Abstract
Background:
Despite the development of several FLT3 inhibitors that have improved outcomes in patients with FLT3-mutant acute myeloid leukemias (AML), drug resistance is frequently observed, which may be associated with the activation of additional pro-survival pathways such as those regulated by BTK, aurora kinases, and potentially others in addition to acquired tyrosine kinase domains (TKD) mutations of FLT3 gene. FLT3may not always be a driver mutation.
Objective:
To evaluate the anti-leukemia efficacy of the novel multi-kinase inhibitor CG-806, which targets FLT3 and other kinases, in order to circumvent drug resistance and target FLT3 wild-type (WT) cells.
Methods:
The anti-leukemia activity of CG-806 was investigated by measuring apoptosis induction and analyzing cell cycle with flow cytometry in vitro, and its anti-leukemia
Results:
CG-806 demonstrated superior anti-leukemia efficacy compared to commercially available FLT3 inhibitors, both in vitro and in vivo, regardless of FLT3 mutational status. The mechanism of action of CG-806 may involve its broad inhibitory profile of FLT3, BTK, and aurora kinases. InFLT3 mutant cells, CG-806 induced G1 phase blockage, while in FLT3WT cells, it resulted in G2/M arrest. Targeting FLT3 and Bcl-2 and/or Mcl-1 simultaneously resulted in a synergistic pro-apoptotic effect in FLT3mutant leukemia cells.
Conclusion:
The results of this study suggest that CG-806 is a promising multi-kinase inhibitor with anti-leukemia efficacy, regardless of FLT3 mutational status. A phase 1 clinical trial of CG-806 for the treatment of AML has been initiated (NCT04477291).
Keywords: Multi-kinase inhibitor, acute myeloid leukemia, FLT3, CG-806
Introduction
Fms-like tyrosine kinase 3 (FLT3)-internal tandem duplication (ITD) mutations occur in approximately 30% of adult acute myeloid leukemia (AML) patients and are associated with poor clinical outcomes (1), particularly in patients with higher mutant-to-wild type (WT) allelic ratios (2). Several putative FLT3 inhibitors (FLT3is) such as sorafenib, midostaurin, quizartinib, crenolanib, and gilteritinib have been investigated alone and in combination with chemotherapeutic drugs or other targeted agents in pre-clinically and clinical trials (3, 4). However, as monotherapies, these inhibitors tend to have limited effectiveness and resistance to the drugs often develops quickly (4–9). The acquisition of secondary mutations in the tyrosine kinase domain (TKD) of FLT3 is one mechanism of such resistance (6, 10), and has been identified in AML patients who relapsed or developed resistance to type-2 FLT3is, such as sorafenib and quizartinib (6, 11).
Moreover, aberrant activation of a number of other receptor and non-receptor tyrosine kinases has been linked to resistance to FLT3 inhibitors, including mitogen-activated protein kinases (MAPKs), signal transducer and activator of transcription 5 (STAT5), cellular myelocytomatosis oncogene (c-Myc) protein and Bruton’s tyrosine kinase (BTK) (12–15). Over-expression of c-Myc has been observed in most human hematopoietic malignancies and is associated with poor prognosis (16). The phosphorylation of c-Myc is regulated by the FLT3 downstream MAPK and PI3K/AKT signaling pathways (1). BTK is also part of the FLT3-ITD signalosome and is activated in an FLT3-ITD-dependent manner to induce proliferation in AML cells (17). In fact, targeting BTK with ibrutinib shows anti-proliferative effects in AML by mediating suppression of FLT3 downstream signaling MAPK, AKT, and NF-κB; and combined inhibition of FLT3 and BTK reportedly has additive anti-leukemia effects (17, 18).
Overexpression of aurora kinases (AURK), a family of serine/threonine kinases, has also been consistently demonstrated in a variety of leukemia cell lines and primary AML samples (19). The aberrant expression of AURK may be associated with poor-risk cytogenetic abnormalities and high blood cell counts in patients with AML (20). It has been reported that these CD34+/CD38− cells aberrantly expressed elevated levels of AURK that are required for cell cycle progression (21, 22), and targeting AURK demonstrated in vitro efficacy against human Myc-overexpressing AML cells (23). Concomitantly targeting AURK and FLT3 exhibited potent cytotoxicity with lower half-maximal inhibitory concentrations (IC50s) values against FLT3-ITD-mutant MV4–11, MOLM13 and MOLM13-resistant AML cells. The latter harbor ITD and D835Y dual mutations (24, 25). Interestingly, Druker’s group recently identified aurora kinase B as an early resistance factor to FLT3 inhibition (26). Therefore, co-targeting FLT3/AURK may be another potential therapeutic strategy in AML treatment. Furthermore, a therapeutic kinase inhibitor directed against all three novel targets, FLT3, BTK, and AURK, may be able to achieve much enhanced anti-leukemia efficacy in the targeted therapy of AML. Furthermore, FLT3 mutations may be acquired late in leukemogenesis and therefore may not be critical driver mutations. Activity against non-FLT3 mutant AML will then present a distinct advantage by targeting several subclones, FLT3 mutant and non-mutant.
The small molecule drug CG-806 (luxeptinib; Aptose, San Diego, CA) is a multi-kinase inhibitor with high activity against FLT3 mutations (27). Here, we evaluated the anti-leukemia activity of CG-806 by investigating its anti-proliferation and survival effects in AML in vitro as well as in AML xenograft models. We found that CG-806 has superior anti-leukemia effects against both FLT3 WT and mutant AML, especially in AML harboring FLT3 and TKD point mutations, which is a potential mechanism of secondary resistance to FLT3i. CG-806 profoundly inhibited phosphorylated (p)-BTK and p-AURK in addition to its FLT3 inhibition in vitro and significantly extended survival in vivo AML murine models. Our findings suggest that co-targeting FLT3, BTK, and AURK with a multi-kinase inhibitor CG-806 may be potent against AML regardless of FLT3 mutational status.
Methods
Compounds
CG-806 was provided by Aptose Biosciences (San Diego, CA). Supplementary Fig. S1 shows the molecular structure of CG-806. Small molecule FLT3 inhibitors quizartinib, gilteritinib and crenolanib, AURK inhibitor SNS-314, Bcl-2 antagonist venetoclax and Mcl-1 inhibitor A1210477 were purchased from Selleckchem (Houston, TX).
Leukemia cell lines and patient samples
AML cell lines were obtained from ATCC (Manassas, VA) or Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ; Braunschweig, Germany) and cultured according to instructions. The FLT3 status of these AML cell lines is shown in Table 1. All the cell lines were validated by STR DNA fingerprinting using the AmpFISTR Identifier kit according to the manufacturer’s instructions (Applied Biosystems cat 4322288). Primary AML patient samples were obtained from The University of Texas MD Anderson Cancer Center under an IRB-approved protocol, with written informed consent and in accordance with the principles of the Declaration of Helsinki. Specific details are provided in the Supplemental Methods and Table S1.
Table 1.
Activities of CG-806 Against FLT3 mutated and FLT3 WT Acute Leukemia Cells
| Cell Line | Characteristics | IC50, nM | 95% Lower/Upper | EC50, nM | 95% Lower/Upper | |
|---|---|---|---|---|---|---|
| Murine Cells | Ba/F3-FLT3 | FLT3 WT | 9.49 | 6.04/14.9 | 23.22 | 15.66/34.43 |
| Ba/F3-ITD | FLT3 ITD | 0.30 | 0.07/1.29 | 5.60 | 2.31/13.58 | |
| Ba/F3-D835G | FLT3 D835G | 0.12 | 0.02/0.89 | 4.30 | 2.20/8.41 | |
| Ba/F3-D835Y | FLT3 D835Y | 8.26 | 4.18/16.30 | 15.46 | 8.22/29.09 | |
| Ba/F3-ITD+691 | ITD+F691L | 0.43 | 0.31/0.61 | 14.65 | 8.84/24.28 | |
| Ba/F3-ITD+842 | ITD+Y842C | 0.73 | 0.42/1.27 | 13.39 | 9.24/19.42 | |
| Ba/F3-ITD+D835Y | FLT3-ITD+D835Y | 9.72 | 5.46/17.30 | 22.01 | 9.51/50.95 | |
| Ba/F3-ITD+D835H | FLT3-ITD+D835H | 6.74 | 3.71/12.26 | 25.82 | 14.25/46.78 | |
| Human Cells | MOLM13 | FLT3 ITD, t(9:11) | 0.82 | 0.79/0.87 | 4.34 | 1.93/9.86 |
| MOLM14 | FLT3 ITD, t(9:11) | 0.92 | 0.76/1.11 | 3.90 | 2.61/5.84 | |
| MV4–11 | FLT3 ITD, t(4:11) | 0.17 | 0.12/0.25 | 1.69 | 1.30/2.20 | |
| OCI/AML3 | FLT3 WT | 11.81 | 6.33/22.01 | 77.24 | 31.84/187.32 | |
| THP-1 | FLT3 WT, t(9:11) | 3.88 | 2.16/6.98 | NA* | NA | |
| Kasumi-1 | FLT3 WT | 21.99 | 16.38/29.53 | NA | NA |
NA = not reachable
Cell viability and apoptosis assays
AML cell lines or primary AML samples were treated with indicated drugs for 48–72 h. The number of viable cells was determined with a Vi-CELL XR Cell Counter (Beckman Coulter Inc., Indianapolis, IN) using the Trypan blue dye exclusion method, and apoptosis induction was determined using flow cytometry by assessing annexin V positivity and propidium iodide (PI) positivity as described previously (28). The IC50s for cell growth inhibition and the half-maximal effective concentrations (EC50s) for apoptosis induction were calculated using CalcuSyn software (BioSoft, Cambridge, UK).
Immunoblot analyses
Protein levels in treated cells were determined by immunoblot analysis as described previously (4). Briefly, treated cells were collected, cell lysates were prepared, resolved by electrophoresis on 10–12% precast sodium dodecyl sulfate-polyacrylamide gels, and transferred to Hybond-P membranes. After immunoblotting with antibodies, signals were detected using the Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE) and semi-quantitative assessment was determined using the Scion Image system and software (beta version 4.03; Scion, Frederick, MD). Details in the Supplemental Methods.
Cell cycle analysis
Cell cycle progression was determined using flow cytometric analysis of cellular DNA content and bromodeoxyuridine (BrdU) incorporation. Details in Supplemental Methods.
c-Myc knockdown with siRNA transfection
The indicated siRNAs and mock control (scramble) siRNAs were purchased from Dharmacon Research, Inc. (Lafayette, CO). Transfections of MOLM14 leukemia cells were carried out by electroporation using the Amaxa Nucleofector system (solution V, program O-017; Lonza, Basel, Switzerland) following the manufacturer’s instructions. The final concentration of siRNA was 300 nM. After 24 h of transfection, the indicated concentrations of CG-806 were added to the cells for an additional 24 h of culture.
Animal studies
An AML model was established by intravenously xenografting GFP-tagged Ba/F3-FLT3-ITD cells (0.5 × 106 cells/mouse), infected with lentivirus expressing firefly luciferase, into NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ mice (eight-week-old female) (The Jackson Laboratory, Bar Harbor, ME). The mice were treated with 10 mg/kg or 100 mg/kg CG-806 (oral gavage daily, 10 mice/group) starting on day 4 after leukemia cell injection, when an unambiguous luciferase signal was recorded. Control mice (n=10) received vehicle once daily for 5 weeks. Mice were noninvasively imaged using an Xenogen-200 in vivo bioluminescence imaging system (Xenogen, Hopkinton, MA) after being injected with 4 mg of luciferin substrate (D-luciferin, GoldBoi, St. Louis, MO). Bioluminescence images were obtained and quantitated as described previously (4). Three mice per group were humanely killed on day 11 after leukemia cell injection, and peripheral blood (PB) and bone marrow (BM) samples were collected to assess leukemia cell engraftment by measuring GFP positivity with flow cytometry. The in vivo studies were performed under approved animal care protocol and the standards of the Association for Assessment and Accreditation of Laboratory Animal Care.
Statistical analyses
The Student t-test was used to analyze immunoblotting and cell apoptosis data. P-values ≤ 0.05 were considered statistically significant. All statistical tests were two-sided, and the results are expressed as the means of triplicate samples/experiments ± standard deviations or as means with 95% confidence intervals. Survival was estimated using the Kaplan–Meier method (29), and log-rank statistics was used to assess differences in survival between groups.
Results
CG-806 exhibits anti-leukemia activity superior to other FLT3i in samples with FLT3 WT or TKD mutations through the inhibition of FLT3/AURK/BTK
Our initial studies indicate that CG-806 has impressive kinase inhibition against WT and mutant FLT3, BTK, and AURK as well at extremely low nanomolar IC50s in a cell-free biochemical kinase inhibition assay (Table S2). Therefore, we first evaluated the anti-leukemia activity of CG-806 in AML cell lines with different FLT3 mutation status. Treatment with extremely low doses (nanomolar to sub-nanomolar) of CG-806 for 72 h profoundly inhibited cell growth via apoptosis induction in both human and murine leukemia cell lines harboring either FLT3-ITD mutations or FLT3-ITD+TKD point mutations (Fig. 1A, B). Cells harboring FLT3 TKD mutations or ITD + TKD dual mutations usually show resistance to most currently available FLT3i in previous studies (6, 10, 30). The IC50s and EC50s of CG-806 against these leukemia cell lines were in the low nanomolar to sub-nanomolar range (Table 1). Interestingly, whereas CG-806 had low IC50s (i.e., 4 to 10 nM) in most human and murine FLT3-WT leukemia cell lines, its EC50s could not be determined in some human FLT3-WT leukemia cell lines, such as THP-1 and Kasumi-1 (Table 1). Most importantly, CG-806 had profound pro-apoptotic effects in primary AML patient samples irrespective of FLT3 mutation status, but did not induce apoptosis in BM cells from healthy donors (Fig. 1C). This suggested that CG-806 has broad and potent anti-cancer activity against AML cells in addition to a potential therapeutic window with respect to toxicity to normal cells.
Figure 1. CG-806 demonstrates an anti-leukemia effect in both FLT3 mutated and WT AML cell lines and primary samples.
(A) Human and (B) murine leukemia cell lines with FLT3WT, FLT3 ITD mutations, or dual FLT3-ITD + different TKD mutations were exposed to increasing doses of CG-806 for 72 h in vitro. Apoptosis induction and cell proliferation inhibition were assessed by measuring annexin V positivity with flow cytometry, and viable cells were assessed with a trypan blue exclusion assay. Experiments were performed in triplicate, and the results are presented as means ± standard deviations. (C) Primary AML mononuclear cell samples with different FLT3 mutation statuses and normal BM samples were exposed to increasing doses (0, 0.1, 0.5, 1 and 3 μM) of CG-806 for 48 h. Apoptosis induction was assessed by measuring annexin V positivity in CD34+ cell population with flow cytometry. Data are presented as percentages of specific apoptosis induction (specific apoptosis (%) = 100 (drug-induced apoptosis - spontaneous apoptosis)/(100 - spontaneous apoptosis)) (57). Primary samples from an AML patient with FLT3-ITD mutations (D) and an AML patient with FLT3-ITD+D835 mutations (E) were treated with CG-806 or quizartinib ex vivo for 48 h, and apoptosis induction was measured with flow cytometry.
To evaluate the anti-leukemia potency of CG-806, we compared the cytotoxic effects of the drug with other currently approved/available FLT3 and multi-kinase inhibitors in FLT3-mutated and FLT3-WT AML cell lines and patient samples. The IC50s of CG-806 were much lower than those of other FLT3is particularly in leukemia cells harboring the “gatekeeper” F691 mutation. The IC50s were 10.0 nM for CG-806 but 115.3 nM, 98.4 nM, and 257.6 nM for quizartinib, gilteritinib, and crenolenib, respectively (Table 2). We further compared the apoptogenic effect of CG-806 with that of the other FLT3i in AML patient samples ex vivo. CG-806 demonstrated markedly greater cytotoxicity than quizartinib in primary peripheral blood mononuclear cells with FLT3-ITD mutations or with FLT3-ITD+TKD mutations (Fig 1D, E).
Table 2.
IC50s comparison of CG-806 and other FLT3 inhibitors in FLT3 WT and mutated cells.
| FLT3 inhibitors | IC50 in Transfected Ba/F3 Cells (nM, n = 3) | ||||
|---|---|---|---|---|---|
| FLT3 WTa | FLT3 ITD | FLT3 D835Y | FLT3ITD+D835Y | FL73 ITD+F691L | |
| CG-806 | 11.3 | 0.5 | 8.8 | 19.3 | 10 |
| Quizartinib | 1956.0 | 2.2 | 2089 | 246.4 | 115.3 |
| Gilteritinib | 500.3 | 26.5 | 472.5 | 6.8 | 98.4 |
| Crenolanib | 2617.0 | 35 | 888.9 | 31.7 | 257.6 |
FLT3 WT cells are IL3-dependent and presented during the inhibitor treatment.
Immunoblot analyses demonstrated that CG-806 at nanomolar concentrations markedly suppressed phosphorylation levels of FLT3, AURK, and BTK, and their downstream signaling partners p-AKT and p-ERK in the leukemia cell lines and primary AML samples harboring FLT3-ITD mutations and/or FLT3-TKD mutations (Fig. 2A, B). CG-806 also upregulated the pro-apoptotic protein Bim in FLT3 WT and ITD mutant AML cell lines after 24 h of treatment, and later triggered the cleavage of caspase-3 and PARP in FLT3-ITD-mutated AML cells (Fig. 2C, Supplementary Fig. S2). However, the treatment only marginally affected Bcl-2 and Bcl-xL levels in the leukemia cells, and even upregulated the anti-apoptotic protein Mcl-1 especially in Ba/F3-ITD mutant and Ba/F3-FLT3-WT cell lines (Fig. 2C).
Figure 2. CG-806 suppresses p-FLT3, -BTK and –AuraK and triggers a pro-apoptotic effect in FLT3mutated AML cells.
Leukemia cell lines (A) and primary AML cells (B) were treated with the indicated concentrations of CG-806 for 4 h. The expression levels of targeting-correlated proteins were measured using immunoblotting. (C) FLT3-ITD-mutated MOLM13 and Ba/F3 cells and FLT3-WT THP-1 and Ba/F3 cells were exposed to CG-806 for 24 h, and apoptosis-related proteins were measured with immunoblotting. GAPDH served as a loading control.
CG-806 blocks leukemia cells in G1 phase in FLT3-ITD-mutated AML cells and triggers G2/M arrest in FLT3-WT AML cells
To further characterize the mechanism(s) underlying the anti-leukemia activity of CG-806, we investigated the impact of CG-806 on cell cycle progression. Results indicated that CG-806 blocked cells in G1 phase in FLT3-ITD-mutated MOLM14 and MV4–11 leukemia cell lines after 24 h of treatment as determined by BrdU incorporation assay (Fig. 3A, B). Immunoblotting analyses showed profound suppression of cell proliferation-related proteins p-mTOR, -S6K, and -RB, upregulation of p27, and reduction of G1 phase checkpoint proteins CDK4, CDK6 and c-Myc as well (Fig. 3C). In terms of cell proliferation, c-Myc has key abilities to control cell cycle progression by promoting transcription of its downstream genes for cell cycle transition from G0/G1 into S phase and antagonizing cell cycle inhibitor activity (31). Therefore, we further determined if c-Myc is critical for CG-806-induced G1 arrest. Knocking down c-Myc with siRNA in MOLM14 cells (Supplementary Fig. S3) triggered more pronounced G1 phase arrest compared to MOLM14 cells without c-Myc knockdown (50.9% vs. 33. 6% in MOLM14-cMyc-siRNA vs. MOLM14-cMyc-scramble cells, respectively, p < 0.05) (Fig. 3D), implying that c-Myc suppression has a role in CG-806-induced inhibition of cell growth through G1 phase arrest in FLT3-mutated leukemia cells.
Figure 3. CG-806 blocks leukemia cells in G1 phase in FLT3 mutant AML cells mainly through suppression of c-Myc.
(A) Human FLT3-ITD-mutated MOLM14 and MV4–11 cells were treated with CG-806 for 24 h. The graphs show a representative figure of propidium iodide (PI)-stained and BrdU-labeled populations. (B) The G1 phase distribution was summarized from 3 independent experiments. Data are presented as the means ± standard deviations. *p ≤ 0.05, **p ≤ 0.01; Student t-test. (C) FLT3-ITD-mutated MOLM14 and MV4–11 cells and FLT3-WT THP-1 cells were treated with CG-806 for 24 h, and the indicated protein levels were measured with immunoblotting. (D) MOLM14 cells were treated with 300 nM c-Myc siRNA or with scrambled siRNA for 48 h, and the cell cycle was determined with flow cytometry after staining with PI.
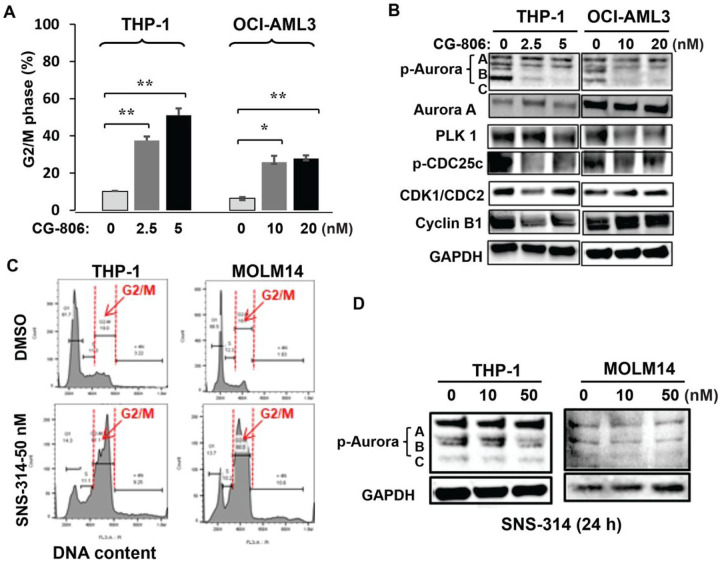
However, we did not observe G1 arrest in FLT3-WT cells. Conversely, CG-806 inhibited the growth of FLT3-WT THP-1 and OCI/AML3 cells by triggering significant G2/M arrest instead (Fig. 4A). Immunoblotting analyses demonstrated that CG-806 profoundly suppressed p-AURK B and C levels and downregulated Polo-like kinase 1 (PLK1), p-CDC25c, and cyclin B1 (Fig. 4B). To confirm that AURK inhibition was associated with G2/M arrest in FLT3-WT cells, we suppressed AURK activity by using an AURK specific inhibitor SNS-314 (32) in either FLT3-WT or -mutant AML cell lines THP-1 or MOLM14. Results showed a similar G2/M arrest accompanied by p-AURK inhibition in both cell lines (Fig. 4C, D), which suggests that AURK suppression and G2/M arrest are interconnected regardless of FLT3 mutation status in AML cells.
Figure 4. CG-806 induces G2/M arrest through p-ARUK inhibition in FLT3-WT AML cells.
(A) FLT3-WT THP-1 and OCI-AML3 cells were exposed to the indicated concentrations of CG-806 for 24 h, and PI staining was used to measure the cell cycle (DNA content) distribution. Data are presented as the means ± standard deviations of 3 independent experiments. *p ≤ 0.05, **p ≤ 0.01; Student t-test. (B) FLT3-WT leukemia cells were treated with CG-806 for 24 h, and cell cycle-related proteins were determined with immunoblotting. FLT3-WT THP-1 cells and FLT3-ITD-mutated MOLM14 cells were treated with the aurora inhibitor SNS-314 (10 or 50 nM) or with DMSO for 24 h. DNA content and cell cycle distribution were determined by flow cytometry after PI staining (C), and p-aurora protein levels were assessed by immunoblotting (D).
CG-806 has marked anti-leukemia efficacy in murine models of FLT3-mutated leukemia
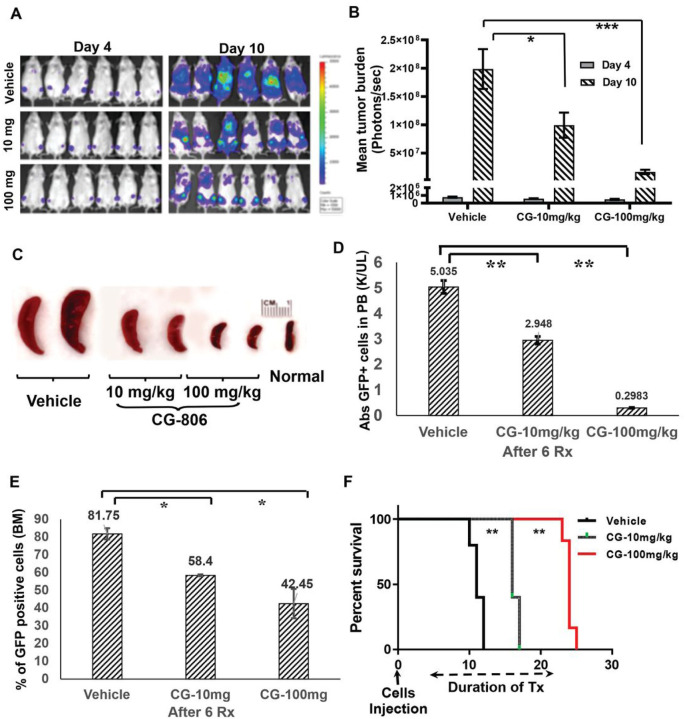
Our preliminary data indicated that mice receiving 100 mg/kg CG-806 had high plasma concentrations 24 h after one dose (Supplementary Fig. S4). Therefore, we established a leukemia model in NSG mice by xenografting Baf3-FLT3-ITD cells and treated mice with 10 or 100 mg/kg doses of CG-806. CG-806 significantly reduced the leukemia burden by 48% (10 mg/kg dose; p < 0.05) and 93% (100 mg/kg dose; p < 0.001) compared to the vehicle group (Fig. 5A, B) and eliminated leukemia-related splenomegaly after 1 week of drug administration (Fig. 5C). CG-806 also eliminated leukemic blasts in both PB and BM in a dose-dependent manner (Fig. 5D, E). In addition, the survival duration of the 10 mg/kg (16 d) and 100 mg/kg CG-806 groups (24 d) was significantly longer than that observed in the vehicle group (11 d; p < 0.01) (Fig. 5F). CG-806 at either dose did not affect mouse body weight (data not shown).
Figure 5. CG-806 exerts profound anti-leukemia effects in a mice model xenografted with FLT3-ITD-mutated leukemia cells.
(A) Serial bioluminescence images of representative mice treated with CG-806 (10 or 100 mg/kg) or vehicle acquired 4 and 10 d after leukemia cell injection are shown. (B) Quantitative analysis of leukemia burden based on the data of serial bioluminescence images. (C) Spleen size from different treatment groups of mice was compared after 1 wk of CG-806 treatment. “Normal” means normal mice without leukemia cells injection and drug treatment. The engraftment of GFP+ leukemia cells was evaluated as the absolute number of GFP+ cells in PB (D) and the percentage of GFP+ leukemia cells in bone marrow (E), as assessed with flow cytometry. (F) The Kaplan-Meier method was used to assess the median survival of each group, and log-rank statistics were used to assess differences in survival. *p ≤0.05, **p ≤ 0.01, ***p ≤ 0.001; Student t-test.
Bcl-2 and/or Mcl-1 inhibition profoundly enhances the cytotoxic effects of CG-806 in AML cells
CG-806 demonstrated much more pronounced anti-proliferative than pro-apoptotic effects, accompanied by only marginal inhibition of Bcl-2, Bcl-xL, and Mcl-1 as well in most of the tested leukemia cell lines, even upregulated the anti-apoptotic protein Mcl-1 in Ba/F3-FLT3-WT and ITD mutant cells (Fig. 2C). In fact, the overexpression of Mcl-1, Bcl-2 and Bcl-2A1 has been associated with therapy resistance of AML cells (33, 34). This finding provides a rationale for combining CG-806 with Mcl-1 and/or Bcl-2 inhibitors to improve anti-leukemia efficacy as previously shown for other FLT3i (35). Therefore, we sought to determine whether the pro-apoptotic effect of CG-806 could be enhanced by combination with Bcl-2 and/or Mcl-1 inhibitors. As expected, all combinations of CG-806 with the Bcl-2 inhibitor venetoclax and/or the Mcl-1 inhibitor A1210477 showed markedly synergistic pro-apoptotic effects in leukemia cells; the combination indexes (CIs) in Ba/F3-ITD, Ba/F3-ITD+F691L and Ba/F3-FLT3-WT cell lines were 0.33 ± 0.04, 0.74 ± 0.08, and 0.36 ± 0.10, respectively, for the combination with Bcl-2 inhibitor; 0.56 ± 0.03, 0.77 ± 0.04, and 0.73 ± 0.07, respectively, for the combination with Mcl-1 inhibitor; and 0.26 ± 0.04, 0.59 ± 0.09, and 0.42 ± 0.04, respectively, for the three-drug combination (Fig. 6A, B, Supplementary Fig. S5).
Figure 6. Bcl-2 and Mcl-1 inhibitors enhance the anti-leukemia effects of CG-806.
FLT3-ITD-mutated Ba/F3 leukemia cells (A) and FLT3-ITD+F691-mutated Ba/F3 leukemia cells (B) were exposed to CG-806 and Bcl-2 antagonist venetoclax and/or Mcl-1 inhibitor A1210477 for 48 h. Cell apoptosis induction was assessed with flow cytometry by measuring annexin V positivity. Data are from three independent experiments and presented as the means ± standard deviations. (C) Immunoblotting was used to assess the targeting-correlated proteins in FLT3-ITD-mutated Ba/F3 cells after being exposed to the combination regimens for 24 h. Bcl-2i (B) = Bcl-2 inhibitor; Mcl-1i (M) = Mcl-1 inhibitor; CG = CG-806.
Immunoblot analyses showed that targeting Bcl-2 and/or Mcl-1 concomitantly with CG-806 profoundly suppressed Mcl-1, reduced p-FLT3, -BTK, and -AURK, and triggered a marked cleavage of caspase-3 (Fig. 6C), suggesting that the combination regimens trigger potent leukemia cell killing which may translate in beneficial effect in relapsed/refractory AML regardless of FLT3 mutational status.
Discussion
Several FLT3i have been developed over the last two decades including sorafenib (4, 5) and FDA-approved gilteritinib and midostaurin (8, 36). A newer generation FLT3i, crenolanib is still under development and showed impressive anti-leukemia effects against resistant AML harboring FLT3 TKD mutations (7). One complicating factor is the oligoclonal nature of AML (37, 38). FLT3 mutant clones co-exist with FLT3 WT clones or dysregulated signaling, which creates a much more complex scenario. Meanwhile, it also provides a window into therapeutic vulnerabilities. Aberrant expression of survival signaling pathways such as MAPK, BTK and AURK is associated with resistance to FLT3i (12, 15, 19). CG-806 as a novel multi-kinase inhibitor of targeting FLT3/BTK/AURK might provide a better pharmacological notion for overcoming resistance than that more specific FLT3i. In the present study, we demonstrated that CG-806 has a superior anti-leukemia efficacy especially against AML harboring “gatekeeper” F691 mutations or FLT3 WT compared to other FLT3i, without detectable toxicity in normal BM samples. Mechanistically, CG-806 profoundly suppresses FLT3, BTK, and AURK activation simultaneously and results in impressive cytotoxicity in these AML cells, particularly against FLT3-WT AML cells which demonstrated abnormally high expression of aurora kinase. Our findings confirmed that CG-806 is a pan-FLT3 inhibitor that may benefit from the simultaneous suppression of FLT3, BTK and AURK activation, leading to a promising anti-leukemia effect against AML regardless of their FLT3 status.
Further, we found that CG-806 achieved its anti-leukemia activity in FLT3-mutated AML and FLT3-WT AML through different mechanisms. Specifically, CG-806 induced G1 arrest in FLT3-mutated MOLM14 and MV4–11 cells but induced G2/M arrest in FLT3-WT THP-1 cells at IC50s of about 1 nM and 5 nM, respectively. In FLT3-mutated cells, G1 cell cycle progression is closely associated with the dominant activation of FLT3 and its downstream effectors, AKT/mTOR/S6K, and MAPK. These signaling cascade proteins are highly expressed and constitutively activated in FLT3-mutated leukemia cells. In addition, BTK, which is expressed in about 80% of human AML, mediates FLT3-ITD-dependent Myc and STAT5 activation (17), and transcriptionally increases the levels of G1 cell cycle checkpoint proteins through c-Myc signaling (39). Thus, by co-targeting FLT3 and BTK, which are dominantly activated in FLT3-mutated AML, CG-806 enhances the downregulation of c-Myc to trigger G1 arrest. In fact, Eriksson et al. reported that targeting FLT3 signaling with the FLT3i AKN-028 or midostaurin for 12–48 h triggered G1 phase arrest in FLT3-mutated MV4–11 AML cells as well (40). In the present study, targeting FLT3 with quizartinib or gilteritinib or targeting BTK with ibrutinib also triggered G1 arrest in FLT3-ITD-mutated AML cells (data not shown). Similarly, c-Myc knockdown elicited G1 arrest in FLT3-mutated MOLM14 cells as well. These findings strongly imply that the CG-806-induced suppression of FLT3 and BTK and their downstream signaling, and the subsequent downregulation of c-Myc play critical roles in G1 arrest in FLT3-mutated AML cells.
We did not observe c-Myc repression in FLT3-WT AML cells treated with CG-806 although it has a high expression in the FLT3-WT cells. In fact, FLT3-WT cells had higher expression of p-AURK, but lower p-FLT3 or -BTK compared to FLT3-mutated AML cells (Supplementary Fig. S6). An investigation of genes essential for proliferation and survival of cancer cells with CERES (computational method to estimate gene-dependency levels from CRISPR–Cas9 essentiality screens) dependency score (lower score indicates a higher likelihood that the gene of interest is essential in a given cell line) indicated that FLT3-WT leukemia cell lines have low CERES scores of AURK-B (−2.0 and −1.7, respectively, on Kasumi-1 and THP-1 cell lines) and of AURK-A (−1.45 and −1.37, respectively, in Kasumi-1 and OCI/AML3 cell lines); which suggests a higher dependency on AURK with regard to proliferation and survival of FLT3-WT leukemia cell lines (41). Actually, the overexpression of AURK and their downstream PLKs were reported to be associated with tumorigenesis of many human tumors, including leukemias (21, 42). AURK signaling plays a fundamental role in regulating cell cycle checkpoints that ensure the timing and order of cell cycle events such as DNA repair, bipolar spindle formation, chromosome segregation and mitotic exit (43–45). Therapeutic targeting of AURK and PLK1 prevents the completion of mitosis and results in G2/M phase arrest and apoptosis (46). In the present study, AURK inhibition by CG-806 and the suppression of PLK1 in FLT3-WT cells THP-1 and OCI-AML3 were accompanied by a reduction in p-CDC25C and the subsequent inactivation of CDC25C, resulting in G2/M arrest. In fact, Jagtap et al. reported that suppressing AURK-B with dual AURK-B/FLT3 inhibitor (Compound-54) can trigger G2/M arrest in FLT3-ITD-mutated MOLM13 cells as well (24). In addition, the suppression of AURK activation by a specific AURK inhibitor SNS-314 (32) also triggered G2/M arrest in both FLT3-WT and mutant leukemia cells, which further supports the notion that AURK suppression is associated with G2/M arrest in leukemia cells. Similarly, in another study, the multi-kinase inhibitor midostaurin triggered G1 arrest in FLT3-mutated leukemia cells and G2/M arrest in FLT3-WT leukemia cells, although the mechanisms involved remain unresolved (47). It has been reported that midostaurin triggers G2/M arrest in breast cancer cell lines through inhibition of the AURK family proteins (48), which supports the notion of an association of AURK suppression and G2/M arrest in FLT3-WT AML. Actually, CG-806 demonstrates much higher anti-AURK activity than midostaurin (IC50s are 3 nM vs. 300 nM, respectively in CG-806 vs. midostaurin) (49), suggesting a more potent against AML with FLT3-WT.
In line with our in vitro results, CG-806 had robust anti-leukemia activity in a murine xenograft model of leukemia created using the aggressive FLT3-ITD-mutated Ba/F3 cells (Fig. 5), and in a PDX AML model as well (50). Compared with vehicle, 100 mg/kg CG-806 significantly reduced leukemia burden and the absolute leukemia cell count in PB by up to 17-fold after 1 wk of treatment. CG-806 also conveyed a remarkable survival benefit, as the survival duration of mice that received 10 mg/kg or 100 mg/kg CG-806 (16 and 24 d, respectively) were markedly longer than that of mice that received vehicle (11 d). Impressively, even at doses of up to 450 mg/kg, the daily oral administration of CG-806 for 14 d elicited no obvious toxicity in Balb/c mice (data not shown). These results suggest that CG-806 could be well-tolerated in a clinical setting as monotherapy or as a combinatorial drug with other targeted agents or chemotherapeutics for AML treatment.
We further observed that combinations of CG-806 with the Bcl-2 antagonist venetoclax and/or the Mcl-1 inhibitor A1210477 had synergistic pro-apoptotic effects not only in FLT3-mutated AML cells (both those with FLT3-ITD mutations and those with FLT3-ITD + “gatekeeper” F691 mutations), but also in FLT3-WT cells, indicating that the combinatorial regimens may have a promising anti-leukemia efficacy in both FLT3-WT and mutant AML. In fact, combinations of venetoclax with various other drugs, including FLT3i, are being actively investigated in AML (35, 51, 52). The anti-apoptotic protein Mcl-1 is one of the main determinants of venetoclax resistance in AML (53). The downregulation of Mcl-1 sensitizes AML to Bcl-2 inhibitor-induced leukemia cell killing (54), and we have also reported a highly synergistic effect of venetoclax with Mcl-1 inhibition recently (33). In the present study, CG-806 by itself showed modest Mcl-1 inhibition, indicating a need for pharmacological inhibition of Mcl-1 which was indeed observed. Also, the benefit was observed in the synergistic efficacy of combination with venetoclax and/or A1210477, through activation of the intrinsic apoptosis pathway, evidenced by the increased level of cleavage caspase-3 cleavage.
Approximately 36 % of patients treated with gilteritinib develop resistance related to the emergence of RAS mutations (55). Ras mutations are also an established resistance factor for Bcl-2 inhibition (56). It remains to be seen if CG-806 can suppress the outgrowth of RAS-mutant subclones in AML, alone or in combination with venetoclax.
Taken together, CG-806 demonstrates superior anti-leukemia activity in vitro and in vivo compared to other FLT3i by suppression of FLT3/BTK/AURK simultaneously. Mechanistically, CG-806 triggers G1 phase arrest in FLT3-mutated AML cells by predominantly targeting FLT3 and BTK, and G2/M phase arrest in FLT3-WT AML cells by predominantly targeting AURK. CG-806 exerts robust anti-leukemia activity by efficiently reducing leukemia burden and doubling survival in a murine xenograft model. Targeting FLT3 mutant and -wild-type cells is a potential additional advantage. Furthermore, our findings provide a potential strategy for CG-806 in combination with Bcl-2 and/or Mcl-1 inhibitors for AML therapy. Currently, CG-806 is in phase 1 clinical trial (NCT04477291) for patients with relapsed or refractory AML and higher-risk myelodysplastic/myeloproliferative neoplasms as well.
Key Points.
Multi-kinase inhibitor CG-806 exerts superior anti-leukemia activity in AML regardless of its FLT3 status.
CG-806 triggers G1 arrest in FLT3 mutated cells and G2/M arrest in FLT3 WT cells through suppression of FLT3/BTK and aurora kinases.
Concomitantly targeting FLT3 and Bcl-2 and/or Mcl-1 exerts synergistic pro-apoptotic effects on both FLT3 WT and mutated AML cells.
Acknowledgments
The authors thank Drs. Donald Small and Neil Shah for providing FLT3-ITD-mutated cells and FLT3-ITD+TKD-mutated cells; Joseph Munch in Scientific Publications in MD Anderson’s Research Medical Library for editing the manuscript.
Funding:
This work was supported in part by a grant from Aptose Biosciences, the Paul and Mary Haas Chair in Genetics, the National Institutes of Health Cancer Center Support Grant (P30CA016672), and CPRIT grant RP130397 (to MA). This work used MD Anderson Cancer Center Flow Cytometry and Cell Imaging, Research Animal Support, and Characterized Cell Line Core Facilities, which were all supported by the National Institutes of Health Cancer Center Support Grant (P30CA016672).
Footnotes
Disclosure of Conflicts of Interest: H.Z. and W.R. are employees of Aptose Biosciences; M.A. serves on the Aptose Biosciences Scientific Advisory Board.
Supplementary Files
Contributor Information
Guopan Yu, The University of Texas MD Anderson Cancer Center.
Weiguo Zhang, University of Texas MD Anderson Cancer Center.
Hongying Zhang, Aptose Biosciences.
Charlie Ly, The University of Texas MD Anderson Cancer Center.
Mahesh Basyal, The University of Texas MD Anderson Cancer Center.
William G. Rice, Aptose Biosciences
Michael Andreeff, The University of Texas MD Anderson Cancer Center.
Data availability statement:
The data that support the findings of this study are available from the corresponding author, [M.A.] upon reasonable request.
References
- 1.Gilliland DG, Griffin JD. The roles of FLT3 in hematopoiesis and leukemia. Blood. 2002;100(5):1532–42. [DOI] [PubMed] [Google Scholar]
- 2.Schnittger S, Bacher U, Haferlach C, Alpermann T, Kern W, Haferlach T. Diversity of the juxtamembrane and TKD1 mutations (exons 13–15) in the FLT3 gene with regards to mutant load, sequence, length, localization, and correlation with biological data. Genes Chromosomes Cancer. 2012;51(10):910–24. [DOI] [PubMed] [Google Scholar]
- 3.Short NJ, Kantarjian H, Ravandi F, Daver N. Emerging treatment paradigms with FLT3 inhibitors in acute myeloid leukemia. Ther Adv Hematol. 2019;10:2040620719827310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang W, Konopleva M, Shi YX, McQueen T, Harris D, Ling X, et al. Mutant FLT3: a direct target of sorafenib in acute myelogenous leukemia. JNatlCancer Inst. 2008;100(3):184–98. [DOI] [PubMed] [Google Scholar]
- 5.Borthakur G, Kantarjian H, Ravandi F, Zhang W, Konopleva M, Wright JJ, et al. Phase I study of sorafenib in patients with refractory or relapsed acute leukemias. Haematologica. 2011;96(1):62–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang W, Gao C, Konopleva M, Chen Y, Jacamo RO, Borthakur G, et al. Reversal of Acquired Drug Resistance in FLT3-Mutated Acute Myeloid Leukemia Cells via Distinct Drug Combination Strategies. ClinCancer Res. 2014;20(9):2363–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith CC, Lasater EA, Lin KC, Wang Q, McCreery MQ, Stewart WK, et al. Crenolanib is a selective type I pan-FLT3 inhibitor. Proc Natl Acad Sci U S A. 2014;111(14):5319–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perl AE, Altman JK, Cortes J, Smith C, Litzow M, Baer MR, et al. Selective inhibition of FLT3 by gilteritinib in relapsed or refractory acute myeloid leukaemia: a multicentre, first-in-human, open-label, phase 1–2 study. Lancet Oncol. 2017;18(8):1061–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cortes JE, Kantarjian H, Foran JM, Ghirdaladze D, Zodelava M, Borthakur G, et al. Phase I study of quizartinib administered daily to patients with relapsed or refractory acute myeloid leukemia irrespective of FMS-like tyrosine kinase 3-internal tandem duplication status. J Clin Oncol. 2013;31(29):3681–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith CC, Lin K, Stecula A, Sali A, Shah NP. FLT3 D835 mutations confer differential resistance to type II FLT3 inhibitors. Leukemia. 2015;29(12):2390–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith CC, Paguirigan A, Jeschke GR, Lin KC, Massi E, Tarver T, et al. Heterogeneous resistance to quizartinib in acute myeloid leukemia revealed by single-cell analysis. Blood. 2017;130(1):48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang W, Borthakur G, Gao C, Chen Y, Mu H, Ruvolo VR, et al. The Dual MEK/FLT3 Inhibitor E6201 Exerts Cytotoxic Activity against Acute Myeloid Leukemia Cells Harboring Resistance-Conferring FLT3 Mutations. Cancer Res. 2016;76(6):1528–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Birkenkamp KU, Geugien M, Lemmink HH, Kruijer W, Vellenga E. Regulation of constitutive STAT5 phosphorylation in acute myeloid leukemia blasts. Leukemia. 2001;15(12):1923–31. [DOI] [PubMed] [Google Scholar]
- 14.Salvatori B, Iosue I, Djodji Damas N, Mangiavacchi A, Chiaretti S, Messina M, et al. Critical Role of c-Myc in Acute Myeloid Leukemia Involving Direct Regulation of miR-26a and Histone Methyltransferase EZH2. Genes Cancer. 2011;2(5):585–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rushworth SA, Murray MY, Zaitseva L, Bowles KM, MacEwan DJ. Identification of Bruton’s tyrosine kinase as a therapeutic target in acute myeloid leukemia. Blood. 2014;123(8):1229–38. [DOI] [PubMed] [Google Scholar]
- 16.Pelengaris S, Khan M, Evan G. c-MYC: more than just a matter of life and death. Nat Rev Cancer. 2002;2(10):764–76. [DOI] [PubMed] [Google Scholar]
- 17.Oellerich T, Mohr S, Corso J, Beck J, Dobele C, Braun H, et al. FLT3-ITD and TLR9 use Bruton tyrosine kinase to activate distinct transcriptional programs mediating AML cell survival and proliferation. Blood. 2015;125(12):1936–47. [DOI] [PubMed] [Google Scholar]
- 18.Pillinger G, Abdul-Aziz A, Zaitseva L, Lawes M, MacEwan DJ, Bowles KM, et al. Targeting BTK for the treatment of FLT3-ITD mutated acute myeloid leukemia. Sci Rep. 2015;5:12949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim SJ, Jang JE, Cheong JW, Eom JI, Jeung HK, Kim Y, et al. Aurora A kinase expression is increased in leukemia stem cells, and a selective Aurora A kinase inhibitor enhances Ara-C-induced apoptosis in acute myeloid leukemia stem cells. Korean J Hematol. 2012;47(3):178–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lucena-Araujo AR, de Oliveira FM, Leite-Cueva SD, dos Santos GA, Falcao RP, Rego EM. High expression of AURKA and AURKB is associated with unfavorable cytogenetic abnormalities and high white blood cell count in patients with acute myeloid leukemia. Leuk Res. 2011;35(2):260–4. [DOI] [PubMed] [Google Scholar]
- 21.Yang J, Ikezoe T, Nishioka C, Nobumoto A, Udaka K, Yokoyama A. CD34(+)/CD38(−) acute myelogenous leukemia cells aberrantly express Aurora kinase A. Int J Cancer. 2013;133(11):2706–19. [DOI] [PubMed] [Google Scholar]
- 22.Goldenson B, Crispino JD. The aurora kinases in cell cycle and leukemia. Oncogene. 2015;34(5):537–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brondfield S, Umesh S, Corella A, Zuber J, Rappaport AR, Gaillard C, et al. Direct and indirect targeting of MYC to treat acute myeloid leukemia. Cancer Chemother Pharmacol. 2015;76(1):35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jagtap AD, Chang PT, Liu JR, Wang HC, Kondekar NB, Shen LJ, et al. Novel acylureidoindolin-2-one derivatives as dual Aurora B/FLT3 inhibitors for the treatment of acute myeloid leukemia. Eur J Med Chem. 2014;85:268–88. [DOI] [PubMed] [Google Scholar]
- 25.Tariq MU, Furqan M, Parveen H, Ullah R, Muddassar M, Saleem RSZ et al. CCT245718, a dual FLT3/Aurora A inhibitor overcomes D835Y-mediated resistance to FLT3 inhibitors in acute myeloid leukaemia cells.Br J Cancer. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joshi SK, Nechiporuk T, Bottomly D, Piehowski PD, Reisz JA, Pittsenbarger J et al. The AML microenvironment catalyzes a stepwise evolution to gilteritinib resistance.Cancer Cell. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rice WG, Howell SB, Zhang H, Rastgoo N, Local A, Kurtz SE et al. Luxeptinib (CG-806) targets FLT3 and clusters of kinases operative in acute myeloid leukemia.Molecular cancer therapeutics. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clodi K, Kliche K-O, Zhao S, Weidner D, Schenk T, Consoli U, et al. Cell-surface exposure of phosphatidylserine correlates with the stage of fludarabine-induced apoptosis in chronic lymphocytic leukemia (CLL) and expression of apoptosis-regulating genes. Cytometry. 2000;40(1):19–25. [DOI] [PubMed] [Google Scholar]
- 29.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 30.Smith CC, Wang Q, Chin CS, Salerno S, Damon LE, Levis MJ, et al. Validation of ITD mutations in FLT3 as a therapeutic target in human acute myeloid leukaemia. Nature. 2012;485(7397):260–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia-Gutierrez L, Delgado MD, Leon J. MYC Oncogene Contributions to Release of Cell Cycle Brakes.Genes (Basel). 2019;10(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.VanderPorten EC, Taverna P, Hogan JN, Ballinger MD, Flanagan WM, Fucini RV. The Aurora kinase inhibitor SNS-314 shows broad therapeutic potential with chemotherapeutics and synergy with microtubule-targeted agents in a colon carcinoma model. Mol Cancer Ther. 2009;8(4):930–9. [DOI] [PubMed] [Google Scholar]
- 33.Carter BZ, Mak PY, Tao W, Warmoes M, Lorenzi PL, Mak D, et al. Targeting MCL-1 dysregulates cell metabolism and leukemia-stroma interactions and resensitizes acute myeloid leukemia to BCL-2 inhibition. Haematologica. 2022;107(1):58–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamatani K, Ai T, Saito K, Suzuki K, Hori A, Kinjo S, et al. Inhibition of BCL2A1 by STAT5 inactivation overcomes resistance to targeted therapies of FLT3-ITD/D835 mutant AML. Transl Oncol. 2022;18:101354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh Mali R, Zhang Q, DeFilippis RA, Cavazos A, Kuruvilla VM, Raman J, et al. Venetoclax combines synergistically with FLT3 inhibition to effectively target leukemic cells in FLT3-ITD + acute myeloid leukemia models. Haematologica. 2021;106(4):1034–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luskin MR, DeAngelo DJ. Midostaurin/PKC412 for the treatment of newly diagnosed FLT3 mutation-positive acute myeloid leukemia. Expert Rev Hematol. 2017;10(12):1033–45. [DOI] [PubMed] [Google Scholar]
- 37.Schmetzer HM, Gerhartz HH. Acute myeloid leukemia (AML) can be oligoclonal. Leukemia. 1993;7(12):1965–70. [PubMed] [Google Scholar]
- 38.Schuringa JJ, Bonifer C. Dissecting Clonal Heterogeneity in AML. Cancer Cell. 2020;38(6):782–4. [DOI] [PubMed] [Google Scholar]
- 39.Kerkhoff E, Houben R, Lofler S, Troppmair J, Lee JE, Rapp UR. Regulation of c-myc expression by Ras/Raf signalling. Oncogene. 1998;16(2):211–6. [DOI] [PubMed] [Google Scholar]
- 40.Eriksson A, Kalushkova A, Jarvius M, Hilhorst R, Rickardson L, Kultima HG, et al. AKN-028 induces cell cycle arrest, downregulation of Myc associated genes and dose dependent reduction of tyrosine kinase activity in acute myeloid leukemia. Biochem Pharmacol. 2014;87(2):284–91. [DOI] [PubMed] [Google Scholar]
- 41.Meyers RM, Bryan JG, McFarland JM, Weir BA, Sizemore AE, Xu H, et al. Computational correction of copy number effect improves specificity of CRISPR-Cas9 essentiality screens in cancer cells. Nat Genet. 2017;49(12):1779–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eckerdt F, Yuan J, Strebhardt K. Polo-like kinases and oncogenesis. Oncogene. 2005;24(2):267–76. [DOI] [PubMed] [Google Scholar]
- 43.Nigg EA. Polo-like kinases: positive regulators of cell division from start to finish. Curr Opin Cell Biol. 1998;10(6):776–83. [DOI] [PubMed] [Google Scholar]
- 44.Dai W, Liu T, Wang Q, Rao CV, Reddy BS. Down-regulation of PLK3 gene expression by types and amount of dietary fat in rat colon tumors. Int J Oncol. 2002;20(1):121–6. [PubMed] [Google Scholar]
- 45.Gruneberg U, Neef R, Honda R, Nigg EA, Barr FA. Relocation of Aurora B from centromeres to the central spindle at the metaphase to anaphase transition requires MKlp2. J Cell Biol. 2004;166(2):167–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sparta AM, Bressanin D, Chiarini F, Lonetti A, Cappellini A, Evangelisti C, et al. Therapeutic targeting of Polo-like kinase-1 and Aurora kinases in T-cell acute lymphoblastic leukemia. Cell Cycle. 2014;13(14):2237–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Furukawa Y, Vu HA, Akutsu M, Odgerel T, Izumi T, Tsunoda S, et al. Divergent cytotoxic effects of PKC412 in combination with conventional antileukemic agents in FLT3 mutation-positive versus - negative leukemia cell lines. Leukemia. 2007;21(5):1005–14. [DOI] [PubMed] [Google Scholar]
- 48.Kawai M, Nakashima A, Kamada S, Kikkawa U. Midostaurin preferentially attenuates proliferation of triple-negative breast cancer cell lines through inhibition of Aurora kinase family. J Biomed Sci. 2015;22:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Manley PW, Caravatti G, Furet P, Roesel J, Tran P, Wagner T, et al. Comparison of the Kinase Profile of Midostaurin (Rydapt) with That of Its Predominant Metabolites and the Potential Relevance of Some Newly Identified Targets to Leukemia Therapy. Biochemistry. 2018;57(38):5576–90. [DOI] [PubMed] [Google Scholar]
- 50.Zhang W, Yu G, Zhang H, Basyal M, Ly C, Yuan B et al. Concomitant targeting of FLT3 and BTK overcomes FLT3 inhibitor resistance in acute myeloid leukemia through inhibition of autophagy.Haematologica. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guerra VA, DiNardo C, Konopleva M. Venetoclax-based therapies for acute myeloid leukemia. Best Pract Res Clin Haematol. 2019;32(2):145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maiti A, DiNardo CD, Daver NG, Rausch CR, Ravandi F, Kadia TM, et al. Triplet therapy with venetoclax, FLT3 inhibitor and decitabine for FLT3-mutated acute myeloid leukemia. Blood Cancer J. 2021;11(2):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bose P, Gandhi V, Konopleva M. Pathways and mechanisms of venetoclax resistance. Leuk Lymphoma. 2017;58(9):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Konopleva M, Contractor R, Tsao T, Samudio I, Ruvolo PP, Kitada S, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10(5):375–88. [DOI] [PubMed] [Google Scholar]
- 55.McMahon CM, Ferng T, Canaani J, Wang ES, Morrissette JJD, Eastburn DJ, et al. Clonal Selection with RAS Pathway Activation Mediates Secondary Clinical Resistance to Selective FLT3 Inhibition in Acute Myeloid Leukemia. Cancer Discov. 2019;9(8):1050–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Q, Riley-Gillis B, Han L, Jia Y, Lodi A, Zhang H, et al. Activation of RAS/MAPK pathway confers MCL-1 mediated acquired resistance to BCL-2 inhibitor venetoclax in acute myeloid leukemia. Signal Transduct Target Ther. 2022;7(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.ten Cate B, Samplonius DF, Bijma T, de Leij LF, Helfrich W, Bremer E. The histone deacetylase inhibitor valproic acid potently augments gemtuzumab ozogamicin-induced apoptosis in acute myeloid leukemic cells. Leukemia. 2007;21(2):248–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, [M.A.] upon reasonable request.