-11,15 ade2-1 trp1 ura3-1 rad52::TRP1 URA3/ADE2(750-bp HaeIII site) |
| YP18-12b | MATα leu2-3,112 his3-11,15 ade2-1 trp1 ura3-1 rad50::KanrVIIL::URA3/ADE2(750-bp HaeIII site) |
| YP18-13a | MATα leu2-3,112 his3-11,15 ade2-1 trp1 ura3-1 rad50::KanrVIIL::URA3/ADE2(750-bp HaeIII site) |
| MBM1-3d | MATa leu2-3,112 his3-11,15 ade2-1 trp1 ura3-1 rad50::KanrVIIL::URA3/ADE2(750 bp HaeIII site) |
| YPM11-3d and -6a | MATα leu2-3,112 HIS3 trp1 ura3-1 VIIL::ura3/ADE2 mre11::URA3 |
| YPM11-6b and -9c | MATα leu2-3,112 his3-11,15 trp1 ura3-1 VIIL::ura3/ADE2 mre11::URA3 |
| YPM11-1b | MATα leu2-3,112 HIS3 trp1 ura3-1 VIIL::ura3/ADE2 MRE11 |
| TVL345de | MATa leu2-Δ1 his3-Δ200 ade2-101 trp1-Δ1 ura3-52 lys2-801 CF+ xrs2::Kanr |
| MBX91-13c | MATa leu2-3,112 his3-11,15 ade2-1 trp1 ura3-1 xrs2::KanrVIIL::URA3/ADE2(750-bp HaeIII site) |
| MBX91-16c | MATα leu2-3,112 his3-11,15 ade2-1 trp1 ura3-1 xrs2::KanrVIIL::URA3/ADE2(750-bp HaeIII site) |
Strains carrying URA3(−)-marked VIIL telomeres with HaeIII sites (the MUH strains) were constructed as follows: The yeast strain YDS/RAP1 was transformed with the 2.5-kb EcoRI/XhoI fragment from pVIIL URA3(−), giving rise to YDS/RAP1/VIIL URA3(−) containing the URA3(−)-marked VIIL telomeres. A plasmid shuffle was subsequently used to replace the wild-type RAP1 on pD130 with the rap1-17 allele on pRS313/rap1-17, yielding YDS/1–17/VIIL URA3(−). The marked telomeres range in size from 400 bp to 3,000 bp greater than the wild-type length (300 bp).
YDS/1–17/VIIL URA3(−) was transformed with the centromeric plasmid pRS314/TLC1-1 in the presence of genomic TLC1 and maintained for 20 to 60 generations before loss of the plasmid. The resulting strains used in this study were MUH4, MUH5, MUH6, and MUH7, containing HaeIII sites at 1,430 bp (HaeIII 1430), 1,880 bp (HaeIII 1880), 1,930 bp (HaeIII 1930), and 2,930 bp (HaeIII 2930) from the subtelomeric junction, respectively. Wild-type RAP1 strains containing HaeIII sites were generated after a plasmid shuffle of an MUH strain carrying a centromeric rap1-17 allele with the plasmid pD130 (Fig. 1A, right panel). This gave rise to MUH15 that contains a HaeIII site 640 bp (HaeIII 640) from the subtelomeric junction.
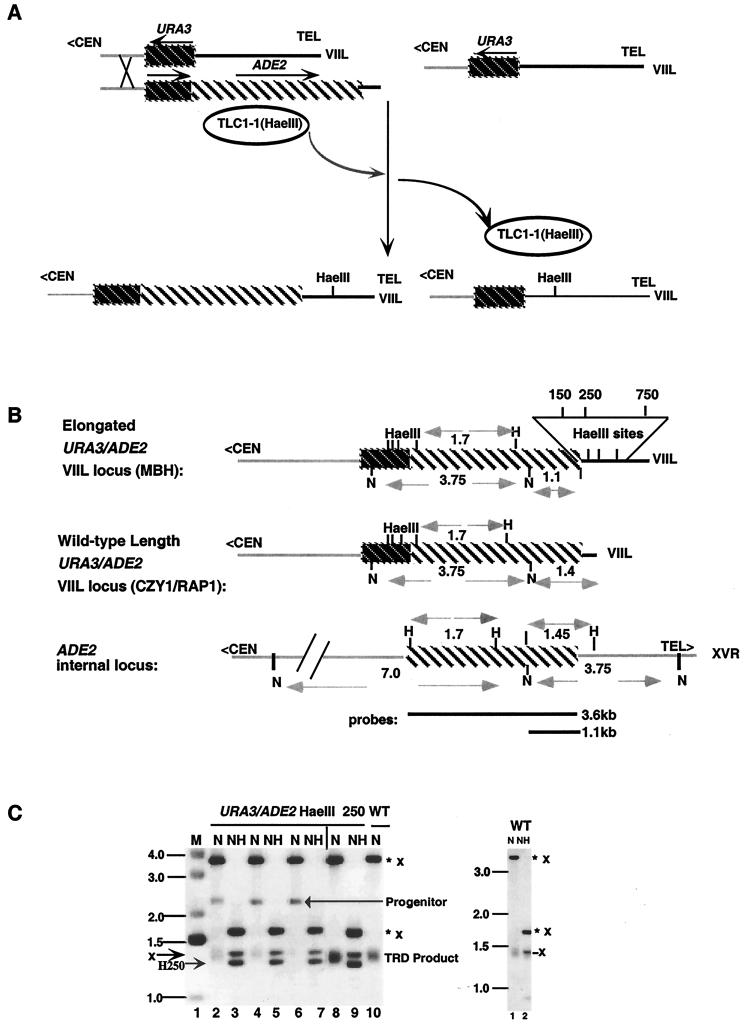
FIG. 1.
Incorporation of HaeIII sites into elongated telomeres. (A, left panel) rap1-17 cells containing newly generated URA3/ADE2-marked VIIL telomeres were derived from a URA3(−)-marked VIIL telomere by recombination with a homologous fragment containing the URA3/ADE2 sequences and 80 bp of telomeric seed sequence. Transformation was conducted in the presence of a plasmid borne copy of TLC1-1. The TLC1-1 RNA contains a HaeIII site within its template region. The telomeric seed sequence was elongated to sizes greater than that of the wild type, which is typical of the telomeres present in rap1-17 cells. As a consequence, HaeIII sites were introduced into the URA3/ADE2-marked VIIL telomere, as well as into other unmarked chromosomal telomeres, during the addition of telomeric repeats. After loss of the plasmid borne TLC1-1 gene, the rap1-17 strains containing the elongated URA3/ADE2 HaeIII-marked telomere were mated to an isogenic wild-type strain, and wild-type RAP1 spore colonies containing elongated telomeres were identified. These spore colonies represent the MBH series of strains. (A, left panel) Elongated URA3(−)-marked VIIL telomeres were grown in the presence of TLC1-1 RNA to generate HaeIII sites within both marked and unmarked chromosomal telomeres in rap1-17 cells. Incorporation of HaeIII sites at multiple positions is presumably due to repetitive cycles of deletion and elongation typical of rap1-17 telomeres. After loss of the mutant telomerase RNA, the plasmid-borne copy of rap1-17 was replaced with a wild-type copy of RAP1 through a plasmid shuffle, giving rise to the MUH series of strains (A, right panel). Dark striped regions, the URA3 gene; light striped regions, the ADE2 gene; chromosomal sequences, gray lines; telomeric sequences, black lines; <CEN, direction toward the centromere; arrow, direction of transcription. (B) Restriction maps of the genomic and telomeric ADE2 genes in the strains used in this study (35). WT refers to the strain CZY1/RAP1 carrying a wild-type length URA3/ADE2-marked telomere. Fragment lengths are shown for NdeI (N), NdeI/HaeIII, and HaeIII (H) sites. Each URA3/ADE2-marked VIIL telomere contains one of the three HaeIII sites. The position of a HaeIII site in the telomere was determined by the size of the NdeI/HaeIII fragment after subtracting the length of subtelomeric sequences (1.1 kb). These sites were positioned at 150 bp (HaeIII 150), 250 bp (HaeIII 250), and 750 bp (HaeIII 750) from the subtelomeric junction. The relative positions of HaeIII 150, HaeIII 250, and HaeIII 750 telomeres are shown in the expanded view. The 3.6- and 1.1-kb ADE2 probes used in the study (top and bottom, respectively) are also shown. Designations were described in the Fig. 1A legend. TEL> refers to the direction toward the telomere. (C, left panel) DNA isolated from MBH22-7b colonies, containing the URA3/ADE2 HaeIII 250-marked VIIL telomere, was digested with NdeI (N) or with NdeI and HaeIII (NH) and then subjected to Southern blotting with the 3.6-kb ADE2 probe indicated in panel B. Progenitor refers to the original marked elongated telomere after digestion of DNA isolated from several MBH22-7b subclones with NdeI (lanes 2, 4, and 6). In some MBH22-7b colonies (lane 8), the progenitor had been deleted before subculturing was done for individual colonies. WT refers to NdeI-cleaved DNA derived from CZY1/RAP1. ✽, Restriction fragments derived from the telomeric copy of ADE2; x, restriction fragments derived from the internal ADE2 locus. Size markers (in kilobases) are shown on the left. The positions of the Progenitor, the TRD product, and the NdeI/HaeIII fragment carrying the HaeIII 250 (H250) site are also indicated. M refers to the molecular mass marker. (C, right panel) As a control, DNA was isolated from CZY1/RAP1 and digested with NdeI or with both NdeI and HaeIII. Note that digestion of CZY1/RAP1 generates an internal 1.45-kb NdeI/HaeIII fragment that overlaps with the telomeric fragment. For presentation purposes, the autoradiogram is a composite of two portions of a single gel, with the junction borders indicated by the vertical line on top of the gel. Similar confirmations of HaeIII incorporation were carried out in MBH11-8b and MBH93-6d, carrying the HaeIII 150- and the HaeIII 750-marked telomeres, respectively.
Strains carrying the URA3/ADE2 HaeIII-marked VIIL telomeres (the MBH strains) were constructed as follows: YDS/1-17/VIIL URA3(−) was transformed with the 3.6-kb SalI/NotI fragment of pADADE2, thereby replacing the URA3(−)-marked VIIL telomere with a URA3/ADE2-marked VIIL telomere (16). The transformation in this case was conducted in the presence of pRS314/TLC1-1 and a genomic copy of TLC1, so that HaeIII sites could be incorporated during telomere lengthening in rap1-17 strains (Fig. 1A, left panel). After loss of the pRS314/TLC1-1 plasmid as described above, three strains were identified.
First, MBH1 contains a HaeIII site at 150 bp from the subtelomeric junction (HaeIII 150). MBH11 is a wild-type RAP1 strain that was derived from a cross between MBH1 and W303a. Second, MBH2 contains an HaeIII site at 250 bp from the subtelomeric junction (HaeIII 250). MBH21 and MBH22 are wild-type RAP1 strains following the first and second backcrosses of MBH2 with W303, respectively. Third, MBH9 has an HaeIII site 750 bp (HaeIII 750) from the subtelomeric junction. The cross between MBH9 and W303a formed the diploid MBH91. MBH91, after sporulation, generated the wild-type RAP1 spore colony MBH91–3. Two additional backcrosses (MBH92 and MBH93) were performed, and wild-type spore products (MBH92–1a and MBH93–6d) were identified. The sizes of the marked telomeric tracts within each progenitor diploid were determined by Southern analysis. MBH9/ura3, containing the ura3/ADE2 HaeIII 750 marked VIIL telomere, was selected after growth of MBH9 on 5-fluoroorotic acid (5-FOA). 5-FOA allows growth of Ura3−, but not Ura3+, cells. MEH9 was the result of transformation of the 14-kb SphI fragment of pUC140, containing the est1::URA3 null allele, into MBH9. The presence of the disrupted allele was confirmed by Southern analysis.
The positions of HaeIII sites within the telomeric tract of elongated URA3/ADE2 marked VIIL telomeres were determined after digestion of DNA with both NdeI, which cleaves within the ADE2 gene, and HaeIII. An identical approach was used to identify strains carrying HaeIII sites in elongated URA3(−)- marked VIIL telomeres, except that DNA was digested with both ApaI, which cleaves uniquely in the URA3 gene, and HaeIII. The presence or absence of multiple sites was determined by partial digestion of genomic DNA. Multiple sites collapse to the HaeIII site closest to the subtelomeric or telomeric junction after complete digestion. HaeIII sites were present at their original position in >99% of mitotically grown cells. Strains containing these elongated progenitor telomeres were used for subsequent site transfer and retention assays.
YP17–24c carries an elongated URA3/ADE2 HaeIII 250-marked VIIL telomere and a wild-type length URA3(−) marked VR telomere. To generate YP17, MBH21-20a was backcrossed with W303 four times to eliminate other elongated and HaeIII-marked telomeres, thereby forming the diploid YP15. The spore colony YP15–2d was subsequently crossed with WUF1 to form YP17. WUF1 was constructed by transformation of W303a with the 3.8-kb EcoRI/SalI fragment of pVR URA3(−).
DJ204-10b carries an elongated ura3/ADE2 HaeIII 750-marked VIIL telomere and a wild-type length URA3(−)-marked VR telomere. A cross between DJ309-1c and WUF1 generated DJ204. To generate DJ309, MBH9/ura3 was backcrossed with W303 four times. Therefore, the VIIL marked telomere is likely to be the sole elongated and HaeIII-marked telomere in DJ204-10b and YP17-24c.
To construct a strain carrying a disruption of the RAD50 gene, the 6.0-kb EcoRI/BglII fragment of pNKY83, carrying the rad50 disruption allele, was transformed into YP20, giving rise to YPR50. YP20 was derived from a cross between W303α and BL27-11a, carrying 50% elongated telomeres including the ura3/ADE2-marked VIIL telomere. Sporulation of YPR50 gave rise to the rad50::URA3 spore colonies YPR50-3b, -3d, -14b, -15c, -15d, -16a, and -16c.
To construct a telomere-marked strain carrying a rad50 null allele in a second genetic background, DVL227-6c was crossed to MBR52-5b, isogenic to the W303 background, and the resulting diploid MBM1 was sporulated. The progeny after sporulation of MBM1 contained rad50 (MBM1-3d) carrying the elongated URA3/ADE2-marked VIIL telomere. YP18 was formed by an independent cross between DVL227-6c and MBR52-5b that gave rise to the rad50 spore colonies YP18-12b and YP18-13a.
To construct a strain carrying a disruption of MRE11, the 2.1-kb PvuII/AseI fragment of pHO27 was transformed into YP20, yielding YPM11. Sporulation of YPM11 gave rise to the spore colonies YPM11-3d, -6a, -6b, and -9c containing the mre11::URA3 disruption allele. Sporulation of MBX91 led to the generation of the xrs2 spore colonies MBX91–13c and MBX91–16c containing the elongated URA3/ADE2-750 HaeIII-marked VIIL telomere. MBX91 is the result of a cross between the nonisogenic strain TVL345 and MBH9.
TRD assays.
Rapid deletion was measured by a change in the size of the telomeric fragment relative to the elongated progenitor. To this end, the progenitor and truncated forms of URA3/ADE2 or URA3 VIIL(−)-marked VIIL telomeres were digested with NdeI and ApaI, respectively, and subjected to Southern analysis.
To ascertain the degree of accumulation of deleted species, cells derived from an initial preculture (s0) were grown for 10 generations in liquid YPAD (s1), serially diluted, and grown for an additional 10 generations (s2). All rate measurements are presented as the degree of precursor retention over a period of 20 generations. The rate of loss of the progenitor form is expressed as [P/(P + D)] over a period of 20 generations, where P is the progenitor signal background and D is the deleted signal background. (The rate of deletion can then be expressed as 1 − the rate of loss.) In each strain, the median of the distribution of values is presented. The use of YPAD media is critical, since elongated ADE2 marked telomeres have low levels of ADE2 expression relative to wild-type-length marked telomeres (54). As a consequence, growth in media containing limiting levels of adenine, such as YPD, selects for cells containing deleted telomeres.
Site retention assay.
The site retention assay measures the presence or absence of the HaeIII site in the marked VIIL telomeres after a screen for cells containing partial deletion events (i.e., a subset of incomplete deletions that are smaller than the distance between the HaeIII site and the extreme terminus). In the case of the URA3(−) HaeIII-marked VIIL telomeres, ApaI and ApaI/HaeIII double digests were used to determine telomere length, the distance between the HaeIII site and the extreme terminus, and the retention or loss of the HaeIII site after Southern blot hybridization with URA3 sequences as a probe. A similar protocol was used with strains containing URA3 (or ura3)/ADE2 HaeIII-marked VIIL telomeres, except that the telomeric length was determined by digestion with NdeI. NdeI/HaeIII double digests were used to measure the distance from the HaeIII site to the extreme terminus and the degree of HaeIII site retention after Southern blots were probed with ADE2 sequences.
Site transfer assay.
The site transfer assay measures the movement of a HaeIII site in elongated URA3/ADE2 HaeIII 250- and ura3/ADE2 HaeIII 750-marked VIIL telomeres to a heterologous wild-type-length URA3(−)-marked VR telomere lacking HaeIII sites in strains YP17-24c and DJ204-10b, respectively.
Independent cells carrying complete deletion products of the URA3/ADE2 HaeIII 250-marked VIIL telomeres in YP17-24c and the ura3/ADE2 HaeIII 750-marked telomeres in DJ204-10b were identified by white colony color, which is indicative of the high ADE2 expression levels as expected for wild-type-length telomeres (54). Southern analysis was used to confirm telomere fragment sizes. The deleted colonies were pooled into batches of 10 before growth and DNA isolation. DNA from 260 independent YP17-24c colonies and 260 DJ204-10b colonies was tested for HaeIII site transfer to the URA3(−) VR-marked telomere by digestion with ApaI and HaeIII. For batch analysis, we confirmed that a ≥10:1 ratio between a telomeric and unique species could be readily detected using PhosphorImager analysis.
In the absence of site transfer, only the URA3/ADE2 HaeIII 250- and ura3/ADE2 HaeIII 750-marked VIIL telomeres should contain the HaeIII site. If site transfer were obligatorily associated with rapid deletion, we would expect a probability of transfer to be 3% for any interacting telomeres, assuming one HaeIII site/telomere and random telomere pairing patterns among the 32 telomeres in a haploid cell. This value may be greater if other telomeres recombine preferentially (see Discussion). This expectation is valid for events occurring in G1, DNA replication, and G2. If TRD occurs in G1, then a single transfer event during rapid deletion will give rise to two identical products. Similarly, the presence of a second independent TRD event occurring during DNA replication or in G2 is highly unlikely. Given this expected value, we estimated that a sample size of 260 independent cells was required for statistical significance as determined by chi-square analysis.
Site acquisition assay.
Conversely, we determined whether the VIIL telomere is capable of acquiring HaeIII sites from heterologous telomeres. To this end, we first crossed AJL 437-1d and MBH9 to give rise to the strain AJL528. The diploid was sporulated, and two spore colonies, AJL 528-1b, and AJL 528-2b, were isolated. These strains carry ≈50% elongated telomeres with a wild-type length ura3/ADE2 VIIL telomere. A total of 221 independent subclones were selected and subsequently subcultured in batches of 10 to 11 as described above.
To estimate the expected frequency of HaeIII site transfer from heterologous telomeres to the ura3/ADE2-marked VIIL telomeres lacking HaeIII sites, we first estimated the likelihood that other telomeres would interact with the marked VIIL telomere. If TRD reflects associations with heterologous telomeres, then the rate of TRD at the VIIL telomere (≈33% after 20 generations; see Table 4) would be equivalent to the combined value for the association of individual heterologous telomeres with the unique marked VIIL telomere. This value therefore reflects the expected rate of site acquisition during TRD if both the marked VIIL telomere and its interacting partner are capable of deletion and if the TRD rates on the marked VIIL telomere and heterologous telomeres were on average comparable. The latter possibility has been verified by the observed rapid deletions at the left arm of chromosome III, a chromosome XI telomere, and the right arm of chromosome V (30, 31). Therefore, the expected percentage of site acquisition is equal to: (% TRD) (% HaeIII-marked elongated telomeres) × 10−2. Among the 50% of telomeres that are elongated in the strains, we estimate that 50% contain a HaeIII site. Therefore, the percentage of site acquisition should equal 8.25%. For the sample size of 221 used in these experiments, we would therefore expect ≈18 events over a period of 20 generations of growth.
TABLE 4.
TRD in rad50, mre11, and xrs2 mutant cells
| Genetic background and straina | Median distribution in strains
|
|||
|---|---|---|---|---|
| % Precursor retainedb | Sample size (n) | No. of spore colonies | Pc | |
| Background 1 | ||||
| Wild type | 67 | 14 | 6 | |
| rad50 | 97 | 18 | 7 | <0.01 |
| mre11 | 97 | 6 | 4 | <0.01 |
| Background 2 | ||||
| Wild type | 55 | 13 | 5 | |
| rad50 | 99.2 | 7 | 3 | <0.01 |
| xrs2 | 73 | 8 | 2 | —d |
All strains listed under background 1 were isogenic to W303a. All strains listed under background 2 were derived from a cross between MBH9 and the nonisogenic strain TVL345 or a cross between MBR52-5b and the nonisogenic strain DVL227-6c. TVL345 is isogenic to the DVL227-6c background. Wild-type spore colonies from these crosses were used to determine the wild-type control value.
That is, the percent precursor retained after 20 generations (s2)/the percent precursor present in initial preculture (s0) calculated as described in Materials and Methods.
P values obtained in comparisons between mutant and wild-type strains determined by the rank sum (Mann-Whitney) test.
—, the hypothesis that xrs2 values are identical to wild-type or to rad50 values cannot be rejected at the 95% confidence level by the Mann-Whitney test.
Statistical analysis.
Chi-square analysis was used for comparisons between expected and observed values in site transfer, retention, and acquisition assays. The rank sum (Mann-Whitney) test was employed to compare the degree of overlap between the distributions of wild-type and mutant cell values.
RESULTS
A physical system to study telomeric dynamics.
We have previously proposed that TRD is the consequence of intrachromosomal recombination. However, further tests of this hypothesis have been limited by the absence of physical markers in telomeric simple sequences. To overcome this obstacle, we introduced HaeIII restriction enzyme sites at defined positions within URA3(−)-marked and URA3/ADE2-marked telomeres on the left arm of chromosome VII (VIIL [35]). This was accomplished by the use of an allele of TLC1, TLC1-1, that acts as a template for the HaeIII sequence (64). Transient expression of TLC1-1 was performed in cells containing both the VIIL marked telomere and the rap1-17 mutation, which confers overelongated telomeres (Fig. 1A; see Materials and Methods). HaeIII sites were kept to a minimum by the coexpression of wild-type and mutant telomerase RNAs. Based on HaeIII digestion of genomic DNA, we estimate that ≈50% of the telomeres incorporated one or more HaeIII sites (data not shown).
We identified rap1-17 cells carrying the URA3/ADE2- or URA3(−)-marked VIIL telomeres with one or more mitotically stable HaeIII sites (see Materials and Methods). The HaeIII sites at the URA3/ADE2-marked VIIL telomere were 150, 250, and 750 bp from the subtelomeric junction (HaeIII 150, HaeIII 250, and HaeIII 750, respectively; Fig. 1B and C). HaeIII 250 and HaeIII 750 sites were present as the sole HaeIII site within the marked telomere, while the HaeIII 150 site consisted of three HaeIII sites located at 150, 250, and 400 bp from the subtelomeric junction (data not shown). At URA3(−)-marked VIIL telomeres, HaeIII sites were identified at distances of 640, 1,430, 1,880, 1,930, and 2,900 bp from the subtelomeric junction (data not shown). After replacement of the rap1-17 allele with wild-type RAP1, both sets of strains were used to distinguish among potential mechanisms of TRD. The presence of the HaeIII sites does not alter the ability of the elongated progenitor telomeres to delete to 300 bp, the wild-type telomeric tract size in these strains (Fig. 1C). Since these telomeres lack the conserved elements found adjacent to most telomeres, our assays measure TRD only in the absence of subtelomeric recombination.
TRD is an end-mediated process.
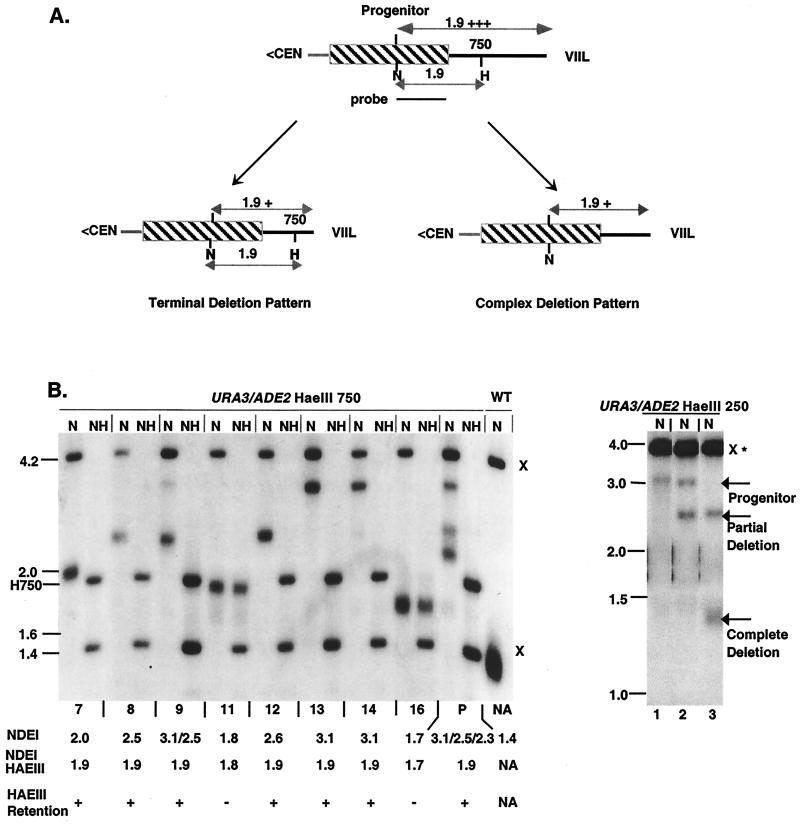
To test whether TRD is mediated through intrachromatid recombination, as postulated previously (35), or through other intra- or interchromosomal pathways, we designed an assay that explores the relationship between the degree of deletion and the retention of the HaeIII site. This site retention assay takes advantage of incomplete TRD events that do not delete to wild-type sizes (see Materials and Methods; Fig. 2A). A subset of incomplete deletions, termed partial deletions in this study, form the basis of the site retention assay. Partial deletions are operationally defined here as incomplete deletions in which the size of the deletion is smaller than the distance from the HaeIII site to the extreme terminus (Fig. 2B, subclones 7, 8, 9, and 12). The behavior of larger incomplete deletions (Fig. 2B, subclones 11 and 16) can be explained by a plethora of mechanisms and are therefore not included in this analysis. Partial deletion products are capable of deletion to wild-type telomeric tract size in a manner indistinguishable from the progenitor (Fig. 2B, right panel). Hence, partial deletions are likely to be mechanistically related to complete deletions in the wild-type TRD pathway (see Discussion).
FIG. 2.
Site retention patterns associated with TRD. (A) Site retention was assayed in partial deletions, representing incomplete deletions that are smaller than the distance between the HaeIII site and the terminus. A terminal deletion pattern (left panel) maintains the HaeIII site. A complex deletion TRD pattern (right panel) would lead to both site loss and retention. The shadings of lines and boxes are described in the legend to Fig. 1A. (B, left panel) MBH91-3 (P), which contains both elongated and deleted forms of the URA3/ADE2 HaeIII 750-marked VIIL telomere, was subcultured, and individual colonies were isolated (lanes 7 to 16). DNAs isolated from these colonies were digested with NdeI to determine the extent of the deletion and with NdeI and HaeIII to measure the retention of the site. The resulting Southern blot was probed with the 1.1-kb NdeI/BamHI ADE2 restriction fragment of pL909. Size markers (in kilobases) are shown to the left of the autoradiogram. Below each lane, the telomeric fragment size (NDEI), the NdeI/HaeIII telomeric fragment size (NDEI/HAEIII), and the presence or absence of the HaeIII site (HAEIII RETENTION) are depicted. HaeIII 750 (H750) denotes the position of the terminal NdeI/HaeIII fragment. x, Restriction sites from the genomic ADE2 locus; NA, not applicable due to absence of HaeIII sites. Other designations are described in the legend to Fig. 1. Subclones 11 and 16 have lost the HaeIII site as a consequence of incomplete deletions larger than the distance between the HaeIII site and the extreme terminus. Partial deletions, on the other hand, (subclones 7, 8, 9, and 12) all displayed the HaeIII 750 site. (B, right panel) DNAs isolated from three subclones of MBH21-20b were subjected to digestion with NdeI (N), and subsequent Southern blots were probed with the 3.6-kb BamHI fragment containing the ADE2 gene. Lane 1, the 3.1-kb precursor representing a telomere tract length of 2.0 kb; lane 2, the 2.5-kb partial deletion product representing a 1.4-kb telomeric tract; lane 3, the 1.35-kb complete deletion product representing a tract length of ≈300 bp. All other designations are indicated in the legend to Fig. 1.
Two possible outcomes of partial deletion can be predicted by analyzing the relationship between the deletion and the retention of the HaeIII site (Fig. 2A). The first class represents telomeres that retain the HaeIII site at its original distance from the subtelomeric junction, regardless of the amount of sequence deleted. This “terminal-deletion” pattern is indicative of mechanisms involving a polar loss of sequences from the telomeric terminus (Fig. 2A, left; Table 2). The second class includes partial deletion products that have lost the HaeIII sites (Fig. 2A, right panel). This “complex-deletion” pattern would be consistent with inter- and intrachromosomal mechanisms that result in telomeric rearrangements (Table 2).
TABLE 2.
Expectations of different mechanisms of TRD
| Event(s)a | Assay
|
|
|---|---|---|
| Site transfer | Terminal deletion pattern (site retention) | |
| Interchromosomal events | ||
| Reciprocal recombination | ||
| Equal | + | NAb |
| Unequal | + | − |
| Nonreciprocal translocation (5, 24) | + | + |
| Break-induced single-strand invasion (53) | − | − |
| DSB gene conversion (68) | + | − |
| Intrachromosomal recombination | ||
| Sister chromatid exchange | ||
| Equal | NAc | NAc |
| Unequal (57) | +/− | − |
| Single-strand annealing (26) | − | − |
| Terminal-strand invasion | − | + |
| Intrachromatid nucleolytic activity | ||
| Endonuclease activity with DNA repair | − | − |
| Endonuclease activity without DNA repair | − | + |
The numbers in parentheses refer to the corresponding reference citation.
NA, not applicable due to absence of partial deletion products.
NA, not applicable due to inability to detect equal sister chromatid exchanges in this assay.
To distinguish between these possibilities, we examined 47 independent partial deletions for both URA3(−) HaeIII-marked and URA3/ADE2 HaeIII-marked VIIL telomeres. Strikingly, none of these deletions lost the HaeIII site. In all cases a terminal deletion pattern was observed (Fig. 2B, left; Table 3). This pattern was observed regardless of whether the HaeIII site was present in end-distal or end-proximal regions. The terminal deletion pattern is consistent with two possible end-mediated processes: (i) invasion of telomeric ends into a heterologous telomere, forming a nonreciprocal translocation (5, 24), or (ii) intrachromatid deletion proceeding from the extreme terminus (Table 2).
TABLE 3.
Site retention patterns associated with TRDa
| URA3/ADE2-HaeIII 750 telomere pattern | No. of deletions for:
|
||
|---|---|---|---|
| RAP1 (NA) | rap1-17 (P < 0.001)b | rap1-17 est1::URA3 (P < 0.025)c | |
| Terminal deletion pattern | 47 | 25 | 19 |
| Complex deletion pattern | 0 | 10 | 0 |
| Total | 47 | 35 | 19 |
Cumulative site retention assays were measured with DNA isolated from individual colonies of MBH11-8b, MBH22-7b, MBH93-6d, and MUH15 wild-type RAP1 strains; MBH1, MBH2, MBH9, MUH4, MUH5, MUH6, and MUH7 rap1-17 strains; and MEH9, carrying the est1 rap1-17 double mutant. The values are represented as the total number of deletions relative to the total sample size. NA, not applicable.
Significance relative to wild-type RAP1 deletion patterns was determined by chi-square analysis.
Significance relative to rap1-17 deletion patterns as determined by chi-square analysis.
Absence of interchromosomal exchange in TRD.
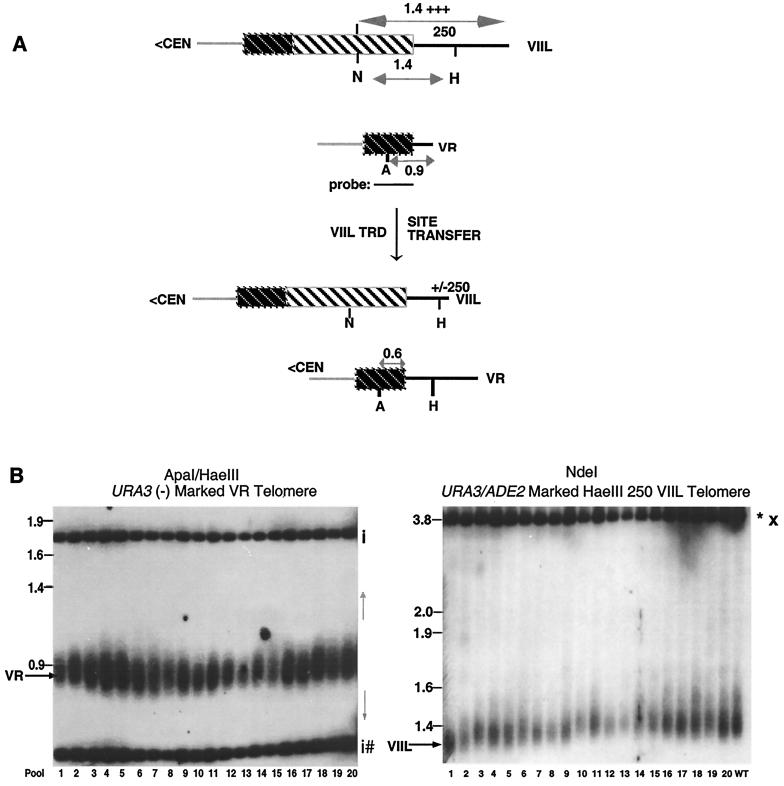
To distinguish between these possibilities, we designed a “site transfer” assay to measure the movement of a HaeIII site between telomeres during rapid deletion. Site transfer measures the ability of the VR URA3(−)-marked telomere that lacks a HaeIII site telomere to acquire a HaeIII site from a heterologous HaeIII-marked VIIL telomere during rapid deletion (Fig. 3A; see Materials and Methods). In most cells, the marked VIIL telomere was the only elongated and HaeIII-marked telomere. To carry out site transfer, we first identified cells carrying a VIIL telomere which had undergone a deletion to the wild-type size (Fig. 3B, right panel). Transfer of HaeIII sites in these cells was subsequently measured by the ability of the VR-marked telomere to be cleaved by HaeIII. Site transfer would be expected to occur in a nonreciprocal translocation, but not in an intrachromatid deletion (Fig. 3A).
FIG. 3.
Relationship between TRD and HaeIII site transfer. (A) Site transfer is assayed by measuring the frequency of HaeIII site movement from the elongated URA3/ADE2 HaeIII 250-marked VIIL telomere to the URA3(−)-marked VR telomere in strain YP17-24c. The shading of lines and boxes has been described in Fig. 1A. (B, left panel) DNA was isolated from 20 batch cultures, each containing 10 independent colonies. DNA was digested with ApaI and HaeIII and probed with a 1.7-kb PstI fragment of pRS316 containing the URA3 gene. The telomeric fragments (VR) were detected in each batch. The position of a unique HaeIII site transferred to the VR telomere (as well as the length of the VR telomere after transfer) is unpredictable and depends on the mechanism leading to the site transfer (gray arrows to the right of the panel). The 1.8-kb fragment represents the left junction of the chromosomal URA3 gene, while the smaller fragments represent two 350-bp HaeIII sites that are common to both telomeric and genomic copies of URA3. The positions of internal URA3 sequences are designated by “i.” Fragments originating from the URA3(−) telomeric fragment are denoted by a hatched mark. Size markers are shown on the left. (B, right panel) DNAs from the same samples were digested with NdeI and Southern blots were probed with the 3.6-kb BamHI fragment containing the ADE2 gene. All samples contained deleted URA3/ADE2-marked VIIL telomeres. WT refers to CZY1/RAP1. Other designations are defined in Fig. 1C.
The site transfer assay was carried out using telomeres carrying either an end-proximal (ura3/ADE2 HaeIII 750) or an end-distal (URA/ADE2 HaeIII 250) HaeIII site and a wild-type-length URA3(−)-marked VR telomere (Fig. 3B, left; data not shown). Among 260 samples for each VIIL telomere, we did not observe a single HaeIII site transfer from the elongated VIIL telomere to the VR-marked telomere. The measured value for both the distal (0 of 260) and proximal (0 of 260) HaeIII sites is significantly different from the expected value (8.7 of 260; χ2 = 6.9; P < 0.01) if deletion and transfer are linked and if the VIIL telomere can randomly recombine with the VR-marked telomere (see Discussion).
For the pooled site transfer results from both set of strains, the expectation of 17.4 site transfers out of 520 samples is clearly different from the experimental results (0 of 520) (χ2 = 16; P < 0.001). The latter result indicates that even a VIIL-VR recombination frequency threefold lower than the expected value would be statistically significant. Our results suggest that site transfer between end-proximal or end-distal HaeIII sites to the VR telomere is not frequently associated with rapid deletion. These data are not consistent with any interchromosomal mechanism, including nonreciprocal translocation, which involves exchange between the telomeres of heterologous chromosomes.
As a reciprocal assay, we determined the frequency of site acquisition from all HaeIII-marked elongated telomeres to a naive wild-type-length ura3/ADE2-marked VIIL telomere, lacking HaeIII sites. If TRD is due to interchromosomal exchange, then the TRD rate is equal to the sum of the rates of the productive associations with heterologous telomeres. Given that TRD was observed in ≈33% of cells after 20 generations of growth (Table 4) and that 25% of telomeres in these strains were both elongated and HaeIII marked, we would expect a site acquisition frequency of 8.25% (see Materials and Methods). We would therefore expect ≈18 acquisition events out of the 221 samples that were tested. Strikingly, we never observed a single site acquisition event (χ2 = 16.7; P < 0.001). Similar results were obtained after analysis of HaeIII site transfer from heterologous telomeres to an elongated marked telomere (data not shown). These data corroborate the site transfer results and suggest the absence of interchromosomal exchange on TRD.
Taken together, our results argue that TRD proceeds unidirectionally from the terminus in the model telomeres used in our study. Our data support a mechanism mediated either through end-mediated intrachromatid recombination or through exo- or endonucleolytic cleavage.
The Rap1p C terminus does not influence TRD.
The 165-amino-acid Rap1p C terminus acts as a negative regulator of telomere addition. The rap1-17 mutant that lacks this domain displays multiple and frequent cycles of rapid loss and overelongation of telomeric tract sequences (30). However, it remains unknown whether this rapid loss of telomeric sequences proceeds through the wild-type mechanism for TRD. We therefore sought to determine the influence of the Rap1p C-terminal domain on TRD by examining the behavior of TRD in rap1-17 strains (30, 35).
One of the hallmarks of TRD in wild-type cells is a terminal deletion pattern of HaeIII site retention among partial deletion products. To test the possibility of a distinct TRD pathway in rap1-17 cells, we conducted the site retention assay in cells carrying either a URA3(−)- or a URA3/ADE2-marked VIIL telomere with HaeIII sites present at distinct telomeric positions (Table 3). Of 35 partial deletions analyzed in rap1-17 cells, 10 lost HaeIII sites, indicating an apparent complex pattern of site retention (Table 3), a result significantly different from that for the wild type (χ2 = 22; P < 0.001).
These observations can be explained in two ways. First, TRD in rap1-17 cells may operate either via a pathway that is mechanistically distinct from that of the wild type or via a mixture of wild-type and alternative pathways. Second, the telomeres of rap1-17 cells may delete to positions beyond the HaeIII site proximal to subtelomeric sequences. Since the formation of the elongated telomeres in rap1-17 cells is dependent on telomerase activity (data not shown), such telomeres could re-elongate after TRD to positions end proximal to the position of the original HaeIII site but lacking the HaeIII site.
To address the latter hypothesis, we examined the effects of the elimination of telomerase activity on TRD in rap1-17 cells that also carried a mutation in one of the telomerase holoenzyme components, Est1p. Est1p is a single-stranded telomeric DNA-binding protein that acts with Cdc13p as an essential component in telomerase loading (12, 39). The est1 mutation alone in a wild-type RAP1 background has no effect on rapid deletion (35). As expected for a loss in telomerase activity, all elongated telomeres were stabilized at distinct sizes in rap1-17 est1::URA3 double mutants (data not shown). If the deletion–re-elongation hypothesis were correct, we would predict a recovery of the wild-type terminal deletion pattern of site retention in double-mutant cells.
To test this possibility, we conducted the site retention assay in rap1-17est1::URA3 cells carrying a URA3/ADE2 HaeIII 750-marked VIIL telomere. Strikingly, we observed that all 19 partial TRD events examined retained the HaeIII site (Table 3). This is a significantly higher frequency of HaeIII retention than observed in rap1-17 cells (χ2 = 4.7; P < 0.025). Hence, the apparent complex deletion pattern in rap1-17 cells is the probable consequence of HaeIII site loss and subsequent re-elongation by telomerase. These data strongly suggest that the C terminus of Rap1p does not influence the mechanism of TRD and thereby unlinks TRD from the process of Rap1p counting (44, 61).
Rad50p and Mre11p are positive regulators of TRD.
The MRX complex participates in a multiplicity of homologous mitotic and meiotic recombinational pathways, nonhomologous end joining, and telomere size control (6, 7, 8, 23, 27, 28, 42, 47, 50, 76). At least one component of this complex, Rad50p, is known to influence the abrupt elongation of telomeres by telomere/telomere recombination in cells that lack telomerase (type II recombination [69]).
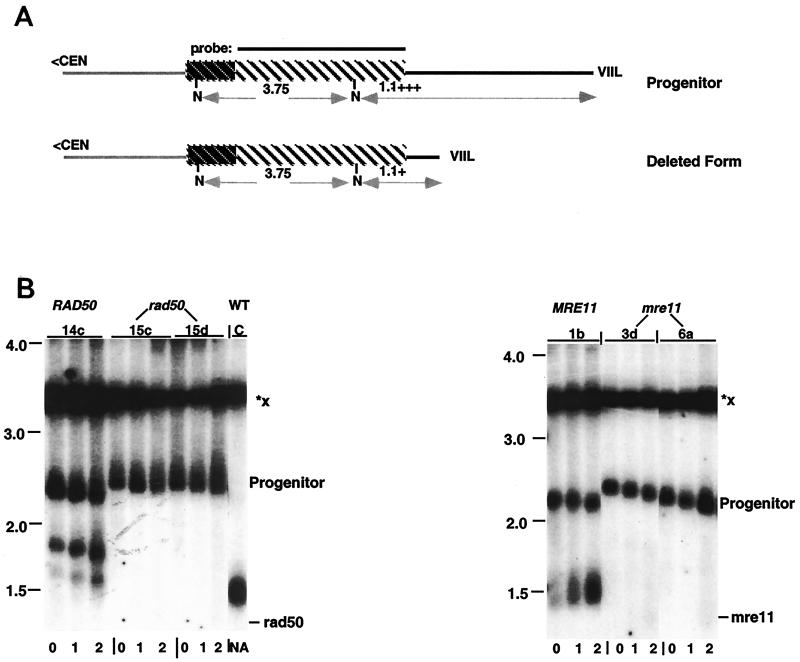
To test whether TRD falls within this pathway for recombination between telomeric repeats, we first tested the possibility of Rad50p involvement in TRD. Relative TRD rates were measured by a decrease in the retention of the undeleted form of URA3/ADE2-marked VIIL telomeres after liquid subculturing of both rad50 and wild-type cells (Table 4; Fig. 4B, left panel; see Materials and Methods). Strikingly, the rad50 mutant strongly reduced the frequency of TRD. The retention of the undeleted form increased from 67% in wild-type strains to 97% in isogenic rad50 cells after 20 generations of growth (P < 0.01; Table 4, background 1 strains). These data suggest that Rad50p is a positive regulator of rapid deletion. Similar results were observed in a second genetic background (Table 4, background 2 strains).
FIG. 4.
rad50 and mre11 mutations inhibit TRD. (A) Diagram of the restriction maps from the progenitor and deleted ura3/ADE2-marked VIIL telomeres. Line and box shadings are defined in Fig. 1A. (B, left panel) DNA was isolated from a wild-type spore colony (YPR50-14c) and from rad50 spore colonies (YPR50-15c and YPR50-15d) after two rounds of liquid subculturing, corresponding to 20 generations of growth (0 to 2, indicated below each lane). All strains contain the elongated ura3/ADE2-marked VIIL telomere. The DNA was digested with NdeI, and the resulting Southern blots were hybridized with the 3.6-kb BamHI fragment of pL909 carrying the ADE2 gene. The expected mobility of the telomeric fragment in rad50 cells is indicated on the right. Note that an incomplete deletion also accumulates during subculturing of wild-type cells. (B, right panel) DNA was isolated from a wild-type spore colony (YPM11-1b) and from mre11 spore colonies (YPM11-3d and YPM11-6a), containing the elongated ura3/ADE2-marked VIIL telomere, after two rounds of liquid subculturing as described above. DNA was digested and subjected to Southern analysis as described in the text. The expected mobility of the telomeric fragment in mre11 cells is indicated on the right. Size markers (in kilobases) are shown on the left of each panel. Vertical bars above the autoradiograms indicate the positions of lanes from the same gel that were spliced for presentation purposes. C refers to the strain CZY1/RAP1. All other designations were described in the legend to Fig. 1C.
If Rad50p acts through the MRX complex in TRD, then we would predict that mre11 and xrs2 mutants would also display a defect in TRD. Indeed, we found that strains carrying an mre11 disruption allele displayed only very low levels of TRD (Table 4; Fig. 4B, right panel). After subculture of the mre11 cells, 97% of the precursor form was retained, compared to the 67% observed in isogenic wild-type cells after 20 generations of growth (P < 0.01). The xrs2 null allele, however, generated values that were not statistically different from values obtained in either wild-type or rad50 cells (Table 4, background 2 strains). Hence, we cannot conclude at present whether Xrs2p participates in TRD. Nonetheless, given the phenotypes of mre11 and rad50 alleles on TRD, these data suggest that TRD is mediated through the MRX complex.
DISCUSSION
In this investigation, we present the first mechanistic insights into TRD, a process that is likely to be a major participant in telomere size control. Our studies have been based on the development of a physical assay for TRD, in which HaeIII sites are introduced into the telomeric tract. The presence of telomeric HaeIII sites does not interfere with telomeric functions including silencing, size control, and TRD (Fig. 1C; also data not shown).
Two physical criteria were used to investigate TRD. The first criterion was the relationship between the loss of sequences and the retention of the HaeIII site. The site retention assay relies on the characteristics of partial TRD events, i.e., incomplete deletions that are smaller than the distance from the HaeIII site to the extreme terminus. Partial deletion follows the same pathway as complete deletions. The evidence for this is twofold: (i) partial deletion species subsequently delete to wild-type-length telomere size (Fig. 2B, right panel), and (ii) complete TRD occurs more frequently as the percentage of wild-type-length telomeres increases (35). Thus, the size of the deletion is dependent on the size of the majority of telomeres rather than being an obligatory intermediate step in TRD. Hence, partial TRD is likely to be the consequence of sizing relative to aberrantly elongated telomeres.
The second criterion is the movement of HaeIII sites between two marked telomeres during rapid deletion. We found no evidence for site transfer in our studies. For statistical evaluation of our data, we made the simplifying assumption that the VIIL telomere associates with VR and other telomeres at similar rates. One important difference, however, is the presence of subtelomeric elements in natural, but not artificially marked, telomeres. Previous studies have revealed the presence of selected groups of telomeres that exhibit preferentially high rates of interchromosomal recombination between conserved Y′ subtelomeric elements (38). Subtelomeric regions that share the greatest degree of homology have the highest rates of recombination, suggesting that the degree of homology dictates the probability of subtelomeric recombination (38). If these preferentially interacting telomeres are functionally sequestered from the marked telomeres, site transfers between the VR and VIIL telomeres may occur at a higher frequency than expected. Hence, the actual significance of the difference between observed and expected values may be greater than we reported. Therefore, associations based on subtelomeric homology are unlikely to interfere with the ability to observe HaeIII site transfer. The lack of ura3/ADE2-marked VIIL telomere site acquisition from heterologous HaeIII-marked elongated telomeres further argues that interchromosomal exchange is not likely to be responsible for TRD.
The presence of a terminal pattern of site retention and the absence of site transfer between nonhomologous telomeres, lacking subtelomeric homology, effectively rule out interchromosomal recombination. These include those processes involving crossing over (see, e.g., references 5 and 24), double-strand break repair (68) and break-induced replication (53). The data are also inconsistent with intrachromosomal events such as sister chromatid exchange (57), single-strand annealing (26, 53), and other mechanisms of internal deletion (Table 2). Rather, the data support a mechanism of intrachromatid loss of telomeric sequences initiated at the extreme terminus. An exonucleolytic activity is an unlikely explanation of our data, given that this activity would be expected to generate heterodispersed telomeric fragments that decrease in size during growth, rather than the discrete TRD products that we observe. In addition, our physical data cannot be explained by endonuclease cleavage, unless cleavage occurs in the absence of DNA repair. We note that any recombinational basis for TRD must involve a homeologous interaction between telomeric sequences, given the limited sequence homology present in telomeric tracts.
TRD is independent of the C terminus of Rap1p. This finding strongly suggests that TRD is also independent of components of telomeric chromatin, including both negative (Rif1p and Rif2p) and positive (Sir3p and Sir4p) regulators of telomere size (10, 25, 52, 77). Additionally, TRD is not linked to the overall structure of subtelomeric regions, since loss of the C-terminal tail and Sir proteins results in an open chromatin state in subtelomeric regions (15, 31).
TRD is likely to be a mechanism that acts in concert with other elements of the telomere sizing system to achieve and maintain homeostasis. In particular, the Rap1p counting mechanism (44, 61), size regulation through Rap1p-telomerase interactions (29, 65), or Cdc13-mediated homeostatic mechanisms (9, 17, 18, 40, 56, 60) present at the terminal 3′ overhang may maintain the size of the TRD product. It is noteworthy that the Rap1p C terminus is essential for the Rap1p counting mechanism, but dispensable for TRD, suggesting that the two processes are mechanistically distinct.
Participation of the MRX complex in TRD.
The strong inhibitory effect of rad50 and mre11 on TRD, coupled with the diminution of TRD in rad52 cells (35; data not shown) supports a recombinational model for this process. However, the relationship between Rad50p stimulation and Rad52p stimulation of TRD is unclear, and the presence of Rad50p-dependent Rad52p-independent deletions remains a distinct possibility.
Rad50p, Mre11p, and Xrs2p are associated in a complex that is required for mitotic homologous recombination, induced by ionizing radiation (47), double-strand break formation (7, 27), nonhomologous end joining (8, 23, 42, 76), and meiotic recombination (23). Rad50p also plays an important, but nonessential, role in single-strand annealing (26). The precise function of Rad50p remains unknown, although it has been suggested to act through the preparation of DNA single strands for recombination (67) or through regulation of chromatin condensation prior to recombination, as observed in meiotic cells (51, 53). Alternatively, the participation of Rad50p in TRD may reflect a specific form of end processing required prior to intramolecular strand invasion. While Rad50p is important for TRD, it does not appear to be essential, since TRD events can be observed under growth conditions that select for the presence of shortened telomeres (54; data not shown; see Materials and Methods).
A second component of the MRX complex, Mre11p, also has a central role in TRD. The exonucleolytic activities of Mre11p may play a role in the processing of ends for strand invasion during mitotic and meiotic recombination (13, 23, 71). However, while null mutations in MRE11 display short telomere tract sizes (6, 50), selective elimination of the Mre11p nuclease activities has no effect on wild-type-length telomeres (48). These data therefore indicate that the function of Mre11p in telomeric size control does not require the nucleolytic activity of Mre11p. Although the relationship of the role of Mre11p in telomere size control and TRD is not known, these data raise the possibility that Mre11p may play a different, possibly structural, role in TRD. The third component of the MRX complex, Xrs2p, may participate in TRD to a lesser extent, if at all. The MRX function in telomere size control is dependent on the presence of the ATM homologue Tel1p (62). However, in rapid deletion studies, we have found that a tel1 null allele confers a wide range of deletion frequencies (data not shown). Therefore, the role of Tel1p in the MRX pathway for TRD remains an open question.
At least two distinct recombinational rescue pathways are present after inactivation of telomerase: Rad50p-independent recombination between subtelomeric Y′ repeats (type I) and Rad50p-dependent recombination between the telomeric simple sequences (type II [69, 70]). The exclusive involvement of Rad50p in the generation of type II recombination is consistent with the participation of telomere-telomere recombination in TRD and may suggest that the generation of type II survivors and TRD are mechanistically related.
TRD and homeologous recombination.
Unlike most homeologous recombination, TRD is not constrained by mismatch repair enzymes (35, 53). Mutations in MSH2, MSH3, or PMS1 have no effect on the rate of TRD (35). This apparent discrepancy has several possible explanations. First, the effect of mismatch repair enzymes on homeologous sequences is dependent on the degree of homology (11). While mismatches of ≈10% are strongly repressed by Msh2p and Msh3p, DNA sequences having lower levels of homology are independent of mismatch repair. Second, cells may have multiple pathways for homeologous recombination, as suggested by the presence of a homeologous recombination pathway independent of mismatch-repair in pms1 cells (4). Third, the chromatin structure at telomeres may influence the telomeric homology requirements or the role of mismatch repair enzymes. For example, among telomeric sequences are multiple copies of the highly conserved 13-bp binding site for Rap1p (14, 74), which, in the context of telomeric chromatin, may play a unique role in telomere-telomere pairing. If Rap1p is involved, however, it must be independent of the Rap1p C terminus, since the rap1-17 mutation does not influence TRD. Similarly, a specific structure formed at telomeres, such as a t-loop (22) or a G-quadraplex structure (75), may act in cis to promote TRD. Distinguishing among these possibilities is a major goal for the future.
A model for telomeric recombination.
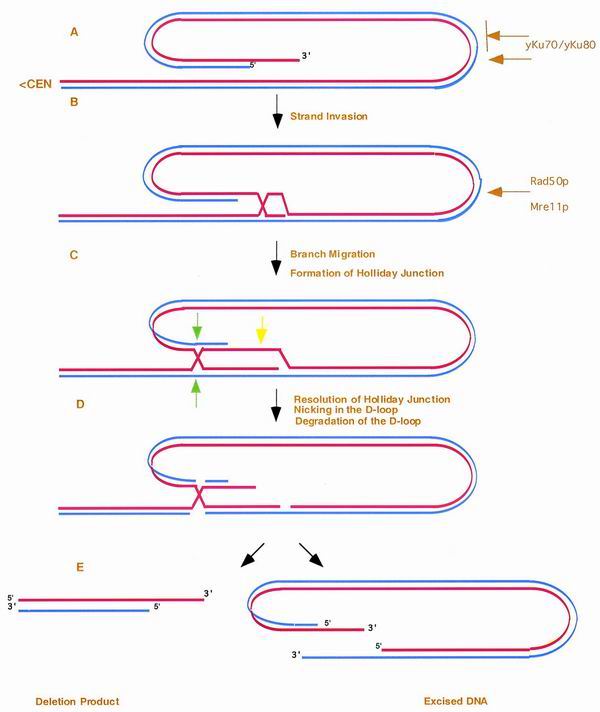
The genetic and physical studies presented here suggest that TRD is a 3′ end-mediated intrachromatid recombinational process (Fig. 5). Specifically, the components of the MRX complex may directly or indirectly prepare the 3′ telomeric terminus for strand invasion. The recombinationally proficient 3′ extreme terminus would subsequently invade distal telomeric DNA, leading to an excision of the intervening sequences through branch migration, D-loop degradation, and resolution of the Holliday junction (Fig. 5). This may result in the transient formation of linear or circular degradation products, depending upon the precise nature of resolution, which would be quickly lost from the population. One intriguing feature of this model is that multiple cycles of both semiconservative replication primed from the invading strand and subsequent reinvasion could give rise to amplified telomeric DNA. This interrelationship raises the possibility that TRD and type II events may be mechanistically related. Interestingly, the proposed TRD intermediate (Fig. 5, step 2) is virtually identical in structure to the stable human t-loops involved in telomere cap function (22). The function and evolutionary relationship between TRD and t-loops, however, remains unknown.
FIG. 5.
Model for telomeric rapid deletion. We propose that the 3′ single strand from the telomeric terminus (A) invades distal telomere tract sequences, leading to the formation of a looped structure (B). (C) After branch migration, the displaced strand forms both a D-loop and Holliday junction. After nicking of the D-loop (yellow arrow), degradation of the D-loop (D), and resolution of the outer strands of the Holliday junction (green arrows), both the TRD product and a linear excision product are produced (E). As shown, we propose that the Ku heterodimer regulates the degree of capping, which is likely to reduce the rate of TRD. Hence, yKu may essentially control the number of telomeres capable of rapid deletion. In this model, the Rad50p and Mre11p components of the MRX complex act to regulate the initial strand invasion either through chromosome condensation or the preparation of ends for recombination. Red line, the leading strand proceeding 5′ to 3′ toward the terminus; blue line, the complementary strand. Dependent upon the direction of resolution and the formation and stability of the D-loop, the intermediate can also give rise to circular forms of the terminal fragment.
Three lines of evidence point to a physiological role for TRD in telomere size regulation. First, telomeric sequences that are ≈400 bp longer than the wild-type length are proficient for TRD, well within the range of telomeric sizes found in different laboratory yeast strains (73). Smaller changes in telomere size cannot be ascribed to TRD, since it is not possible, under our assay conditions, to determine whether such decreases in size are due to slow attrition or rapid deletion of telomeric sequences. Second, the efficiency and precision of TRD events suggest that any overelongated telomere is inherently susceptible to rapid deletion to wild-type size. Third, the presence of rapid deletion of telomeric tracts in a multiplicity of organisms, including Tetrahymena spp. (33), trypanosomes (55), and human cells (45, 49), suggests that the rapid deletion of telomeric tracts is a conserved process through evolution.
A further mechanistic understanding of TRD will provide paradigms for the control of telomere size in yeast and higher systems. The characterization of TRD and other size control regulatory systems in yeast and higher eukaryotes will ultimately lead to an ability to understand and to regulate the factors involved in telomere size control.
ACKNOWLEDGMENTS
M.B. and Y.P. contributed equally to this study.
We thank Charles Zhang for his excellent technical contribution to this study. We also thank E. B. Hoffman, M. Clancy, T. de Lange, D. Gottschling, M. A. Osley, T. D. Petes, M. Wellinger, and members of our laboratory for critical comments during the course of these studies and D. Gottschling, E. Louis, V. Lundblad, T. D. Petes, M. Resnick, and L. Symington for providing strains and plasmids.
This study was supported by NIH grant GM56526 and matching funds from the Tulane Cancer Center. Initial studies were funded by the National Science Foundation.
Footnotes
Dedicated to E. B. Hoffman.
REFERENCES
- 1.Aguilera A, Klein H L. Genetic and molecular analysis of recombination events in Saccharomyces cerevisiae occurring in the presence of the hyper-recombination mutation hpr1. Genetics. 1989;122:503–517. doi: 10.1093/genetics/122.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alani E, Cao L, Kleckner N. A method for gene disruption that allows repeated use of URA3 selection in the construction of multiply disrupted yeast strains. Genetics. 1987;116:541–545. doi: 10.1534/genetics.112.541.test. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bianchi A, de Lange T. Ku binds telomeric DNA in vitro. J Biol Chem. 1999;274:21223–21227. doi: 10.1074/jbc.274.30.21223. [DOI] [PubMed] [Google Scholar]
- 4.Borts R H, Leung W Y, Kramer W, Kramer B, Williamson M, Fogel S, Haber J E. Mismatch repair-induced meiotic recombination requires the pms1 gene product. Genetics. 1990;124:573–584. doi: 10.1093/genetics/124.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bosco G, Haber J E. Chromosome break-induced DNA replication leads to nonreciprocal translocations and telomere capture. Genetics. 1998;150:1037–1047. doi: 10.1093/genetics/150.3.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boulton S J, Jackson S P. Components of the Ku-dependent non-homologous end-joining pathway are involved in telomeric length maintenance and telomeric silencing. EMBO J. 1998;17:1819–1828. doi: 10.1093/emboj/17.6.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bressan D A, Baxter B K, Petrini J H. The Mre11-Rad50-Xrs2 protein complex facilitates homologous recombination-based double-strand break repair in Saccharomyces cerevisiae. Mol Cell Biol. 1999;19:7681–7687. doi: 10.1128/mcb.19.11.7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chamankhah M, Xiao W. Formation of the yeast Mre11-Rad50-Xrs2 complex is correlated with DNA repair and telomere maintenance. Nucleic Acids Res. 1999;27:2072–2079. doi: 10.1093/nar/27.10.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chandra A, Hughes T R, Nugent C I, Lundblad V. Cdc13 both positively and negatively regulates telomere replication. Genes Dev. 2001;15:404–414. doi: 10.1101/gad.861001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cockell M, Gotta M, Palladino F, Martin S G, Gasser S M. Targeting Sir proteins to sites of action: a general mechanism for regulated repression. Cold Spring Harbor Symp Quant Biol. 1998;63:401–412. doi: 10.1101/sqb.1998.63.401. [DOI] [PubMed] [Google Scholar]
- 11.Datta A, Adjiri A, New L, Crouse G F, Jinks Robertson S. Mitotic crossovers between diverged sequences are regulated by mismatch repair proteins in Saccaromyces cerevisiae. Mol Cell Biol. 1996;16:1085–1093. doi: 10.1128/mcb.16.3.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans S K, Lundblad V. Est1 and Cdc13 as comediators of telomerase access. Science. 1999;286:117–120. doi: 10.1126/science.286.5437.117. [DOI] [PubMed] [Google Scholar]
- 13.Furuse M, Nagase Y, Tsubouchi H, Murakami-Murofushi K, Shibata T, Ohta K. Distinct roles of two separable in vitro activities of yeast Mre11 in mitotic and meiotic recombination. EMBO J. 1998;17:6412–6425. doi: 10.1093/emboj/17.21.6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilson E, Roberge M, Giraldo R, Rhodes D, Gasser S M. Distortion of the DNA double helix by RAP1 at silencers and multiple telomeric binding sites. J Mol Biol. 1993;231:293–310. doi: 10.1006/jmbi.1993.1283. [DOI] [PubMed] [Google Scholar]
- 15.Gottschling D E. Telomere-proximal DNA in Saccharomyces cerevisiae is refractory to methyltransferase activity in vivo. Proc Natl Acad Sci USA. 1992;89:4062–4065. doi: 10.1073/pnas.89.9.4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gottschling D E, Aparicio O M, Billington B L, Zakian V A. Position effect at Saccharomyces cerevisiae telomeres: reversible repression of Pol II transcription. Cell. 1990;63:751–762. doi: 10.1016/0092-8674(90)90141-z. [DOI] [PubMed] [Google Scholar]
- 17.Grandin N, Damon C, Charbonneau M. Cdc13 cooperates with the yeast Ku proteins and Stn1 to regulate telomerase recruitment. Mol Cell Biol. 2000;20:8397–8408. doi: 10.1128/mcb.20.22.8397-8408.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grandin N, Damon C, Charbonneau M. Ten1 functions in telomere end protection and length regulation in association with Stn1 and Cdc13. EMBO J. 2001;20:1173–1183. doi: 10.1093/emboj/20.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gravel S, Larrivee M, Labrecque P, Wellinger R J. Yeast Ku as a regulator of chromosomal DNA end structure. Science. 1998;280:741–744. doi: 10.1126/science.280.5364.741. [DOI] [PubMed] [Google Scholar]
- 20.Greider C W. Telomerase biochemistry and regulation. Plainview, N.Y: Cold Spring Harbor Laboratory; 1995. [Google Scholar]
- 21.Greider C W, Blackburn E H. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature. 1989;337:331–337. doi: 10.1038/337331a0. [DOI] [PubMed] [Google Scholar]
- 22.Griffith J D, Comeau L, Rosenfield S, Stansel R M, Bianchi A, Moss H, de Lange T. Mammalian telomeres end in a large duplex loop. Cell. 1999;97:503–514. doi: 10.1016/s0092-8674(00)80760-6. [DOI] [PubMed] [Google Scholar]
- 23.Haber J E. The many interfaces of Mre11. Cell. 1998;95:583–586. doi: 10.1016/s0092-8674(00)81626-8. [DOI] [PubMed] [Google Scholar]
- 24.Haber J E, Hearn M. Rad52-independent mitotic gene conversion in Saccharomyces cerevisiae frequently results in chromosomal loss. Genetics. 1985;111:7–22. doi: 10.1093/genetics/111.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hardy C F, Sussel L, Shore D. A RAP1-interacting protein involved in transcriptional silencing and telomere length regulation. Genes Dev. 1992;6:801–814. doi: 10.1101/gad.6.5.801. [DOI] [PubMed] [Google Scholar]
- 26.Ivanov E L, Sugawara N, Fishman-Lobell J, Haber J E. Genetic requirements for the single-strand annealing pathway of double-strand break repair in Saccharomyces cerevisiae. Genetics. 1996;142:693–704. doi: 10.1093/genetics/142.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ivanov E L, Sugawara N, White C I, Fabre F, Haber J E. Mutations in XRS2 and RAD50 delay but do not prevent mating-type switching in Saccharomyces cerevisiae. Mol Cell Biol. 1994;14:3414–3425. doi: 10.1128/mcb.14.5.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kironmai K M, Muniyappa K. Alteration of telomeric sequences and senescence caused by mutations in RAD50 of Saccharomyces cerevisiae. Genes Cells. 1997;2:443–455. doi: 10.1046/j.1365-2443.1997.1330331.x. [DOI] [PubMed] [Google Scholar]
- 29.Krauskopf A, Blackburn E H. Rap1 protein regulates telomere turnover in yeast. Proc Natl Acad Sci USA. 1998;95:12486–12491. doi: 10.1073/pnas.95.21.12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kyrion G, Boakye K A, Lustig A J. C-terminal truncation of RAP1 results in the deregulation of telomere size, stability, and function in Saccharomyces cerevisiae. Mol Cell Biol. 1992;12:5159–5173. doi: 10.1128/mcb.12.11.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kyrion G, Liu K, Liu C, Lustig A J. RAP1 and telomere structure regulate telomere position effects in Saccharomyces cerevisiae. Genes Dev. 1993;7:1146–1159. doi: 10.1101/gad.7.7a.1146. [DOI] [PubMed] [Google Scholar]
- 32.Laroche T, Martin S G, Gotta M, Gorham H C, Pryde F E, Louis E J, Gasser S M. Mutation of yeast Ku genes disrupts the subnuclear organization of telomeres. Curr Biol. 1998;8:653–656. doi: 10.1016/s0960-9822(98)70252-0. [DOI] [PubMed] [Google Scholar]
- 33.Larson D D, Spangler E A, Blackburn E H. Dynamics of telomere length variation in Tetrahymena thermophila. Cell. 1987;50:477–483. doi: 10.1016/0092-8674(87)90501-0. [DOI] [PubMed] [Google Scholar]
- 34.Le S, Moore J K, Haber J E, Greider C W. RAD50 and RAD51 define two pathways that collaborate to maintain telomeres in the absence of telomerase. Genetics. 1999;152:143–152. doi: 10.1093/genetics/152.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li B, Lustig A J. A novel mechanism for telomere size control in Saccharomyces cerevisiae. Genes Dev. 1996;10:1310–1326. doi: 10.1101/gad.10.11.1310. [DOI] [PubMed] [Google Scholar]
- 36.Lingner J, Cech T R, Hughes T R, Lundblad V. Three ever shorter telomere (EST) genes are dispensable for in vitro yeast telomerase activity. Proc Natl Acad Sci USA. 1997;94:11190–11195. doi: 10.1073/pnas.94.21.11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lingner J, Hughes T R, Shevchenko A, Mann M, Lundblad V, Cech T R. Reverse transcriptase motifs in the catalytic subunit of telomerase. Science. 1997;276:561–567. doi: 10.1126/science.276.5312.561. [DOI] [PubMed] [Google Scholar]
- 38.Louis E J, Borts R H. A complete set of marked telomeres in Saccharomyces cerevisiae for physical mapping and cloning. Genetics. 1995;139:125–136. doi: 10.1093/genetics/139.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lundblad V, Szostak J W. A mutant with a defect in telomere elongation leads to senescence in yeast. Cell. 1989;57:633–643. doi: 10.1016/0092-8674(89)90132-3. [DOI] [PubMed] [Google Scholar]
- 40.Lustig A J. Cdc13 subcomplexes regulate multiple telomere functions. Nat Struct Biol. 2001;8:297–299. doi: 10.1038/86157. [DOI] [PubMed] [Google Scholar]
- 41.Lustig A J. The identification of telomerase subunits: catalyzing telomere research. Trends Cell Biol. 1997;7:299–302. doi: 10.1016/S0962-8924(97)01110-0. [DOI] [PubMed] [Google Scholar]
- 42.Lustig A J. The Kudos of non-homologous end-joining. Nat Genet. 1999;23:130–131. doi: 10.1038/13755. [DOI] [PubMed] [Google Scholar]
- 43.Marcand S, Brevet V, Gilson E. Progressive cis-inhibition of telomerase upon telomere elongation. EMBO J. 1999;18:3509–3519. doi: 10.1093/emboj/18.12.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marcand S, Gilson E, Shore D. A protein-counting mechanism for telomere length regulation in yeast. Science. 1997;275:986–990. doi: 10.1126/science.275.5302.986. [DOI] [PubMed] [Google Scholar]
- 45.Martens U M, Chavez E A, Poon S S, Schmoor C, Lansdorp P M. Accumulation of short telomeres in human fibroblasts prior to replicative senescence. Exp Cell Res. 2000;256:291–299. doi: 10.1006/excr.2000.4823. [DOI] [PubMed] [Google Scholar]
- 46.McAinsh A D, Scott-Drew S, Murray J A, Jackson S P. DNA damage triggers disruption of telomeric silencing and Mec1p-dependent relocation of Sir3p. Curr Biol. 1999;9:963–966. doi: 10.1016/s0960-9822(99)80424-2. [DOI] [PubMed] [Google Scholar]
- 47.McKee R H, Lawrence C W. Genetic analysis of gamma-ray mutagenesis in yeast. III. Double-mutant strains. Mutat Res. 1980;70:37–48. doi: 10.1016/0027-5107(80)90056-1. [DOI] [PubMed] [Google Scholar]
- 48.Moreau S, Ferguson J R, Symington L S. The nuclease activity of Mre11 is required for meiosis but not for mating type switching, end joining, or telomere maintenance. Mol Cell Biol. 1999;19:556–566. doi: 10.1128/mcb.19.1.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murnane J P, Sabatier L, Marder B A, Morgan W F. Telomere dynamics in an immortal human cell line. EMBO J. 1994;13:4953–4962. doi: 10.1002/j.1460-2075.1994.tb06822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nugent C I, Bosco G, Ross L O, Evans S K, Salinger A P, Moore J K, Haber J E, Lundblad V. Telomere maintenance is dependent on activities required for end repair of double-strand breaks. Curr Biol. 1998;8:657–660. doi: 10.1016/s0960-9822(98)70253-2. [DOI] [PubMed] [Google Scholar]
- 51.Ohta K, Nicolas A, Furuse M, Nabetani A, Ogawa H, Shibata T. Mutations in the MRE11, RAD50, XRS2, and MRE2 genes alter chromatin configuration at meiotic DNA double-stranded break sites in premeiotic and meiotic cells. Proc Natl Acad Sci USA. 1998;95:646–651. doi: 10.1073/pnas.95.2.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palladino F, Laroche T, Gilson E, Axelrod A, Pillus L, Gasser S M. SIR3 and SIR4 proteins are required for the positioning and integrity of yeast telomeres. Cell. 1993;75:543–555. doi: 10.1016/0092-8674(93)90388-7. [DOI] [PubMed] [Google Scholar]
- 53.Paques F, Haber J E. Multiple pathways of recombination induced by double-strand breaks in Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 1999;63:349–404. doi: 10.1128/mmbr.63.2.349-404.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park Y, Lustig A J. Telomere structure regulates the heritability of repressed subtelomeric chromatin in Saccharomyces cerevisiae. Genetics. 2000;154:587–598. doi: 10.1093/genetics/154.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pays E, Laurent M, Delinte K, Van Meirvenne N, Steinert M. Differential size variations between transcriptionally active and inactive telomeres of Trypanosoma brucei. Nucleic Acids Res. 1983;11:8137–8147. doi: 10.1093/nar/11.23.8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pennock E, Buckley K, Lundblad V. Cdc13 delivers separate complexes to the telomere for end protection and replication. Cell. 2001;104:387–396. doi: 10.1016/s0092-8674(01)00226-4. [DOI] [PubMed] [Google Scholar]
- 57.Petes T D. Unequal meiotic recombination within tandem arrays of yeast ribosomal DNA genes. Cell. 1980;19:765–774. doi: 10.1016/s0092-8674(80)80052-3. [DOI] [PubMed] [Google Scholar]
- 58.Polotnianka R M, Li J, Lustig A J. The yeast Ku heterodimer is essential for protection of the telomere against nucleolytic and recombinational activities. Curr Biol. 1998;8:831–834. doi: 10.1016/s0960-9822(98)70325-2. [DOI] [PubMed] [Google Scholar]
- 59.Porter S E, Greenwell P W, Ritchie K B, Petes T D. The DNA-binding protein Hdf1p (a putative Ku homologue) is required for maintaining normal telomere length in Saccharomyces cerevisiae. Nucleic Acids Res. 1996;24:582–585. doi: 10.1093/nar/24.4.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qi H, Zakian V A. The Saccharomyces telomere-binding protein Cdc13p interacts with both the catalytic subunit of DNA polymerase alpha and the telomerase-associated Est1 protein. Genes Dev. 2000;14:1777–1788. [PMC free article] [PubMed] [Google Scholar]
- 61.Ray A, Runge K W. The yeast telomere length counting machinery is sensitive to sequences at the telomere-nontelomere junction. Mol Cell Biol. 1999;19:31–45. doi: 10.1128/mcb.19.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ritchie K B, Petes T D. The Mre11p/Rad50p/Xrs2p complex and the Tel1p function in a single pathway for telomere maintenance in yeast. Genetics. 2000;155:475–479. doi: 10.1093/genetics/155.1.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shampay J, Blackburn E H. Generation of telomere-length heterogeneity in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1988;85:534–538. doi: 10.1073/pnas.85.2.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Singer M S, Gottschling D E. TLC1: template RNA component of Saccharomyces cerevisiae telomerase. Science. 1994;266:404–409. doi: 10.1126/science.7545955. [DOI] [PubMed] [Google Scholar]
- 65.Smith C D, Blackburn E H. Uncapping and deregulation of telomeres lead to detrimental cellular consequences in yeast. J Cell Biol. 1999;145:203–214. doi: 10.1083/jcb.145.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smogorzewska A, van Steensel B, Bianchi A, Oelmann S, Schaefer M R, Schnapp G, de Lange T. Control of human telomere length by TRF1 and TRF2. Mol Cell Biol. 2000;20:1659–1668. doi: 10.1128/mcb.20.5.1659-1668.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sugawara N, Haber J E. Characterization of double-strand break-induced recombination: homology requirements and single-stranded DNA formation. Mol Cell Biol. 1992;12:563–575. doi: 10.1128/mcb.12.2.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Szostak J W, Orr-Weaver T L, Rothstein R J, Stahl F W. The double-strand-break repair model for recombination. Cell. 1983;33:25–35. doi: 10.1016/0092-8674(83)90331-8. [DOI] [PubMed] [Google Scholar]
- 69.Teng S, Chang J, McCowan B, Zakian V A. Telomerase-independent lengthening of yeast telomeres occurs by an abrupt Rad50p-dependent, Rif-inhibited recombinational process. Mol Cell. 2000;6:947–952. doi: 10.1016/s1097-2765(05)00094-8. [DOI] [PubMed] [Google Scholar]
- 70.Teng S C, Zakian V A. Telomere-telomere recombination is an efficient bypass pathway for telomere maintenance in Saccharomyces cerevisiae. Mol Cell Biol. 1999;19:8083–8093. doi: 10.1128/mcb.19.12.8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Usui T, Ohta T, Oshiumi H, Tomizawa J, Ogawa H, Ogawa T. Complex formation and functional versatility of Mre11 of budding yeast in recombination. Cell. 1998;95:705–716. doi: 10.1016/s0092-8674(00)81640-2. [DOI] [PubMed] [Google Scholar]
- 72.van Steensel B, de Lange T. Control of telomere length by the human telomeric protein TRF1. Nature. 1997;385:740–743. doi: 10.1038/385740a0. [DOI] [PubMed] [Google Scholar]
- 73.Walmsley R M, Petes T D. Genetic control of chromosome length in yeast. Proc Natl Acad Sci USA. 1985;82:506–510. doi: 10.1073/pnas.82.2.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang S S, Zakian V A. Sequencing of Saccharomyces telomeres cloned using T4 DNA polymerase reveals two domains. Mol Cell Biol. 1990;10:4415–4419. doi: 10.1128/mcb.10.8.4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Williamson J R, Raghuraman M K, Cech T R. Monovalent cation-induced structure of telomeric DNA: the G-quartet model. Cell. 1989;59:871–880. doi: 10.1016/0092-8674(89)90610-7. [DOI] [PubMed] [Google Scholar]
- 76.Wilson S, Warr N, Taylor D L, Watts F Z. The role of Schizosaccharomyces pombe Rad32, the Mre11 homologue, and other DNA damage response proteins in non-homologous end joining and telomere length maintenance. Nucleic Acids Res. 1999;27:2655–2661. doi: 10.1093/nar/27.13.2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wotton D, Shore D. A novel Rap1p-interacting factor, Rif2p, cooperates with Rif1p to regulate telomere length in Saccharomyces cerevisiae. Genes Dev. 1997;11:748–760. doi: 10.1101/gad.11.6.748. [DOI] [PubMed] [Google Scholar]
- 78.Zhu X D, Kuster B, Mann M, Petrini J H, de Lange T. Cell-cycle-regulated association of RAD50/MRE11/NBS1 with TRF2 and human telomeres. Nat Genet. 2000;25:347–352. doi: 10.1038/77139. [DOI] [PubMed] [Google Scholar]