ABSTRACT
The phagolysosome is an antimicrobial and degradative organelle that plays key roles in macrophage-mediated inflammatory and homeostatic functions. Whereas mature phagolysosomes are known to sequester and degrade their contents into basic nutrients, they were not previously assigned an active role in amplifying inflammation. We have described a novel macrophage process in which partially digested immunostimulatory PAMPs are released extracellularly from the mature phagolysosome via discrete events we term eructophagy. Eructophagy is induced by proinflammatory stimuli, negatively regulated by IL4 and MTOR, and is dependent on key autophagy proteins, including fusion machinery of degradative and secretory autophagy. We propose that macrophages use eructophagy to release processed PAMPs/DAMPs to amplify local inflammation.
KEYWORDS: Autophagy, eructophagy, inflammation, macrophage, PAMPs, phagolysosome, phagosome, secretion
Macrophages are integral to the innate immune response by coordinating inflammation through communication with other leukocytes, and by removing microbes, debris, and dying cells via phagocytosis. Following phagocytosis, the phagosome matures into the antimicrobial and degradative hybrid organelle named the phagolysosome. While it is well established that protein antigens can be processed into linear oligopeptides of suitable length to be presented as T cell antigens, little attention has been paid to the potential for phagosomes to process engulfed PAMPs and DAMPs. However, similar to T cell antigens, many PAMPs and DAMPs in their native macromolecular complexes are not immunostimulatory or freely diffusible, and must be partially digested to activate the immune system optimally. While the phagolysosome has the hydrolytic machinery to partially digest potential PAMPs and DAMPs into more stimulatory forms, without a mechanism for their release, phagocytosed PAMPs and DAMPs would be sequestered in the terminal phagolysosomes until they are completely digested and recycled as inert primary building blocks. We recently described a process whereby proinflammatory macrophages can release partially digested material directly from the phagolysosome to the extracellular space through discrete, controlled events [1]. Moreover, we demonstrated that phagosomally processed PAMPs released from macrophages through this process can activate local immune cells in a paracrine fashion. Because particulate material is retained within the phagolysosome during the release of soluble material, we have named this process eructophagy – derived from eructare (Latin: to burp or belch), and the suffix -phagy to acknowledge the connection with phagocytosis and autophagy.
Eructophagy was observed during a study of phagolysosome heterogeneity using experimental particles labeled with proteolysis and pH reporters. Loss of soluble products of digestion is observed in some phagolysosomes, corresponding to temporary neutralization of lumenal pH. Following the events, phagolysosomes quickly reacidify and resume proteolysis within minutes. As we observed no cytosolic fluorescence increase, we hypothesized the phagolysosomes release the fluorescent peptides generated by phagosomal proteolysis extracellularly. We developed a live-imaging assay to test this hypothesis, which we later adopted as the gold standard for detecting eructophagy. In brief, after phagocytosis of cellulase-conjugated particles, macrophages are exposed to a cell-impermeable cellulase substrate that cannot interact with cellulase without bidirectional exchange between mature phagolysosomes and the extracellular environment. Fluorescence is generated when cellulase cleaves its substrate, and, indeed, we observe localized fluorescent events indicative of extracellular communication.
After first describing eructophagy in bone marrow-derived murine macrophages, we investigated whether the process occurs in other phagocytic cell types. Eructophagy can be readily observed in monocyte-derived human macrophages, murine macrophages derived from conditionally immortalized myeloid precursors, and to a lesser degree in bone marrow-derived dendritic cells. Eructophagy is not detected in traditional phagocyte-like cell lines including RAW264.7, J774, THP-1, and DC2.4. Despite being most prevalent in macrophages, eructophagy is relatively infrequent in unstimulated macrophages. We found that classically activating macrophages with IFNG/IFN-γ (interferon gamma) significantly increases eructophagy, but alternatively activating them to a tissue-reparative phenotype with IL4 essentially abolishes it (Figure 1). Because IFNG inhibits MTORC1 in macrophages, we investigated the relationship between eructophagy and MTOR. Pharmacological inhibition of MTOR increases eructophagy to a point where almost every phagolysosome goes through at least one event over a 5-h period post-phagocytosis. Conversely stimulation of MTOR with super-physiological levels of amino acids inhibits eructophagy almost completely.
Figure 1.
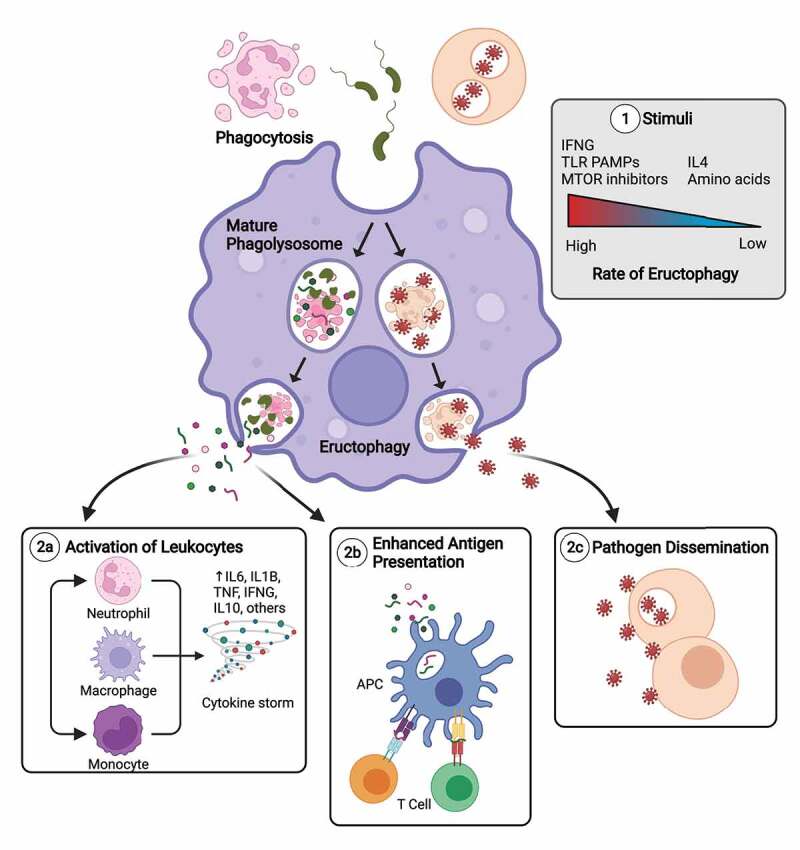
Induction of eructophagy in macrophages and its potential outcomes. To date we know that eructophagy is induced by proinflammatory stimuli, turned off by IL4, negatively regulated by MTOR, and is dependent on key autophagy proteins, including fusion machinery of degradative and secretory autophagy (1). It has been shown that eructophagy can lead to the release of phagosomally processed PAMPs and DAMPs that can activate vicinal cells (2a). However, because phagolysosomes can contain a variety of bioactive molecules and complexes, including hydrolases, antimicrobial peptides, exogenous and endogenous protein antigens, exosomes, and potentially pathogenicity factors, toxins, and infectious virions or prions, the impact of eructophagy on health and disease may be widespread, affecting antigen presentation and cross-presentation (2b), humoral immune responses, tissue damage, activation of protease-activated receptors, and the potential to enhance the spread or pathogenicity of certain pathogens (2c). APC, antigen-processing cell. This figure was created with Biorender.com.
We next examined the involvement of autophagy in eructophagy and found that deficiencies in key autophagic genes, notably Becn1, Atg7, Atg5, and the phosphatidylinositol 3-kinase complex (Pik3cg/Pi3kγ), reduce the level of eructophagy. Cybb/Nox2 and Rubcn/Rubicon genes, which are implicated in LC3-associated phagocytosis/LAP, do not alter rates of eructophagy, thus distinguishing these two processes. Eructophagy is, however, reduced by deficiencies in the membrane fusion complexes involved in degradative (STX17-SNAP29-VAMP8) and secretory autophagy (SEC22B-STX3/STX4-SNAP23/SNAP29). We are currently investigating whether this fusion machinery localizes directly to the phagolysosomal membrane for fusion with the plasma membrane or whether autophagosomes themselves are required for eructophagy. Interestingly, LC3 was observed to be robustly recruited to the phagolysosomal membrane during, but not preceding, eructophagy events. Nevertheless, we concluded that eructophagy proceeds via distinct fusion pathways and involves degradative and secretory autophagy machinery.
We further hypothesized that proinflammatory macrophages can use eructophagy to amplify local inflammation. Indeed, not only did we find that TLR (toll-like receptor) ligand PAMPs immediately upregulate eructophagy in macrophages, we demonstrated that macrophages themselves can activate nearby cells by releasing phagosomally processed PAMPs via eructophagy. Based on these findings, we propose that proinflammatory macrophages can process and redeploy hidden or complexed PAMPs and DAMPs as secondary messengers to amplify local inflammation through eructophagy. Conversely, in repair-focused environments, IL4-activated macrophages sequester potentially inflammatory cargoes within their phagolysosomes leading to complete digestion of ingested PAMPs and DAMPs into inert building blocks. While amplification of inflammatory responses can be advantageous in localized disease processes, it is not yet known whether dysregulated eructophagy can accelerate acute systemic inflammatory disorders such as systemic inflammatory response syndrome/SIRS and sepsis (Figure 1).
In conclusion, eructophagy is a newly discovered macrophage process that uses key autophagic machinery to transiently release soluble matter from phagolysosomes to the extracellular environment while retaining particulate cargo. Thus far, we have focused on the potential for eructophagy to propagate inflammation through the controlled release of processed PAMPs and DAMPs. However, we recognize that eructophagy would also mediate the release of a host of soluble factors found in phagolysosomes (Figure 1). While the study of eructophagy is still embryonic, its potential physiological significance and impact on inflammation and human disease may be profound.
Funding Statement
This work was supported by the Canadian Institutes of Health Research [152888]; Natural Sciences and Engineering Research Council of Canada [04147-2017].
Disclosure statement
No potential conflict of interest was reported by the author(s).
Reference
- [1].Greene CJ, Nguyen JA, Cheung SM, et al. Macrophages disseminate pathogen associated molecular patterns through the direct extracellular release of the soluble content of their phagolysosomes. Nat Commun. 2022;13(1):3072. [DOI] [PMC free article] [PubMed] [Google Scholar]


