Abstract
Objective
To estimate the prevalence of chronic kidney disease (CKD) among patients with type 2 diabetes mellitus (T2DM) and determine the sociodemographic and clinical risk factors associated with CKD.
Design and settings
Cross-sectional study among diabetic outpatients of a tertiary hospital in Nepal.
Participants
201 patients with T2DM above 18 years of age.
Intervention
Participants completed a questionnaire regarding their socioeconomic information and underwent pertinent physical and haematological examinations.
Primary and secondary outcomes measure
The prevalence and risk factors of CKD among patients with T2DM.
Results
The prevalence of CKD in T2DM was 86.6%. In univariable analysis, the variables like age (p=0.026), hypertension status (p=0.002), duration of diabetes (p=0.009) and haemoglobin levels (p=0.027) were significantly associated with CKD among the participants with T2DM. Kruskal-Wallis H test showed that age was significantly different between various CKD stages. Multivariate analysis demonstrated a significant relationship between CKD with age (Adjusted odds ratio (AOR) 3, 95% CI 1.1 to 8.8) and literacy status (AOR 5.8, 95% CI 1.4 to 24.6)
Conclusion
Advancing age, concomitant hypertension, increasing duration of T2DM and presence of anaemia were found to be important risk factors of CKD. Age is the most important predictor of CKD showing increasing prevalence in the elderly population. Periodic screening tests are essential at an early age to identify kidney diseases at incipient stages, thereby preventing progression to end-stage renal disease.
Keywords: internal medicine, nephrology, diabetes & endocrinology
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This is one of the very few studies that has staged chronic kidney disease (CKD) in the diabetic population based on estimated glomerular filtration rate.
Multivariate logistic regression following univariate analysis helped better predict the association of risk factors and CKD.
This study did not incorporate albuminuria which is indeed an important early predictor of renal damage.
Unlike prospective studies, this study being a cross-sectional type, could not study the causal relationship of risk factors with CKD.
The failure of inclusion of other comorbidities and systemic conditions has led to confounding bias in the association of CKD and anaemia.
Introduction
Diabetes mellitus (DM) is a global health burden of the 21st century with rapidly increasing prevalence.1 Type 2 DM (T2DM) accounts for 90% of overall diabetes.2 Diabetic nephropathy is a major microvascular complication accounting for 40% of patients with T2DM.3 DM is a leading cause of chronic kidney disease (CKD).3 The presence of albuminuria and a decrease in glomerular filtration rate (GFR) are the key clinical markers of any pathological damage to the kidney. An estimated GFR (eGFR) of less than 60 mL/min per 1.73 m2 body surface area (BSA) on at least two occasions 3 months apart is an important diagnostic parameter of CKD.4 The spectrum of CKD, however, comprises of five stages corresponding to eGFR per 1.73 m² BSA: more than 90 mL/min (stage 1), 60–89 mL/min (stage 2), 30–44 mL/min (stage 3a), 45–59 mL/min (stage 3b), 15–29 mL/min (stage 4) and less than 15 mL/min (stage 5).5 This staging is based on the concept that renal impairment is a gradual process and hyperfiltration is an early marker of kidney injury.6
With the progression of different stages of CKD, there is a significant increase in the risk of complications including cardiovascular diseases, anaemia, metabolic bone disease and electrolyte disturbances.7 Considering the high morbidity and mortality due to CKD and its complications, the government of Nepal under the ‘Impoverished Citizens Service Scheme’ of social health security section has the provision of free dialysis service along with additional financial aids pretransplant and post-transplant.8
Routine screening with serum creatinine and urinary albumin level helps in its identification at an early stage and assists to take essential measures in preventing the further progression of renal impairment toward end stage renal disease (stages 4 and 5). Patients with DM are found to be nearly twice more likely to develop CKD than non-diabetic patients, with the OR varying between 1.3 and 4.6 globally.9 Early recognition of renal impairment in the diabetic population helps policy makers and health practitioners to prepare and implement the screening guidelines and management protocols accordingly. Although there have been some studies regarding the prevalence of CKD in Nepal, there is a paucity of research that has studied the risk factors of CKD in the diabetic population and the association of those factors with the eGFR level. This study was conducted with the aim to estimate the prevalence of CKD in patients with T2DM and determine the associated risk factors thereof.
Methodology
Study design and population
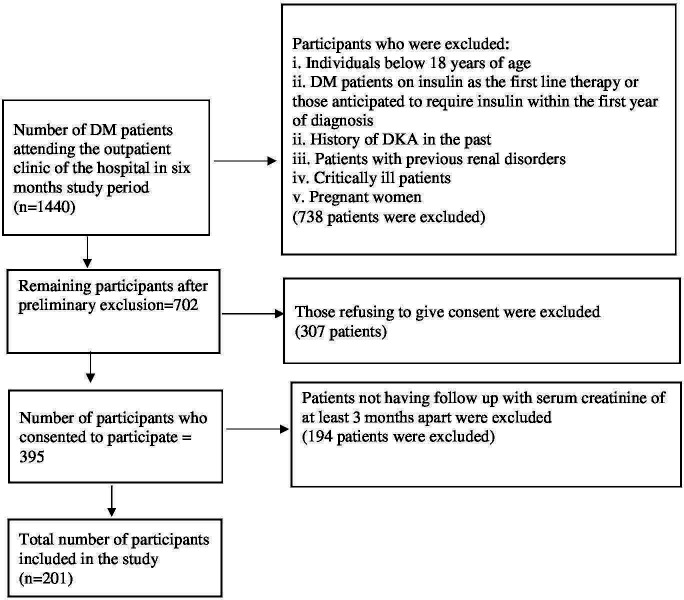
This cross-sectional study was conducted among patients attending the outpatient department of the Department of Internal Medicine of Shree Birendra Hospital, Kathmandu, Nepal from 1 November 2020 to 30 May 2021. Patients above 18 years of age on antidiabetes medications or recently diagnosed DM based on American Diabetes Association criteria10 were included in the study. The patients excluded in the study were patients with diabetes on insulin as the first-line treatment or those anticipated to require insulin within the first year of diagnosis, history of diabetic ketoacidosis any history suggestive of previous renal disorders or documented renal pathology, pregnant women and critically ill patients. A total of 201 patients were enrolled in our study based on such criteria (figure 1). This study was conducted in accordance with the guidelines of Strengthening of the Reporting of Observational Studies in Epidemiology.
Figure 1.
Participants characteristics for individuals on antidiabetes medications or recently diagnosed DM. DKA, diabetic ketoacidosis; DM, diabetes mellitus.
Data collection
Sociodemographic information from the participants was collected. Fasting blood sugar (FBS), 2 hours post prandial blood sugar (PPBS), glycated haemoglobin (HbA1c), haemoglobin and serum creatinine were recorded during the follow-up. Body weight was measured to the nearest 0.1 kilograms (kg) using a digital weighing machine (Nureca, New York, USA). The eGFR was calculated using the Cockcroft-Gault equation.11
(Where, X=1 for male and 0.85 for female)
Staging of renal disease was done based on eGFR as per National Kidney Foundation guidelines, New York, USA.5 During analysis of CKD with other parameters, only stages 3, 4 and 5 are considered as the presence of CKD while stages 1 and 2 were considered as absence of CKD, thereby aligning to the definition that CKD accounts to a reduction of eGFR less than 60 mL/min.4
Data analysis
Descriptive analyses were conducted and expressed as frequency while continuous data as mean±SD. The Kruskal-Wallis H test was used to assess the association of continuous variables like age, haemoglobin, HbA1c, FBS and PPBS with different stages of CKD. Post-hoc analysis was performed for findings that were significant in the Kruskal-Wallis H test to assess the group difference for each continuous variable. The differences in participants’ characteristics and risk factors for CKD were analysed using the χ2 test and unpaired t-test (two tailed) as applicable. The linearity of the continuous variables with the outcome was checked using the Box-Tidwell method after assuming the linear relationship between continuous predictors and natural log (log odds). The interactions of continuous predictors and their logs were included in the model. A deviation of >0.05 from the linearity for haemoglobin, HbA1c, FBS and PPBS was >0.05 was significant, suggesting the linear relationship between those parameters and CKD status. The multivariable logistic regression was then performed to estimate the unique relationship of the included variables with the CKD status. A p<0.05 was taken as statistically significant. Data were entered in Microsoft Excel 2019 V.16.0 (Microsoft, Washington, USA) and data analysis was done using Statistical Packages for Social Sciences V.21 (IBM SPSS).
Patient and public statement
Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Results
The average age of the study population was 58.9 years with most of the patients belonging the age greater than 60 years (53.3%), followed by 40–59 years (34.8%). Almost two-thirds of the patients (73.1%) had concomitant hypertension. More than half of the participants had DM of 11–15 years duration (53.7%). The mean FBS and PPBS values were 149.6±59.9 and 211.7±72.6 mg/dL, respectively. The mean eGFR was 33.9±28.2 mL/min per 1.73 m2 BSA ranging from 3.4 to 178.6 mL/min per 1.73 m2 BSA. This study demonstrated that 86.6% of patients with DM had CKD. Table 1 summarises the sociodemographic and clinical characteristics of study participants.
Table 1.
Background characteristics of the participants
| Characteristics* | Measures | |
| Frequency (percentage) | ||
| Gender | Female | 102 (50.7) |
| Male | 99 (49.3) | |
| Age (years) | <40 | 24 (11.9) |
| 40–59 | 70 (34.8) | |
| ≥60 | 107 (53.3) | |
| Education | Informal/Illiterate | 23 (11.4) |
| Literate | 178 (88.6) | |
| Occupation | Homemaker | 78 (38.8) |
| Job (government or private) | 21 (10.4) | |
| Farmer | 55 (27.4) | |
| Retired from job | 47 (23.4) | |
| Hypertension | No | 54 (26.9) |
| Yes | 147 (73.1) | |
| Family history of DN | No | 153 (76.1) |
| Yes | 48 (23.9) | |
| Duration of DM (years) | ≤5 years | 57 (28.4) |
| 6–10 years | 17 (8.5) | |
| 11–15 years | 108 (53.7) | |
| ≥16 years | 19 (9.5) | |
| Chronic kidney disease | No | 27 (13.4) |
| Yes | 174 (86.6) | |
| Mean±SD or (median (range)) | ||
| Age (years) | 58.9±13.9 | |
| Haemoglobin (g/dL) | 9.5±1.6 | |
| HbA1c (%) | 6.8±1.1 | |
| Fasting blood sugar (mg/dL) | 149.6±59.9 | |
| 2-hour post prandial sugar (mg/dL) | 211.7±72.6 | |
| eGFR (mL/min/1.73 m2) | 33.9±28.2 (25.3 (3.4–178.6)) | |
*Continuous characteristics are presented as mean±SD or median (range).
DM, diabetes mellitus; DN, Diabetic nephropathy; eGFR, estimated glomerular filtration rate; HbA1c, glycated haemoglobin.
Most of the participants belonged to stage 5 (30.8%), followed by stage 4 (23.4%) and stage 3a (16.91 %) (figure 2).
Figure 2.
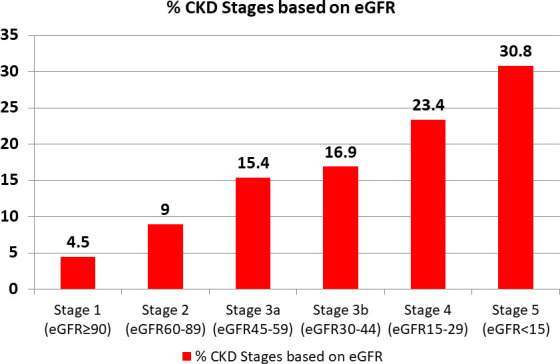
Frequency of different stages of chronic kidney disease (CKD) based on estimated glomerular filtration rate (eGFR) among participants with T2DM. T2DM, type 2 diabetes mellitus.
Age was found to be statistically significant among different CKD stages (p=0.003) using the Kruskal Wallis H test (table 2).
Table 2.
Clinical and background variables of each eGFR category
| Stage eGFR† | 5 eGFR <15 (n=62) | 4 eGFR 15–29 (n=47) | 3b eGFR 30–44 (n=34) | 3a eGFR 45–59 (n=31) | 2 eGFR 60–89 (n=18) | 1 eGFR ≥90 (n=9) | P value* |
| Variables | |||||||
| Age (years) | 55.1±14.4 | 63.7±15.4 | 63.1±12.5 | 60.6±10.5 | 54.4±9.5 | 49.4±12.4 | 0.003 |
| Hb (g/dL) | 9.0±1.8 | 9.6±1.1 | 9.8±1.6 | 9.6±1.9 | 10.0±1.1 | 9.8±1.2 | 0.085 |
| HbA1c % | 6.8±1.2 | 7.0±0.9 | 7.0±1.1 | 6.5±0.5 | 6.6±0.8 | 6.3±0.7 | 0.138 |
| FBS (mg/dL) | 135.2±39.2 | 161.3±88.7 | 160.3±48.2 | 140.4±51.1 | 146.2±39.8 | 185.9±72.8 | 0.104 |
| 2-hour PPBS (mg/dL) | 195.8±65.6 | 215.4±70.7 | 246.0±85.3 | 205.7±73.7 | 208.1±62.3 | 201.3±72.5 | 0.080 |
*Kruskal-Wallis H test.
†eGFR in mL/min per 1.73 m2 BSA.
BSA, body surface area; eGFR, estimated glomerular filtration rate; FBS, fasting blood sugar; Hb, haemoglobin; HbA1c, glycated haemoglobin; PPBS, post prandial blood sugar.
In univariable analysis, the variables like age (p=0.026), hypertension status (p=0.002), duration of diabetes (p=0.009) and haemoglobin levels (p=0.027) were significantly associated with CKD among the participants with T2DM.
The multivariable regression model was conducted to assess factors associated with CKD. The DM participants with age 60 or more years had three times high odds of CKD (AOR 3, 95% CI 1.1 to 8.8, adjusted p=0.042) than those of less than 60 years of age. The literate participants had five times higher odds of CKD (AOR 5.8, 95% CI 1.4 to 24.6, adjusted p=0.018) than those illiterate participants. Low haemoglobin level (AOR 0.8, 95% CI 0.6 to 1.1, adjusted p=0.194) was associated with increased odds of CKD but was not statistically significant (table 3).
Table 3.
Comparison between participants with chronic kidney disease and preserved eGFR (n=201)
| Variables | Chronic kidney disease (CKD) | Multivariable results | ||||
| No (%) | Yes (%) | P value * | Adjusted P value |
AOR (95% CI) | ||
| Gender | Female | 14 (13.7) | 88 (86.3) | 0.902 | 1 (Ref.) | |
| Male | 13 (13.1) | 86 (86.9) | 0.329 | 0.6 (0.2 to 1.7) | ||
| Age (years) | <60 | 18 (19.1) | 76 (80.9) | 0.026 | 1 (Ref.) | |
| ≥60 | 9 (8.4) | 98 (91.6) | 0.042 | 3.0 (1.1 to 8.8) | ||
| Education | Illiterate | 6 (26.1) | 17 (73.9) | 0.095 | 1 (Ref.) | |
| Literate | 21 (11.8) | 157 (88.2) | 0.018 | 5.8 (1.4 to 24.6) | ||
| Occupation | Homemaker and/or retired | 17 (13.6) | 108 (86.4) | 0.929 | 1 (Ref.) | |
| All other† | 10 (13.2) | 66 (86.8) | 0.696 | 0.8 (0.3 to 2.3) | ||
| Hypertension | No | 14 (25.9) | 40 (74.1) | 0.002 | 1 (Ref.) | |
| Yes | 13 (8.8) | 134 (91.2) | 0.200 | 1.9 (0.7 to 5.3) | ||
| Family history of CKD | No | 24 (15.7) | 129 (84.3) | 0.094 | 1 (Ref.) | |
| Yes | 3 (6.3) | 45 (93.8) | 0.249 | 2.4 (0.5 to 11.0) | ||
| Duration of DM | ≤10 years | 16 (21.6) | 58 (78.4) | 0.009 | 1 (Ref.) | |
| ≥11 years | 11 (8.7) | 116 (91.3) | 0.181 | 2.0 (0.7 to 5.3) | ||
| Haemoglobin (g/dL) | 10.0±1.1 | 9.4±1.6 | 0.027 | 0.194 | 0.8 (0.6 to 1.1) | |
| HbA1c (%) | 6.5±0.7 | 6.8±1.0 | 0.086 | 0.470 | 1.3 (0.6 to 2.7) | |
| Fasting blood sugar (mg/dL) | 159.4±55.0 | 148.1±60.6 | 0.362 | 0.112 | 0.9 (0.9 to 1.0) | |
| 2-hour post prandial sugar (mg/dL | 205.8±64.6 | 212.7±73.9 | 0.649 | 0.613 | 1.0 (0.9 to 1.0) | |
*Χ2 test and independent t-test.
†All other: job and farmer.
AOR, Adjusted odds ratio; DM, diabetes mellitus; HbA1c, glycated haemoglobin.
Discussion
The prevalence of CKD in T2DM was 81.6% in this study. There has been a wide range of variations (23.6%–83.7%) in the prevalence of CKD in T2DM in other studies.12–14 The variation in prevalence rate could be due to differences in the definitions adopted and variability in health-seeking behaviour among people of different places. Most of the patients in our study belonged to stages 3, 4 and 5 and very few patients were in stages 1 and 2 of CKD. This is in sheer contrast with a similar study conducted in Tanzania where around two-thirds of the patients were in stages 1 and 2 and less than one-third belonged to stages 3 and above.13 In lower-income and middle-income countries (LMICs) such as Nepal, asymptomatic people rarely go for a routine health check-up and patients visit the hospital only after developing severe symptoms or complications. Although the complications of T2DM take a few years to develop, often those complications are identified only at the time of diagnosis. Therefore, delayed hospital presentation in DM explains the higher prevalence of CKD. Thus, the current guidelines recommend at least annual screening for possible nephropathy after the diagnosis of T2DM.15
Advancing age was a risk factor for the progression of CKD in our study, the prevalence being greater in diabetics above 60 years of age. A study done among patients with CKD in a tertiary hospital in Nepal showed that almost half of the patients were above 58 years of age.16 The significant relationship of age with CKD can be explained by the age-related loss of renal mass and steady decline in GFR with ageing.17 The other reason for this association can be due to the fact that older people are at a greater risk of comorbidities such as diabetes, hypertension and obesity.18 Study among disease-matched subjects is essential to assess the independent association of age with CKD. Though there was no gender association in the prevalence of CKD in our study, previous literature suggests that the prevalence of CKD is higher in males than in females.15 16 Renal function is thought to decline more rapidly in men than women due to the damaging effects of testosterone and protective effects of oestrogen.19 20 This study showed that literate people were at a higher risk of developing CKD. This finding is quite unusual as education is usually linked to better health literacy, healthier lifestyle and better compliance with various treatment modalities. However, increased comparative prevalence among literate people could also be higher detection rates among them due to better health seeking behaviour as compared with illiterate ones where the disease remains masked.
Patients with diabetes with concomitant hypertension were found to be twice more likely to develop CKD than non-hypertensive patients. Hypertension is not only a cause but also a consequence of CKD.21 High systolic blood pressure is considered a better predictor of renal damage than high diastolic blood pressure.22 ACE inhibitors are considered the first-line therapy for the treatment of hypertension in diabetics as they help optimise blood pressure as well as reduce proteinuria.23
Our study showed that the probability of progression to CKD increases with the duration of diabetes. Previous studies also suggest that the duration of diabetes is an independent risk factor for the progression of diabetic kidney disease.24 25 Prolonged duration of diabetes is associated with the accumulation of advanced glycation end products, which indeed are implicated in the pathophysiology of diabetic nephropathy as well as other microvascular complications.26
Decreased haemoglobin level was found to be associated with CKD in this study. Anaemia is common in CKD and can be both a cause and consequence of CKD.27 Previous studies show a linear relationship between a fall in haemoglobin and eGFR in CKD.28 This highlights the importance of screening for haemoglobin levels in the follow-up visit in patients with diabetes for the early address with the appropriate treatment strategy.
None of the markers of glycaemic control including HbA1c, FBS and PPBS directly correlated with eGFR levels in this study. The development of microvascular complications including diabetic nephropathy is thought to be related to glycaemic control.29 30 HbA1c level reflects glycaemic control of the preceding 3 months and is minimally affected by diurnal variation, acute changes in diet and physical activity.31 Most of the previous studies show that higher mean HbA1c and higher HbA1c variability are associated with a higher prevalence of CKD in patients with T2DM14 32 However, a similar study conducted among the adult Palestinian diabetic population reports no association of CKD with glycaemic markers including HbA1c.12
The prevalence and progression of CKD are associated with multiple factors. It is important to assess and control the modifiable risk factors at an early stage to prevent nephropathy and its progression from stage 1 to ESRD.29
Although there have been many studies on diabetic kidney diseases and diabetic nephropathy in Nepal, most of them are based on the presence of albuminuria; there are very few studies which have actually staged CKD based on eGFR specifically in diabetic population. Rigorous statistic tools have been used in our study. Multivariate regression was used after univariate analysis. This helped to adjust for the potential bias and better predict the risk factors of CKD in the study population. Kidney Disease Improving Global Outcomes has staged CKD based on eGFR and albuminuria. While we have included eGFR, we have failed to incorporate albuminuria which is one of the major shortcomings of this study. The failure of inclusion of other comorbidities could have led to potential confounding bias in the association of CKD with other risk factors. Although we have been able to identify and estimate the risks associated with CKD, the cause-and-effect relationship cannot be fully studied unlike cohort studies. Our study has shown that there is an association between hypertension and CKD, but whether hypertension is a cause or an effect of CKD cannot be ascertained by this study.
Conclusion
The prevalence of CKD is high among the diabetic population in Nepal. Advancing age, illiteracy, concomitant hypertension, increasing duration of diabetes and the presence of anaemia were found to be important risk factors. Age is the most important predictor of CKD, with an increasing prevalence in the elderly population. It is strongly recommended that all countries, including LMICs such as Nepal, must implement a guideline for periodic renal assessment for early detection and management of renal complications of DM. This, overall, helps decrease the global health burden due to such chronic diseases.
Supplementary Material
Acknowledgments
We are grateful to the staff of the Department of Medicine of Shree Birendra Hospital. We acknowledge all the participants for their voluntary involvement in this study.
Footnotes
Twitter: @drgopalr, @SRKBroNepal
Contributors: RJ and SK designed the study and all authors contributed to questionnaire development. PS, GKY and RJ contributed to the experimental study, data collection and data curation. GKY contributed to the statistical analysis. KA, SR, TR and PS conducted literature search. RJ and PS prepared the manuscript with contributions from KA and SR. SK and TR reviewed the manuscript. SK is responsible for the overall content as guarantor. The corresponding author attests that all listed authors meet ICMJE authorship criteria and that no others meeting the criteria have been omitted.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available on reasonable request.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
Ethics approval
The study was approved by the Institutional Review Board of the Nepalese Army Institute of Health Sciences (Reg. No. 352) and abided by the Declaration of Helsinki. All the participants were explained the purpose of the study and its associated risks and benefits. They were assured that their participation was entirely voluntary and that they could withdraw at any point of time of the study. Only those patients who were willing and consented to participate were enrolled.
References
- 1.Standl E, Khunti K, Hansen TB, et al. The global epidemics of diabetes in the 21st century: current situation and perspectives. Eur J Prev Cardiol 2019;26:7–14. 10.1177/2047487319881021 [DOI] [PubMed] [Google Scholar]
- 2.Selby NM, Taal MW. An updated overview of diabetic nephropathy: diagnosis, prognosis, treatment goals and latest guidelines. Diabetes Obes Metab 2020;22:3–15. 10.1111/dom.14007 [DOI] [PubMed] [Google Scholar]
- 3.Noble R, Taal MW. Epidemiology and causes of chronic kidney disease. Medicine 2019;47:562–6. 10.1016/j.mpmed.2019.06.010 [DOI] [Google Scholar]
- 4.Vaidya SR, Aeddula NR. Chronic renal failure. In: Statpearls [online]. Treasure Island, FL: StatPearls Publishing, 2022. [Google Scholar]
- 5.National Kidney Foundation . How to classify CKD [internet]. 2015. Available: https://www.kidney.org/professionals/explore-your-knowledge/how-to-classify-ckd
- 6.Palatini P. Glomerular hyperfiltration: a marker of early renal damage in pre-diabetes and pre-hypertension. Nephrol Dial Transplant 2012;27:1708–14. 10.1093/ndt/gfs037 [DOI] [PubMed] [Google Scholar]
- 7.Thomas R, Kanso A, Sedor JR. Chronic kidney disease and its complications. Prim Care 2008;35:329–44, 10.1016/j.pop.2008.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Annual report FY 2077/78 (2020/21) [online]. n.d. Available: https://dohs.gov.np/annual-report-fy-2077-78-2019-20/
- 9.Koye DN, Magliano DJ, Nelson RG, et al. The global epidemiology of diabetes and kidney disease. Adv Chronic Kidney Dis 2018;25:121–32. 10.1053/j.ackd.2017.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diagnosis | ADA [online]. 2022. Available: https://www.diabetes.org/diabetes/a1c/diagnosis
- 11.Ferreira JP, Girerd N, Pellicori P, et al. Renal function estimation and cockroft-gault formulas for predicting cardiovascular mortality in population-based, cardiovascular risk, heart failure and post-myocardial infarction cohorts: the heart “ omics ” in ageing (homage) and the high-risk myocardial infarction database initiatives. BMC Med 2016;14:181. 10.1186/s12916-016-0731-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nazzal Z, Hamdan Z, Masri D, et al. Prevalence and risk factors of chronic kidney disease among Palestinian type 2 diabetic patients: a cross-sectional study. BMC Nephrol 2020;21:484. 10.1186/s12882-020-02138-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janmohamed MN, Kalluvya SE, Mueller A, et al. Prevalence of chronic kidney disease in diabetic adult out-patients in Tanzania. BMC Nephrol 2013;14:183. 10.1186/1471-2369-14-183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Low SK, Sum CF, Yeoh LY, et al. Prevalence of chronic kidney disease in adults with type 2 diabetes mellitus. Ann Acad Med Singap 2015;44:164–71. 10.47102/annals-acadmedsg.V44N5p164 [DOI] [PubMed] [Google Scholar]
- 15.Bakris GL. Recognition, pathogenesis, and treatment of different stages of nephropathy in patients with type 2 diabetes mellitus. Mayo Clin Proc 2011;86:444–56. 10.4065/mcp.2010.0713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pakhrin S, Shrestha S, Karki R, et al. Drug dosage adjustment of chronic kidney disease patients at nephrology ward in tertiary care hospital of Nepal. Europasian J Med Sci 2020;2:41–50. 10.46405/ejms.v2i1.39 [DOI] [Google Scholar]
- 17.Weinstein JR, Anderson S. The aging kidney: physiological changes. Adv Chronic Kidney Dis 2010;17:302–7. 10.1053/j.ackd.2010.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stevens LA, Viswanathan G, Weiner DE. Chronic kidney disease and end-stage renal disease in the elderly population: current prevalence, future projections, and clinical significance. Adv Chronic Kidney Dis 2010;17:293–301. 10.1053/j.ackd.2010.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carrero JJ, Hecking M, Chesnaye NC, et al. Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat Rev Nephrol 2018;14:151–64. 10.1038/nrneph.2017.181 [DOI] [PubMed] [Google Scholar]
- 20.Chudek J, Wieczorowska-Tobis K, Zejda J, et al. The prevalence of chronic kidney disease and its relation to socioeconomic conditions in an elderly Polish population: results from the National population-based study polsenior. Nephrol Dial Transplant 2014;29:1073–82. 10.1093/ndt/gft351 [DOI] [PubMed] [Google Scholar]
- 21.Hall ME, do Carmo JM, da Silva AA, et al. Obesity, hypertension, and chronic kidney disease. Int J Nephrol Renovasc Dis 2014;7:75–88. 10.2147/IJNRD.S39739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bakris GL, Weir MR, Shanifar S, et al. Effects of blood pressure level on progression of diabetic nephropathy: results from the RENAAL study. Arch Intern Med 2003;163:1555–65. 10.1001/archinte.163.13.1555 [DOI] [PubMed] [Google Scholar]
- 23.Strippoli GFM, Bonifati C, Craig M, et al. Angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists for preventing the progression of diabetic kidney disease. Cochrane Database Syst Rev 2006;2006:CD006257. 10.1002/14651858.CD006257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alrawahi AH, Rizvi SGA, Al-Riyami D, et al. Prevalence and risk factors of diabetic nephropathy in Omani type 2 diabetics in al-dakhiliyah region. Oman Med J 2012;27:212–6. 10.5001/omj.2012.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Unnikrishnan RI, Rema M, Pradeepa R, et al. Prevalence and risk factors of diabetic nephropathy in an urban South Indian population: the Chennai urban rural epidemiology study (cures 45). Diabetes Care 2007;30:2019–24. 10.2337/dc06-2554 [DOI] [PubMed] [Google Scholar]
- 26.Busch M, Franke S, Rüster C, et al. Advanced glycation end-products and the kidney. Eur J Clin Invest 2010;40:742–55. 10.1111/j.1365-2362.2010.02317.x [DOI] [PubMed] [Google Scholar]
- 27.Mehdi U, Toto RD. Anemia, diabetes, and chronic kidney disease. Diabetes Care 2009;32:1320–6. 10.2337/dc08-0779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.New JP, Aung T, Baker PG, et al. The high prevalence of unrecognized anaemia in patients with diabetes and chronic kidney disease: a population-based study. Diabet Med 2008;25:564–9. 10.1111/j.1464-5491.2008.02424.x [DOI] [PubMed] [Google Scholar]
- 29.Faselis C, Katsimardou A, Imprialos K, et al. Microvascular complications of type 2 diabetes mellitus. Curr Vasc Pharmacol 2020;18:117–24. 10.2174/1570161117666190502103733 [DOI] [PubMed] [Google Scholar]
- 30.Kawazu S, Tomono S, Shimizu M, et al. The relationship between early diabetic nephropathy and control of plasma glucose in non-insulin-dependent diabetes mellitus. The effect of glycemic control on the development and progression of diabetic nephropathy in an 8-year follow-up study. J Diabetes Complications 1994;8:13–7. 10.1016/1056-8727(94)90005-1 [DOI] [PubMed] [Google Scholar]
- 31.Speeckaert M, Van Biesen W, Delanghe J, et al. Are there better alternatives than haemoglobin A1c to estimate glycaemic control in the chronic kidney disease population? Nephrol Dial Transplant 2014;29:2167–77. 10.1093/ndt/gfu006 [DOI] [PubMed] [Google Scholar]
- 32.Luk AOY, Ma RCW, Lau ESH, et al. Risk association of HbA1c variability with chronic kidney disease and cardiovascular disease in type 2 diabetes: prospective analysis of the Hong Kong diabetes registry. Diabetes Metab Res Rev 2013;29:384–90. 10.1002/dmrr.2404 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on reasonable request.